Micropropagation Protocols for Three Elite Genotypes of Stevia rebaudiana Bertoni
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection and Collection of Plant Material
2.2. Disinfection of Explants
2.3. Shoot Multiplication
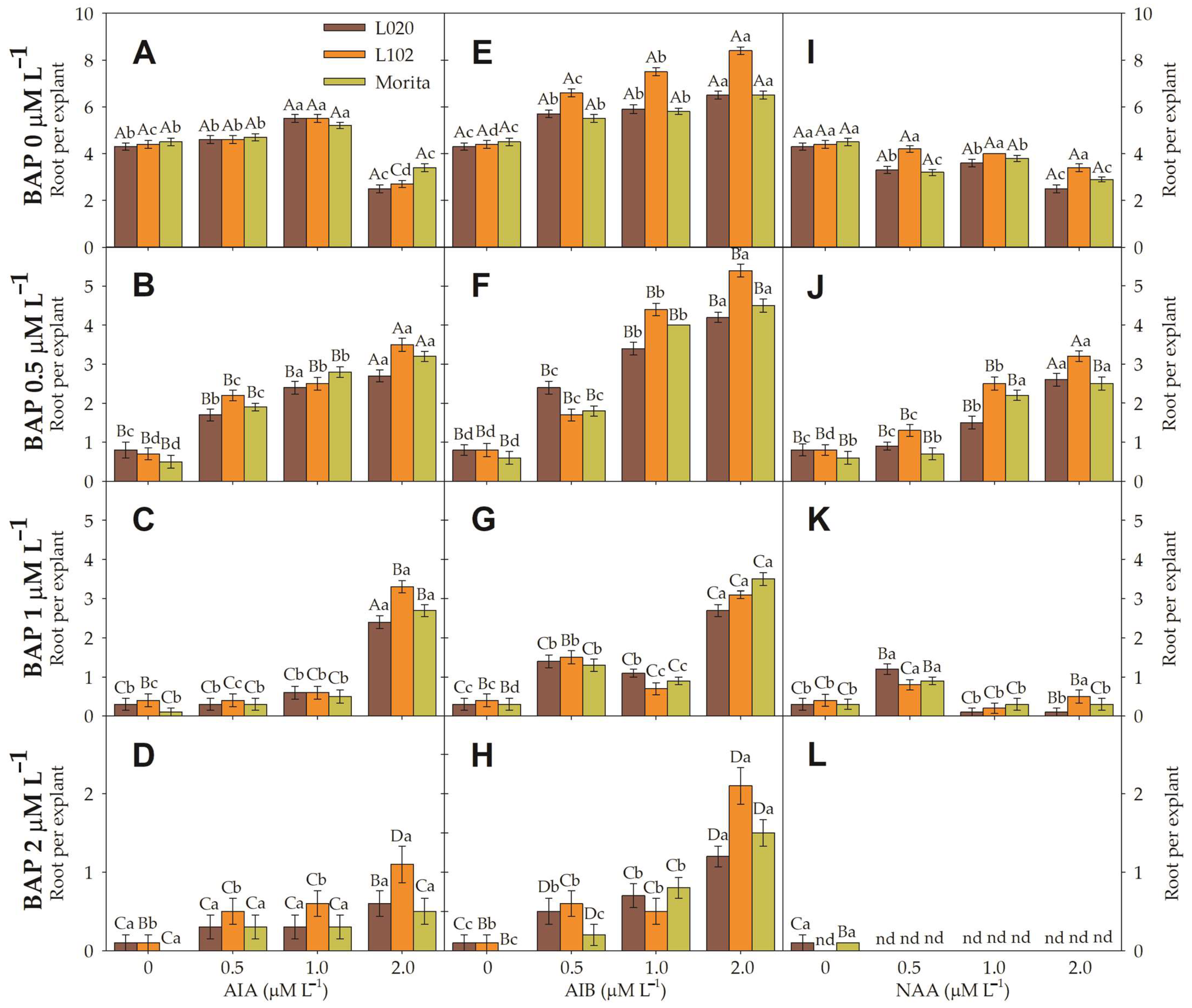
2.4. Root Induction
2.5. Experimental Design and Statistical Analyses
3. Results
3.1. Explant Disinfection
3.2. Shoot Multiplication
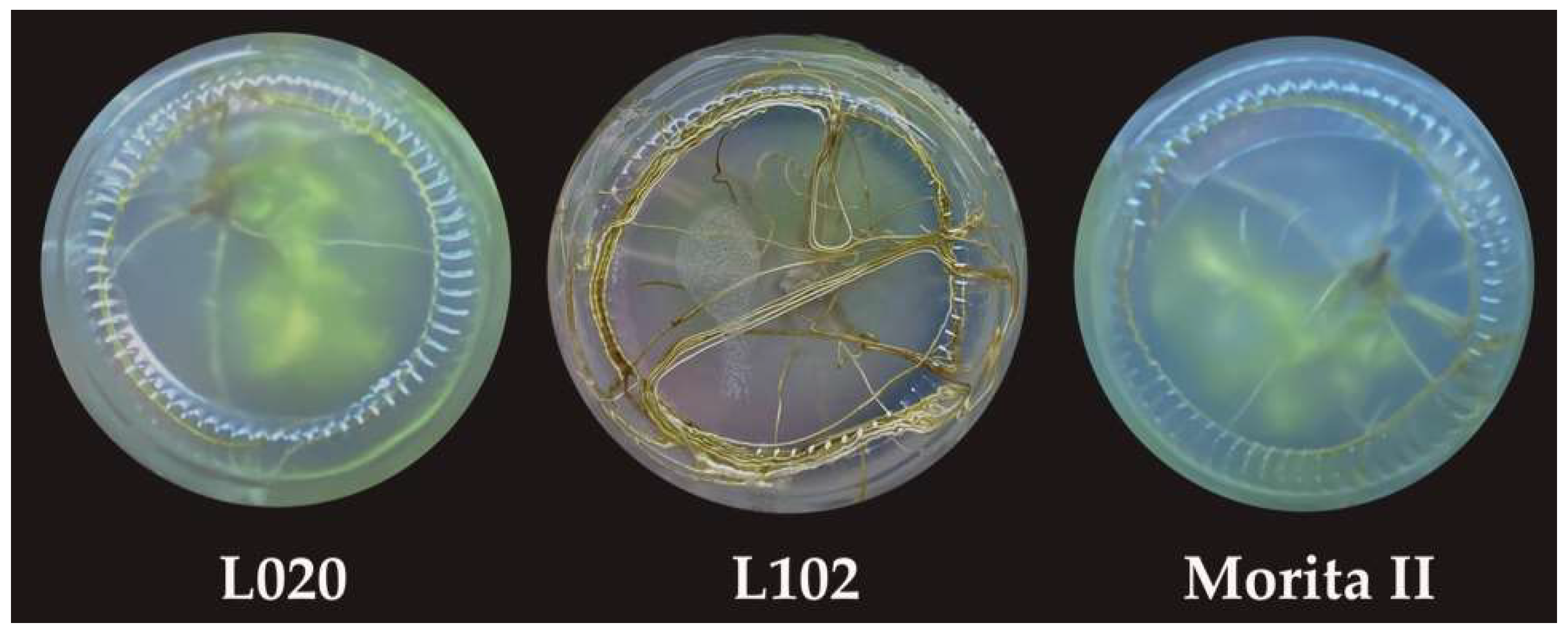
3.3. Root Induction
3.4. Acclimatization and Transference of Plantlets to Greenhouse
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soejarto, D.D.; Compadre, C.M.; Kinghorn, D. Ethnobotanical notes in Stevia. Bot. Mus. Leafl. 1983, 29, 1–25. [Google Scholar] [CrossRef]
- Soejarto, D.D. Botany of Stevia and Stevia rebaudiana. In Stevia: The Genus Stevia; Kinghorn, A.D., Ed.; CRC Press: London, UK, 2001; pp. 22–28. [Google Scholar]
- Pande, S.S.; Gupta, P. Plant tissue culture of Stevia rebaudiana (Bertoni): A review. J. Pharmacogn. Phytother. 2013, 5, 26–33. [Google Scholar]
- Miyagawa, H.; Fujioka, N.; Kohda, H.; Yamasaki, K.; Taniguchi, K.; Tanaka, R. Studies on the tissue culture of Stevia rebaudiana and its components; (II). Induction of shoot primordia. Planta Med. 1986, 4, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, M.; Dahot, M.U.; Mangrio, S.M.; Naqvi, H.A.; Qarshi, I.A. In vitro clonal propagation and biochemical analysis of field established Stevia rebaudiana Bertoni. Pak. J. Bot. 2007, 39, 2467–2474. [Google Scholar]
- Dey, A.; Kundu, S.; Bandyopadhyay, A.; Bhattacharjee, A. Efficient micropropagation and chlorocholine chloride induced stevioside production of Stevia rebaudiana Bertoni. C. R. Biol. 2013, 336, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Brandle, J.E.; Rosa, N. Heritability for yield, leaf: Stem ratio and stevioside content estimated from a landrace cultivar of Stevia rebaudiana. Can. J. Plant Sci. 1992, 72, 1263–1266. [Google Scholar] [CrossRef]
- Wibbens, K. Executive Review of the Stevia Food System: Plan B Project. Market Research Future. 2023. Available online: https://n9.cl/steviamarket (accessed on 11 April 2024).
- Martyn, D.; Darch, M.; Roberts, A.; Lee, H.Y.; Yaqiong Tian, T.; Kaburagi, N.; Belmar, P. Low-/No-Calorie Sweeteners: A Review of Global Intakes. Nutrients 2018, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Avilez, J.A.; Jarma-Orozco, A.; Pompelli, M.F. Stevia rebaudiana Bertoni: The interaction of night interruption on gas exchange, flowering delay, and steviol glycosides synthesis. Horticulturae 2021, 7, 543. [Google Scholar] [CrossRef]
- Iatridis, N.; Kougioumtzi, A.; Vlataki, K.; Papadaki, S.; Magklara, A. Anti-cancer properties of Stevia rebaudiana; More than a sweetener. Molecules 2022, 27, 1362. [Google Scholar] [CrossRef]
- Liu, J.C.; Kao, P.K.; Chan, P.; Hsu, Y.H.; Hou, C.C.; Lien, G.S.; Hsieh, M.H.; Chen, Y.J.; Cheng, J.T. Mechanism of the antihypertensive effect of stevioside in anesthetized dogs. Pharmacology 2003, 67, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Planas, G.M.; Kuć, J. Contraceptive properties of Stevia rebaudiana. Science 1968, 162, 1007. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, F.; Margarita, C. Antimicrobial potential of extracts from Stevia rebaudiana leaves against bacteria of importance in dental caries. Acta Odontol. Latinoam. 2012, 25, 171–175. [Google Scholar] [PubMed]
- Debnath, M.; Ashwath, N.; Midmore, D.J. Physiological and morphological responses to abiotic stresses in two cultivars of Stevia rebaudiana (Bert.) Bertoni. S. Afr. J. Bot. 2019, 123, 124–132. [Google Scholar] [CrossRef]
- Gantait, S.; Das, A.; Banerjee, J. Geographical distribution, botanical discription and self-incompatibility mechanism of genus Stevia. Sugar Tech. 2018, 20, 1–10. [Google Scholar] [CrossRef]
- Yang, Y.-W.; Hsing, Y.-I.; Chang, W.-C. Clonal propagation of Stevia rebaudiana Bertoni through axillary shoot proliferation in vitro. Bot. Bull. Acad. Sin. 1981, 22, 57–62. [Google Scholar]
- Vives, K.; Andújar, I.; Lorenzo, J.C.; Concepción, O.; Hernández, M.; Escalona, M. Comparison of different in vitro micropropagation methods of Stevia rebaudiana B. including temporary immersion bioreactor (BIT®). Plant Cell Tiss. Organ. Cult. 2017, 131, 195–199. [Google Scholar] [CrossRef]
- Felippe, G.M.; Lucas, N.M.C.; Behar, l.; Oliveira, M.A.C. Observações a respeito de germinação de Stevia rebaudiana Bert. Hoehnea 1971, 1, 81–93. [Google Scholar]
- Jitendra, M.; Monika, S.; Ratan, S.D.; Priyanka, G.; Priyanka, S.; Kiran, D.J. Micropropagation of an Anti diabetic Plant—Stevia rebaudiana Bertoni, (Natural Sweetener) in Hadoti Region of South-East Rajasthan, India. J. Biol. Sci. 2012, 1, 37–42. [Google Scholar]
- Ferreira, C.M.; Handro, W. Micropropagation of Stevia rebaudiana through leaf explants from adult plants. Planta Med. 1988, 54, 157–160. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Salahin, M.; Karim, R.; Razvy, M.A.; Hannan, M.M.; Sultana, R.; Hossain, M.; Islam, R. An efficient method for in vitro clonal propagation of a newly introduced sweetener plant (Stevia rebaudiana Bertoni.) in Bangladesh. Am. Eurasian J. Sci. Res. 2007, 2, 121–125. [Google Scholar]
- Sivaram, L.; Mukundan, U. In vitro culture studies on Stevia rebaudiana. In Vitro Cell. Dev. Biol.-Plant 2003, 39, 520–523. [Google Scholar] [CrossRef]
- Medorio-García, H.P.; Hernández-Domínguez, E.; Andueza-Noh, R.H.; López-Aguilar, D.R.; Ramírez-Mosqueda, M.A. Micropropagation of Stevia (Stevia rebaudiana Bert.) in RITA. In Micropropagation Methods in Temporary Immersion Systems. Methods in Molecular Biology; Ramírez-Mosqueda, M.A., Cruz-Cruz, C.A., Eds.; Humana: New York, NY, USA, 2024; Volume 2759. [Google Scholar]
- Razak, U.N.A.A.; Ong, C.B.; Yu, S.T.; Lau, L.K. In Vitro Micropropagation of Stevia rebaudiana Bertoni in Malaysia. Braz. Arch. Biol. Technol. 2014, 57, 23–28. [Google Scholar] [CrossRef]
- Chugh, S.; Guha, S.; Rao, I.U. Micropropagation of orchids: A review on the potential of different explants. Sci. Hort. Amst. 2009, 122, 507–520. [Google Scholar] [CrossRef]
- Beruto, M.; Lanteri, L.; Portogallo, C. Micropropagation of tree peony (Paeonia suffruticosa). Plant Cell Tiss. Org. Cult. 2004, 79, 249–255. [Google Scholar] [CrossRef]
- Wojciechowicz, M.K. Comparison of regenerative potential of petals, stamens and pistils of five Sedum species in vitro. Biodiv Res. Conserv. 2007, 5–8, 87–94. [Google Scholar]
- Khatun, R.; Islam, S.M.S.; Miah, M.A.B. Studies on plant regeneration efficiency through in vitro micropropagation and anther culture of twenty five rice cultivars in Bangladesh. J. Appl. Sci. Res. 2010, 6, 1705–1711. [Google Scholar]
- Sharma, V. Orchid micropropagation: Regeneration competence of anther culture. J. Biotechnol. Biomater. 2012, 2, 1000149. [Google Scholar] [CrossRef]
- Tamura, Y.; Nakamura, S.; Fukui, H.; Tabata, M. Comparison of Stevia plants grown from seeds, cuttings and stem tip cultures for growth and sweet diterpene glycosides. Plant Cell Rep. 1984, 3, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Linsmaier, E.M.; Skoog, F. Organic growth requirements of tobacco tissue cultures. Physiol. Plant 1965, 18, 100–127. [Google Scholar] [CrossRef]
- Zou, Z.; Yang, L.; Gong, J.; Mo, Y.; Wang, J.; Cao, J.; An, F.; Xie, G. Genome-wide identification of Jatropha curcas aquaporin genes and the comparative analysis provides insights into the gene family expansion and evolution in Hevea brasiliensis. Front. Plant Sci. 2016, 7, 395. [Google Scholar] [CrossRef]
- Ramírez-Mosqueda, M.A.; Iglesias-Andreu, L.G.; Ramírez-Madero, G.; Hernández-Rincón, E.U. Micropropagation of Stevia rebaudiana Bert. in temporary immersion systems and evaluation of genetic fidelity. S. Afr. J. Bot. 2016, 106, 238–243. [Google Scholar] [CrossRef]
- Naranjo, E.J.; Fernandez Betin, O.; Urrea Trujillo, A.I.; Callejas Posada, R.; Atehortúa Garcés, L. Effect of genotype on the in vitro regeneration of Stevia rebaudiana via somatic embryogenesis. Acta Biol. Col. 2015, 21, 87–98. [Google Scholar] [CrossRef]
- Tavarini, S.; Passera, B.; Angelini, L.G. Crop and steviol glycoside improvement in Stevia by breeding. In Food Chemistry, Function and Analysis; Wölwer-Rieck, U., Ed.; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 1–31. [Google Scholar]
- Rodriguez-Paez, L.A.; Jimenez-Ramirez, A.M.; Pompelli, M.F.; Pineda-Rodriguez, Y.Y.; Jarma-Orozco, A.; Jaraba-Navas, J.D.; Aramendiz-Tatis, H.; Combatt-Caballero, E.; Oloriz-Ortega, M.I.; Rodríguez, N.V. Physiological and enzymatic evaluation of selected genotypes of Stevia rebaudiana Bertoni. Agronomy 2023, 13, 403. [Google Scholar] [CrossRef]
- Rodriguez-Paez, L.A.; Jaraba-Navas, J.D.D.; Pineda-Rodriguez, Y.Y.; Begambre-Hernandez, M.; Pompelli, M.F.; Jimenez-Ramirez, A.M.; Gil-Rocha, A.; Jarma-Orozco, A.; Combatt-Caballero, E.; Aviña-Padilla, K.; et al. Natural biocontrol of Athelia rolfsii isolate INVEPAR-05 in Stevia rebaudiana Bertoni: Exploring the biocontrol potential of native Trichoderma spp. Strains. Preprints 2023, 2023, 2023081062. [Google Scholar] [CrossRef]
- Jimenez Ramirez, A. Caracterización Morfoagronómica de Clones de Estevia (Stevia rebaudiana Bert.) en la Región Caribe de Colombia. Master Thesis, Universidad de Córdoba, Córdoba, Spain, 2023. Available online: https://repositorio.unicordoba.edu.co/server/api/core/bitstreams/c2194056-8603-4da7-8b1d-e4c4723ebd2c/content (accessed on 22 February 2024).
- Ariza-González, A.R.; Jarma-Orozco, A.; Jaraba-Navas, J.D.; Pico-González, A.I.; Herazo-Cárdenas, D.S.; Vegliante Arrieta, D.; Vallejo-Isaza, A.; Pineda-Rodriguez, Y.Y.; Rodriguez-Paez, L.A.; Pompelli, M.F. Net photosynthesis and biomass production in stevia, eggplant, and cowpea can be improved by fertilization with cyanobacteria (Limnospira maxima). Horticulturae 2023, 9, 1309. [Google Scholar] [CrossRef]
- Jaraba-Navas, J.D.; Combatt-Caballero, E.M.; Jarma-Orozco, A.; Pineda-Rodriguez, Y.Y.; Rodriguez-Paez, L.A. Trichoderma harzianum isolate INVEPAR-T01 5.8S ribosomal RNA gene, partial sequence; internal transcribed spacer 2, complete sequence; and large subunit ribosomal RNA gene, partial sequence. GenBank 2021. Preprints. [Google Scholar]
- Combatt-Caballero, E.; Hernández-Burgos, J.; Jarma-Orozco, A.; Jaraba-Navas, J.; Rodríguez-Páez, L. Macroelements and mcroelements in the soil and their relationship with the content of steviol glucosides in Stevia rebaudiana Bert from five regions of Colombia. Horticulturae 2021, 7, 547. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Rivero, A.M.; Ramírez-Mosqueda, M.A.; Frómeta, O.M.; Morgado, M.M.E.; Paneca, M.R.; Escriba, R.C.R.; Gradaille, M.A.D.; Bello-Bello, J.J. Influence of Vitrofural® on sugarcane micropropagation using temporary immersion system. Plant Cell Tiss. Org. Cult. 2020, 141, 447–453. [Google Scholar] [CrossRef]
- Likert, R. A technique for the measurement of attitudes. Arch. Psychol. 1932, 140, 44–53. [Google Scholar]
- Pompelli, M.F.; Guerra, M.P. Ex situ conservation of Dyckia distachya: An endangered bromeliad from South Brazil. Crop Breed. Appl. Biotechnol. 2004, 4, 273–279. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Guerra, M.P. Micropropagation enables the mass propagation and conservation of Dyckia distachya Hassler. Crop Breed. Appl. Biotechnol. 2005, 5, 117–126. [Google Scholar] [CrossRef]
- Smith, K.; Guo, S.; Zhu, Q.; Dong, X.; Liu, S. An evaluation of the environmental benefit and energy footprint of China’s stricter wastewater standards: Can benefit be increased? J. Clean. Prod. 2019, 219, 723–733. [Google Scholar] [CrossRef]
- Pais, A.K.; da Silva, A.P.; de Souza, J.C.; Teixeira, S.L.; Ribeiro, J.M.; Peixoto, A.R.; da Paz, C.D. Sodium hypochlorite sterilization of culture medium in micropropagation of Gerbera hybrida cv. Essandre. Afr. J. Biotechnol. 2016, 15, 1995–1998. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Tripathi, S.; Lal, M.; Mishra, S. Screening of some chemical disinfectants for media sterilization during in vitro micropropagation of sugarcane. Sugar Tech. 2012, 14, 364–369. [Google Scholar] [CrossRef]
- Peiris, S.E.; De Silva, E.D.U.D.; Edussuriya, M.; Attanayake, A.M.U.R.K.; Peiris, B.C.N. CSUP technique: A low cost sterilization method using sodium hypochlorite to replace the use of expensive equipment in micropropagation. J. Natl. Sci. Found. Sri Lanka 2012, 40, 49–54. [Google Scholar] [CrossRef]
- Jawad, H.A.; Hussein, N.H.; Salef, F.F. Effect of sodium chloride on stevia plant growth in vitro. Int. J. Appl. Sci. Technol. 2022, 4, 171. [Google Scholar] [CrossRef]
- Shaafi, B.; Mosavi, S.; Abdollahi, M.R.; Sarikhani, H. The optimized protocols for production, adaptation and keeping of the produced artificial seeds from encapsulated lateral buds in Stevia Rebaudiana (Bertoni). Agrotech. Ind. Crops 2021, 1, 24–35. [Google Scholar] [CrossRef]
- Jadid, N.; Anggraeni, S.; Ramadani, M.R.N.; Arieny, M.; Mas’ud, F. In Vitro propagation of Indonesian stevia (Stevia rebaudiana) genotype using axenic nodal segments. BMC Res. Notes 2024, 17, 45. [Google Scholar] [CrossRef]
- Ghose, A.K.; Abdullah, S.N.A.; Md Hatta, M.A.; Megat Wahab, P.E. In Vitro regeneration of stevia (Stevia rebaudiana Bertoni) and evaluation of the impacts of growth media nutrients on the biosynthesis of steviol glycosides (SGs). Agronomy 2022, 12, 1957. [Google Scholar] [CrossRef]
- Ahmed, K.Z.; Osman, S.A.-M.; Saber, S.M.; Abdel-Hamed, A.A. An efficient in vitro culture protocol of stevia plants (Stevia rebaudiana Bertoni var. chaine.2) with cytogenetical studies. J. Agric. Res. Dev. 2018, 38, 93–113. [Google Scholar] [CrossRef]
- Vilariño, S.; Florido, M.D.C.; García, J.L.; Cantos, M. Effects of culture system and substrate composition on micropropagated plantlets of two varieties of Stevia rebaudiana Bert. Physiologia 2023, 3, 74–85. [Google Scholar] [CrossRef]
- Das, A.; Gantait, S.; Mandal, N. Micropropagation of an elite medicinal plant: Stevia rebaudiana Bert. Int. J. Agric. Res. 2011, 6, 40–48. [Google Scholar] [CrossRef]
- Hwang, S.J. Rapid in vitro propagation and enhanced stevioside accumulation in Stevia rebaudiana Ber. J. Plant Biol. 2006, 49, 267–270. [Google Scholar] [CrossRef]
- Espinal de Rueda, D.; Delvalle, W.; Cifuentes, E.; Ramia, N. Propagación in vitro de Stevia rebaudiana B: A partir de segmentos nodales. Ceiba 2006, 47, 11–18. [Google Scholar] [CrossRef]
- Kumar, N.; Reddy, M.P. In Vitro plant propagation: A review. J. Envirom Sci. 2011, 27, 61–72. [Google Scholar] [CrossRef]
- Jangid, V.K.; Senthil-Kumar, M.; Chandran, D.; Sinharoy, S. Callus induction and efficient in vitro plant regeneration protocol for Chickpea. Plant Cell Tiss. Organ. Cult. 2024, 156, 21. [Google Scholar] [CrossRef]
- Raha, P.; Saha, G.; Khatua, I.; Bandyopadhyay, T.K. Direct shoot from root and true-to-type micropropagation of Limonium “Misty Blue” in partially immersed culture on an aluminum mesh raft. In Micropropagation Methods in Temporary Immersion Systems. Methods in Molecular Biology; Ramírez-Mosqueda, M.A., Cruz-Cruz, C.A., Eds.; Humana: New York, NY, USA, 2024; Volume 2759. [Google Scholar]
- Alhady, M.R.A.A. Micropropagation of Stevia rebaudiana Bertoni.: A new sweetening crop in Egypt. Glob. J. Biotechnol. Biochem. 2011, 6, 178–182. [Google Scholar]
- Patel, R.M.; Shah, R.R. Regeneration of Stevia plant through callus culture. Indian J. Pharm. Sci. 2009, 71, 46–50. [Google Scholar] [CrossRef]




| NaOCl (%) | Time (min) | Contamination | Survival Rate |
|---|---|---|---|
| 0 | 0 | 2.6 ± 0.0 Aa | 1.0 ± 0.0 Ab |
| 5 | 2.7 ± 0.1 Ca | 1.2 ± 0.1 Cab | |
| 10 | 2.8 ± 0.0 Ba | 1.5 ± 0.1 Da | |
| 15 | 2.9 ± 0.1 Ba | 1.8 ± 0.2 Da | |
| 0.5 | 0 | 2.6 ± 0.0 Ab | 1.0 ± 0.0 Ad |
| 5 | 2.9 ± 0.2 Bb | 2.1 ± 0.1 Bc | |
| 10 | 5.0 ± 0.0 Aa | 4.9 ± 0.1 Aa | |
| 15 | 5.0 ± 0.0 Aa | 2.5 ± 0.2 Ab | |
| 1 | 0 | 2.6 ± 0.0 Ac | 1.0 ± 0.0 Ac |
| 5 | 3.0 ± 0.1 Bb | 2.3 ± 0.1 Bb | |
| 10 | 4.9 ± 0.1 Aa | 4.0 ± 0.1 Ba | |
| 15 | 5.0 ± 0.1 Aa | 2.1 ± 0.1 Bb | |
| 1.5 | 0 | 2.6 ± 0.0 Ac | 1.0 ± 0.0 Ad |
| 5 | 3.9 ± 0.3 Ab | 3.8 ± 0.1 Aa | |
| 10 | 5.0 ± 0.0 Aa | 2.6 ± 0.2 Cb | |
| 15 | 5.0 ± 0.0 Aa | 2.1 ± 0.2 Bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguéz-Páez, L.A.; Pineda-Rodriguez, Y.Y.; Pompelli, M.F.; Jimenez-Ramirez, A.M.; Genes-Avilez, O.J.; Jaraba-Navas, J.d.D.; Jarma-Orozco, A.; Combatt-Caballero, E.; Oviedo Zumaqué, L.E.; Suarez-Padron, I.E.; et al. Micropropagation Protocols for Three Elite Genotypes of Stevia rebaudiana Bertoni. Horticulturae 2024, 10, 404. https://doi.org/10.3390/horticulturae10040404
Rodriguéz-Páez LA, Pineda-Rodriguez YY, Pompelli MF, Jimenez-Ramirez AM, Genes-Avilez OJ, Jaraba-Navas JdD, Jarma-Orozco A, Combatt-Caballero E, Oviedo Zumaqué LE, Suarez-Padron IE, et al. Micropropagation Protocols for Three Elite Genotypes of Stevia rebaudiana Bertoni. Horticulturae. 2024; 10(4):404. https://doi.org/10.3390/horticulturae10040404
Chicago/Turabian StyleRodriguéz-Páez, Luis Alfonso, Yirlis Yadeth Pineda-Rodriguez, Marcelo F. Pompelli, Ana Melisa Jimenez-Ramirez, Osmin José Genes-Avilez, Juan de Dios Jaraba-Navas, Alfredo Jarma-Orozco, Enrique Combatt-Caballero, Luis Eliécer Oviedo Zumaqué, Isidro Elias Suarez-Padron, and et al. 2024. "Micropropagation Protocols for Three Elite Genotypes of Stevia rebaudiana Bertoni" Horticulturae 10, no. 4: 404. https://doi.org/10.3390/horticulturae10040404







