Mycorrhizal Symbiosis Enhances P Uptake and Indole-3-Acetic Acid Accumulation to Improve Root Morphology in Different Citrus Genotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Culture
2.2. Experimental Design
2.3. Variables Measurement
2.4. Gene Expressions Analysis
2.5. Statistical Analysis
3. Results
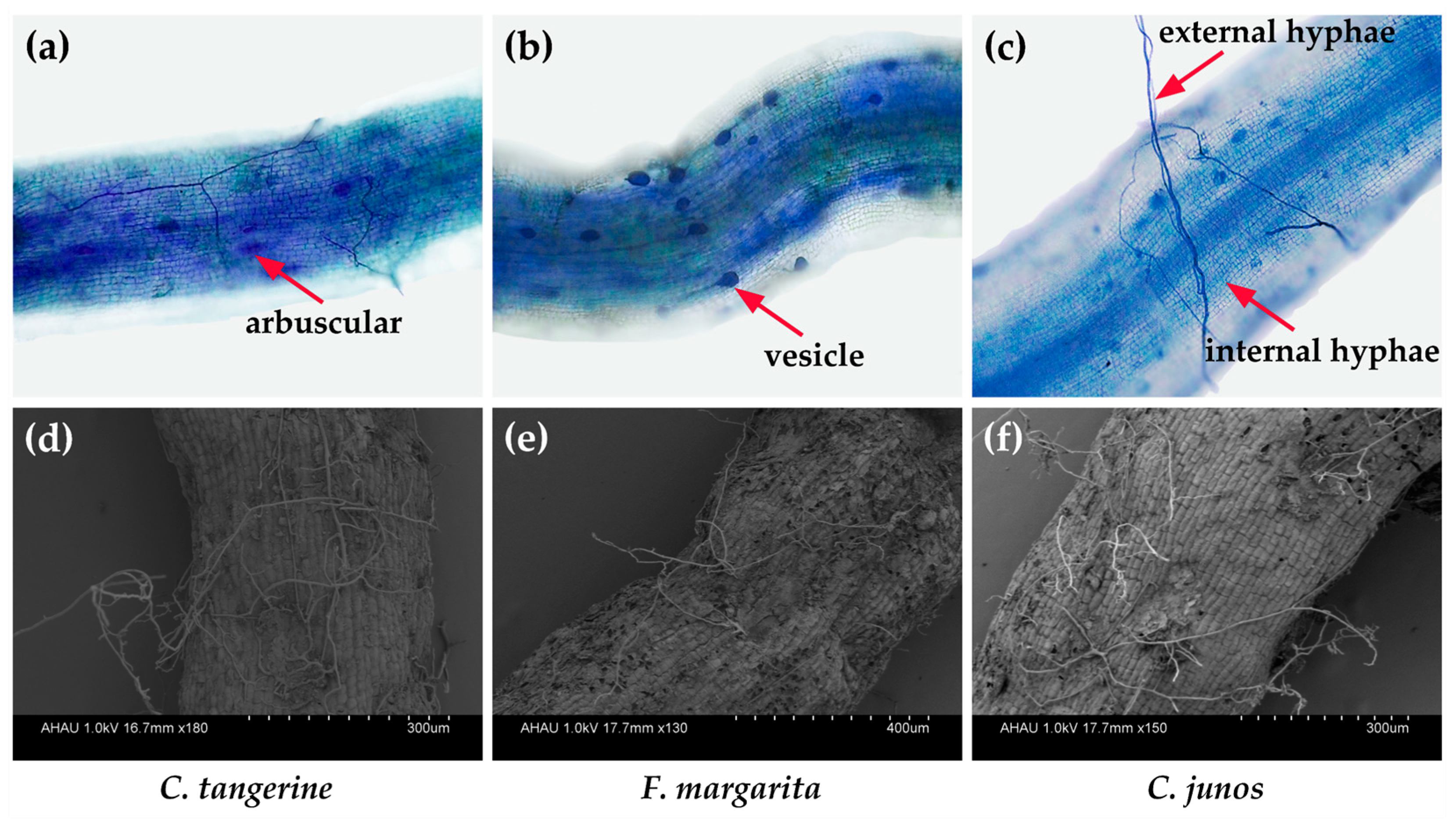
3.1. Mycorrhizal Colonization
3.2. Plant Growth
3.3. Root System Architecture
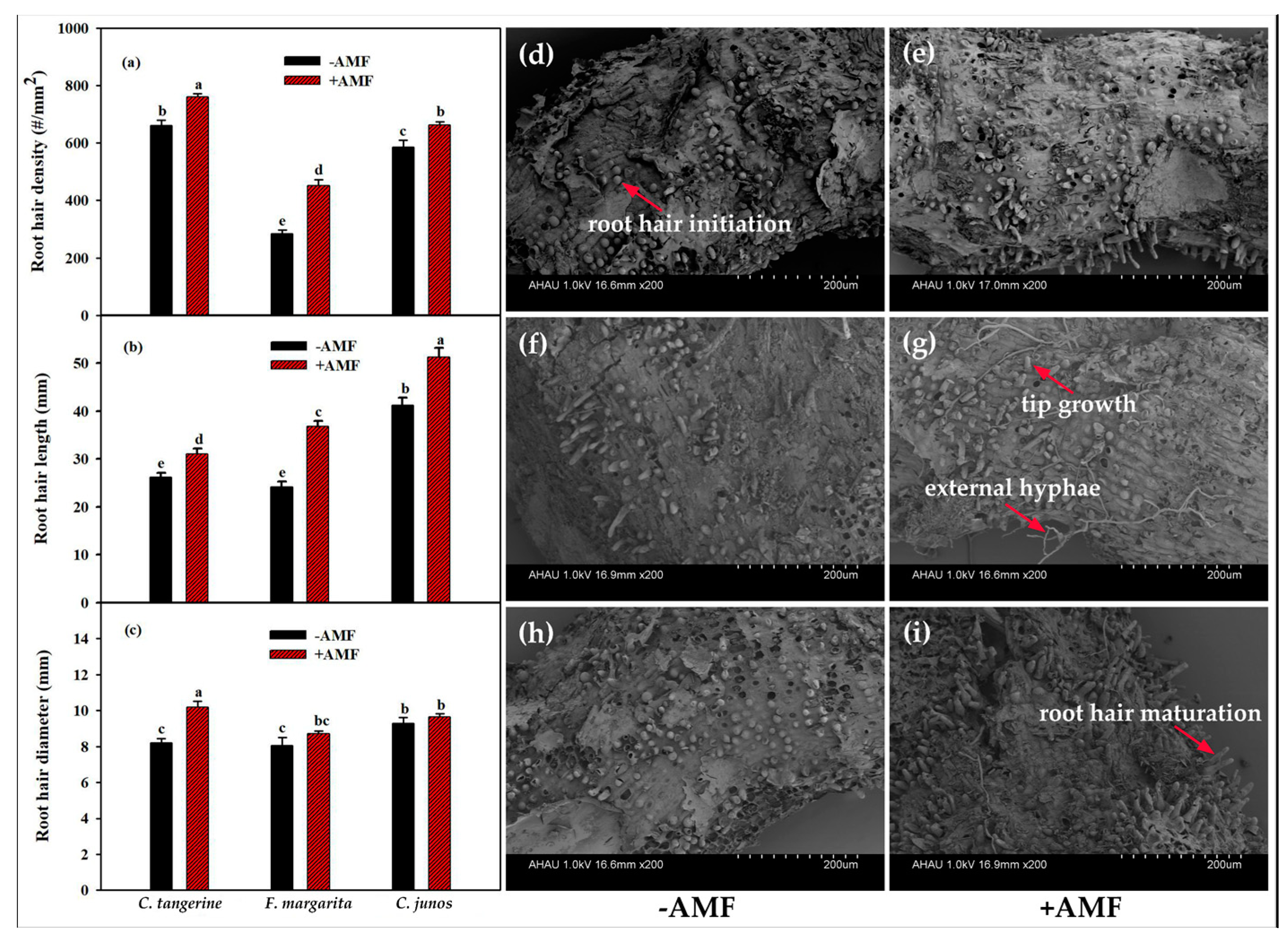
3.4. Root Hair Growth
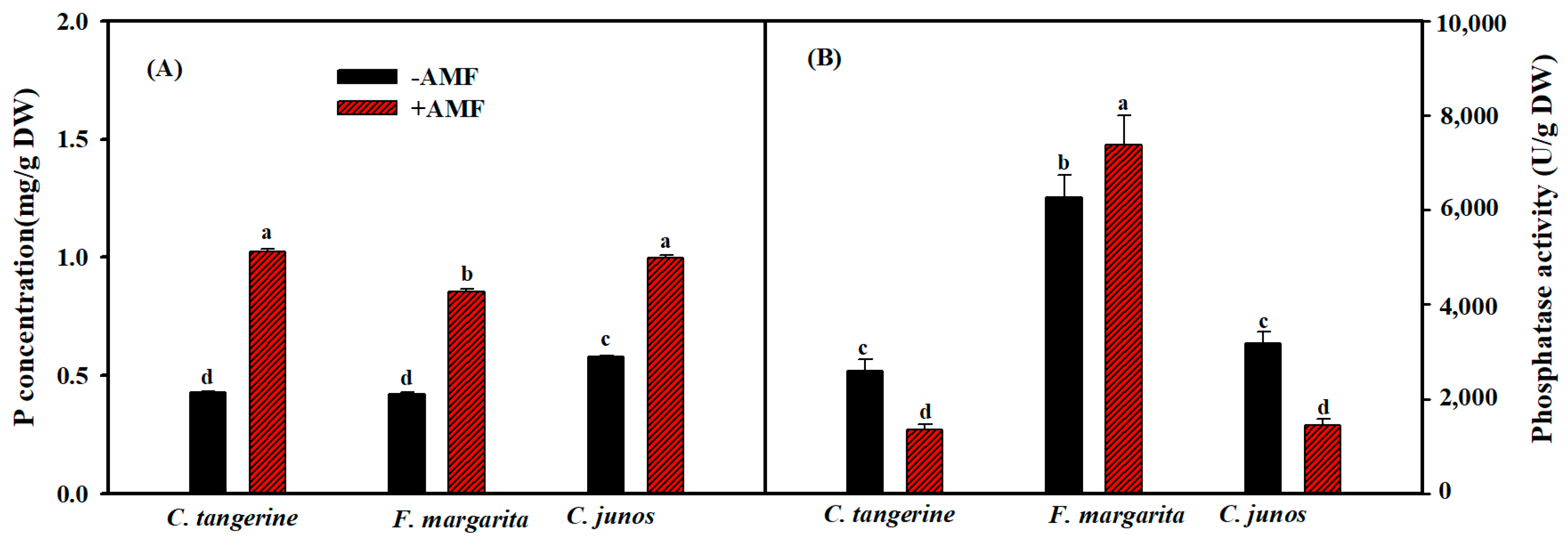
3.5. P Content and Phosphatase Activity
3.6. IAA Content
3.7. Root EXP Expression
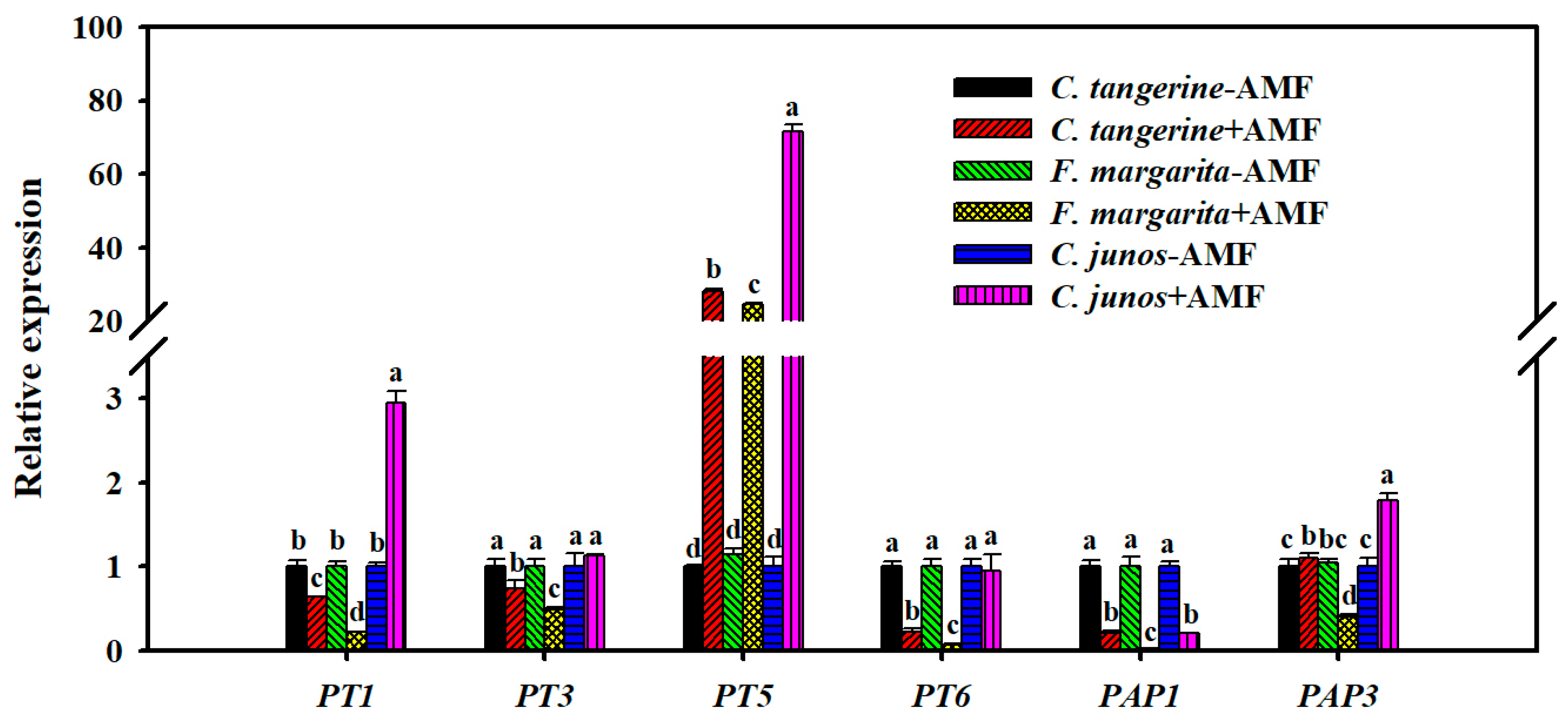
3.8. P Transporter and Phosphatase Gene Expression
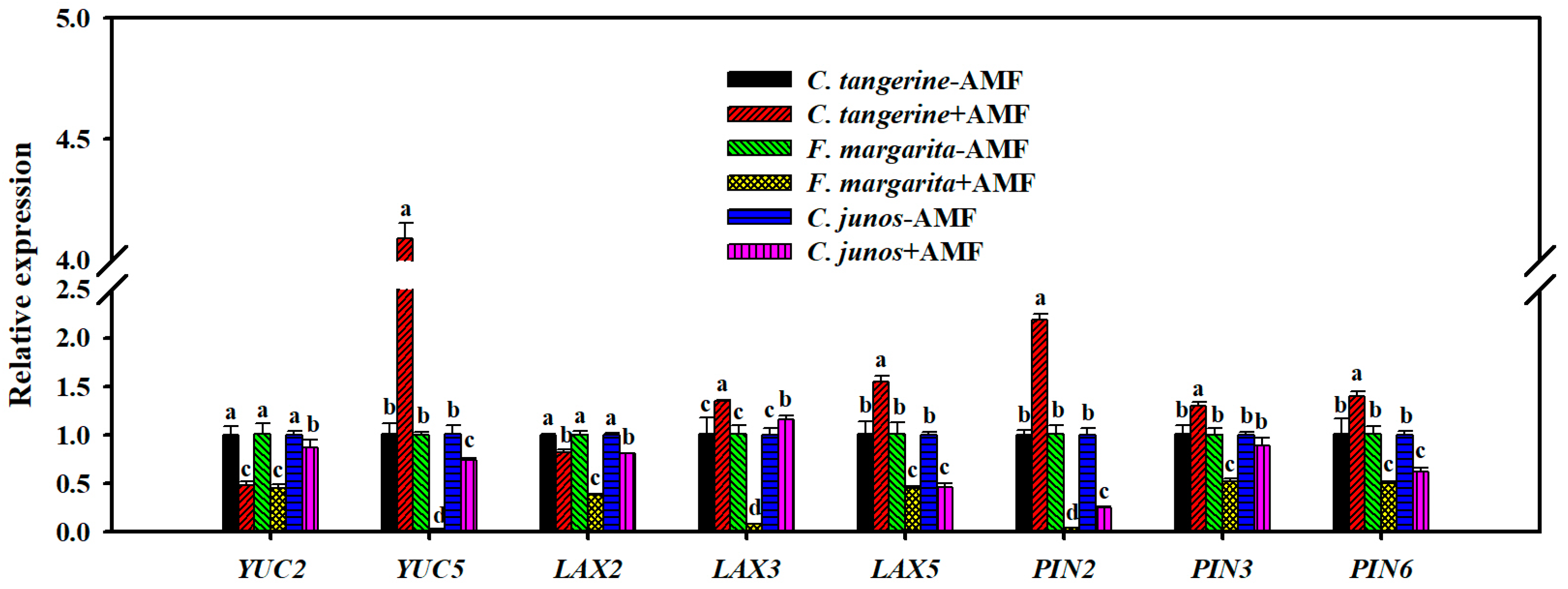
3.9. Root Auxin Related Gene Expression
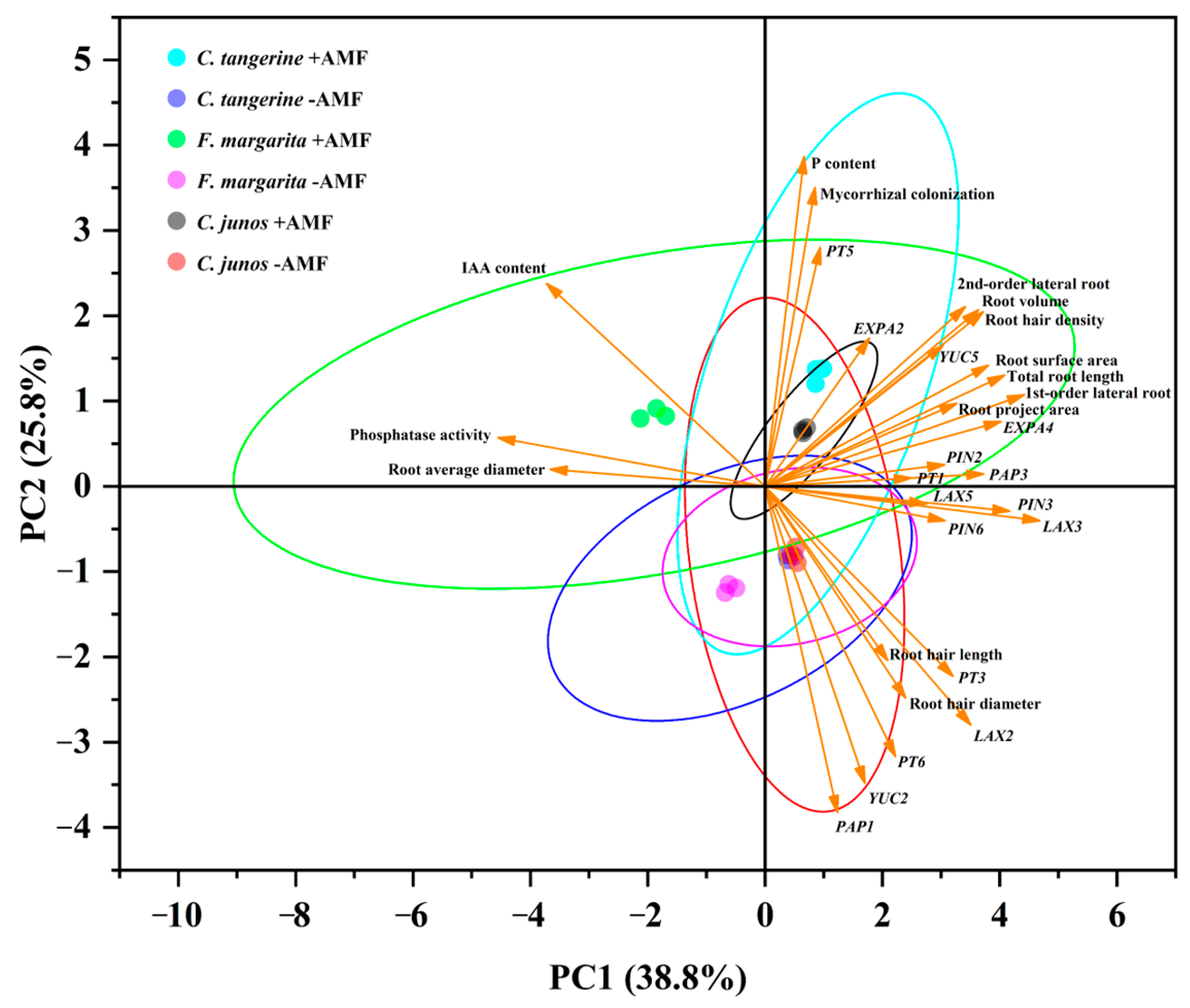
3.10. Principal Component Analysis (PCA)
3.11. Pearson Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zayed, A.; Badawy, M.T.; Farag, M.A. Valorization and extraction optimization of citrus seeds for food and functional food applications. Food Chem. 2021, 355, 129609. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Wang, S.; Cao, M.-Q.; Zou, Y.-N.; Yao, Y.-X. Tempo-spatial distribution and related functionings of root glomalin and glomalin-related soil protein in a citrus rhizosphere. J. Anim. Plant Sci. 2014, 24, 245–251. [Google Scholar] [CrossRef]
- Florian, K.; Florian, V.; Li, X.; Hinrich, B.; Günter, N.; Benjamin, N.; Frank, h.; Uwe, L. Estimating the importance of maize root hairs in low phosphorus conditions and under drought. Ann. Bot. 2019, 124, 961–968. [Google Scholar] [CrossRef]
- Berger, F.; Gutjahr, C. Factors affecting plant responsiveness to arbuscular mycorrhiza. Curr. Opin. Plant Biol. 2021, 59, 101994. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chang, H.; Feng, Z.; Liu, X.; Zhu, H.; Yao, Q. Growth and photosynthetic responses of litchi seedlings to arbuscular mycorrhizal fungal inoculation: Differences between two genotypes. Not. Bot. Horti Agrobo. 2018, 46, 466–473. [Google Scholar] [CrossRef]
- Zhou, L.-J.; Wang, Y.; Alqahtani, M.-D.; Wu, Q.-S. Positive changes in fruit quality, leaf antioxidant defense system, and soil fertility of beni-madonna tangor citrus (Citrus nanko × C. amakusa) after field AMF inoculation. Horticulturae 2023, 9, 1324. [Google Scholar] [CrossRef]
- Li, Q.-S.; Srivastava, A.-K.; Zou, Y.-N.; Wu, Q.-S. Field inoculation responses of arbuscular mycorrhizal fungi versus endophytic fungi on sugar metabolism associated changes in fruit quality of lane late navel orange. Sci. Hortic. 2023, 308, 111587. [Google Scholar] [CrossRef]
- Chen, W.; Li, J.; Zhu, H.; Xu, P.; Chen, J.; Yao, Q. The differential and interactive effects of arbuscular mycorrhizal fungus and phosphorus on the lateral root formation in Poncirus trifoliata (L.). Sci. Horti. 2017, 217, 258–265. [Google Scholar] [CrossRef]
- Caruso, T.; Mafrica, R.; Bruno, M.; Vescio, R.; Sorgonàb, A. Root architectural traits of rooted cuttings of two fig cultivars: Treatments with arbuscular mycorrhizal fungi formulation. Sci. Hortic. 2021, 283, 110083. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, H.; Twagirayezu, G.; Zhang, F.; Shi, Y.; Luo, C.; Yan, F.; Wang, Z.; Xing, D. Arbuscular mycorrhizal fungi adjusts root architecture to promote leaf nitrogen accumulation and reduce leaf carbon–nitrogen ratio of mulberry seedlings. Forests 2023, 14, 2448. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Liu, C.-Y.; Zhang, D.-J.; Zou, Y.-N.; He, X.-H.; Wu, Q.-H. Mycorrhiza alters the profile of root hairs in trifoliate orange. Mycorrhiza 2016, 26, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Y.; Hao, Y.; Wu, X.-L.; Dai, F.-J.; Abd-Allah, E.-F.; Wu, Q.-S.; Liu, S.-R. Arbuscular mycorrhizal fungi improve drought tolerance of tea plants via modulating root architecture and hormones. Plant Growth Regul. 2024, 102, 13–22. [Google Scholar] [CrossRef]
- Zhang, D.-J.; Xia, R.-X.; Cao, X. Ethylene modulates root hair development in trifoliate orange through auxin-signaling pathway. Sci. Horti. 2016, 213, 252–259. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Guo, X.-N.; Wu, X.-L.; Dai, F.-J.; Wu, Q.-S. The comprehensive effects of Rhizophagus intraradices and P on root system architecture and P transportation in Citrus limon L. Agriculture 2022, 12, 317. [Google Scholar] [CrossRef]
- Mohanty, S.-K.; Arthikala, M.-K.; Nanjareddy, K.; Lara, M. Plant-symbiont interactions: The functional role of expansins. Symbiosis 2018, 74, 1–10. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Wang, P.; Zhang, D.-J.; Zou, Y.-N.; Kuca, K.; Wu, Q.-S. Mycorrhiza-induced change in root hair growth is associated with IAA accumulation and expression of EXPs in trifoliate orange under two P levels. Sci. Hortic. 2018, 234, 227–235. [Google Scholar] [CrossRef]
- Chen, W.; Li, J.; Zhu, H.; Xu, P.; Chen, J.; Yao, Q. Arbuscular mycorrhizal fungus enhances lateral root formation in Poncirus trifoliata (L.) as revealed by RNA-Seq analysis. Front. Plant Sci. 2017, 8, 2039. [Google Scholar] [CrossRef]
- Cao, X.; Chen, C.-L.; Zhang, D.-J.; Shu, B.; Xiao, J.; Xia, R.-X. Influence of nutrient deficiency on root architecture and root hair morphology of trifoliate orange (Poncirus trifoliate L. Raf.) seedlings under sand culture. Sci. Hortic. 2013, 162, 100–105. [Google Scholar] [CrossRef]
- Phillips, J.-M.; Hayman, D.-S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- McLachlan, K.-D.; Elliot, D.-E.; Marco, D.; Garran, J.-H.; De, M.-D. Leaf acid phosphatase isozymes in the diagnosis of phosphorus status in field-grown wheat. Aust. J. Agric. Res. 1987, 38, 1–13. [Google Scholar] [CrossRef]
- Dobrev, P.-I.; Kaminek, M. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J. Chromatogr. A 2002, 950, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-W.; Yuan, F.-R.; Long, G.-Y.; Qin, L.; Deng, Z.-N. Selection of reference genes for quantitative real-time RT-PCR analysis in citrus. Mol. Biol. Rep. 2012, 39, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Livak, L.-J.; Schmittgen, T.-D. Analysis of relative gene expression data using real-time quantitative PCR and 2−∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Erdinc, C.; Durak, E.-D.; Ekincialp, A.; Şensoy, S.; Demir, S. Variations in response of determinate common bean (Phaseolus vulgaris L.) genotypes to arbuscular mycorrhizal fungi (AMF) inoculation. Turk. J. Agric. For. 2017, 41, 1–9. [Google Scholar] [CrossRef]
- Felföldi, Z.; Vidican, R.; Stoian, V.; Roman, I.-A.; Sestras, A.-F.; Rusu, T.; Sestras, R.-E. Arbuscular mycorrhizal fungi and fertilization influence yield, growth and root colonization of different tomato genotype. Plants 2022, 11, 1743. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zou, Y.-N.; Wu, Q.-S. Effects of Diversispora spurca inoculation on growth, root system architecture and chlorophyll contents of four citrus genotypes. Int. J. Agric. Biol. 2013, 15, 342–346. [Google Scholar]
- Youpensuk, S.; Lordkaew, S.; Rerkasem, B. Genotypic variation in responses of Citrus spp. to arbuscular mycorrhizal fungi. J. Agric. Sci. 2009, 1, 59. [Google Scholar] [CrossRef]
- Satria, B.; Fadli, M.; Herawati, N. Utilization of arbuskular mikoriza fungi [AMF] for growth and ready to release of three genotype gaharu [Aquilaria spp.]. IOP Conf. Ser. Earth Environ. Sci. 2021, 741, 012049. [Google Scholar] [CrossRef]
- Pang, J.; Bansal, R.; Zhao, H.; Bohuon, E.; Lambers, H.; Ryan, M.-H.; Ranathunge, K.; Siddique, K.-H.-M. The carboxylate-releasing phosphorus-mobilizing strategy can be proxied by foliar manganese concentration in a large set of chickpea germplasm under low phosphorus supply. New Phytol. 2018, 219, 518–529. [Google Scholar] [CrossRef]
- Rigas, S.; Ditengou, F.-A.; Ljung, K.; Daras, G.; Tietz, O.; Palme, K.; Hatzopoulos, P. Root gravitropism and root hair development constitute coupled developmental responses regulated by auxin homeostasis in the Arabidopsis root apex. New Phytol. 2013, 197, 1130–1141. [Google Scholar] [CrossRef]
- Balestrini, R.; Cosgrove, D.-J.; Bonfante, P. Differential location of α-expansin proteins during the accommodation of root cells to an arbuscular mycorrhizal fungus. Planta 2005, 220, 889–899. [Google Scholar] [CrossRef]
- Plassard, C.; Becquer, A.; Garcia, K. Phosphorus transport in mycorrhiza: How far are we? Trends Plant Sci. 2019, 24, 794–801. [Google Scholar] [CrossRef]
- De Oliveira, V.-H.; Mazzafera, P.; de Andrade, S.-A.-L. Alleviation of low phosphorus stress in Eucalyptus grandis by arbuscular mycorrhizal symbiosis and excess Mn. Plant Stress 2022, 5, 100104. [Google Scholar] [CrossRef]
- Sané, A.-K.; Diallo, B.; Kane, A.; Ngom, M.; Cissoko, M.; Sy, M.-O. Response to inoculation with arbuscular mycorrhizal fungi of two tomato (Solanum lycopersicum L.) varieties subjected to water stress under semi-controlled conditions. Agric. Sci. 2022, 13, 790–819. [Google Scholar] [CrossRef]
- El-Sherbeny, T.-M.-S.; Mousa, A.-M.; El-Sayed, E.-S.-R. Use of mycorrhizal fungi and phosphorus fertilization to improve the yield of onion (Allium cepa L.) plant. Saudi J. Biol. Sci. 2022, 29, 331–338. [Google Scholar] [CrossRef]
- Golubkina, N.; Amagova, Z.; Matsadze, V.; Zamana, S.; Tallarita, A.; Caruso, G. Effects of arbuscular mycorrhizal fungi on yield, biochemical characteristics, and elemental composition of garlic and onion under selenium supply. Plants 2020, 9, 84. [Google Scholar] [CrossRef]
- Shu, B.; Xia, R.-X.; Wang, P. Differential regulation of Pht1 phosphate transporters from trifoliate orange (Poncirus trifoliata L. Raf) seedlings. Sci. Hortic. 2012, 146, 115–123. [Google Scholar] [CrossRef]
- Chen, J.; van Groenigen, K.-J.; Hungate, B.-A.; Terrer, C.; van Groenigen, J.-W.; Maestre, F.-T.; Ying, S.-C.; Luo, Y.; Jorgensen, U.; Sinsabaugh, R.-L.; et al. Long-term nitrogen loading alleviates phosphorus limitation in terrestrial ecosystems. Glob. Chang. Biol. 2020, 26, 5077–5086. [Google Scholar] [CrossRef]
- Li, C.-C.; Gui, S.-H.; Yang, T.; Walk, T.; Wang, X.-R.; Liao, H. Identification of soybean purple acid phosphatase genes and their expression responses to phosphorus availability and symbiosis. Ann. Bot. 2012, 109, 275–285. [Google Scholar] [CrossRef]
- Gutjahr, C.; Sawers, R.-J.-H.; Marti, G.; Andrés-Hernández, L.; Yang, S.-Y.; Casieri, L. Transcriptome diversity among rice root types during asymbiosis and interaction with arbuscular mycorrhizal fungi. Proc. Natl. Acad. Sci. USA 2015, 112, 6754–6759. [Google Scholar] [CrossRef]
- Yao, Q.; Zhu, H.-H.; Chen, J.-Z. Growth responses and endogenous IAA and iPAs changes of litchi (Litchi chinensis Sonn.) seedlings induced by arbuscular mycorrhizal fungal inoculation. Sci. Hortic. 2005, 105, 145–151. [Google Scholar] [CrossRef]
- Pacheco-Villalobos, D.; Sankar, M.; Ljung, K.; Hardtke, C.-S. Disturbed local auxin homeostasis enhances cellular anisotropy and reveals alternative wiring of auxin-ethylene crosstalk in Brachypodium distachyon seminal roots. PLoS Genet. 2013, 9, e1003564. [Google Scholar] [CrossRef] [PubMed]
- Geisler, M.; Aryal, B.; Donato, M.-D.; Hao, P.-C. A critical view on ABC transporters and their interacting partners in auxin transport. Plant Cell Physiol. 2017, 58, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-J.; Luo, J. The PIN-FORMED auxin efflux carriers in plants. Int. J. Mol. Sci. 2018, 19, 2759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Swarup, R.; Bennett, M.; Schaller, G.-E.; Kieber, J.-J. Cytokinin induces cell division in the quiescent center of the Arabidopsis root apical merstem. Curr. Biol. 2013, 23, 1979–1989. [Google Scholar] [CrossRef]
- Petrášek, J.; Friml, J. Auxin transport routes in plant development. Development 2009, 136, 2675–2688. [Google Scholar] [CrossRef]



| Gene Name | Accession No. | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| 18SrRNA | FJ356261.1 | TCGGGTGTTTTCACGTCTCA | CGAAGGGTCGCCGTAGGT |
| YUC2 | XM_006475554.2 | ATAGACGCACCAAGCAGC | TCCTCGGATGGAGTTACC |
| YUC5 | XM_006480095.2 | CACAAGCCACTGGTCTCAT | TTCTGCCAACTGCCTAAAC |
| PIN2 | XM_006474238.1 | AGGGAGGAGCAAGAGTGG | AAACAGGAGAAGCGGTAGA |
| PIN3 | XM_006469105.2 | ACCTGCCACCTGAAAGCG | AGTGTTATGACCCGTCTGATT |
| PIN6 | XM_006472856.3 | CTTGCCTACCTCCTTGATACTTC | TCCTCGGATGCCTACTCG |
| LAX2 | XM_006480924.2 | GCCAAACGACCAGTAAACG | CACGCTATCACAGTGGAAAT |
| LAX3 | XM_006472665.1 | CACTCCCTCAACCTGTCC | TGTTCATCCCTTCATACC |
| LAX5 | XM_006486704.2 | GGAAGATGGGTAGGGACG | GCGGTGGTGTTGAAAGAGT |
| EXPA2 | NM_001288851.1 | GTGGTGCTTGTGGGTATGG | ACCGCCGTTGTCGTTAGA |
| EXPA4 | XM_006486407.2 | AGGGTGGCAAGCAATGTC | GCTGGTAATAGTTCTTCCGTCA |
| PT1 | JQ666156 | GCTGCTCTTACTTACTACTGGC | TGCTACCTTGTCCTCCTGA |
| PT3 | JQ666158 | ACTCTGTTTCTTTCGCTTCTG | TCTTCTTGTTGGCATACTCG |
| PT5 | JQ666160 | GGGGTTCCTCTGCTCTTT | ATGCTTTCCGTTGGTTGC |
| PT6 | JQ666161 | AACCCTCGTTGCTCTATGTGG | CAGTGGTGATACGGAATGG |
| PAP1 | JQ666163 | GATTTAGTCGTGGCTGGTCAT | GATAGACTGGAGCAGAAGGGT |
| PAP3 | JQ666165 | TACAACCTCAACAGTCAGTCACA | CCTTCCCCAATAATCCCAAC |
| Citrus Genotype | AMF Inoculation | Mycorrhizal Colonization (%) | Plant Height (cm) | Shoot Biomass (g DW/Plant) | Root Biomass (g DW/Plant) |
|---|---|---|---|---|---|
| C. tangerine | −AMF | 0 ± 0 d | 9.73 ± 0.71 d | 0.53 ± 0.04 e | 0.24 ± 0.02 d |
| +AMF | 43.7 ± 1.7 a | 15.95 ± 0.35 b | 1.82 ± 0.11 b | 0.61 ± 0.04 b | |
| F. margarita | −AMF | 0 ± 0 d | 10.31 ± 0.68 d | 0.46 ± 0.05 f | 0.26 ± 0.01 d |
| +AMF | 21.1 ± 1.87 c | 14.23 ± 1.18 c | 0.90 ± 0.07 c | 0.38 ± 0.03 c | |
| C. junos | −AMF | 0 ± 0 d | 13.89 ± 1.39 c | 0.75 ± 0.07 d | 0.39 ± 0.04 c |
| +AMF | 30.9 ± 1.93 b | 29.54 ± 2.09 a | 2.45 ± 0.17 a | 1.11 ± 0.09 a |
| Citrus Genotype | AMF Inoculation | Total Length (cm) | Projected Area (cm2) | Surface Area (cm2) | Volume (cm3) | Average Diameter (cm) | Lateral Root Numbers (#/Plant) | |
|---|---|---|---|---|---|---|---|---|
| 1st-Order | 2nd-Order | |||||||
| C. tangerine | −AMF | 158 ± 10 b | 10.6 ± 0.7 b | 14.8 ± 0.5 a | 0.60 ± 0.01 c | 0.47 ± 0.03 d | 39 ± 2 a | 12 ± 1 d |
| +AMF | 168 ± 13 a | 11.3 ± 0.9 ab | 15.0 ± 0.9 a | 0.65 ± 0.02 bc | 0.74 ± 0.04 b | 41 ± 4 a | 44 ± 4 b | |
| F. margarita F. margarita | −AMF | 102 ± 5 d | 9.1 ± 0.5 c | 11.2 ± 0.7 d | 0.82 ± 0.03 a | 0.26 ± 0.02 f | 13 ± 1 c | 7 ± 1 e |
| +AMF | 122 ± 19 c | 9.8 ± 1.0 c | 12.2 ± 0.9 c | 0.81 ± 0.07 a | 0.34 ± 0.03 e | 16 ± 1 c | 11 ± 1 d | |
| C. junos | −AMF | 159 ± 5 b | 11.9 ± 0.7 a | 13.4 ± 0.7 b | 0.63 ± 0.06 c | 0.60 ± 0.05 c | 33 ± 3 b | 32 ± 2 c |
| +AMF | 173 ± 11 a | 11.7 ± 1.2 a | 14.6 ± 1.0 a | 0.71 ± 0.01 b | 0.96 ± 0.05 a | 42 ± 3 a | 65 ± 5 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-Y.; Guo, X.-N.; Dai, F.-J.; Wu, Q.-S. Mycorrhizal Symbiosis Enhances P Uptake and Indole-3-Acetic Acid Accumulation to Improve Root Morphology in Different Citrus Genotypes. Horticulturae 2024, 10, 339. https://doi.org/10.3390/horticulturae10040339
Liu C-Y, Guo X-N, Dai F-J, Wu Q-S. Mycorrhizal Symbiosis Enhances P Uptake and Indole-3-Acetic Acid Accumulation to Improve Root Morphology in Different Citrus Genotypes. Horticulturae. 2024; 10(4):339. https://doi.org/10.3390/horticulturae10040339
Chicago/Turabian StyleLiu, Chun-Yan, Xiao-Niu Guo, Feng-Jun Dai, and Qiang-Sheng Wu. 2024. "Mycorrhizal Symbiosis Enhances P Uptake and Indole-3-Acetic Acid Accumulation to Improve Root Morphology in Different Citrus Genotypes" Horticulturae 10, no. 4: 339. https://doi.org/10.3390/horticulturae10040339
APA StyleLiu, C. -Y., Guo, X. -N., Dai, F. -J., & Wu, Q. -S. (2024). Mycorrhizal Symbiosis Enhances P Uptake and Indole-3-Acetic Acid Accumulation to Improve Root Morphology in Different Citrus Genotypes. Horticulturae, 10(4), 339. https://doi.org/10.3390/horticulturae10040339







