Application of Procyanidins from Aronia melanocarpa (Michx.) Elliott in Fresh-Cut Apple Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments and Equipment
2.2. Preparation of DESs
2.3. Single-Factor Test
2.4. Determination of Proanthocyanidin Content and Extraction Rate
2.5. Orthogonal Test
2.6. Purify
2.7. Antioxidant Capacity
2.7.1. DPPH Radical Scavenging Activity
2.7.2. Hydroxyl Radical Scavenging Activity
2.7.3. ABTS Radical Scavenging Activity
2.7.4. Superoxide Anion Radical Scavenging Activity
2.8. Bacteriostatic Activity
2.8.1. Inhibition Circle
2.8.2. Minimum Inhibitory Concentration (MIC)
2.8.3. Growth Inhibition Curve
2.9. Fresh-Cut Apple Preservation
2.9.1. Pretreatment
2.9.2. Weight Loss Rate
2.9.3. Firmness
2.9.4. Appearances
2.9.5. Total Number of Bacterial Colonies
3. Results
3.1. Preparation of Proanthocyanidin Extract
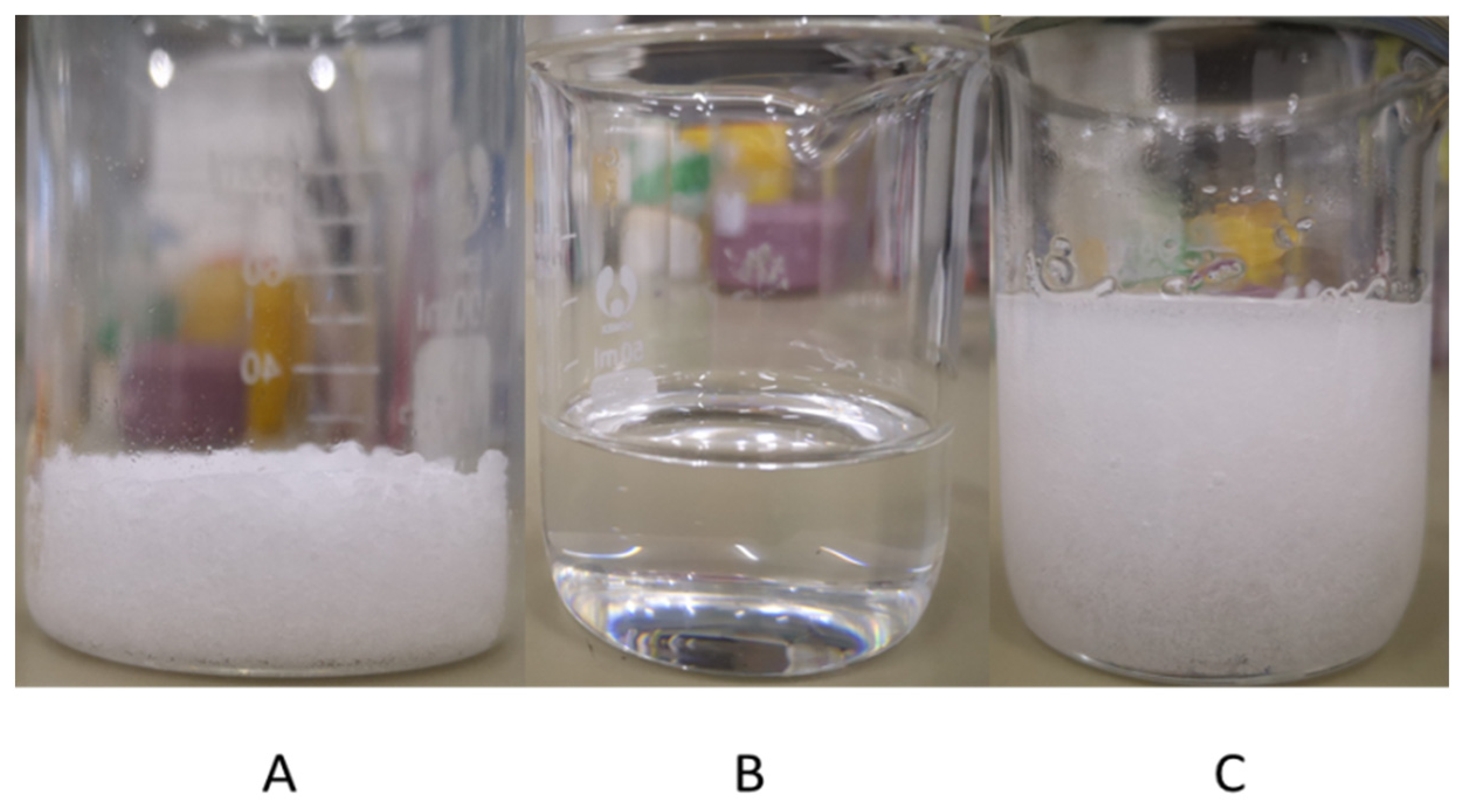
3.1.1. Preparation of DESs
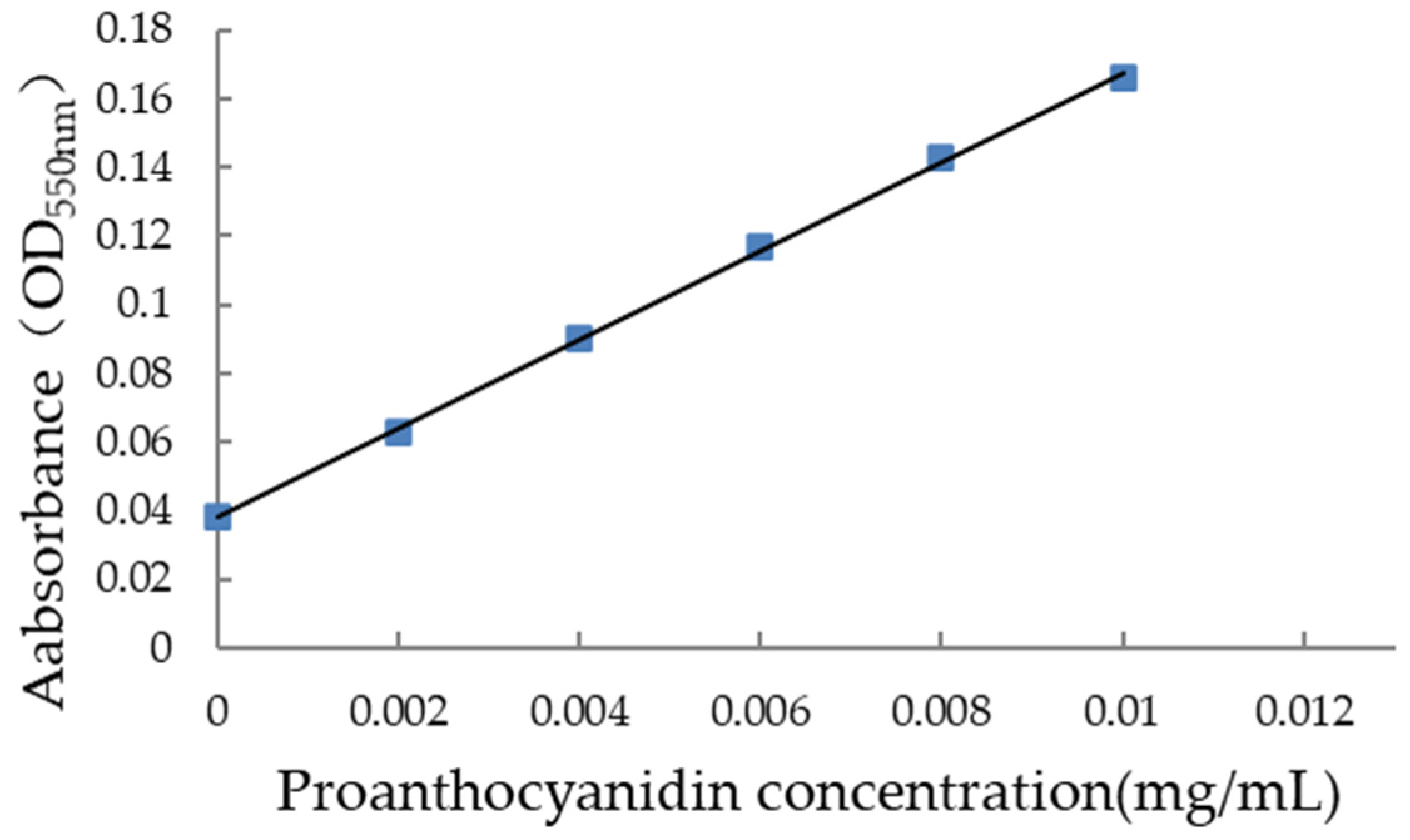
3.1.2. Standard Curve
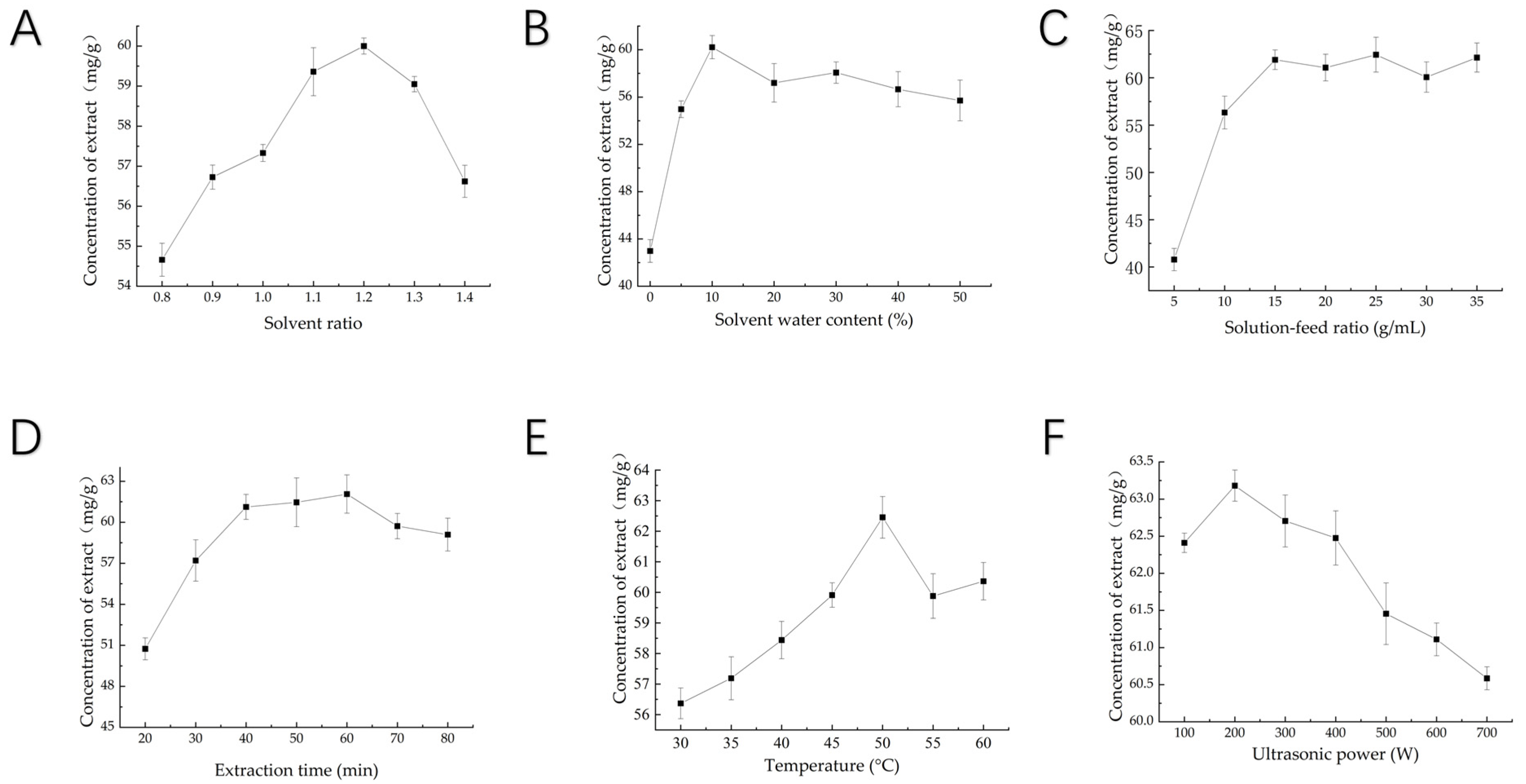
3.1.3. Single-Factor Test
3.1.4. Orthogonal Test
3.1.5. Purify
3.2. In Vitro Antioxidant Capacity
3.3. In Vitro Bacteriostatic Activity
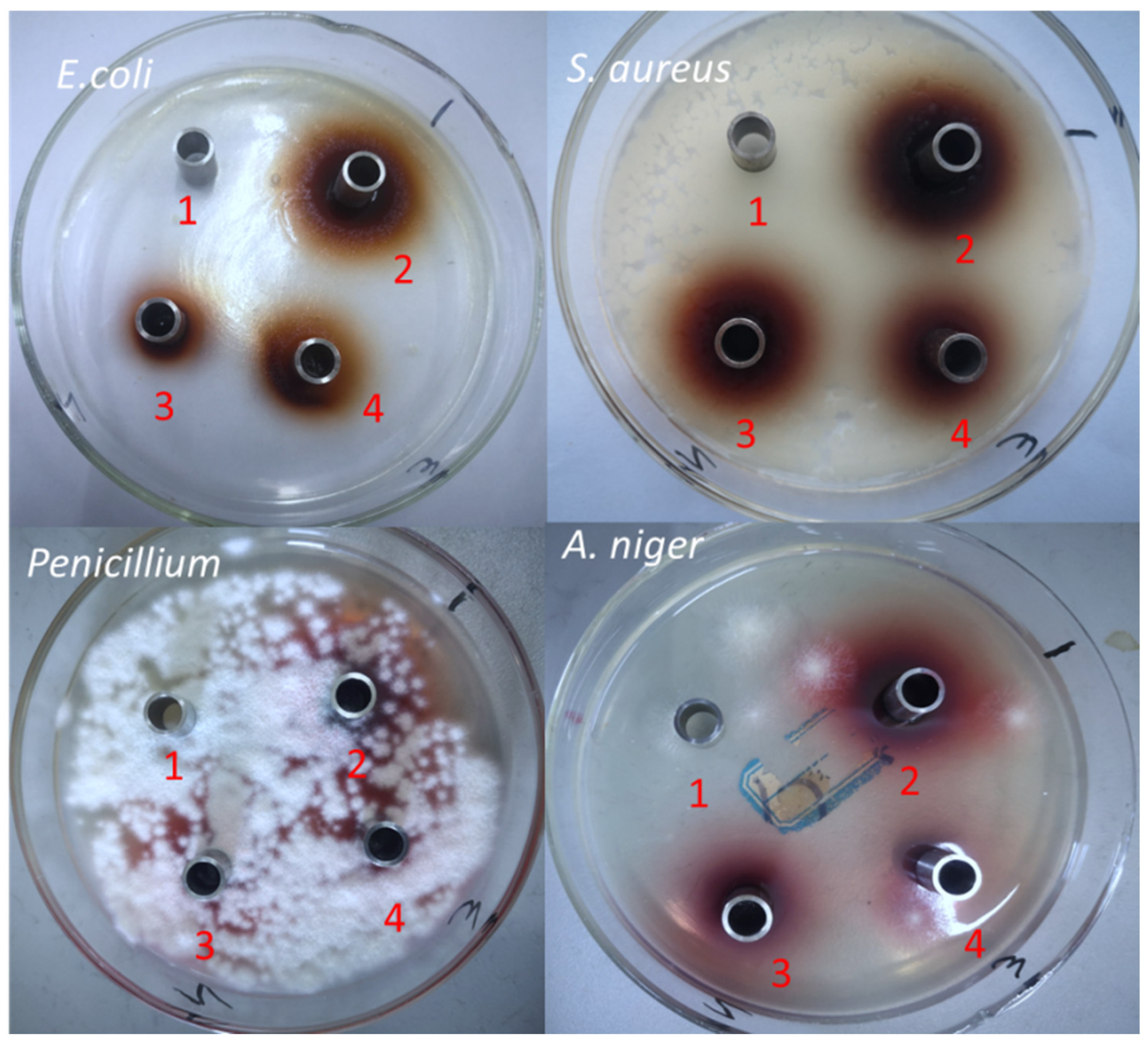
3.3.1. Circle of Inhibition Size
3.3.2. Determination of Minimum Inhibitory Concentration (MIC)
3.3.3. Growth Inhibition Curve
3.4. Fresh-Cut Apple Preservation
3.4.1. Weight Loss Rate
3.4.2. Firmness
3.4.3. Appearance Changes
3.4.4. Determination of Total Bacterial Count
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kobus, Z.; Nadulski, R.; Wilczyński, K.; Kozak, M.; Guz, T.; Rydzak, L. Effect of the black chokeberry (Aronia melanocarpa (Michx.) Elliott) juice acquisition method on the content of polyphenols and antioxidant activity. PLoS ONE 2019, 14, e0219585. [Google Scholar] [CrossRef] [PubMed]
- Wawer, I.; Wolniak, M.; Paradowska, K. Solid state NMR study of dietary fiber powders from aronia, bilberry, black currant and apple. Solid State Nucl. Magn. Reson. 2006, 30, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Krenn, L.; Steitz, M.D.; Schlicht, C.; Kurth, H.; Gaedcke, F. Anthocyanin- and proanthocyanidin-rich extracts of berries in food supplements–analysis with problems. Die Pharm. 2007, 62, 803–812. [Google Scholar]
- Slimestad, R.; Torskangerpoll, K.; Nateland, H.S.; Johannessen, T.C.; Giske, N.H. Flavonoids from black chokeberries, Aronia melanocarpa. J. Food Compos. Anal. 2005, 18, 61–68. [Google Scholar] [CrossRef]
- Kim, J.H.; Auger, C.; Kurita, I.; Anselm, E.; Rivoarilala, L.O.; Lee, H.J.; Lee, K.W.; Schini-Kerth, V.B. Aronia melanocarpa juice, a rich source of polyphenols, induces endothelium-dependent relaxations in porcine coronary arteries via the redox-sensitive activation of endothelial nitric oxide synthase. Nitric Oxide Biol. Chem. 2013, 35, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Šeruga, M.; Medvidović-Kosanović, M.; Novak, I. Antioxidant Activity and Polyphenols of Aronia in Comparison to other Berry Species. Agric. Conspec. Sci. 2007, 72, 301–306. [Google Scholar]
- Olas, B.; Wachowicz, B.; Nowak, P.; Kędzierska, M.; Tomczak, A.; Stochmal, A.; Oleszek, W.; Jeziorski, A.; Piekarski, J.H. Studies on antioxidant properties of polyphenol-rich extract from berries of Aronia melanocarpa in blood platelets. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2008, 59, 823–835. [Google Scholar]
- Howell, A.B. Cranberry Proanthocyanidins and the Maintenance of Urinary Tract Health. Crit. Rev. Food Sci. Nutr. 2002, 42, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.-X.; Yanagita, T.; Uto, T.; Masuzaki, S.; Fujii, M. Anthocyanidins inhibit cyclooxygenase-2 expression in LPS-evoked macrophages: Structure-activity relationship and molecular mechanisms involved. Biochem. Pharmacol. 2005, 70, 417–425. [Google Scholar] [CrossRef]
- Handeland, M.; Grude, N.; Torp, T.; Slimestad, R. Black chokeberry juice (Aronia melanocarpa) reduces incidences of urinary tract infection among nursing home residents in the long term—A pilot study. Nutr. Res. 2014, 34, 518–525. [Google Scholar] [CrossRef]
- Gąsiorowski, K.; Szyba, K.; Brokos, B.; Oszmiański, J. Antimutagenic activity of anthocyanins isolated from Aronia melanocarpa fruits. Cancer Lett. 1997, 119, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, K.; Ilieva, I.B.; Shiratori, K.; Koyama, Y.; Jin, X.-H.; Yoshida, K.; Kase, S.; Kitaichi, N.; Suzuki, Y.; Tanaka, T.; et al. Anti-inflammatory effects of aronia extract on rat endotoxin-induced uveitis. Investig. Ophthalmol. Vis. Sci. 2005, 46, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Xue, Y.; Lu, X.; Shao, Q.; Cao, Y.; Wu, Z.; Chen, G. The effects of different degrees of procyanidin polymerization on the nutrient absorption and digestive enzyme activity in mice. Molecules 2018, 23, 2916. [Google Scholar] [CrossRef] [PubMed]
- Aires, A. Phenolics in foods: Extraction, analysis and measurements. Phenolic Compd. 2017, 61–88. [Google Scholar] [CrossRef]
- Sun, X.; Jin, Z.; Yang, L.; Hao, J.; Zu, Y.; Wang, W.; Liu, W. Ultrasonic-assisted extraction of procyanidins using ionic liquid solution from Larix gmelinii bark. J. Chem. 2013, 2013, 541037. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Natolino, A. Water and ethanol as co-solvent in supercritical fluid extraction of proanthocyanidins from grape marc: A comparison and a proposal. J. Supercrit. Fluids 2014, 87, 1–8. [Google Scholar] [CrossRef]
- King, J.W. Supercritical fluid extraction: Present status and prospects. Grasas Y Aceites 2002, 53, 8–21. [Google Scholar] [CrossRef]
- Biernacki, K.; Souza, H.i.K.; Almeida, C.u.M.; Magalhães, A.L.; Gonçalves, M.P. Physicochemical properties of choline chloride-based deep eutectic solvents with polyols: An experimental and theoretical investigation. ACS Sustain. Chem. Eng. 2020, 8, 18712–18728. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, T. Applications of Green Deep Eutectic Solvents (DESs) in Synthetic Transformations. Green Solvents Org. Synth. 2024, 199–236. [Google Scholar] [CrossRef]
- Oliva, E.; Mir-Cerdà, A.; Sergi, M.; Granados, M.; Sentellas, S.; Saurina, J. Green extraction of phenolic compounds from strawberry waste based on natural deep eutectic solvents. Int. J. Food Sci. Technol. 2024, 59, 3967–3977. [Google Scholar] [CrossRef]
- Pareek, S. Fresh-cut fruits and vegetables: Technology, physiology, and safety. Science 2004, 71, S615–S620. [Google Scholar]
- Martín-Belloso, O.; Soliva-Fortuny, R.; Oms-Oliu, G. Fresh-cut fruits. Handb. Fruits Fruit Process. 2006, 129–144. [Google Scholar] [CrossRef]
- Rux, G.; Efe, E.; Ulrichs, C.; Huyskens-Keil, S.; Hassenberg, K.; Herppich, W.B. Effects of pre-processing hot-water treatment on aroma relevant VOCs of fresh-cut apple slices stored in sugar syrup. Foods 2020, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Curutchet, A.; Dellacassa, E.; Ringuelet, J.A.; Chaves, A.R.; Viña, S.Z. Nutritional and sensory quality during refrigerated storage of fresh-cut mints (Mentha × piperita and M. spicata). Food Chem. 2014, 143, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Alam, M. Preservation of fresh-cut fruits and vegetables: Current status and emerging technologies. Stewart Postharvest Rev. 2012, 8, 1–10. [Google Scholar]
- Lebelo, K.; Malebo, N.; Mochane, M.J.; Masinde, M. Chemical contamination pathways and the food safety implications along the various stages of food production: A review. Int. J. Environ. Res. Public Health 2021, 18, 5795. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Chang, S.; Liu, J.; Li, H.; Xu, P.; Wang, P.; Wang, X.; Zhao, M.; Zhao, B.; Wang, L.; et al. Physicochemical Properties, Antioxidant and Antidiabetic Activities of Polysaccharides from Quinoa (Chenopodium quinoa Willd.) Seeds. Molecules 2020, 25, 3840. [Google Scholar] [CrossRef] [PubMed]
- Jirasuteeruk, C.; Theerakulkait, C. Ultrasound-assisted extraction of phenolic compounds from mango (Mangifera indica cv. Chok Anan) peel and its inhibitory effect on enzymatic browning of potato puree. Food Technol. Biotechnol. 2019, 57, 350. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Sokorai, K.J.; Liao, C.H.; Cooke, P.; Zhang, H.Q. Antibrowning and antimicrobial properties of sodium acid sulfate in apple slices. J. Food Sci. 2009, 74, M485–M492. [Google Scholar] [CrossRef]
- Lai, W.-F. Design and application of self-healable polymeric films and coatings for smart food packaging. NPJ Sci. Food 2023, 7, 11. [Google Scholar] [CrossRef]
- Unusan, N. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Neto, R.T.; Santos, S.A.O.; Oliveira, J.; Silvestre, A.J.D. Tuning of Proanthocyanidin Extract’s Composition through Quaternary Eutectic Solvents Extraction. Antioxidants 2020, 9, 1124. [Google Scholar] [CrossRef]
- Musdzalifah, M.; Fahrurrozi, M.; Sediawan, W.; Susanti, D. Separation of proanthocyanidin from red sorghum seed extract using macroporous resin. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 963, p. 012043. [Google Scholar]
- Yao, J.; Chen, W.; Fan, K. Novel Efficient Physical Technologies for Enhancing Freeze Drying of Fruits and Vegetables: A Review. Foods 2023, 12, 4321. [Google Scholar] [CrossRef] [PubMed]
- Rico, D.; Martin-Diana, A.B.; Barat, J.; Barry-Ryan, C. Extending and measuring the quality of fresh-cut fruit and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 373–386. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Tsironi, T.N. Application of processing and packaging hurdles for fresh-cut fruits and vegetables preservation. Foods 2021, 10, 830. [Google Scholar] [CrossRef]
- Li, X.; He, C.; Song, L.; Li, T.; Cui, S.; Zhang, L.; Jia, Y. Antimicrobial activity and mechanism of Larch bark procyanidins against Staphylococcus aureus. Acta Biochim. Biophys. Sin. 2017, 49, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Pinzón-Arango, P.A.; Holguin, K.; Camesano, T.A. Impact of cranberry juice and proanthocyanidins on the ability of Escherichia coli to form biofilms. Food Sci. Biotechnol. 2011, 20, 1315–1321. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Betts, G.; Hoskins, N.; Edwards, M.; Ercolini, D.; Mauriello, G. Membrane toxicity of antimicrobial compounds from essential oils. J. Agric. Food Chem. 2007, 55, 4863–4870. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Losa, R.; Zweifel, B.; Wallace, R.J. Sensitivity of pathogenic and commensal bacteria from the human colon to essential oils. Microbiology 2012, 158, 2870–2877. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Junior, M.R.M. Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. [Google Scholar]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Del-Valle, V.; Hernández-Muñoz, P.; Guarda, A.; Galotto, M.J. Development of a cactus-mucilage edible coating (Opuntia ficus indica) and its application to extend strawberry (Fragaria ananassa) shelf-life. Food Chem. 2005, 91, 751–756. [Google Scholar] [CrossRef]
- Liang, L.; Qiu, H.; Liu, Y.; Liu, Y.; Weng, L.; Zhong, W.; Meng, F. Exploring the potential of ume-derived proanthocyanidins: Novel applications for blueberry preservation. Front. Microbiol. 2023, 14, 1265993. [Google Scholar] [CrossRef] [PubMed]

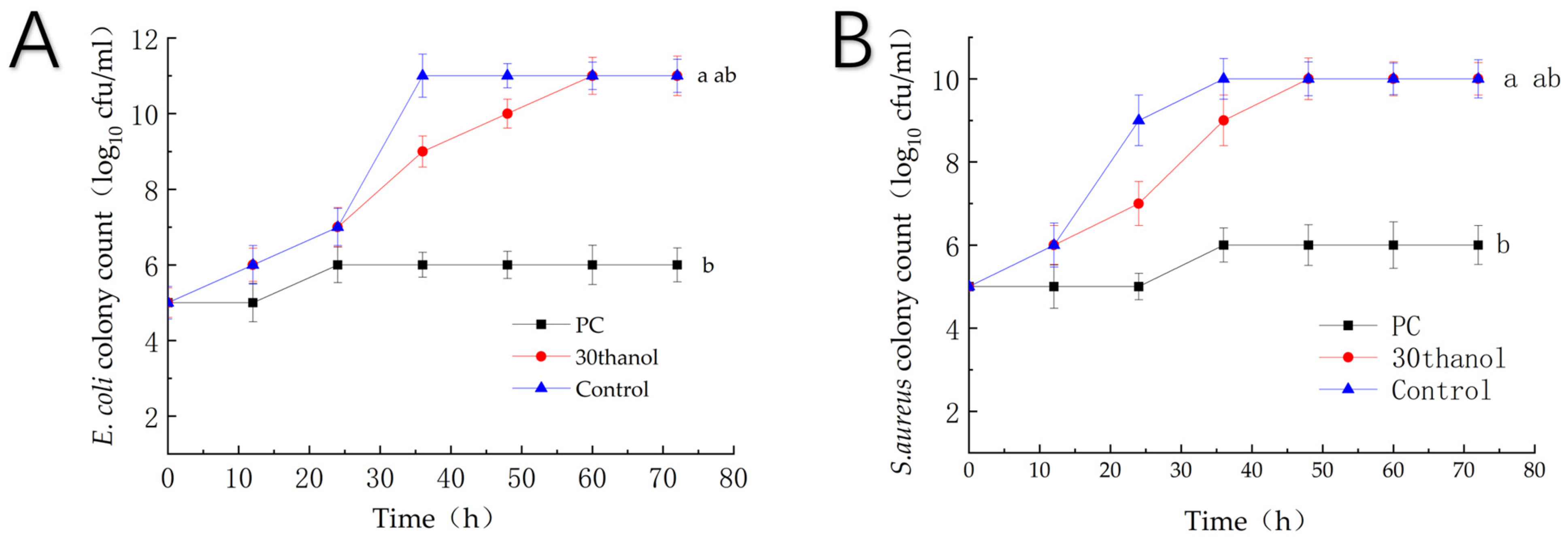
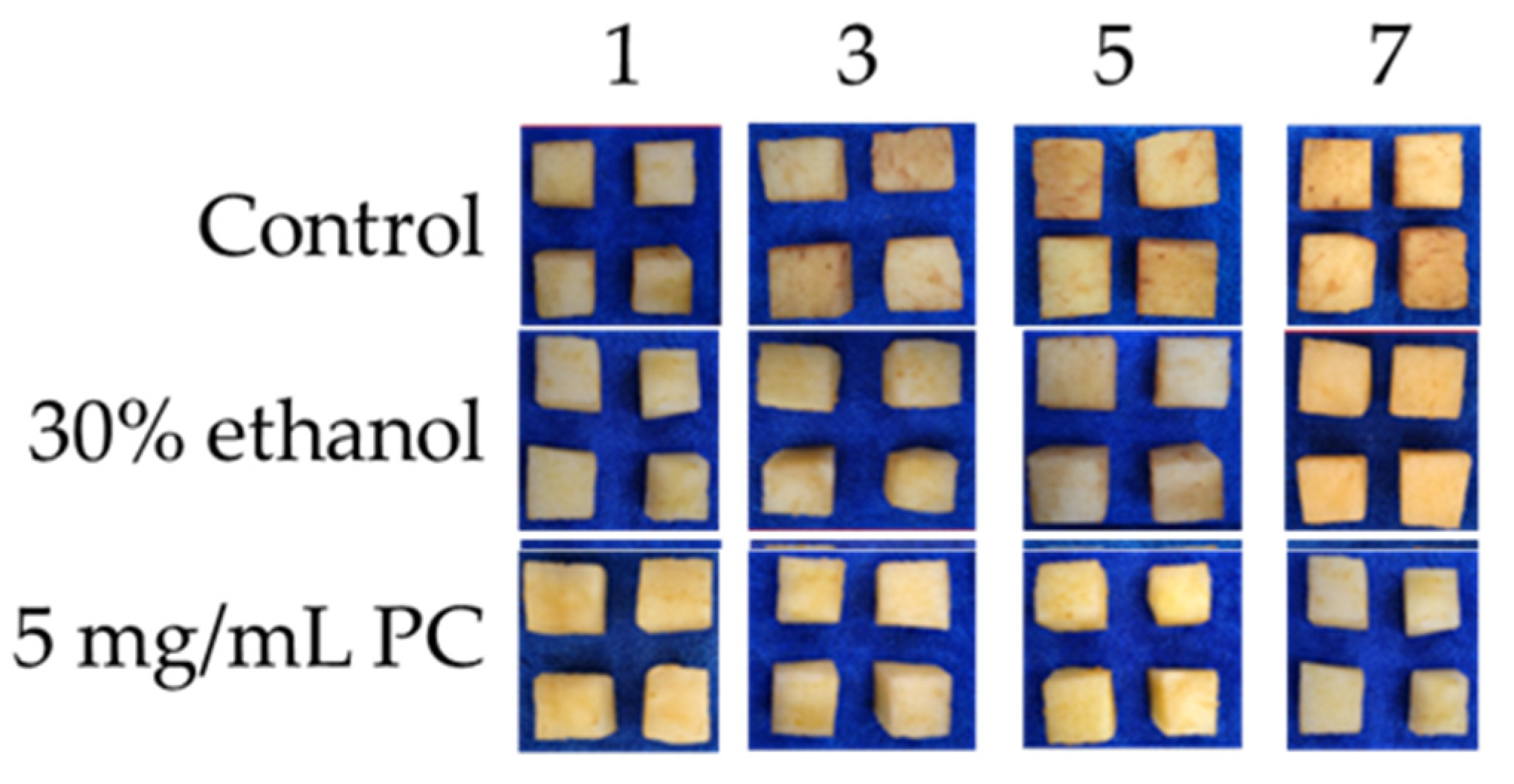

| No. | Solvent Ratio A | Water Content (%) B | S-F Ratio D | Extraction Time (min) C | Temperature (°C) E | Ultrasonic Power (W) F | Concentration (mg/g) |
|---|---|---|---|---|---|---|---|
| 1 | 1.1 | 5 | 10 | 30 | 45 | 100 | 40.01 |
| 2 | 1.1 | 5 | 15 | 40 | 55 | 300 | 59.48 |
| 3 | 1.1 | 10 | 20 | 30 | 55 | 200 | 61.21 |
| 4 | 1.1 | 10 | 10 | 50 | 50 | 300 | 56.6 |
| 5 | 1.1 | 15 | 20 | 40 | 50 | 100 | 59.12 |
| 6 | 1.1 | 15 | 15 | 50 | 45 | 200 | 61.48 |
| 7 | 1.2 | 5 | 20 | 30 | 50 | 300 | 60.29 |
| 8 | 1.2 | 5 | 10 | 50 | 55 | 200 | 56.12 |
| 9 | 1.2 | 10 | 15 | 40 | 50 | 200 | 63.72 |
| 10 | 1.2 | 10 | 20 | 50 | 45 | 100 | 62.09 |
| 11 | 1.2 | 15 | 15 | 30 | 55 | 100 | 52.98 |
| 12 | 1.2 | 15 | 10 | 40 | 45 | 300 | 58.16 |
| 13 | 1.3 | 5 | 20 | 40 | 45 | 200 | 51.65 |
| 14 | 1.3 | 5 | 15 | 50 | 50 | 100 | 59.64 |
| 15 | 1.3 | 10 | 15 | 30 | 45 | 300 | 60.65 |
| 16 | 1.3 | 10 | 10 | 40 | 55 | 100 | 58.79 |
| 17 | 1.3 | 15 | 10 | 30 | 50 | 200 | 57.59 |
| 18 | 1.3 | 15 | 20 | 50 | 55 | 300 | 56.53 |
| K1 | 337.90 | 327.19 | 332.73 | 327.27 | 334.04 | 332.63 | |
| K2 | 353.36 | 363.06 | 350.92 | 357.95 | 356.96 | 351.77 | |
| K3 | 344.85 | 345.86 | 352.46 | 350.89 | 345.11 | 351.71 | |
| k1 | 112.63 | 109.06 | 110.91 | 109.09 | 111.35 | 110.88 | |
| k2 | 117.79 | 121.02 | 116.97 | 119.32 | 118.99 | 117.26 | |
| k3 | 114.95 | 115.29 | 117.49 | 116.96 | 115.04 | 117.24 | |
| R | 5.15 | 11.96 | 6.58 | 10.23 | 7.64 | 6.38 | |
| Factor weight | B > D > E > C > F > A | ||||||
| Best combination | A2B2C3D2E2F2 | ||||||
| Optimum level | A2 (1:1.2) | B2 (10%) | C3 (40 min) | D2 (1:15 g/mL) | E2 (50 °C) | F2 (200 w) | |
| Strains | Inhibition Circle Diameter (mm) | |||
|---|---|---|---|---|
| 30% Ethanol | 100 mg/mL | 50 mg/mL | 25 mg/mL | |
| E. coli | - | 16 ± 0.11 | 14 ± 0.14 | 10 ± 0.21 |
| S. aureus | - | 18 ± 0.09 | 16 ± 0.17 | 12 ± 0.24 |
| Penicillium sp. | - | - | - | - |
| A. niger | - | - | - | - |
| Sample | MIC (mg/mL) | |||
|---|---|---|---|---|
| E. coli | S. aureus | Penicillium sp. | A. niger | |
| proanthocyanidin | 25 | 5 | - | - |
| 30% ethanol | - | - | - | - |
| Groups | Weight Loss Rate (%) | |||
|---|---|---|---|---|
| 1 Day | 3 Day | 5 Day | 7 Day | |
| 5 mg/mL PC | 1.12 ± 0.47 a | 2.46 ± 0.56 a | 3.48 ± 0.47 a | 6.76 ± 0.68 a |
| Control | 1.93 ± 0.55 a | 4.76 ± 0.83 b | 6.71 ± 0.91 b | 9.19 ± 0.85 b |
| 30% ethanol | 1.25 ± 0.35 a | 3.84 ± 0.71 b | 5.38 ± 1.21 ab | 8.85 ± 0.83 b |
| Groups | Firmness (gf) | |||
|---|---|---|---|---|
| 1 Day | 3 Day | 5 Day | 7 Day | |
| 5 mg/mL PC | 243.78 ± 8.43 a | 225.32 ± 5.43 b | 218.43 ± 6.47 c | 208.43 ± 7.68 a |
| Control | 247.51 ± 6.89 a | 212.76 ± 5.07 a | 190.75 ± 6.23 ab | 178.33 ± 6.12 b |
| 30% ethanol | 245.57 ± 7.32 a | 215.14 ± 5.02 ab | 196.03 ± 6.54 b | 182.71 ± 7.46 b |
| Groups | L* | |||
|---|---|---|---|---|
| 1 Day | 3 Day | 5 Day | 7 Day | |
| 5 mg/mL PC | 76.43 ± 7.68 a | 67.34 ± 5.43 a | 64.45 ± 6.47 a | 62.56 ± 4.3 a |
| Control | 75.13 ± 3.89 a | 68.63 ± 3.47 a | 67.13 ± 2.54 a | 66.23 ± 2.22 a |
| 30% ethanol | 70.33 ± 3.92 a | 67.87 ± 2.64 a | 66.8 ± 2.63 a | 64.13 ± 3.93 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Chen, J.; Sarengaowa; Chen, C.; Hu, W. Application of Procyanidins from Aronia melanocarpa (Michx.) Elliott in Fresh-Cut Apple Preservation. Horticulturae 2024, 10, 556. https://doi.org/10.3390/horticulturae10060556
Li S, Chen J, Sarengaowa, Chen C, Hu W. Application of Procyanidins from Aronia melanocarpa (Michx.) Elliott in Fresh-Cut Apple Preservation. Horticulturae. 2024; 10(6):556. https://doi.org/10.3390/horticulturae10060556
Chicago/Turabian StyleLi, Shangjian, Jiajia Chen, Sarengaowa, Chen Chen, and Wenzhong Hu. 2024. "Application of Procyanidins from Aronia melanocarpa (Michx.) Elliott in Fresh-Cut Apple Preservation" Horticulturae 10, no. 6: 556. https://doi.org/10.3390/horticulturae10060556
APA StyleLi, S., Chen, J., Sarengaowa, Chen, C., & Hu, W. (2024). Application of Procyanidins from Aronia melanocarpa (Michx.) Elliott in Fresh-Cut Apple Preservation. Horticulturae, 10(6), 556. https://doi.org/10.3390/horticulturae10060556








