The Decomposition Dynamics and Substrate Component Potential of Biomass from the Seagrass Posidonia oceanica (L.) Delile
Abstract
:1. Introduction
- (1)
- Assess the capability of forest microbiome to accelerate the degradation of P. oceanica debris;
- (2)
- Identify the optimal application rate of P. oceanica debris to support the growth of ornamental and horticultural species;
- (3)
- Understand the need for mineral fertilization for efficient use of P. oceanica debris as a growing substrate.
2. Materials and Methods
2.1. Study Site and Substrate Collection
2.2. P. oceanica Debris Chemical Analysis
2.3. Decomposition Experiment
2.4. The Use of P. oceanica as a Growth Substrate
2.5. Data Analysis
3. Results
3.1. Posidonia Chemical Analysis
3.2. Decomposition of P. oceanica Debris
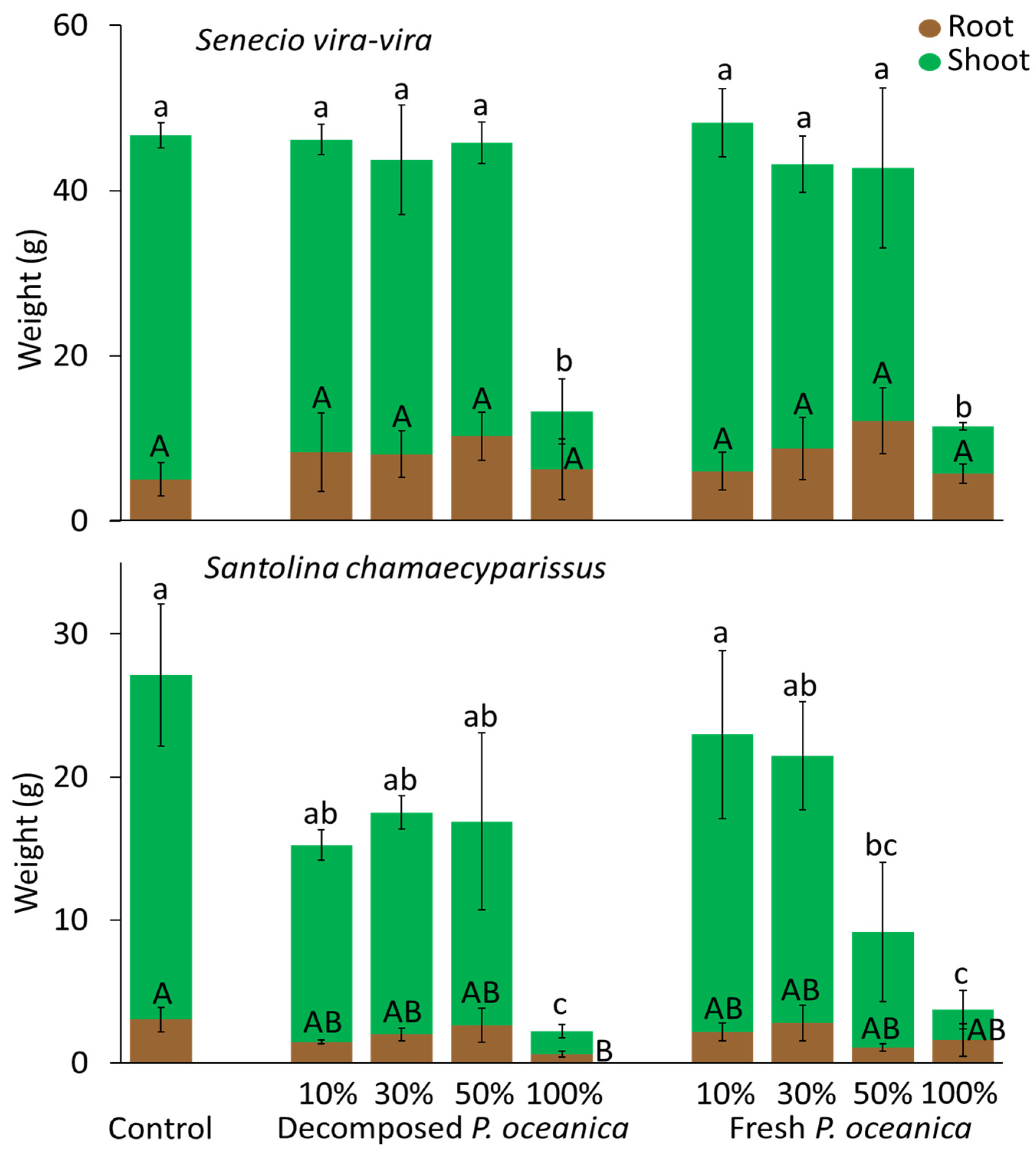
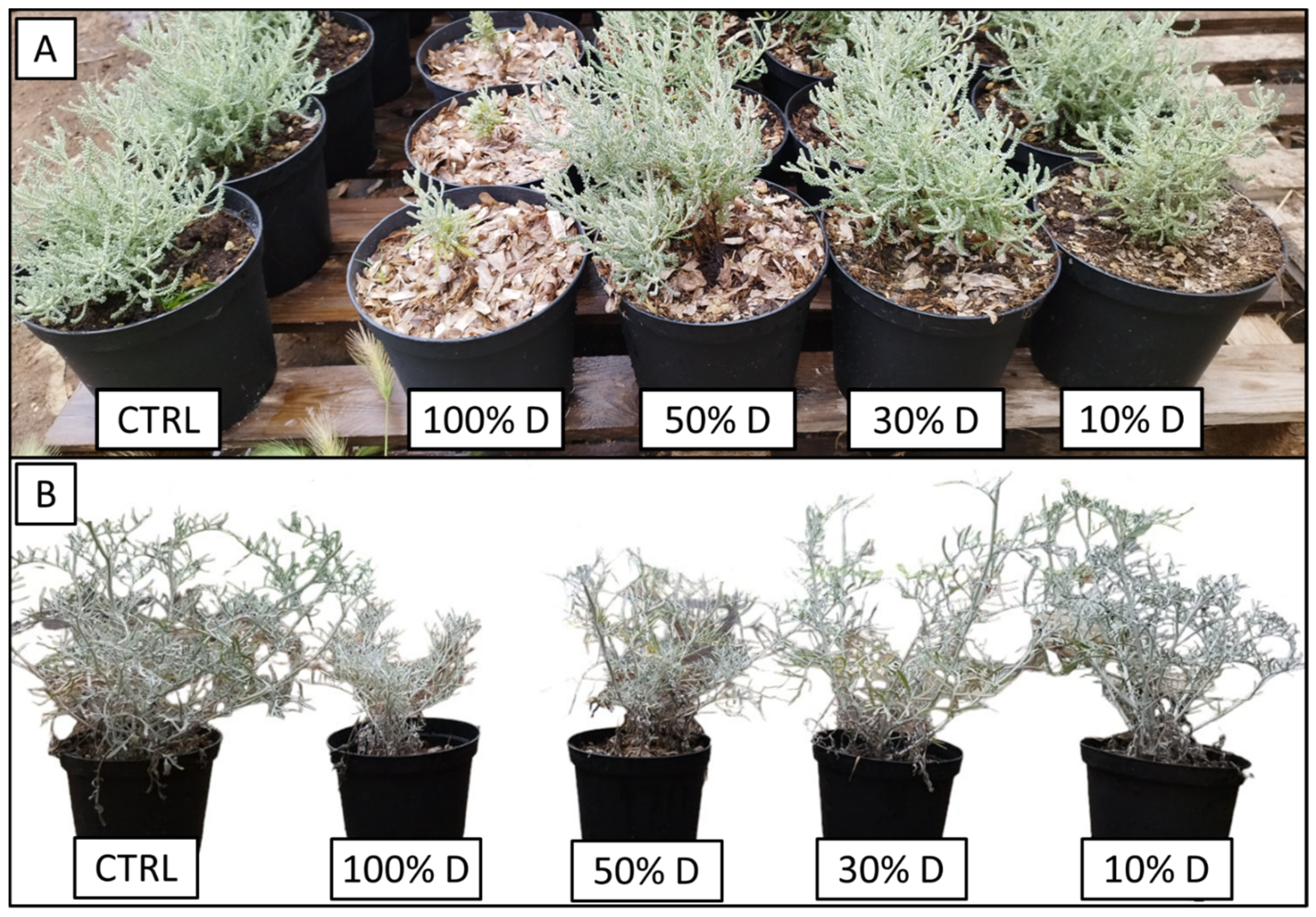
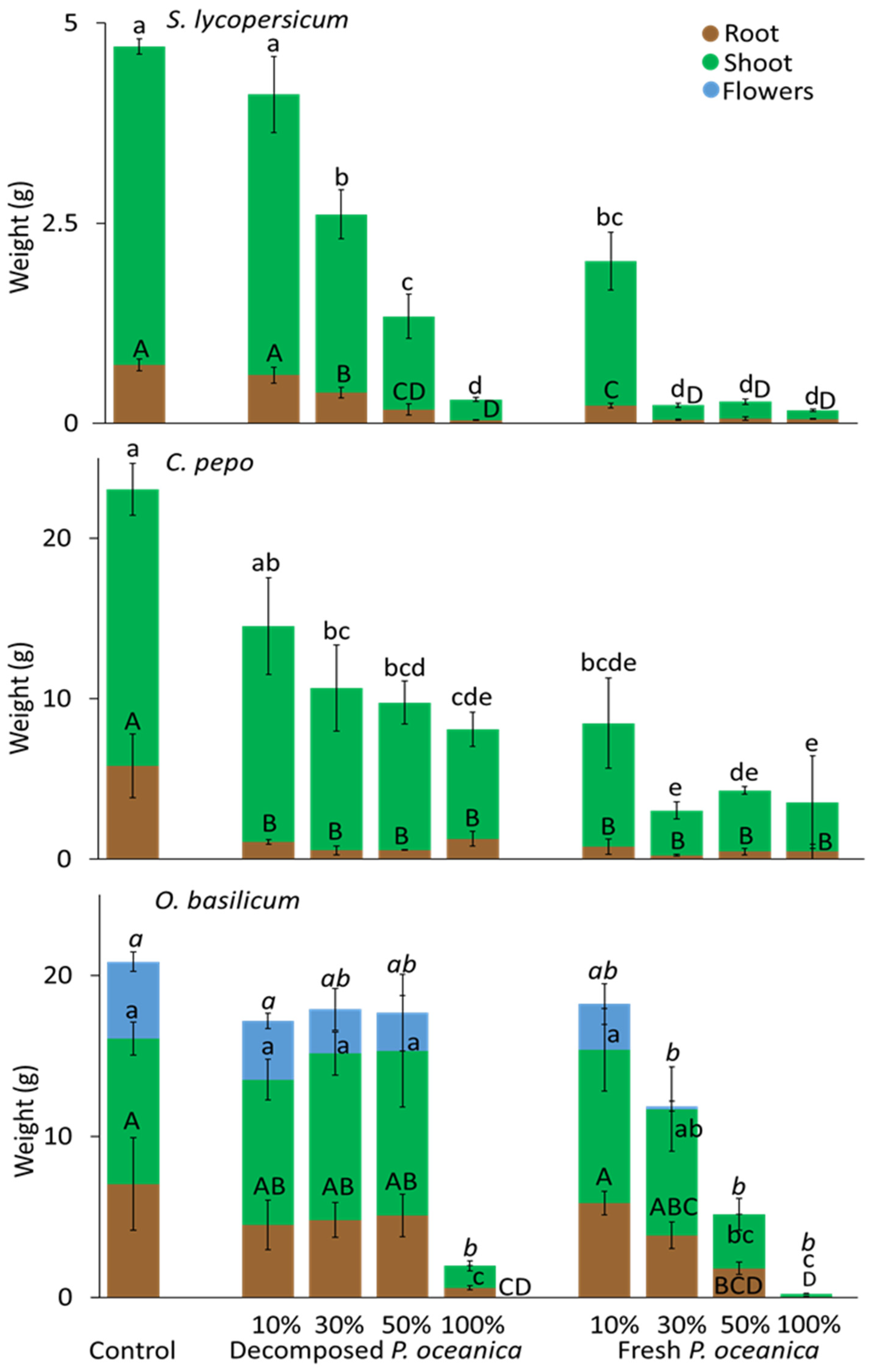
3.3. P. oceanica as a Growth Substrate without Mineral Fertilizer
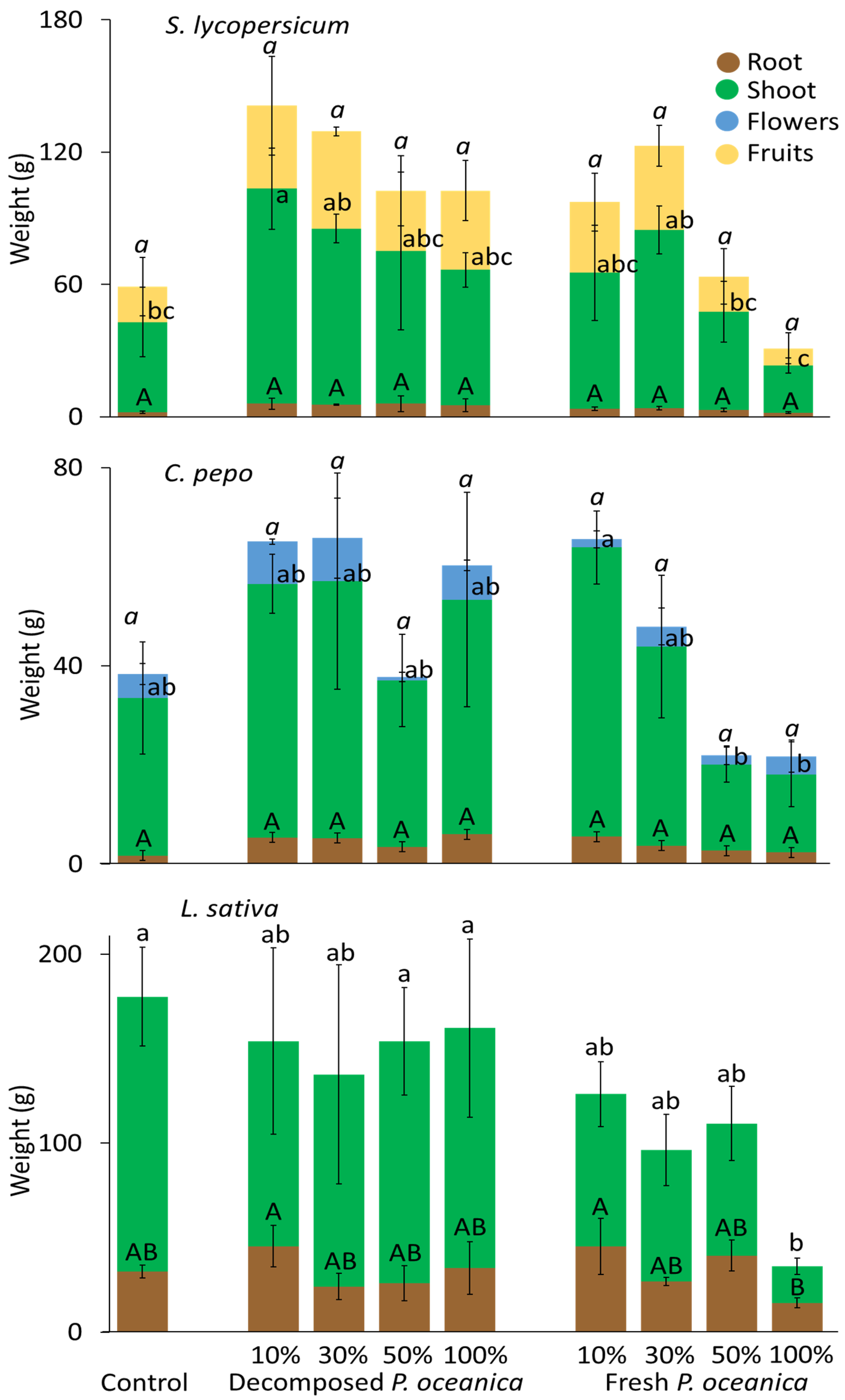
3.4. P. oceanica as a Growth Substrate with Mineral Fertilizer
3.5. Shoot-to-Root Ratios
4. Discussion
4.1. Decomposition of P. oceanica Debris
4.2. P. oceanica Debris as Growing Substrate
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rotini, A.; Chiesa, S.; Manfra, L.; Borrello, P.; Piermarini, R.; Silvestri, C.; Cappucci, S.; Parlagreco, L.; Devoti, S.; Pisapia, M.; et al. Effectiveness of the “Ecological Beach” Model: Beneficial Management of Posidonia Beach Casts and Banquette. Water 2020, 12, 3238. [Google Scholar] [CrossRef]
- Apostolaki, E.T.; Marbà, N.; Holmer, M.; Karakassis, I. Fish Farming Impact on Decomposition of Posidonia oceanica Litter. J. Exp. Mar. Biol. Ecol. 2009, 369, 58–64. [Google Scholar] [CrossRef]
- Kaal, J.; Serrano, O.; Nierop, K.G.J.; Schellekens, J.; Martínez Cortizas, A.; Mateo, M.Á. Molecular Composition of Plant Parts and Sediment Organic Matter in a Mediterranean Seagrass (Posidonia oceanica) Mat. Aquat. Bot. 2016, 133, 50–61. [Google Scholar] [CrossRef]
- Cocozza, C.; Parente, A.; Zaccone, C.; Mininni, C.; Santamaria, P.; Miano, T. Chemical, Physical and Spectroscopic Characterization of Posidonia oceanica (L.) Del. Residues and Their Possible Recycle. Biomass Bioenergy 2011, 35, 799–807. [Google Scholar] [CrossRef]
- Ecol, M.; Ser, P.; Dalla Via, J.; Sturmbauer, C.; Schonweger, G.; Sotz, E.; Mathekowitsch, S.; Stifter, M.; Rieger, R. Ecology Progress Series Light Gradients and Meadow Structure in Posidonia oceanica: Ecomorphological and Functional Correlates; Inter-Research: Oldendorf/Luhe, Germany, 1998; Volume 163. [Google Scholar]
- Astudillo, C.; Gracia, V.; Sierra, J.P.; Cáceres, I.; Sánchez-arcilla, A. Posidonia Beach-Cast and Banquette: Evaluation of Sediment Trapping and Characterisation for Coastal Protection. In Coastal Sediments; World Scientific: New Orleans, LA, USA, 2023; pp. 2265–2277. [Google Scholar]
- Fernández-Torquemada, Y.; Sánchez-Lizaso, J.L. Effects of Salinity on Leaf Growth and Survival of the Mediterranean Seagrass Posidonia oceanica (L.) Delile. J. Exp. Mar. Biol. Ecol. 2005, 320, 57–63. [Google Scholar] [CrossRef]
- Delgado, O.; Ruiz, J.; Pérez, M.; Romero, J.; Ballesteros, E. Effects of Fish Farming on Seagrass (Posidonia oceanica) in a Mediterranean Bay: Seagrass Decline after Organic Loading Cessation. Oceanol. Acta 1999, 22, 109–117. [Google Scholar] [CrossRef]
- Moltó, J.; Montalbán, M.G.; Núñez, S.S.; Jordá, J.D. Revalorization of Posidonia oceanica Waste for the Thermochemical Production of Biochar. Appl. Sci. 2022, 12, 7422. [Google Scholar] [CrossRef]
- Ravaglioli, C.; Lardicci, C.; Pusceddu, A.; Arpe, E.; Bianchelli, S.; Buschi, E.; Bulleri, F. Ocean Acidification Alters Meiobenthic Assemblage Composition and Organic Matter Degradation Rates in Seagrass Sediments. Limnol. Oceanogr. 2020, 65, 37–50. [Google Scholar] [CrossRef]
- Ogden, J. Seagrasses: Biology, Ecology and Conservation. Mar. Ecol. 2006, 27, 431–432. [Google Scholar] [CrossRef]
- Castillo, C.; Mantecón, A.R.; Sotillo, J.; Benedito, J.L.; Abuelo, A.; Gutiérrez, C.; Hernández, J. The Use of Banquettes of Posidonia oceanica as a Source of Fiber and Minerals in Ruminant Nutrition. An Observational Study. Animal 2014, 8, 1663–1666. [Google Scholar] [CrossRef]
- Gómez-Pujol, L.; Orfila, A.; Álvarez-Ellacuría, A.; Terrados, J.; Tintoré, J. Posidonia oceanica Beach-Cast Litter in Mediterranean Beaches: A Coastal Videomonitoring Study. J. Coast. Res. 2013, 165, 1768–1773. [Google Scholar] [CrossRef]
- Cardona, L.; García, M. Beach-Cast Seagrass Material Fertilizes the Foredune Vegetation of Mediterranean Coastal Dunes. Acta Oecologica 2008, 34, 97–103. [Google Scholar] [CrossRef]
- Mateo, M.A.; Cebrián, J.; Dunton, K.; Mutchler, T. Carbon Flux in Seagrass Ecosystems. In Seagrasses: Biology, Ecology and Conservation; Springer: Berlin/Heidelberg, Germany, 2006; pp. 159–192. [Google Scholar]
- Pedersen, M.Ø.; Serrano, O.; Mateo, M.Á.; Holmer, M. Temperature Effects on Decomposition of a Posidonia oceanica Mat. Aquat. Microb. Ecol. 2011, 65, 169–182. [Google Scholar] [CrossRef]
- Bonanomi, G.; Cesarano, G.; Iacomino, G.; Cozzolino, A.; Motti, R.; Idbella, M. Decomposition of Posidonia oceanica (L.) Delile Leaf Blade and Rhizome in Terrestrial Conditions: Effect of Temperature and Substrate Fertility. Waste Biomass Valorization 2023, 14, 1869–1878. [Google Scholar] [CrossRef]
- Legislative Decree No. 75 (Circular 8838/2019). Gestione degli Accumuli di Posidonia oceanica spiaggiati. m_amte. RIN.REGISTRO UFFICIALE USCITA.Prot.0008838.20-05-2019. Ministero dell’Ambiente e della Sicurezza Energetica. Available online: www.mase.gov.it (accessed on 3 January 2024).
- Borrello, P.; De Angelis, R.; Pallottini, E.; Saccomandi, F. Formazione e Gestione Delle Banquettes Di Posidonia oceanica Sugli Arenili; ISPRA: Ispra, Italy, 2010; ISBN 978-88-448-0426-8. [Google Scholar]
- Pizzanelli, S.; Maisano, S.; Pinzino, C.; Manariti, A.; Chiodo, V.; Pitzalis, E.; Forte, C. The Effect of Activation on the Structure of Biochars Prepared from Wood and from Posidonia oceanica: A Spectroscopic Study. Physchem 2022, 2, 286–304. [Google Scholar] [CrossRef]
- Karberg, N.J.; Scott, N.A.; Giardina, C.P. Methods for Estimating Litter Decomposition. In Field Measurements for Forest Carbon Monitoring; Springer: Dordrecht, The Netherlands, 2008; pp. 103–111. [Google Scholar]
- Bonanomi, G.; De Filippis, F.; Cesarano, G.; La Storia, A.; Zotti, M.; Mazzoleni, S.; Incerti, G. Linking Bacterial and Eukaryotic Microbiota to Litter Chemistry: Combining next Generation Sequencing with 13 C CPMAS NMR Spectroscopy. Soil. Biol. Biochem. 2019, 129, 110–121. [Google Scholar] [CrossRef]
- Iacomino, G.; Sarker, T.C.; Ippolito, F.; Bonanomi, G.; Vinale, F.; Staropoli, A.; Idbella, M. Biochar and Compost Application Either alone or in Combination Affects Vegetable Yield in a Volcanic Mediterranean Soil. Agronomy 2022, 12, 1996. [Google Scholar] [CrossRef]
- Serrano, O.; Lavery, P.S.; Rozaimi, M.; Mateo, M.Á. Influence of Water Depth on the Carbon Sequestration Capacity of Seagrasses. Global Biogeochem. Cycles 2014, 28, 950–961. [Google Scholar] [CrossRef]
- Lopez, N.I.; Duarte, C.M.; Vallespinos, F.; Romero, J.; Alcoverro, T. The Effect of Nutrient Additions on Bacterial Activity in Seagrass (Posidonia oceanica) Sediments. J. Exp. Mar. Biol. Ecol. 1998, 224, 155–166. [Google Scholar] [CrossRef]
- Gacia, E.; Duarte, C.M.; Middelburg, J.J. Carbon and Nutrient Deposition in a Mediterranean Seagrass (Posidonia oceanica) Meadow. Limnol. Oceanogr. 2002, 47, 23–32. [Google Scholar] [CrossRef]
- Pergent, G.; Rico-Raimondino, V.; Pergent-Martini, C. Fate of Primary Production in Posidonia oceanica Meadows of the Mediterranean. Aquat. Bot. 1997, 59, 307–321. [Google Scholar] [CrossRef]
- Enriquez, S.; Duarte, C.M.; Sand-Jensen, K. Patterns in Decomposition Rates among Photosynthetic Organisms: The Importance of Detritus C:N:P Content. In Oecologia; Springer: Berlin/Heidelberg, Germany, 1993; Volume 94, pp. 457–471. [Google Scholar]
- Costa, V.; Mazzola, A.; Rossi, F.; Vizzini, S. Decomposition Rate and Invertebrate Colonization of Seagrass Detritus along a Hydrodynamic Gradient in a Mediterranean Coastal Basin: The Stagnone Di Marsala (Italy) Case Study. Mar. Ecol. 2019, 40, e12570. [Google Scholar] [CrossRef]
- Arnstadt, T.; Hoppe, B.; Kahl, T.; Kellner, H.; Krüger, D.; Bauhus, J.; Hofrichter, M. Dynamics of Fungal Community Composition, Decomposition and Resulting Deadwood Properties in Logs of Fagus Sylvatica, Picea Abies and Pinus Sylvestris. For. Ecol. Manag. 2016, 382, 129–142. [Google Scholar] [CrossRef]
- Madadi, M.; Abbas, A. Lignin Degradation by Fungal Pretreatment: A Review. J. Plant Pathol. Microbiol. 2017, 8, 1000398. [Google Scholar] [CrossRef]
- Goodell, B.; Qian, Y.; Jellison, J. Fungal Decay of Wood: Soft Rot-Brown Rot-White Rot; ACS Symposium Series; American Chemical Society (ACS): Washington, DC, USA, 2008; Volume 982, pp. 9–31. [Google Scholar]
- Fioretto, A.; Papa, S.; Pellegrino, A.; Fuggi, A. Decomposition Dynamics of Myrtus Communis and Quercus Ilex Leaf Litter: Mass Loss, Microbial Activity and Quality Change. Appl. Soil. Ecol. 2007, 36, 32–40. [Google Scholar] [CrossRef]
- Tagger, S.; Périssol, C.; Gil, G.; Vogt, G.; Le Petit, J. Phenoloxidases of the White-Rot Fungus Marasmius Quercophilus Isolated from an Evergreen Oak Litter (Quercus ilex L.). Enzym. Microb. Technol. 1998, 23, 372–379. [Google Scholar] [CrossRef]
- Cocozza, C.; Parente, A.; Zaccone, C.; Mininni, C.; Santamaria, P.; Miano, T. Comparative Management of Offshore Posidonia Residues: Composting vs. Energy Recovery. Waste Manag. 2011, 31, 78–84. [Google Scholar] [CrossRef]
- Vannucchi, F.; Macci, C.; Doni, S.; Longo, V.; Ugolini, F.; Masciandaro, G.; Peruzzi, E. Posidonia-Based Compost and Dredged Sediment in Growing Media Improve Tolerance and Nutrient Uptake in Ornamental Plants. Sustainability 2022, 14, 14419. [Google Scholar] [CrossRef]
- Briggs, D. Genecological Studies of Salt Tolerance In Groundsel (Senecio vulgaris L.) With Particular Reference to Roadside Habitats. New Phytol. 1978, 81, 381–389. [Google Scholar] [CrossRef]
- Nooh, A.; Khattab, M.; Koreish, E.; El-Tanbouly, R. Effect of Saline Irrigation Water on The Landscaping Potentials of Santolina chamaecyparissus,L, Plants. Alex. Sci. Exch. J. 2014, 35, 23–28. [Google Scholar]
- Hodge, A.; Robinson, D.; Fitter, A. Are Microorganisms More Effective than Plants at Competing for Nitrogen? Trends Plant Sci. 2000, 5, 304–308. [Google Scholar] [CrossRef]
- Mininni, C.; Santamaria, P.; Abdelrahman, H.M.; Cocozza, C.; Miano, T.; Montesano, F.; Parente, A. Posidonia-Based Compost as a Peat Substitute for Lettuce Transplant Production. HortScience 2012, 47, 1438–1444. [Google Scholar] [CrossRef]
- Mininni, C.; Grassi, F.; Traversa, A.; Cocozza, C.; Parente, A.; Miano, T.; Santamaria, P. Posidonia oceanica (L.) Based Compost as Substrate for Potted Basil Production. J. Sci. Food Agric. 2015, 95, 2041–2046. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, W.D.; Hartung, W. Root-Shoot Interactions in Mineral Nutrition. Plant Soil 2000, 226, 57–69. [Google Scholar] [CrossRef]
- Lopez, G.; Ahmadi, S.H.; Amelung, W.; Athmann, M.; Ewert, F.; Gaiser, T.; Gocke, M.I.; Kautz, T.; Postma, J.; Rachmilevitch, S.; et al. Nutrient Deficiency Effects on Root Architecture and Root-to-Shoot Ratio in Arable Crops. Front. Plant Sci. 2023, 13, 5385. [Google Scholar] [CrossRef] [PubMed]
- Mašková, T.; Herben, T. Root:Shoot Ratio in Developing Seedlings: How Seedlings Change Their Allocation in Response to Seed Mass and Ambient Nutrient Supply. Ecol. Evol. 2018, 8, 7143–7150. [Google Scholar] [CrossRef]
- Kang, J.-G.; Van Iersel, M.W. Nutrient Solution Concentration Affects Shoot: Root Ratio, Leaf Area Ratio, and Growth of Subirrigated Salvia (Salvia splendens). HortScience 2004, 39, 49–54. [Google Scholar] [CrossRef]
- D’Imperio, M.; Montesano, F.F.; Montemurro, N.; Parente, A. Posidonia Natural Residues as Growing Substrate Component: An Ecofriendly Method to Improve Nutritional Profile of Brassica Microgreens. Front. Plant Sci. 2021, 12, 580596. [Google Scholar] [CrossRef]
- Ferrández-Gómez, B.; Jordá, J.D.; Cerdán, M.; Sánchez, A. Valorization of Posidonia oceanica Biomass: Role on Germination of Cucumber and Tomato Seeds. Waste Manag. 2023, 171, 634–641. [Google Scholar] [CrossRef]
- Serio, F.; De Gara, L.; Caretto, S.; Leo, L.; Santamaria, P. Influence of an Increased NaCl Concentration on Yield and Quality of Cherry Tomato Grown in Posidonia (Posidonia ooeanica (L.) Delile). J. Sci. Food Agric. 2004, 84, 1885–1890. [Google Scholar] [CrossRef]
- Ledent, G.; Mateo, M.; Warnau, M.; Temara, A.; Dubois, P. Element Losses Following Distilled Water Rinsing of Leaves of the Seagrass Posidonia oceanica (L.) Delile. Aquat. Bot. 1995, 52, 229–235. [Google Scholar] [CrossRef]
- Lafabrie, C.; Pergent-Martini, C.; Pergent, G. Metal Contamination of Posidonia oceanica Meadows along the Corsican Coastline (Mediterranean). Environ. Pollut. 2008, 151, 262–268. [Google Scholar] [CrossRef] [PubMed]



| P. oceanica | ||
|---|---|---|
| Decomposed | Fresh | |
| CSC (meq/100 g) | 58.0 | 45.1 |
| P (mg/kg) | 133.2 | 163.5 |
| K (mg/kg) | 698.0 | 806.9 |
| Ca (mg/kg) | 31,567.0 | 25,224.7 |
| Na (mg/kg) | 681.5 | 1742.0 |
| Mg (mg/kg) | 4889.0 | 5017.7 |
| N (%) | 1.8 | 1.1 |
| C (%) | 38.9 | 38.3 |
| C/N | 22.1 | 35.1 |
| C/P | 2921.7 | 2342.8 |
| B (mg/kg) | 3297.2 | 3120.4 |
| Fe (mg/kg) | 3818.9 | 3874.2 |
| Mn (mg/kg) | 35.4 | 31.9 |
| Zn (mg/kg) | 116.1 | 108.1 |
| Se (mg/kg) | 0.9 | 0.7 |
| Mo (mg/kg) | 0.6 | 0.5 |
| Al (mg/kg) | 1665.3 | 1521.7 |
| Cu (mg/kg) | 18.0 | 19.3 |
| Pb (mg/kg) | 7.7 | 8.1 |
| Hg (mg/kg) | <0.1 | <0.1 |
| Cr (mg/kg) | 6.5 | 6.5 |
| Ni (mg/kg) | 20.7 | 22.4 |
| Cd (mg/kg) | 0.2 | 0.3 |
| As (mg/kg) | 2.5 | 2.4 |
| Bulk Density (kg/m3) | ||
|---|---|---|
| P. oceanica Application Rate | Mixture | |
| Soil | Peat | |
| 0% | 1121.21 | 125.00 |
| 10% | 1009.12 | 117.27 |
| 30% | 798.33 | 100.68 |
| 50% | 582.73 | 82.91 |
| 100% | 42.40 | 42.40 |
| Cultivation System | Species | Mixture | P. oceanica Application Rate |
|---|---|---|---|
| Horticoltural | S. lycopersicum | Fresh P. oceanica-soil | |
| Decomposed P. oceanica-soil | |||
| L. sativa | Fresh P. oceanica-soil | ||
| Decomposed P. oceanica-soil | |||
| O. basilicum | Fresh P. oceanica-soil | ||
| Decomposed P. oceanica-soil | |||
| C. pepo | Fresh P. oceanica-soil | (100%; 50%; 30%; 10%; 0%) | |
| Decomposed P. oceanica-soil | |||
| Ornamental | S. vira vira | Fresh P. oceanica-peat | |
| Decomposed P. oceanica-peat | |||
| S. chamaecyparissus | Fresh P. oceanica-peat | ||
| Decomposed P. oceanica-peat |
| Effect | dF | F | p-Value |
|---|---|---|---|
| Inocula | 3 | 7.732 | <0.001 |
| Time | 2 | 121.914 | <0.001 |
| Inocula × time | 6 | 0.627 | 0.708 |
| Error | 48 |
| P. oceanica Type | P. oceanica Application Rate | S. vira-vira | S. chamaecyparissus | O. basilicum | L. sativa | S. lycopersicum | S. lycopersicum Fertilized | C. pepo | C. pepo Fertilized | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 8.30 a | 7.92 a | 1.28 a | 4.53 a | 5.47 abc | 20.41 a | ** | 2.97 c | 18.58 a | ** | |
| decomposed | 10% | 4.55 a | 9.39 ab | 2.01 a | 2.39 a | 5.81 ab | 16.39 a | ** | 12.52 abc | 9.49 b | |
| 30% | 4.41 a | 7.76 ab | 2.16 a | 4.66 a | 5.81 ab | 14.67 a | * | 18.39 a | 9.85 b | ** | |
| 50% | 3.46 a | 5.43 ab | 2.00 a | 4.95 a | 6.76 ab | 11.64 a | * | 16.22 ab | 9.57 b | * | |
| 100% | 1.11 a | 2.61 b | 2.28 a | 3.76 a | 6.33 ab | 11.63 a | 5.36 bc | 7.82 b | |||
| fresh | 10% | 6.99 a | 9.65 ab | 1.62 a | 1.78 a | 8.23 a | 16.78 a | * | 9.92 abc | 10.50 b | |
| 30% | 3.95 a | 6.74 ab | 2.04 a | 2.60 a | 4.06 bc | 20.25 a | ** | 11.56 abc | 10.73 b | ||
| 50% | 2.53 a | 7.28 ab | 1.88 a | 1.73 a | 3.57 bc | 14.14 a | * | 8.10 bc | 6.42 b | ||
| 100% | 1.01 a | 1.34 b | 3.77 a | 1.25 a | 1.96 c | 11.45 a | * | 6.52 bc | 6.69 b | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amoroso, G.; Cozzolino, A.; Idbella, M.; Iacomino, G.; Motti, R.; Bonanomi, G. The Decomposition Dynamics and Substrate Component Potential of Biomass from the Seagrass Posidonia oceanica (L.) Delile. Horticulturae 2024, 10, 58. https://doi.org/10.3390/horticulturae10010058
Amoroso G, Cozzolino A, Idbella M, Iacomino G, Motti R, Bonanomi G. The Decomposition Dynamics and Substrate Component Potential of Biomass from the Seagrass Posidonia oceanica (L.) Delile. Horticulturae. 2024; 10(1):58. https://doi.org/10.3390/horticulturae10010058
Chicago/Turabian StyleAmoroso, Giandomenico, Alessia Cozzolino, Mohamed Idbella, Giuseppina Iacomino, Riccardo Motti, and Giuliano Bonanomi. 2024. "The Decomposition Dynamics and Substrate Component Potential of Biomass from the Seagrass Posidonia oceanica (L.) Delile" Horticulturae 10, no. 1: 58. https://doi.org/10.3390/horticulturae10010058








