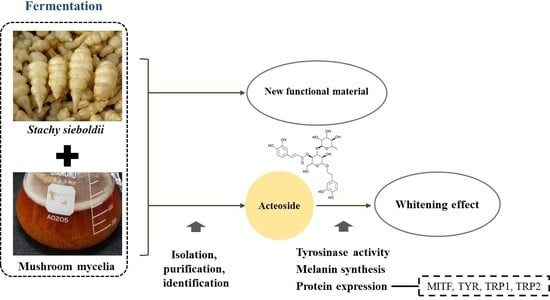
Whitening Activity of Acteoside from Stachys sieboldii Fermented with Hericium erinaceus Mycelia on Melanocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of S. sieboldii
2.3. Extraction and Fermentation
2.4. Liquid–Liquid Partition
2.5. Isolation and Purification
2.6. NMR Analysis
2.7. Cell Culture
2.8. Cell Viability Assay (MTS Assay)
2.9. Mushroom Tyrosinase Activity Assay
2.10. Intracellular Tyrosinase Activity Assay
2.11. Measurement of Melanin Content
2.12. Western Blotting
2.13. Statistical Analysis
3. Results
3.1. Effects of Fermentation Using Different Mushroom Mycelia on Extract Yields
3.2. Effect of S. sieboldii Extract Fermented with Mushroom Mycelia
3.3. Cytotoxic and Whitening Effects of Solvent Fractions of S. sieboldii Extract Fermented by H. erinaceus Mycelia
3.4. Isolation and Purification of the Major Compounds in the Ethyl Acetate Extract Sub-Fractions
3.5. Structural Characterization of the Isolated Compound
3.6. Effects of Acteoside on Cell Viability and Whitening Effect
3.7. Effects of Acteoside on the Expression of Melanogenesis-Related Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krutmann, J.; Humbert, P. Nutrition for Healthy Skin; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Wu, P.-Y.; Lin, T.-Y.; Hou, C.-W.; Chang, Q.-X.; Wen, K.-C.; Lin, C.-Y.; Chiang, H.-M. 1, 2-bis [(3-methoxyphenyl) methyl] ethane-1, 2-dicarboxylic acid reduces uvb-induced photodamage in vitro and in vivo. Antioxidants 2019, 8, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penna, I.; Albanesi, E.; Bertorelli, R.; Bandiera, T.; Russo, D. Cytoprotective, anti-inflammatory, and antioxidant properties of high-molecular-weight hyaluronan enriched with red orange extract in human fibroblasts exposed to ultra violet light B irradiation. Biotechnol. Appl. Biochem. 2019, 66, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Durai, P.C.; Thappa, D.M.; Kumari, R.; Malathi, M. Aging in elderly: Chronological versus photoaging. Indian J. Dermatol. 2012, 57, 343. [Google Scholar] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Brown, D.A. Skin pigmentation enhancers. Compr. Ser. Photosciences 2001, 3, 637–675. [Google Scholar]
- Chiang, H.-M.; Chien, Y.-C.; Wu, C.-H.; Kuo, Y.-H.; Wu, W.-C.; Pan, Y.-Y.; Su, Y.-H.; Wen, K.-C. Hydroalcoholic extract of Rhodiola rosea L.(Crassulaceae) and its hydrolysate inhibit melanogenesis in B16F0 cells by regulating the CREB/MITF/tyrosinase pathway. Food Chem. Toxicol. 2014, 65, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Sarna, M.; Zadlo, A.; Hermanowicz, P.; Madeja, Z.; Burda, K.; Sarna, T. Cell elasticity is an important indicator of the metastatic phenotype of melanoma cells. Exp. Dermatol. 2014, 23, 813–818. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Medrano, E.E. Melanin accumulation accelerates melanocyte senescence by a mechanism involving p16INK4a/CDK4/pRB and E2F1. Ann. N. Y. Acad. Sci. 2000, 908, 71–84. [Google Scholar] [CrossRef]
- Bonaventure, J.; Domingues, M.J.; Larue, L. Cellular and molecular mechanisms controlling the migration of melanocytes and melanoma cells. Pigment Cell Melanoma Res. 2013, 26, 316–325. [Google Scholar] [CrossRef]
- Gillbro, J.; Olsson, M. The melanogenesis and mechanisms of skin-lightening agents–existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef]
- Thody, A.J.; Higgins, E.M.; Wakamatsu, K.; Ito, S.; Burchill, S.A.; Marks, J.M. Pheomelanin as well as eumelanin is present in human epidermis. J. Investig. Dermatol. 1991, 97, 340–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sõukand, R.; Pieroni, A.; Biró, M.; Dénes, A.; Dogan, Y.; Hajdari, A.; Kalle, R.; Reade, B.; Mustafa, B.; Nedelcheva, A. An ethnobotanical perspective on traditional fermented plant foods and beverages in Eastern Europe. J. Ethnopharmacol. 2015, 170, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Bleotu, A.; Mandravel, C.; Ciuculescu, C. Characterization of some glycoside iridoids by mass spectrometry. Rom. Biotechnol. Lett. 2006, 11, 2643. [Google Scholar]
- Chung, H.-J.; Kim, W.K.; Park, H.J.; Cho, L.; Kim, M.-r.; Kim, M.J.; Shin, J.-S.; Lee, J.H.; Ha, I.-H.; Lee, S.K. Anti-osteoporotic activity of harpagide by regulation of bone formation in osteoblast cell culture and ovariectomy-induced bone loss mouse models. J. Ethnopharmacol. 2016, 179, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Bujak, T.; Zagórska-Dziok, M.; Wójciak, M.; Sowa, I. Effect of fermentation time on the content of bioactive compounds with cosmetic and dermatological properties in Kombucha Yerba Mate extracts. Sci. Rep. 2021, 11, 18792. [Google Scholar] [CrossRef]
- Chen, L.; Song, P.; Jia, F.; Wang, J.S. Reducing the allergenicity from food by microbial fermentation. Adv. Mater. Res. 2012, 524, 2302–2305. [Google Scholar] [CrossRef]
- El-Ghaish, S.; Ahmadova, A.; Hadji-Sfaxi, I.; El Mecherfi, K.E.; Bazukyan, I.; Choiset, Y.; Rabesona, H.; Sitohy, M.; Popov, Y.G.; Kuliev, A.A. Potential use of lactic acid bacteria for reduction of allergenicity and for longer conservation of fermented foods. Trends Food Sci. Technol. 2011, 22, 509–516. [Google Scholar] [CrossRef]
- Benson, K.F.; Stamets, P.; Davis, R.; Nally, R.; Taylor, A.; Slater, S.; Jensen, G.S. The mycelium of the Trametes versicolor (Turkey tail) mushroom and its fermented substrate each show potent and complementary immune activating properties in vitro. BMC Complement. Altern. Med. 2019, 19, 342. [Google Scholar] [CrossRef] [Green Version]
- Matsui, T. Development of functional foods by mushroom fermentation. Mushroom Sci. Biotechnol. 2017, 24, 169–175. [Google Scholar]
- Yin, J.; Yang, G.; Wang, S.; Chen, Y. Purification and determination of stachyose in Chinese artichoke (Stachys Sieboldii Miq.) by high-performance liquid chromatography with evaporative light scattering detection. Talanta 2006, 70, 208–212. [Google Scholar] [CrossRef]
- Jeon, K.-S.; Lee, N.-H.; Park, S.-I. Quality characteristics of white pan bread with Chinese artichoke (Stachys sieboldii MIQ) powder. Culin. Sci. Hosp. Res. 2015, 21, 1–15. [Google Scholar]
- Lee, J.E.; Jin, S.Y.; Han, Y.S. Antioxidant activities and quality characteristics of tofu supplemented with Chinese artichoke powder. Korean J. Food Nutr. 2014, 27, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Goren, A.C.; Piozzi, F.; Akcicek, E.; Kılıç, T.; Çarıkçı, S.; Mozioğlu, E.; Setzer, W.N. Essential oil composition of twenty-two Stachys species (mountain tea) and their biological activities. Phytochem. Lett. 2011, 4, 448–453. [Google Scholar] [CrossRef]
- Na, B.-R.; Lee, J.-H. Antioxidative capacities of Stachys sieboldii MIQ and ginseng powders and their effects on quality characteristics of cookies. J. Korean Soc. Food Sci. Nutr. 2017, 46, 68–76. [Google Scholar] [CrossRef]
- Slobodianiuk, L.; Budniak, L.; Marchyshyn, S.; Demydiak, O. Investigation of the anti-inflammatory effect of the dry extract from the herb of Stachys sieboldii Miq. Pharmacologyonline 2021, 2, 590–597. [Google Scholar]
- Baek, H.; Song, S.; Na, Y.; Ryu, B. Antioxidant activities of Stachys sieboldii MIQ. stalks. Korean J. Biotechnol. Bioeng. 2003, 18, 266–271. [Google Scholar]
- Kim, H.; Choi, J.; Cho, J.K.; Kim, S.Y.; Lee, Y.-S. Solid-phase synthesis of kojic acid-tripeptides and their tyrosinase inhibitory activity, storage stability, and toxicity. Bioorg. Med. Chem. Lett. 2004, 14, 2843–2846. [Google Scholar] [CrossRef]
- Lin, C.-H.V.; Ding, H.-Y.; Kuo, S.-Y.; Chin, L.-W.; Wu, J.-Y.; Chang, T.-S. Evaluation of in vitro and in vivo depigmenting activity of raspberry ketone from Rheum officinal e. Int. J. Mol. Sci. 2011, 12, 4819–4835. [Google Scholar] [CrossRef] [Green Version]
- Hart, S.N.; Therneau, T.M.; Zhang, Y.; Poland, G.A.; Kocher, J.-P. Calculating sample size estimates for RNA sequencing data. J. Comput. Biol. 2013, 20, 970–978. [Google Scholar] [CrossRef] [Green Version]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Barwick, B.; Abramovitz, M.; Kodani, M.; Moreno, C.; Nam, R.; Tang, W.; Bouzyk, M.; Seth, A.; Leyland-Jones, B. Prostate cancer genes associated with TMPRSS2–ERG gene fusion and prognostic of biochemical recurrence in multiple cohorts. Br. J. Cancer 2010, 102, 570–576. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Tan, J.; Gou, Y.; Liao, Y.; Xu, F.; Li, G.; Cao, J.; Yao, J.; Ye, J.; Tang, N. The biocontrol of postharvest decay of table grape by the application of kombucha during cold storage. Sci. Hortic. 2019, 253, 134–139. [Google Scholar] [CrossRef]
- Wu, A.Y.T.; Ueda, K.; Lai, C.P.K. Proteomic analysis of extracellular vesicles for cancer diagnostics. Proteomics 2019, 19, 1800162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Paul, S.; Andrew, M.; Owen, O. Baliga Nitin S, Wang Jonathan T, Ramage Daniel, Amin Nada, Schwikowski Benno, Ideker Trey. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar]
- Guo, H.; Liu, A.-H.; Li, L.; Guo, D.-A. Simultaneous determination of 12 major constituents in Forsythia suspensa by high performance liquid chromatography—DAD method. J. Pharm. Biomed. Anal. 2007, 43, 1000–1006. [Google Scholar] [CrossRef]
- Ashraf, Z.; Rafiq, M.; Nadeem, H.; Hassan, M.; Afzal, S.; Waseem, M.; Afzal, K.; Latip, J. Carvacrol derivatives as mushroom tyrosinase inhibitors; synthesis, kinetics mechanism and molecular docking studies. PLoS ONE 2017, 12, e0178069. [Google Scholar] [CrossRef] [Green Version]
- MTH, Z.S.B.A.K. Munoz-munoz j. Garcia-molina f. Garcia-canoras f. Sabour aa j. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar]
- Song, H.S.; Sim, S.S. Acetoside inhibits α-MSH-induced melanin production in B16 melanoma cells by inactivation of adenyl cyclase. J. Pharm. Pharmacol. 2009, 61, 1347–1351. [Google Scholar] [CrossRef]
- Wu, S.-C.; Chen, R.-J.; Lee, K.-W.; Tung, C.-C.; Lin, W.-P.; Yi, P. Angioembolization as an effective alternative for hemostasis in intractable life-threatening maxillofacial trauma hemorrhage: Case study. Am. J. Emerg. Med. 2007, 25, 988.e1–988.e5. [Google Scholar] [CrossRef] [PubMed]






| Parameter | Condition | ||
|---|---|---|---|
| Instrument | Agilent Technologies 6410 Triple Quad | ||
| Column | Kromasil C18 (3.0 mm × 150 mm, 3.0 μm) | ||
| Solvent * | A: Distilled water B: Acetonitrile | ||
| Gradient | Time (min) | B% | |
| 1 | 0 | 5 | |
| 2 | 30 | 100 | |
| 3 | 32 | 100 | |
| Flow rate | 0.4 mL/min | ||
| Injection volume | 5 µL | ||
| Sample (1) | Extract Yield (%) | |
|---|---|---|
| S. sieboldii hot water extract | 5.44 | |
| S. sieboldii hot water extract | H. erinaceus mycelia | 7.65 |
| W. extensa mycelia | 6.3 | |
| L. edodes mycelia | 8.06 | |
| G. lucidum mycelia | 5.91 | |
| Compound | ||
|---|---|---|
| Position | δH | δC |
| 1 | - | 129.9 |
| 2 | 6.68 (d, J = 1.8 Hz) | 114.7 |
| 3 | - | 143.1 |
| 4 | - | 145.3 |
| 5 | 6.66 (d, J = 7.8 Hz) | 115.6 |
| 6 | 6.55 (dd, J = 7.8, 1.8 Hz) | 119.7 |
| 7 | 2.79 (m) | 35.0 |
| 8 | 4.04 (m) | 70.8 |
| 1′ | - | 126.1 |
| 2′ | 7.04 (d, J = 1.8 Hz) | 113.7 |
| 3′ | - | 144.6 |
| 4′ | - | 148.2 |
| 5′ | 6.70 (d, J = 7.8 Hz) | 114.9 |
| 6′ | 6.94 (dd, J = 7.8, 1.8 Hz) | 121.7 |
| 7′ | 7.58 (d, J = 15.6 Hz) | 146.5 |
| 8′ | 6.26 (d, J = 15.6 Hz) | 113.1 |
| c = 0 | - | 166.7 |
| 1″ | 4.36 (d, J = 7.8 Hz) | 102.7 |
| 2″ | 3.54 (m) | 74.5 |
| 3″ | 3.80 (t-like, J = 8.9 Hz) | 80.1 |
| 4″ | 4.91 (t-like, J = 8.9 Hz) | 69.0 |
| 5″ | 3.38 (t-like, J = 8.9 Hz) | 74.7 |
| 6″ | 3.70 (m), 3.60 (m) | 60.8 |
| 1‴ | 5.17 (d, J = 1.9 Hz) | 101.5 |
| 2‴ | 3.71 (m) | 70.5 |
| 3‴ | 3.90 (m) | 70.7 |
| 4‴ | 3.28 (t-like, J = 9.5 Hz) | 72.2 |
| 5‴ | 3.55 (m) | 68.9 |
| 6‴ | 1.08 (d, J = 5.9 Hz) | 16.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, S.-B.; Mun, S.-K.; Ha, N.-I.; Jang, H.-Y.; Kang, K.-Y.; Park, K.-W.; Seo, K.-S.; Kim, K.-J.; Yee, S.-T. Whitening Activity of Acteoside from Stachys sieboldii Fermented with Hericium erinaceus Mycelia on Melanocytes. Fermentation 2023, 9, 697. https://doi.org/10.3390/fermentation9080697
Im S-B, Mun S-K, Ha N-I, Jang H-Y, Kang K-Y, Park K-W, Seo K-S, Kim K-J, Yee S-T. Whitening Activity of Acteoside from Stachys sieboldii Fermented with Hericium erinaceus Mycelia on Melanocytes. Fermentation. 2023; 9(8):697. https://doi.org/10.3390/fermentation9080697
Chicago/Turabian StyleIm, Seung-Bin, Seul-Ki Mun, Neul-I Ha, Ho-Yeol Jang, Kyung-Yun Kang, Kyung-Wuk Park, Kyoung-Sun Seo, Kyung-Je Kim, and Sung-Tae Yee. 2023. "Whitening Activity of Acteoside from Stachys sieboldii Fermented with Hericium erinaceus Mycelia on Melanocytes" Fermentation 9, no. 8: 697. https://doi.org/10.3390/fermentation9080697