Abstract
The productivity of many tree crops is limited by low yield, partly due to high rates of fruitlet abscission during early fruit development. Early studies suggested that cross-pollinated fruitlets may be selectively retained during fruit development, although paternity testing of fruitlets to test this hypothesis was technically challenging. We used MassARRAY genotyping to determine the effects of pollen parentage on fruitlet retention and fruit quality of Hass avocado. We identified the paternity of abscised and retained fruitlets at 6 and 10 weeks, and mature fruit at 36 weeks, after peak anthesis. We measured the embryo mass, pericarp mass, total mass and nutrient concentrations of fruitlets, and the seed mass, flesh mass, total mass, diameter, length, nutrient concentrations and fatty-acid composition of mature fruit. The percentages of progeny on the tree that were cross-fertilised increased from 4.6% at 6 weeks after peak anthesis to 10.7% at fruit maturity. Only 1.0% of freshly abscised fruitlets on the ground at 10 weeks after peak anthesis were cross-pollinated even though 6.5% of retained fruitlets on the tree were cross-pollinated. At this stage, cross-pollinated fruitlets had similar nutrient concentrations to self-pollinated fruitlets, but they had higher total contents of P, K, Al, Ca, Fe, Mn and Zn due to having greater fruitlet mass. At maturity, cross-pollinated fruit were 6% heavier and had 2% greater diameter than self-pollinated fruit, without significant differences in flesh nutrient concentrations or fatty acid composition. The results demonstrate that Hass avocado trees selectively retain cross-pollinated fruitlets, which are larger than self-pollinated fruitlets and ultimately produce larger mature fruit. Avocado growers can increase fruit size and yield by improving the opportunities for cross-pollination, possibly by closely interplanting type A and type B cultivars and introducing more beehives into orchards.
1. Introduction
Tree crops contribute about 10% of global food production and they provide many fruits and nuts of high nutritional value [1,2]. High tree-crop yields can increase the total production of nutrient-rich food and strengthen food security [3]. However, tree yields can be limited by premature fruit drop, with many fruitlets abscising before they can reach maturity [4,5,6,7,8,9]. Premature fruit drop is often observed in mass-flowering tree crops even after successful pollination [8,9,10].
Selective abscission of inferior genotypes could be a cause of premature fruit abscission in tree crops [4,10]. Self-pollinated fruitlets can be shed selectively during the period of premature fruit drop due to either late-acting self-incompatibility or inbreeding depression [11,12,13,14,15]. Late-acting self-incompatibility is a genetic self-rejection mechanism that prevents the growth of self-pollen tubes from the lower style into the ovule or that arrests embryo development following self-fertilisation [13,14,15,16]. Self-fertilised embryos may also experience inbreeding depression due to an excess of non-lethal, deleterious, recessive alleles that results in slower development and poor capacity to compete with larger embryos [4,12,17]. Cross-fertilised fruitlets may outcompete self-pollinated fruitlets for maternal resources, allowing the cross-pollinated fruitlets to be retained selectively on trees until maturity [4,10,11]. Self-pollinated cashew, lychee and mango fruitlets appear to abscise at higher rates than cross-pollinated fruitlets [5,6,8,18,19]. However, many early studies could not definitively determine embryo paternity, especially during early fruitlet development.
The genetic contribution from the pollen parent can affect the “discernible characteristics, especially of size, color, shape, chemical composition, and developmental timing, of parts of seeds and other fruits, including the embryo, endosperm, and maternal tissues”, a phenomenon termed “xenia” [20]. Thus, pollen-parent effects are not confined to the early stages of endosperm and embryo development but can also result in differences in the final size or quality of fruit [20,21]. Cross-pollinated mature fruit often have greater final mass, firmness, skin colour, sugar content, or oil content than self-pollinated mature fruit [8,22,23,24,25,26]. However, the influence of embryo paternity on early fruitlet growth and how this is translated into final fruit size has not been studied in most tropical crops.
Avocado is a mass-flowering tree crop that produces mature fruit from less than 1% of its flowers [27,28,29,30,31,32]. Many avocado fruitlets abscise during the first two months after peak flowering [28,29,32,33]. Avocado flowers are hermaphrodite but functionally unisexual, with a separation of the female and male phases within the one flower that functions to reduce the incidence of self-pollination [28,34,35]. The flowers of Type A cultivars such as Hass often open as females on the morning of one day, close for the afternoon and the following morning, and re-open as males in the afternoon [28,34,35]. The flowers of Type B cultivars such as Shepard often open as females in the afternoon, close overnight, and re-open as males for the morning of the next day [28,34,35]. This alternation of genders between Type A and Type B avocado cultivars can promote cross-pollination (i.e., pollination between cultivars) as there is a long overlap each day between the female-phase flowers of one cultivar and the male-phase flowers of alternate-type cultivars [28,34,35]. Avocado flowers are not autogamous (i.e., they are not capable of self-pollination within the same flower without a pollen vector). However, there can be a short overlap each day between female-phase and male-phase flowers within the one cultivar, allowing self-pollination (i.e., pollination within the same clonally propagated cultivar) by pollinators such as honeybees [28,34,35]. Avocado flowers are self-compatible; thus, they can set fruit through either self-pollination or cross-pollination [29,36,37,38,39,40]. Early studies on premature fruit drop in avocado using isozyme methods indicated that cross-pollinated fruitlets were selectively retained over self-pollinated fruitlets [40,41,42]. These early studies often could not definitely determine paternity, especially in small fruitlets, due to limitations of the existing technology. However, later research using DNA-microsatellite methods confirmed that self-fertilised fruitlets were selectively abscised, possibly because they were fertilised late in the flowering season and were, therefore, smaller than cross-fertilised fruitlets [43]. The percentage of cross- and self-pollinated avocado fruitlets during early fruit development and how paternity affects fruitlet retention during the early stages of fruit set require further study, especially for the most widely grown cultivar, Hass.
In addition, xenia effects have been observed on avocado fruit-quality characteristics including whole-fruit mass, flesh mass, calcium concentration and fatty acid composition [33,36,37,39,40]. Cross-fertilisation of Fuerte by Ettinger, Teague, or Topa-Topa increases whole-fruit mass and pericarp mass by 9–17% and 5–13%, respectively, when compared with self-fertilisation [36]. Cross-fertilisation of Hass by Shepard can increase whole-fruit mass by 12% [37] and flesh calcium concentration by 10% [39] while reducing the ratio of unsaturated to saturated fatty acids by 4% [39]. However, there is little understanding of paternity effects on the size and nutrient concentrations of avocado fruitlets during premature fruit drop, and whether these effects may be related to selective abscission of self-pollinated fruitlets. A better understanding of embryo paternity and fruitlet development may help to distinguish between late-acting self-incompatibility and inbreeding depression as the underlying causes of premature fruit drop from avocado trees.
Here, we aimed to determine how embryo paternity influences fruitlet retention and fruit quality in Hass avocado. We used novel markers, based on single nucleotide polymorphisms (SNPs) identified by high-throughput genotyping [39,44], to assign paternity to embryos. Specifically, we assessed: (a) whether the percentages of progeny in the tree that were cross-fertilised changed during fruit development; (b) whether there were differences in the percentages of progeny that were cross-fertilised among the abscised fruitlets vs. the fruitlets that were retained on the tree; and (c) how embryo paternity affects the size and nutrient composition of the fruitlets and mature fruit. We hypothesised that cross-fertilised fruitlets would be retained selectively during the period of premature fruit drop and that embryo paternity would affect the size and nutrient composition of the fruitlets and mature fruit.
2. Materials and Methods
2.1. Study Site
The experiment was conducted in Eastridge avocado orchard (25°13′25″ S 152°18′54″ E), near Childers, Queensland, Australia. The orchard contained cultivars Hass, Maluma Hass and Shepard in large single-cultivar blocks [37]. We selected 30 trees in the 82nd and 83rd rows of a 132-row-wide block of Hass trees, which were planted 5 years prior to the experiment, as described previously [29]. The nearest other cultivar was a block of Shepard trees at the northern end of the Hass rows, approximately 190 m from the experimental trees. The experimental trees were part of a boron-fertiliser experiment, with 30 experimental trees located in ten plots, with each of the three experimental trees within a plot being allocated randomly to one of three boron treatments (0, 15, or 30 g B per tree). The boron treatments had no significant effect on fruit paternity [29].
2.2. Experimental Design and Sample Processing
Peak anthesis in the experimental trees occurred in late August 2018. We collected ten retained fruitlets from one side of each tree, selecting five fruitlets from the outside of the canopy and five fruitlets from the inside of the canopy, at each of 6 and 10 weeks after peak anthesis; i.e., 10 fruitlets per tree × 30 trees × 2 time points = 600 retained fruitlets from the canopy. We also collected ten freshly-abscised fruitlets under each tree, selecting five fruitlets close to the tree trunk and five distant from the trunk, at each of 6 and 10 weeks after peak anthesis; i.e., 10 fruitlets per tree × 30 trees × 2 time points = 600 freshly-abscised fruitlets from the ground. All fruitlets were stored at −18 °C until further processing. We harvested 16 mature fruit per tree at 36 weeks after peak anthesis using a stratified sampling design, with each tree divided into eight sectors, four on each side of the canopy. Two fruit were sampled per sector, one from the outside and one from the inside of the canopy; i.e., 16 fruit per tree × 30 trees = 480 mature fruit. Mature fruit were stored in a cold room at 4 °C until further processing.
We weighed all fruitlets that were collected at 6 and 10 weeks after peak anthesis. The fruitlets were then dissected longitudinally into two halves (Figure 1). We measured the mass of the following sub-samples from retained fruitlets collected from the canopy: (1) total embryo, possibly with endosperm, hereafter referred to as the embryo (E1 + E2); (2) a combined half-fruitlet comprising pericarp and embryo (M1 + E1); and (3) the remaining half of the pericarp without embryo (M2).

Figure 1.
A dissected Hass avocado fruitlet at 10 weeks after peak anthesis, with E1 and E2 (embryo, possibly including endosperm), and M1 and M2 (maternal tissue).
We randomly selected 8 of the 16 mature fruit per tree after 7–21 days at 4 °C, weighed each fruit individually, and recorded length and diameter. Each fruit was dissected, and the flesh (without skin) and the seed were weighed. The other eight mature fruit per tree were stored for a total of 22–28 days at 4 °C and then stored at 21 °C until ripe. Ripeness was measured using an FR-5120 fruit hardness tester with an 8 mm head (Lutron Electronic, Taipei, Taiwan). A fruit was considered ripe if the maximum force required to press the 8 mm head of the hardness tester approximately 1 mm deep was less than 20 N. We measured the length, diameter and mass of each ripe fruit, then dissected the fruit and weighed the flesh (without skin) and the seed.
2.3. Paternity Analysis
We used one half of the embryo (E2) of each retained fruitlet and each abscised fruitlet collected at 6 and 10 weeks after peak anthesis, and approximately 70 mg fresh mass of each mature seed, for genotyping. DNA extraction from embryos and mature seeds followed the glass-fibre plate DNA extraction protocol for plants [45]. We added disposable 2.3 mm and 0.1 mm zirconia/silica beads and liquid nitrogen to the samples prior to shaking on a TissueLyser II (Qiagen, Hilden, Germany). Multiplex PCR reactions were conducted for each extracted DNA sample to amplify regions with unique homozygous SNPs (Table 1) that we identified from ten avocado cultivars [39,44]. High-throughput genotyping was performed using the Agena MassARRAY platform (Agena Bioscience, San Diego, CA, USA) to assign paternity using 28 recently designed assays for avocado [39,44].

Table 1.
Examples of 8 of the 52 DNA sequences used for identification of pollen parents by MassARRAY analysis of unique homozygous SNPs from each avocado cultivar [44].
2.4. Mineral Nutrient Analysis
We used the following subsamples to analyse mineral nutrient concentrations: (1) half-fruitlets collected from the canopy, comprising the pericarp and embryo (M1 + E1); (2) representative subsamples of at least 300 mg from the seed of each ripe fruit; and (3) representative subsamples of at least 300 mg from the flesh of each ripe fruit. Nitrogen (N) concentrations in the seed and the flesh of ripe fruit were determined by combustion analysis using a LECO CNS 928 analyser (TruSpec®, LECO Corporation, St. Joseph, MI, USA) [46,47]. Aluminium (Al), boron (B), calcium (Ca), iron (Fe), magnesium (Mg), manganese (Mn), phosphorus (P), potassium (K), sodium (Na), sulphur (S) and zinc (Zn) concentrations in fruitlets and the seed and flesh of ripe fruit were analysed by inductively coupled plasma–optical emission spectroscopy (Vista Pro®, Varian Incorporation, Palo Alto, CA, USA) after digestion with a 5:1 mixture of nitric and perchloric acids [48,49].
We calculated the total content of each mineral nutrient in 6- and 10-week-old fruitlets using Equation (1):
where [M1 + E1] was the nutrient concentration in subsample M1 + E1.
Nutrient content in total fruitlet = [M1 + E1] × total fruitlet mass
The contents of each nutrient in the seed (Contentseed), flesh (Contentflesh) and whole mature fruit without skin (Contentfruit) were calculated using Equations (2)–(4):
where [Seed] and [Flesh] were the nutrient concentrations, and Massseed and Massflesh were the masses, of the seed and the flesh of mature fruit, respectively.
Contentseed = [Seed] × Massseed
Contentflesh = [Flesh] × Massflesh
Contentfruit = Contentseed + Contentflesh
2.5. Fatty Acid Analysis
We finely mashed one half of the flesh from each of the eight ripe fruit per tree to extract oil. Fatty acid methyl esters were derivatised from the extracted oil, and fatty acid composition was determined by gas chromatography–mass spectrometry (PerkinElmer Clarus 580 GC coupled to a PerkinElmer Clarus SQ8S MS) using methods described previously [50,51]. Quantitation of each compound was via integration of the peak area on the total ion current chromatogram. We calculated the relative abundance of individual fatty acids in each sample by dividing the peak area of each individual fatty acid by the total peak area of all fatty acids in the sample and multiplying by 100%.
2.6. Statistical Analysis
We compared the percentages of retained progeny that were cross-pollinated at 6 weeks after peak anthesis vs. 10 weeks after peak anthesis vs. maturity (36 weeks after peak anthesis) using a generalised linear mixed model (GLMM) with a Gaussian distribution and identity link function. Time (6 vs. 10 vs. 36 weeks after peak anthesis), boron treatment and plot were included as fixed effects. We also compared the percentages of progeny at 6 and 10 weeks after peak anthesis that were cross-pollinated among the abscised fruitlets on the ground vs. the retained fruitlets in the canopy using generalised linear models (GLMs) with a Gaussian distribution and identity link function. Sample location (ground or canopy), boron treatment and plot were included as fixed effects. We analysed pollen-parent effects on (1) embryo mass, pericarp mass and total mass of fruitlets at 6 and 10 weeks after peak anthesis; (2) diameter, length, seed mass, flesh mass and total mass of mature fruit; (3) nutrient levels in fruitlets, seeds, flesh or whole fruit; and (4) relative abundances of fatty acids in flesh, using GLMs. Pollen parent, boron treatment and plot were included as fixed effects. Boron application increased the diameter and flesh mass of mature fruit [29] but partitioning of the data into the three boron treatments accounted for potential effects of boron application on fruitlet or fruit parameters. We compared differences between means using a pairwise comparison procedure, with sequential Šidák corrections when significant differences were detected. Differences between means were considered significant at p ˂ 0.05. Means are reported with standard errors. All statistical analyses were performed using SPSS version 26.0 (IBM, Armonk, NY, USA).
3. Results
3.1. Percentages of Retained Fruitlets That Were Cross-Fertilised
We identified selective retention of cross-fertilised Hass avocado fruitlets because the percentage of retained progeny that were cross-fertilised more than doubled during fruit development, from 4.6% at 6 weeks after peak anthesis to 10.7% at fruit maturity (Table 2). Cross-fertilised fruitlets represented 4.6% of the retained fruitlets at 6 weeks after peak anthesis, but only 1.6% of the freshly-abscised fruitlets at this stage were cross-fertilised (Table 2). Similarly, 6.5% of the retained fruitlets at 10 weeks after peak anthesis were cross-fertilised whereas only 1.0% of the freshly-abscised fruitlets were cross-fertilised (Table 2). All cross-fertilised fruitlets and cross-fertilised mature fruit were pollinated by the nearest other cultivar, viz. Shepard.

Table 2.
Percentages of Hass avocado fruitlets or fruit, collected from the ground vs. from the canopy at 6, 10, or 36 weeks after peak anthesis that were cross-fertilised.
3.2. Cross-Fertilisation Effects on Fruitlet or Fruit Size
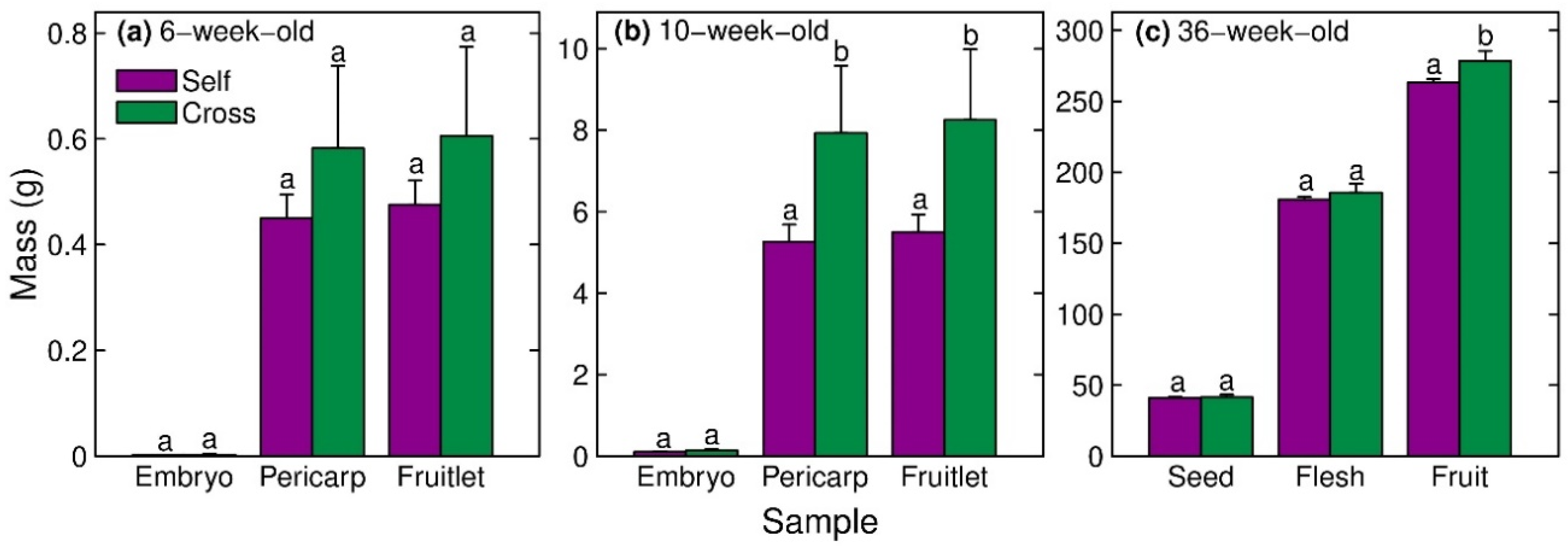
Embryo, pericarp and whole-fruitlet masses at 6 weeks after peak anthesis did not differ significantly between cross-fertilised and self-fertilised fruitlets (Figure 2a). However, the pericarp and whole-fruitlet masses at 10 weeks after peak anthesis were 51% and 50% higher, respectively, in cross-fertilised fruitlets than self-fertilised fruitlets (Figure 2b). Cross-fertilised fruit at maturity were 6% heavier (Figure 2c) and had 2% greater diameter than self-fertilised mature fruit (Figure 2c, Table 3).
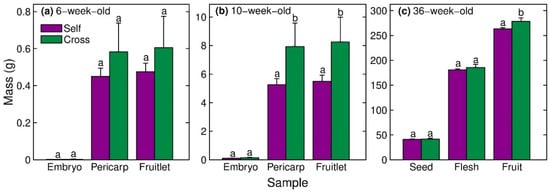
Figure 2.
Mass of the (i) embryo or seed, (ii) pericarp or flesh, and (iii) total fruitlet or fruit of self-fertilised vs. cross-fertilised progeny of Hass avocado at (a) 6 weeks, (b) 10 weeks and (c) 36 weeks after peak anthesis. Means (±SE) with different letters within a sample type and time point are significantly different (GLM, p < 0.05, n = 95–325 selfs and n = 13–50 crosses).

Table 3.
Length and diameter of self- and cross-fertilised mature fruit of Hass avocado.
3.3. Cross-Fertilisation Effects on Mineral Nutrient Levels and Fatty Acid Composition
The concentrations of most mineral nutrients did not differ significantly between cross-fertilised and self-fertilised fruitlets at either 6 or 10 weeks after peak anthesis (Table 4). The total content of each mineral nutrient did not differ significantly between cross-fertilised and self-fertilised fruitlets at 6 weeks after peak anthesis (Table 5). However, the total contents of P, K, Al, Ca, Fe, Mn and Zn were higher in cross-fertilised fruitlets than self-fertilised fruitlets at 10 weeks after peak anthesis (Table 5).

Table 4.
Mineral nutrient concentrations (mg/100 g) in self- and cross-fertilised fruitlets of Hass avocado at 6 and 10 weeks after peak anthesis.

Table 5.
Mineral nutrient contents (mg) in self- and cross-fertilised fruitlets of Hass avocado at 6 and 10 weeks after peak anthesis.
The concentrations of most mineral nutrients in the flesh or seed did not differ significantly between cross-fertilised and self-fertilised mature Hass fruit (Table 6). Similarly, the total content of each mineral nutrient in the seed, flesh, or whole fruit did not differ significantly between cross-fertilised and self-fertilised Hass fruit (Table 7). Relative abundances of the six predominant fatty acids, the total unsaturated fatty acids (UFAs) and the total saturated fatty acids (SFAs) also did not differ significantly (Table 8).

Table 6.
Mineral nutrient concentrations (mg/100 g) in the seed and flesh of self- and cross-fertilised mature fruit of Hass avocado.

Table 7.
Mineral nutrient contents (mg) in the seed, flesh and whole fruit of self- and cross-fertilised Hass avocado.

Table 8.
Relative abundances of individual fatty acids, total unsaturated fatty acids (UFAs) and total saturated fatty acids (UFAs) in self- and cross-fertilised Hass avocado fruit.
4. Discussion
The results of this study support the hypotheses that cross-fertilised avocado fruitlets would be retained selectively during premature fruit drop and that embryo paternity would affect the size of the fruitlets and fruit. Only 1.0% of the abscised fruitlets at 10 weeks after peak anthesis were cross-fertilised, while 99.0% of the abscised fruitlets were self-fertilised; thus, the percentage of progeny on the tree that were cross-fertilised more than doubled between 6 weeks after peak anthesis and fruit maturity. Cross-fertilised fruitlets were larger than self-fertilised fruit at 10 weeks after peak anthesis and cross-fertilised mature fruit were ultimately heavier, with greater diameter, than self-fertilised mature fruit. However, our results, for the most part, do not support the hypothesis that embryo paternity would affect the nutrient composition of the fruitlets and mature fruit. Differences in total nutrient accumulation between cross-fertilised fruitlets and self-fertilised fruitlets at 10 weeks after peak anthesis were not due to differences in nutrient concentrations but were due solely to differences in fruitlet mass. In addition, the flesh of cross-fertilised and self-fertilised mature fruit did not differ significantly in mineral nutrient concentrations, total contents of each mineral nutrient, or fatty acid composition.
Our results suggest that inbreeding depression and inter-fruit competition, rather than a strong post-zygotic self-incompatibility mechanism, may be involved in the selective abscission of self-fertilised avocado fruitlets during the period of premature fruit drop. The self-fertilised fruitlets were smaller than the cross-fertilised fruitlets; thus, they accrued a lower total mass of many mineral nutrients. Accumulation of non-lethal deleterious alleles can reduce the growth of selfed progeny, which then have a reduced capacity to compete with larger outcrossed progeny for maternal resources during the period of premature fruit drop [11,15,17]. Mineral nutrient transport to fruitlets occurs down a concentration gradient that follows, rather than regulates, the mass flow of water through the xylem and phloem [52,53,54,55]. This transport via mass flow explains why the self-fertilised fruitlets, which were smaller than the cross-fertilised fruitlets, did not have lower nutrient concentrations despite having lower total contents of many nutrients. The ultimately high levels of selfing among mature avocado fruit in this study and previous studies [36,37,39,40,41,43,56,57,58] also suggest that the selective abscission of self-fertilised fruitlets during premature fruit drop was not driven by a rigorous post-zygotic self-incompatibility mechanism. This type of genetic self-rejection system can cause embryo abortion at various stages during fruit development, although it usually operates soon after fertilisation [12,14,15,16,59].
The cross-fertilised mature fruit of Hass, which were all pollinated by the neighbouring cultivar Shepard, had 6% higher mass and 2% greater diameter than self-fertilised fruit. Similar xenia effects on avocado have been observed recently in Spain, with cross-fertilisation by Fuerte also increasing the mass of Hass fruit [33]. In addition, cross-fertilisation by Ettinger, Teague, or Topa-Topa has increased the mass of Fuerte fruit in Israel [36]. Larger fruit provide higher financial returns to avocado growers because premiums are paid for cartons that contain fruit of greater diameter [60,61,62,63]. The cross-fertilised Hass fruit in this study and a previous study [39] appeared to have the same nutritional quality as self-fertilised fruit, possessing similar flesh nutrient concentrations and fatty acid profiles. Both types of fruit had the characteristic fatty acid profile of avocado, with high levels of unsaturated oleic, elaidic, linoleic and palmitoleic acids, that are reported to make the fruit a valuable component of a healthy diet [64,65,66,67,68].
Increasing the opportunities for cross-pollination in avocado orchards can, therefore, provide larger fruit with higher commercial value for growers. We have shown recently that raising the levels of cross-fertilisation can also improve avocado yield by increasing the number of mature fruit produced by each tree [37]. Outcrossing rates often decline with increasing distance to trees of another avocado cultivar [37,39,40,41,43]. The Hass trees in the current study were located at least 190 m from the polliniser, Shepard, which explains why only 11% of mature Hass fruit were cross-fertilised. Much higher rates of outcrossing (30–52%) have been observed among Hass trees in the same orchard that were located only 20–40 m from the Shepard trees [37]. Cross-pollination and yield can be increased by closer interplanting of type A and type B cultivars [37,38,43] and by increasing the number and distribution of beehives in orchards [30,69,70,71,72]. Managing the surrounding habitat to enhance pollinator abundance and pollinator diversity may also increase the frequency of successful pollination of avocado flowers [72,73,74,75].
5. Conclusions
This study demonstrated that cross-fertilised Hass avocado fruitlets are retained selectively during the period of premature fruit drop. Only 1.0% of freshly-abscised fruitlets on the ground at 10 weeks after peak anthesis were cross-fertilised, while 99.0% of the freshly-abscised fruitlets at this stage were self-fertilised. Cross-fertilised fruitlets at this stage were larger than self-fertilised fruitlets, which indicates that inbreeding depression resulted in the self-fertilised fruitlets growing more slowly and having reduced capacity to compete for maternal resources during the period of premature fruit drop. Furthermore, cross-fertilised mature fruit were heavier and had greater diameter than self-fertilised fruit; thus, they can provide higher financial returns. Avocado growers might increase fruit size and yield by improving the opportunities for cross-pollination. This could be achieved by closely interplanting type A and type B cultivars, introducing more beehives into orchards and maintaining habitat that sustains an abundance and diversity of wild pollinators.
Author Contributions
Conceptualization, W.K., S.H.B., S.M.O., H.M.W. and S.J.T.; formal analysis, N.S.H.; funding acquisition, S.H.B., S.M.O., H.M.W. and S.J.T.; investigation, N.S.H., W.K. and J.N.; methodology, N.S.H., W.K., S.H.B., S.M.O., J.N., H.M.W. and S.J.T.; project administration, S.J.T.; supervision, W.K., S.H.B., S.M.O., H.M.W. and S.J.T.; writing—original draft, N.S.H.; writing—review and editing, W.K., S.H.B., S.M.O., J.N., H.M.W. and S.J.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by project PH16001 of the Hort Frontiers strategic partnership initiative developed by Hort Innovation, with co-investment from Griffith University, Plant & Food Research Ltd., University of the Sunshine Coast, and contributions from the Australian Government. Nimanie Hapuarachchi was supported by a Griffith University International Postgraduate Research Scholarship and a Griffith University Postgraduate Research Scholarship.
Data Availability Statement
The data will be made available upon request with permission from Hort Innovation.
Acknowledgments
We thank the growers for assistance and access to the orchard. We thank Anushika De Silva, Marta Gallart, Sascha Kämper, Tsvakai Gama, Tracey McMahon, Rachele Wilson, Peter Brooks, Jack Royle and David Appleton for field and laboratory assistance.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; or in the writing of the manuscript; however, they approved the publication of the results.
References
- Food and Agriculture Organisation. Fruit and Vegetables—Your Dietary Essentials; FAO: Rome, Italy, 2020. [Google Scholar]
- Production Volume of the Most Produced Food Commodities Worldwide in 2019, by Product. Available online: https://www.statista.com/statistics/1003455/most-produced-crops-and-livestock-products-worldwide/ (accessed on 25 March 2024).
- Ickowitz, A.; McMullin, S.; Rosenstock, T.; Dawson, I.; Rowland, D.; Powell, B.; Mausch, K.; Djoudi, H.; Sunderland, T.; Nurhasan, M.; et al. Transforming food systems with trees and forests. Lancet Planet. Health 2022, 6, e632–e639. [Google Scholar] [CrossRef] [PubMed]
- Pallardy, S.G. Reproductive growth. In Physiology of Woody Plants; Pallardy, S.G., Ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 87–103. [Google Scholar]
- Dag, A.; Eisenstein, D.; Gazit, S.; El-Batsri, R.; Degani, C. Effect of pollinizer distance and selective fruitlet abscission on outcrossing rate and yield in ‘Tommy Atkins’ mango. J. Am. Soc. Hortic. Sci. 1998, 123, 618–622. [Google Scholar] [CrossRef]
- Holanda-Neto, J.P.; Freitas, B.M.; Bueno, D.M.; Araújo, Z.B. Low seed/nut productivity in cashew (Anacardium occidentale): Effects of self-incompatibility and honeybee (Apis mellifera) foraging behavior. J. Hortic. Sci. Biotechnol. 2002, 77, 226–231. [Google Scholar] [CrossRef]
- Trueman, S.J.; Kämper, W.; Nichols, J.; Ogbourne, S.M.; Hawkes, D.; Peters, T.; Bai, S.H.; Wallace, H.M. Pollen limitation and xenia effects in a cultivated mass-flowering tree, Macadamia integrifolia (Proteaceae). Ann. Bot. 2022, 129, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Degani, C.; Stern, R.A.; El-Batsri, R.; Gazit, S. Pollen-parent effect on the selective abscission of ‘Mauritius’ and ‘Floridian’ Lychee Fruitlets. J. Am. Soc. Hortic. Sci. 1995, 120, 523–526. [Google Scholar] [CrossRef]
- Pérez, V.; Herrero, M.; Hormaza, J.I. Self-fertility and preferential cross-fertilization in mango (Mangifera indica). Sci. Hortic. 2016, 213, 373–378. [Google Scholar] [CrossRef]
- Stephenson, A.G. Flower and fruit abortion proximate causes and ultimate functions. Annu. Rev. Ecol. Syst. 1981, 12, 253–279. [Google Scholar] [CrossRef]
- Xiong, H.; Zou, F.; Guo, S.; Yuan, D.; Niu, G. Self-sterility may be due to prezygotic late-acting self-incompatibility and early-acting inbreeding depression in Chinese chestnut. J. Am. Soc. Hortic. Sci. 2019, 144, 172–181. [Google Scholar] [CrossRef]
- Hao, Y.-Q.; Zhao, X.-F.; She, D.-Y.; Xu, B.; Zhang, D.-Y.; Liao, W.-J. The role of late-acting self-incompatibility and early-acting inbreeding depression in governing female fertility in monkshood, Aconitum kusnezoffii. PLoS ONE 2012, 7, e47034. [Google Scholar] [CrossRef]
- Gibbs, P.E. Late-acting self-incompatibility—The pariah breeding system in flowering plants. New Phytol. 2014, 203, 717–734. [Google Scholar] [CrossRef]
- Seavey, S.R.; Bawa, K.S. Late-acting self-incompatibility in angiosperms. Bot. Rev. 1986, 52, 196–213. [Google Scholar] [CrossRef]
- Sage, T.L.; Sampson, F.B. Evidence for ovarian self-incompatibility as a cause of self-sterility in the relictual woody angiosperm, Pseudowintera axillaris (Winteraceae). Ann. Bot. 2003, 91, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.B.; Meagher, T.R.; Gibbs, P.E. Do s genes or deleterious recessives control late-acting self-incompatibility in Handroanthus heptaphyllus (Bignoniaceae)? A diallel study with four full-sib progeny arrays. Ann. Bot. 2021, 127, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Husband, B.C.; Schemske, D.W. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 1996, 50, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Dag, A.; Degani, C.; Gazit, S. Gene flow in mango orchards and its impact on yield. Acta Hortic. 2009, 820, 347–350. [Google Scholar] [CrossRef]
- Raz, A.; Goldway, M.; Sapir, G.; Stern, R.A. “Hong Long” lychee (Litchi chinensis Sonn.) is the optimal pollinizer for the main lychee cultivars in Israel. Plants 2022, 11, 1996. [Google Scholar] [CrossRef] [PubMed]
- Denney, J.O. Xenia includes metaxenia. HortScience 1992, 27, 722–727. [Google Scholar] [CrossRef]
- Yang, Q.; Fu, Y.; Liu, Y.; Zhang, T.; Peng, S.; Deng, J. Novel classification forms for xenia. HortScience 2020, 55, 980–987. [Google Scholar] [CrossRef]
- Wallace, H.M.; Lee, L.S. Pollen source, fruit set and xenia in mandarins. J. Hortic. Sci. Biotechnol. 1999, 74, 82–86. [Google Scholar] [CrossRef]
- Militaru, M.; Butac, M.; Sumedrea, D.; Chiţu, E. Effect of metaxenia on the fruit quality of scab resistant apple varieties. Agric. Agric. Sci. Procedia 2015, 6, 151–156. [Google Scholar] [CrossRef]
- Dung, C.D.; Wallace, H.M.; Bai, S.H.; Ogbourne, S.M.; Trueman, S.J. Cross-pollination affects fruit colour, acidity, firmness and shelf life of self-compatible strawberry. PLoS ONE 2021, 16, e0256964. [Google Scholar] [CrossRef] [PubMed]
- Kämper, W.; Thorp, G.; Wirthensohn, M.; Brooks, P.; Trueman, S.J. Pollen paternity can affect kernel size and nutritional composition of self-incompatible and new self-compatible almond cultivars. Agronomy 2021, 11, 326. [Google Scholar] [CrossRef]
- Trueman, S.J.; Penter, M.G.; Malagodi-Braga, K.S.; Nichols, J.; De Silva, A.L.; Ramos, A.T.M.; Moriya, L.M.; Ogbourne, S.M.; Hawkes, D.; Peters, T.; et al. High outcrossing levels among global macadamia cultivars: Implications for nut quality, orchard designs and pollinator management. Horticulturae 2024, 10, 203. [Google Scholar] [CrossRef]
- Lahav, E.; Zamet, D. Flowers, fruitlets and fruit drop in avocado trees. Rev. Chapingo Ser. Hortic. 1999, 5, 95–100. [Google Scholar]
- Gazit, S.; Degani, C. Reproductive biology. In The Avocado: Botany, Production and Uses; Whiley, A.W., Schaffer, B., Wolstenholme, B.N., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 101–133. [Google Scholar]
- Hapuarachchi, N.S.; Kämper, W.; Wallace, H.M.; Bai, S.H.; Ogbourne, S.M.; Nichols, J.; Trueman, S.J. Boron effects on fruit set, yield, quality and paternity of Hass avocado. Agronomy 2022, 12, 1479. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Hormaza, J.I. Inadequate pollination is a key factor determining low fruit-to-flower ratios in avocado. Horticulturae 2024, 10, 140. [Google Scholar] [CrossRef]
- Toukem, N.K.; Dubois, T.; Mohamed, S.A.; Lattorff, H.M.G.; Jordaens, K.; Yusuf, A.A. The effect of annual flower strips on pollinator visitation and fruit set of avocado (Persea americana Mill.) in Kenya. Arthropod–Plant Interact. 2023, 17, 19–29. [Google Scholar] [CrossRef]
- D’Asaro, A.; Reig, C.; Martínez-Fuentes, A.; Mesejo, C.; Farina, V.; Agustí, M. Hormonal and carbohydrate control of fruit set in avocado ‘Lamb Hass’. A question of the type of inflorescence? Sci Hortic. 2021, 282, 110046. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Hormaza, J.I. Fruit set in avocado: Pollen limitation, pollen load size, and selective fruit abortion. Agronomy 2021, 11, 1603. [Google Scholar] [CrossRef]
- Solares, E.; Morales-Cruz, A.; Balderas, R.F.; Focht, E.; Ashworth, V.E.T.M.; Wyant, S.; Minio, A.; Cantu, D.; Arpaia, M.L.; Gaut, B.S. Insights into the domestication of avocado and potential genetic contributors to heterodichogamy. G3 2023, 13, jkac323. [Google Scholar] [CrossRef]
- Scholefield, P.B. A scanning electron microscope study of flowers of avocado, litchi, macadamia and mango. Sci. Hortic. 1982, 16, 263–272. [Google Scholar] [CrossRef]
- Degani, C.; Goldring, A.; Adato, I.; El-Batsri, R. Pollen-parent effect on outcrossing rate, yield, and fruit characteristics of ‘Fuerte’ avocado. HortScience 1990, 25, 471–473. [Google Scholar] [CrossRef]
- Trueman, S.J.; Nichols, J.; Farrar, M.B.; Wallace, H.M.; Hosseini Bai, S. Outcrossing rate and fruit yield of Hass avocado trees decline at increasing distance from a polliniser cultivar. Agronomy 2024, 14, 122. [Google Scholar] [CrossRef]
- Stahl, P.; Mirom, Y.L.; Stern, R.A.; Goldway, M. Comparing ‘Iriet’ and ‘Ettinger’ avocado cultivars as pollinators of ‘Hass’ using SNPs for paternal identification. Sci. Hortic. 2019, 248, 50–57. [Google Scholar] [CrossRef]
- Kämper, W.; Ogbourne, S.M.; Hawkes, D.; Trueman, S.J. SNP markers reveal relationships between fruit paternity, fruit quality and distance from a cross-pollen source in avocado orchards. Sci. Rep. 2021, 11, 20043. [Google Scholar] [CrossRef]
- Degani, C.; El-Batsri, R.; Gazit, S. Outcrossing rate, yield, and selective fruit abscission in ‘Ettinger’ and ‘Ardith’ avocado plots. J. Am. Soc. Hortic. Sci. 1997, 122, 813–817. [Google Scholar] [CrossRef]
- Degani, C.; Goldring, A.; Gazit, S. Pollen-parent effect on outcrossing rate in ‘Hass’ and ‘Fuerte’ avocado plots during fruit development. J. Am. Soc. Hortic. Sci. 1989, 114, 106–111. [Google Scholar] [CrossRef]
- Degani, C.; Goldring, A.; Gazit, S.; Lavi, U. Genetic selection during the abscission of avocado fruitlets. HortScience 1986, 21, 1187–1192. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Hormaza, J.I. Influence of physical distance between cultivars on yield, outcrossing rate and selective fruit drop in avocado (Persea americana, Lauraceae). Ann. Appl. Biol. 2011, 158, 354–361. [Google Scholar] [CrossRef]
- Kämper, W.; Trueman, S.J.; Cooke, J.; Kasinadhuni, N.; Brunton, A.J.; Ogbourne, S.M. Single nucleotide polymorphisms (SNPs) that uniquely identify cultivars of avocado (Persea americana). Appl. Plant Sci. 2021, 9, e11440. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Fazekas, A.J.; Hebert, P.D.N. Semi-automated, membrane-based protocol for DNA isolation from plants. Plant Mol. Biol. Rep. 2008, 26, 186–198. [Google Scholar] [CrossRef]
- McGeehan, S.L.; Naylor, D.V. Automated instrumental analysis of carbon and nitrogen in plant and soil samples. Commun. Soil Sci. Plant Anal. 1988, 19, 493–505. [Google Scholar] [CrossRef]
- Rayment, G.E.; Higginson, F.R. Australian Laboratory Handbook of Soil and Water Chemical Methods; Inkata: Melbourne, Australia, 1992. [Google Scholar]
- Martinie, G.D.; Schilt, A.A. Investigation of the wet oxidation efficiencies of perchloric acid mixtures for various organic substances and the identities of residual matter. Anal. Chem. 1976, 48, 70–74. [Google Scholar] [CrossRef]
- Munter, R.C.; Grande, R.A. Plant tissue and soil extract analysis by ICP-atomic emission spectrometry. In Developments in Atomic Plasma Spectrochemical Analysis; Byrnes, R.M., Ed.; Heyden: London, UK, 1981; pp. 653–672. [Google Scholar]
- Kämper, W.; Trueman, S.J.; Tahmasbian, I.; Bai, S.H. Rapid determination of nutrient concentrations in Hass avocado fruit by Vis/NIR hyperspectral imaging of flesh or skin. Remote Sens. 2020, 12, 3409. [Google Scholar] [CrossRef]
- Richards, T.E.; Kämper, W.; Trueman, S.J.; Wallace, H.M.; Ogbourne, S.M.; Brooks, P.R.; Nichols, J.; Bai, S.H. Relationships between nut size, kernel quality, nutritional composition and levels of outcrossing in three macadamia cultivars. Plants 2020, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Tagliavini, M.; Zavalloni, C.; Rombolà, A.D.; Quartieri, M.; Malaguti, D.; Mazzanti, F.; Millard, P.; Marangoni, B. Mineral nutrient partitioning to fruits of deciduous trees. Acta Hortic. 2000, 512, 131–140. [Google Scholar] [CrossRef]
- Karley, A.J.; White, P.J. Moving cationic minerals to edible tissues: Potassium, magnesium, calcium. Curr. Opin. Plant Biol. 2009, 12, 291–298. [Google Scholar] [CrossRef]
- Ruan, Y.-L.; Patrick, J.W.; Bouzayen, M.; Osorio, S.; Fernie, A.R. Molecular regulation of seed and fruit set. Trends Plant Sci. 2012, 17, 656–665. [Google Scholar] [CrossRef]
- Dung, C.D.; Wallace, H.M.; Bai, S.H.; Ogbourne, S.M.; Trueman, S.J. Biomass and mineral nutrient partitioning among self-pollinated and cross-pollinated fruit on the same strawberry plant. PLoS ONE 2022, 17, e0269485. [Google Scholar] [CrossRef]
- Vrecenar-Gadus, M.; Ellstrand, N.C. The effect of planting design on out-crossing rate and yield in the ‘Hass’ avocado. Sci. Hortic. 1985, 27, 215–221. [Google Scholar] [CrossRef]
- Garner, L.C.; Ashworth, V.E.T.M.; Clegg, M.T.; Lovatt, C.J. The impact of outcrossing on yields of ‘Hass’ avocado. J. Am. Soc. Hortic. Sci. 2008, 133, 648–652. [Google Scholar] [CrossRef]
- Kobayashi, M.; Lin, J.-Z.; Davis, J.; Francis, L.; Clegg, M.T. Quantitative analysis of avocado outcrossing and yield in California using RAPD markers. Sci. Hortic. 2000, 86, 135–149. [Google Scholar] [CrossRef]
- Bittencourt, N.S. Evidence for post-zygotic self-incompatibility in Handroanthus impetiginosus (Bignoniaceae). Plant Reprod. 2017, 30, 69–79. [Google Scholar]
- Whiley, A.W. Crop management. In The Avocado: Botany, Production and Uses; Whiley, A.W., Schaffer, B., Wolstenholme, B.N., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 231–253. [Google Scholar]
- Lovatt, C.J. Hass Avocado Nutrition Research in California; University of California: Riverside, CA, USA, 2013. [Google Scholar]
- Lovatt, C.; Zheng, Y.; Khuong, T.; Campisi-Pinto, S.; Crowley, D.; Rolshausen, P. Yield characteristics of ‘Hass’ avocado trees under California growing conditions. In Proceedings of the VIII World Avocado Congress: Management and Techniques of Cultivation; ProHass: Lima, Peru, 2015; pp. 336–341. [Google Scholar]
- Avocados Australia. Avocados Australia Best Practice Resource—Packaging for Export; Avocados Australia: Rocklea, Australia, 2018. [Google Scholar]
- Fulgoni, V.L.; Dreher, M.; Davenport, A.J. Avocado consumption is associated with better diet quality and nutrient intake, and lower metabolic syndrome risk in US adults: Results from the national health and nutrition examination survey (NHANES) 2001–2008. Nutr. J. 2013, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.L.; Cheng, F.W.; Ford, N.A. A comprehensive review of Hass avocado clinical trials, observational studies, and biological mechanisms. Nutrients 2021, 13, 4376. [Google Scholar] [CrossRef] [PubMed]
- Stolp, L.J.; Kodali, D.R. Naturally occurring high-oleic oils: Avocado, macadamia, and olive oils. In High Oleic Oils. Development, Properties, and Uses; Flider, F.J., Ed.; Elsevier: London, UK, 2021; pp. 7–52. [Google Scholar]
- Pacheco, L.S.; Li, Y.; Rimm, E.B.; Manson, J.E.; Sun, Q.; Rexrode, K.; Hu, F.B.; Guasch-Ferré, M. Avocado consumption and risk of cardiovascular disease in US adults. J. Am. Heart Assoc. 2022, 11, e024014. [Google Scholar] [CrossRef]
- Monge, A.; Stern, D.; Cortés-Valencia, A.; Catzín-Kuhlmann, A.; Lajous, M.; Denova-Gutiérrez, E. Avocado consumption is associated with a reduction in hypertension incidence in Mexican women. Br. J. Nutr. 2023, 129, 1976–1983. [Google Scholar] [CrossRef]
- Read, S.F.J.; Howlett, B.G.; Jesson, L.K.; Pattemore, D.E. Insect visitors to avocado flowers in the Bay of Plenty, New Zealand. N. Z. Plant Prot. 2017, 70, 38–44. [Google Scholar] [CrossRef]
- Peña, J.F.; Carabalí, A. Effect of honey bee (Apis mellifera L.) density on pollination and fruit set of avocado (Persea americana Mill.) cv. Hass. J. Apic. Sci. 2018, 62, 5–14. [Google Scholar] [CrossRef]
- Sagwe, R.N.; Peters, M.K.; Dubois, T.; Steffan-Dewenter, I.; Lattorff, H.M.G. Insect pollination and pollinator supplementation enhances fruit weight, quality, and marketability of avocado (Persea americana). Arthropod–Plant Interact. 2023, 17, 753–763. [Google Scholar] [CrossRef]
- Dymond, K.; Celis-Diez, J.L.; Potts, S.G.; Howlett, B.G.; Wilcox, B.K.; Garratt, M.P.D. The role of insect pollinators in avocado production: A global review. J. Appl. Entomol. 2021, 145, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Faber, B.; Frankie, G.; Pawelek, J.; Chase, M.; Witt, S. Native pollinators of avocado as affected by constructed pollinator habitat gardens in southern California. Acta Hortic. 2020, 1299, 329–332. [Google Scholar] [CrossRef]
- Celis-Diez, J.L.; García, C.B.; Armesto, J.J.; Abades, S.; Garratt, M.P.D.; Fontúrbel, F.E. Wild floral visitors are more important than honeybees as pollinators of avocado crops. Agronomy 2023, 13, 1722. [Google Scholar] [CrossRef]
- Muñoz, A.E.; Plantegenest, M.; Amouroux, P.; Zavieso, T. Native flower strips increase visitation by non-bee insects to avocado flowers and promote yield. Basic Appl. Ecol. 2021, 56, 369–378. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).