A Comparative Study of Calcium Sulfate Alternatives in Compost Production for White Button Mushroom (Agaricus bisporus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analysis of Basic Compost Parameters
2.2. Industrial Scale Composting Experiments
2.3. Mushroom Cultivation Experiments in Bags
2.4. Statistical Analysis
3. Results
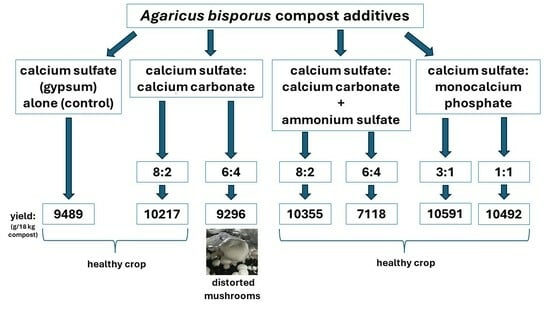
3.1. Full Replacement of Calcium Sulfate with Calcium Carbonate
3.2. Mixture of Calcium Sulfate and Calcium Carbonate in Ratios of 8:2 and 6:4
3.3. Mixture of Calcium Sulfate and Calcium Carbonate in Ratios of 8:2 and 6:4, Combined with Ammonium Sulfate Solution
3.4. Mixture of Calcium Sulfate and Monocalcium Phosphate in Ratios of 75:25 and 50:50
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arya, A.; Rusevska, K. (Eds.) Biology, Cultivation and Applications of Mushrooms; Springer Verlag: Singapore, 2022; ISBN 9789811662560. [Google Scholar]
- Singhal, S.; Rasane, P.; Kaur, S.; Garba, U.; Singh, J.; Raj, N.; Gupta, N. Mushroom cultivation, processing and value-added products: A patent based review. Recent Pat. Food Nutr. Agric. 2019, 10, 3–19. [Google Scholar] [CrossRef]
- Sánchez, C. Modern aspects of mushroom culture technology. Appl. Microbiol. Biotechnol. 2004, 64, 756–762. [Google Scholar] [CrossRef]
- Noble, R.; Gaze, R.H. Preparation of mushroom (Agaricus bisporus) composts in controlled environments: Factors influencing compost bulk density and productivity. Int. Biodeterior. Biodegr. 1996, 37, 93–100. [Google Scholar] [CrossRef]
- Kariaga, M.G.; Nyongesa, H.W.; Keya, N.C.O.; Tsingalia, H.M. Compost physico-chemical factors that impact on yield in button mushrooms, Agaricus bisporus (Lge) and Agaricus bitorquis (Quel) Saccardo. J. Agric. Sci. 2012, 3, 49–54. [Google Scholar] [CrossRef]
- Lyons, G.A.; Sharma, H.S.S.; Kilpatrick, M.; Cheung, L.; Moore, S. Monitoring of changes in substrate characteristics during mushroom compost production. J. Agric. Food Chem. 2006, 54, 4658–4667. [Google Scholar] [CrossRef]
- Straatsma, G.; Gerrits, J.P.G.; Thissen, J.T.N.M.; Amsing, J.G.M.; Loeffen, H.; Van Griensven, L.J.L.D. Adjustment of the composting process for mushroom cultivation based on initial substrate composition. Biores. Technol. 2000, 72, 67–74. [Google Scholar] [CrossRef]
- Gerrits, J.P.G. The significance of gypsum applied to mushroom compost, in particular in relation to the ammonia content. Neth. J. Agric. Sci. 1977, 25, 288–302. [Google Scholar] [CrossRef]
- Mouthier, T.M.B.; Kilic, B.; Vervoort, P.; Gruppen, H.; Kabel, M.A. Potential of a gypsum-free composting process of wheat straw for mushroom production. PLoS ONE 2017, 12, e0185901. [Google Scholar] [CrossRef] [PubMed]
- Gąsecka, M.; Magdziak, Z.; Siwulski, M.; Mleczek, M. Profile of phenolic and organic acids, antioxidant properties and ergosterol content in cultivated and wild growing species of Agaricus. Eur. Food Res. Technol. 2018, 244, 259–268. [Google Scholar] [CrossRef]
- Kredics, L.; Hatvani, L.; Allaga, H.; Büchner, R.; Cai, F.; Vágvölgyi, C.; Druzhinina, I.S.; Naeimi, S. Trichoderma green mould disease of cultivated mushrooms. In Advances in Trichoderma Biology for Agricultural Applications; Fungal Biology Series; Amaresan, N., Sankaranarayanan, A., Dwivedi, M.K., Druzhinina, I.S., Eds.; Springer: Cham, Switzerland, 2022; pp. 559–606. [Google Scholar] [CrossRef]
- Tang, Z.-X.; Shi, L.-E.; Jiang, Z.-B.; Bai, X.-L.; Ying, R.-F. Calcium enrichment in edible mushrooms: A review. J. Fungi 2023, 9, 338. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, M.; Song, C.; Liu, J.; Xu, Z.; Shang, X. Effects of Fe2+, Zn2+ and Ca2+ on mycelium growth and its biological enrichment in mycelia of three edible mushrooms. Acta Edulis Fungi 2017, 24, 27–33. [Google Scholar]
- Zhang, T.; Qian, L.; Wang, W.; Li, C.; Li, F. Study on calcium enrichment in mycelia of Hypsizygus marmoreus. N. Hortic. 2022, 4, 104–109. [Google Scholar]
- Zhang, J.; Xin, Z. Effect of exogenous calcium on Lentinula edodes mycelium growth. N. Hortic. 2016, 18, 143–145. [Google Scholar]
- Choi, U.K.; Lee, O.; Kim, Y. Effect of calcinated oyster shell powder on growth, yield, spawn run, and primordial formation of king oyster mushroom (Pleurotus eryngii). Molecules 2011, 16, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Royse, D.J.; Sanchez-Vazquez, J.E. Influence of precipitated calcium carbonate (CaCO3) on shiitake (Lentinula edodes) yield and mushroom size. Biores. Technol. 2003, 90, 225–228. [Google Scholar] [CrossRef]
- Fan, L.; Jin, Q.; Feng, W.; Song, T.; Shen, Y.; Cai, W. Effects of different calcium sources and amounts on the growth and yield of Flammulina velutipes. Edible Med. Mushrooms 2018, 26, 91–93. [Google Scholar]
- Ghareeb, B.A. Impact different level of calcium carbonate (CaCO3) on growth and yield of oyster mushroom (Pleurotus spp.). Int. J. Eng. Technol. 2019, 11, 785–792. [Google Scholar] [CrossRef]
- Feng, G.; Shi, H.; Zheng, J.; Shi, Y. Research process of metal ion on the growth and development of edible fungi. Food Res. Dev. 2021, 42, 193–198. [Google Scholar]
- Melse, R.W.; Ogink, N.W.M. Air scrubbing techniques for ammonia and odor reduction at livestock operations: Review of on-farm research in the Netherlands. Trans. Am. Soc. Agric. Eng. 2005, 48, 2303–2313. [Google Scholar] [CrossRef]
- Zied, D.C.; Pardo-Giménez, A.; Minhoni, M.A.; Villas, R.L.; Álvarez-Ortí, M.; Pardo-González, J.E. Characterization, feasibility and optimization of Agaricus subrufescens growth based on chemical elements on casing layer. Saudi J. Biol. Sci. 2012, 19, 343–347. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, S.; Chen, Z.; Ma, L.; Liu, Q. Effect of calcium salt on the growth of Lentinan mycelium in course of shaking flask fermentation. Food Mach. 2013, 29, 58–61. [Google Scholar]
| Treatment | Ratio in % | Ammonium Sulfate Solution (kg per 1 kg Calcium Carbonate) | ||
|---|---|---|---|---|
| Calcium Sulfate | Calcium Carbonate | Monocalcium Phosphate | ||
| Basic technology | 100 | - | - | - |
| 1 | - | 100 | - | - |
| 2 | 80 | 20 | - | - |
| 3 | 60 | 40 | - | - |
| 4 | 80 | 20 | - | 0.7 |
| 5 | 60 | 40 | - | 0.85 |
| 6 | 75 | - | 25 | - |
| 7 | 50 | - | 50 | - |
| Compost Sample | Phase of Composting | pH | Moisture Content (%) | Total N (%) | NH4+ (%) | Organic Matter (%) | C/N Ratio | Hemicellulose (%) | Cellulose (%) | Lignin (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Technological optimum (CaSO4) | End of Phase I | 7.8–8.3 | 73–75 | 1.8–2.2 | 0.25–0.5 | 76–79 | 19–21 | 14–18 | 32–42 | 8–12 |
| End of Phase II | 7.4–7.8 | 66–70 | 2.0–2.4 | <0.1 | 73–76 | 16–17 | 10–15 | 26–33 | 11–14 | |
| End of Phase III | 6.2–6.7 | 61–65 | 2.2–2.6 | 0.05–0.15 | 70–73 | 14–15 | 8–12 | 25–29 | 9–11 | |
| Full replacement of CaSO4 with CaCO3 | End of Phase I | 8.63 ± 0.27 | 73.02 ± 0.10 | 2.08 ± 0.08 | 0.27 ± 0.02 | 78.18 ± 1.15 | 18.84 ± 1.11 | 15.72 ± 0.04 | 42.02 ± 0.41 | 8.45 ± 0.15 |
| End of Phase II | 8.54 ± 0.06 * | 67.20 ± 0.46 | 2.16 ± 0.04 | 0.01 ± 0.00 | 78.13 ± 0.25 * | 18.27 ± 0.35 * | 12.01 ± 0.07 | 36.65 ± 1.03 * | 12.53 ± 0.33 | |
| End of Phase III | - | - | - | - | - | - | - | - | - | |
| Mixture of CaSO4 and CaCO3 in ratio of 8:2 | End of Phase I | 8.36 ± 0.02 | 73.22 ± 0.18 | 1.74 ± 0.05 | 0.22 ± 0.01 | 73.79 ± 0.38 * | 21.45 ± 0.50 | 17.25 ± 0.31 | 39.50 ± 1.04 | 6.62 ± 0.50 * |
| End of Phase II | 7.57 ± 0.03 | 68.65 ± 0.24 | 2.27 ± 0.05 | 0.05 ± 0.00 | 72.30 ± 0.69 | 16.06 ± 0.27 | 13.52 ± 0.63 | 29.09 ± 0.43 | 11.62 ± 0.36 | |
| End of Phase III | 6.47 ± 0.03 | 62.95 ± 0.49 | 2.14 ± 0.09 | 0.10 ± 0.01 | 69.83 ± 1.06 | 16.32 ± 0.84 * | 8.27 ± 0.17 | 26.96 ± 0.43 | 9.55 ± 0.25 | |
| Mixture of CaSO4 and CaCO3 in ratio of 6:4 | End of Phase I | 8.31 ± 0.02 | 73.51 ± 0.26 * | 1.90 ± 0.03 | 0.22 ± 0.01 | 76.05 ± 0.98 | 20.39 ± 0.43 | 16.94 ± 0.72 | 38.54 ± 1.17 | 8.43 ± 0.74 |
| End of Phase II | 7.97 ± 0.03 * | 68.19 ± 0.67 | 1.99 ± 0.02 | 0.00 ± 0.00 | 73.60 ± 0.92 | 18.51 ± 0.42 | 13.42 ± 0.07 | 32.03 ± 0.88 | 12.12 ± 0.39 | |
| End of Phase III | 6.43 ± 0.06 | 67.10 ± 0.40 | 2.23 ± 0.05 | 0.07 ± 0.01 | 69.29 ± 1.61 | 15.62 ± 0.64 | 9.35 ± 0.23 | 25.73 ± 1.07 | 10.10 ± 0.21 | |
| Mixture of CaSO4 and CaCO3 in ratio of 8:2, with (NH4)2SO4 solution | End of Phase I | 8.19 ± 0.06 | 73.72 ± 0.15 | 2.03 ± 0.09 | 0.24 ± 0.01 | 77.59 ± 1.19 | 19.45 ± 0.91 | 17.90 ± 0.83 | 37.19 ± 0.91 | 8.57 ± 0.65 |
| End of Phase II | 7.92 ± 0.04 * | 68.76 ± 0.06 | 2.23 ± 0.04 | 0.01 ± 0.00 | 74.70 ± 0.28 | 16.79 ± 0.35 | 14.51 ± 0.36 | 30.33 ± 0.35 | 10.97 ± 0.38 | |
| End of Phase III | 6.67 ± 0.07 | 66.75 ± 0.77 * | 2.45 ± 0.05 | 0.07 ± 0.00 | 72.82 ± 0.50 | 14.88 ± 0.22 | 10.13 ± 0.78 | 26.75 ± 0.58 | 10.05 ± 0.26 | |
| Mixture of CaSO4 and CaCO3 in ratio of 6:4, with (NH4)2SO4 solution | End of Phase I | 8.23 ± 0.07 | 73.04 ± 0.37 | 2.03 ± 0.04 | 0.27 ± 0.01 | 78.24 ± 0.28 | 19.53 ± 0.39 | 17.09 ± 0.51 | 34.19 ± 0.98 | 11.20 ± 0.37 |
| End of Phase II | 7.73 ± 0.04 | 68.40 ± 0.31 | 2.48 ± 0.04 * | 0.00 ± 0.00 | 74.39 ± 0.16 | 15.03 ± 0.24 * | 12.70 ± 0.42 | 29.26 ± 0.62 | 12.58 ± 0.40 | |
| End of Phase III | 6.52 ± 0.02 | 63.76 ± 0.24 | 2.39 ± 0.07 | 0.06 ± 0.01 | 73.91 ± 0.34 * | 15.45 ± 0.46 | 10.08 ± 0.40 | 28.02 ± 0.38 | 9.28 ± 0.36 | |
| Mixture of CaSO4 and Ca(H₂PO₄)₂ in ratio of 75:25 | End of Phase I | 8.28 ± 0.01 | 74.85 ± 0.26 | 1.81 ± 0.06 | 0.28 ± 0.03 | 78.71 ± 0.32 | 22.04 ± 0.66 * | 19.32 ± 0.96 * | 41.97 ± 0.68 | 5.60 ± 0.17 * |
| End of Phase II | 7.82 ± 0.08 | 70.81 ± 0.24 * | 2.33 ± 0.06 | 0.01 ± 0.00 | 75.31 ± 0.07 | 16.42 ± 0.22 | 14.78 ± 0.39 | 28.63 ± 0.16 | 12.03 ± 0.38 | |
| End of Phase III | 6.49 ± 0.02 | 64.31 ± 0.43 | 2.44 ± 0.03 | 0.08 ± 0.00 | 72.59 ± 1.00 | 14.92 ± 0.16 | 9.18 ± 0.95 | 27.08 ± 0.28 | 7.58 ± 0.67 * | |
| Mixture of CaSO4 and Ca(H₂PO₄)₂ in ratio of 50:50 | End of Phase I | 8.09 ± 0.06 | 75.06 ± 0.31 | 1.95 ± 0.04 | 0.28 ± 0.01 | 77.76 ± 0.42 | 20.17 ± 0.48 | 19.08 ± 0.74 * | 39.19 ± 0.93 | 8.03 ± 0.55 |
| End of Phase II | 7.98 ± 0.06 * | 70.78 ± 0.22 * | 2.28 ± 0.02 | 0.01 ± 0.01 | 74.19 ± 0.33 | 16.26 ± 0.14 | 14.61 ± 0.30 | 29.33 ± 0.41 | 12.80 ± 0.39 | |
| End of Phase III | 6.49 ± 0.06 | 66.22 ± 0.32 * | 2.27 ± 0.07 | 0.03 ± 0.01 * | 72.52 ± 1.16 | 15.98 ± 0.32 * | 9.83 ± 0.27 | 26.19 ± 0.80 | 8.10 ± 0.35 * |
| Treatment | Mushroom Yield (g/18 kg Compost) | ||
|---|---|---|---|
| 1st Flush | 2nd Flush | Total | |
| Control: 100% CaSO4 | 5077 ± 187 | 4412 ± 139 | 9489 ± 180 |
| 8:2 mixture of CaSO4 and CaCO3 | 6256 ± 220 * | 3961 ± 141 * | 10,217 ± 120 * |
| 6:4 mixture of CaSO4 and CaCO3 | 4699 ± 164 | 4597 ± 148 | 9296 ± 99 * |
| 8:2 mixture of CaSO4 and CaCO3, with (NH4)2SO4 | 5907 ± 180 * | 4448 ± 114 | 10,355 ± 130 * |
| 6:4 mixture of CaSO4 and CaCO3, with (NH4)2SO4 | 2651 ± 92 * | 4467 ± 118 | 7118 ± 90 * |
| 75:25 mixture of CaSO4 and Ca(H₂PO₄)₂ | 5377 ± 140 | 5214 ± 161 * | 10,591 ± 125 * |
| 50:50 mixture of CaSO4 and Ca(H₂PO₄)₂ | 4970 ± 116 | 5522 ± 132 * | 10,492 ± 106 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misz, A.; Szőke, A.S.; Bajzát, J.; Kökény, D.; Visnyei, M.; Kredics, L.; Allaga, H.; Szűcs, A.; Kocsubé, S.; Csutorás, C.; et al. A Comparative Study of Calcium Sulfate Alternatives in Compost Production for White Button Mushroom (Agaricus bisporus). Horticulturae 2024, 10, 378. https://doi.org/10.3390/horticulturae10040378
Misz A, Szőke AS, Bajzát J, Kökény D, Visnyei M, Kredics L, Allaga H, Szűcs A, Kocsubé S, Csutorás C, et al. A Comparative Study of Calcium Sulfate Alternatives in Compost Production for White Button Mushroom (Agaricus bisporus). Horticulturae. 2024; 10(4):378. https://doi.org/10.3390/horticulturae10040378
Chicago/Turabian StyleMisz, András, Amanda Sándorné Szőke, Judit Bajzát, Dániel Kökény, Marianna Visnyei, László Kredics, Henrietta Allaga, Attila Szűcs, Sándor Kocsubé, Csaba Csutorás, and et al. 2024. "A Comparative Study of Calcium Sulfate Alternatives in Compost Production for White Button Mushroom (Agaricus bisporus)" Horticulturae 10, no. 4: 378. https://doi.org/10.3390/horticulturae10040378
APA StyleMisz, A., Szőke, A. S., Bajzát, J., Kökény, D., Visnyei, M., Kredics, L., Allaga, H., Szűcs, A., Kocsubé, S., Csutorás, C., & Vágvölgyi, C. (2024). A Comparative Study of Calcium Sulfate Alternatives in Compost Production for White Button Mushroom (Agaricus bisporus). Horticulturae, 10(4), 378. https://doi.org/10.3390/horticulturae10040378