Biomass Source of Biochar and Genetic Background of Tomato Influence Plant Growth and Development and Fruit Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. BC Source
2.2. SEM and EDX Analysis
2.3. Plant Growth Conditions
2.4. Experimental Design
2.5. Plant Growth Parameters and Assessment of Fruit Quality
2.6. Statistical Analysis
2.6.1. Three-Way and Two-Way ANOVA
2.6.2. Correlation Plot
3. Results and Discussion
3.1. Qualitative Characterization of BC Using Scanning Electron Microscopy and EDX
3.2. Agronomic Traits: Plant Dry Weight and Yield Per Plant
3.3. Fruit Quality: Glucose, Fructose, Citrate, and Malate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cotching, W.E. Organic Matter in the Agricultural Soils of Tasmania, Australia—A Review. Geoderma 2018, 312, 170–182. [Google Scholar] [CrossRef]
- Hewitt, S.; Ghogare, R.; Troxel, W.; Tenic, E.; Isaac, D.; Dhingra, A. Metatranscriptomic Analysis of Tomato Rhizospheres Reveals Insight into Plant-Microbiome Molecular Response to Biochar-Amended Organic Soil. Front. Anal. Sci. 2023, 3, 1205583. [Google Scholar] [CrossRef]
- Lal, R. Soil Health and Carbon Management. Food Energy Secur. 2016, 5, 212–222. [Google Scholar] [CrossRef]
- Novotny, E.H.; Hayes, M.H.B.; Madari, B.E.; Bonagamba, T.J.; deAzevedo, E.R.; de Souza, A.A.; Song, G.; Nogueira, C.M.; Mangrich, A.S. Lessons from the Terra Preta de Índios of the Amazon Region for the Utilisation of Charcoal for Soil Amendment. J. Braz. Chem. Soc. 2009, 20, 1003–1010. [Google Scholar] [CrossRef]
- Steiner, C.; Teixeira, W.G.; Lehmann, J.; Nehls, T.; De MacÊdo, J.L.V.; Blum, W.E.H.; Zech, W. Long Term Effects of Manure, Charcoal and Mineral Fertilization on Crop Production and Fertility on a Highly Weathered Central Amazonian Upland Soil. Plant Soil 2007, 291, 275–290. [Google Scholar] [CrossRef]
- Zech, W.; Haumaier, L.; Reinhold, H. Ecological Aspects of Soil Organic Matter in Tropical Land Use. In Humic Substances in Soil and Crop Sciences: Selected Readings; Wiley Online: Hoboken, NJ, USA, 1990; pp. 187–202. [Google Scholar]
- Basso, B.; Dumont, B.; Maestrini, B.; Shcherbak, I.; Robertson, G.P.; Porter, J.R.; Smith, P.; Paustian, K.; Grace, P.R.; Asseng, S.; et al. Soil Organic Carbon and Nitrogen Feedbacks on Crop Yields under Climate Change. Agric. Environ. Lett. 2018, 3, 180026. [Google Scholar] [CrossRef]
- Magdoff, F. Ecological Agriculture: Principles, Practices, and Constraints. Renew. Agric. Food Syst. 2007, 22, 109–117. [Google Scholar] [CrossRef]
- Doran, J.W.; Zeiss, M.R. Soil Health and Sustainability: Managing the Biotic Component of Soil Quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G.A. An Underground Revolution: Biodiversity and Soil Ecological Engineering for Agricultural Sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J.; et al. Biochar: A Synthesis of Its Agronomic Impact beyond Carbon Sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef]
- Laird, D.A. The Charcoal Vision: A Win-Win-Win Scenario for Simultaneously Producing Bioenergy, Permanently Sequestering Carbon, While Improving Soil and Water Quality. Agron. J. 2008, 100, 178–181. [Google Scholar] [CrossRef]
- Gurwick, N.P.; Moore, L.A.; Kelly, C.; Elias, P. A Systematic Review of Biochar Research, with a Focus on Its Stability In Situ and Its Promise as a Climate Mitigation Strategy. PLoS ONE 2013, 8, e75932. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H. Wood Biochar Impacts Soil Phosphorus Dynamics and Microbial Communities in Organically-Managed Croplands. Soil. Biol. Biochem. 2018, 126, 144–150. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.; Chen, Z.; Zhu, L.; Schnoor, J.L. Insight into Multiple and Multilevel Structures of Biochars and Their Potential Environmental Applications: A Critical Review. Envrion. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef] [PubMed]
- Tenic, E.; Ghogare, R.; Dhingra, A. Biochar—A Panacea for Agriculture or Just Carbon? Horticulturae 2020, 6, 37. [Google Scholar] [CrossRef]
- Flowers, M.D.; Lal, R. Axle Load and Tillage Effects on Soil Physical Properties and Soybean Grain Yield on a Mollic Ochraqualf in Northwest Ohio. Soil. Tillage Res. 1998, 48, 21–35. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, X.; Tang, C.; Muhammad, N.; Wu, J.; Brookes, P.C.; Xu, J. Potential Role of Biochars in Decreasing Soil Acidification-A Critical Review. Sci. Total Environ. 2017, 581, 601–611. [Google Scholar] [CrossRef]
- Thi Thu Hien, T.; Shinogi, Y.; Taniguchi, T.; Hien, T.T.T.; Shinogi, Y.; Taniguchi, T. The Different Expressions of Draft Cherry Tomato Growth, Yield, Quality under Bamboo and Rice Husk Biochars Application to Clay Loamy Soil. Agric. Sci. 2017, 08, 934–948. [Google Scholar] [CrossRef]
- Woldetsadik, D.; Drechsel, P.; Marschner, B.; Itanna, F.; Gebrekidan, H. Effect of Biochar Derived from Faecal Matter on Yield and Nutrient Content of Lettuce (Lactuca Sativa) in Two Contrasting Soils. Environ. Syst. Res. 2018, 6, 2. [Google Scholar] [CrossRef]
- Velez, T.I.; Moonilall, N.I.; Reed, S.; Jayachandran, K.; Scinto, L.J. Impact of Melaleuca Quinquenervia Biochar on Phaseolus Vulgaris Growth, Soil Nutrients, and Microbial Gas Flux. J. Environ. Qual. 2018, 47, 1487–1495. [Google Scholar] [CrossRef]
- Nzediegwu, C.; Prasher, S.; Elsayed, E.; Dhiman, J.; Mawof, A.; Patel, R. Effect of Biochar on Heavy Metal Accumulation in Potatoes from Wastewater Irrigation. J. Environ. Manag. 2019, 232, 153–164. [Google Scholar] [CrossRef]
- Li, S.; Shangguan, Z. Positive Effects of Apple Branch Biochar on Wheat Yield Only Appear at a Low Application Rate, Regardless of Nitrogen and Water Conditions. J. Soils Sediments 2018, 18, 3235–3243. [Google Scholar] [CrossRef]
- Faloye, O.T.; Alatise, M.O.; Ajayi, A.E.; Ewulo, B.S. Effects of Biochar and Inorganic Fertiliser Applications on Growth, Yield and Water Use Efficiency of Maize under Deficit Irrigation. Agric. Water Manag. 2019, 217, 165–178. [Google Scholar] [CrossRef]
- Liu, B.; Cai, Z.; Zhang, Y.; Liu, G.; Luo, X.; Zheng, H. Comparison of Efficacies of Peanut Shell Biochar and Biochar-Based Compost on Two Leafy Vegetable Productivity in an Infertile Land. Chemosphere 2019, 224, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Laghari, M.; Naidu, R.; Xiao, B.; Hu, Z.; Mirjat, M.S.; Hu, M.; Kandhro, M.N.; Chen, Z.; Guo, D.; Jogi, Q.; et al. Recent Developments in Biochar as an Effective Tool for Agricultural Soil Management: A Review. J. Sci. Food Agric. 2016, 96, 4840–4849. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Lang, K.; Snyder, D. Impact of Biochar and Fertility Management on Potato Production. 2018. Available online: https://www.iastatedigitalpress.com/farmreports/article/id/1151/ (accessed on 2 February 2024).
- Muhammad, N.; Dai, Z.; Xiao, K.; Meng, J.; Brookes, P.C.; Liu, X.; Wang, H.; Wu, J.; Xu, J. Changes in Microbial Community Structure Due to Biochars Generated from Different Feedstocks and Their Relationships with Soil Chemical Properties. Geoderma 2014, 226–227, 270–278. [Google Scholar] [CrossRef]
- Jiang, Z.; Lian, F.; Wang, Z.; Xing, B. The Role of Biochars in Sustainable Crop Production and Soil Resiliency. J. Exp. Bot. 2020, 71, 520–542. [Google Scholar] [CrossRef]
- Smith, P. Soil Carbon Sequestration and Biochar as Negative Emission Technologies. Glob. Chang. Biol. 2016, 22, 1315–1324. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A.; Salvi, B.L. Comprehensive Review on Production and Utilization of Biochar. SN Appl. Sci. 2019, 1, 168. [Google Scholar] [CrossRef]
- Bruun, E.W.; Petersen, C.T.; Hansen, E.; Holm, J.K.; Hauggaard-Nielsen, H. Biochar Amendment to Coarse Sandy Subsoil Improves Root Growth and Increases Water Retention. Soil. Use Manag. 2014, 30, 109–118. [Google Scholar] [CrossRef]
- Chen, Q.; Qin, J.; Sun, P.; Cheng, Z.; Shen, G. Cow Dung-Derived Engineered Biochar for Reclaiming Phosphate from Aqueous Solution and Its Validation as Slow-Release Fertilizer in Soil-Crop System. J. Clean. Prod. 2018, 172, 2009–2018. [Google Scholar] [CrossRef]
- Haider, G.; Steffens, D.; Moser, G.; Müller, C.; Kammann, C.I. Biochar Reduced Nitrate Leaching and Improved Soil Moisture Content without Yield Improvements in a Four-Year Field Study. Agric. Ecosyst. Environ. 2017, 237, 80–94. [Google Scholar] [CrossRef]
- Murtaza, G.; Ahmed, Z.; Usman, M.; Tariq, W.; Ullah, Z.; Shareef, M.; Iqbal, H.; Waqas, M.; Tariq, A.; Wu, Y. Biochar induced modifications in soil properties and its impacts on crop growth and production. J. Plant Nutr. 2021, 44, 1677–1691. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar Application to Low Fertility Soils: A Review of Current Status, and Future Prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to Improve Soil Fertility. A Review. Agron. Sustain. Dev. 2016, 36, 1–18. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and Its Effects on Plant Productivity and Nutrient Cycling: A Meta-Analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Schmidt, H.P.; Kammann, C.; Hagemann, N.; Leifeld, J.; Bucheli, T.D.; Sánchez Monedero, M.A.; Cayuela, M.L. Biochar in agriculture—A systematic review of 26 global meta-analyses. GCB Bioenergy 2021, 13, 1708–1730. [Google Scholar] [CrossRef]
- Takaragawa, H.; Yabuta, S.; Watanabe, K.; Kawamitsu, Y. Effects of Application of Bagasse- and Sunflower Residue-Derived Biochar to Soil on Growth and Yield of Oilseed Sunflower. Trop. Agric. Dev. 2017, 61, 32–39. [Google Scholar] [CrossRef]
- Vaccari, F.P.; Maienza, A.; Miglietta, F.; Baronti, S.; Di Lonardo, S.; Giagnoni, L.; Lagomarsino, A.; Pozzi, A.; Pusceddu, E.; Ranieri, R.; et al. Biochar Stimulates Plant Growth but Not Fruit Yield of Processing Tomato in a Fertile Soil. Agric. Ecosyst. Environ. 2015, 207, 163–170. [Google Scholar] [CrossRef]
- Beauchamp, E.G.; Kannenberg, L.W.; Hunter, R.B. Nitrogen Accumulation and Translocation in Corn Genotypes Following Silking1. Agron. J. 1976, 68, 418. [Google Scholar] [CrossRef]
- Siciliano, S.D.; Fortin, N.; Mihoc, A.; Wisse, G.; Labelle, S.; Beaumier, D.; Ouellette, D.; Roy, R.; Whyte, L.G.; Banks, M.K.; et al. Selection of Specific Endophytic Bacterial Genotypes by Plants in Response to Soil Contamination. Appl. Environ. Microbiol. 2001, 67, 2469–2475. [Google Scholar] [CrossRef]
- Richard-Molard, C.; Krapp, A.; Brun, F.; Ney, B.; Daniel-Vedele, F.; Chaillou, S. Plant Response to Nitrate Starvation Is Determined by N Storage Capacity Matched by Nitrate Uptake Capacity in Two Arabidopsis Genotypes. J. Exp. Bot. 2008, 59, 779–791. [Google Scholar] [CrossRef]
- Choi, K.; Lee, G.; Han, Y.J.; Bunn, J.M. Tomato Maturity Evaluation Using Color Image Analysis. Trans. ASAE 1995, 38, 171–176. [Google Scholar] [CrossRef]
- López Camelo, A.F.; Gómez, P.A. Comparison of Color Indexes for Tomato Ripening. Hortic. Bras. 2004, 22, 534–537. [Google Scholar] [CrossRef]
- Hewitt, S.L.; Ghogare, R.; Dhingra, A. Glyoxylic Acid Overcomes 1-MCP-Induced Blockage of Fruit Ripening in Pyrus communis L. var. ‘D’Anjou’. Sci. Rep. 2020, 10, 7084. [Google Scholar] [CrossRef] [PubMed]
- Ince, P.J. PULPING|Fiber Resources. In Encyclopedia of Forest Sciences; Elsevier: Amsterdam, The Netherlands, 2004; pp. 877–883. [Google Scholar] [CrossRef]
- Gondim, R.S.; Muniz, C.R.; Lima, C.E.P.; Santos, C.L.A. Explaining the water-holding capacity of biochar by scanning electron microscope images. Rev. Caatinga 2018, 31, 972–979. [Google Scholar] [CrossRef]
- Mukhina, I.M.; Rizhiya, E.Y.; Buchkina, N.P.; Balashov, E.V. Changes in Soil Conditions after Application of Biochar. IOP Conf. Ser. Earth Environ. Sci. 2019, 368, 012037. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Echlin, P.; Joy, D.C.; Lyman, C.E.; Lifshin, E.; Sawyer, L.; Michael, J.R.; Goldstein, J.I.; Newbury, D.E.; et al. X-ray Spectral Measurement: EDS and WDS. In Scanning Electron Microscopy and X-ray Microanalysis; Springer: New York, NY, USA, 2003; pp. 297–353. [Google Scholar]
- Ma, X.; Zhou, B.; Budai, A.; Jeng, A.; Hao, X.; Wei, D.; Zhang, Y.; Rasse, D. Study of Biochar Properties by Scanning Electron Microscope—Energy Dispersive X-ray Spectroscopy (SEM-EDX). Commun. Soil. Sci. Plant Anal. 2016, 47, 593–601. [Google Scholar] [CrossRef]
- Gascó, G.; Cely, P.; Paz-Ferreiro, J.; Plaza, C.; Méndez, A. Relation between biochar properties and effects on seed germination and plant development. Biol. Agric. Hortic. 2016, 32, 237–247. [Google Scholar] [CrossRef]
- Prakongkep, N.; Gilkes, R.J.; Wiriyakitnateekul, W. Forms and Solubility of Plant Nutrient Elements in Tropical Plant Waste Biochars. J. Plant Nutr. Soil Sci. 2015, 178, 732–740. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.A.; Cayuela, M.L.; Sánchez-García, M.; Vandecasteele, B.; D’Hose, T.; López, G.; Martínez-Gaitán, C.; Kuikman, P.J.; Sinicco, T.; Mondini, C. Agronomic Evaluation of Biochar, Compost and Biochar-Blended Compost across Different Cropping Systems: Perspective from the European Project FERTIPLUS. Agronomy 2019, 9, 225. [Google Scholar] [CrossRef]
- Dorais, M.; Gagnon, F.; Laurin-Lanctôt, S.; Thériault, M.; Ménard, C.; Pepin, S. Short-Term Improvement of Soil Biological Activity in Biochar-Amended Organic Greenhouse Tomato Crops. Acta Hortic. 2017, 1164, 249–256. [Google Scholar] [CrossRef]
- Graber, E.; Meller Harel, Y.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar Impact on Development and Productivity of Pepper and Tomato Grown in Fertigated Soilless Media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Bhattacharjya, S.; Chandra, R.; Pareek, N.; Raverkar, K.P. Biochar and Crop Residue Application to Soil: Effect on Soil Biochemical Properties, Nutrient Availability and Yield of Rice (Oryza sativa L.) and Wheat (Triticum aestivum L.). Arch. Agron Soil Sci. 2016, 62, 1095–1108. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. The Biochar Dilemma. Soil Res. 2014, 52, 217–230. [Google Scholar] [CrossRef]
- Sharma, S.; Rana, V.S.; Rana, N.; Prasad, H.; Sharma, U.; Patiyal, V. Biochar from Fruit Crops Waste and Its Potential Impact on Fruit Crops. Sci. Hortic. 2022, 299, 111052. [Google Scholar] [CrossRef]
- Yu, H.; Zou, W.; Chen, J.; Chen, H.; Yu, Z.; Huang, J.; Tang, H.; Wei, X.; Gao, B. Biochar Amendment Improves Crop Production in Problem Soils: A Review. J. Environ. Manag. 2019, 232, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Al-Rabaiai, A.; Menezes-Blackburn, D.; Al-Ismaily, S.; Janke, R.; Pracejus, B.; Al-Alawi, A.; Al-Kindi, M.; Bol, R. Customized biochar for soil applications in arid land: Effect of feedstock type and pyrolysis temperature on soil microbial enumeration and respiration. J. Anal. Appl. Pyrolysis 2022, 168, 105693. [Google Scholar] [CrossRef]
- Ayari, O.; Dorais, M.; Gosselin, A. Daily Variations of Photosynthetic Efficiency of Greenhouse Tomato Plants during Winter and Spring. J. Am. Soc. Hortic. Sci. 2000, 125, 235–241. [Google Scholar] [CrossRef]
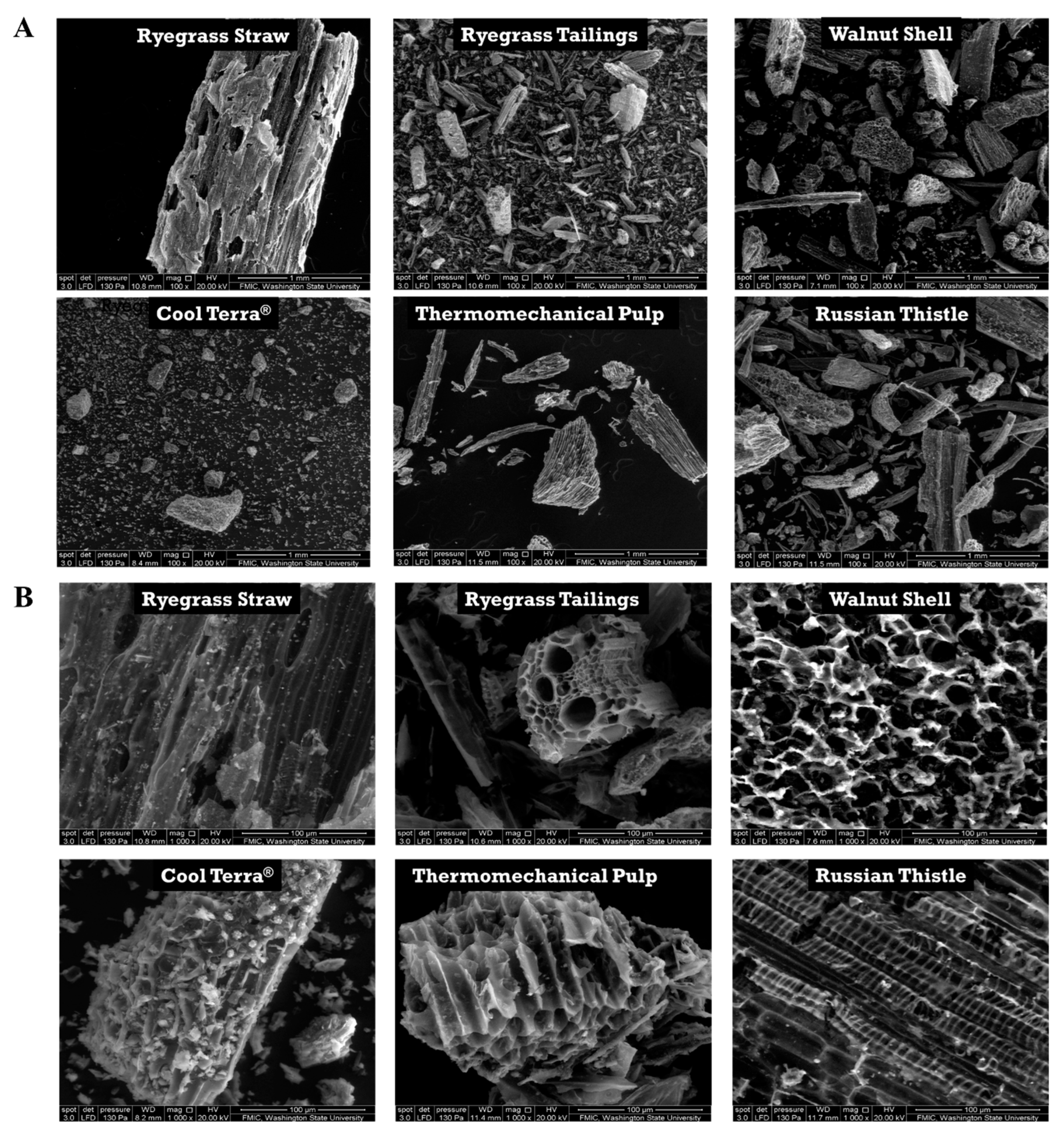
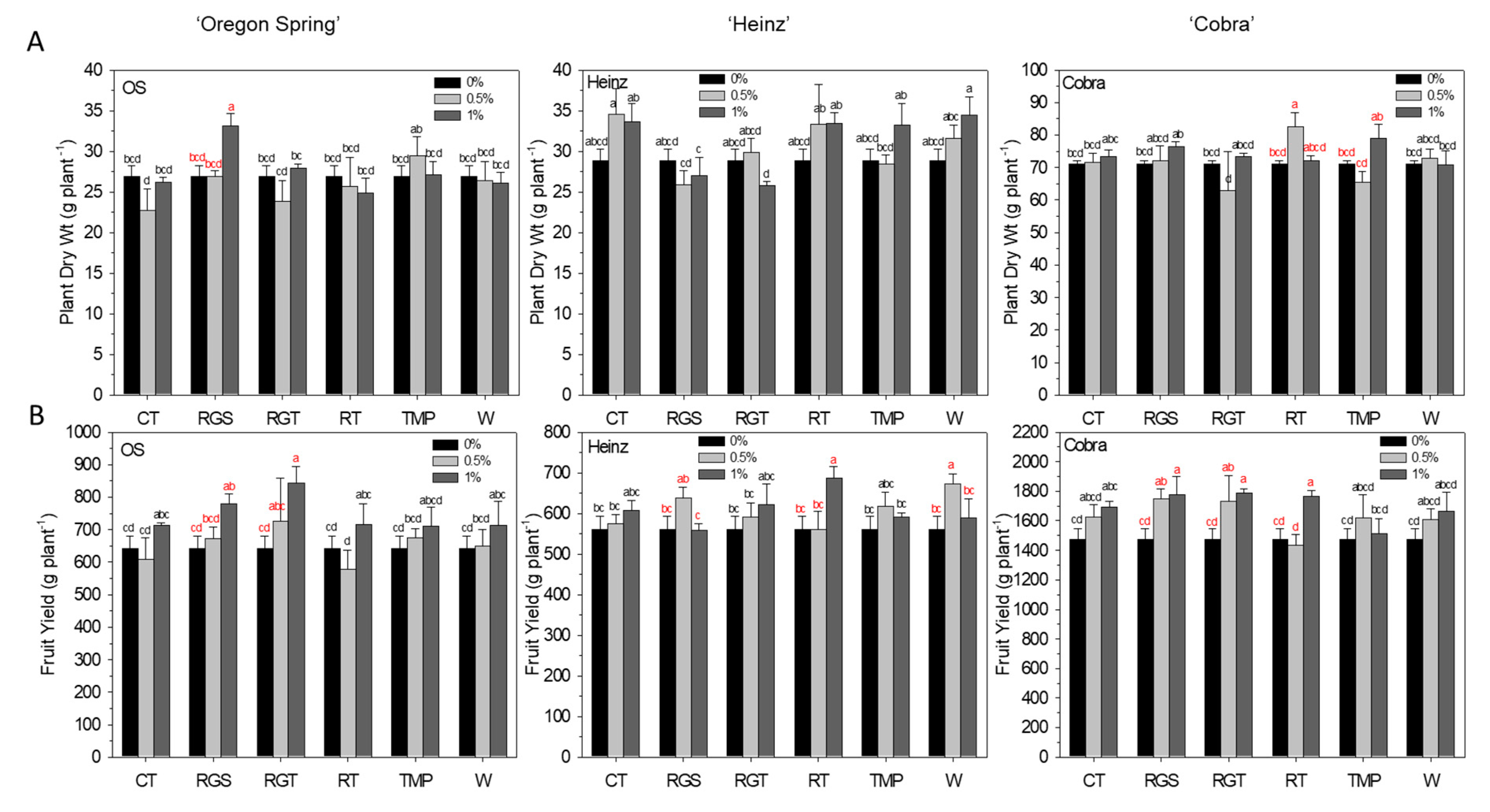

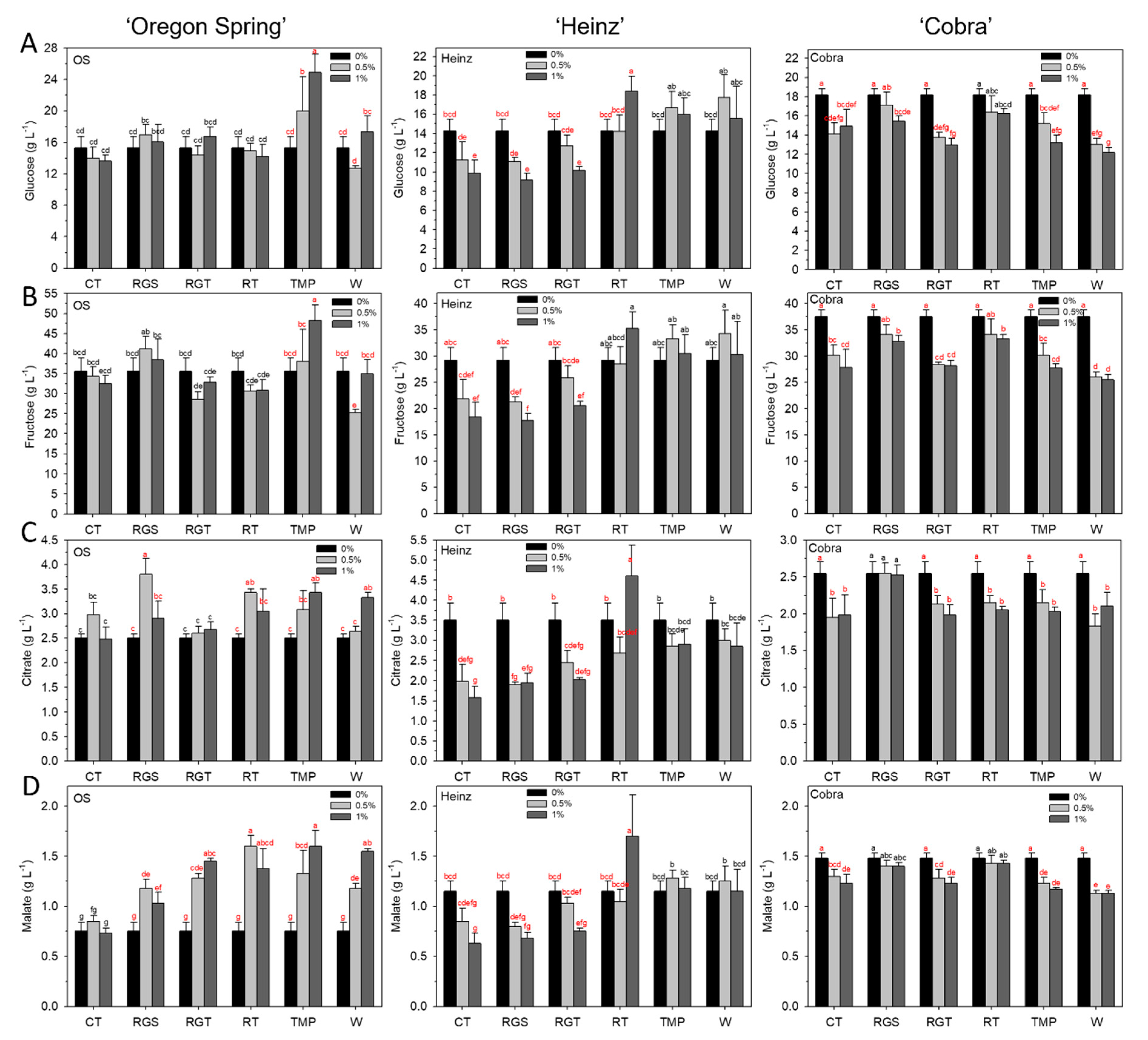

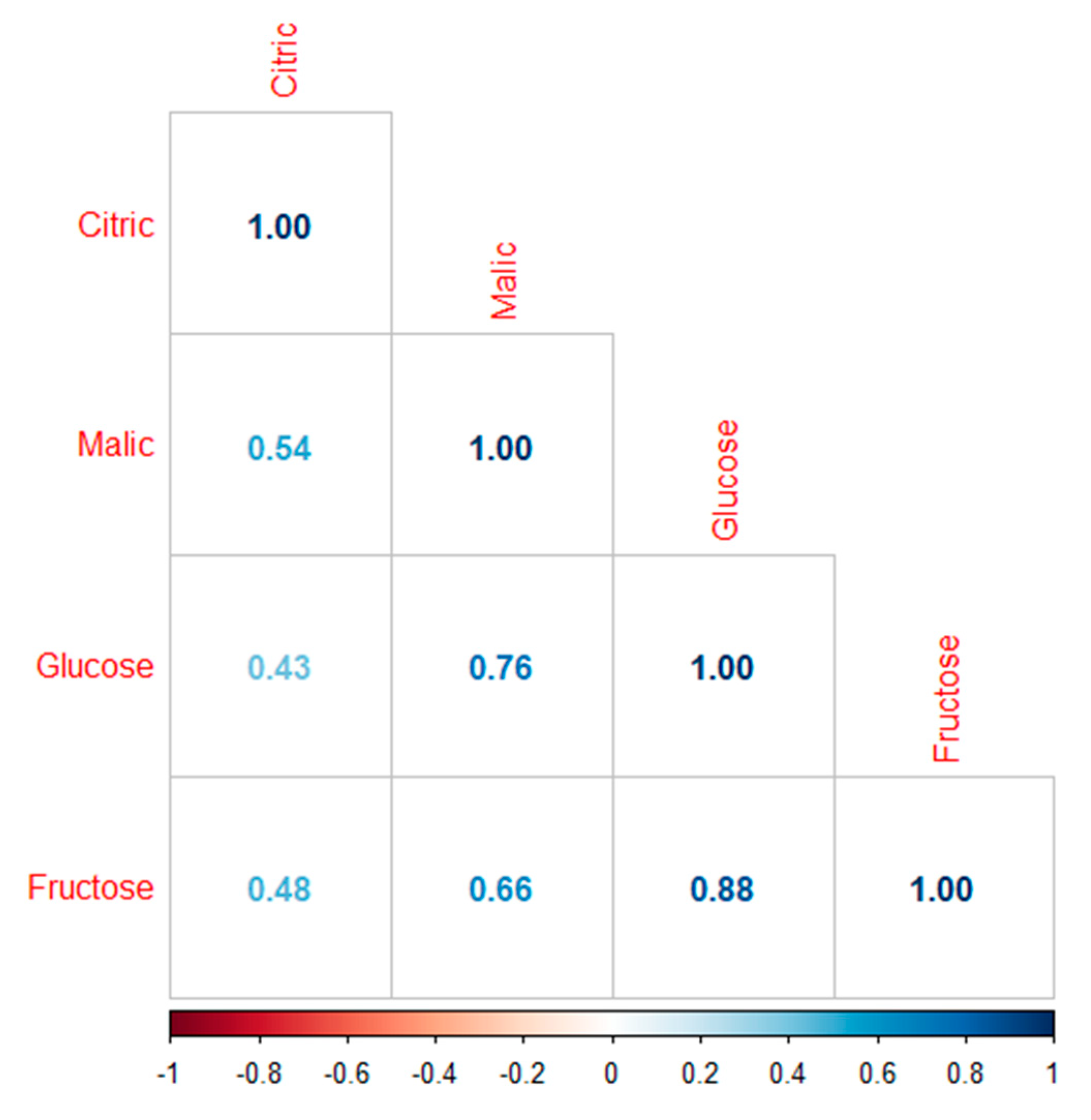
| Experiment | Cultivar | Date Planted | Date Harvested |
|---|---|---|---|
| 1 | ‘Oregon Spring’ | 17 February 2017 | 3 June 2017 |
| 2 | ‘Oregon Spring’ | 20 January 2018 | 7 May 2018 |
| 1 | ‘Heinz’ | 17 February 2017 | 3 June 2017 |
| 2 | ‘Heinz’ | 15 May 2017 | 30 August 2017 |
| 1 | ‘Cobra’ | 16 May 2017 | 10 November 2017 |
| 2 | ‘Cobra’ | 8 November 2017 | 9 May 2018 |
| Treatments | BC | Control | CT | RGS | RGT | TMP | RT | W | ||||||
| % | 0 | 0.5 | 1.0 | 0.5 | 1.0 | 0.5 | 1.0 | 0.5 | 1.0 | 0.5 | 1.0 | 0.5 | 1.0 | |
| n | 8 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | |
| Biochar Feedstock | N | P | K | Ca | S | Mg | Mo | Si | Cl | Na | Al |
| CoolTerra® (CT) | Y | – | Y | – | – | – | – | Y | – | Y | Y |
| Ryegrass straw (RGS) | Y | Y | Y | Y | Y | – | – | Y | Y | – | – |
| Ryegrass tailings (RGT) | Y | Y | Y | Y | Y | Y | Y | Y | – | – | Y |
| Thermomechanical pulp waste (TMP) | Y | Y | Y | Y | – | – | – | Y | – | – | – |
| Russian thistle (RT) | Y | Y | Y | Y | Y | – | Y | Y | Y | – | – |
| Walnut (W) | Y | Y | – | Y | – | – | – | – | – | – | Y |
| Trial Dates | Sources of Variation | Plant Wt | YPP 1 | Citrate | Malate | Glc | Fru |
|---|---|---|---|---|---|---|---|
| 17 February 2017 to 10 November 2017 | Cultivar (C) | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Biochar (B) | ns 2 | ns | 0.001 | 0.001 | 0.001 | 0.001 | |
| Rate (R) | 0.05 | 0.001 | 0.002 | ns | 0.04 | 0.003 | |
| C × B | ns | ns | 0.001 | 0.001 | 0.001 | 0.002 | |
| C × R | ns | 0.005 | 0.001 | 0.001 | 0.001 | 0.001 | |
| B × R | ns | ns | 0.02 | 0.001 | ns | ns | |
| C × B × R | ns | ns | 0.002 | 0.001 | 0.04 | 0.04 | |
| 15 May 2017 to 9 May 2018 | Cultivar (C) | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Biochar (B) | ns | ns | 0.02 | 0.001 | 0.001 | 0.001 | |
| Rate (R) | ns | 0.03 | 0.03 | 0.002 | ns | ns | |
| C × B | ns | ns | ns | 0.001 | 0.03 | 0.001 | |
| C × R | ns | 0.008 | 0.001 | 0.005 | 0.003 | ns | |
| B × R | ns | ns | 0.001 | 0.001 | 0.001 | 0.001 | |
| C × B × R | ns | ns | ns | 0.001 | 0.001 | 0.001 |
| Trial Dates | Cultivar | Sources of Variation | Plant Wt | YPP 1 | Citrate | Malate | Glc | Fru |
|---|---|---|---|---|---|---|---|---|
| 17 February 2017 to 3 June 2017 | OS | Biochar (B) | ns 2 | ns | 0.06 | 0.001 | 0.002 | 0.008 |
| Rate | ns | 0.001 | 0.001 | 0.001 | ns | ns | ||
| B × Rate | ns | ns | 0.03 | 0.002 | 0.09 | ns | ||
| 17 February 2017 to 3 June 2017 | Heinz | Biochar (B) | 0.03 | ns | 0.001 | 0.001 | 0.001 | 0.001 |
| Rate | ns | 0.02 | 0.001 | ns | ns | 0.08 | ||
| B × Rate | ns | 0.09 | 0.007 | 0.004 | 0.03 | 0.03 | ||
| 16 May 2017 to 10 November 2017 | Cobra | Biochar (B) | ns | ns | 0.02 | 0.001 | 0.009 | 0.001 |
| Rate | ns | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | ||
| B × Rate | ns | ns | ns | 0.07 | ns | ns | ||
| 20 January 2018 to 7 May 2018 | OS | Biochar (B) | ns | ns | 0.04 | 0.001 | 0.005 | 0.001 |
| Rate | ns | ns | 0.009 | 0.001 | 0.007 | ns | ||
| B × Rate | ns | ns | 0.05 | 0.001 | 0.07 | 0.03 | ||
| 15 May 2017 to 30 August 2017 | Heinz | Biochar (B) | ns | ns | ns | 0.001 | 0.07 | 0.07 |
| Rate | 0.02 | ns | 0.003 | ns | ns | ns | ||
| B × Rate | ns | ns | 0.002 | 0.001 | 0.001 | 0.001 | ||
| 8 November 2017 to 9 May 2018 | Cobra | Biochar (B) | ns | ns | ns | 0.05 | 0.02 | 0.001 |
| Rate | ns | 0.006 | 0.007 | ns | 0.02 | 0.03 | ||
| B × Rate | ns | 0.08 | ns | ns | 0.10 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isaac, D.; Labbancz, J.; Knowles, N.R.; Tenic, E.; Horgan, A.; Ghogare, R.; Dhingra, A. Biomass Source of Biochar and Genetic Background of Tomato Influence Plant Growth and Development and Fruit Quality. Horticulturae 2024, 10, 368. https://doi.org/10.3390/horticulturae10040368
Isaac D, Labbancz J, Knowles NR, Tenic E, Horgan A, Ghogare R, Dhingra A. Biomass Source of Biochar and Genetic Background of Tomato Influence Plant Growth and Development and Fruit Quality. Horticulturae. 2024; 10(4):368. https://doi.org/10.3390/horticulturae10040368
Chicago/Turabian StyleIsaac, Daylen, June Labbancz, Norman Richard Knowles, Elvir Tenic, Andrew Horgan, Rishikesh Ghogare, and Amit Dhingra. 2024. "Biomass Source of Biochar and Genetic Background of Tomato Influence Plant Growth and Development and Fruit Quality" Horticulturae 10, no. 4: 368. https://doi.org/10.3390/horticulturae10040368
APA StyleIsaac, D., Labbancz, J., Knowles, N. R., Tenic, E., Horgan, A., Ghogare, R., & Dhingra, A. (2024). Biomass Source of Biochar and Genetic Background of Tomato Influence Plant Growth and Development and Fruit Quality. Horticulturae, 10(4), 368. https://doi.org/10.3390/horticulturae10040368






