Improvement of ε-Poly-l-lysine Production by Co-Culture Fermentation Strategy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Culture and Fermentation Media Composition
2.3. Single Factor Experiment in Co-Culture Fermentation
2.4. Response Surface Design of Co-Culture Fermentation Process
2.5. Fermentation in a 2-L Stirred Tank Bioreactor
2.6. Analytical Methods
2.7. Statistical Analysis
3. Results
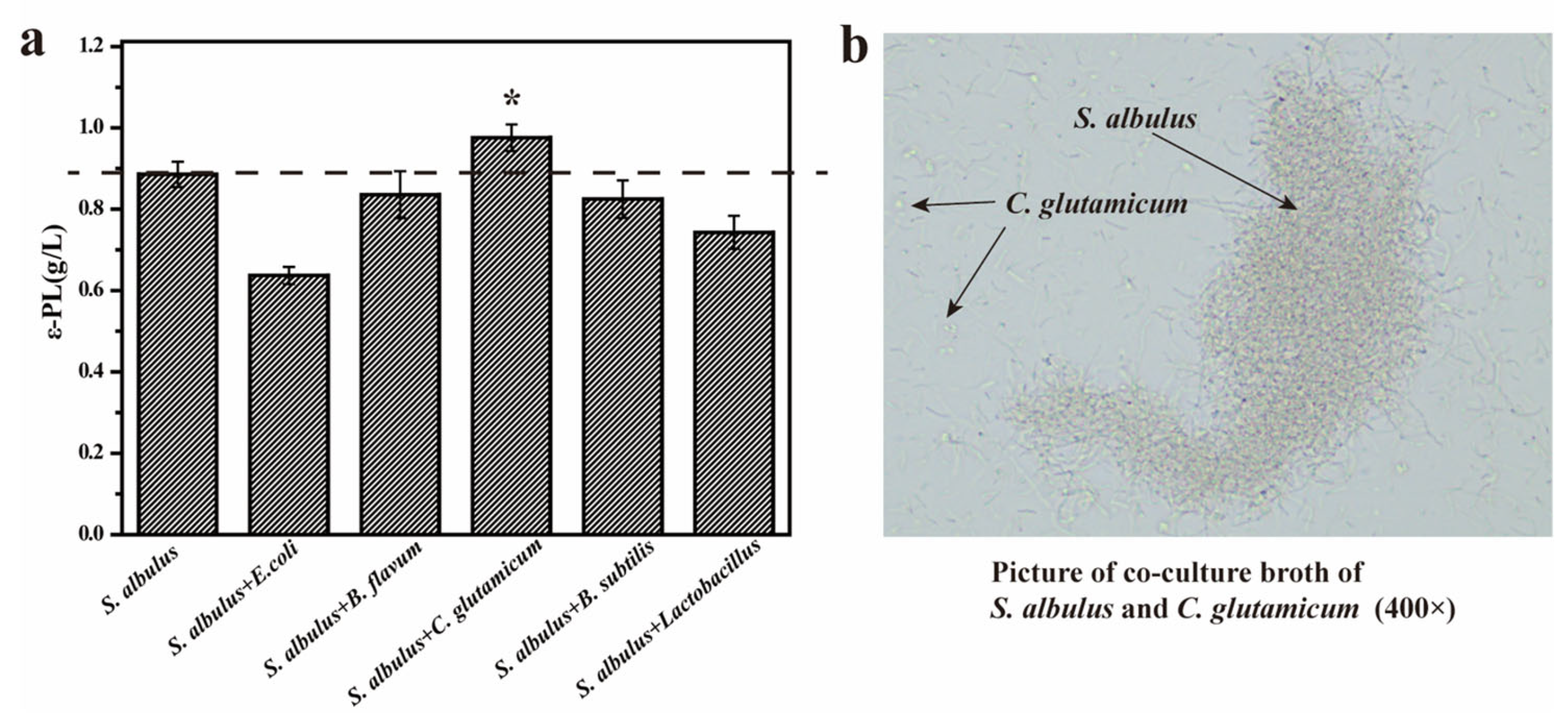
3.1. Improving the Production of ε-PL by Co-Culture with S. albulus IFO 14147 and C. glutamicum CICC 10064
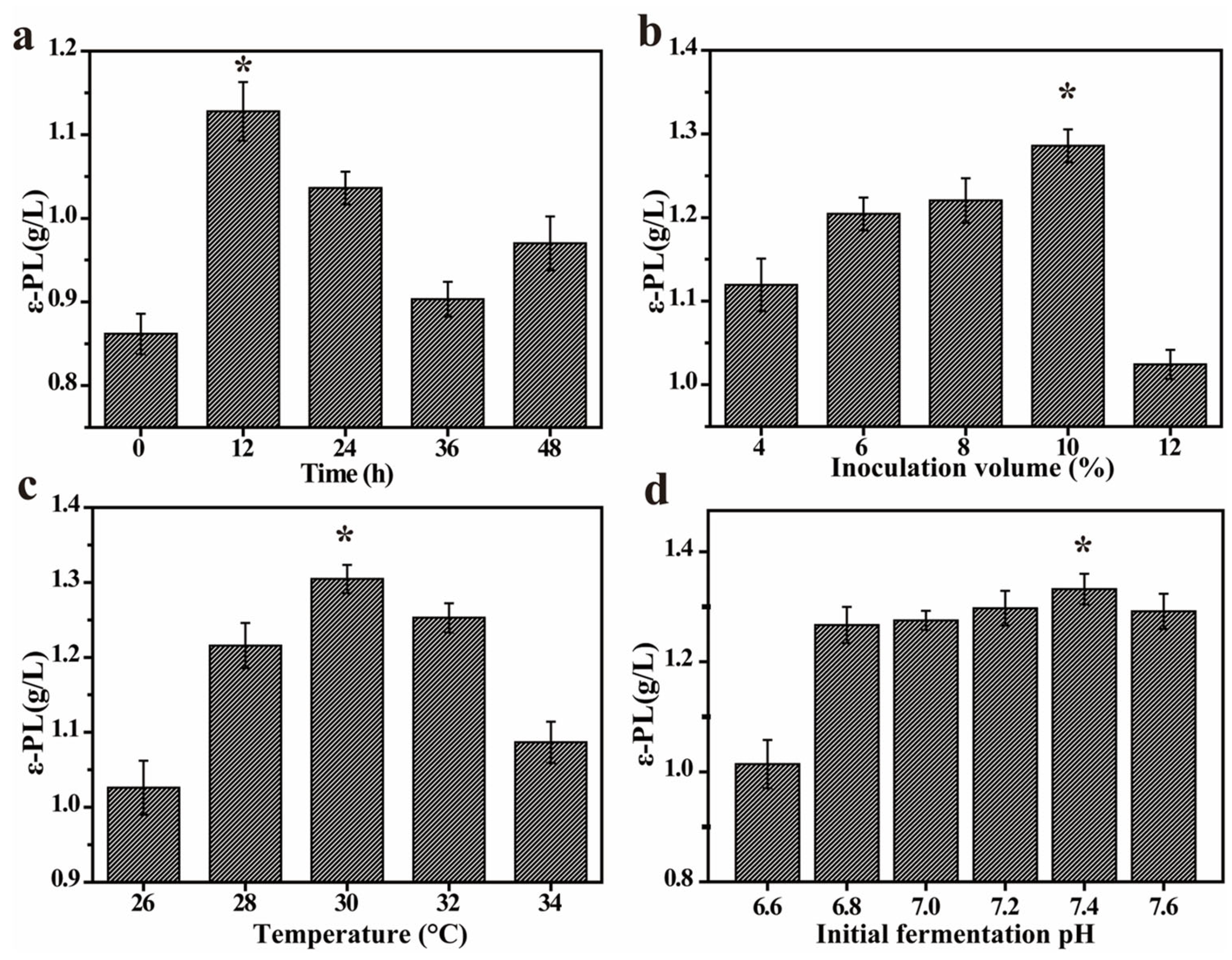
3.2. Single Factor Experiment on Co-Culture Fermentation
3.2.1. Effect of Inoculum Time Conditions on ε-PL Production
3.2.2. Effect of Inoculum Volume on ε-PL Production
3.2.3. Effect of Fermentation Temperature on ε-PL Production
3.2.4. Effect of Initial pH on ε-PL Production
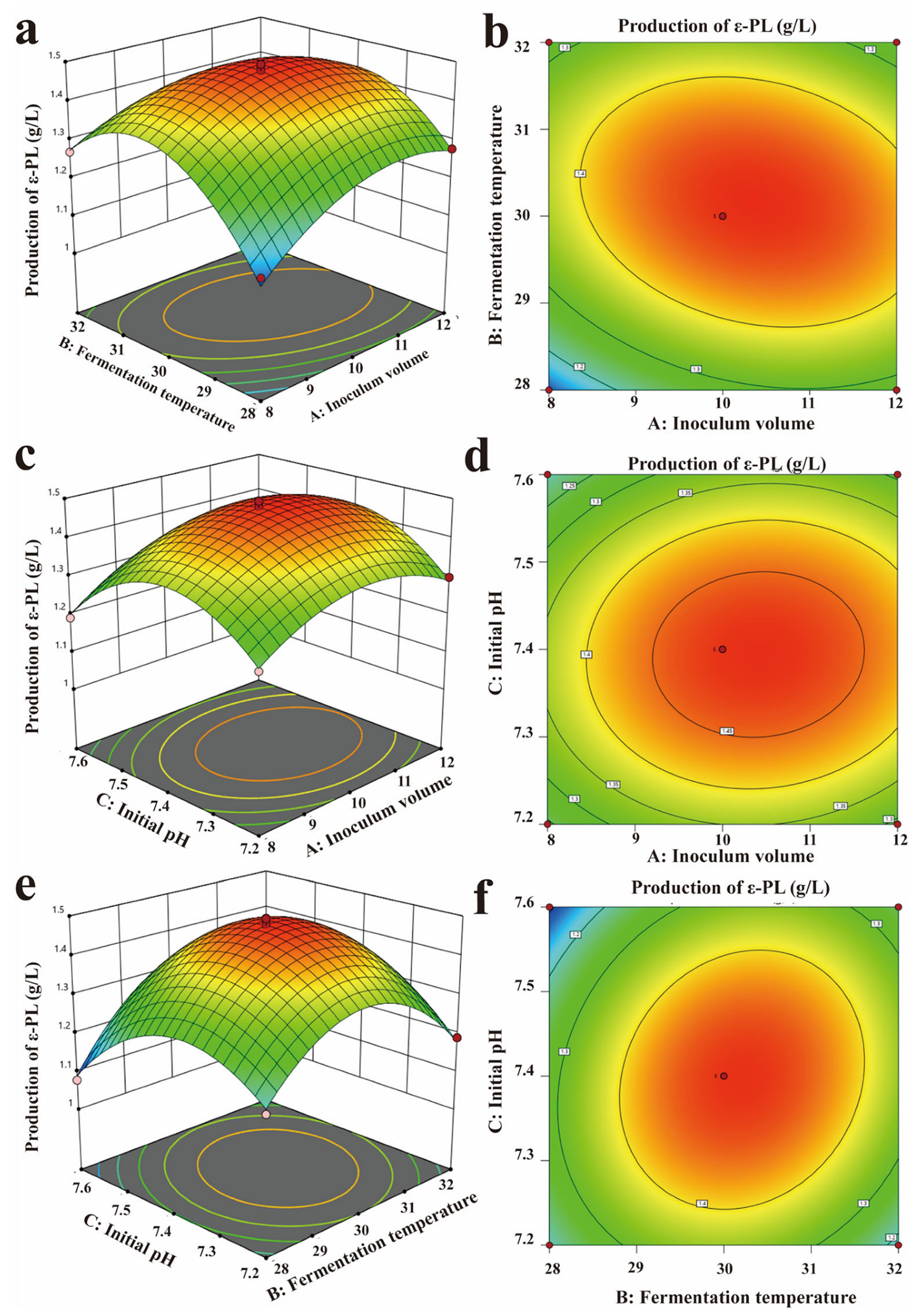
3.3. Response Surface Design to Optimize the Optimal Fermentation Conditions of Co-Culture
3.4. Verification Test with the Optimal Fermentation Conditions of Co-Culture
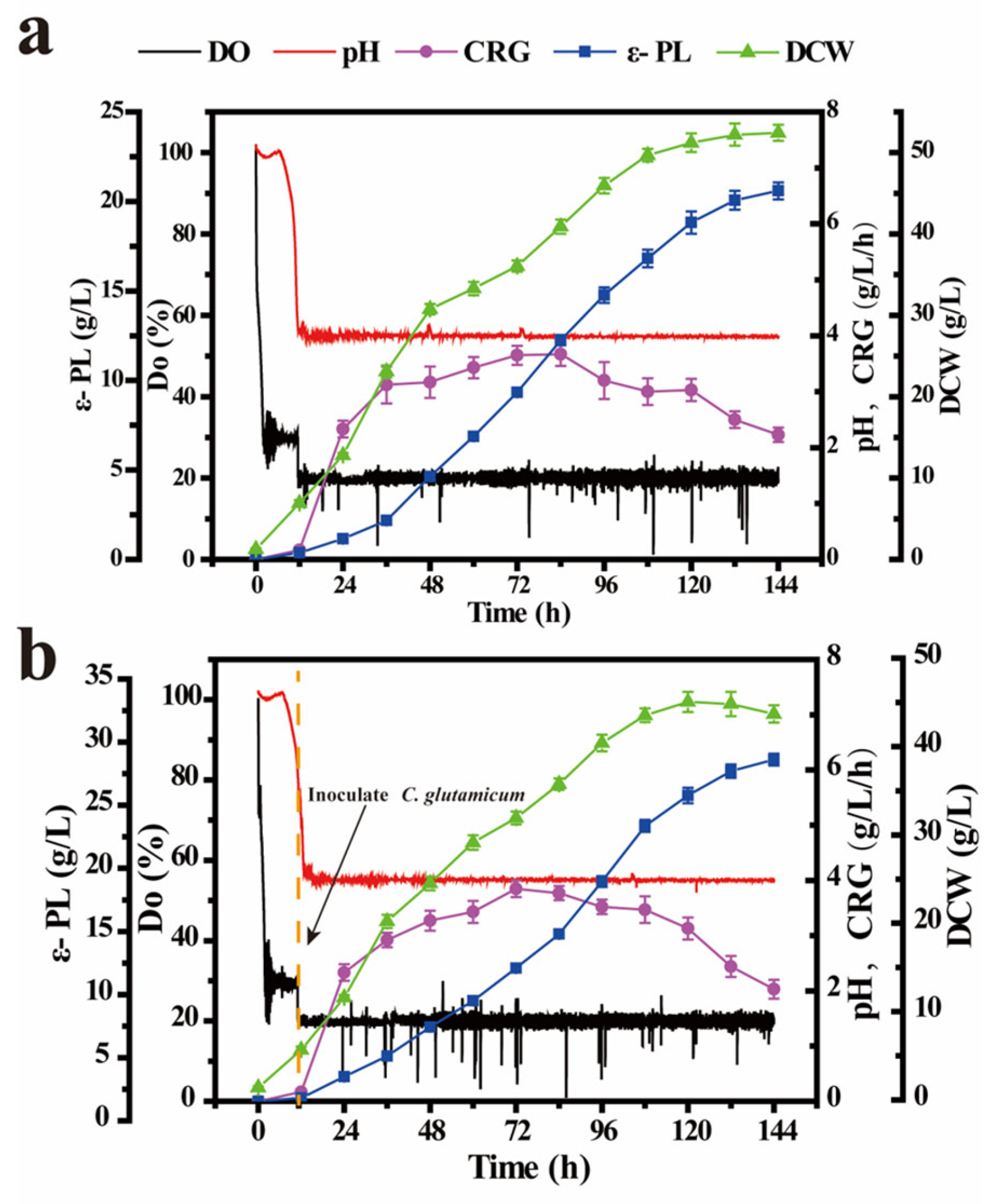
3.5. Production of ε-PL with Co-Culture Fermentation Strategy in a 2-L Fermenter
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saimura, M.; Takehara, M.; Mizukami, S.; Kataoka, K.; Hirohara, H. Biosynthesis of nearly monodispersed poly(ε-l-lysine) in Streptomyces species. Biotechnol. Lett. 2008, 30, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-S.; Ren, X.-D.; Zeng, X.; Zhao, F.-L.; Tang, L.; Zhang, H.-J.; Zhang, J.-H.; Mao, Z.-G. Enhancement of ε-poly-l-lysine production coupled with precursor l-lysine feeding in glucose–glycerol co-fermentation by Streptomyces sp. M-Z18. Bioprocess Biosyst. Eng. 2013, 36, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Nagasawa, T. ε-Poly-l-lysine: Microbial production, biodegradation and application potential. Appl. Microbiol. Biotechnol. 2003, 62, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-S.; Ren, X.-D.; Dong, N.; Li, S.; Li, F.; Zhao, F.-L.; Tang, L.; Zhang, J.-H.; Mao, Z.-G. Culture medium containing glucose and glycerol as a mixed carbon source improves ε-poly-l-lysine production by Streptomyces sp. M-Z18. Bioprocess Biosyst. Eng. 2012, 35, 469–475. [Google Scholar] [CrossRef]
- Xu, Z.; Feng, X.; Sun, Z.; Cao, C.; Li, S.; Xu, Z.; Xu, Z.; Bo, F.; Xu, H. Economic process to co-produce poly(ε-l-lysine) and poly(l-diaminopropionic acid) by a pH and dissolved oxygen control strategy. Bioresour. Technol. 2015, 187, 70–76. [Google Scholar] [CrossRef]
- Pan, L.; Chen, X.-S.; Wang, K.-F.; Mao, Z.-G. Mechanisms of response to pH shock in microbial fermentation. Bioprocess Biosyst. Eng. 2020, 43, 361–372. [Google Scholar] [CrossRef]
- Liu, S.; Wu, Q.; Zhang, J.; Mo, S. Production of ε-poly-L-lysine by Streptomyces sp. using resin-based, in situ product removal. Biotechnol. Lett. 2011, 33, 1581–1585. [Google Scholar] [CrossRef]
- Kahar, P.; Kobayashi, K.; Iwata, T.; Hiraki, J.; Kojima, M.; Okabe, M. Production of ε-polylysine in an airlift bioreactor (ABR). J. Biosci. Bioeng. 2002, 93, 274–280. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.; Xu, H.; Yao, Z.; Ouyang, P. ε-Poly-L-lysine production by immobilized cells of Kitasatospora sp. MY 5-36 in repeated fed-batch cultures. Bioresour. Technol. 2010, 101, 5523–5527. [Google Scholar] [CrossRef]
- Xu, D.; Yao, H.; Xu, Z.; Wang, R.; Xu, Z.; Li, S.; Feng, X.; Liu, Y.; Xu, H. Production of ε-poly-lysine by Streptomyces albulus PD-1 via solid-state fermentation. Bioresour. Technol. 2017, 223, 149–156. [Google Scholar] [CrossRef]
- Kahar, P.; Iwata, T.; Hiraki, J.; Park, E.Y.; Okabe, M. Enhancement of ε-polylysine production by Streptomyces albulus strain 410 using pH control. J. Biosci. Bioeng. 2001, 91, 190–194. [Google Scholar] [CrossRef]
- Ren, X.-D.; Chen, X.-S.; Zeng, X.; Wang, L.; Tang, L.; Mao, Z.-G. Acidic pH shock induced overproduction of ε-poly-l-lysine in fed-batch fermentation by Streptomyces sp. M-Z18 from agro-industrial by-products. Bioprocess Biosyst. Eng. 2015, 38, 1113–1125. [Google Scholar] [CrossRef]
- Rodríguez, M.E.; Lopes, C.A.; Barbagelata, R.J.; Barda, N.B.; Caballero, A.C. Influence of Candida pulcherrima Patagonian strain on alcoholic fermentation behaviour and wine aroma. Int. J. Food Microbiol. 2010, 138, 19–25. [Google Scholar] [CrossRef]
- Casey, E.; Mosier, N.S.; Adamec, J.; Stockdale, Z.; Ho, N.; Sedlak, M. Effect of salts on the Co-fermentation of glucose and xylose by a genetically engineered strain of Saccharomyces cerevisiae. Biotechnol. Biofuels 2013, 6, 83. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Kaur, K.; Bhushan, B.; Kaur, M.; Hans, M. Inoculum Size and Age Studies on Single and Mixed Strain Fermentation of Grape Juice. J. Pure Appl. Microbiol. 2020, 14, 2137–2145. [Google Scholar] [CrossRef]
- Huan, L.I.; Zhou, L.; Chen, Y.; Wei, P.; Ouyang, P.K. Studies on multi-bacterium fermentation of L-ornithine. Ind. Microbiol. 2006. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, J.; Tan, F.; Yin, H.; Yang, C.; Lu, K. Improvement of nisin production by using the integration strategy of co-cultivation fermentation, foam fractionation and pervaporation. LWT Food Sci. Technol. 2021, 142, 111093. [Google Scholar] [CrossRef]
- Pan, L.; Chen, X.-S.; Liu, M.-M.; Liu, Y.-J.; Mao, Z.-G. Efficient production of ε-poly-l-lysine from glucose by two-stage fermentation using pH shock strategy. Process. Biochem. 2017, 63, 8–15. [Google Scholar] [CrossRef]
- Pan, L.; Chen, X.; Wang, K.; Mao, Z. A temporal transcriptomic dynamics study reveals the reason of enhanced ε-poly-L-lysine production in Streptomyces albulus M-Z18 by pH shock. Process. Biochem. 2019, 85, 1–11. [Google Scholar] [CrossRef]
- VanBogelen, R.A.; Kelley, P.M.; Neidhardt, F.C. Differential induction of heat shock, SOS, and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. J. Bacteriol. 1987, 169, 26–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Q.-G.; Zeng, B.-D.; Liang, L.; Wu, S.-G.; Huang, J.-Z. Genome shuffling and high-throughput screening of Brevibacterium flavum MDV1 for enhanced l-valine production. World J. Microbiol. Biotechnol. 2018, 34, 121. [Google Scholar] [CrossRef] [PubMed]
- Vallino, J.J.; Stephanopoulos, G. Metabolic flux distributions inCorynebacterium glutamicum during growth and lysine overproduction. Biotechnol. Bioeng. 2010, 41, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Voelker, U.; Dufour, A.; Haldenwang, W.G. The Bacillus subtilis rsbU gene product is necessary for RsbX-dependent regulation of sigma B. J. Bacteriol. 2019, 177, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Pan, M.; Wan, C.; Shah, N.P.; Tao, X.; Wei, H. Physiological and transcriptional responses and cross protection of Lactobacillus plantarum ZDY2013 under acid stress. J. Dairy Sci. 2016, 99, 1002–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.-D.; Chen, X.-S.; Tang, L.; Zeng, X.; Wang, L.; Mao, Z.-G. Physiological mechanism of the overproduction of ε-poly-l-lysine by acidic pH shock in fed-batch fermentation. Bioprocess Biosyst. Eng. 2015, 38, 2085–2094. [Google Scholar] [CrossRef]
- Santisawadi, S.; Chaiseri, S.; Jinda, N.; Klinkesorn, U. Process optimization using response surface design for diacylglycerol synthesis from palm fatty acid distillate by enzymatic esterification. Songklanakarin J. Sci. Technol. 2013, 35, 23–32. [Google Scholar]
- Ren, X.-D.; Chen, X.-S.; Tang, L.; Sun, Q.-X.; Zeng, X.; Mao, Z.-G. Efficient production of ε-poly-l-lysine from agro-industrial by-products by Streptomyces sp. M-Z18. Ann. Microbiol. 2014, 65, 733–743. [Google Scholar] [CrossRef]
- Itzhaki, R.F. Colorimetric method for estimating polylysine and polyarginine. Anal. Biochem. 1972, 50, 569–574. [Google Scholar] [CrossRef]
- Buruleanu, C.L.; Nicolescu, C.L.; Avram, D.; Manea, I.; Bratu, M.G. Effects of yeast extract and different amino acids on the dynamics of some components in cabbage juice during fermentation with Bifidobacterium lactis BB-12. Food Sci. Biotechnol. 2012, 21, 691–699. [Google Scholar] [CrossRef]
- Xin, W.; Shao, C.; Lian, L.; Xing, G.; Xu, Y.; Xin, L. Optimization, partial characterization and antioxidant activity of an exopolysaccharide from Lactobacillus plantarum KX041. Int. J. Biol. Macromol. 2017, 103, 1173–1184. [Google Scholar]
- Bandaiphet, C.; Prasertsan, P. Effect of aeration and agitation rates and scale-up on oxygen transfer coefficient, kLa in exopolysaccharide production from Enterobacter cloacae WD7. Carbohydr. Polym. 2006, 66, 216–228. [Google Scholar] [CrossRef]
- He, Q.; Hemme, C.L.; Jiang, H.; He, Z.; Zhou, J. Mechanisms of enhanced cellulosic bioethanol fermentation by co-cultivation of Clostridium and Thermoanaerobacter spp. Bioresour. Technol. 2011, 102, 9586–9592. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Inoculums volume (%) | 8% | 10% | 12% |
| Fermentation temperature (°C) | 28 | 30 | 32 |
| Initial pH | 7.2 | 7.4 | 7.6 |
| Run No | A | B | C |
|---|---|---|---|
| 1 | −1 | −1 | 0 |
| 2 | −1 | 1 | 0 |
| 3 | 1 | −1 | 0 |
| 4 | 1 | 1 | 0 |
| 5 | 0 | −1 | −1 |
| 6 | 0 | −1 | 1 |
| 7 | 0 | 1 | −1 |
| 8 | 0 | 1 | 1 |
| 9 | −1 | 0 | −1 |
| 10 | 1 | 0 | −1 |
| 11 | −1 | 0 | 1 |
| 12 | 1 | 0 | 1 |
| 13 | 0 | 0 | 0 |
| 14 | 0 | 0 | 0 |
| 15 | 0 | 0 | 0 |
| 16 | 0 | 0 | 0 |
| 17 | 0 | 0 | 0 |
| Source | Sum of Square | df | Mean Square | F Value | p Value | Significant |
|---|---|---|---|---|---|---|
| Model | 0.2796 | 9 | 0.0311 | 50.16 | <0.0001 | ** |
| A | 0.0096 | 1 | 0.0096 | 15.51 | 0.0056 | ** |
| B | 0.0097 | 1 | 0.0097 | 15.72 | 0.0054 | ** |
| C | 0.0008 | 1 | 0.0008 | 1.36 | 0.2825 | |
| AB | 0.0077 | 1 | 0.0077 | 12.38 | 0.0097 | ** |
| AC | 0.0005 | 1 | 0.0005 | 0.7847 | 0.4051 | |
| BC | 0.0073 | 1 | 0.0073 | 11.77 | 0.0110 | * |
| A2 | 0.0231 | 1 | 0.0231 | 47.50 | 0.0002 | ** |
| B2 | 0.0930 | 1 | 0.0930 | 181.14 | <0.0001 | ** |
| C2 | 0.0646 | 1 | 0.0646 | 127.18 | <0.0001 | ** |
| Residual | 0.0043 | 7 | 0.0272 | |||
| Lack of fit | 0.0016 | 3 | 0.0005 | 5.91 | 0.7120 | |
| Pure Error | 0.0027 | 2 | 0.0010 | |||
| Cor Total | 0.2840 | 14 | ||||
| R2 | 0.9847 | |||||
| Adj-R2 | 0.9651 | |||||
| C.V.% | 1.92 |
| Parameters | Control Group | Experimental Group | Comparison (%) |
|---|---|---|---|
| Fermentation time (h) | 144 | 144 | 0 |
| ε-PL production (g/L) | 20.59 ± 0.39 | 27.07 ± 0.47 | 31.47 |
| DCW (g/L) | 43.84 ± 0.98 | 52.44 ± 1.18 | 19.62 |
| Yield (g/g) | 0.46 ± 0.01 | 0.52 ± 0.02 | 13.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.; Zhang, C.; Yuan, X.; Zhang, Y.; Chen, X.; Tian, C.; Zhang, Z.; Tian, M.; Liao, A.; Yu, G.; et al. Improvement of ε-Poly-l-lysine Production by Co-Culture Fermentation Strategy. Fermentation 2023, 9, 626. https://doi.org/10.3390/fermentation9070626
Pan L, Zhang C, Yuan X, Zhang Y, Chen X, Tian C, Zhang Z, Tian M, Liao A, Yu G, et al. Improvement of ε-Poly-l-lysine Production by Co-Culture Fermentation Strategy. Fermentation. 2023; 9(7):626. https://doi.org/10.3390/fermentation9070626
Chicago/Turabian StylePan, Long, Cunjin Zhang, Xinyu Yuan, Yu Zhang, Xusheng Chen, Cuizhu Tian, Zishan Zhang, Mengqing Tian, Aimei Liao, Guanghai Yu, and et al. 2023. "Improvement of ε-Poly-l-lysine Production by Co-Culture Fermentation Strategy" Fermentation 9, no. 7: 626. https://doi.org/10.3390/fermentation9070626
APA StylePan, L., Zhang, C., Yuan, X., Zhang, Y., Chen, X., Tian, C., Zhang, Z., Tian, M., Liao, A., Yu, G., Hui, M., Zeng, X., & Huang, J. (2023). Improvement of ε-Poly-l-lysine Production by Co-Culture Fermentation Strategy. Fermentation, 9(7), 626. https://doi.org/10.3390/fermentation9070626







