Lactic Acid Production from Cow Manure: Experimental Process Conditions Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material (Cow Manure)
2.2. Cow Manure Analysis
2.3. Microorganism
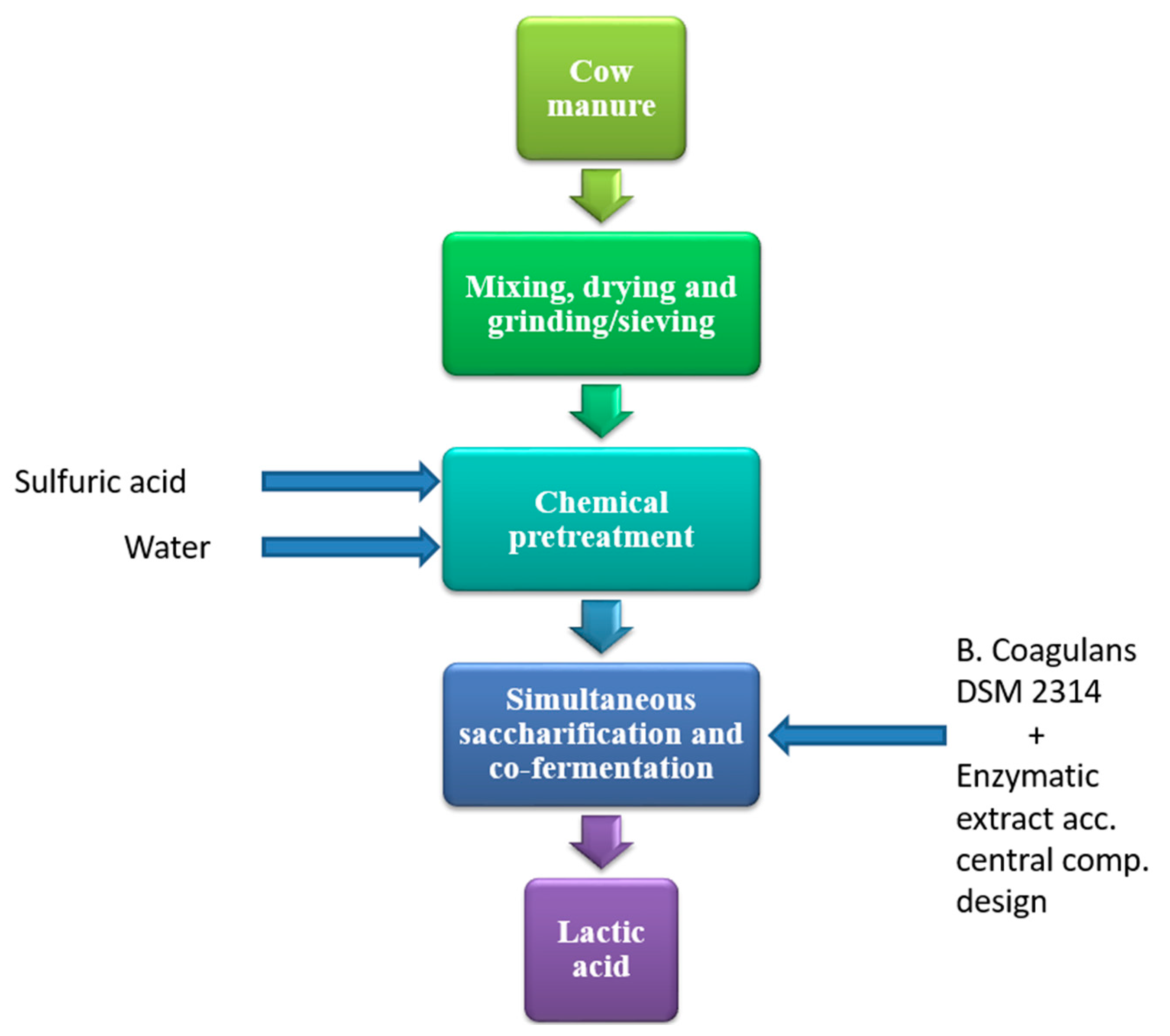
2.4. Process Flow
2.4.1. Experimental Design
2.4.2. Analysis of Monomeric Sugars, Lactic Acid, and Byproducts
2.5. Experimental Results Calculation
3. Results and Discussion
3.1. Results
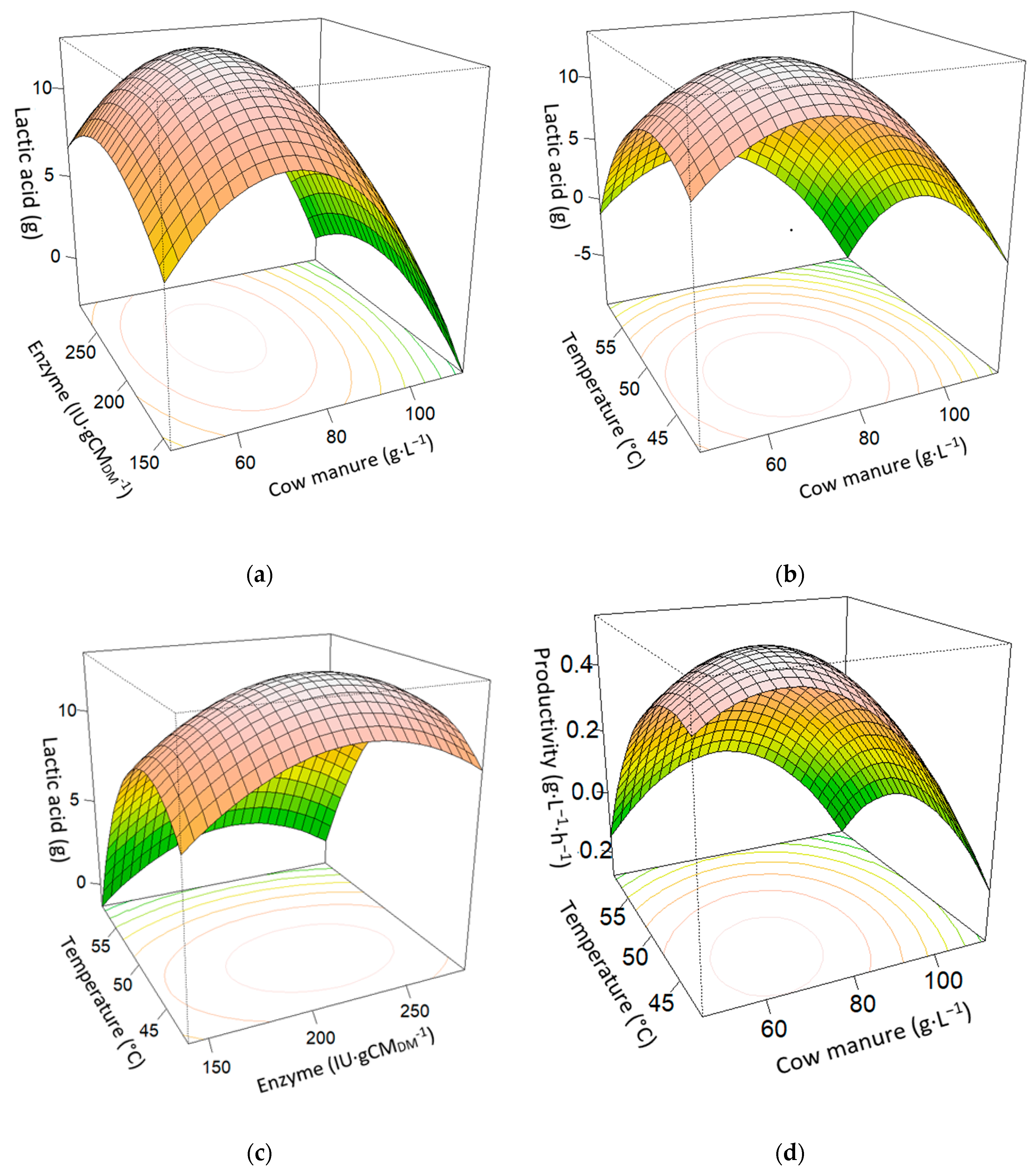
3.2. Statistical Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wee, Y.J.; Kim, J.N.; Ryu, H.W. Biotechnological production of lactic acid and its recent applications. Food Technol. Biotechnol. 2006, 44, 163–172. [Google Scholar]
- Datta, R.; Henry, M. Lactic acid: Recent advances in products, processes and technologies—A review. J. Chem. Technol. Biotechnol. 2006, 81, 1119–1129. [Google Scholar] [CrossRef]
- Datta, R. Technological and economic potential of poly(lactic acid) and lactic acid derivatives. FEMS Microbiol. Rev. 1995, 16, 221–231. Available online: https://www.academia.edu/25459908/Technological_and_economic_potential_of_poly_lactic_acid_and_lactic_acid_derivatives (accessed on 7 January 2021). [CrossRef]
- Timbuntam, W.; Sriroth, K.; Tokiwa, Y. Lactic acid production from sugar-cane juice by a newly isolated Lactobacillus sp. Biotechnol. Lett. 2006, 28, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Garrido, R.; Cabeza, L.F.; Falguera, V. An Overview of Bioplastic Research on Its Relation to National Policies. Sustainability 2021, 13, 7848. [Google Scholar] [CrossRef]
- Södegard, A.; Stolt, M. Properties of polylactic acid fiber based polymers and their correlation with composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, X.; Wang, Y.; Li, H.; Li, Z.; Zhou, L.; Qu, Y.; Li, Z.; Bao, X. Cow manure as a lignocellulosic substrate for fungal cellulase expression and bioethanol production. AMB Express 2018, 8, 190. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Zi, L.H.; Bai, F.W.; Lin, H.L.; Hao, X.M.; Yue, G.J.; Ho, N.W. Bioethanol from Lignocellulosic Biomass; Bai, F.-W., Liu, C.-G., Huang, H., Tsao, G.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 25–51. [Google Scholar]
- Maas, R.H.; Bakker, R.R.; Jansen, M.L.; Visser, D.; De Jong, E.; Eggink, G.; Weusthuis, R.A. Lactic acid production from lime-treated wheat straw by Bacillus coagulans: Neutralization of acid by fed-batch addition of alkaline substrate. Appl. Microbiol. Biotechnol. 2008, 78, 751–758. [Google Scholar] [CrossRef] [Green Version]
- van der Pol, E.C.; Eggink, G.; Weusthuis, R.A. Production of l(+)-lactic acid from acid pretreated sugarcane bagasse using Bacillus coagulans DSM2314 in a simultaneous saccharification and fermentation strategy. Biotechnol. Biofuels 2016, 9, 248. [Google Scholar] [CrossRef] [Green Version]
- van der Pol, E.C.; Bakker, R.R.; Baets, P.; Eggink, G. By-products resulting from lignocellulose pretreatment and their inhibitory effect on fermentations for (bio)chemicals and fuels. Appl. Microbiol. Biotechnol. 2014, 98, 9579–9593. [Google Scholar] [CrossRef]
- Garrido, R.; Cabeza, L.F.; Falguera, V.; Navarro, O.P. Potential Use of Cow Manure for Poly(Lactic Acid) Production. Sustainability 2022, 14, 16753. [Google Scholar] [CrossRef]
- Castro, Y.P. Aprovechamiento de Biomasa Lignocelulósica: Algunas Experiencias de Investigación en Colombia; UTadeo: Bogotá, Colombia, 2014. [Google Scholar]
- Miller, C.; Fosmer, A.; Rush, B.; McMullin, T.; Beacom, D.; Suominen, P. Industrial Production of Lactic Acid, 2nd ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2011; Volume 3. [Google Scholar]
- Singhvi, M.; Gokhale, D. Biomass to biodegradable polymer (PLA). RSC Adv. 2013, 3, 13558. [Google Scholar] [CrossRef]
- Van Der Pol, E.C. Development of a Lactic Acid Production Process Using Lignocellulosic Biomass as Feedstock. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2016. [Google Scholar]
- Sluiter, J.S.A.; Hames, B.; Ruiz, R.; Scarlata, C.; Templeton, D. Determination of Ash in Biomass, 2005th ed.; NREL/TP-510-42622; NREL: Golden, CO, USA, 2005; Available online: https://www.nrel.gov/docs/gen/fy08/42622.pdf (accessed on 3 January 2021).
- Sahito, A.R.; Mahar, R.; Siddiqui, Z.; Brohi, K.M. Estimating Calorific Values of Lignocellulosic Biomass from Volatile and Fixed Solids. Int. J. Biomass Renew. 2013, 2, 1–6. [Google Scholar]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Kalavathy, M.H.; Regupathi, I.; Pillai, M.G.; Miranda, L.R. Modelling, analysis and optimization of adsorption parameters for H3PO4 activated rubber wood sawdust using response surface methodology (RSM). Colloids Surfaces B Biointerfaces 2009, 70, 35–45. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022; Available online: https://www.r-project.org (accessed on 3 January 2021).
- Lenth, R.V. Response-Surface Methods in R, Using rsm. J. Stat. Softw. 2009, 32, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Fan, B.; Li, T.; Song, X.; Wu, C.; Qian, C. A rapid, accurate and sensitive method for determination of monosaccharides in different varieties of Osmanthus fragrans Lour by pre-column derivatization with HPLC-MS/MS. Int. J. Biol. Macromol. 2019, 125, 221–231. [Google Scholar] [CrossRef]
- Xia, Y.-G.; Wang, T.-L.; Sun, L.-M.; Liang, J.; Yang, B.-Y.; Kuang, H.-X. A New UPLC-MS/MS Method for the Characterization and Discrimination of Polysaccharides from Genus Ephedra Based on Enzymatic Digestions. Molecules 2017, 22, 1992. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Wang, H.; Han, X.; Chen, S.; Zhu, S.; Dai, J. Fingerprint analysis of polysaccharides from different Ganoderma by HPLC combined with chemometrics methods. Carbohydr. Polym. 2014, 114, 432–439. [Google Scholar] [CrossRef]
- Gao, Y.-Y.; Jiang, Y.; Chen, G.-C.; Li, S.-S.; Yang, F.; Ma, Q. A Sensitive and Rapid UPLC-MS/MS Method for Determination of Monosaccharides and Anti-Allergic Effect of the Polysaccharides Extracted from Saposhnikoviae Radix. Molecules 2018, 23, 1924. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Q.; Shi, X.; Liu, Y.; Liu, X.; Dou, S.; Ning, C.; Liu, Z.Q.; Sun, S.; Chen, X.; Ren, X. Production of nisin and lactic acid from corn stover through simultaneous saccharification and fermentation. Biotechnol. Biotechnol. Equip. 2018, 32, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Pol, E.; Bakker, R.; van Zeeland, A.; Garcia, D.S.; Punt, A.; Eggink, G. Analysis of by-product formation and sugar monomerization in sugarcane bagasse pretreated at pilot plant scale: Differences between autohydrolysis, alkaline and acid pretreatment. Bioresour. Technol. 2015, 181, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Zou, Y.; Gu, Z.; Li, Z.; Jiang, Z.; Cheng, L.; Hong, Y.; Li, C. Liquefaction concentration impacts the fine structure of maltodextrin. Ind. Crops Prod. 2018, 123, 687–697. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Modig, T.; Lidén, G.; Taherzadeh, M.J. Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem. J. 2002, 363, 769–776. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Niklasson, C.; Lidén, G. Acetic acid-friend or foe in anaerobic batch conversion of glucose to ethanol by Saccharomyces cerevisiae? Chem. Eng. Sci. 1997, 52, 2653–2659. [Google Scholar] [CrossRef]
- Yankov, D. Fermentative Lactic Acid Production From Lignocellulosic Feedstocks: From Source to Purified Product. Front. Chem. 2022, 10, 823005. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M.; Aroca, R. Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- González-Leos, A.; Bustos-Vázquez, M.G.; Rodríguez-Castillejos, G.C.; Rodríguez-Durán, L.V.; Del Ángel-Del Ángel, A. Kinetics of Lactic Acid Fermentation from Sugarcane Bagasse by Lactobacillus Pentosus. Rev. Mex. Ing. Quim. 2019, 19, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Huo, W.; Wang, B.; Wang, Y.; Wen, H.; Cai, D.; Zhang, C.; Wu, Y.; Qin, P. L-lactic acid production by simultaneous saccharification and fermentation of dilute ethylediamine pre-treated rice straw. Ind. Crops Prod. 2019, 141, 111749. [Google Scholar] [CrossRef]
- Lee, K.H.; Jang, Y.W.; Lee, J.; Kim, S.; Park, C.; Yoo, H.Y. Statistical Optimization of Alkali Pretreatment to Improve Sugars Recovery from Spent Coffee Grounds and Utilization in Lactic Acid Fermentation. Processes 2021, 9, 494. [Google Scholar] [CrossRef]
- Kim, J.; Jang, J.H.; Yeo, H.J.; Seol, J.; Kim, S.R.; Jung, Y.H. Lactic Acid Production from a Whole Slurry of Acid-Pretreated Spent Coffee Grounds by Engineered Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 2019, 189, 206–216. [Google Scholar] [CrossRef] [PubMed]


| Independent Variables | Symbol | Range and Levels | ||||
|---|---|---|---|---|---|---|
| −α | −1 | 0 | 1 | +α | ||
| Cow manure (g·L−1) | X1 | 46.36 | 60 | 80 | 100 | 113.63 |
| Enzyme (IU·gCMDM−1) | X2 | 141.02 | 170 | 212.5 | 255 | 283.97 |
| Temperature (°C) | X3 | 41.59 | 45 | 50 | 55 | 58.41 |
| Run | Codified Variables | Experimental Levels | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Cow Manure (g·L−1) | Enzyme (IU·gCMDM−1) | Temperature (°C) | |
| 1 | 1 | −1 | −1 | 100 | 170 | 45 |
| 2 | 1 | 1 | 1 | 100 | 255 | 55 |
| 3 | 1 | −1 | 1 | 100 | 170 | 55 |
| 4 | 0 | 0 | 0 | 80 | 212.5 | 50 |
| 5 | 1.682 | 0 | 0 | 113.636 | 212.5 | 50 |
| 6 | −1 | 1 | 1 | 60 | 255 | 55 |
| 7 | 1 | 1 | −1 | 100 | 255 | 45 |
| 8 | −1 | −1 | 1 | 60 | 170 | 55 |
| 9 | −1 | −1 | −1 | 60 | 170 | 45 |
| 10 | −1.682 | 0 | 0 | 46.364 | 212.5 | 50 |
| 11 | −1 | 1 | −1 | 60 | 255 | 45 |
| 12 | 0 | 1.682 | 0 | 80 | 283.976 | 50 |
| 13 | 0 | 0 | 1.682 | 80 | 212.5 | 58.41 |
| 14 | 0 | 0 | 0 | 80 | 212.5 | 50 |
| 15 | 0 | −1.682 | 0 | 80 | 141.024 | 50 |
| 16 | 0 | 0 | 0 | 80 | 212.5 | 50 |
| 17 | 0 | 0 | 0 | 80 | 212.5 | 50 |
| 18 | 0 | 0 | −1.682 | 80 | 212.5 | 41.59 |
| Sample | Hemicellulose (%) | Cellulose (%) | Lignin (%) | Ash (%) | Humidity (%) |
|---|---|---|---|---|---|
| Manure heap A * | 27.3 | 24.5 | 3.8 | 17.69 | 2.32 |
| Manure heap B * | 26.3 | 25 | 3.8 | 18.85 | 2.5 |
| Farm 1A | 28.3 | 23.4 | 3.5 | 18.04 | 2.32 |
| Farm 1B | 30.2 | 21.7 | 3.1 | 17.43 | 2.11 |
| Farm 2A | 28.4 | 23.6 | 4.1 | 17.13 | 2.76 |
| Farm 2B | 28.3 | 22.9 | 5.3 | 16.5 | 2.23 |
| Average | 28.1 | 23.5 | 3.9 | 17.6 | 2.4 |
| Cow Manure (g/L) | Mannose % | Glucose % | Galactose % | Arabinose % | Xylose % | Total % | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 46.4 | 0.08 | 10.09 | 6.36 | 18.63 | 48.30 | 44.56 | ||||||||||||
| 60 | 0.06 | 4.32 | - | 4.39 | 3.02 | - | 3.44 | 10.66 | - | 11.14 | 22.75 | - | 25.06 | 22.36 | - | 23.10 | ||
| 80 | 0.04 | - | 0.53 | 5.85 | - | 52.89 | 3.55 | - | 6.40 | 8.84 | - | 15.68 | 26.35 | - | 31.77 | 25.53 | - | 47.19 |
| 100 | 0.04 | - | 0.32 | 8.93 | - | 47.84 | 4.23 | - | 4.48 | 9.53 | - | 10.34 | 28.58 | - | 31.10 | 29.12 | - | 45.00 |
| 113.6 | 0.22 | 8.34 | 4.50 | 11.04 | 28.62 | 27.98 | ||||||||||||
| Run | Mannose (g·L−1) | Glucose (g·L−1) | Galactose (g·L−1) | Arabinose (g·L−1) | Xylose (g·L−1) | Total (g·L−1) |
|---|---|---|---|---|---|---|
| 1 | 0.48 | 21.23 | 1.35 | 2.82 | 11.59 | 32.47 |
| 2 | 0.61 | 40.53 | 2.09 | 3.4 | 11.68 | 53.31 |
| 3 | 0.29 | 24.76 | 1.37 | 2.36 | 8.23 | 32.01 |
| 4 | 0.31 | 12.9 | 2.32 | 2.2 | 9.7 | 22.43 |
| 5 | 0.55 | 29.07 | 3.91 | 3.4 | 11.64 | 43.57 |
| 6 | 0.31 | 15.86 | 0.75 | 1.9 | 6.96 | 20.78 |
| 7 | 0.45 | 20.81 | 1.13 | 2.34 | 9.65 | 29.38 |
| 8 | 0.19 | 12.89 | 0.55 | 1.49 | 5.79 | 15.91 |
| 9 | 0.21 | 15.29 | 0.11 | 1.52 | 6.77 | 18.9 |
| 10 | 0.2 | 7.21 | 1.13 | 1.52 | 5.91 | 10.97 |
| 11 | 0.21 | 17.25 | 0.12 | 0.93 | 9.15 | 22.66 |
| 12 | 0.42 | 18.37 | 3.21 | 2.41 | 9.33 | 28.74 |
| 13 | 0.27 | 24.66 | 1.33 | 2.45 | 8.07 | 31.78 |
| 14 | 0.29 | 12.43 | 2.33 | 2.14 | 8.53 | 20.72 |
| 15 | 0.41 | 25.26 | 2.98 | 2.71 | 10.15 | 36.51 |
| 16 | 0.48 | 27.15 | 1.29 | 2.88 | 11.16 | 37.96 |
| 17 | 0.37 | 18.15 | 1.11 | 2.1 | 8.44 | 25.17 |
| 18 | 0.23 | 14.6 | 0.94 | 2.0 | 8.15 | 20.92 |
| Run | Acetic acid (g·L−1) | Furfural (g·L−1) | 5-HMF (g·L−1) | Acetic Acid (g·L−1) |
|---|---|---|---|---|
| Chemical Pretreatment | SSAF | |||
| 1 | 1.44 | 0.74 | 0.94 | 1.80 |
| 2 | 2.00 | 1.26 | 5.00 | 2.20 |
| 3 | 2.00 | 1.26 | 5.00 | 1.72 |
| 4 | 0.96 | 1.43 | 0.33 | 1.08 |
| 5 | 2.14 | 1.14 | 1.38 | 1.90 |
| 6 | 0.58 | 0.42 | 0.48 | 0.90 |
| 7 | 1.44 | 0.74 | 0.94 | 1.80 |
| 8 | 0.58 | 0.42 | 0.48 | 0.96 |
| 9 | 0.62 | 0.80 | 0.38 | 1.24 |
| 10 | 0.81 | 1.00 | 0.33 | 0.81 |
| 11 | 0.62 | 0.80 | 0.38 | 1.00 |
| 12 | 2.14 | 1.14 | 1.38 | 1.05 |
| 13 | 1.36 | 0.74 | 0.54 | 1.38 |
| 14 | 0.96 | 1.43 | 0.33 | 1.20 |
| 15 | 0.94 | 0.68 | 0.61 | 1.70 |
| 16 | 1.04 | 0.76 | 3.92 | 2.00 |
| 17 | 1.14 | |||
| 18 | 1.36 | 0.74 | 0.54 | 1.92 |
| Average | 1.33 | 1.04 | 1.35 | 1.45 |
| Run | 24 h | 48 h | ||||
|---|---|---|---|---|---|---|
| LA (g·L−1) | Productivity (g·L−1·h−1) | Yield wt.% (LA/Lignocellulosic) | LA (g·L−1) | Productivity (g·L−1·h−1) | Yield wt.% (LA/ Lignocellulosic) | |
| 1 | 3.21 | 0.13 | 6.22 | 15.06 | 0.31 | 29.19 |
| 2 | 2.04 | 0.09 | 3.95 | 2.52 | 0.05 | 4.88 |
| 3 | 1.89 | 0.08 | 3.66 | 2.16 | 0.05 | 4.19 |
| 4 | 12.45 | 0.52 | 30.16 | 13.2 | 0.28 | 31.98 |
| 5 | 2.13 | 0.09 | 3.63 | 2.43 | 0.05 | 4.14 |
| 6 | 6.36 | 0.27 | 20.54 | 11.82 | 0.25 | 38.18 |
| 7 | 5.34 | 0.22 | 10.35 | 14.34 | 0.30 | 27.79 |
| 8 | 4.80 | 0.20 | 15.50 | 7.38 | 0.15 | 23.84 |
| 9 | 11.10 | 0.46 | 35.85 | 14.31 | 0.30 | 46.22 |
| 10 | 9.18 | 0.38 | 38.37 | 11.94 | 0.25 | 49.91 |
| 11 | 11.55 | 0.48 | 37.31 | 12.51 | 0.26 | 40.41 |
| 12 | 11.01 | 0.46 | 26.67 | 12.9 | 0.27 | 31.25 |
| 13 | 1.83 | 0.08 | 4.43 | 2.01 | 0.04 | 4.87 |
| 14 | 11.13 | 0.46 | 26.96 | 12.06 | 0.25 | 29.22 |
| 15 | 9.90 | 0.41 | 23.98 | 10.44 | 0.22 | 25.29 |
16 | 13.65 | 0.57 | 33.07 | 15.09 | 0.31 | 36.56 |
| 17 | 11.13 | 0.46 | 26.96 | 12.45 | 0.26 | 30.16 |
| 18 | 13.41 | 0.56 | 32.49 | 13.65 | 0.28 | 33.07 |
| Time | Model | Intercept | Cow Manure Content (CM) | Enzyme E | Temperature (T) | CM:T | CM2 | E2 | T2 | Adjusted R2 | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | LA | −2.2024 × 102 | -- | -- | 7.4187 | -- | −6.6988 × 10−3 | −5.4426 10−4 | −7.9398 × 10−2 | 0.8537 | <0.0001 |
| Y | −2.5511 × 102 | −2.3562 | 5.7394 × 10−1 | 1.4827 | 3.5200 | -- | −1.2879 10−3 | −1.9009 × 10−1 | 0.8647 | <0.0001 | |
| P | −5.5552 | 1.8012 × 10−2 | -- | 2.4129 × 10−1 | 3.6250 × 10−4 | −2.5738 × 10−4 | -- | −2.9163 × 10−3 | 0.818 | <0.0001 | |
| 48 h | LA | −2.0978 × 102 | 1.6774 | 7.1460 | -- | −2.1375 × 10−2 | −4.4435 × 10−3 | -- | −6.1962 × 10−2 | 0.8755 | <0.0001 |
| Y | −320.523 | 0.87509 | -- | 15.3092 | −0.02912 | -- | -- | −0.14735 | 0.9039 | <0.0001 | |
| P | −4.3588 | 3.4893 × 10−2 | -- | 1.4845 × 10−1 | −4.4500 × 10−4 | −9.2354 × 10−5 | -- | −1.2865 × 10−3 | 0.8745 | <0.0001 |
| Process Time (h) | Cow Manure (g·L−1) | Enzyme (IU·gCM DM−1) | Temperature (°C) | Lactic Acid (g·L−1) | Productivity (g·L−1·h−1) | Yield (wt. %) | Desirability |
|---|---|---|---|---|---|---|---|
| 24 h | 71 | 220 | 45 | 13.4 | 0.53 | 36.28 | 0.952 |
| 48 h | 86 | 140 | 42.5 | 15.27 | 0.317 | 32.76 | 0.917 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido, R.; Falguera, V.; Pérez Navarro, O.; Acosta Solares, A.; Cabeza, L.F. Lactic Acid Production from Cow Manure: Experimental Process Conditions Analysis. Fermentation 2023, 9, 604. https://doi.org/10.3390/fermentation9070604
Garrido R, Falguera V, Pérez Navarro O, Acosta Solares A, Cabeza LF. Lactic Acid Production from Cow Manure: Experimental Process Conditions Analysis. Fermentation. 2023; 9(7):604. https://doi.org/10.3390/fermentation9070604
Chicago/Turabian StyleGarrido, Ricard, Víctor Falguera, Omar Pérez Navarro, Amanda Acosta Solares, and Luisa F. Cabeza. 2023. "Lactic Acid Production from Cow Manure: Experimental Process Conditions Analysis" Fermentation 9, no. 7: 604. https://doi.org/10.3390/fermentation9070604
APA StyleGarrido, R., Falguera, V., Pérez Navarro, O., Acosta Solares, A., & Cabeza, L. F. (2023). Lactic Acid Production from Cow Manure: Experimental Process Conditions Analysis. Fermentation, 9(7), 604. https://doi.org/10.3390/fermentation9070604








