Phosphorus-Containing Catalyst Impact on Furfural and Glucose Production during Consecutive Hydrothermal Pretreatment and Enzymatic Hydrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Feedstock
2.2. Characterization of Feedstock
2.3. Hydrothermal Pretreatment System and Experimental Procedure
2.4. Enzymatic Hydrolysis and Experimental Design
3. Results and Discussion
3.1. Hydrothermal Pretreatment
3.2. Effect of Hydrothermal Pretreatment on the Composition of Birch Wood
3.3. Preliminary Study of Enzymatic Hydrolysis
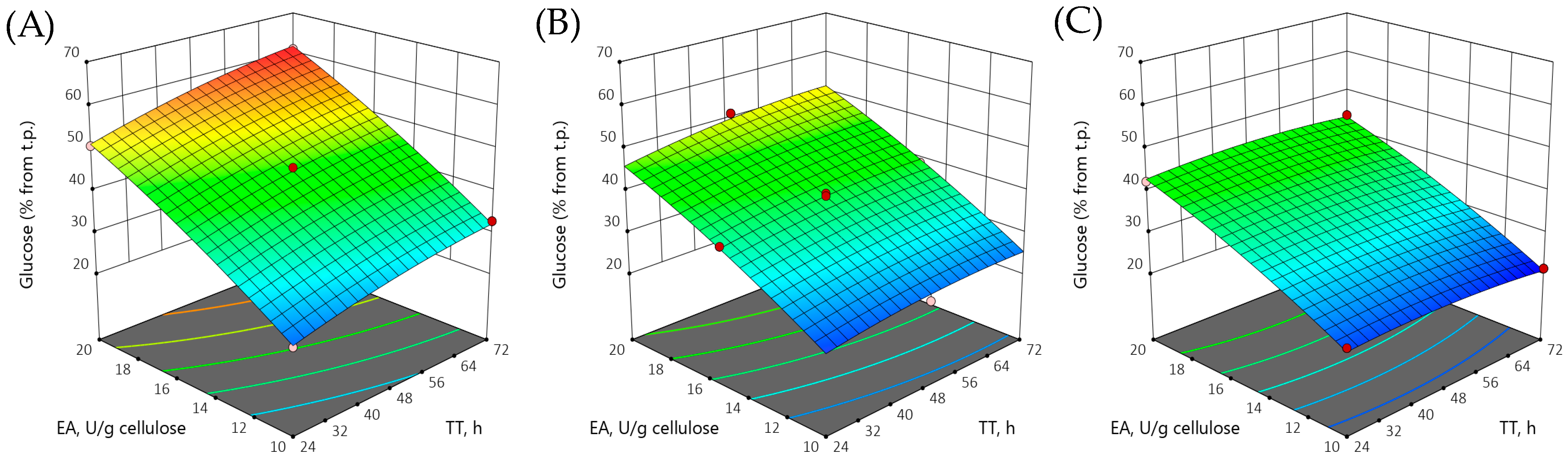
3.4. Optimization of Enzymatic Hydrolysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stegmann, P.; Londo, M.; Junginger, M. The Circular Bioeconomy: Its Elements and Role in European Bioeconomy Clusters. Resour. Conserv. Recycl. X 2020, 6, 100029. [Google Scholar] [CrossRef]
- Daioglou, V.; Doelman, J.C.; Wicke, B.; Faaij, A.; van Vuuren, D.P. Integrated Assessment of Biomass Supply and Demand in Climate Change Mitigation Scenarios. Glob. Environ. Change 2019, 54, 88–101. [Google Scholar] [CrossRef]
- Mawhood, R.; Gazis, E.; de Jong, S.; Hoefnagels, R.; Slade, R. Production Pathways for Renewable Jet Fuel: A Review of Commercialization Status and Future Prospects. Biofuels Bioprod. Biorefining 2016, 10, 462–484. [Google Scholar] [CrossRef]
- Gunasekaran, M.; Kumar, G.; Karthikeyan, O.P.; Varjani, S. Lignocellulosic Biomass as an Optimistic Feedstock for the Production of Biofuels as Valuable Energy Source: Techno-Economic Analysis, Environmental Impact Analysis, Breakthrough and Perspectives. Environ. Technol. Innov. 2021, 24, 102080. [Google Scholar] [CrossRef]
- Velvizhi, G.; Goswami, C.; Shetti, N.P.; Ahmad, E.; Kishore Pant, K.; Aminabhavi, T.M. Valorisation of Lignocellulosic Biomass to Value-Added Products: Paving the Pathway towards Low-Carbon Footprint. Fuel 2022, 313, 122678. [Google Scholar] [CrossRef]
- Hernández-Beltrán, J.U.; Hernández-De Lira, I.O.; Cruz-Santos, M.M.; Saucedo-Luevanos, A.; Hernández-Terán, F.; Balagurusamy, N. Insight into Pretreatment Methods of Lignocellulosic Biomass to Increase Biogas Yield: Current State, Challenges, and Opportunities. Appl. Sci. 2019, 9, 3721. [Google Scholar] [CrossRef]
- Puke, M.; Godina, D.; Kirpluks, M.; Brazdausks, P.; Rizikovs, J. Characterization of Birch Wood Residue after 2-Furaldehyde Obtaining, for Further Integration in Biorefinery Processing. Polymers 2021, 13, 4366. [Google Scholar] [CrossRef]
- Wu, W.; Li, P.; Huang, L.; Wei, Y.; Li, J.; Zhang, L.; Jin, Y. The Role of Lignin Structure on Cellulase Adsorption and Enzymatic Hydrolysis. Biomass 2023, 3, 96–107. [Google Scholar] [CrossRef]
- Huang, L.-Z.; Ma, M.-G.; Ji, X.-X.; Choi, S.-E.; Si, C. Recent Developments and Applications of Hemicellulose From Wheat Straw: A Review. Front. Bioeng. Biotechnol. 2021, 9, 690773. [Google Scholar] [CrossRef]
- Zhou, X.; Li, W.; Mabon, R.; Broadbelt, L.J. A Critical Review on Hemicellulose Pyrolysis. Energy Technol. 2017, 5, 52–79. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef]
- Grgas, D.; Rukavina, M.; Bešlo, D.; Štefanac, T.; Crnek, V.; Šikić, T.; Habuda-Stanić, M.; Landeka Dragičević, T. The Bacterial Degradation of Lignin—A Review. Water 2023, 15, 1272. [Google Scholar] [CrossRef]
- Wohlert, M.; Benselfelt, T.; Wågberg, L.; Furó, I.; Berglund, L.A.; Wohlert, J. Cellulose and the Role of Hydrogen Bonds: Not in Charge of Everything. Cellulose 2022, 29, 1–23. [Google Scholar] [CrossRef]
- Antunes, F.A.F.; Chandel, A.K.; Terán-Hilares, R.; Ingle, A.P.; Rai, M.; dos Santos Milessi, T.S.; da Silva, S.S.; dos Santos, J.C. Overcoming Challenges in Lignocellulosic Biomass Pretreatment for Second-Generation (2G) Sugar Production: Emerging Role of Nano, Biotechnological and Promising Approaches. 3 Biotech 2019, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, R.; Tang, W.; Zheng, Y.; Meng, X. Improve Enzymatic Hydrolysis of Lignocellulosic Biomass by Modifying Lignin Structure via Sulfite Pretreatment and Using Lignin Blockers. Fermentation 2022, 8, 558. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Maurya, D.P.; Singla, A.; Negi, S. An Overview of Key Pretreatment Processes for Biological Conversion of Lignocellulosic Biomass to Bioethanol. 3 Biotech 2015, 5, 597–609. [Google Scholar] [CrossRef]
- Brazdausks, P.; Godina, D.; Puke, M. Direct Furfural Production from Deciduous Wood Pentosans Using Different Phosphorus-Containing Catalysts in the Context of Biorefining. Molecules 2022, 27, 7353. [Google Scholar] [CrossRef]
- Sharma, S.; Tsai, M.-L.; Sharma, V.; Sun, P.-P.; Nargotra, P.; Bajaj, B.K.; Chen, C.-W.; Dong, C.-D. Environment Friendly Pretreatment Approaches for the Bioconversion of Lignocellulosic Biomass into Biofuels and Value-Added Products. Environments 2022, 10, 6. [Google Scholar] [CrossRef]
- K.N, Y.; T.M, M.U.; Sachdeva, S.; Thakur, S.; S, A.K.; J, R.B. Lignocellulosic Biorefinery Technologies: A Perception into Recent Advances in Biomass Fractionation, Biorefineries, Economic Hurdles and Market Outlook. Fermentation 2023, 9, 238. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Huzaifah, M.R.M.; Farid, M.A.A.; Shazleen, S.S.; Misenan, M.S.M.; Yasim-Anuar, T.A.T.; Naveen, J.; Nurazzi, N.M.; Rani, M.S.A.; Hakimi, M.I.; et al. Greener Pretreatment Approaches for the Valorisation of Natural Fibre Biomass into Bioproducts. Polymers 2021, 13, 2971. [Google Scholar] [CrossRef]
- Denault, L.J.; Allen, W.G.; Boyer, E.W.; Collins, D.; Kramme, D.; Spradlin, J.E. A Simple Reducing Sugar Assay for Measuring β-Glucanase Activity in Malt, and Various Microbial Enzyme Preparations. J. Am. Soc. Brew. Chem. 1978, 36, 18–23. [Google Scholar] [CrossRef]
- Dygert, S.; Li, L.H.; Thoma, J.A. Determination of Reducing Sugar with Improved Precision. Anal. Biochem. 1965, 13, 367–374. [Google Scholar] [CrossRef]
- Bittencourt, G.A.; Vandenberghe, L.P.d.S.; Valladares-Diestra, K.K.; Soccol, C.R. Soybean Hull Valorization for Sugar Production through the Optimization of Citric Acid Pretreatment and Enzymatic Hydrolysis. Ind. Crops Prod. 2022, 186, 115178. [Google Scholar] [CrossRef]
- Djajadi, D.T.; Jensen, M.M.; Oliveira, M.; Jensen, A.; Thygesen, L.G.; Pinelo, M.; Glasius, M.; Jørgensen, H.; Meyer, A.S. Lignin from Hydrothermally Pretreated Grass Biomass Retards Enzymatic Cellulose Degradation by Acting as a Physical Barrier Rather than by Inducing Nonproductive Adsorption of Enzymes. Biotechnol. Biofuels 2018, 11, 85. [Google Scholar] [CrossRef]
- Li, H.; Pu, Y.; Kumar, R.; Ragauskas, A.J.; Wyman, C.E. Investigation of Lignin Deposition on Cellulose during Hydrothermal Pretreatment, Its Effect on Cellulose Hydrolysis, and Underlying Mechanisms. Biotechnol. Bioeng. 2014, 111, 485–492. [Google Scholar] [CrossRef]
- Assabjeu, A.C.; Noubissié, E.; Desobgo, S.C.Z.; Ali, A. Optimization of the Enzymatic Hydrolysis of Cellulose of Triplochiton Scleroxylon Sawdust in View of the Production of Bioethanol. Sci. Afr. 2020, 8, e00438. [Google Scholar] [CrossRef]



| Parameter | Symbol | Factor Level | ||
|---|---|---|---|---|
| Treatment time (TT) | h | 24 | 48 | 72 |
| Enzyme load (EL) | U/g cellulose | 10 | 15 | 20 |
| Solid load/buffer ratio (S/B) | % | 10 | 15 | 20 |
| Components | Untreated | H3PO4/NaH2PO4 (1:2) | H3PO4/NaH2PO4 (1:1) | H3PO4/NaH2PO4 (2:1) | H3PO4 |
|---|---|---|---|---|---|
| Extractives * | 4.2 ± 0.2 | n.d. | n.d. | n.d. | n.d. |
| Glucan | 38.7 ± 0.5 | 48.0 ± 0.5 | 48.2 ± 0.3 | 48.5 ± 0.6 | 48.2 ± 0.1 |
| Xylan | 18.6 ± 0.4 | 5.8 ± 0.0 | 4.4 ± 0.2 | 3.9 ± 0.1 | 2.9 ± 0.0 |
| Arabinan | 0.5 ± 0.0 | 0.1 ± 0.0 | 0.5 ± 0.1 | 0.6 ± 0.0 | 0.4 ± 0.0 |
| Galactan | 1.0 ± 0.1 | 0.8 ± 0.0 | 1.1 ± 0.1 | 1.3 ± 0.1 | 1.9 ± 0.1 |
| Mannan | 0.9 ± 0.1 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.0 |
| Acetyl groups | 4.6 ± 0.0 | 0.5 ± 0.0 | 0.4 ± 0.1 | 0.2 ± 0.0 | 0.1 ± 0.0 |
| Acid-insoluble residue | 19.6 ± 0.4 | 39.7 ± 0.2 | 40.3 ± 0.1 | 40.2 ± 0.3 | 41.8 ± 0.3 |
| Run | Treatment Time | Enzyme Load | Solid/Buffer Ratio | Glucose |
|---|---|---|---|---|
| (TT) | (EA) | (S/B) | ||
| h | U/g Cellulose | % | % of the t.p.y. | |
| 1 | 24 | 20 | 20 | 42.27 |
| 2 | 72 | 10 | 10 | 32.80 |
| 3 | 72 | 20 | 20 | 43.72 |
| 4 | 48 | 15 | 15 | 39.50 |
| 5 | 24 | 20 | 10 | 50.69 |
| 6 | 48 | 15 | 20 | 33.48 |
| 7 | 24 | 10 | 10 | 23.48 |
| 8 | 48 | 15 | 10 | 45.62 |
| 9 | 72 | 15 | 15 | 38.94 |
| 10 | 48 | 10 | 15 | 23.30 |
| 11 | 24 | 15 | 15 | 35.88 |
| 12 | 48 | 20 | 15 | 51.21 |
| 13 | 24 | 10 | 20 | 23.71 |
| 14 | 72 | 20 | 10 | 60.86 |
| 15 | 72 | 10 | 20 | 21.21 |
| 16 | 48 | 15 | 15 | 38.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brazdausks, P.; Godina, D.; Puke, M. Phosphorus-Containing Catalyst Impact on Furfural and Glucose Production during Consecutive Hydrothermal Pretreatment and Enzymatic Hydrolysis. Fermentation 2023, 9, 803. https://doi.org/10.3390/fermentation9090803
Brazdausks P, Godina D, Puke M. Phosphorus-Containing Catalyst Impact on Furfural and Glucose Production during Consecutive Hydrothermal Pretreatment and Enzymatic Hydrolysis. Fermentation. 2023; 9(9):803. https://doi.org/10.3390/fermentation9090803
Chicago/Turabian StyleBrazdausks, Prans, Daniela Godina, and Maris Puke. 2023. "Phosphorus-Containing Catalyst Impact on Furfural and Glucose Production during Consecutive Hydrothermal Pretreatment and Enzymatic Hydrolysis" Fermentation 9, no. 9: 803. https://doi.org/10.3390/fermentation9090803
APA StyleBrazdausks, P., Godina, D., & Puke, M. (2023). Phosphorus-Containing Catalyst Impact on Furfural and Glucose Production during Consecutive Hydrothermal Pretreatment and Enzymatic Hydrolysis. Fermentation, 9(9), 803. https://doi.org/10.3390/fermentation9090803






