Effects of Progressive Drought Stress on the Growth, Ornamental Values, and Physiological Properties of Begonia semperflorens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Drought Treatment
2.3. Determination of the Photosynthetic Indexes
2.4. Determination of the Osmotic Adjustment Substances and Indicators Related to Antioxidant Ability
2.5. Statistical Analysis
3. Results
3.1. Effect of Gradient Drought Stress on the Growth of B. semperflorens
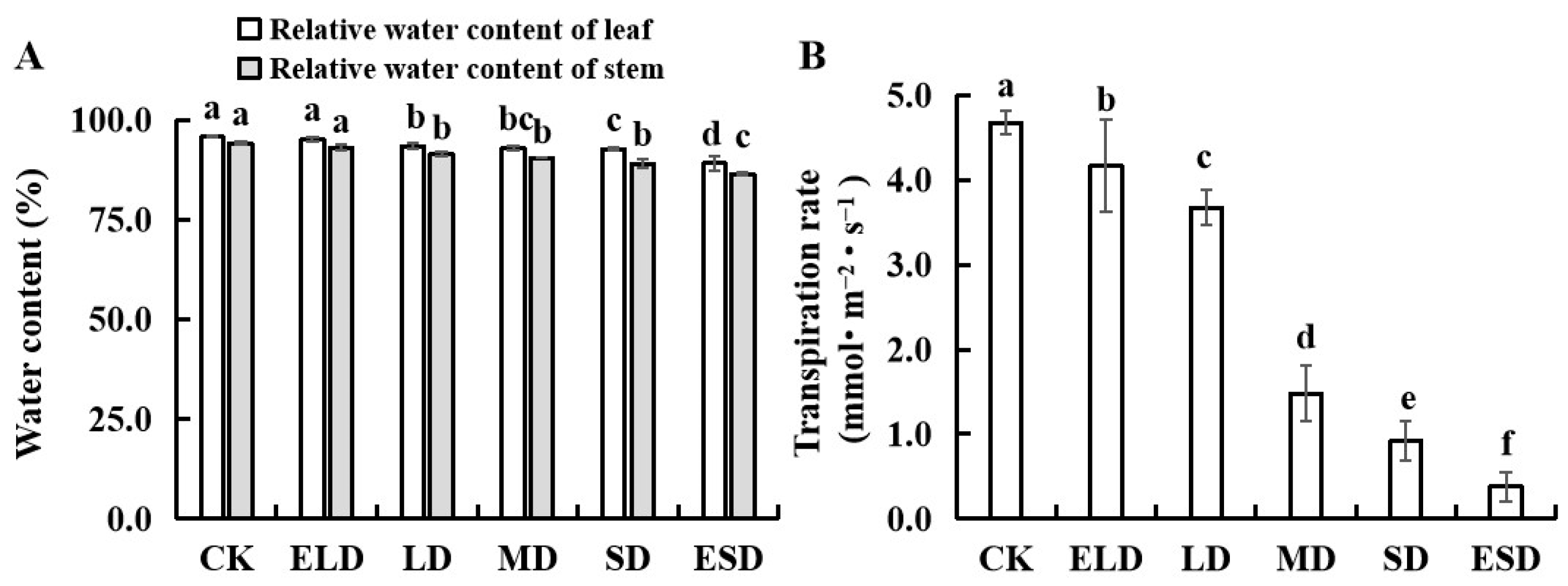
3.2. Effect of Gradient Drought Stress on the Water Content and Transpiration Rates in the Leaves and Stems of B. semperflorens
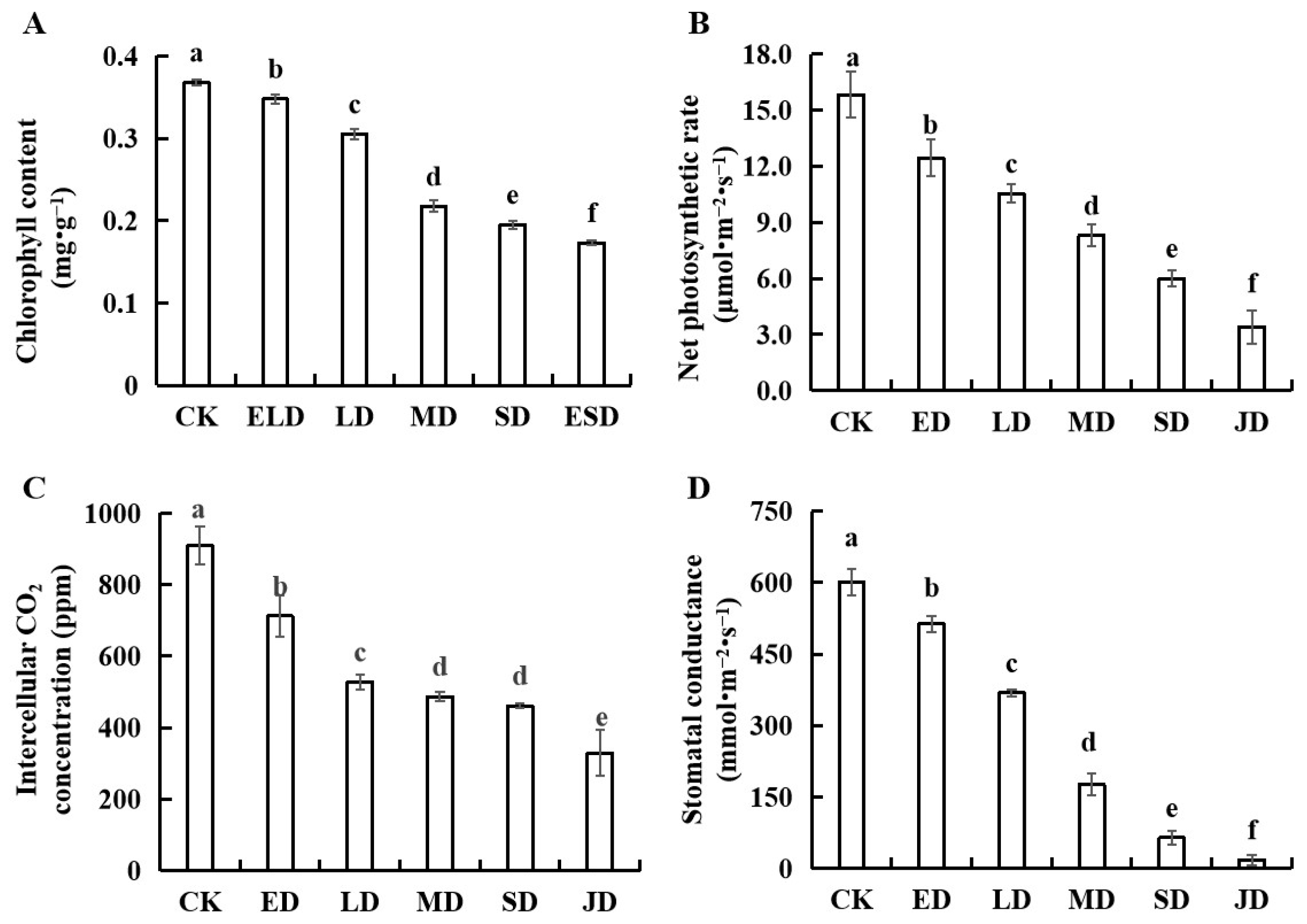
3.3. Effect of Gradient Drought Stress on the Photosynthetic Indexes of B. semperflorens Leaves
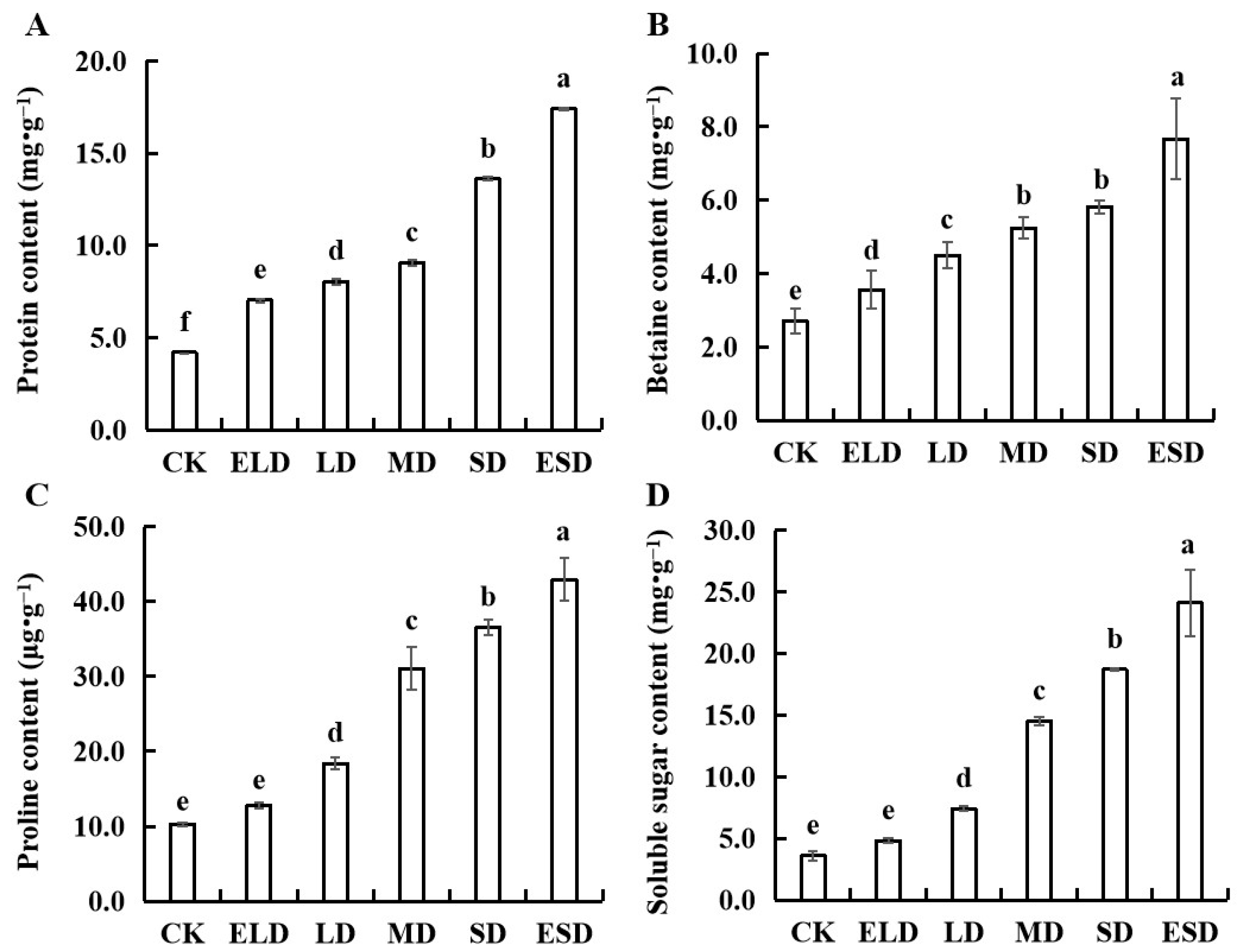
3.4. Effect of Gradient Drought Stress on the Osmotic Adjustment Substances in B. semperflorens Leaves
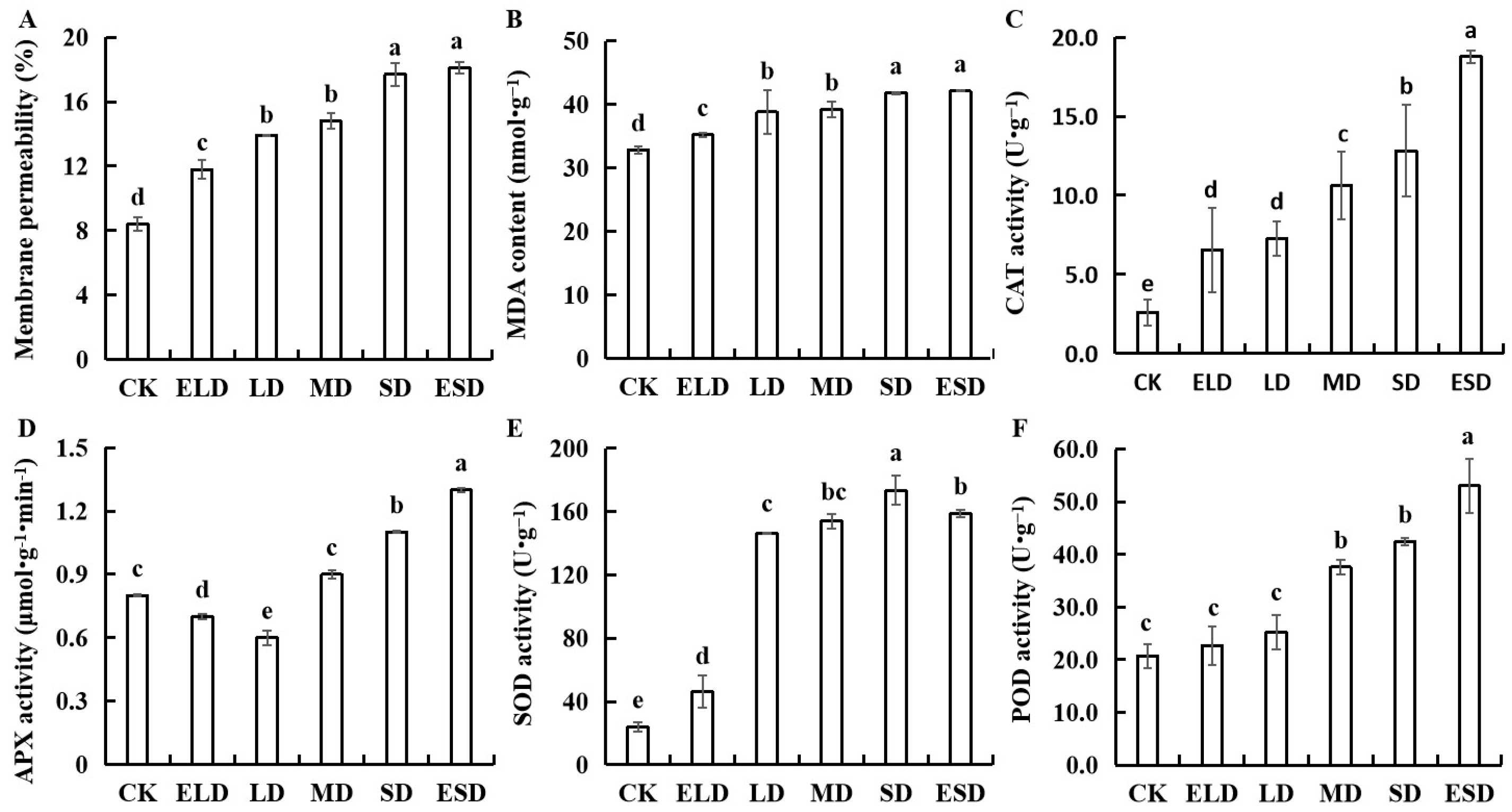
3.5. Effect of Gradient Drought Stress on the Antioxidant Activities in B. semperflorens Leaves
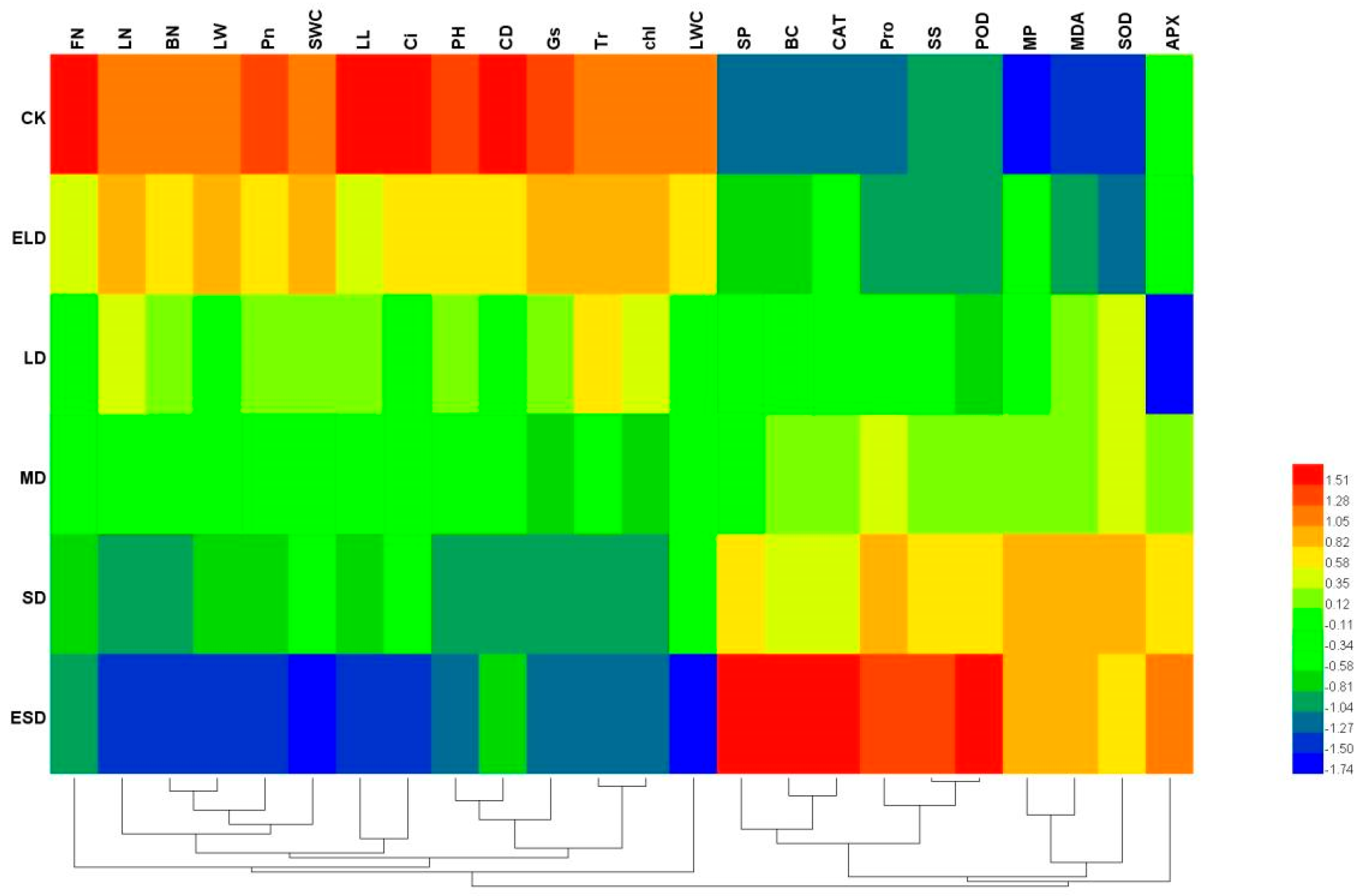
3.6. Correlation Analysis between the Indexes of Drought Resistance of B. semperflorens
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, J.; Zhao, X.; Gu, L.; Liu, P.; Zhao, B.; Zhang, J.; Ren, B. The effects of high temperature, drought, and their combined stresses on the photosynthesis and senescence of summer maize. Agric. Water Manag. 2023, 289, 108525. [Google Scholar] [CrossRef]
- Zhang, X.; Rademacher, T.; Liu, H.; Wang, L.; Manzanedo, R.D. Fading regulation of diurnal temperature ranges on drought-induced growth loss for drought-tolerant tree species. Nat. Commun. 2023, 14, 6916. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.T.; Pershey, N.A.; Andresen, J.A.; Cregg, B.M. Water conserving irrigation practices, plant growth, seasonal crop coefficients, and nutrition of container-grown woody ornamentals. Water 2019, 11, 2070. [Google Scholar] [CrossRef]
- Azizi, S.; Tabari, M.; Abad, A.R.F.N.; Ammer, C.; Guidi, L.; Bader, M.K.F. Soil inoculation with beneficial microbes buffers negative drought effects on biomass, nutrients, and water relations of common myrtle. Front. Plant Sci. 2022, 13, 892826. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Ou, J.; Tian, A.; Li, W.; Lin, S.; Wang, H.; Zhou, Y. Effects of drought stress on growth and photosynthetic physiological characteristics of Rhododendron delavayi. Southwest China J. Agric. Sci. 2023, 36, 2670–2677. [Google Scholar]
- Wu, S.; Ding, X. Effects of drought stress on photosynthetic and physiological characteristics of Chrysanthemum morifolium Ramat. J. Changchun Univ. 2022, 32, 39–44. [Google Scholar]
- Wen, Z.; Feng, Y.; Liu, Q.; Chen, Y.; Chen, Y.; Liu, B.; Wang, Z. Changes in seed germination, seedling growth and physiology of 3 herbaceous species in response to drought stress. J. Fujian Agric. For. Univ. 2022, 51, 562–569. [Google Scholar]
- Wang, J.; Fu, B.; Li, S.; Wang, X.; Song, W.; Ye, Y.; Hu, P.; Wang, T. Effects of exogenous melatonin on growth and physiological characteristics of Agropyron mongolicum seedlings under drought stress. Chin. J. Appl. Ecol. 2023, 34, 2947–2957. [Google Scholar]
- Huang, Y.; Deng, M.; Peng, C.; Wen, J. Studies on the response of lily petal antioxidant enzyme system to drought stress. Acta Hortic. Sin. 2020, 47, 788–796. [Google Scholar]
- Zong, H.; Jia, X.; Li, X.; He, R.; Bao, H.; Yu, Z. Physiological response and drought resistance evaluation of interspecific hybrids of Elymus L. under drought stress. Mol. Plant Breed. 2024, 12, 1–16. [Google Scholar]
- Fu, Y.; Li, P.; Mounkaila Hamani, A.K.; Wan, S.; Gao, Y.; Wang, X. Effects of single and combined drought and salinity stress on the root morphological characteristics and root hydraulic conductivity of different winter wheat varieties. Plants 2023, 12, 2694. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, J.; Wang, M.; Cao, X.; Liu, L.; Xu, H.; Li, W. Effect of drought stress on the growth and physiological characteristics of nine species of wild ornamental plants in Lanzhou. Pratacultural Sci. 2018, 35, 2865–2871. [Google Scholar]
- Xue, W.; Zheng, T.; Zhang, L. Effects of N, P and K on the growth of tissue culture seedlings of Begonia semperflorens. J. Southwest For. Univ. 2020, 40, 166–170. [Google Scholar]
- Crutchfield, E.F.; Mcgiffen, M.E., Jr.; Merhaut, D.J. Effects of biochar on nutrient leaching and Begonia plant growth. J. Environ. Hortic. 2018, 36, 126–132. [Google Scholar] [CrossRef]
- Dong, Y.; Qu, Y.; Qi, R.; Bai, X.; Tian, G.; Wang, Y.; Wang, J.; Zhang, K. Transcriptome analysis of the biosynthesis of anthocyanins in Begonia semperflorens under low-temperature and high-light conditions. Forests 2018, 9, 87. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, A.; Zhang, X.; Huang, S. Effects of shading on some morphological and physiological characteristics of Begonia semperflorens. Pak. J. Bot. 2018, 50, 2173–2179. [Google Scholar]
- Yao, K.; Wang, R.; Liu, J.; Bai, X.; Qi, R.; Liu, S.; Li, Y.; Zhang, K. Composition of anthocyanin components and related gene expression in leaves of Begonia semperflorens under low temperature stress. J. Northeast. For. Univ. 2022, 50, 57–62+82. [Google Scholar]
- Liu, B.; Zhang, Y.; Yao, K.; Shi, Z.; Li, F.; Li, Y.; Zhang, K. Drought tolerance functions of BsMYB62 in Begonia semperflorens. J. Northwest AF Univ. 2023, 12, 1–10. [Google Scholar]
- Wang, X.; Huang, J. Principles and Techniques of Plant Physiology and Biochemistry Experiment; Higher Education Press: Beijing, China, 2015. [Google Scholar]
- Li, Z.; Gong, M. Comprehensive and Designed Experiments in Plant Physiology; Huazhong University of Science and Technology Press: Wuhan, China, 2014. [Google Scholar]
- Zhang, J.; Wei, G.; Peng, Y.; Wang, H.; Huang, X.; Wu, J.; Huang, M.; Li, Y. Drought resistance evaluation of eight strong gluten wheat varieties. J. Triticeae Crops 2023, 1–11. [Google Scholar]
- Xi, L.; Gou, Q.; Wang, G.; Song, B. The responses of typical annual herbaceous plants to drought stress in a desert-oasis ecotone. Acta Ecol. Sin. 2021, 41, 5425–5434. [Google Scholar]
- Yu, S.; Luo, Y.; Tang, F.; Qiu, Y.; Li, Z. Effects of drought stress on growth and chlorophyll fluorescence characteristics of Medicago sativa L. Acta Agrestia Sinia 2023, 31, 1762–1771. [Google Scholar]
- Xie, Z.; Zhang, W.; Liu, X. Growth and physiological characteristics of Xanthoceras sorbifolia seedlings under soil drought stress. Acta Bot. Boreali-Occident. Sin. 2010, 30, 948–954. [Google Scholar]
- Zhao, L.; Jin, H.; Cao, X.; Deng, W.; Du, L. Physiological response to drought stress and drought resistance of six Helleborus orientlis cultivars. Chin. J. Appl. Ecol. 2023, 34, 2644–2654. [Google Scholar]
- Jin, S.; Peng, Z. Research progress on drought stress on Robinia pseudoacacia and Pinus tabuliformis. J. Northwest For. Univ. 2022, 37, 79–91. [Google Scholar]
- Sun, X.; Zhang, X.; Ding, S.; Li, W.; Guo, C.; Wang, D.; Liu, X.; Tang, X. Responses of physiological and photosynthetic characteristics in leaves of two Malus cultivars to drought stress. J. Northeast For. Univ. 2019, 47, 28–32+66. [Google Scholar]
- Tan, K.; Liu, X.; Ma, D.; Wang, L. Physiological response to drought stress and drought resistance evaluation of Taxodium distichum var. imbricatum (Nuttall) cropm seedlings from three provenances. Shandong Agric. Sci. 2022, 54, 30–37. [Google Scholar]
- Xian, X.; Zhang, D.; Zhang, Z.; Wang, S.; Gao, Y.; Wang, Y. Effects of 2,4-Epibrassinolide on physiological characteristics of Malus halliana under drought stress. Agric. Res. Arid Areas 2022, 40, 37–45. [Google Scholar]
- Devi, M.; Reddy, V.; Timlin, D. Drought-induced responses in maize under different vapor pressure deficit conditions. Plants 2022, 11, 2771. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Bai, S.; Li, M.; Qu, Y. Effects of exogenous abscisic acid on photosynthetic and fluorescence indexes of Camellia reticulata under drought stress. Mol. Plant Breed. 2023, 14, 1–13. [Google Scholar]
- Qi, Y.; Ma, L.; Ghani, M.I.; Peng, Q.; Fan, R.; Hu, X.; Chen, X. Effects of drought stress induced by hypertonic polyethylene glycol (PEG-6000) on Passiflora edulis sims physiological properties. Plants 2023, 12, 2296. [Google Scholar] [CrossRef]
- Iqbal, N.; Ashraf, Y.; Ashraf, M. Modulation of endogenous levels of some key organic metabolites by exogenous application of glycine betaine in drought stressed plants of sunflower (Helianthus annuus L.). Plant Growth Regul. 2011, 63, 7–12. [Google Scholar] [CrossRef]
- An, Y.; Hao, W.; Gong, C.; Han, R.; Liang, Z. Effects of drying and re-watering on the photosynthesis and active oxygen metabolism of Periploca sepium seedlings. Chin. J. Appl. Ecol. 2010, 21, 3047–3055. [Google Scholar]
- Huang, S. Harm by drought and mechanism of drought resistance of plant. J. Anhui Agric. Sci. 2009, 37, 10370–10372. [Google Scholar]
- Niu, X.; Ma, R. Effects of drought stress on leaf physiology of Reaumuria soongorica seedlings during the growing season. Pratacultural Sci. 2023, 40, 2483–2492. [Google Scholar]
- Hoekstra, F.A.; Golovina, E.A.; Buitink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Han, Y.; Qiao, Y.; Xie, K.; Li, S.; Dong, Y.; Li, S.; Zhang, S. Effects of drought stress on the growth and physiological characteristics of Sect. Aigeiros clones. J. Beijing For. Univ. 2023, 45, 81–89. [Google Scholar]
- Li, M.; Ma, J. Influences of salt and drought stress on proline content in three poplar varieties. Hunan Agric. Sci. 2013, 1, 105–107+110. [Google Scholar]
- Singh, T.; Sandhu, P.S.; Chahal, G.K.; Walia, S.S. Foliar thiourea confers moisture stress tolerance in rainfed maize through elevated antioxidative defence system, osmolyte accumulation and starch synthesis grown under different planting methods. J. Plant Growth Regul. 2023, 42, 199–217. [Google Scholar] [CrossRef]
- Wang, N.; Liang, Q.; Zhou, Z.; Guo, W.; Wang, L.; Wu, W. Physiological response of begonia ‘Orococo’ to drought stress. Anhui Agric. Sci. 2023, 51, 131–134. [Google Scholar]
- Wang, Y.; Yu, S.; Wang, J.; Song, H.; Lei, Y.; Zhang, H.; Liu, B. Effects of drought stress and rehydration on physiological characteristics of Hemerocallis fulva. Chin. Agric. Sci. Bull. 2023, 39, 58–64. [Google Scholar]
- Li, C.; Wan, Y.; Shang, X.; Fang, S. Responses of microstructure, ultrastructure and antioxidant enzyme activity to PEG-induced drought stress in Cyclocarya paliurus seedlings. Forests 2022, 13, 836. [Google Scholar] [CrossRef]
- Guan, C.; Jiao, M.; Zhang, Y. Comprehensive evaluation and analysis of drought resistance of 8 Petunia cultivars. J. Northwest For. Univ. 2018, 33, 63–69. [Google Scholar]
- Li, C.; Yang, Y.; Yang, Z.; Wang, J.; Liao, W. Comparison on the drought-resistance of five ornamental crabapple cultivars. Acta Agric. Zhejiangsis 2017, 29, 782–790. [Google Scholar]
- Liu, M.; Guo, L.; Yue, Y.; Wu, J.; Fan, X.; Xiao, G.; Teng, K. Physiological and antioxidant enzyme gene expression differences between female and male Buchloe dactyloides plants under drought stress. Acta Prataculturae Sin. 2023, 32, 93–103. [Google Scholar]
- Zhang, Y.; Wei, M.; Sun, O.; Cai, J. Physiology responses and drought mechanisms of different Hydrangea varieties under drought stress. J. Northwest For. Univ. 2018, 33, 90–97. [Google Scholar]
- Wang, C.; Li, Q.; Zhang, X. Physiological response of three ornamental grasses under drought stress. Anhui Agric. Sci. Bull. 2023, 29, 74–81. [Google Scholar]
- Zomorrodi, N.; Rezaei Nejad, A.; Mousavi-Fard, S.; Feizi, H.; Tsaniklidis, G.; Fanourakis, D. Potency of titanium dioxide nanoparticles, sodium hydrogen sulfide and salicylic acid in ameliorating the depressive effects of water deficit on periwinkle ornamental quality. Horticulturae 2022, 8, 675. [Google Scholar] [CrossRef]


| Treatment | FN | LN | BN | LW (cm) | LL (cm) | PH (cm) | CD (cm) |
|---|---|---|---|---|---|---|---|
| CK | 73 ± 2.05 a | 197 ± 0.82 a | 29 ± 0.47 a | 5.4 ± 0.12 a | 5.5 ± 0.04 a | 21.4 ± 0.52 a | 35.3 ± 0.21 a |
| ELD | 52 ± 1.63 b | 179 ± 2.16 b | 26 ± 0.47 b | 4.8 ± 0.08 b | 5.2 ± 0.12 b | 18.1 ± 0.16 b | 34.8 ± 0.58 b |
| LD | 31 ± 0.47 c | 159 ± 0.81 c | 24 ± 0.81 c | 4.4 ± 0.04 c | 4.3 ± 0.08 c | 15.9 ± 0.43 c | 32.6 ± 0.21 c |
| MD | 23 ± 2.05 d | 142 ± 0.81 d | 21 ± 0.81 d | 3.9 ± 0.08 d | 4.1 ± 0.21 d | 13.6 ± 0.29 d | 27.1 ± 0.33 d |
| SD | 11 ± 0.82 e | 105 ± 0.81 e | 17 ± 0.47 e | 3.4 ± 0.08 e | 3.3 ± 0.08 e | 11.2 ± 0.21 e | 23.9 ± 0.33 e |
| ESD | 5 ± 0.82 f | 88 ± 2.05 f | 13 ± 0.94 f | 2.7 ± 0.08 f | 2.9 ± 0.21 f | 9.5 ± 0.43 f | 20.6 ± 0.90 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Liu, A.; Zhang, Y.; Yang, X.; Yang, S.; Zhao, K. Effects of Progressive Drought Stress on the Growth, Ornamental Values, and Physiological Properties of Begonia semperflorens. Horticulturae 2024, 10, 405. https://doi.org/10.3390/horticulturae10040405
Zhao Z, Liu A, Zhang Y, Yang X, Yang S, Zhao K. Effects of Progressive Drought Stress on the Growth, Ornamental Values, and Physiological Properties of Begonia semperflorens. Horticulturae. 2024; 10(4):405. https://doi.org/10.3390/horticulturae10040405
Chicago/Turabian StyleZhao, Zhimin, Airong Liu, Yuanbing Zhang, Xiaodong Yang, Shuyue Yang, and Kunkun Zhao. 2024. "Effects of Progressive Drought Stress on the Growth, Ornamental Values, and Physiological Properties of Begonia semperflorens" Horticulturae 10, no. 4: 405. https://doi.org/10.3390/horticulturae10040405




