Widely Targeted Metabonomic Analysis to Study Effect of GSH on Metabolites of Chardonnay Wine during Simulated Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Preparation
2.3. Widely Targeted Metabolome Analysis
2.4. LC-MS/MS Analysis
2.5. Metabolome Analysis of Volatiles
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of GSH on Non-Volatile Metabolites in Chardonnay Wine during Simulated Oxidation
3.1.1. Overall Analysis of Metabolic Components in White Wine
3.1.2. Screening and Analysis of Differential Metabolites
3.2. Effect of GSH on Volatile Metabolites in Chardonnay Wine during Simulated Oxidation
3.2.1. Effect of GSH on Volatile Metabolites in Chardonnay Wine
3.2.2. Effect of Key Non-Volatile Metabolites on Volatile Metabolites in Chardonnay Wine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nikolantonaki, M.; Julien, P.; Coelho, C.; Roullier-Gall, C.; Ballester, J.; Schmitt-Kopplin, P.; Gougeon, R.D. Impact of glutathione on Wines Oxidative Stability: A Combined Sensory and Metabolomic Study. Front. Chem. 2018, 6, 182. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, N.E.C.; Lund, M.N.; Andersen, M.L.; Cardoso, D.R. Beer Thiol-containing compounds and redox stability: Kinetic study of 1-hydroxyethyl radical scavenging ability. J. Agric. Food Chem. 2013, 61, 9444–9452. [Google Scholar] [CrossRef] [PubMed]
- El Hosry, L.; Auezova, L.; Sakr, A.; Hajj-Moussa, E. Browning susceptibility of white wine and antioxidant effect of glutathione. Int. J. Food Sci. Technol. 2009, 44, 2459–2463. [Google Scholar] [CrossRef]
- Andújar-Ortiz, I.; Chaya, C.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Pozo-Bayón, M. Impact of using new commercial glutathione enriched inactive dry yeast oenological preparations on the aroma and sensory properties of wines. Int. J. Food Prop. 2014, 17, 987–1001. [Google Scholar] [CrossRef]
- Roussis, I.G.; Lambropoulos, I.; Tzimas, P. Protection of volatiles in a Wine with Low Sulfur Dioxide by Caffeic Acid or Glutathione. Am. J. Enol. Vitic. 2007, 58, 274–278. [Google Scholar] [CrossRef]
- Ugliano, M.; Kwiatkowski, M.; Vidal, S.; Capone, D.; Siebert, T.; Dieval, J.-B.; Aagaard, O.; Waters, E.J. Evolution of 3-Mercaptohexanol, hydrogen Sulfide, and Methyl Mercaptan during Bottle Storage of Sauvignon blanc Wines. Effect of Glutathione, Copper, Oxygen Exposure, and Closure-Derived Oxygen. J. Agric. Food Chem. 2011, 59, 2564–2572. [Google Scholar] [CrossRef]
- Webber, V.; Dutra, S.V.; Spinelli, F.R.; Carnieli, G.J.; Cardozo, A.; Vanderlinde, R. Effect of glutathione during bottle storage of sparkling wine. Food Chem. 2017, 216, 254–259. [Google Scholar] [CrossRef]
- Marchante, L.; Marquez, K.; Contreras, D.; Izquierdo-Cañas, P.M.; García-Romero, E.; Díaz-Maroto, M.C. Potential of Different natural antioxidant Substances to Inhibit the 1-Hydroxyethyl Radical in SO2-Free Wines. J. Agric. Food Chem. 2020, 68, 1707–1713. [Google Scholar] [CrossRef]
- Bahut, F.; Liu, Y.; Romanet, R.; Coelho, C.; Sieczkowski, N.; Alexandre, H.; Schmitt-Kopplin, P.; Nikolantonaki, M.; Gougeon, R.D. Metabolic diversity conveyed by the process leading to glutathione accumulation in inactivated dry yeast: A synthetic media study. Food Res. Int. 2019, 123, 762–770. [Google Scholar] [CrossRef]
- Comuzzo, P.; Battistutta, F.; Vendrame, M.; Páez, M.S.; Luisi, G.; Zironi, R. Antioxidant properties of different products and additives in white wine. Food Chem. 2015, 168, 107–114. [Google Scholar] [CrossRef]
- Kritzinger, E.C.; Bauer, F.F.; du Toit, W.J. Role of glutathione in winemaking: A review. J. Agric. Food Chem. 2012, 61, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, M.; Aleixandre-Tudo, J.; Kilmartin, P.; Sieczkowski, N.; du Toit, W. Additions of glutathione or Specific Glutathione-rich Dry inactivated Yeast Preparation (DYP) to Sauvignon blanc Must: Effect on Wine Chemical and Sensory Composition. S. Afr. J. Enol. Vitic. 2017, 38, 18–28. [Google Scholar] [CrossRef]
- Romanini, E.; Colangelo, D.; Lucini, L.; Lambri, M. Identifying chemical parameters and discriminant phenolic compounds from metabolomics to gain insight into the oxidation status of bottled white wines. Food Chem. 2019, 288, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Silvia, M.; Tirelli, A.G.; Cravero, M.C.; Massimo, G.; Antonella, B. Effect of SO2, glutathione and gallotannins on the shelf-life of a Cortese white wine bottled with different oxygen intakes. OENO ONE 2022, 56, 221–235. [Google Scholar] [CrossRef]
- Liang, H.M.; Gao, D.Y.; Quan, Q.L.; Wang, C.; Guo, Y.Y.; Shi, H.M. Effect of GSH on Metabolites of Chardonnay Wine Based on Metabonomics Analysis. Food Sci. 2023, 1–18. Available online: http://kns.cnki.net/kcms/detail/11.2206.TS.20230131.0910.009.html (accessed on 30 May 2023).
- Zhang, M.; Yang, Y.; Yuan, H.; Hua, J.; Deng, Y.; Jiang, Y.; Wang, J. Contribution of addition theanine/sucrose on the formation of chestnut-like aroma of green tea. LWT 2020, 129, 109512. [Google Scholar] [CrossRef]
- Nikolantonaki, M.; Magiatis, P.; Waterhouse, A.L. Measuring protection of aromatic wine thiols from oxidation by competitive reactions vs wine preservatives with ortho-quinones. Food Chem. 2014, 163, 61–67. [Google Scholar] [CrossRef]
- Romanet, R.; Bahut, F.; Nikolantonaki, M.; Gougeon, R.D. Molecular characterization of white wines antioxidant metabolome by ultra high performance liquid chromatography high-resolution mass spectrometry. Antioxidants 2020, 9, 115. [Google Scholar] [CrossRef]
- Martínez, J.; González-Arenzana, L. Use of glutathione in the winemaking of white grape varieties. In White Wine Technology; Academic Press: Cambridge, MA, USA, 2022; pp. 29–38. [Google Scholar] [CrossRef]
- Panero, L.; Motta, S.; Petrozziello, M.; Guaita, M.; Bosso, A. Effect of SO2, reduced glutathione and ellagitannins on the shelf life of bottled white wines. Eur. Food Res. Technol. 2014, 240, 345–356. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Allegro, G.; Pastore, C.; Valentini, G.; Filippetti, I. The Evolution of Phenolic Compounds in Vitis vinifera L. Red Berries during Ripening: Analysis and Role on Wine Sensory—A Review. Agronomy 2021, 11, 999. [Google Scholar] [CrossRef]
- Zhao, Q.; Du, G.; Zhao, P.; Guo, A.; Cao, X.; Cheng, C.; Liu, H.; Wang, F.; Zhao, Y.; Liu, Y.; et al. Investigating wine astringency profiles by characterizing tannin fractions in Cabernet Sauvignon wines and model wines. Food Chem. 2023, 414, 135673. [Google Scholar] [CrossRef] [PubMed]
- Buchner, N.; Krumbein, A.; Rohn, S.; Kroh, L.W. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun. Mass Spectrom. 2006, 20, 3229–3235. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.C. Biochemistry of Tea, 3rd ed.; China Agricultural Publishing House: Beijing, China, 2003; pp. 41–45. [Google Scholar]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; Moridani, M.Y.; Chan, T.S.; O’brien, P.J. Peroxidative metabolism of apigenin and naringenin versus luteolin and quercetin: Glutathione oxidation and conjugation. Free Radic. Biol. Med. 2001, 30, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Ivanova-Petropulos, V.; Duan, C.; Yan, G. Effect of unsaturated Fatty Acids on Intra-Metabolites and Aroma Compounds of Saccharomyces cerevisiae in Wine Fermentation. Foods 2021, 10, 277. [Google Scholar] [CrossRef]
- Lin, J.; Blank, I. Odorants Generated by Thermally induced Degradation of Phospholipids. J. Agric. Food Chem. 2003, 51, 4364–4369. [Google Scholar] [CrossRef]
- Lavigne-Cruège, V.; Dubourdieu, D. Role of glutathione on development of aroma defects in dry white wines. In Proceedings of the 13th International Enology Symposium, Montpellier, France, 9–12 June 2002; pp. 331–347. [Google Scholar]
- Wang, S.; Zhang, Q.; Zhao, P.; Ma, Z.; Zhang, J.; Ma, W.; Wang, X. Investigating the effect of three phenolic fractions on the volatility of floral, fruity, and aged aromas by HS-SPME-GC-MS and NMR in model wine. Food Chem. X 2022, 13, 100281. [Google Scholar] [CrossRef]
- Pittari, E.; Moio, L.; Piombino, P. Interactions between Polyphenols and Volatile Compounds in Wine: A Literature Review on Physicochemical and Sensory Insights. Appl. Sci. 2021, 11, 1157. [Google Scholar] [CrossRef]
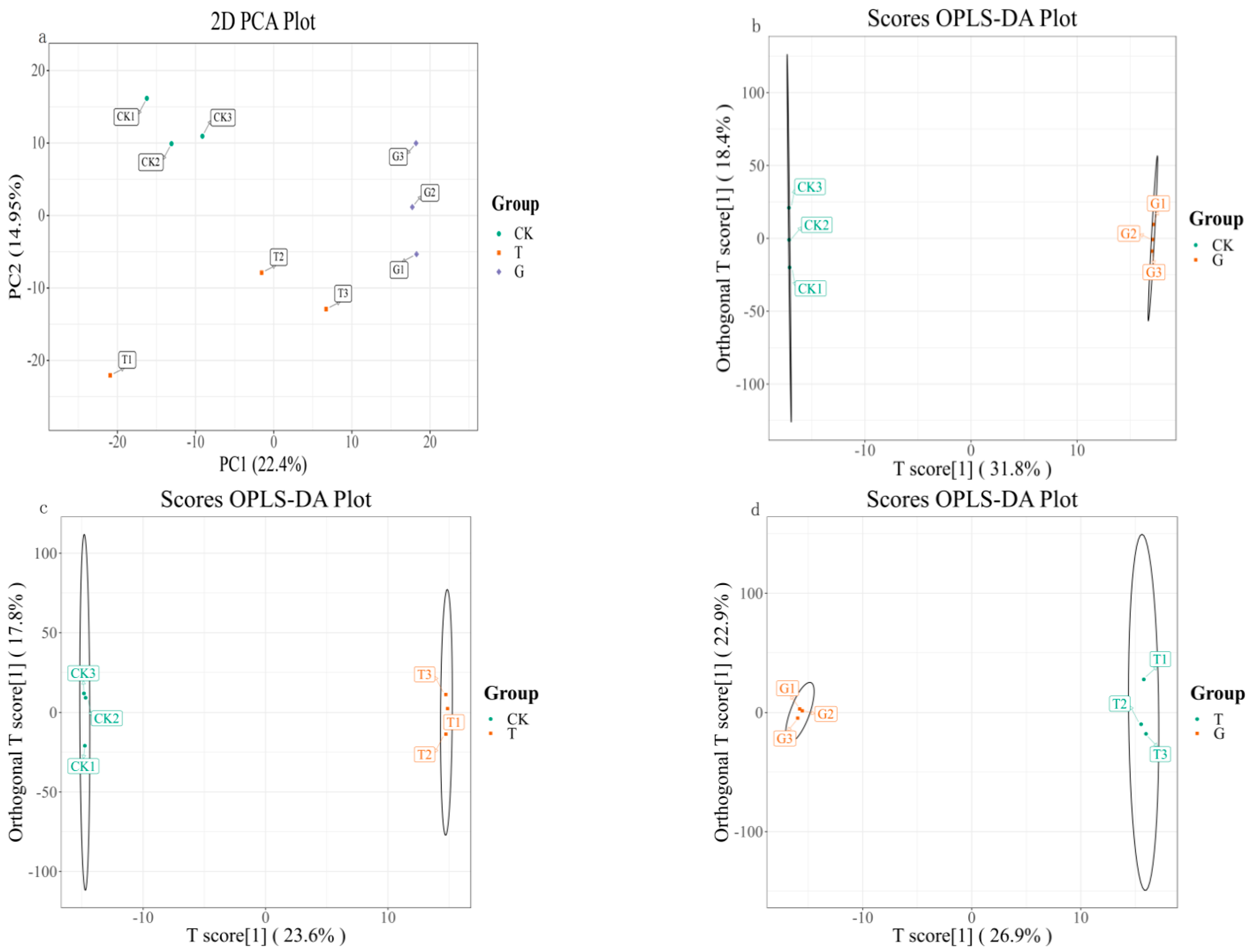
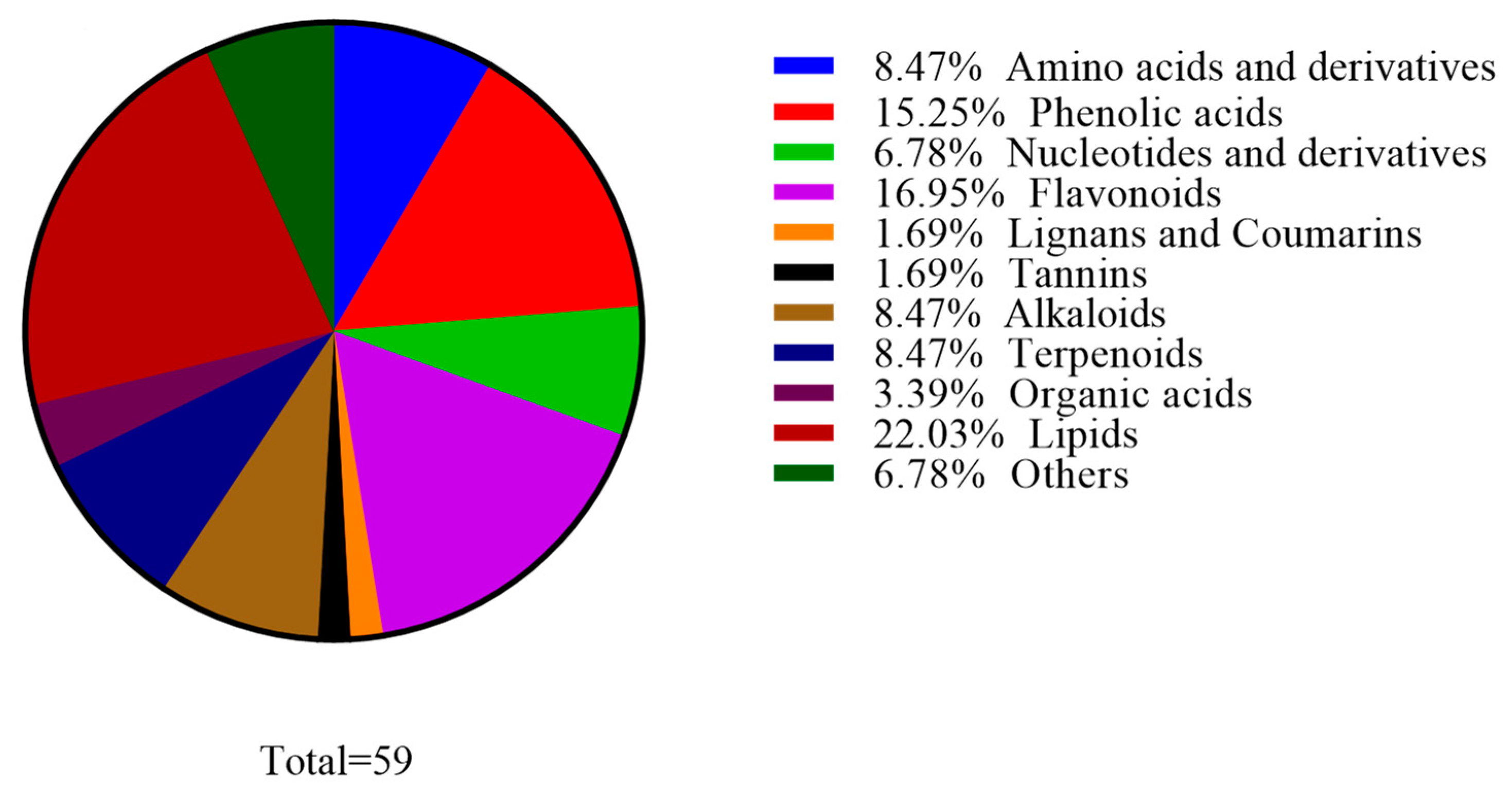
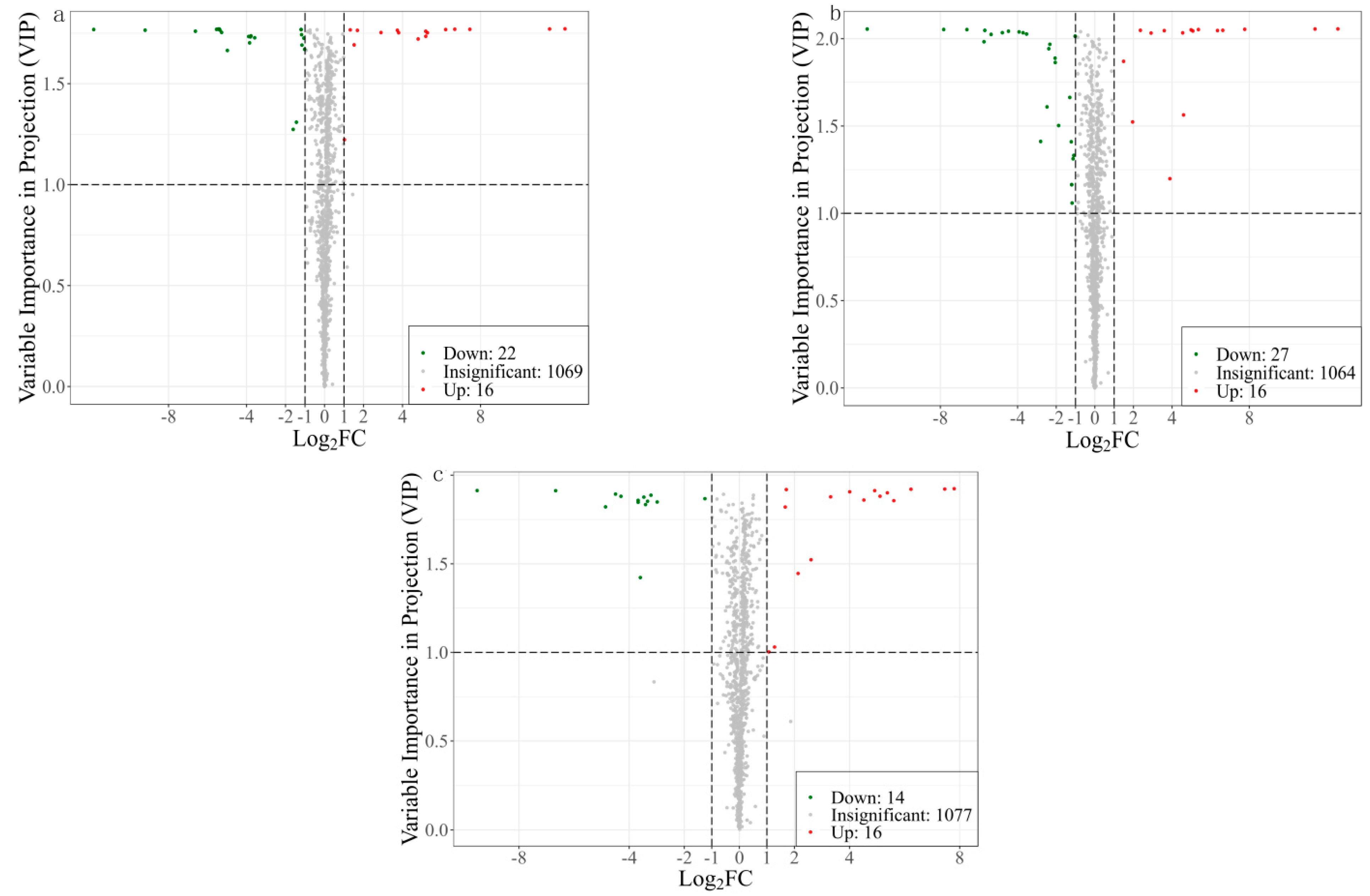
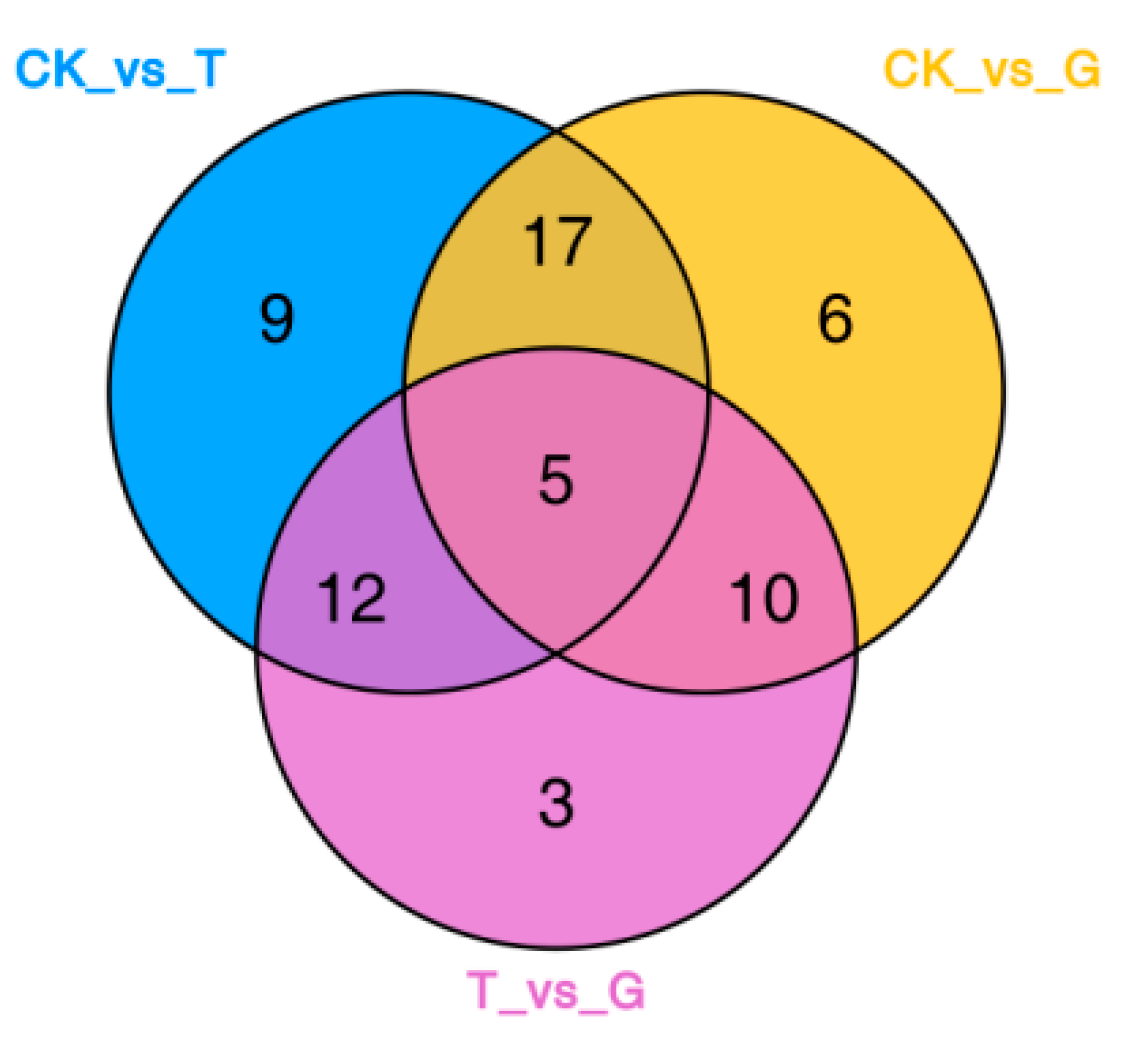


| Code | Compound | Class I | CK vs. T | CK vs. G | ||
|---|---|---|---|---|---|---|
| Fold Change | Up/Down | Fold Change | Up/Down | |||
| 1 | L-Leucine | Amino acids and derivatives | 6157.155 | Up | 5157.34 | Up |
| 2 | Oxiglutatione | Amino acids and derivatives | 31.665 | Up | 13.319 | Up |
| 3 | 3,4-Dimethylbenzoic acid | Phenolic acids | 24.153 | Up | 36.448 | Up |
| 4 | Vnilloyltartaric acid | Phenolic acids | 217.094 | Up | 175.151 | Up |
| 5 | 1-O-caffeoyl-3,4-di-O-galloyl-β-D-glucose | Phenolic acids | 41.167 | Up | 36.542 | Up |
| 6 | Adenosine 5′-monophosphate | Nucleotides and derivatives | 81.771 | Up | 74.159 | Up |
| 7 | 1-Beta-D-arabinofuranosyluracil | Nucleotides and derivatives | 2708.792 | Up | 2983.773 | Up |
| 8 | Riboflavin 5′-adenosine diphosphate | Nucleotides and derivatives | 0.0759 | Down | 0.067 | Down |
| 9 | kaempferol-3-O-(2″-p-coumaroyl)galactoside | Flavonoids | 33.753 | Up | 38.789 | Up |
| 10 | Diosmetin-7-O-neohesperidoside (Neodiosmin) | Flavonoids | 98.80 | Up | 102.440 | Up |
| 11 | Quercetin-3-O-sophorotrioside-7-O-arabinoside | Flavonoids | 0.429 | Down | 0.368 | Down |
| 12 | Quercetin-3-O-(2″,3″-O-digalloyl)-glucoside | Flavonoids | 0.458 | Down | 0.328 | Down |
| 13 | N′,N″,N′′′-p-coumaroyl-cinnamoyl-caffeoyl spermidine | Alkaloids | 5.128 | Up | 3.217 | Up |
| 14 | 3-Epiursolic acid | Terpenoids | 0.192 | Down | 0.024 | Down |
| 15 | Ursolic acid | Terpenoids | 0.241 | Down | 0.023 | Down |
| 16 | Mangiferolic acid | Terpenoids | 0.199 | Down | 0.022 | Down |
| 17 | Isomangiferolic acid | Terpenoids | 0.240 | Down | 0.024 | Down |
| 18 | Dihydrosphingosine-1-phosphate | Lipids | 7.529 | Up | 7.443 | Up |
| 19 | 13-hydroperoxy-9Z,11E-octadecadienoic acid | Lipids | 0.493 | Down | 0.442 | Down |
| 20 | 7S,8S-DiHODE; (9Z,12Z)-(7S,8S)-dihydroxyoctadeca-9,12-dienoic acid | Lipids | 0.493 | Down | 0.435 | Down |
| 21 | LysoPE 18:2(2n isomer) | Lipids | 14.798 | Up | 13.934 | Up |
| 22 | 16-Methylheptadecanoic acid | Lipids | 0.001 | Down | 0.001 | Down |
| Code | Compound | Class I | Fold Change | CK vs. T |
|---|---|---|---|---|
| 1 | N-acetyl-L-tryptophan | Amino acids and derivatives | 0.470 | Down |
| 2 | Glutathione reduced form | Amino acids and derivatives | 2.803 | Up |
| 3 | Isochlorogenic acid B | Phenolic acids | 0.436 | Down |
| 4 | Isochlorogenic acid C | Phenolic acids | 0.436 | Down |
| 5 | 1-O-galloyl-6-O-feruloyl-β-D-glucose | Phenolic acids | 0.036 | Down |
| 6 | 1-O-galloyl-4-O-feruloyl-β-D-glucose | Phenolic acids | 0.045 | Down |
| 7 | Myricetin | Flavonoids | 3.882 | Up |
| 8 | Quercetin-3-O-rhamnoside (Quercitrin) | Flavonoids | 0.407 | Down |
| 9 | Quercetin-3,7-Di-O-glucoside | Flavonoids | 0.180 | Down |
| 10 | Dihydrodehydrodiconiferyl alcohol | Lignans and coumarins | 0.272 | Down |
| 11 | Ellagic acid-4-O-glucoside | Tannins | 0.010 | Down |
| 12 | 4-Hydroxymandelonitrile | Alkaloids | 0.143 | Down |
| 13 | 2,4-Dihydroxyquinoline | Alkaloids | 0.004 | Down |
| 14 | Caffeoylcholine-4-O-glucoside | Alkaloids | 0.019 | Down |
| 15 | Benzoylformic acid | Organic acids | 0.019 | Down |
| 16 | Methyl dihydrojasmonate | Organic acids | 0.024 | Down |
| 17 | Docosapentaenoic acid | Lipids | 23.43 | Up |
| 18 | 12-Hydroxyoctadecanoic acid | Lipids | 14.80 | Up |
| 19 | LysoPE 18:2 | Lipids | 0.086 | Down |
| 20 | LysoPC 17:0 | Lipids | 0.066 | Down |
| 21 | Glucarate O-Phosphoric acid | Others | 0.442 | Down |
| Code | Compound | Class I | Fold Change | CK vs. G |
|---|---|---|---|---|
| 1 | S-(5′-Adenosyl)-L-methionine | Amino acids and derivatives | 27.87 | Up |
| 2 | 4-Hydroxy-3,5-diisopropylbenzaldehyde | Phenolic acids | 0.032 | Down |
| 3 | 2-Phenoxyethanol | Phenolic acids | 0.448 | Down |
| 4 | 3′-Adenylic Acid | Nucleotides and derivatives | 0.072 | Down |
| 5 | Quercetin-7-O-glucoside | Flavonoids | 0.010 | Down |
| 6 | 5-Hydroxy-6,7,3′,4′-tetramethoxyflavone | Flavonoids | 0.026 | Down |
| 7 | 5,6,7,4′-Tetramethoxyflavone | Flavonoids | 0.084 | Down |
| 8 | N-Oleoylethanolamine | Alkaloids | 0.480 | Down |
| 9 | Maslinic acid | Terpenoids | 2.494 | Up |
| 10 | 13-Hydroxy-6,9,11-octadecatrienoic acid | Lipids | 0.442 | Down |
| 11 | 9,10-DHOME; (12Z)-9,10-Dihydroxyoctadec-12-enoic acid | Lipids | 0.073 | Down |
| 12 | Cis-4,7,10,13,16,19-Docosahexaenoic Acid | Lipids | 0.070 | Down |
| 13 | LysoPC 18:0 | Lipids | 0.493 | Down |
| 14 | Rhapontigenin | Others | 2.863 | Up |
| 15 | Xylitol | Others | 0.002 | Down |
| 16 | D-Threose | Others | 2.030 | Up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, D.; Wang, C.; Shi, H.; Liang, H. Widely Targeted Metabonomic Analysis to Study Effect of GSH on Metabolites of Chardonnay Wine during Simulated Oxidation. Fermentation 2023, 9, 815. https://doi.org/10.3390/fermentation9090815
Gao D, Wang C, Shi H, Liang H. Widely Targeted Metabonomic Analysis to Study Effect of GSH on Metabolites of Chardonnay Wine during Simulated Oxidation. Fermentation. 2023; 9(9):815. https://doi.org/10.3390/fermentation9090815
Chicago/Turabian StyleGao, Deyan, Cong Wang, Hongmei Shi, and Hongmin Liang. 2023. "Widely Targeted Metabonomic Analysis to Study Effect of GSH on Metabolites of Chardonnay Wine during Simulated Oxidation" Fermentation 9, no. 9: 815. https://doi.org/10.3390/fermentation9090815
APA StyleGao, D., Wang, C., Shi, H., & Liang, H. (2023). Widely Targeted Metabonomic Analysis to Study Effect of GSH on Metabolites of Chardonnay Wine during Simulated Oxidation. Fermentation, 9(9), 815. https://doi.org/10.3390/fermentation9090815





