Influence of Intercropping Arisaema amurense with Acanthopanax senticosus on Soil Microbial Community and the Effective Ingredients of A. senticosus
Abstract
1. Introduction
2. Materials and Methods
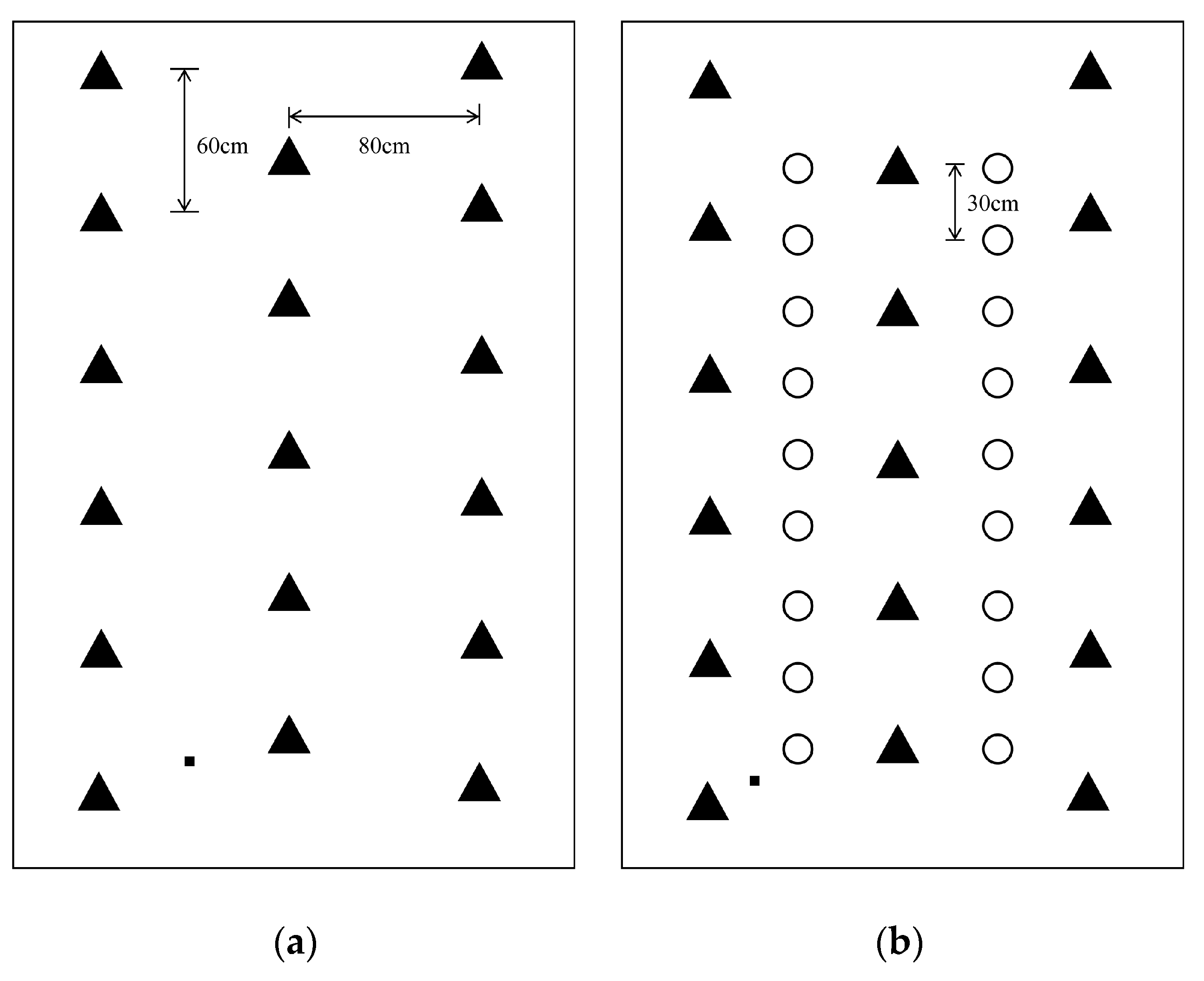
2.1. Site Description and Experimental Design
2.2. Plant and Soil Sampling
2.3. Analysis of Soil Properties and Enzyme Activities
2.4. Determining the Active Ingredients of A. senticosus
2.5. Soil Microbial Analysis
2.6. Statistical Analysis
3. Results
3.1. Active Ingredients of A. senticosus
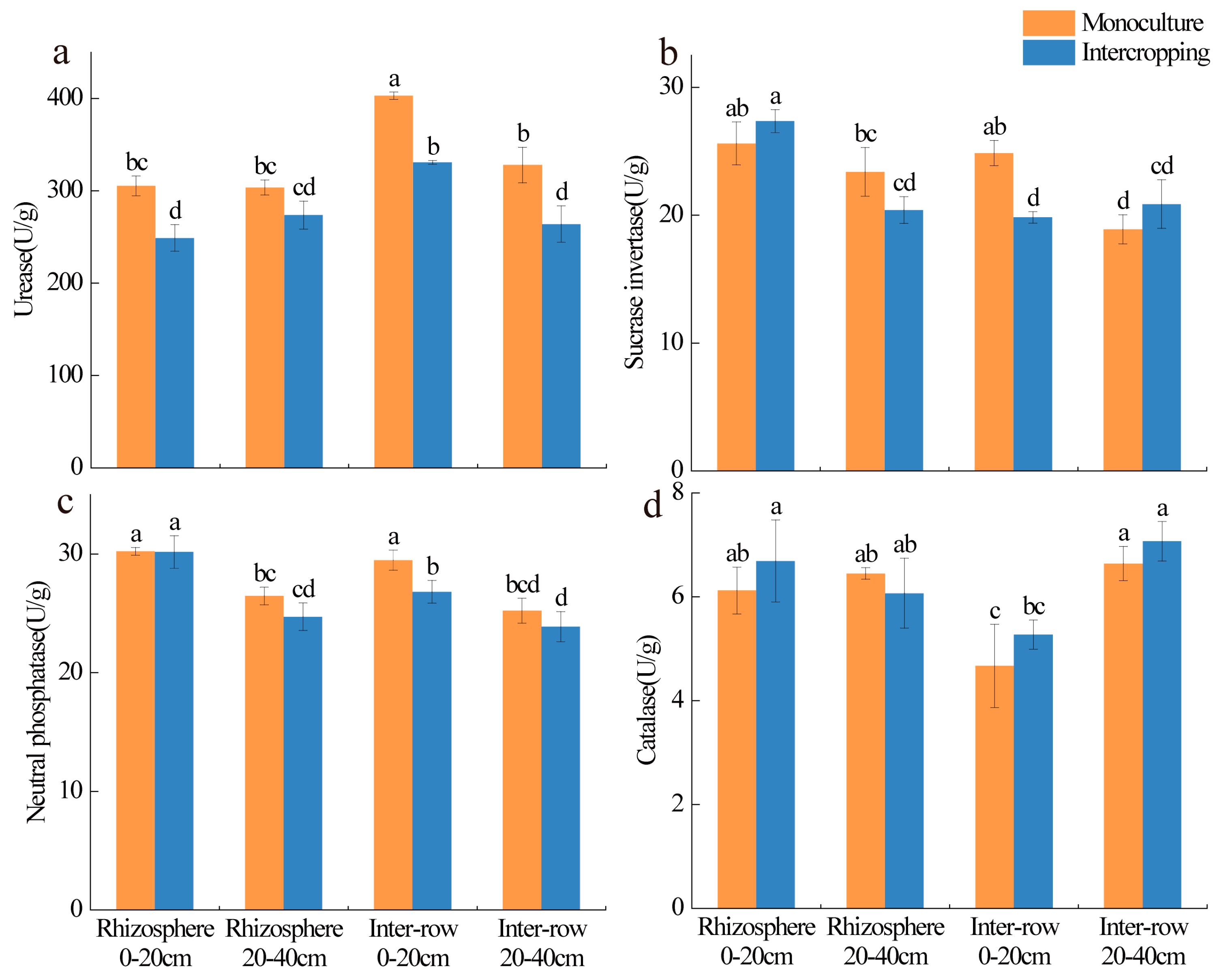
3.2. Soil Properties and Soil Enzymes
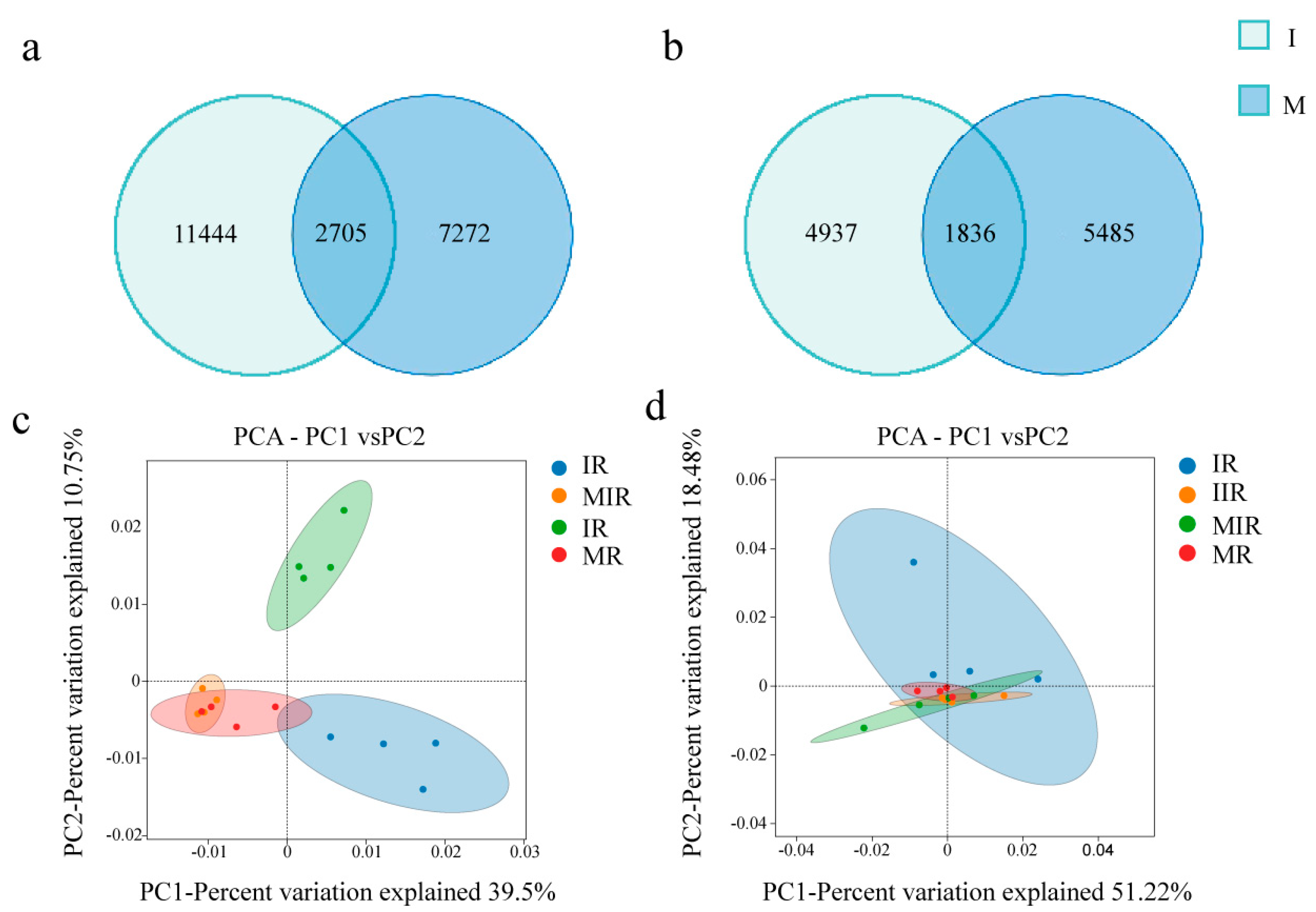
3.3. Soil Microbial Community Structure and Diversity
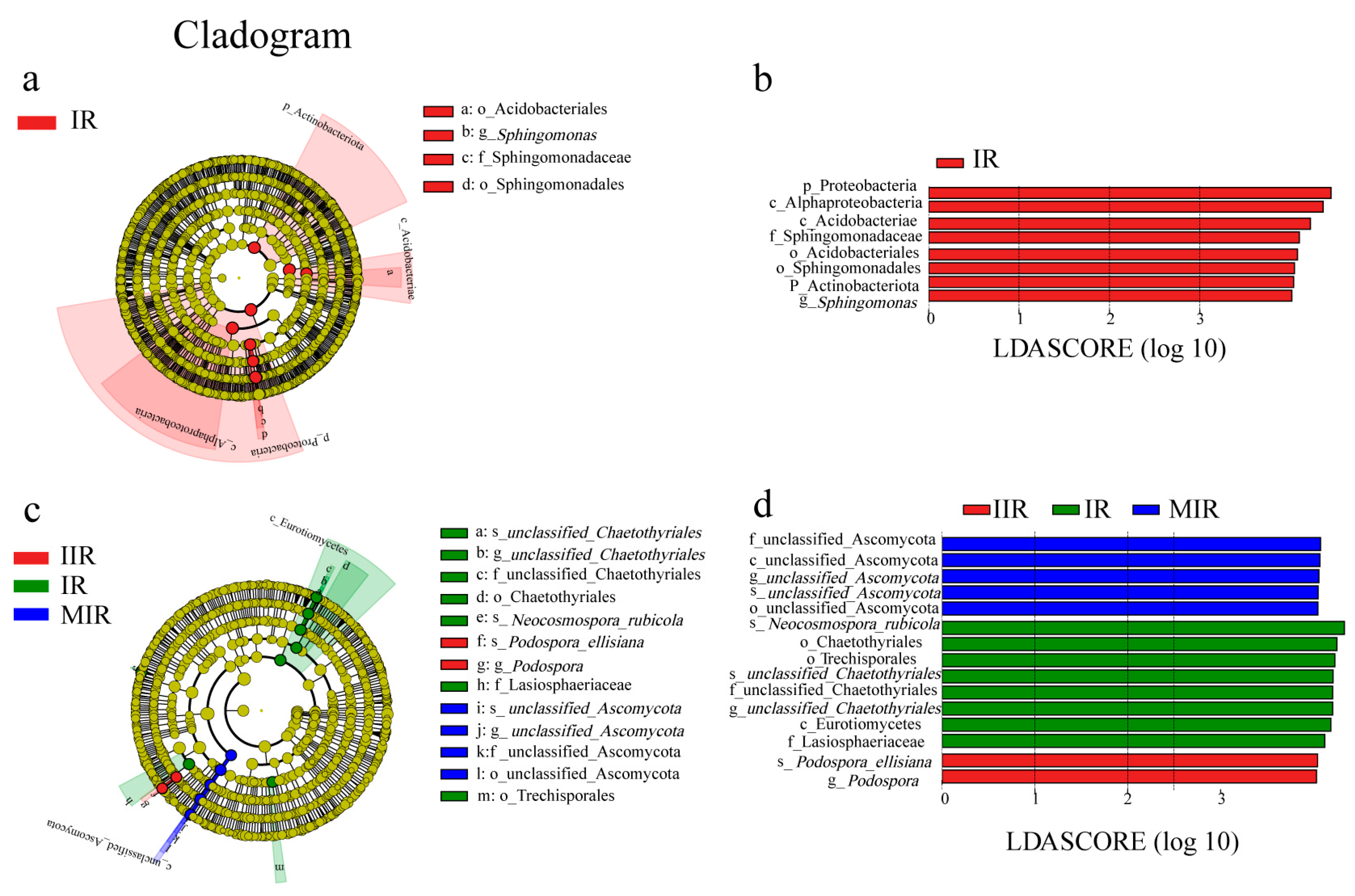
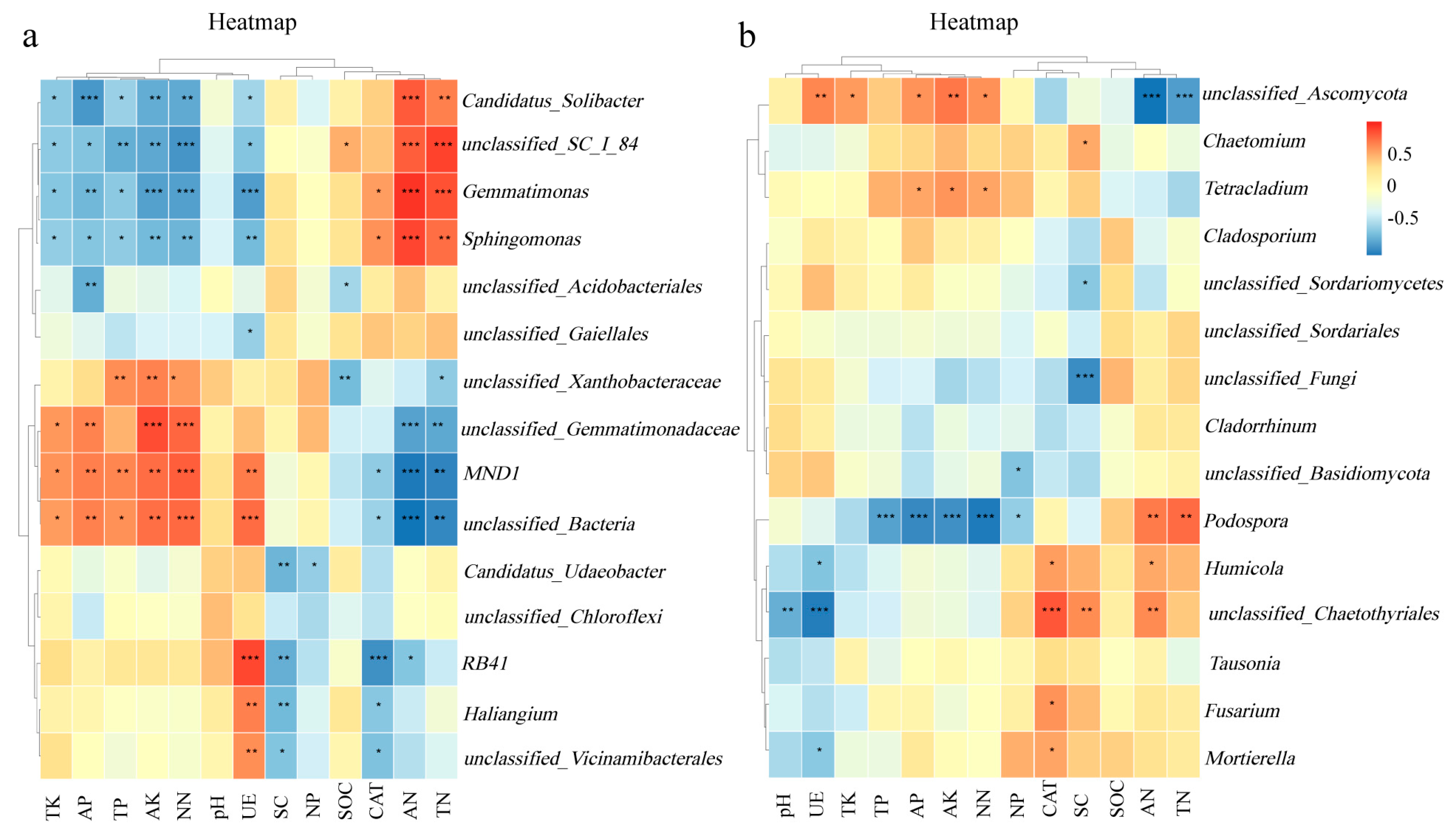
3.4. Composition of Soil Microbial Communities
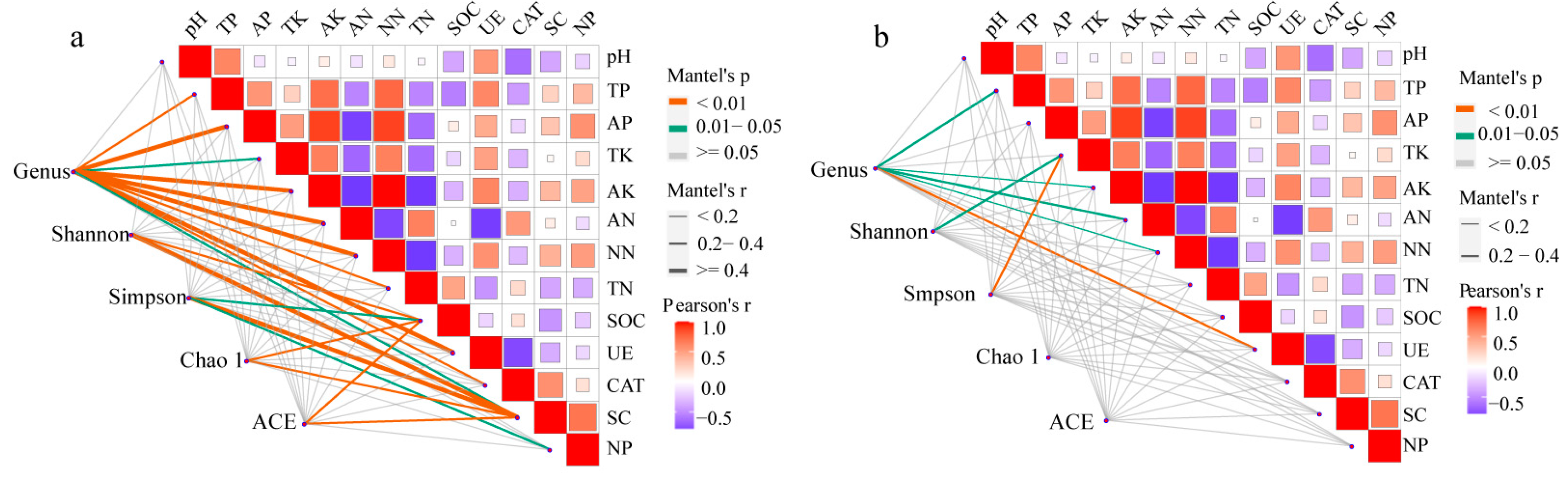
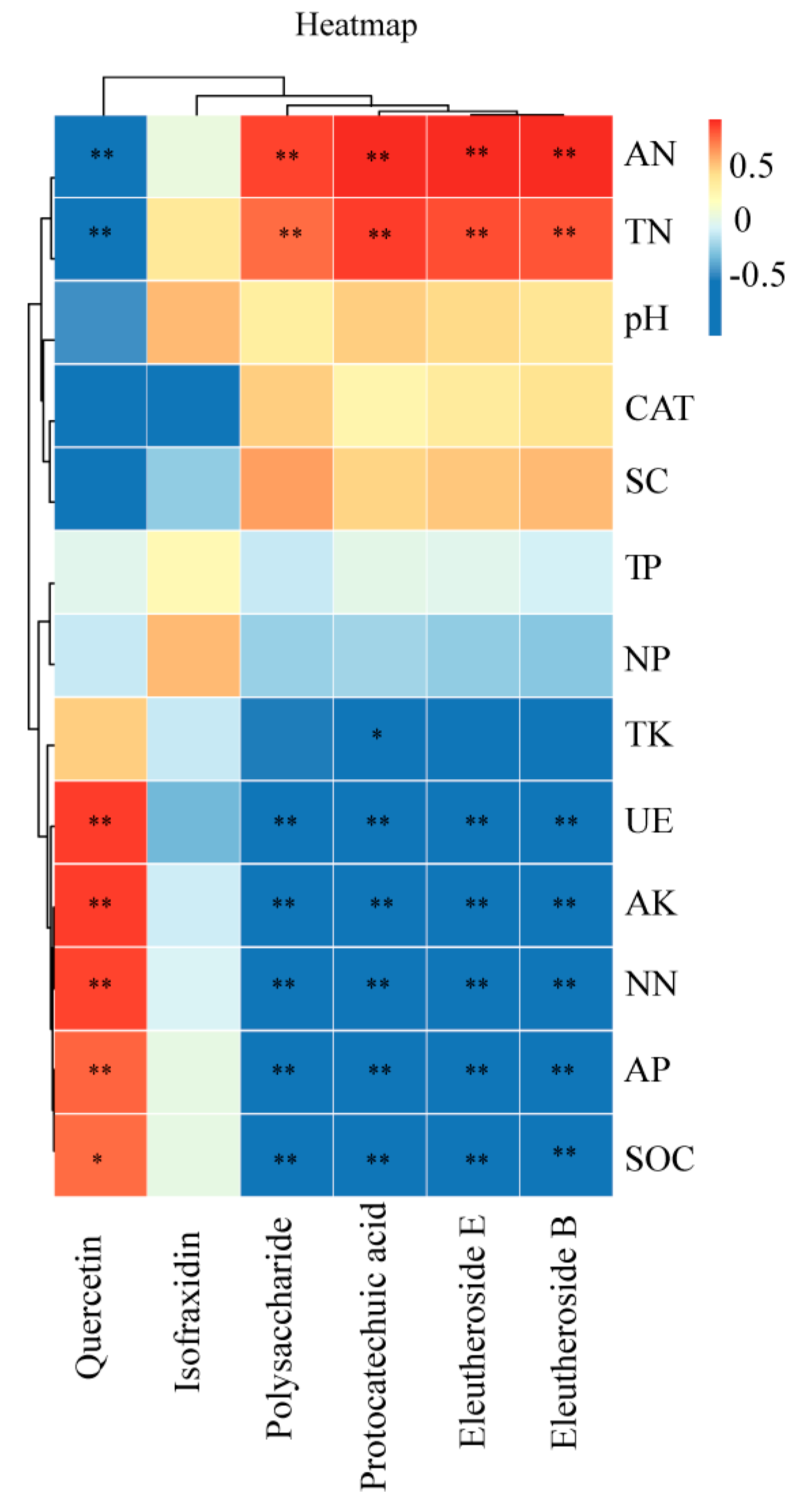
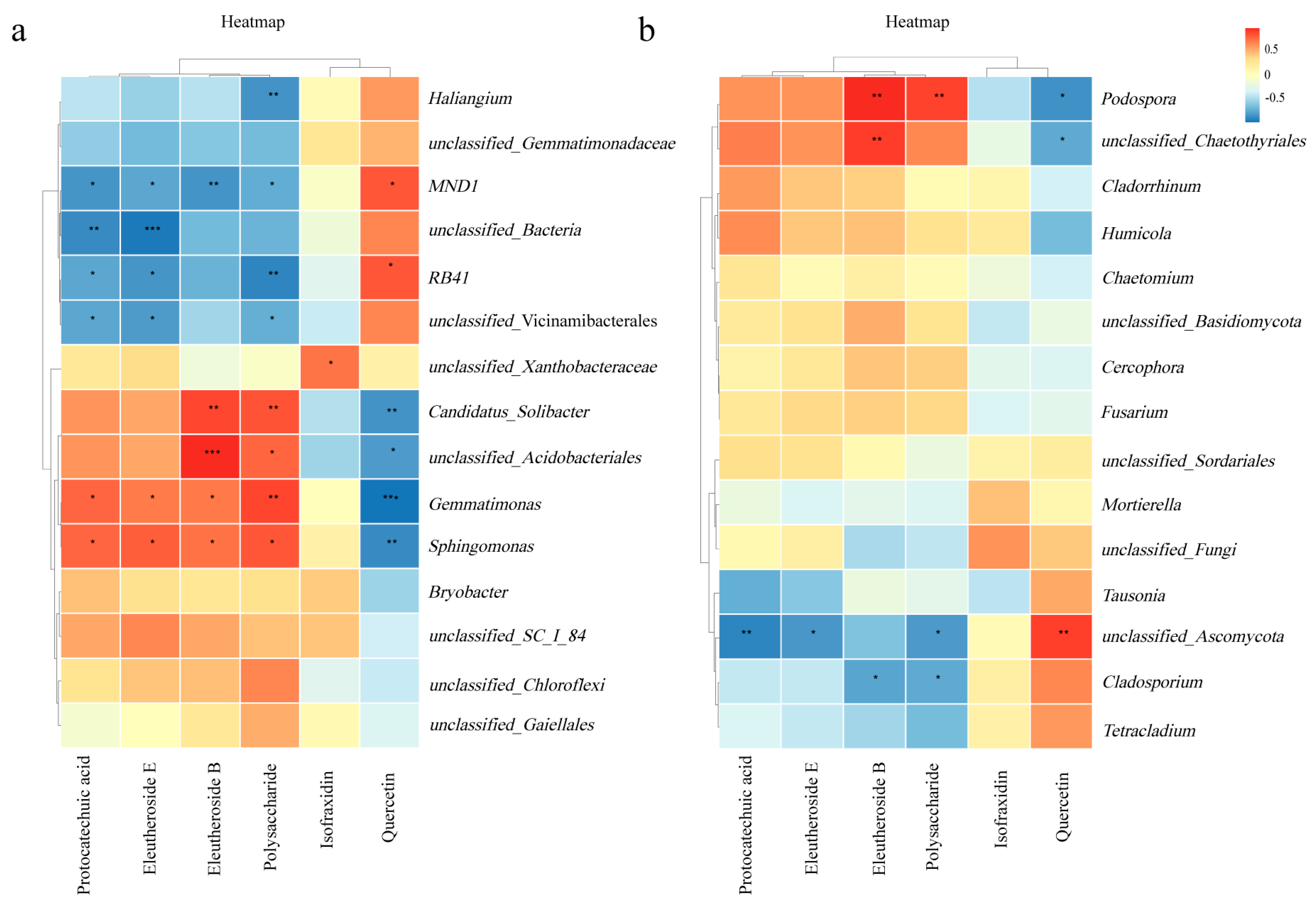
3.5. Correlation Analysis
4. Discussion
4.1. Effect of Intercropping on Soil Properties and Soil Enzyme Activities
4.2. Effect of Intercropping on Soil Microorganisms
4.3. Effect of Intercropping on the Active Ingredients of A. senticosus
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, L.M. Theory and Technology Frontiers of Ecological Planting of Chinese Medicinal Materials. J. Jilin Agric. Univ. 2020, 42, 355–363. [Google Scholar]
- Dong, L.L.; Xu, J.; Li, Y.; Fang, H.L.; Niu, W.H.; Li, X.W.; Zhang, Y.J.; Ding, W.L.; Chen, S.L. Manipulation of microbial community in the rhizosphere alleviates the replanting issues in Panax ginseng. Soil Biol. Biochem. 2018, 125, 64–67. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.Y.; Niu, J.F.; Dang, K.K.; Zhang, S.K.; Wang, S.Q.; Wang, Z.Z. Changes in physicochemical properties, enzymatic activities, and the microbial community of soil significantly influence the continuous cropping of Panax quinquefolius L. (American ginseng). Plant Soil. 2021, 463, 427–446. [Google Scholar] [CrossRef]
- Yuan, Y.D.; Zuo, J.J.; Zhang, H.Y.; Zu, M.T.; Liu, S. The Chinese medicinal plants rhizosphere: Metabolites, microorganisms, and interaction. Rhizosphere 2022, 22, 100540. [Google Scholar] [CrossRef]
- Maitra, S.; Hossain, A.; Brestic, M.; Skalicky, M.; Ondrisik, P.; Gitari, H.; Brahmachari, K.; Shankar, T.; Bhadra, P.; Palai, J.B.; et al. Intercropping-A Low Input Agricultural Strategy for Food and Environmental Security. Agronomy 2021, 11, 343. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Ros, G.H.; Furtak, K.; Iqbal, H.M.N.; Parra-Saldívar, R. Soil carbon sequestration-An interplay between soil microbial community and soil organic matter dynamics. Sci. Total Environ. 2022, 815, 152928. [Google Scholar] [CrossRef] [PubMed]
- Nsabimana, D.; Haynes, R.J.; Wallis, F.M. Size, activity and catabolic diversity of the soil microbial biomass as affected by land use. Appl. Soil Ecol. 2004, 26, 81–92. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Lachouani, P.; Knudsen, M.T.; Ambus, P.; Boelt, B.; Gislum, R. Productivity and carbon footprint of perennial grass-forage legume intercropping strategies with high or low nitrogen fertilizer input. Sci. Total Environ. 2016, 557, 917–918. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, L.J.; Lin, W.F. Calla lily intercropping in rubber tree plantations changes the nutrient content, microbial abundance, and enzyme activity of both rhizosphere and non-rhizosphere soil and calla lily growth. Ind. Crop. Prod. 2019, 132, 344–351. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, X.M.; Tian, X.P.; Yang, T.; Deng, R.; Huang, J. Effects of continuous cropping of Pinellia ternata (Thunb.) Breit. on soil physicochemical properties, enzyme activities, microbial communities and functional genes. Chem. Biol. Technol. Agric. 2021, 8, 43. [Google Scholar] [CrossRef]
- Wang, X.S.; Lian, Y.H.; Guo, H.; Ren, Y.Z.; Xin, Z.Y.; Lin, T.B.; Wang, Z.Q. Effects of wheat/safflower intercropping on rhizosphere microbial community function and structure. Chin. J. Eco-Agric. 2023, 31, 516–529. [Google Scholar]
- Jiaduo, W.; Jiashi, A.; Ma, H.Y.; Ye, G.S.; Chen, C.; Gong, K.J.; Ren, Y.; La, M.J.; Zeng, R. Effect of Different Cultivation Modes on Microbial Diversity and Secondary Metabolites of Rhizosphere Soil of Gentiana crassicaulis. Chin. J. Exp. Tradit. Med. Formulae 2023, 29, 192–201. [Google Scholar]
- Wu, J.X.; Liu, T.; Cun, M.Z.; Xu, Z.Y.; Luo, Z.; Zi, S.H. Effects of Intercropping Maize with Aconitum vilmorinianum Kom. on Rhizosphere Microbial Community Diversity. Mol. Plant Breed. 2024, 22, 2281–2289. [Google Scholar]
- Zhang, J.E.; Gao, A.X.; Xu, H.Q.; Luo, M.Z. Effects of maize/peanut intercropping on rhizosphere soil microbes and nutrient contents. Chin. J. Appl. Ecol. 2009, 20, 1597–1602. [Google Scholar]
- Huang, L.Z.; Huang, B.K.; Ye, Q.; Qin, L.P. Bioactivity-guided fractionation for anti-fatigue property of Acanthopanax senticosus. J. Ethnopharmacol. 2011, 133, 213–219. [Google Scholar] [CrossRef]
- Li, X.J.; Tang, S.Q.; Huang, H.; Luo, J.; Zhang, X.D.; Yook, C.S.; Whang, W.K.; Kim, Y.C.; Liu, X.Q. Acanthopanax henryi: Review of Botany, Phytochemistry and Pharmacology. Molecules 2021, 26, 2215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Li, X.L. The development and utilization of Acanthopanax senticosus. China Food Drug Adm. Mag. 2021, 5, 108–111. [Google Scholar]
- Chamoli, M.; Varshney, V.K.; Srivastava, P.K.; Pandey, R.; Dayal, R. TLC-densitometric evaluation of three major bioactive diterpene lactones in Andrographis paniculata intercropped with Morus alba. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 2258–2274. [Google Scholar] [CrossRef]
- Zeng, J.R.; Liu, J.Z.; Lu, C.H.; Ou, X.H.; Luo, K.K.; Li, C.M.; He, M.L.; Zhang, H.Y.; Yan, H.J. Intercropping with Turmeric or Ginger Reduce the Continuous Cropping Obstacles That Affect Pogostemon cablin (Patchouli). Front. Microbiol. 2020, 11, 579719. [Google Scholar] [CrossRef]
- Li, Y.J.; Ma, J.W.; Li, Y.Q.; Shen, X.Y.; Xia, X.H. Responses of soil microbial community to global climate change: A review. Microbiol. China 2023, 50, 1700–1719. [Google Scholar]
- Wang, Z.L. Optimization of the Extraction Process of the Main Active Components from Acanthopanax senticosus and Their Preliminary Activity Evaluation. Master’s Thesis, Northeast Forestry University, Harbin, China, 2022. [Google Scholar]
- Houba, V.J.G.; Temminghoff, E.J.M.; Gaikhorst, G.A.; van Vark, W. Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun. Soil Sci. Plant Anal. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Wang, Z.L.; Pan, H.Y.; Xu, J.; Chang, Y.H.; Liu, C.; Zhang, Y.; Yang, H.; Duan, C.J.; Huang, J.; Fu, Y.J. A sustainable and integrated natural surfactant mediated microwave-assisted extraction technique enhances the extraction of phytochemicals from plants. Ind. Crop. Prod. 2022, 184, 115043. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Y.Y.; Sun, C.W.; Gao, M.; He, Y.P.; Wang, J.H. Effects of different manure treatments on heavy metals content (Cr, Cu, Pb and Zn) and their availability in ginseng cultivated soil. J. Jilin Agric. Univ. 2011, 33, 411–417. [Google Scholar]
- Xu, Y.B.; Qiu, W.W.; Sun, J.P.; Christoph, M.; Lei, B.K. Effects of wheat/faba bean intercropping on soil nitrogen transformation processes. J. Soils Sediments 2018, 19, 1724–1734. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Wang, Q.Z.; Sun, Z.M.; Niu, S.B.; Liu, J.; Ma, W.Q.; Yang, X.Z. Effects of yam/leguminous crops intercropping on soil chemical and biological properties of yam field. Chin. J. Appl. Ecol. 2018, 29, 4071–4079. [Google Scholar]
- Haichar, F.E.; Marol, C.; Berge, O.; Rangel-Castro, J.I.; Prosser, J.I.; Balesdent, J.; Heulin, T.; Achouak, W. Plant host habitat and root exudates shape soil bacterial community structure. ISME J. 2008, 2, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yan, P.D.; Liu, Z.Y.; Zhang, J.L.; Zhang, G.Y.; Cui, L.J. Strip intercropping with local crops increased Aconitum carmichaeli yield and soil quality. Front. Plant Sci. 2023, 14, 1147671. [Google Scholar] [CrossRef] [PubMed]
- Cong, R.H.; Wang, X.J.; Xu, M.G.; Zhang, W.J.; Xie, L.J.; Yang, X.Y.; Huang, S.M.; Wang, B.R. Dynamics of soil carbon to nitrogen ratio changes under long-term fertilizer addition in wheat-corn double cropping systems of China. Eur. J. Soil Sci. 2012, 63, 341–350. [Google Scholar] [CrossRef]
- Zhu, L.Z.; He, J.; Tian, Y.; Li, X.Y.; Li, Y.H.; Wang, F.; Qin, K.; Wang, J. Intercropping Wolfberry with Gramineae plants improves productivity and soil quality. Sci. Hortic. 2021, 292, 110632. [Google Scholar] [CrossRef]
- Jing, Y.L.; Zhang, Y.H.; Han, I.; Wang, P.; Mei, Q.W.; Huang, Y.J. Effects of different straw biochars on soil organic carbon, nitrogen, available phosphorus, and enzyme activity in paddy soil. Sci. Rep. 2020, 10, 8837. [Google Scholar] [CrossRef]
- Chen, F.H.; Xie, Y.J.; Jia, Q.W.; Li, S.Y.; Li, S.Y.; Shen, N.K.; Jiang, M.G.; Wang, Y.B. Effects of the Continuous Cropping and Soilborne Diseases of Panax Ginseng C. A. Meyer on Rhizosphere Soil Physicochemical Properties, Enzyme Activities, and Microbial Communities. Agronomy 2023, 13, 210. [Google Scholar] [CrossRef]
- Curtright, A.J.; Tiemann, L.K. Intercropping increases soil extracellular enzyme activity: A meta-analysis. Agric. Ecosyst. Environ. 2021, 319, 107489. [Google Scholar] [CrossRef]
- Sun, N.; Liu, J.Y.; Yu, W.Y.; Liu, S.S.; Ren, Y.L.; Fang, K.X.; Shu, P.H. Research progress on chemical constituents and biological activities of Arisaematis Rhizoma. China J. Chin. Mater. Med. 2021, 46, 5194–5220. [Google Scholar]
- Zhang, H.L.; Wang, R.S.; Gao, J.H.; Zhang, W.; Zhang, G.C.; Song, C.J.; Song, C.H. Inhibition effect of four kinds of low-toxin insecticides to Cytospora chrysosperma. J. Northeast For. Univ. 2010, 38, 110–111. [Google Scholar]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Hang, Y.; Luo, F.L.; Tang, C.L.; Lin, H.; Li, Q.W.; Deng, C. Effect of Pinellia ternata with Different Intercropping Crops on Soil Microorganism, Nutrient and Enzyme Activity. J. Chin. Med. Mater. 2018, 41, 1522–1528. [Google Scholar]
- Gong, X.W.; Liu, C.J.; Li, J.; Luo, Y.; Yang, Q.H.; Zhang, W.L.; Yang, P.; Feng, B.L. Responses of rhizosphere soil properties, enzyme activities and microbial diversity to intercropping patterns on the Loess Plateau of China. Soil Tillage Res. 2019, 195, 104355. [Google Scholar] [CrossRef]
- Duchene, O.; Vian, J.F.; Celette, F. Intercropping with legume for agroecological cropping systems: Complementarity and facilitation processes and the importance of soil microorganisms. A review. Agric. Ecosyst. Environ. 2017, 240, 148–161. [Google Scholar] [CrossRef]
- Diakhaté, S.; Gueye, M.; Chevallier, T.; Diallo, N.H.; Assigbetse, K.; Abadie, J.; Diouf, M.; Masse, D.; Sembène, M.; Ndour, Y.B.; et al. Soil microbial functional capacity and diversity in a millet-shrub intercropping system of semi-arid Senegal. J. Arid. Environ. 2016, 129, 71–79. [Google Scholar] [CrossRef]
- Mouhamadou, B.; Puissant, J.; Personeni, E.; Desclos-Theveniau, M.; Kastl, E.M.; Schloter, M.; Zinger, L.; Roy, J.; Geremia, R.A.; Lavorel, S. Effects of two grass species on the composition of soil fungal communities. Biol. Fertil. Soils 2013, 49, 1131–1139. [Google Scholar] [CrossRef]
- Ali, A.; Shaheen, S.M.; Guo, D.; Li, Y.M.; Xiao, R.; Wahid, F.; Azeem, M.; Sohail, K.; Zhang, T.; Rinklebe, J.; et al. Apricot shell- and apple tree-derived biochar affect the fractionation and bioavailability of Zn and Cd as well as the microbial activity in smelter contaminated soil. Environ. Pollut. 2020, 264, 114773. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.N.; Yao, S.; Yang, X.L.; Jiang, X. Correlations between soil metabolomics and bacterial community structures in the pepper rhizosphere under plastic greenhouse cultivation. Sci. Total Environ. 2020, 728, 138439. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Huang, J.; Lu, J.; Sun, Y. Study on the influence of soil microbial community on the long-term heavy metal pollution of different land use types and depth layers in mine. Ecotoxicol. Environ. Saf. 2019, 170, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, F.Z. Diversity and Co-occurrence Patterns of Soil Bacterial and Fungal Communities in Seven Intercropping Systems. Front. Microbiol. 2018, 9, 1521. [Google Scholar] [CrossRef] [PubMed]
- Li, N.H.; Gao, D.M.; Zhou, X.G.; Chen, S.C.; Li, C.X.; Wu, F.Z. Intercropping with Potato-Onion Enhanced the Soil Microbial Diversity of Tomato. Microorganisms 2020, 8, 834. [Google Scholar] [CrossRef]
- Fontaine, S.; Henault, C.; Aamor, A.; Bdioui, N.; Bloor, J.M.G.; Maire, V.; Mary, B.; Revaillot, S.; Maron, P.A. Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol. Biochem. 2011, 43, 86–96. [Google Scholar] [CrossRef]
- Guo, L.P.; Zhou, L.Y.; Kang, C.Z.; Wang, H.Y.; Zhang, W.J.; Wang, S.; Wang, R.S.; Wang, X.; Han, B.X.; Zhou, T.; et al. Strategies for medicinal plants adapting environmental stress and “simulative habitat cultivation” of Dao-di herbs. China J. Chin. Mater. Med. 2020, 45, 1969–1974. [Google Scholar]
- Jaakola, L.; Hohtola, A. Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef]
- Wang, W.T.; Hu, S.Y.; Zhang, C.J.; Yang, J.; Zhang, T.; Wang, D.H.; Cao, X.Y.; Wang, Z.Z. Systematic Analysis and Functional Characterization of R2R3-MYB Genes in Scutellaria baicalensis Georgi. Int. J. Mol. Sci. 2022, 23, 9342. [Google Scholar] [CrossRef]
- Mañero, F.J.G.; Algar, E.; Gómez, M.S.M.; Sierra, M.D.S.; Solano, B.R. Elicitation of secondary metabolism in Hypericum perforatum by rhizosphere bacteria and derived elicitors in seedlings and shoot cultures. Pharm. Biol. 2012, 50, 1201–1209. [Google Scholar] [CrossRef]


| Treatment | Protocatechuic Acid μg/g | Eleutheroside E μg/g | Isofraxidin μg/g | Quercetin μg/g | Eleutheroside B μg/g | Polysaccharide mg/g |
|---|---|---|---|---|---|---|
| monoculture | 103.23 ± 0.42 b | 36.92 ± 0.84 b | 21.07 ± 0.13 a | 0.135 ± 0.004 b | 0.79 ± 0.05 b | 11.27 ± 0.34 b |
| intercropping | 131.84 ± 0.64 a | 56.38 ± 0.36 a | 21.08 ± 0.41 a | 0.148 ± 0.001 a | 5.14 ± 0.05 a | 14.20 ± 0.73 a |
| 0–20 cm Soil A. senticosus Monocropping | A. senticosus/A. amurense Intercropping | 20–40 cm Soil A. senticosus Monocropping | A. senticosus/A. amurense Intercropping | |||||
|---|---|---|---|---|---|---|---|---|
| Rhizosphere | Inter-Row | Rhizosphere | Inter-Row | Rhizosphere | Inter-Row | Rhizosphere | Inter-Row | |
| pH | 6.23 ± 0.26 cd | 6.62 ± 0.14 a | 6.38 ± 0.15 abc | 6.50 ± 0.14 ab | 6.16 ± 0.07 d | 6.25 ± 0.02 cd | 6.31 ± 0.04 bcd | 6.42 ± 0.02 abc |
| TN (g/kg) | 1.54 ± 0.06 b | 1.39 ± 0.01 b | 2.05 ± 0.25 a | 2.29 ± 0.59 a | 1.26 ± 0.03 b | 1.26 ± 0.04 b | 1.35 ± 0.01 b | 1.18 ± 0.01 b |
| TP (g/kg) | 0.92 ± 0.07 ab | 1.04 ± 0.03 a | 0.93 ± 0.01 ab | 0.89 ± 0.04 ab | 0.90 ± 0.10 ab | 0.87 ± 0.08 b | 0.88 ± 0.09 ab | 0.84 ± 0.08 b |
| TK (g/kg) | 51.41 ± 1.94 ab | 52.27 ± 1.96 ab | 48.74 ± 2.16 b | 49.57 ± 2.38 ab | 53.46 ± 2.11 a | 51.60 ± 2.02 ab | 49.29 ± 2.11 ab | 49.92 ± 1.21 ab |
| SOC (g/kg) | 15.10 ± 0.66 a | 12.66 ± 0.34 b | 13.13 ± 0.37 b | 10.91 ± 1.34 c | 10.73 ± 0.48 c | 10.60 ± 0.58 c | 10.22 ± 0.09 c | 9.30 ± 0.03 d |
| AK (mg/kg) | 301.69 ± 5.43 b | 350.31 ± 3.73 a | 199.75 ± 2.02 e | 169.95 ± 5.94 f | 214.97 ± 6.59 d | 264.15 ± 6.05 c | 166.59 ± 5.22 f | 134.86 ± 4.12 e |
| AP (mg/kg) | 73.07 ± 4.30 a | 69.47 ± 1.29 a | 53.07 ± 3.07 cd | 52.53 ± 6.05 cd | 64.80 ± 5.00 ab | 58.47 ± 2.00 bc | 45.20 ± 6.63 de | 38.93 ± 1.40 e |
| AN (mg/kg) | 9.44 ± 1.66 cd | 6.61 ± 1.32 d | 21.07 ± 0.95 a | 14.81 ± 1.73 b | 14.73 ± 1.22 b | 7.15 ± 1.01 d | 12.62 ± 2.08 bc | 21.54 ± 2.48 a |
| NN (mg/kg) | 35.52 ± 2.34 b | 42.73 ± 1.26 a | 20.14 ± 0.81 d | 13.31 ± 1.95 e | 28.65 ± 0.68 c | 28.83 ± 0.82 c | 27.73 ± 0.42 c | 13.11 ± 0.30 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Zhang, Y.; Shao, C.; Lv, B.; Liang, H.; Cao, W.; Zhang, G.; Sun, H. Influence of Intercropping Arisaema amurense with Acanthopanax senticosus on Soil Microbial Community and the Effective Ingredients of A. senticosus. Horticulturae 2024, 10, 592. https://doi.org/10.3390/horticulturae10060592
Zhu J, Zhang Y, Shao C, Lv B, Liang H, Cao W, Zhang G, Sun H. Influence of Intercropping Arisaema amurense with Acanthopanax senticosus on Soil Microbial Community and the Effective Ingredients of A. senticosus. Horticulturae. 2024; 10(6):592. https://doi.org/10.3390/horticulturae10060592
Chicago/Turabian StyleZhu, Jiapeng, Yayu Zhang, Cai Shao, Bochen Lv, Hao Liang, Weiyu Cao, Guojia Zhang, and Hai Sun. 2024. "Influence of Intercropping Arisaema amurense with Acanthopanax senticosus on Soil Microbial Community and the Effective Ingredients of A. senticosus" Horticulturae 10, no. 6: 592. https://doi.org/10.3390/horticulturae10060592
APA StyleZhu, J., Zhang, Y., Shao, C., Lv, B., Liang, H., Cao, W., Zhang, G., & Sun, H. (2024). Influence of Intercropping Arisaema amurense with Acanthopanax senticosus on Soil Microbial Community and the Effective Ingredients of A. senticosus. Horticulturae, 10(6), 592. https://doi.org/10.3390/horticulturae10060592






