Bioacetoin Production by Bacillus subtilis subsp. subtilis Using Enzymatic Hydrolysate of Lignocellulosic Biomass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of Bacterial Strain
2.2. Production of Bioacetoin by Bacillus subtilis subsp. subtilis JJBS250
2.3. Estimation of Bioacetoin
2.4. Optimization of the Culture Conditions for Bioacetoin Production in Submerged Fermentation
2.5. Analysis of the Presence of Bioacetoin by Thin Layer Chromatography
2.6. Physico-Chemical Pretreatment of Lignocellulosic Biomass for Bioacetoin Production
2.7. Cellulase Production and Enzymatic Saccharification of Pretreated Lignocellulosic Biomass for Bioacetoin Production
2.8. Fermentation of Enzymatic Hydrolysate of Rice Straw and Sugarcane Bagasse for Bioacetoin Production by Bacterial Culture
2.9. Statistical Analysis
3. Results and Discussion
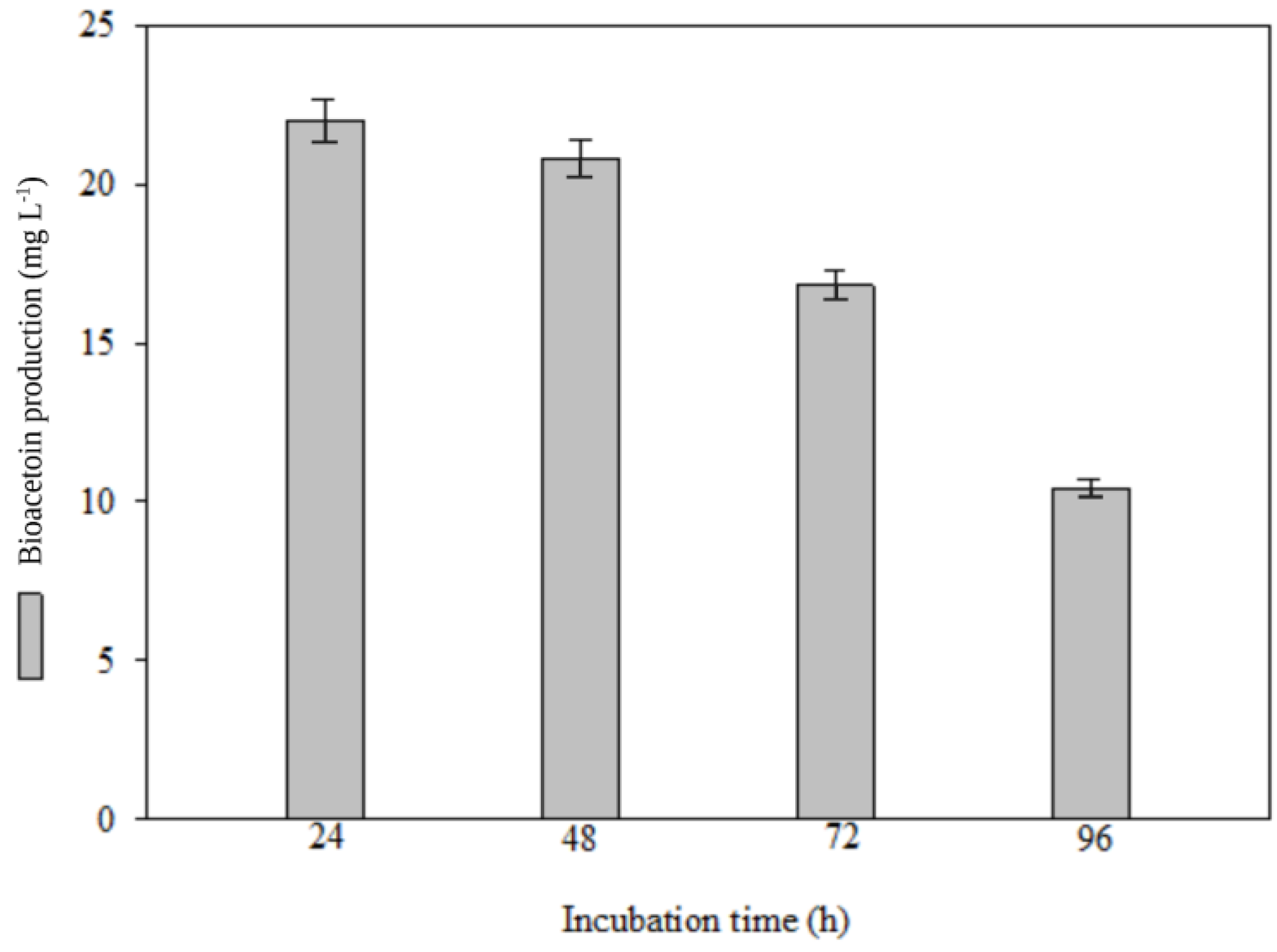
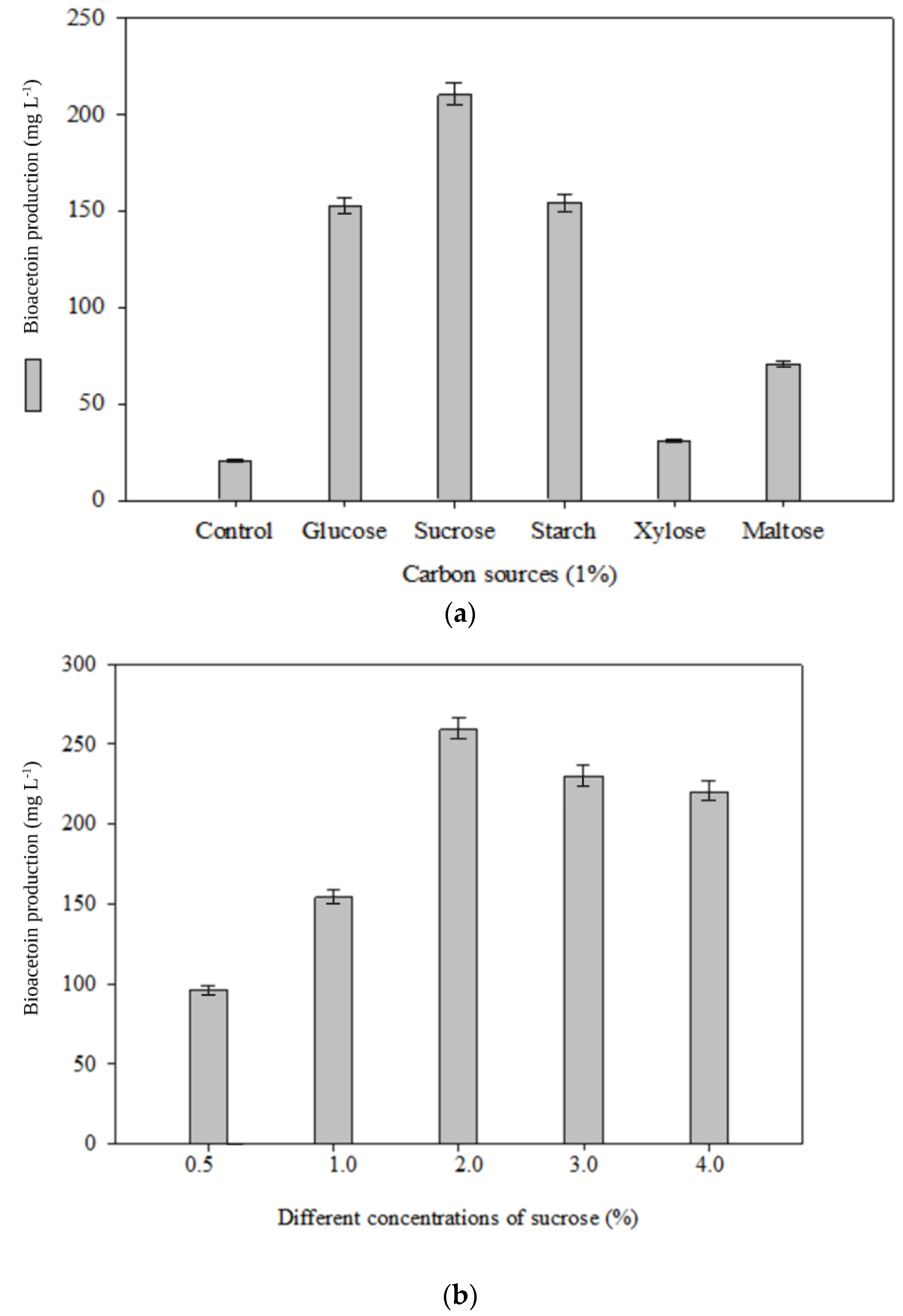
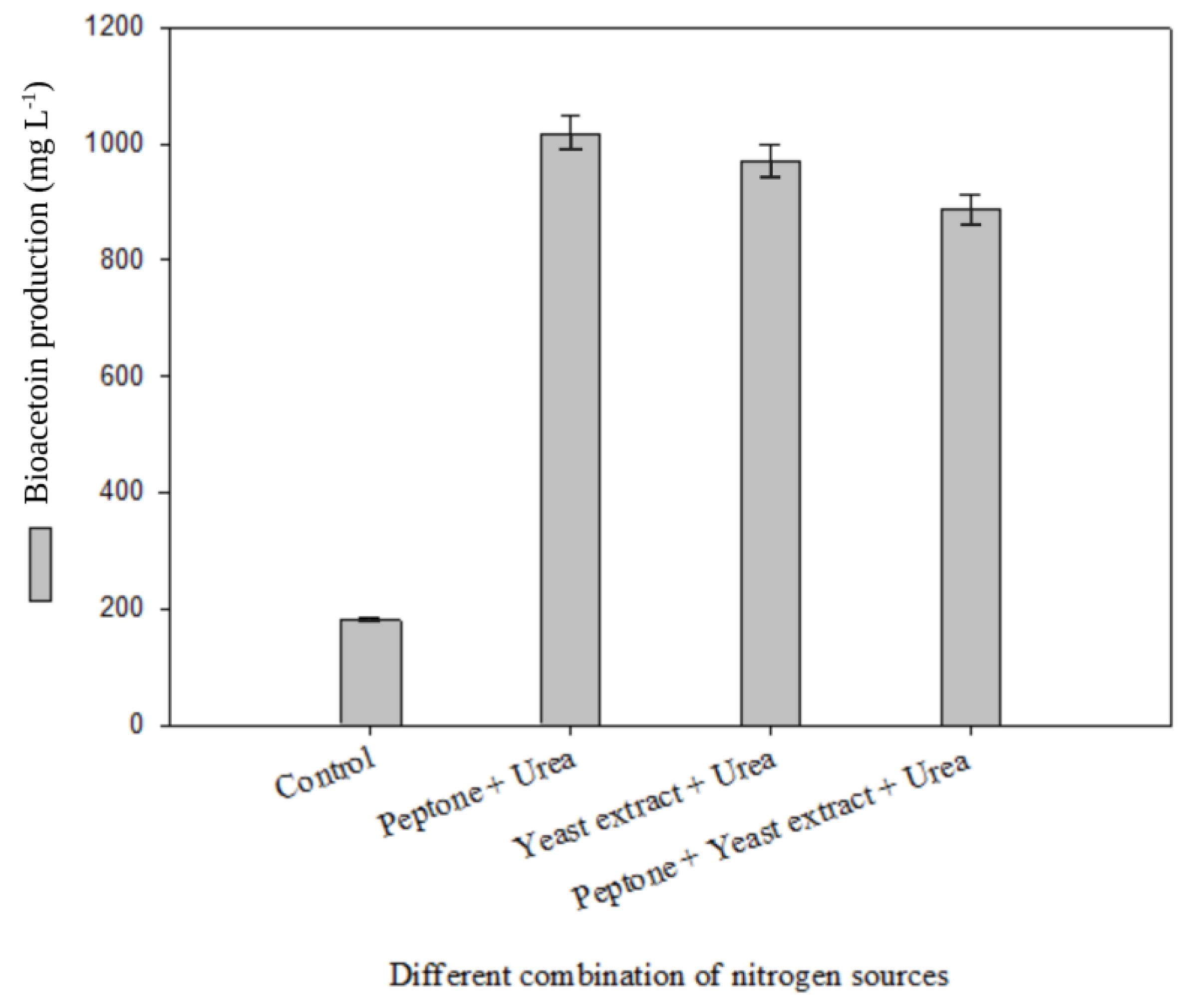
3.1. Optimization of Bioacetoin Production by Bacterial Culture
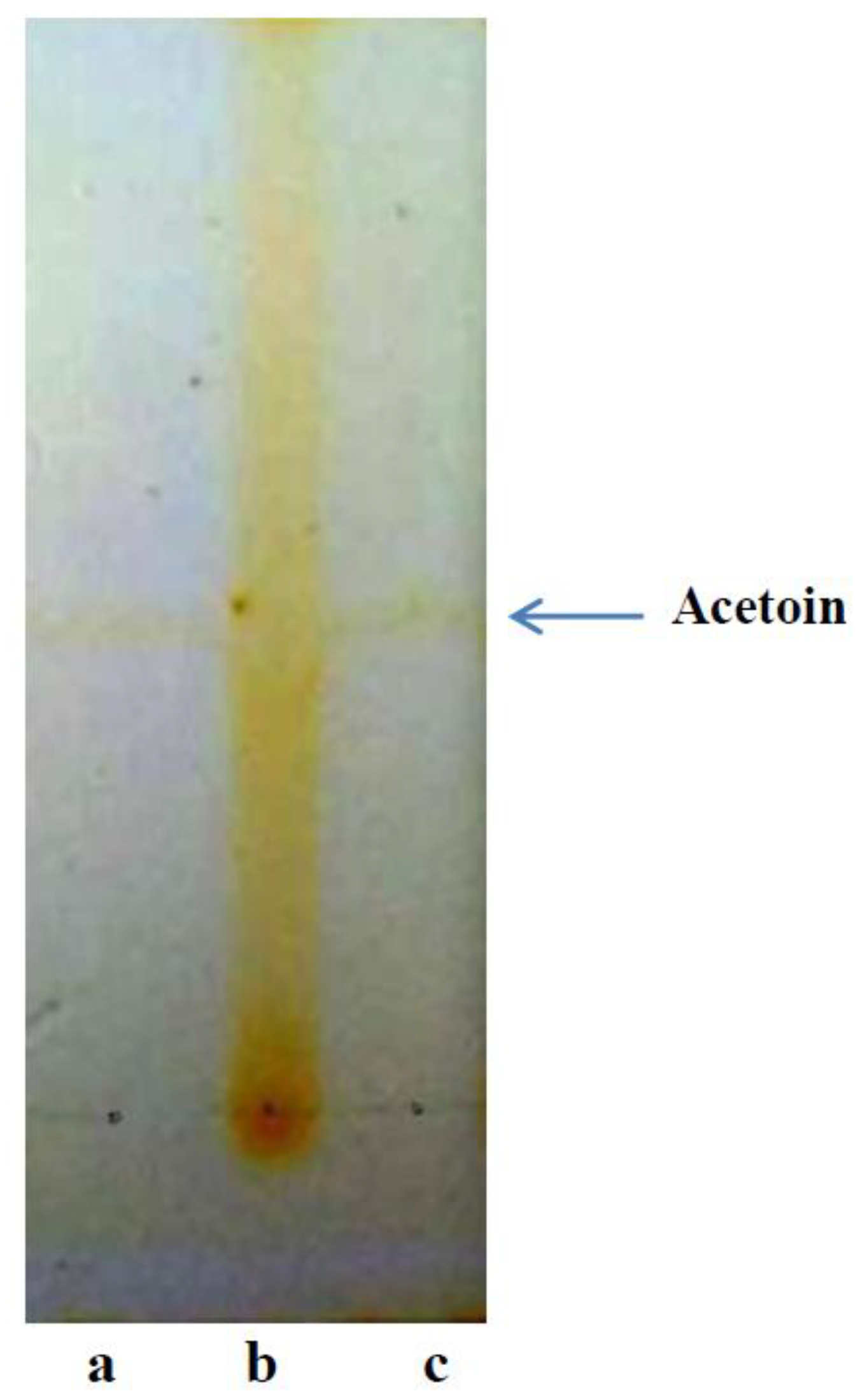
3.2. Analysis of Bioacetoin by Thin Layer Chromatography
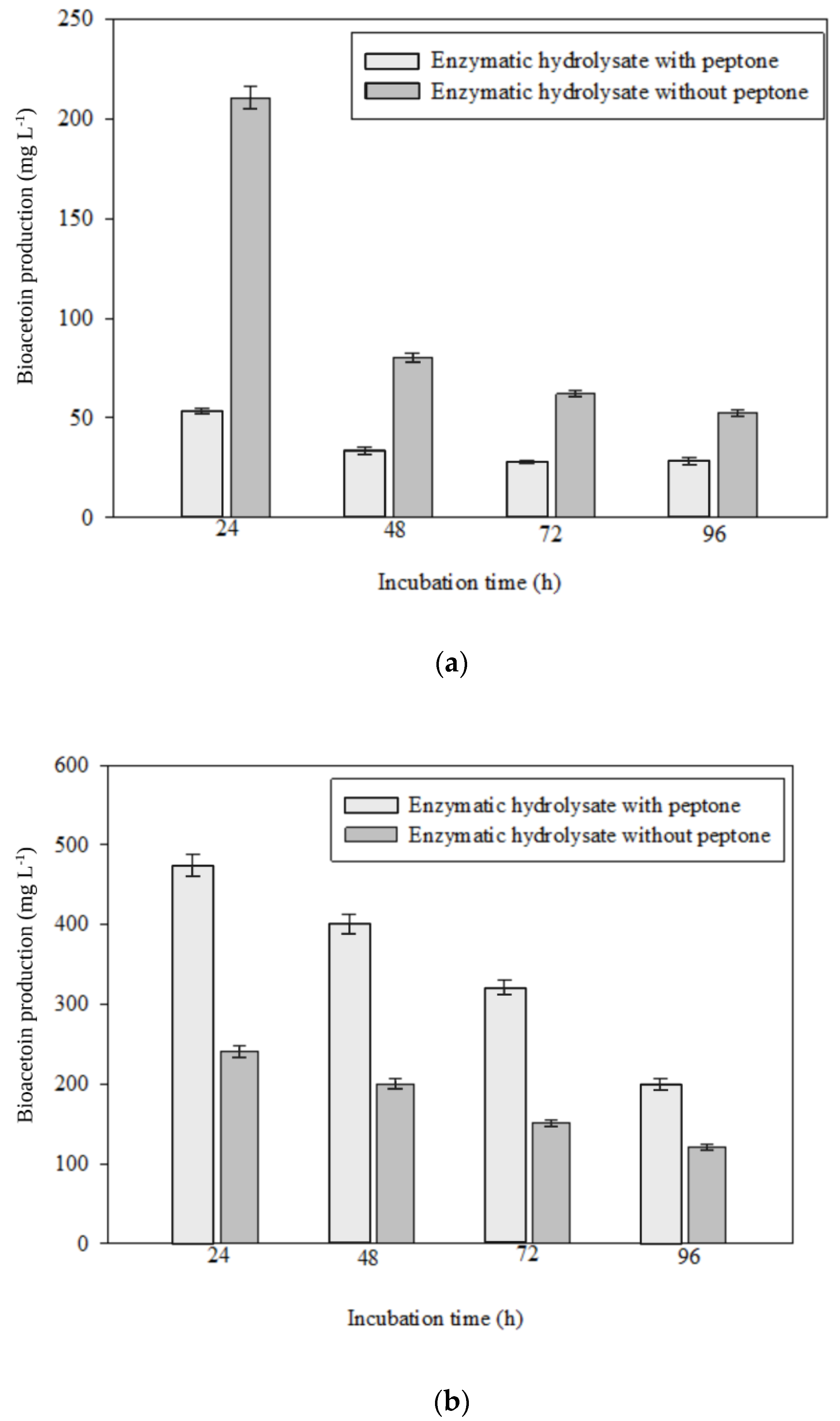
3.3. Fermentation of Enzymatic Hydrolysate of Lignocellulosic Biomass for Bioacetoin Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, T.; Liu, P.; Guo, G.; Liu, Z.; Zhong, L.; Guo, L.; Chen, C.; Hao, N.; Ouyang, P. Production of acetoin and its derivative tetramethylpyrazine from okara hydrolysate with Bacillus subtilis. AMB Express 2023, 13, 25. [Google Scholar] [CrossRef]
- Petrov, K.; Petrova, P. Current Advances in Microbial Production of Acetoin and 2,3-Butanediol by Bacillus spp. Fermentation 2021, 7, 307. [Google Scholar] [CrossRef]
- Bae, S.J.; Kim, S.; Hahn, J.S. Efficient production of bioacetoin in Saccharomyces cerevisiae by disruption of 2, 3-butanediol dehydrogenase and expression of NADH oxidase. Sci. Rep. 2016, 6, 27667. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.; Wang, Z.; Zheng, M.; Chen, T. Advances in biological production of acetoin: A comprehensive overview. Crit. Rev. Biotechnol. 2022, 42, 1135–1156. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Shen, T.; Liu, D.; Wu, J.; Chen, Y.; Wang, L.; Guo, K.; Ying, H.; Ouyang, P. Production of liquid hydrocarbon fuels with acetoin and platform molecules derived from lignocellulose. Green Chem. 2016, 18, 2165–2174. [Google Scholar] [CrossRef]
- Xiao, Z.; Lu, J.R. Strategies for enhancing fermentative production of acetoin: A review. Biotechnol. Adv. 2014, 32, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, X.; Ren, K.; Han, R.; Lu, R.; Bao, T.; Rao, Z. Acetoin production from lignocellulosic biomass hydrolysates with a modular metabolic engineering system in Bacillus subtilis. Biotechnol Biofuels 2022, 15, 87. [Google Scholar] [CrossRef]
- Dai, J.Y.; Cheng, L.; He, Q.F.; Xiu, Z.L. High acetoin production by a newly isolated marine Bacillus subtilis strain with low requirement of oxygen supply. Process Biochem. 2015, 50, 1730–1734. [Google Scholar] [CrossRef]
- Windhorst, C.; Gescher, J. Efficient biochemical production of acetoin from carbon dioxide using Cupriavidus necator H16. Biotechnol. Biofuels. 2019, 12, 1–11. [Google Scholar] [CrossRef]
- Xiao, Z.; Lu, J.R. Generation of acetoin and its derivatives in foods. J. Agric. Food Chem. 2014, 62, 6487–6497. [Google Scholar] [CrossRef]
- Su, H.Y.; Lin, W.H.; Liang, Y.L.; Chou, H.H.; Wu, S.W.; Shi, H.L.; Chen, J.Y.; Cheng, K.K. Co-production of acetoin and succinic acid using corncob hydrolysate by engineered Enterobacter cloacae. Chem. Eng. Sci. 2022, 252, 117511. [Google Scholar] [CrossRef]
- Jonsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Yumei, J.; Hu, L.; Zhen, F. Lignin-derived layered 3D biochar with controllable acidity for enhanced catalytic upgrading of Jatropha oil to biodiesel. Catal. Today 2022, 15, 35–48. [Google Scholar] [CrossRef]
- Caporusso, A.; De Bari, I.; Giuliano, A.; Liuzzi, F.; Albergo, R.; Pietrafesa, R.; Siesto, G.; Romanelli, A.; Braccio, G.; Capece, A. Optimization of wheat straw conversion into microbial lipids by Lipomyces tetrasporus DSM 70314 from bench to pilot scale. Fermentation 2023, 9, 180. [Google Scholar] [CrossRef]
- Vedernikovs, N.; Khroustalyova, G.; Muiznieks, I.; Rapoport, A. New concept for conversion of lignocellulose to ethanol and furfural. Appl. Microbiol. Biotechnol. 2023, 107, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, M.; Kim, B.S. Lignin valorization using biological approach. Biotechnol. Appl. Biochem. 2021, 68, 459–468. [Google Scholar] [CrossRef]
- Singhvi, M.; Kim, B.S. Current developments in lignocellulosic biomass conversion into biofuels using nanobiotechology approach. Energies 2020, 13, 5300. [Google Scholar] [CrossRef]
- Rozenfelde, L.; Puke, M.; Vedernikovs, N.; Scherbaka, R.; Rapoport, A. Catalytic treatment of rapeseed straw for enhanced production of furfural and glucose for bioethanol production. Process Biochem. 2021, 102, 102–107. [Google Scholar] [CrossRef]
- Jain, J.; Singh, B. Phytase production and development of an ideal dephytinization process for amelioration of food nutrition using microbial phytases. Appl. Biochem. Biotechnol. 2017, 18, 1485–1495. [Google Scholar] [CrossRef]
- Taiwo, A.E.; Madzimbamuto, T.N.; Ojumu, T.V. Optimization of process variables for acetoin production in a bioreactor using Taguchi orthogonal array design. Heliyon 2020, 6, e05103. [Google Scholar] [CrossRef]
- Anu; Singh, B.; Kumar, A. Process development for sodium carbonate pretreatment and enzymatic saccharification of rice straw for bioethanol production. Biomass Bioenergy 2020, 138, 105574. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Z.; Gao, C.; Sun, Q.; Liu, J.; She, D. Synthesis of honeycomb lignin-based biochar and its high-efficiency adsorption of norfloxacin. Bioresour. Technol. 2023, 369, 128402. [Google Scholar] [CrossRef]
- Bala, A.; Singh, B. Concomitant production of cellulase and xylanase by thermophilic mould Sporo trichum thermophile in solid state fermentation and their applicability in bread making. World J. Microbiol. Biotechnol. 2017, 33, 109. [Google Scholar] [CrossRef]
- Bala, A.; Singh, B. Development of an environmental-benign process for efficient pretreatment and saccharification of Saccharum biomasses for bioethanol production. Renew Energy 2019, 130, 12–24. [Google Scholar] [CrossRef]
- Anu; Kumar, A.; Singh, D.; Kumar, V.; Singh, B. Production of cellulolytic enzymes by Myceliophthora thermophila and their applicability in saccharification of rice straw. Biomass Convers. Biorefinery 2022, 12, 2649–2662. [Google Scholar] [CrossRef]
- Bibra, M.; Kunreddy, V.R.; Sani, R.K. Thermostable xylanase production by Geobacillus sp. strain DUSELR13, and its application in ethanol production with lignocellulosic biomass. Microorganisms 2018, 6, 93. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Fan, Y.; Liu, J.; Zhao, X.; Chen, W. Effect of nitrogen, carbon sources and agitation speed on acetoin production of Bacillus subtilis SF4-3. Electron. J. Biotechnol. 2016, 19, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Peng, X.; Liu, Y.; Han, Y. Conversion of cellulose and hemicellulose of biomass simultaneously to acetoin by thermophilic simultaneous saccharification and fermentation. Biotechnol. Biofuels 2017, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Noronha, S. Comparative assessment of factors involved in bioacetoin synthesis by Bacillus subtilis 168. Int. Sch. Res. Not. 2014, 2014, 578682. [Google Scholar]
- Xiao, Z.; Wang, X.; Huang, Y.; Huo, F.; Zhu, X.; Xi, L.; Lu, J.R. Thermophilic fermentation of acetoin and 2, 3-butanediol by a novel Geobacillus strain. Biotechnol. Biofuels 2012, 5, 1–11. [Google Scholar] [CrossRef]
- Xiao, Z.; Qiao, S.; Ma, C.; Xu, P. Acetoin production associated with the increase of cell biomass in Bacillus pumilus ATCC 14884. Afr. J. Microbiol. Res. 2010, 4, 1997–2003. [Google Scholar]
- Sharifi, R.; Kiani, H.; Ahmadzadeh, M.; Behboudi, K. Optimization of acetoin production by biological control strain Bacillus Subtilis GB03 using statistical experimental design. Biol. J. Microorg. 2019, 8, 47–57. [Google Scholar]
- Zhang, B.; Li, X.L.; Fu, J.; Li, N.; Wang, Z.; Tang, Y.J.; Chen, T. Production of acetoin through simultaneous utilization of glucose, xylose, and arabinose by engineered Bacillus subtilis. PLoS ONE 2016, 11, e0159298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Wu, H.; Jia, Z.; Li, G.; Li, Q.; Chen, N.; Xie, X. Metabolic engineering of Bacillus subtilis for the co-production of uridine and acetoin. Appl. Microbiol. Biotechnol. 2018, 102, 8753–8762. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.J.; Liu, P.H.; Qin, J.Y.; Xu, P. Statistical optimization of medium components for enhanced acetoin production from molasses and soybean meal hydrolysate. Appl. Microbiol. Biotechnol. 2007, 74, 61–68. [Google Scholar] [CrossRef]
- Yusoff, M.Z.M.; Akita, H.; Hassan, M.A.; Fujimoto, S.; Yoshida, M.; Nakashima, N.; Hoshino, T. Production of acetoin from hydrothermally pretreated oil mesocarp fiber using metabolically engineered Escherichia coli in a bioreactor system. Bioresour. Technol. 2017, 245, 1040–1048. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, L.; Zhao, J.Y.; Xiao, Z. Fermentative production of chiral acetoin by wild-type Bacillus strains. Prep. Biochem. Biotechnol. 2020, 50, 116–122. [Google Scholar] [CrossRef]
- Yang, S.; Mohagheghi, A.; Franden, M.A.; Chou, Y.C.; Chen, X.; Dowe, N.; Himmel, M.E.; Zhang, M. Metabolic engineering of Zymomonas mobilis for 2, 3-butanediol production from lignocellulosic biomass sugars. Biotechnol. Biofuels 2016, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Mazumdar, S.; Lee, J.; Oh, M.K. Microbial production of 2, 3 butanediol from seaweed hydrolysate using metabolically engineered Escherichia coli. Bioresour. Technol. 2013, 136, 329–336. [Google Scholar] [CrossRef]

| Microorganisms Involved | Type of Microorganism | Biomass Used | Fermentation Conditions | Acetoin Production (g L−1) | References |
|---|---|---|---|---|---|
| Bacillus subtilis 168 | Recombinant | Pretreated okara | 37 °C, 36 h | 11.79 | [1] |
| Bacillus subtilis IPE5–4-UD-4 | Mutant | Pretreated corncob | 50 °C, 60 h | 22.76 | [28] |
| Escherichia coli | Recombinant | Pretreated oil palm mesocarp fiber | 37 °C, 24 h, 1000 rpm | 15.5 | [36] |
| Zymomonas mobilis 22C–BC5 | Recombinant | Pretreated corn stover | 33 °C, 24 h, 200 rpm | 10.0 | [38] |
| Bacillus subtilis ZB02 | Recombinant | Corn stover and corn powder hydrolysate | 37 °C, 30 h | 11.2 | [33] |
| E. coli DSM02-B | Recombinant | Pretreated brown seaweed | 37 °C, 96 h, 200 rpm | 4.80 | [39] |
| Bacillus subtilis 168 | Recombinant | Glucose | pH 7.5, 100 rpm, 37 °C, 36 h | 0.08 | [29] |
| B. subtilis subsp. subtilis JJBS250 | Wild | Pretreated rice straw Pretreated sugarcane bagasse | 30 °C, 24 h | 0.21 0.47 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saini, M.; Anu; Rapoport, A.; Tiwari, S.K.; Singh, D.; Malik, V.; Kumar, S.; Singh, B. Bioacetoin Production by Bacillus subtilis subsp. subtilis Using Enzymatic Hydrolysate of Lignocellulosic Biomass. Fermentation 2023, 9, 698. https://doi.org/10.3390/fermentation9080698
Saini M, Anu, Rapoport A, Tiwari SK, Singh D, Malik V, Kumar S, Singh B. Bioacetoin Production by Bacillus subtilis subsp. subtilis Using Enzymatic Hydrolysate of Lignocellulosic Biomass. Fermentation. 2023; 9(8):698. https://doi.org/10.3390/fermentation9080698
Chicago/Turabian StyleSaini, Meenaxi, Anu, Alexander Rapoport, Santosh Kumar Tiwari, Davender Singh, Vinay Malik, Sandeep Kumar, and Bijender Singh. 2023. "Bioacetoin Production by Bacillus subtilis subsp. subtilis Using Enzymatic Hydrolysate of Lignocellulosic Biomass" Fermentation 9, no. 8: 698. https://doi.org/10.3390/fermentation9080698
APA StyleSaini, M., Anu, Rapoport, A., Tiwari, S. K., Singh, D., Malik, V., Kumar, S., & Singh, B. (2023). Bioacetoin Production by Bacillus subtilis subsp. subtilis Using Enzymatic Hydrolysate of Lignocellulosic Biomass. Fermentation, 9(8), 698. https://doi.org/10.3390/fermentation9080698






