Effect of Different Vinification Techniques on the Concentration of Volatile Aroma Compounds and Sensory Profile of Malvazija Istarska Wines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Setting up the Experiment
2.3. Standard Physico-Chemical Analysis
2.4. Analysis of Volatile Aroma Compounds
2.5. Sensory Analysis
2.6. Statistical Data Analysis
3. Results and Discussion
3.1. Standard Physico-Chemical Analysis
3.2. Evaluation of Volatile Aroma Compounds
3.3. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arroyo, T.; Lozano, J.; Cabellos, J.M.; Gil-Diaz, M.; Santos, J.; Horrillo, M.C. Evaluation of Wine Aromatic Compounds by a Sensory Human Panel and an Electronic Nose. J. Agric. Food Chem. 2009, 57, 11543–11549. [Google Scholar] [CrossRef] [PubMed]
- Ayestarán, B.; Martínez-Lapuente, L.; Guadalupe, Z.; Canals, C.; Adell, E.; Vilanova, M. Effect of the Winemaking Process on the Volatile Composition and Aromatic Profile of Tempranillo Blanco Wines. Food Chem. 2019, 276, 187–194. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science: Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-381469-2. [Google Scholar]
- Francis, I.L.; Newton, J.L. Determining Wine Aroma from Compositional Data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Vilanova, M.; Genisheva, Z.; Masa, A.; Oliveira, J.M. Correlation between Volatile Composition and Sensory Properties in Spanish Albariño Wines. Microch. J. 2010, 95, 240–246. [Google Scholar] [CrossRef]
- Radeka, S.; Herjavec, S.; Peršurić, Đ.; Lukić, I.; Sladonja, B. Effect of Different Maceration Treatments on Free and Bound Varietal Aroma Compounds in Wine of Vitis vinifera L. cv. Malvazija Istarska Bijela. Food Technol. Biotechnol. 2008, 46, 86–92. [Google Scholar]
- Styger, G.; Prior, B.; Bauer, F.F. Wine Flavor and Aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef]
- Olejar, K.J.; Fedrizzi, B.; Kilmartin, P.A. Antioxidant Activity and Phenolic Profiles of Sauvignon Blanc Wines Made by Various Maceration Techniques. Aust. J. Grape Wine Res. 2015, 21, 57–68. [Google Scholar] [CrossRef]
- Casassa, L.F.; Bolcato, E.A.; Sari, S.E.; Barda, N. Effects of Maceration Length after Prefermentative Cold Soak: Detailed Chromatic, Phenolic and Sensory Composition of Cabernet Sauvignon, Malbec and Merlot Wines. J. Food Compos. Anal. 2021, 104, 104168. [Google Scholar] [CrossRef]
- Sancho-Galán, P.; Amores-Arrocha, A.; Jiménez-Cantizano, A.; Palacios, V. Influence of the Presence of Grape Skins during White Wine Alcoholic Fermentation. Agronomy 2021, 11, 452. [Google Scholar] [CrossRef]
- Olejar, K.J.; Fedrizzi, B.; Kilmartin, P.A. Enhancement of Chardonnay Antioxidant Activity and Sensory Perception through Maceration Technique. LWT Food Sci. Technol. 2016, 65, 152–157. [Google Scholar] [CrossRef]
- Selli, S.; Canbas, A.; Cabaroglu, T.; Erten, H.; Lepoutre, J.-P.; Gunata, Z. Effect of Skin Contact on the Free and Bound Aroma Compounds of the White Wine of Vitis vinifera L. cv. Narince. Food Control 2006, 17, 75–82. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud-Funel, A.; Glories, Y.; Maujean, A.; Towey, J. Handbook of Enology, 3rd ed.; Wiley: Hoboken, NJ, USA, 2020; ISBN 978-1-119-58468-1. [Google Scholar]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative Study of Aromatic Compounds in Two Young White Wines Subjected to Pre-Fermentative Cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Weightman, C.; Panzeri, V.; Nieuwoudt, H.H.; du Toit, W.J. Effect of Skin Contact before and during Alcoholic Fermentation on the Chemical and Sensory Profile of South African Chenin Blanc White Wines. S. Afr. J. Enol. Vitic. 2015, 36, 366–377. [Google Scholar] [CrossRef]
- Žulj Mihaljević, M.; Maletić, E.; Preiner, D.; Zdunić, G.; Bubola, M.; Zyprian, E.; Pejić, I. Genetic Diversity, Population Structure, and Parentage Analysis of Croatian Grapevine Germplasm. Genes 2020, 11, 737. [Google Scholar] [CrossRef]
- Crespo, J.; Romero, V.; García, M.; Arroyo, T.; Cabellos, J.M. Influence of Skin-Contact Treatment on Aroma Profile of Malvasia Aromatica Wines in D.O. “Vinos de Madrid.”. In Grapes and Wine; Morata, A., Loira, I., González, C., Eds.; IntechOpen: London, UK, 2022; ISBN 978-1-83969-641-1. [Google Scholar]
- Pereira, A.; Fraga, M.; Garcia-Oliveira, P.; Carpena, M.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Barros, L.; Ferreira, C.F.R.I.; Angel Prieto, M.; Simal-Gandara, J. Management of Wine Aroma Compounds: Principal Basis and Future Perspectives. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; Cosme, F., Nunes, M.F., Filipe-Ribeiro, L., Eds.; IntechOpen: London, UK, 2021; ISBN 978-1-83962-575-6. [Google Scholar]
- Murray, J.M.; Delahunty, C.M.; Baxter, I.A. Descriptive Sensory Analysis: Past, Present and Future. Food Res.Int. 2001, 34, 461–471. [Google Scholar] [CrossRef]
- Stone, H.; Sidel, J.L. Quantitative Descriptive Analysis: Developments, Applications and the Future. Food Technol. 1998, 52, 48–52. [Google Scholar]
- Moyano, L.; Zea, L.; Moreno, J.; Medina, M. Analytical Study of Aromatic Series in Sherry Wines Subjected to Biological Aging. J. Agric. Food Chem. 2002, 50, 7356–7361. [Google Scholar] [CrossRef]
- Guld, Z.; Nyitrainé Sárdy, D.; Gere, A.; Rácz, A. Comparison of Sensory Evaluation Techniques for Hungarian Wines. J. Chemom. 2020, 34, e3219. [Google Scholar] [CrossRef] [Green Version]
- Palomo, E.S.; González-Viñas, M.A.; Díaz-Maroto, M.C.; Soriano-Pérez, A.; Pérez-Coello, M.S. Aroma Potential of Albillo Wines and Effect of Skin-Contact Treatment. Food Chem. 2007, 103, 631–640. [Google Scholar] [CrossRef]
- Cabaroglu, T.; Canbas, A.; Baumes, R.; Bayonove, C.; Lepoutre, J.P.; Günata, Z. Aroma Composition of a White Wine of Vitis vinifera L. cv. Emir as Affected by Skin Contact. J. Food Sci. 1997, 62, 680–683. [Google Scholar] [CrossRef]
- OIV (Intwrnational Organization of Vine and Wine). Compendium of International Methods of Wine and Must Analysis; International Organisation of Vine and Wine (OIV): Paris, France, 2018; Volume 1, Available online: http://www.oiv.int/en/technical-standards-and-documents/methods-of-analysis/compendium-of-international-methods-of-analysis-of-wines-and-musts (accessed on 29 April 2023).
- Bubola, M.; Lukić, I.; Radeka, S.; Sivilotti, P.; Grozić, K.; Vanzo, A.; Bavčar, D.; Lisjak, K. Enhancement of Istrian Malvasia Wine Aroma and Hydroxycinnamate Composition by Hand and Mechanical Leaf Removal: Enhancement of Wine Aroma and Hydroxycinnamate Composition by Leaf Removal. J. Sci. Food Agric. 2019, 99, 904–914. [Google Scholar] [CrossRef]
- Lukić, I.; Horvat, I. Differentiation of Commercial PDO Wines Produced in Istria (Croatia) According to Variety and Harvest Year Based on HS-SPME-GC/MS Volatile Aroma Compound Profiling. Food Technol. Biotechnol. 2017, 55, 95–108. [Google Scholar] [CrossRef]
- Bestulić, E.; Rossi, S.; Plavša, T.; Horvat, I.; Lukić, I.; Bubola, M.; Ilak Peršurić, A.S.; Jeromel, A.; Radeka, S. Comparison of Different Maceration and Non-Maceration Treatments for Enhancement of Phenolic Composition, Colour Intensity, and Taste Attributes of Malvazija Istarska (Vitis vinifera L.) White Wines. J. Food Compos. Anal. 2022, 109, 104472. [Google Scholar] [CrossRef]
- Rossi, S.; Bestulić, E.; Horvat, I.; Plavša, T.; Lukić, I.; Bubola, M.; Ganić, K.K.; Ćurko, N.; Jagatić Korenika, A.-M.; Radeka, S. Comparison of Different Winemaking Processes for Improvement of Phenolic Composition, Macro- and Microelemental Content, and Taste Sensory Attributes of Teran (Vitis vinifera L.) Red Wines. LWT 2022, 154, 112619. [Google Scholar] [CrossRef]
- ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. International Standards Organization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/66912.html (accessed on 30 June 2023).
- Official Gazzette—Regulation on Wine Production, No. 2, 2005. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2005_01_2_17.html (accessed on 29 June 2023).
- ISO8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. International Standards Organization: Geneva Switzerland, 2007. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/03/63/36385.html (accessed on 29 June 2023).
- SIST ISO 3591:1997; Sensory Analysis—Apparatus Wine—Tasting Glass. The Slovenian Institute for Standardization (SIST): Ljubljana, Slovenia, 1997. Available online: https://www.iso.org/standard/9002.html (accessed on 29 June 2023).
- OIV/CONCOURS 332A/2009; OIV Standard for International Wine and Spirituous Beverages of Vitivinicultural Origin Competitions. International Organisation of Vine and Wine: Paris, France, 2009.
- Handbook of Enology, Volume 2: The Chemistry of Wine—Stabilization and Treatments, 2nd ed.; Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. (Eds.) Wiley: Chichester, UK; Hoboken, NJ, USA, 2006; ISBN 978-0-470-01037-2. [Google Scholar]
- Rizzon, L.A.; Miele, A. Características analíticas de vinhos Merlot da Serra Gaúcha. Cienc. Rural 2009, 39, 1913–1916. [Google Scholar] [CrossRef] [Green Version]
- Francesca, N.; Romano, R.; Sannino, C.; Le Grottaglie, L.; Settanni, L.; Moschetti, G. Evolution of Microbiological and Chemical Parameters during Red Wine Making with Extended Post-Fermentation Maceration. Int. J. Food Microbiol. 2014, 171, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Yilmaztekin, M.; Kocabey, N.; Hayaloglu, A.A. Effect of Maceration Time on Free and Bound Volatiles of Red Wines from cv. Karaoğlan (Vitis Vinifera L.) Grapes Grown in Arapgir, Turkey. J. Food Sci. 2015, 80, C556–C563. [Google Scholar] [CrossRef]
- Herjavec, S.; Jeromel, A.; Prusina, T.; Maslov, L. Utjecaj Hladne Maceracije NA Kemijski Sastav Vina ŽIlavka. J. Cent. Eur. Agric. 2008, 9, 505–510. [Google Scholar]
- European Commission (EC). Commission Regulation (EC) No 606/2009 of 10 July 2009 Laying down Certain Detailed Rules for Implementing Council Regulation (EC) No 479/2008 as Regards the Categories of Grapevine Products, Oenological Practices and the Applicable Restrictions; European Commission: Brussels, Belgium, 2009; Volume 193. [Google Scholar]
- Lukić, I.; Horvat, I.; Radeka, S.; Damijanić, K.; Staver, M. Effect of Different Levels of Skin Disruption and Contact with Oxygen during Grape Processing on Phenols, Volatile Aromas, and Sensory Characteristics of White Wine. J. Food Process.Preserv. 2019, 43, e13969. [Google Scholar] [CrossRef]
- Baron, M.; Prusova, B.; Tomaskova, L.; Kumsta, M.; Sochor, J. Terpene Content of Wine from the Aromatic Grape Variety ‘Irsai Oliver’ (Vitis vinifera L.) Depends on Maceration Time. Open Life Sci. 2017, 12, 42–50. [Google Scholar] [CrossRef]
- Šikuten, I.; Štambuk, P.; Karoglan Kontić, J.; Maletić, E.; Tomaz, I.; Preiner, D. Optimization of SPME-Arrow-GC/MS Method for Determination of Free and Bound Volatile Organic Compounds from Grape Skins. Molecules 2021, 26, 7409. [Google Scholar] [CrossRef] [PubMed]
- Radeka, S.; Lukić, I.; Peršurić, Đ. Influence of Different Maceration Treatments on the Aroma Profile of Rosé and Red Wines from Croatian Aromatic cv. Muškat Ruža Porečki (Vitis vinifera L.). Food Technol. Biotechnol. 2012, 50, 442–453. [Google Scholar]
- Lasanta, C.; Cejudo, C.; Gómez, J.; Caro, I. Influence of Prefermentative Cold Maceration on the Chemical and Sensory Properties of Red Wines Produced in Warm Climates. Processes 2023, 11, 374. [Google Scholar] [CrossRef]
- Lukic, I.; Radeka, S.; Grozaj, N.; Staver, M.; Persuric, D. Changes in Physico-Chemical and Volatile Aroma Compound Composition of Gewurztraminer Wine as a Result of Late and Ice Harvest. Food Chem. 2016, 196, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Selli, S.; Canbas, A.; Cabaroglu, T.; Erten, H.; Günata, Z. Aroma Components of cv. Muscat of Bornova Wines and Influence of Skin Contact Treatment. Food Chem. 2006, 94, 319–326. [Google Scholar] [CrossRef]
- Rocha, S.M.; Rodrigues, F.; Coutinho, P.; Delgadillo, I.; Coimbra, M.A. Volatile Composition of Baga Red Wine: Assessment of the Identification of the Would-Be Impact Odourants. Anal. Chim. Acta 2004, 513, 257–262. [Google Scholar] [CrossRef]
- Cai, J.; Zhu, B.-Q.; Wang, Y.-H.; Lu, L.; Lan, Y.-B.; Reeves, M.J.; Duan, C.-Q. Influence of Pre-Fermentation Cold Maceration Treatment on Aroma Compounds of Cabernet Sauvignon Wines Fermented in Different Industrial Scale Fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef]
- Delač Salopek, D.; Horvat, I.; Hranilović, A.; Plavša, T.; Radeka, S.; Pasković, I.; Lukić, I. Diversity of Volatile Aroma Compound Composition Produced by Non-Saccharomyces Yeasts in the Early Phase of Grape Must Fermentation. Foods 2022, 11, 3088. [Google Scholar] [CrossRef]
- Mihnea, M.; González-SanJosé, M.L.; Ortega-Heras, M.; Pérez-Magariño, S. A Comparative Study of the Volatile Content of Mencía Wines Obtained Using Different Pre-Fermentative Maceration Techniques. LWT Food Sci. Technol. 2015, 64, 32–41. [Google Scholar] [CrossRef]
- Herjavec, S.; Majdak, A. The Influence of Maceration on the Composition of Some Volatile Compounds and Sensory Properties of Traminer Wines. Agric. Conspec. Sci. 2002, 67, 11–17. [Google Scholar]
- Gómez García-Carpintero, E.; Gómez Gallego, M.A.; Sánchez-Palomo, E.; González Viñas, M.A. Impact of Alternative Technique to Ageing Using Oak Chips in Alcoholic or in Malolactic Fermentation on Volatile and Sensory Composition of Red Wines. Food Chem. 2012, 134, 851–863. [Google Scholar] [CrossRef]
- Álvarez, I.; Aleixandre, J.L.; García, M.J.; Lizama, V. Impact of Prefermentative Maceration on the Phenolic and Volatile Compounds in Monastrell Red Wines. Anal. Chim. Acta 2006, 563, 109–115. [Google Scholar] [CrossRef]
- Benucci, I.; Luziatelli, F.; Cerreti, M.; Liburdi, K.; Nardi, T.; Vagnoli, P.; Ruzzi, M.; Esti, M. Pre-Fermentative Cold Maceration in the Presence of Non-Saccharomyces Strains: Effect on Fermentation Behaviour and Volatile Composition of a Red Wine. Aust. J. Grape Wine Res. 2018, 24, 267–274. [Google Scholar] [CrossRef]
- Câmara, J.S.; Herbert, P.; Marques, J.C.; Alves, M.A. Varietal Flavour Compounds of Four Grape Varieties Producing Madeira Wines. Anal. Chim. Acta 2004, 513, 203–207. [Google Scholar] [CrossRef]
- Radeka, S.; Lukić, I.; Bavčar, D.; Vanzo, A.; Lisjak, K. Characterization of different wine styles of Istrian Malvasia produced in Croatian and Slovenian Istria on the basis of descriptive sensory analysis of wine. In Proceedings of the 50th Croatian and 10th International Symposium on Agriculture, Opatija, Hrvatska, 16–20 February 2015; pp. 510–515. [Google Scholar]
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascués, E.; Calderón, F.; Benito, A.; Rauhut, D.; Santos, A.; et al. Effects on Varietal Aromas during Wine Making: A Review of the Impact of Varietal Aromas on the Flavor of Wine. Appl. Microbiol. Biotechnol. 2019, 103, 7425–7450. [Google Scholar] [CrossRef]
- Petronilho, S.; Lopez, R.; Ferreira, V.; Coimbra, M.A.; Rocha, S.M. Revealing the Usefulness of Aroma Networks to Explain Wine Aroma Properties: A Case Study of Portuguese Wines. Molecules 2020, 25, 272. [Google Scholar] [CrossRef] [Green Version]
- Jagatić Korenika, A.-M.; Maslov, L.; Jakobović, S.; Palčić, I.; Jeromel, A. Comparative Study of Aromatic and Polyphenolic Profiles of Croatian White Wines Produced by Cold Maceration. Czech J. Food Sci. 2018, 36, 459–469. [Google Scholar] [CrossRef]
- Bestulić, E.; Rossi, S.; Plavša, T.; Bubola, M.; Ilak Peršurić, A.S.; Jeromel, A.; Radeka, S. Relationship between Some Sensory Attributes and Overall Impression of Malvazija Istarska Wines Produced with Different Vinification Techniques. In Proceedings of the 56th Croatian & 16th International Symposium on Agriculture, Vodice, Hrvatska, 5–10 September 2021; pp. 682–686. [Google Scholar]
- Fariña, L.; Villar, V.; Ares, G.; Carrau, F.; Dellacassa, E.; Boido, E. Volatile Composition and Aroma Profile of Uruguayan Tannat Wines. Food Res. Int. 2015, 69, 244–255. [Google Scholar] [CrossRef]
- Flanzy, C. Oenologie: Fondements Scientifiques et Technologiques; Collection Sciences et Techniques Agroalimentaires; Tec & doc-Lavoisier: London, UK; Paris, France; New York, NY, USA, 1998; ISBN 978-2-7430-0243-5. [Google Scholar]
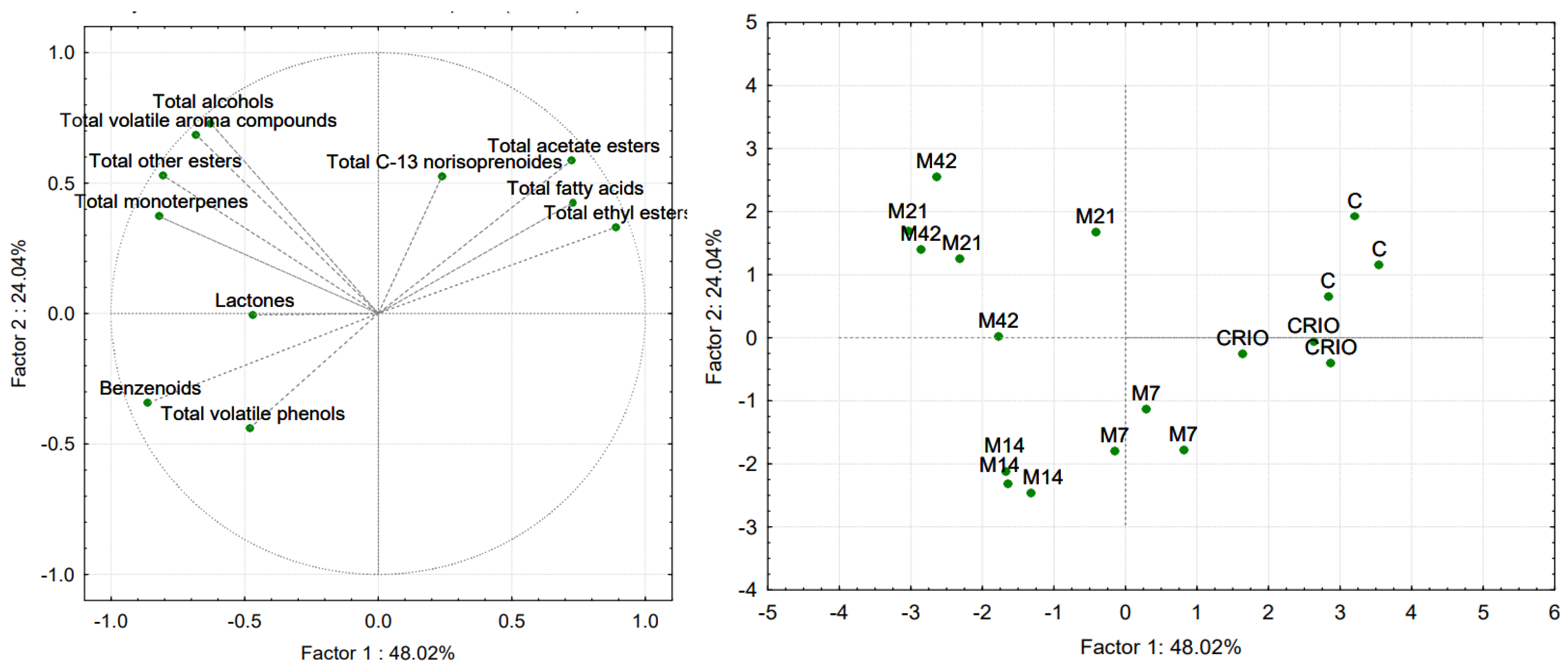


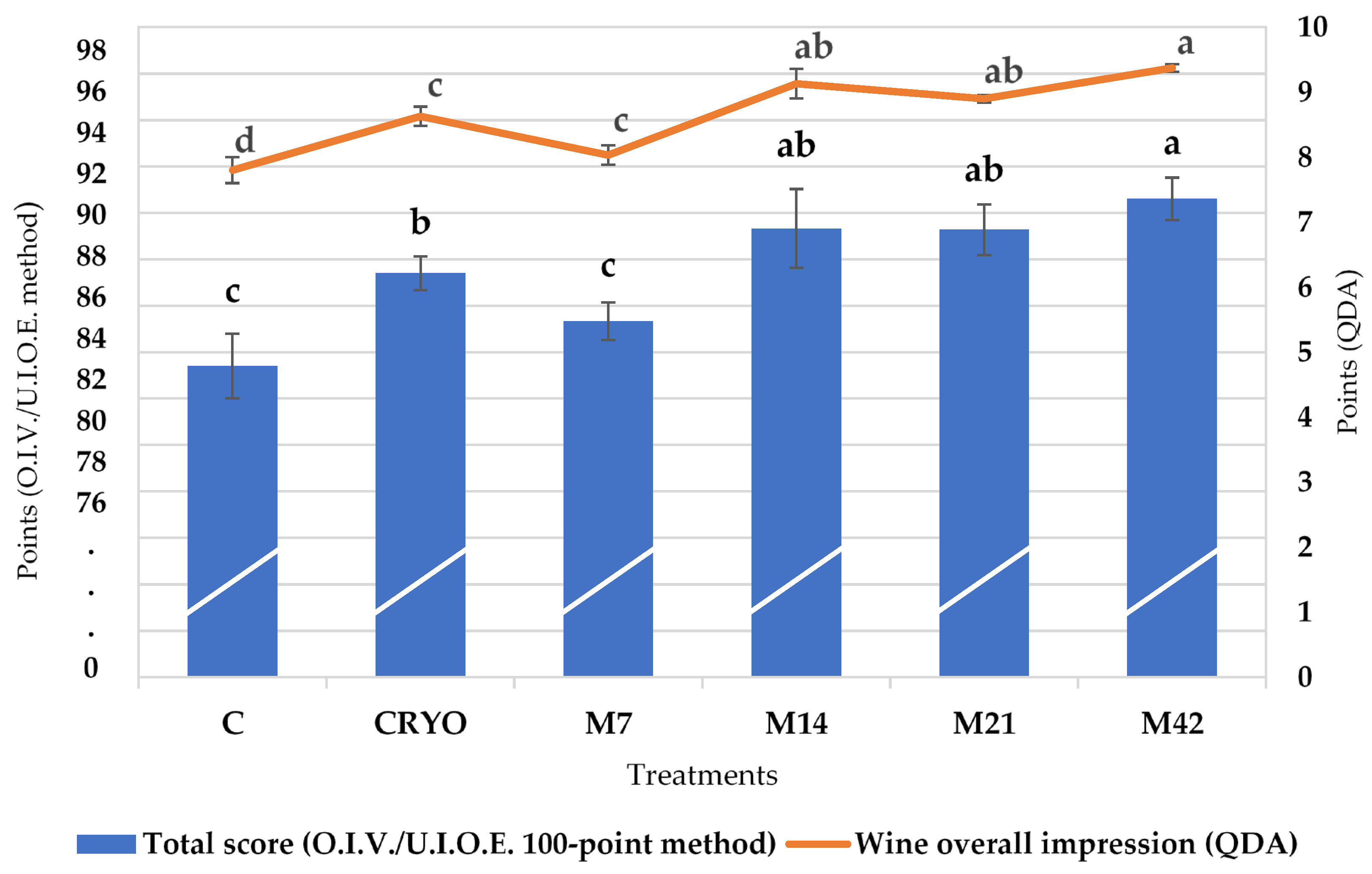
| Standard Physico-Chemical Parameters | Treatment | |||||
|---|---|---|---|---|---|---|
| C | CRYO | M7 | M14 | M21 | M42 | |
| Alcohol (vol%) | 12.69 ± 0.07 a | 12.65 ± 0.03 a | 11.74 ± 0.08 b | 11.69 ± 0.08 b | 11.68 ± 0.08 b | 11.63 ± 0.05 b |
| Total dry extract (g/L) | 19.9 ± 0.10 d | 20.04 ± 0.35 d | 22.6 ± 0.00 a | 21.83 ± 0.25 b | 20.73 ± 0.12 c | 21.67 ± 0.12 b |
| Reducing sugars (g/L) | 1.77 ± 0.06 e | 2.20 ± 0.00 cd | 2.67 ± 0.06 a | 2.43 ± 0.06 b | 2.13 ± 0.06 d | 2.23 ± 0.06 c |
| Extract without reducing sugars (g/L) | 17.13 ± 0.06 d | 16.84 ± 0.35 d | 18.93 ± 0.06 a | 18.40 ± 0.30 b | 17.6 ± 0.10 c | 18.43 ± 0.06 b |
| Ash (g/L) | 2.71 ± 0.05 c | 2.84 ± 0.06 bc | 3.11 ± 0.08 a | 3.13 ± 0.01 a | 2.88 ± 0.21 bc | 2.91 ± 0.03 b |
| pH | 3.48 ± 0.01 c | 3.60 ± 0.07 a | 3.55 ± 0.02 b | 3.62 ± 0.01 a | 3.62 ± 0.02 a | 3.63 ± 0.00 a |
| Total acidity 1 (g/L) | 5.00 ± 0.00 ab | 4.40 ± 0.36 c | 5.37 ± 0.12 a | 4.43 ± 0.06 c | 4.57 ± 0.29 bc | 4.97 ± 0.40 ab |
| Volatile acidity 2 (g/L) | 0.44 ± 0.04 b | 0.42 ± 0.10 b | 0.53 ± 0.25 ab | 0.48 ± 0.11 b | 0.60 ± 0.21 ab | 0.85 ± 0.33 a |
| Volatile Compounds | Treatments | ||||||
|---|---|---|---|---|---|---|---|
| C | CRYO | M7 | M14 | M21 | M42 | ||
| Monoterpenes | |||||||
| Limonene | 2.50 ± 0.53 | 4.04 ± 0.33 | 4.60 ± 0.30 | 3.79 ± 2.69 | 3.11 ± 3.40 | 4.98 ± 0.68 | n.s. |
| Eucalyptol | 0.20 ± 0.17 b | 1.63 ± 2.34 a | 0.48 ± 0.17 b | 0.50 ± 0.07 b | 4.25 ± 2.95 a | 2.34 ± 2.22 ab | |
| β-pinene | 2.83 ± 2.09 b | 5.55 ± 4.65 ab | 10.15 ± 0.49 ab | 12.42 ± 1.61 a | 10.28 ± 7.34 ab | 9.35 ± 6.93 ab | |
| Linalool | 45.06 ± 2.73 c | 73.72 ± 6.91 b | 69.90 ± 0.80 b | 78.89 ± 4.55 b | 113.91 ± 9.33 a | 111.65 ± 20.50 a | |
| 4-Terpineol | 0.43 ± 0.04 d | 0.57 ± 0.04 bc | 0.89 ± 0.05 a | 0.44 ± 0.04 cd | 0.66 ± 0.15 b | 0.67 ± 0.07 b | |
| Menthol | 13.69 ± 3.54 b | 6.94 ± 0.26 c | 18.98 ± 1.66 a | 6.78 ± 0.59 c | 11.18 ± 1.99 b | 12.28 ± 1.16 b | |
| α-Terpineol | 33.12 ± 0.5 b | 33.45 ± 1.69 b | 39.81 ± 2.84 b | 46.52 ± 1.92 b | 76.68 ± 13.77 a | 68.46 ± 12.17 a | |
| Citronellol | 6.05 ± 1.44 cd | 4.62 ± 0.26 d | 8.86 ± 0.97 bc | 11.52 ± 0.27 b | 19.53 ± 2.32 a | 18.54 ± 2.68 a | |
| Geraniol | 37.68 ± 7.32 b | 71.66 ± 5.92 a | 44.21 ± 13.58 b | 46.62 ± 2.95 b | 40.22 ± 12.90 b | 44.79 ± 5.36 b | |
| Geranyl acetone | 2.90 ± 1.37 a | 2.18 ± 0.15 ab | 1.99 ± 0.37 ab | 0.96 ± 0.18 b | 2.06 ± 0.17 ab | 2.80 ± 1.47 a | |
| trans-Nerolidol | 7.26 ± 1.16 a | 5.95 ± 1.18 a | 2.87 ± 0.44 b | 2.47 ± 0.75 b | 2.98 ± 0.24 b | 1.87 ± 0.67 b | |
| trans-Rose oxide | 0.58 ± 0.02 d | 0.58 ± 0.04 d | 0.99 ± 0.04 c | 0.96 ± 0.07 c | 1.34 ± 0.05 a | 1.16 ± 0.19 b | |
| Total monoterpenes | 152.31 ± 12.60 c | 210.89 ± 13.92 b | 203.73 ± 20.67 b | 211.89 ± 11.19 b | 286.18 ± 47.82 a | 278.87 ± 42.35 a | |
| C13-norisoprenoides | |||||||
| Vitispirane I | 2.92 ± 0.05 d | 4.04 ± 0.67 cd | 5.26 ± 0.17 bc | 5.39 ± 0.37 b | 6.38 ± 0.49 ab | 6.78 ± 1.53 a | |
| Vitispirane II | 2.25 ± 0.12 c | 2.73 ± 0.46 bc | 3.17 ± 0.26 bc | 3.27 ± 0.35 bc | 5.58 ± 1.21 a | 3.92 ± 0.92 b | |
| β-Damascenone | 18.76 ± 1.78 ab | 23.22 ± 0.61 a | 15.89 ± 4.05 bc | 12.07 ± 3.18 c | 19.27 ± 5.39 ab | 11.08 ± 1.42 c | |
| β-Ionone | 2.41 ± 1.27 | 1.18 ± 0.64 | 3.43 ± 0.61 | 1.73 ± 0.24 | 2.94 ± 0.49 | 3.28 ± 20.89 | |
| α-Isomethyl ionone | 6.78 ± 6.50 | 2.94 ± 0.71 | 3.65 ± 1.05 | 2.88 ± 1.22 | 1.98 ± 0.71 | 2.76 ± 1.38 | n.s. |
| Total C13-norisoprenoides | 33.12 ± 8.21 ab | 34.11 ± 2.84 ab | 31.40 ± 5.42 ab | 25.34 ± 1.84 b | 36.15 ± 5.06 ab | 27.83 ± 4.92 ab | |
| Alcohols | |||||||
| 1-Hexanol | 3006 ± 79 b | 1646 ± 26 d | 2123 ± 110 cd | 2221 ± 41 c | 4329 ± 195 a | 4752 ± 746 a | |
| trans-3-Hexen-1-ol | 207.04 ± 6.33 a | 65.76 ± 3.48 c | 51.58 ± 0.22 d | 53.45 ± 1.23 cd | 97.61 ± 3.45 b | 89.28 ± 16.21 b | |
| cis-3-Hexen-1-ol | 169.67 ± 10.77 a | 84.38 ± 5.69 b | 46.74 ± 2.08 d | 38.55 ± 1.15 d | 75.17 ± 11.11 bc | 69.91 ± 10.26 c | |
| 2-Phenylethyl Alcohol | 51,271 ± 1578 b | 28,495 ± 1114 c | 38,950 ± 1747 bc | 41,021 ± 951 bc | 77,248 ± 5958 a | 79,674 ± 17,382 a | |
| Total alcohols | 54,654 ± 1631 b | 30,291 ± 1091 c | 41,172 ± 1652 bc | 43,334 ± 927 bc | 81,750 ± 5817 a | 84,585 ± 18,133 a | |
| Fatty acids | |||||||
| Butanoic acid | 4015 ± 324 a | 2425 ± 42 b | 1421 ± 98 d | 1062 ± 28 d | 1854 ± 152 c | 1818 ± 339 c | |
| Hexanoic acid | 12,527 ± 1491 a | 5956 ± 615 abc | 2138 ± 144 bx | 1285 ± 115 c | 3820 ± 1595 bc | 9834 ± 11,287 ab | |
| Octanoic Acid | 11,821 ± 1317 a | 9532 ± 407 b | 2460 ± 117 cd | 1550 ± 73 d | 2703 ± 169 c | 2824 ± 110 c | |
| Nonanoic acid | 84.36 ± 104.91 | 64.33 ± 101.50 | 24.61 ± 19.54 | 19.81 ± 3.12 | 46.85 ± 65.00 | 51.31 ± 51.91 | n.s. |
| n-Decanoic acid | 3558 ± 644 a | 3212 ± 405 a | 526 ± 4 b | 271 ± 21 b | 441 ± 111 b | 339 ± 59 b | |
| Total fatty acids | 32,006 ± 3466 a | 21,189 ± 538 b | 6569 ± 282 cd | 4188 ± 194 d | 8865 ± 1458 cd | 14,866 ± 11,098 bc | |
| Ethyl esters | |||||||
| Ethyl butanoate | 853.76 ± 59.49 a | 561.38 ± 10.47 b | 232.87 ± 22.28 cd | 174.34 ± 4.82 d | 273.98 ± 34.40 c | 242.07 ± 38.89 c | |
| Ethyl 2-methylbutanoate | 26.13 ± 2.29 a | 14.20 ± 1.17 c | 19.72 ± 2.01 bc | 16.86 ± 0.42 c | 28.70 ± 4.85 a | 25.17 ± 5.05 ab | |
| Ethyl 3-methylbutanoate | 53.08 ± 6.90 a | 28.58 ± 3.00 b | 34.47 ± 3.01 b | 30.40 ± 1.90 b | 53.14 ± 9.96 a | 46.74 ± 9.09 a | |
| Ethyl pentanoate | 2.27 ± 0.14 c | 4.93 ± 0.31 a | 2.16 ± 0.21 c | 2.56 ± 0.07 c | 3.65 ± 0.44 b | 3.85 ± 0.48 b | |
| Ethyl hexanoate | 1566 ± 110 a | 1463 ± 10 a | 482 ± 45 b | 340 ± 8 c | 481 ± 44 b | 467 ± 87 b | |
| Ethyl octanoate | 4348 ± 555 a | 3908 ± 239 a | 773 ± 70 b | 505 ± 41 b | 871 ± 108 b | 732 ± 187 b | |
| Ethyl 3-furoate | 188.41 ± 20.3 a | 115.19 ± 12.48 b | 77.12 ± 10.64 c | 63.81 ± 6.06 c | 90.33 ± 18.34 bc | 78.93 ± 19.00 c | |
| Ethyl hex-4-enoate | 9.3 ± 1.55 a | 5.86 ± 1.0 b | 2.78 ± 0.22 d | 3.28 ± 0.08 cd | 6.38 ± 2.1 b | 5.22 ± 0.96 bc | |
| Ethyl 2-hexenoate | 232.1 ± 11.36 a | 64.5 ± 1.65 c | 49.84 ± 4.18 d | 50.2 ± 0.13 d | 82.05 ± 8.18 b | 70.55 ± 12.6 bc | |
| Ethyl cinnamate | 10.02 ± 0.83 ab | 11.42 ± 2 a | 3.92 ± 6.32 ab | 2.81 ± 4.02 b | 8.88 ± 6.54 ab | 7.04 ± 3.42 ab | |
| Total ethyl esters | 7290 ± 724 a | 6177 ± 227 b | 1678 ± 128 cd | 1189 ± 49 d | 1899 ± 196 c | 1679 ± 356 cd | |
| Acetate esters | |||||||
| Butyl acetate | 0.12 ± 0.03 ab | 0.08 ± 0.02 b | 0.15 ± 0.01 a | 0.07 ± 0.01 b | 0.14 ± 0.06 a | 0.16 ± 0.02 a | |
| Isoamyl acetate | 2870 ± 258 a | 1452 ± 404 b | 617 ± 317 c | 440 ± 25 c | 1062 ± 528 bc | 1030 ± 522 bc | |
| Hexyl acetate | 91.88 ± 14.37 a | 39.81 ± 36.17 b | 3.78 ± 2.12 c | 3.49 ± 0.32 c | 6.35 ± 3.63 c | 8.07 ± 4.05 c | |
| 2-Phenethyl acetate | 164.6 ± 21.99 a | 83.41 ± 29.13 b | 30.79 ± 8.60 c | 25.31 ± 0.60 c | 43.27 ± 8.58 c | 47.89 ± 15.84 c | |
| Isobornyl acetate | 14.14 ± 10.90 a | 6.45 ± 0.26 ab | 13.48 ± 1.12 ab | 5.69 ± 0.50 b | 6.41 ± 1.35 ab | 5.90 ± 1.01 b | |
| Total acetate esters | 3141 ± 294 a | 1582 ± 466 b | 667 ± 328 c | 475 ± 25 c | 1118 ± 542 bc | 1092 ± 542 bc | |
| Other esters | |||||||
| Ethyl lactate | 54,251 ± 1501 c | 50,485 ± 32073 c | 43,406 ± 2542 c | 97,682 ± 90 b | 160,331 ± 2903 a | 152,140 ± 23,248 a | |
| Diethyl succinate | 10,966 ± 469 c | 4609 ± 101 d | 4608 ± 442 d | 5398 ± 83 cd | 18,615 ± 5155 b | 36,258 ± 5934 a | |
| Isoamyl propanoate | 0.11 ± 0.01 c | 0.08 ± 0.02 d | 0.21 ± 0.03 ab | 0.17 ± 0.00 b | 0.23 ± 0.02 a | 0.17 ± 0.04 b | |
| Isoamyl lactate | 2340 ± 95 c | 2047 ± 1469 c | 2571 ± 100 c | 9588 ± 232 b | 16,057 ± 1824 a | 15,660 ± 1916 a | |
| n-Hexyl salicylate | 14.54 ± 4.37 | 17.64 ± 2.50 | 18.40 ± 4.94 | 18.26 ± 3.62 | 11.10 ± 0.01 | 15.95 ± 10.56 | n.s. |
| Total other esters | 67,573 ± 1156 c | 57,159 ± 33,450 c | 50,603 ± 2206 c | 112,686 ± 337 b | 195,015 ± 5677 a | 204,075 ± 30,942 a | |
| Volatile phenols | |||||||
| 4-Ethylguaiacol | 2.38 ± 1.88 c | 1.25 ± 0.43 c | 2.22 ± 0.10 c | 75.76 ± 13.71 a | 40.90 ± 42.06 b | 5.79 ± 0.88 c | |
| Eugenol | 0.81 ± 0.05 | 0.65 ± 0.40 | 1.22 ± 1.17 | 1.48 ± 0.36 | 0.82 ± 0.80 | 1.71 ± 2.23 | n.s. |
| 4-Ethylphenol | 13.21 ± 1.28 | 14.51 ± 2.40 | 14.13 ± 2.88 | 17.14 ± 6.22 | 12.74 ± 2.83 | 11.83 ± 0.66 | n.s. |
| 4-Vinylguaiacol | 23.36 ± 2.50 ab | 26.31 ± 4.29 ab | 19.54 ± 14.28 ab | 29.79 ± 5.21 a | 15.01 ± 11.88 b | 21.8 ± 0.74 ab | |
| Total volatile phenols | 39.76 ± 2.15 bc | 42.72 ± 6.76 bc | 37.11 ± 18.42 c | 124.18 ± 14.68 a | 69.47 ± 36.07 b | 41.14 ± 2.74 bc | |
| Benzenoids | |||||||
| Benzaldehyde | 1.99 ± 0.14 c | 3.74 ± 0.84 c | 11.99 ± 1.04 b | 20.64 ± 1.12 a | 11.46 ± 2.30 b | 19.86 ± 3.96 a | |
| Lactones | |||||||
| γ -Nonalactone | 24.57 ± 3.24 | 21.69 ± 1.47 | 26.31 ± 5.20 | 25.82 ± 3.45 | 24.86 ± 8.35 | 27.80 ± 1.84 | n.s. |
| Total volatile compounds | 164,915 ± 3351 b | 116,711 ± 31,492 c | 101,000 ± 989 c | 162,279 ± 1431 b | 289,075 ± 9415 a | 306,692 ± 39,715 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radeka, S.; Bestulić, E.; Rossi, S.; Orbanić, F.; Bubola, M.; Plavša, T.; Lukić, I.; Jeromel, A. Effect of Different Vinification Techniques on the Concentration of Volatile Aroma Compounds and Sensory Profile of Malvazija Istarska Wines. Fermentation 2023, 9, 676. https://doi.org/10.3390/fermentation9070676
Radeka S, Bestulić E, Rossi S, Orbanić F, Bubola M, Plavša T, Lukić I, Jeromel A. Effect of Different Vinification Techniques on the Concentration of Volatile Aroma Compounds and Sensory Profile of Malvazija Istarska Wines. Fermentation. 2023; 9(7):676. https://doi.org/10.3390/fermentation9070676
Chicago/Turabian StyleRadeka, Sanja, Ena Bestulić, Sara Rossi, Fumica Orbanić, Marijan Bubola, Tomislav Plavša, Igor Lukić, and Ana Jeromel. 2023. "Effect of Different Vinification Techniques on the Concentration of Volatile Aroma Compounds and Sensory Profile of Malvazija Istarska Wines" Fermentation 9, no. 7: 676. https://doi.org/10.3390/fermentation9070676
APA StyleRadeka, S., Bestulić, E., Rossi, S., Orbanić, F., Bubola, M., Plavša, T., Lukić, I., & Jeromel, A. (2023). Effect of Different Vinification Techniques on the Concentration of Volatile Aroma Compounds and Sensory Profile of Malvazija Istarska Wines. Fermentation, 9(7), 676. https://doi.org/10.3390/fermentation9070676








