Boosting Tomato Resilience in Tanzania: Grafting to Combat Bacterial Wilt and Abiotic Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experiments
2.2.1. Experiment 1: Response of Ungrafted and Grafted Tomato Plants to Different Fertilization Regimes
2.2.2. Experiment 2: Response of Ungrafted and Grafted Tomato Plants to the Soil Infested with Bacterial Wilt Pathogen
2.2.3. Experiment 3: Response of Ungrafted and Grafted Tomato Plants to Water Deficit Soil Infested by Bacterial Wilt
2.3. Molecular Typing of Bacterial Wilt Strains
2.4. Statistical Analyses
3. Results
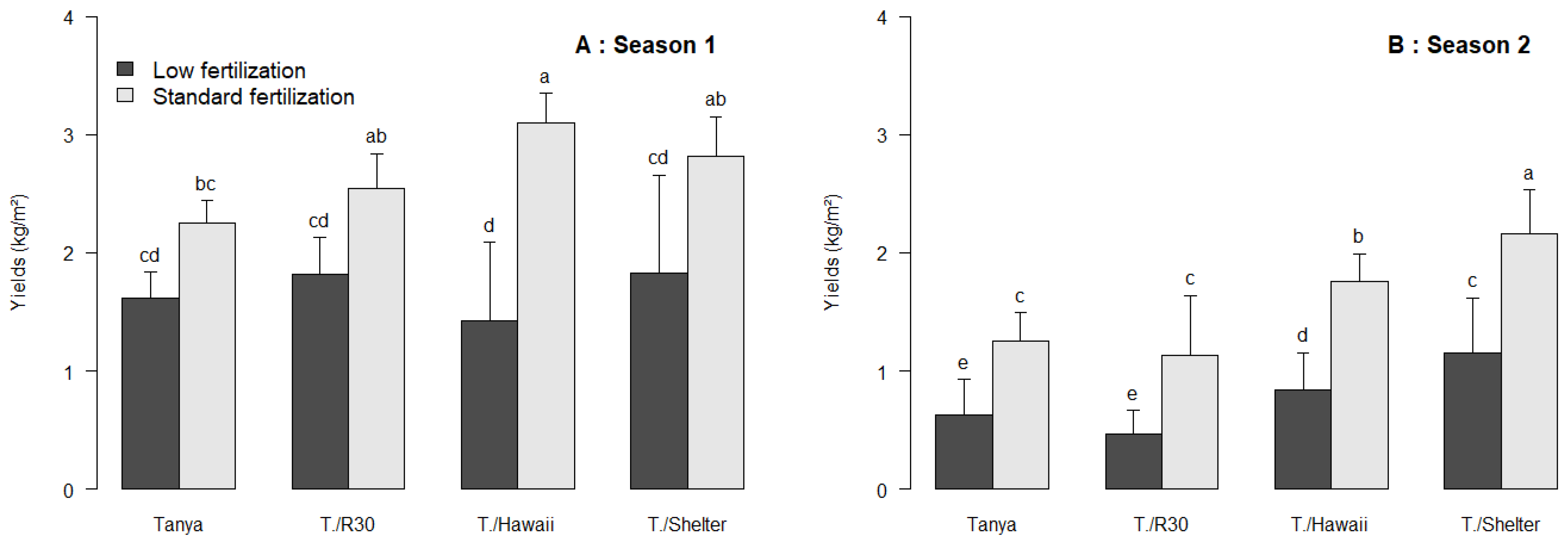
3.1. The Response of Grafted Tomatoes to Different Fertilization Treatments
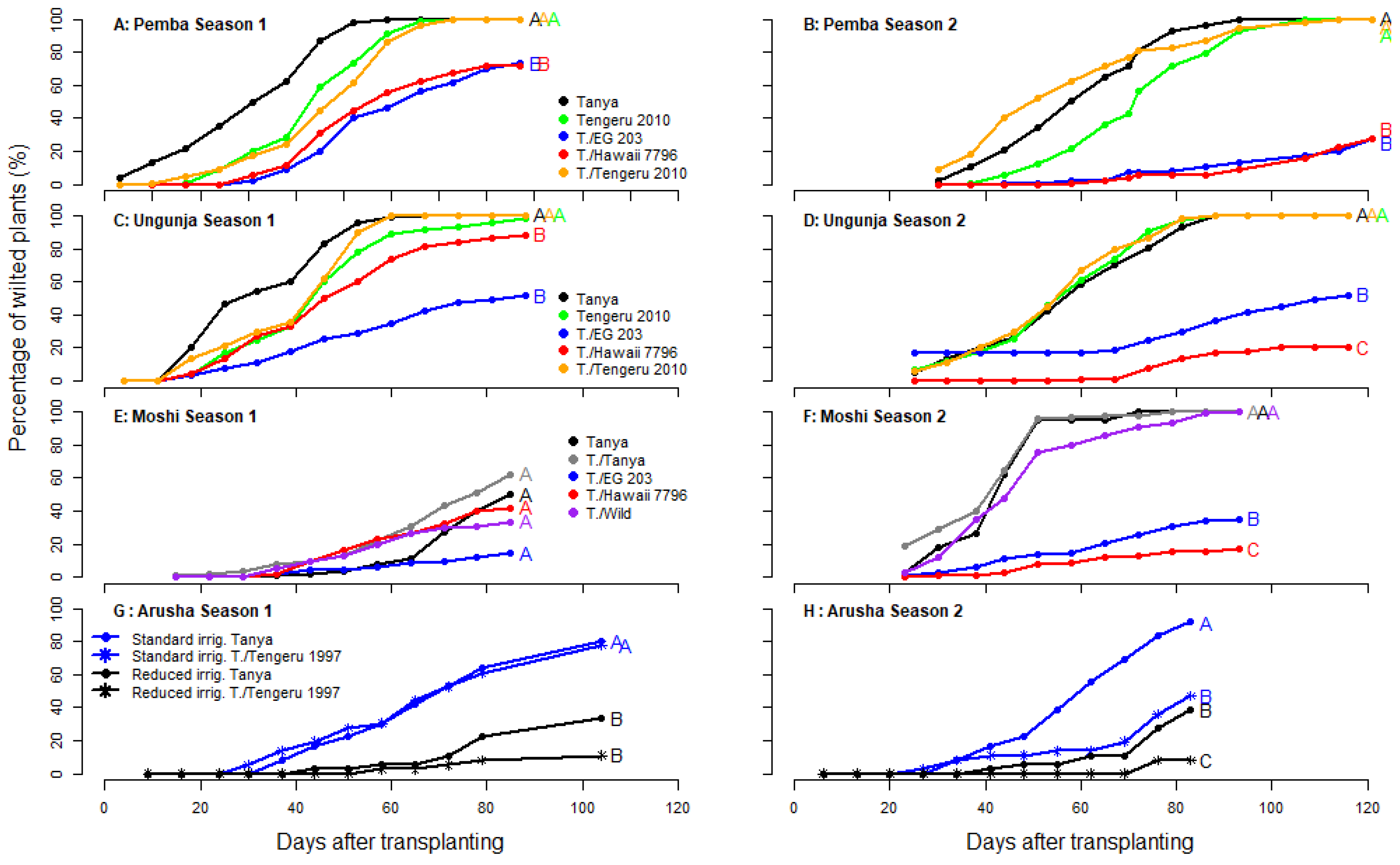
3.2. Performances of Grafted Tomato in Soil Infested by Bacterial Wilt
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schreinemachers, P.; Simmons, E.B.; Wopereis, M.C.S. Tapping the Economic and Nutritional Power of Vegetables. Glob. Food Secur. 2018, 16, 36–45. [Google Scholar] [CrossRef]
- Fakayode, S.; Rahji, M.; Adeniyi, S. Economic Analysis of Risks in Fruit and Vegetable Farming in Osun State, Nigeria. Bangladesh J. Agric. Res 2012, 37, 473–491. [Google Scholar] [CrossRef]
- Cellier, G.; Nordey, T.; Cortada, L.; Gauche, M.; Rasoamanana, H.; Yahiaoui, N.; Rébert, E.; Prior, P.; Chéron, J.J.; Poussier, S.; et al. Molecular Epidemiology of Ralstonia pseudosolanacearum Phylotype I Strains in the Southwest Indian Ocean Region and Their Relatedness to African Strains. Phytopathology® 2023, 113, 423–435. [Google Scholar] [CrossRef] [PubMed]
- De Bon, H.; Huat, J.; Parrot, L.; Sinzogan, A.; Martin, T.; Malézieux, E.; Vayssières, J.-F. Pesticide Risks from Fruit and Vegetable Pest Management by Small Farmers in Sub-Saharan Africa. A Review. Agron. Sustain. Dev. 2014, 34, 723–736. [Google Scholar] [CrossRef]
- Ngowi, A.V.F.; Mbise, T.J.; Ijani, A.S.M.; London, L.; Ajayi, O.C. Smallholder Vegetable Farmers in Northern Tanzania: Pesticides Use Practices, Perceptions, Cost and Health Effects. Crop Prot. 2007, 26, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, E.; Turrero, N.; Kolia, M.; Konaté, Y.; De Alencastro, L.F. Dietary Risk Assessment of Pesticides from Vegetables and Drinking Water in Gardening Areas in Burkina Faso. Sci. Total Environ. 2017, 601–602, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Mutengwe, M.T.; Aneck-Hahn, N.H.; Korsten, L.; Van Zijl, M.C.; De Jager, C. Pesticide Residues and Estrogenic Activity in Fruit and Vegetables Sampled from Major Fresh Produce Markets in South Africa. Food Addit. Contam. Part A 2015, 33, 95–104. [Google Scholar] [CrossRef]
- Doré, T.; Makowski, D.; Malézieux, E.; Munier-Jolain, N.; Tchamitchian, M.; Tittonell, P. Facing up to the Paradigm of Ecological Intensification in Agronomy: Revisiting Methods, Concepts and Knowledge. Eur. J. Agron. 2011, 34, 197–210. [Google Scholar] [CrossRef]
- Wezel, A.; Casagrande, M.; Celette, F.; Vian, J.-F.; Ferrer, A.; Peigné, J. Agroecological Practices for Sustainable Agriculture. A Review. Agron. Sustain. Dev. 2014, 34, 1–20. [Google Scholar] [CrossRef]
- Nordey, T.; Schwarz, D.; Kenyon, L.; Manickam, R.; Huat, J. Tapping the Potential of Grafting to Improve the Performance of Vegetable Cropping Systems in Sub-Saharan Africa. A Review. Agron. Sustain. Dev. 2020, 40, 23. [Google Scholar] [CrossRef]
- Louws, F.J.; Rivard, C.L.; Kubota, C. Grafting Fruiting Vegetables to Manage Soilborne Pathogens, Foliar Pathogens, Arthropods and Weeds. Sci. Hortic. 2010, 127, 127–146. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Colla, G. Vegetable Grafting: A Toolbox for Securing Yield Stability under Multiple Stress Conditions. Front. Plant Sci. 2018, 8, 2255. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.; Rouphael, Y.; Colla, G.; Venema, J.H. Grafting as a Tool to Improve Tolerance of Vegetables to Abiotic Stresses: Thermal Stress, Water Stress and Organic Pollutants. Sci. Hortic. 2010, 127, 162–171. [Google Scholar] [CrossRef]
- Grieneisen, M.L.; Aegerter, B.J.; Scott Stoddard, C.; Zhang, M. Yield and Fruit Quality of Grafted Tomatoes, and Their Potential for Soil Fumigant Use Reduction. A Meta-Analysis. Agron. Sustain. Dev. 2018, 38, 29. [Google Scholar] [CrossRef]
- Ganiyu, S.; Popoola, R.; Enikuomehin, O.; Goke, B. Tube Grafting Reduces Incidence and Severity of Bacterial Wilt in Two Tomato Cultivars in Abeokuta, Nigeria. J. Agric. Sci. Environ. 2017, 16, 96–104. [Google Scholar] [CrossRef]
- Ganiyu, S.A.; Popoola, A.R.; Enikuomehin, O.A.; Bodunde, J.G. Influence of Grafting on Growth and Yield Performance of Two Tomato Cultivars Grown in Open Field in Nigeria. J. Plant Pathol. 2018, 100, 43–50. [Google Scholar] [CrossRef]
- Lin, L.J.; Luther, G.C.; Hanson, P. Raising Healthy Tomato Seedlings; AVRDC—The World Vegetable Center: Shanhua, Taiwan, 2015; pp. 15–795. [Google Scholar]
- Mpinga, L.E. Tomato Grafting for Low-Resource Open-Field Tomato Production in Tanzania. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2013. [Google Scholar]
- Nordey, T.; Shem, E.; Huat, J. Impacts of Temperature and Rootstocks on Tomato Grafting Success Rates. HortScience 2020, 55, 136–140. [Google Scholar] [CrossRef]
- Black, L.L.; Wu, D.L.; Wang, J.F.; Kalb, T.; Abbass, D.; Chen, J.H. Grafting Tomatoes for Production in the Hot-Wet Season; Asian Vegetable Research & Development Center (AVRDC Publication): Shanhua, Taiwan, 2003. [Google Scholar]
- Hanson, P.; Chen, J.T.; Kuo, C.G.; Morris, R.; Opeña, R.T. Suggested Cultural Practices for Tomato. Int. Coop. Guide AVRDC 2000, 1–8. [Google Scholar]
- Nordey, T.; Deletre, E.; Mlowe, N.; Martin, T. Nethouses Protect Cucumber Plants from Insect Pests and Increase Yields in Eastern Africa. J. Hortic. Sci. Biotechnol. 2020, 95, 673–678. [Google Scholar] [CrossRef]
- Nordey, T.; Deletre, E.; Mlowe, N.; Martin, T. Small Mesh Nets Protect Tomato Plants from Insect Pests and Increase Yields in Eastern Africa. J. Hortic. Sci. Biotechnol. 2020, 95, 222–228. [Google Scholar] [CrossRef]
- Do Rosário, G.; Oliveira, M.; Calado, A.M.; Portas, C.A.M. Tomato Root Distribution under Drip Irrigation. J. Am. Soc. Hortic. Sci. 1996, 121, 644–648. [Google Scholar] [CrossRef]
- N’Guessan, C.A.; Abo, K.; Fondio, L.; Chiroleu, F.; Lebeau, A.; Poussier, S.; Wicker, E.; Koné, D. So Near and Yet so Far: The Specific Case of Ralstonia solanacearum Populations from Côte d’Ivoire in Africa. Phytopathology® 2012, 102, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, A.; Daunay, M.-C.; Frary, A.; Palloix, A.; Wang, J.-F.; Dintinger, J.; Chiroleu, F.; Wicker, E.; Prior, P. Bacterial Wilt Resistance in Tomato, Pepper, and Eggplant: Genetic Resources Respond to Diverse Strains in the Ralstonia solanacearum Species Complex. Phytopathology® 2011, 101, 154–165. [Google Scholar] [CrossRef] [PubMed]
- King, S.R.; Davis, A.R.; Liu, W.; Levi, A. Grafting for Disease Resistance. HortScience 2008, 43, 1673–1676. [Google Scholar] [CrossRef]
- Kanyagha, H. Characterization of Ralstonia spp. in Tanzania and Potential Intergrated Pest Management Strategies for Managing Bacterial Wilt in Tomatoes. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2021. [Google Scholar]
- Herman, R.; Perl-Treves, R. Characterization and Inheritance of a New Source of Resistance to Fusarium oxysporum f. sp. melonis Race 1.2 in Cucumis melo. Plant Dis. 2007, 91, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Rivard, C.L.; O’Connell, S.; Peet, M.M.; Welker, R.M.; Louws, F.J. Grafting Tomato to Manage Bacterial Wilt Caused by Ralstonia solanacearum in the Southeastern United States. Plant Dis. 2012, 96, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Genova, C.; Schreinemachers, P.; Afari-Sefa, V. An Impact Assessment of AVRDC’s Tomato Grafting in Vietnam; AVRDC—The World Vegetable Center: Shanhua, Taiwan, 2013. [Google Scholar]
- Kitundu, J.A.; Dinssa, F.F.; Manickam, R.; Macharia, J.; Mbwambo, O.; Aloyce, A.; Ramasamy, S. Effect of Grafting on Yield of Commercial Tomato Cultivars Grown under Bacterial Wilt (Ralstonia solanacearum) Infested Soil. Fruits 2022, 77, 20220152857. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.M.; Toyota, K. Effect of Moisture Conditions and Pre-Incubation at Low Temperature on Bacterial Wilt of Tomato Caused by Ralstonia solanacearum. Microb. Environ. 2004, 19, 244–247. [Google Scholar] [CrossRef]
- Biswas, S.; Akter, K.T.; Rakib, A.; Rhanom, M.S.R.; Rahman, M.M.; Ahmed, S. Performance of Tomato Varieties Grafted with Eggplant under Rain Shelter Condition. Int. J. Nat. Soc. Sci. 2019, 6, 63–70. [Google Scholar]
- Davis, A.R.; Perkins-Veazie, P.; Hassell, R.; Levi, A.; King, S.R.; Zhang, X. Grafting Effects on Vegetable Quality. HortScience 2008, 43, 1670–1672. [Google Scholar] [CrossRef]
- Meya, A.I.; Mamiro, D.P.; Kusolwa, P.M.; Maerere, A.P.; Sibuga, K.P.; Erbaugh, M.; Miller, S.A.; Mtui, H.D. Management of Tomato Late Blight Disease Using Reduced Fungicide Spray Regimes in Morogoro, Tanzania. J. Agric. Sci. 2014, 13, 2. [Google Scholar]
- Rivard, C.L.; Louws, F.J. Grafting to Manage Soilborne Diseases in Heirloom Tomato Production. HortScience 2008, 43, 2104–2111. [Google Scholar] [CrossRef]
- Savvas, D.; Öztekin, G.B.; Tepecik, M.; Ropokis, A.; Tüzel, Y.; Ntatsi, G.; Schwarz, D. Impact of Grafting and Rootstock on Nutrient-to-Water Uptake Ratios during the First Month after Planting of Hydroponically Grown Tomato. J. Hortic. Sci. Biotechnol. 2017, 92, 294–302. [Google Scholar] [CrossRef]
- Schwarz, D.; Öztekin, G.B.; Tüzel, Y.; Brückner, B.; Krumbein, A. Rootstocks Can Enhance Tomato Growth and Quality Characteristics at Low Potassium Supply. Sci. Hortic. 2013, 149, 70–79. [Google Scholar] [CrossRef]
- Djidonou, D.; Zhao, X.; Simonne, E.H.; Koch, K.E.; Erickson, J.E. Yield, Water-, and Nitrogen-use Efficiency in Field-grown, Grafted Tomatoes. HortScience 2013, 48, 485–492. [Google Scholar] [CrossRef]
- Suchoff, D.H.; Gunter, C.C.; Schultheis, J.R.; Hassell, R.L.; Louws, F.J. The Effect of Grafting on Nitrogen Use in Determinate Field-Grown Tomatoes. J. Hortic. Sci. Biotechnol. 2019, 94, 102–109. [Google Scholar] [CrossRef]
- Ullah, F.; Saqib, S.; Khan, W.; Ayaz, A.; Batool, A.; Wang, W.-Y.; Xiong, Y.-C. The Multifaceted Role of Sodium Nitroprusside in Plants: Crosstalk with Phytohormones under Normal and Stressful Conditions. Plant Growth Regul. 2024, 1–18. [Google Scholar] [CrossRef]
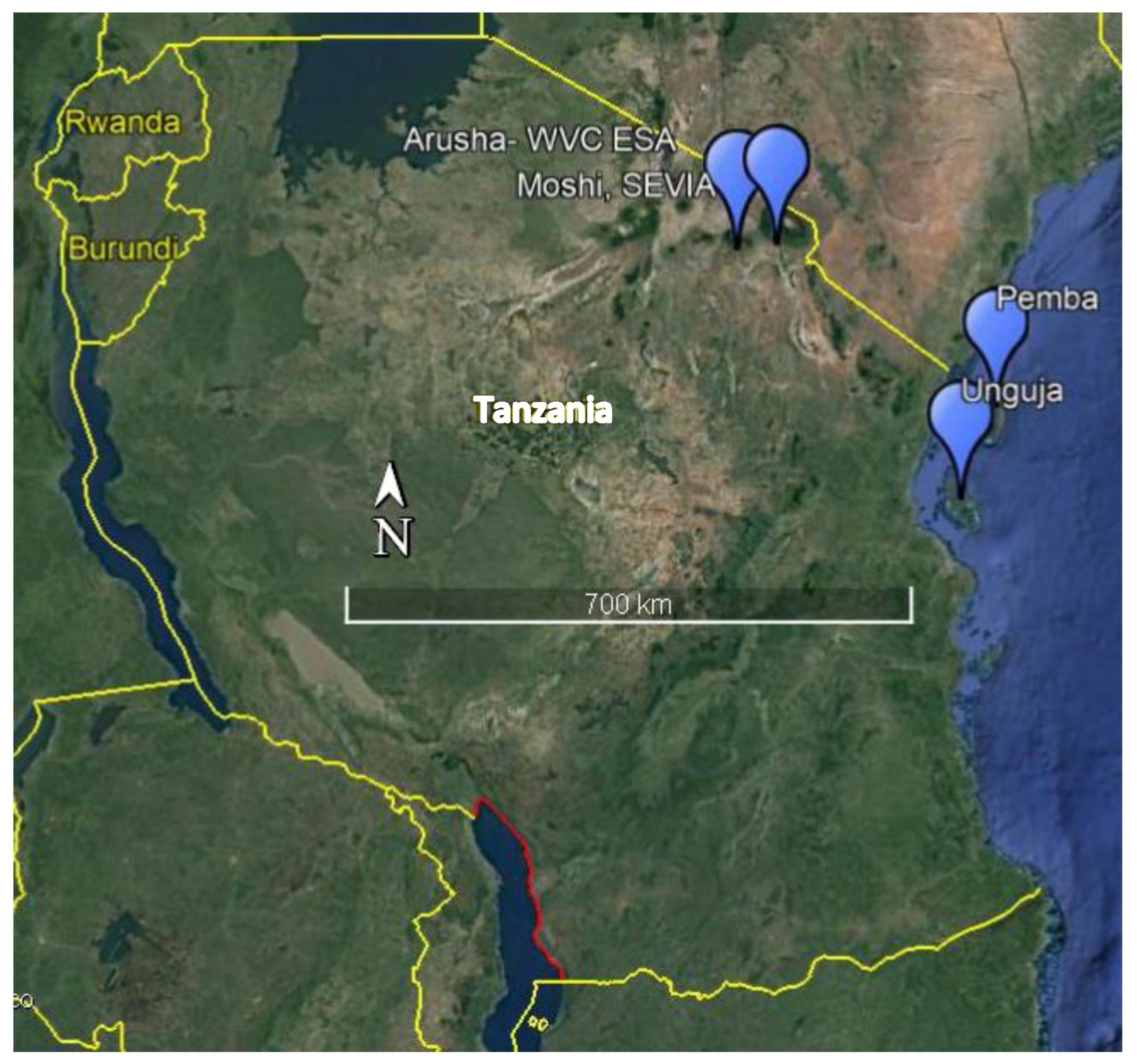
| Varieties | Species | Type | Remarks | Use | Origin | Experiments |
|---|---|---|---|---|---|---|
| Tanya | S. lycopersicum L. | Open-pollinated, determinate | Extended shelf-life, highly sensitive to nematodes and bacterial wilt | Scion and ungrafted control | WorldVeg | 1, 2, and 3 |
| Tengeru 1997 | S. lycopersicum L. | Open-pollinated, semi-indeterminate | Partial resistance to bacterial wilt | Rootstock | WorldVeg | 2 and 3 |
| Tengeru 2010 | S. lycopersicum L. | Open-pollinated, semi-indeterminate | Partial resistance to bacterial wilt | Rootstock and ungrafted control | WorldVeg | 2 |
| Hawaii 7796 | S. lycopersicum L. | Open-pollinated, determinate | Used as a rootstock in Asia to improve resistance to bacterial wilt | Rootstock | INRA | 1 and 2 |
| R3034 | S. melongena | Open-pollinated, determinate | Used as a rootstock in Asia to improve resistance to bacterial wilt | Rootstock | WorldVeg | 1 |
| Shelter | S. lycopersicum L. | Hybrid | Commercial rootstock | Rootstock | Rijk Zwaan | 1 |
| EG 203 | S. melongena | Open-pollinated | Used as a rootstock in Asia to improve resistance to bacterial wilt and flooding. | Rootstock | WorldVeg | 1 and 2 |
| Silverleaf nightshade | S. elaeagnifolium | Wild Solanum species widespread in Tanzania | Resistance to bacterial wilt is not known. | Rootstock | Wild species | 1 and 2 |
| Experiments | Sites | Active Ingredients | Number of Applications in the First Season | Number of Applications in the Second Season | Targets |
|---|---|---|---|---|---|
| 1 | Arusha | Thiocyclam (C7H13NO4S3, Evisect, Arysta LifeScience, Noguères, France) | 3 | 1 | Whiteflies |
| Lambda-cyhalothrin (C23H19ClF3NO3 Ninja, Posit. Inter. Limited, Daresalaam, Tanzania) | 2 | 1 | Whiteflies | ||
| Emamectin benzoate (C49H75NO13, Prove, Equatorial Africa Ltd., Daresalaam, Tanzania) | 2 | 1 | Tuta absoluta | ||
| Metalaxyl and mancozeb (Ridomyl, Syngenta, Basel, Switzerland) | 3 | 5 | Early and late blight | ||
| Copper (Mocrops, Daresalaam, Tanzania) | 0 | 1 | Bacterial spot | ||
| 2 | Pemba | Metalaxyl and mancozeb (Ridomyl, Syngenta, Basel, Switzerland) | 4 | 4 | Early and late blights |
| Chlorothalonil (Daconil, Syngenta, Basel, Switzerland) | 3 | 5 | Powdery mildew | ||
| Imidacloprid (Confidor, Bayer, Leverkusen, Germany) | 4 | 4 | Whiteflies | ||
| Esfenvalerate (Sumi-alpha, Philagro, Pretoria, South Africa) | 4 | 4 | Whiteflies | ||
| Azinphos-methyl (Gusathion, Bayer, Leverkusen, Germany) | 1 | 2 | Tomato fruit worms | ||
| Methomyl (Lannate, Dupont, Tel Aviv, Israel) | 1 | 2 | Tomato fruit worms | ||
| Emamectin benzoate (C49H75NO13, Prove, Equatorial Africa, Daresalaam, Tanzania) | 3 | 4 | Tuta absoluta | ||
| Unguja | Metalaxyl and mancozeb (Ridomyl, Syngenta, Basel, Switzerland) | 5 | 4 | Early and late blight | |
| Chlorothalonil (Daconil, Syngenta, Basel, Switzerland) | 4 | 4 | Powdery mildew | ||
| Imidacloprid (Confidor, Bayer, Leverkusen, Germany) | 4 | 2 | Whiteflies | ||
| Esfenvalerate (Sumi-alpha, Philagro, Tokyo, Japan) | 4 | 2 | Whiteflies | ||
| Azinphos-methyl (Gusathion, Bayer, Leverkusen, Germany) | 2 | 3 | Tomato fruit worms | ||
| Methomyl (Lannate, Dupont, Tel Aviv, Israel) | 2 | 3 | Tomato fruit worms | ||
| Emamectin benzoate (Prove, Equatorial Africa, Daresalaam, Tanzania) | 2 | 6 | Tuta absoluta | ||
| Moshi | Metalaxyl and mancozeb (Ridomyl, Syngenta, Basel, Switzerland) | 3 | 7 | Early and late blight | |
| Chlorothalonil (Daconil, Syngenta, Basel, Switzerland) | 2 | 5 | Powdery mildew | ||
| Imidacloprid (Confidor, Bayer, Leverkusen, Germany) | 5 | 3 | Whiteflies | ||
| Esfenvalerate (Sumi-alpha, Philagro, Tokyo, Japan) | 5 | 3 | Whiteflies | ||
| Azinphos-methyl (Gusathion, Bayer, Leverkusen, Germany) | 3 | 1 | Tomato fruit worms | ||
| Methomyl (Lannate, Dupont, Tel Aviv, Israel) | 3 | 1 | Tomato fruit worms | ||
| Emamectin benzoate (C49H75NO13, Prove, Equatorial Africa, Daresalaam, Tanzania) | 8 | 2 | Tuta absoluta | ||
| 3 | Arusha | Metalaxyl and mancozeb (Ridomyl, Syngenta, Basel, Switzerland) | 1 | 2 | Early and late blight |
| Chlorothalonil (Daconil, Syngenta, Basel, Switzerland) | 3 | 0 | Powdery mildew | ||
| Imidacloprid (Confidor, Bayer, Leverkusen, Germany) | 3 | 4 | Whiteflies | ||
| Emamectin Benzoate (C49H75NO13, Prove, Equatorial Africa, Daresalaam, Tanzania) | 4 | 3 | Tuta absoluta |
| Experiment | Location | Season | Date of Transplanting | Date of the Last Harvest | Average Temperature (°C) | Rainfall (mm) | Average Relative Humidity (%) |
|---|---|---|---|---|---|---|---|
| 1 | Arusha (open field) | 1 | 14 November 2018 | 15 February 2019 | 22.4 | 87.9 | 71.3 |
| 2 | 8 March 2019 | 7 July 2019 | 20.1 | 287.4 | 82.3 | ||
| 2 | Moshi (open field) | 1 | 31 May 2018 | 11 September 2018 | 20.6 | 17.2 | |
| 2 | 19 November 2018 | 19 February 2019 | 25.6 | 101.6 | |||
| 2 | Unguja (open field) | 1 | 1 November 2018 | 31 January 2019 | 27.8 | 266.9 | |
| 2 | 4 July 2019 | 15 October 2019 | 26.1 | 350.8 | |||
| 2 | Pemba (open field) | 1 | 25 October 2018 | 25 January 2019 | 26.6 | 177.9 | |
| 2 | 28 June 2019 | 15 Oct 2019 | 24.4 | 4.8 | |||
| 3 | Arusha (greenhouse) | 1 | 3 December 2018 | 5 March 2019 | 28.1 | 0 | 64.5 |
| 2 | 27 March 2019 | 18 June 2019 | 22.1 | 0 | 77.1 |
| Season | Fertilization | Plants | Plant Fresh Weight (g) | Plant Leaf Surface (cm2) |
|---|---|---|---|---|
| 1 | Low | Tanya | 144.3 ± 18.6 ab | 1108.2 ± 291.8 ab |
| Tanya/Hawaii | 101.1 ± 11.3 cd | 993.3 ± 336.6 ab | ||
| Tanya/R30 | 94.9 ± 11.1 d | 1030.9 ± 355.3 ab | ||
| Tanya/Shelter | 124.6 ± 9.1 bc | 848.8 ± 74.9 b | ||
| Standard | Tanya | 164.4 ± 32.0 a | 1319.9 ± 57.5 a | |
| Tanya/Hawaii | 100.0 ± 17.0 cd | 972.3 ± 105.9 ab | ||
| Tanya/R30 | 108.3 ± 14.0 cd | 805.6 ± 82.5 b | ||
| Tanya/Shelter | 127.4 ± 22.9 bc | 986.3 ± 317.2 ab | ||
| 2 | Low | Tanya | 26.5 ± 9.5 bcd | 1028.7 ± 23.7 ab |
| Tanya/Hawaii | 17.4 ± 1.1 e | 861.5 ± 160.6 bcd | ||
| Tanya/R30 | 18.6 ± 2.5 de | 819.8 ± 73.0 cd | ||
| Tanya/Shelter | 21.9 ± 7.6 cde | 1009.1 ± 36.6 abc | ||
| Standard | Tanya | 37.8 ± 7.8 a | 1162.2 ± 179.3 a | |
| Tanya/Hawaii | 28.9 ± 3.1 bc | 940.1 ± 104.3 bcd | ||
| Tanya/R30 | 24.3 ± 4.4 bcde | 747.1 ± 112.7 d | ||
| Tanya/Shelter | 32.0 ± 8.3 ab | 929.5 ± 86.9 bcd |
| Sites | Treatment | Plants | Marketable Yields (kg/m2) Season 1 | Marketable Yields (kg/m2) Season 2 |
|---|---|---|---|---|
| Pemba | Normal irrigation | Tanya | 0.00 ± 0.00 c | 0.00 ± 0.0 b |
| Normal irrigation | Tengeru 2010 | 0.05 ± 0.06 abc | 0.12 ± 0.11 b | |
| Normal irrigation | Tanya/EG 203 | 0.18 ± 0.04 a | 0.52 ± 0.17 a | |
| Normal irrigation | Tanya/Hawaii 7796 | 0.10 ± 0.08 ab | 0.55 ± 0.20 a | |
| Normal irrigation | Tanya/Tengeru 2010 | 0.01 ± 0.02 bc | 0.04 ± 0.07 b | |
| Unguja | Normal irrigation | Tanya | 0.00 ± 0.00 b | 0.01 ± 0.02 c |
| Normal irrigation | Tengeru 2010 | 0.02 ± 0.04 b | 0.07 ± 0.05 bc | |
| Normal irrigation | Tanya/EG 203 | 0.28 ± 0.02 a | 0.53 ± 0.18 a | |
| Normal irrigation | Tanya/Hawaii 7796 | 0.04 ± 0.01 ab | 0.33 ± 0.09 ab | |
| Normal irrigation | Tanya/Tengeru 2010 | 0.00 ± 0.00 b | 0.01 ± 0.01 c | |
| Moshi | Normal irrigation | Tanya | 3.59 ± 1.85 a | 0.00 ± 0.00 b |
| Normal irrigation | Tanya/EG 203 | 3.59 ± 1.91 a | 0.56 ± 0.11 a | |
| Normal irrigation | Tanya/Hawaii 7796 | 2.05 ± 1.13 a | 0.85 ± 0.34 a | |
| Normal irrigation | Tanya/Tanya | 2.46 ± 1.16 a | 0.00 ± 0.00 b | |
| Normal irrigation | Tanya/Wild | 2.32 ± 1.01 a | 0.00 ± 0.01 b | |
| Arusha | Normal irrigation | Tanya | 0.49 ± 0.12 c | 0.43 ± 0.05 c |
| Normal irrigation | Tanya/Tengeru 1997 | 0.54 ± 0.07 c | 0.55 ± 0.14 c | |
| Half-reduced irrigation | Tanya | 1.02 ± 0.24 b | 1.39 ± 0.36 b | |
| Half-reduced irrigation | Tanya/Tengeru 1997 | 1.22 ± 0.24 a | 2.11 ± 0.47 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Msabila, S.E.; Nordey, T.; Ernest, Z.; Mlowe, N.; Manickam, R.; Ramasamy, S.; Huat, J. Boosting Tomato Resilience in Tanzania: Grafting to Combat Bacterial Wilt and Abiotic Stress. Horticulturae 2024, 10, 338. https://doi.org/10.3390/horticulturae10040338
Msabila SE, Nordey T, Ernest Z, Mlowe N, Manickam R, Ramasamy S, Huat J. Boosting Tomato Resilience in Tanzania: Grafting to Combat Bacterial Wilt and Abiotic Stress. Horticulturae. 2024; 10(4):338. https://doi.org/10.3390/horticulturae10040338
Chicago/Turabian StyleMsabila, Shem Elias, Thibault Nordey, Zablon Ernest, Nickson Mlowe, Ravishankar Manickam, Srinivasan Ramasamy, and Joël Huat. 2024. "Boosting Tomato Resilience in Tanzania: Grafting to Combat Bacterial Wilt and Abiotic Stress" Horticulturae 10, no. 4: 338. https://doi.org/10.3390/horticulturae10040338








