Optimizing Green Globular Body Induction for Micropropagation of Microsorum pteropus ‘Windeløv’
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Sterilization
2.2. Green Globular Bodies (GGBs) Induction
2.3. Green Globular Bodies’ Proliferation
2.4. Regeneration of Sporophyte
2.5. Acclimatization
2.6. Statistical Analysis
3. Results
3.1. Sterilization Stage
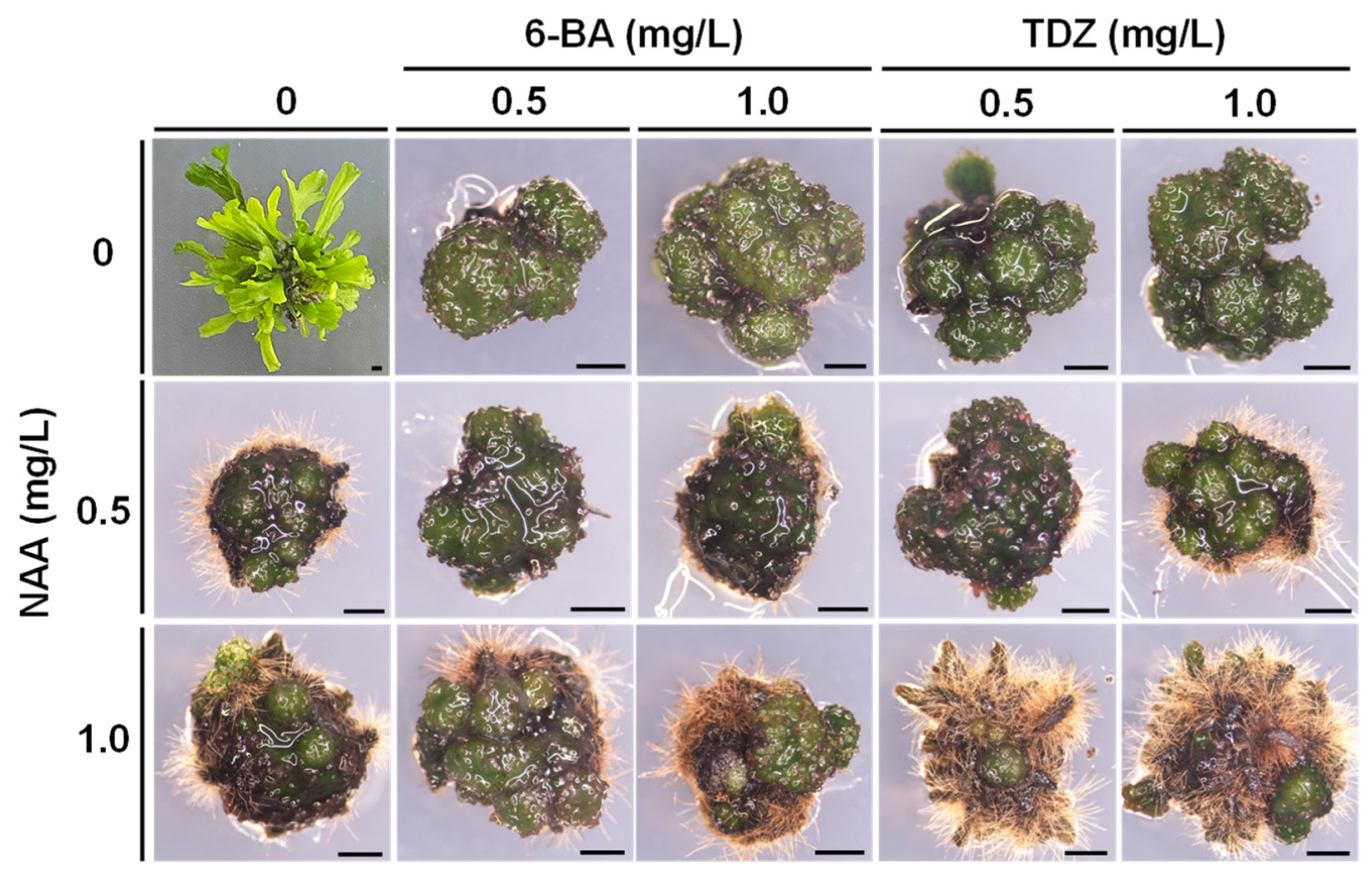
3.2. Green Globular Bodies’ Induction
3.3. Green Globular Bodies’ Proliferation
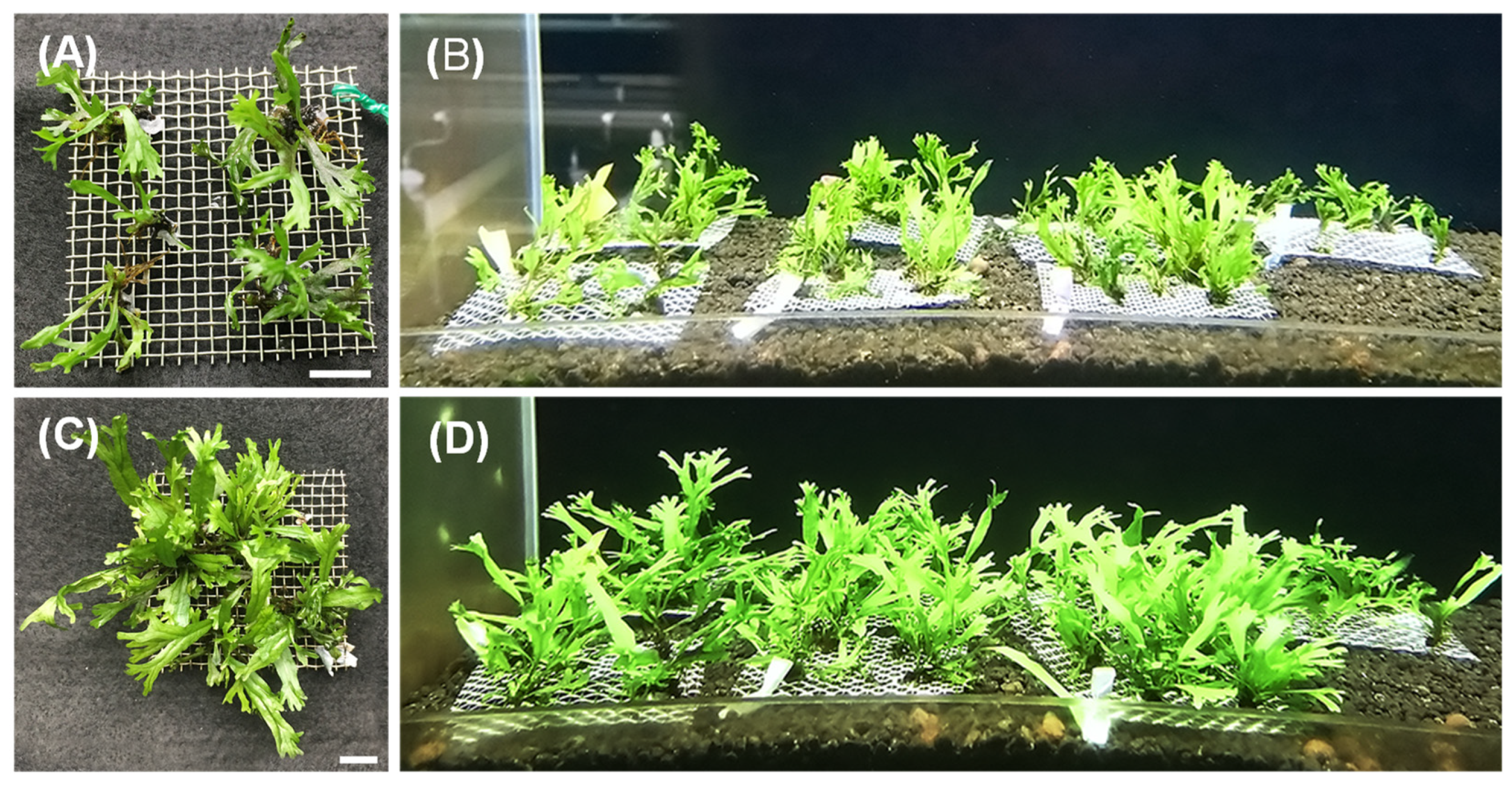
3.4. The Regeneration of Sporophytes
3.5. Acclimatization
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brix, H. Do macrophytes play a role in constructed treatment wetlands? Water Sci. Technol. 1997, 35, 11–17. [Google Scholar] [CrossRef]
- Engelhardt, K.A.M.; Ritchie, M.E. Effects of macrophyte species richness on wetland ecosystem functioning and services. Nature 2001, 411, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.A.; O’Hare, M.T.; McDonald, C.; Searle, K.R.; Daunt, F.; Stillman, R.A. Herbivore regulation of plant abundance in aquatic ecosystems. Biol. Rev. 2017, 92, 1128–1141. [Google Scholar] [CrossRef] [PubMed]
- Alavijeh, M.; Ebadi, A.; Zarei, A.; Omidi, M. Somatic embryogenesis from anther, whole flower, and leaf explants of some grapevine cultivars. Plant Tissue Cult. Biotechnol. 2016, 26, 219. [Google Scholar] [CrossRef]
- Karimi Alavijeh, M.; Safi, S.; Zarei, A. An efficient method for economic micropropagation of three aquatic plant species (Lobelia cardinalis, Staurogyne repens, and Alternanthera reineckii). Aquac. Int. 2023, 31, 1623–1636. [Google Scholar] [CrossRef]
- Mansour, A.T.; Ashour, M.; Alprol, A.E.; Alsaqufi, A.S. Aquatic plants and aquatic animals in the context of sustainability: Cultivation techniques, integration, and blue revolution. Sustainability 2022, 14, 3257. [Google Scholar] [CrossRef]
- Hiscock, P. Encyclopedia of Aquarium Plants; Interpet: London, UK, 2003. [Google Scholar]
- Yu, R.; Li, F.; Wang, G.; Ruan, J.; Wu, L.; Wu, M.; Yang, C.; Shan, Q. In vitro regeneration of the colorful fern Pteris aspericaulis var. tricolor via green globular bodies system. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 225–234. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, G.; Li, H.; Cao, H.; Mo, X.; Gui, M.; Zhou, X.; Jiang, Y.; Li, S.; Wang, J. In vitro propagation of the endangered tree fern Cibotium barometz through formation of green globular bodies. Plant Cell Tissue Organ Cult. (PCTOC) 2017, 128, 369–379. [Google Scholar] [CrossRef]
- Nofal, E.M.S.; Sayed, S.S.; Hassan, H.H.M. Micropropagation protocol of rabbit foot fern Davallia fejeensis Hook. Appl. Ecol. Environ. 2022, 20, 699–709. [Google Scholar] [CrossRef]
- Rai, P.K. Heavy metal phytoremediation from aquatic ecosystems with special reference to macrophytes. Crit. Rev. Environ. Sci. Technol. 2009, 39, 697–753. [Google Scholar] [CrossRef]
- Dhote, S.; Dixit, S. Water quality improvement through macrophytes—A review. Environ. Monit. Assess. 2009, 152, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.R.; Prasad, M. Copper toxicity in Ceratophyllum demersum L. (Coontail), a free floating macrophyte: Response of antioxidant enzymes and antioxidants. Plant Sci. 1998, 138, 157–165. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, H.; Yu, H.; Wang, X.; Wu, J.; Xue, Y. Bioaccumulation and physiological effects of tetrabromobisphenol A in coontail Ceratophyllum demersum L. Chemosphere 2008, 70, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Duman, F.; Koca, F.D.; Sahan, S. Antagonist effects of sodium chloride on the biological responses of an aquatic plant (Ceratophyllum demersum L.) exposed to hexavalent chromium. Water Air Soil Pollut. 2014, 225, 1865. [Google Scholar] [CrossRef]
- Khamushi, M.; Dehestani-Ardakani, M.; Zarei, A.; Kamali Aliabad, K. An efficient protocol for micropropagation of old cypress of Abarkuh (Cupressus sempervirens var. horizontalis [Mill.]) under in vitro condition. Plant Cell Tissue Organ Cult. 2019, 138, 597–601. [Google Scholar] [CrossRef]
- Ozcan, E.; Onlu, S.; Sezgin, M.; Barpete, S. The effect of improvised media and sugar concentration on in vitro shoot multiplication of Riccia fluitans L.: An amphibious liverwort. Fresenius Environ. Bull. 2021, 30, 1696–1702. [Google Scholar]
- Liao, Y.; Wu, Y. In vitro propagation of Platycerium bifurcatum (Cav.) C. Chr. via green globular body initiation. Bot. Stud. 2011, 52, 455–463. [Google Scholar]
- Pence, V. Propagation and cryopreservation of Asplenium scolopendrium var. americanum, the American Hart’s-Tongue fern. Am. Fern J. 2015, 105, 211–225. [Google Scholar] [CrossRef]
- Li, X.; Fang, Y.-H.; Han, J.-D.; Bai, S.-N.; Rao, G.-Y. Isolation and characterization of a novel somatic embryogenesis receptor kinase gene expressed in the fern Adiantum capillus-veneris during shoot regeneration in vitro. Plant Mol. Biol. Report. 2015, 33, 638–647. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with Tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Haque, S.M.; Ghosh, B. A submerged culture system for rapid micropropagation of the commercially important aquarium plant, ‘Amazon sword’ (Echinodorus ‘Indian Red’). Vitr. Cell. Dev. Biol.-Plant 2019, 55, 81–87. [Google Scholar] [CrossRef]
- Yildiz, M. The prerequisite of the success in plant tissue culture: High frequency shoot regeneration. In Recent Advances in Plant In Vitro Culture; Leva, A., Rinaldi, L., Eds.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Higuchi, H.; Amaki, W.; Suzuki, S. In vitro propagation of Nephrolepis cordifolia Prsel. Sci. Hortic. 1987, 32, 105–113. [Google Scholar] [CrossRef]
- Higuchi, H.; Amaki, W. Effects of 6-benzylaminopurine on the organogenesis of Asplenium nidus L. through in vitro propagation. Sci. Hortic. 1989, 37, 351–359. [Google Scholar] [CrossRef]
- Bertrand, A.M.; Albuerne, M.A.; Fernández, H.; González, A.; Sánchez-Tamés, R. In vitro organogenesis of Polypodium cambricum. Plant Cell Tissue Organ Cult. 1999, 57, 65–69. [Google Scholar] [CrossRef]
- Lu, D.; Huang, Q.; Deng, C.; Zheng, Y. Phytoremediation of copper pollution by eight aquatic plants. Pol. J. Environ. Stud. 2018, 27, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Shih, C.-H.; Chang, H.-C.; Kao, C.-Y. Green Globular Body Induction and Plantlet Regeneration of Endangered Fern Adantum Reniforme var. sinense. Indian J. Agric. Res. 2024, 58, 56–62. [Google Scholar]
- Amaki, W.; Higuchi, H. A possible propagation system of Nephrolepis, Asplenium, Pteris, Adiantum and Rumohra (Arachniodes) through tissue culture. Acta Hortic. 1992, 300, 237–244. [Google Scholar] [CrossRef]
- Cárdenas-Aquino, M.d.R.; Camas-Reyes, A.; Valencia-Lozano, E.; López-Sánchez, L.; Martínez-Antonio, A.; Cabrera-Ponce, J.L. The cytokinins BAP and 2-iP modulate different molecular mechanisms on shoot proliferation and root development in lemongrass (Cymbopogon citratus). Plants 2023, 12, 3637. [Google Scholar] [CrossRef]
- Shelikhan, L.A. In vitro regeneration of fern via green globular bodies. Russ. J. Plant Physiol. 2023, 70, 16. [Google Scholar] [CrossRef]
- Fernandez, H.; Bertrand, A.M.; Sanchez-Tames, R. Influence of tissue culture conditions on apogamy in Dryopteris affinis sp. affinis. Plant Cell Tissue Organ Cult. 1996, 45, 93–97. [Google Scholar] [CrossRef]
- Hsu, W. In vitro plant regeneration from sporophytic root explants of Platycerium hillii and Platycerium alcicorne. Master’s Thesis, National Ilan University, Yilan, Taiwan, 2011. [Google Scholar]


| NaOCl (%) | Duration Time (Min) | % Vital Aseptic Explants * |
|---|---|---|
| 1.0 | 10.0 | 0.0 ± 0.0 c |
| 20.0 | 47.5 ± 12.6 a | |
| 30.0 | 15.0 ± 12.9 c | |
| 2.0 | 10.0 | 12.5 ± 9.6 c |
| 20.0 | 10.0 ± 8.2 c | |
| 30.0 | 15.0 ± 5.8 c | |
| 3.0 | 10.0 | 2.5 ± 5.0 bc |
| 20.0 | 0.0 ± 0.0 c | |
| 30.0 | 0.0 ± 0.0 c | |
| 4.0 | 10.0 | 0.0 ± 0.0 c |
| 20.0 | 0.0 ± 0.0 c | |
| 30.0 | 0.0 ± 0.0 c |
| Cytokinin (mg/L) | NAA (mg/L) | % GGB Formation | GGB No. per Explant | |
|---|---|---|---|---|
| 0.0 | 0.0 | 0.0 ± 0.0 e | 0.0 ± 0.0 f | |
| 1.0 | 5.0 ± 8.7 e | 0.2 ± 0.2 f | ||
| 5.0 | 65.0 ± 8.7 c | 1.0 ± 0.4 de | ||
| 10.0 | 75.0 ± 8.7 bc | 1.3 ± 0.1 d | ||
| 6-BA | 0.5 | 0.0 | 95.0 ± 8.7 ab | 2.8 ± 0.8 abc |
| 1.0 | 90.0 ± 10 ab | 1.5 ± 0.1d | ||
| 5.0 | 100.0 ± 0.0 a | 4.0 ± 0.8 a | ||
| 10.0 | 100.0 ± 0.0 a | 2.5 ± 0.3 b | ||
| 1.0 | 0.0 | 100.0 ± 0.0 a | 4.0 ± 0.8 a | |
| 1.0 | 100.0 ± 0.0 a | 3.1 ± 0.7 ab | ||
| 5.0 | 95 ± 8.7 ab | 2.0 ± 0.2 c | ||
| 10.0 | 65 ± 8.7 c | 1.2 ± 0.4 de | ||
| 5.0 | 0.0 | 30.0 ± 10.0 d | 0.7 ± 0.1 e | |
| 1.0 | 15.0 ± 8.7 de | 0.1 ± 0.1 f | ||
| 5.0 | 50.0 ± 10.0 cd | 1.2 ± 0.2 d | ||
| 10.0 | 55.0 ± 8.7 c | 1.6 ± 0.6 cde | ||
| TDZ | 0.5 | 0.0 | 70 ± 10.0 bc | 1.5 ± 0.1 d |
| 1.0 | 55 ± 8.7 c | 1.2 ± 0.4 de | ||
| 5.0 | 75 ± 8.7 bc | 1.3 ± 0.3 d | ||
| 10.0 | 85 ± 8.7 b | 1.2 ± 0.4 de | ||
| 1.0 | 0.0 | 90 ± 10.0 ab | 1.7 ± 0.3 cd | |
| 1.0 | 95 ± 8.7 ab | 1.9 ± 0.1 c | ||
| 5.0 | 80 ± 14.1 bc | 1.5 ± 0.5 cd | ||
| 10.0 | 85 ± 8.7 b | 1.8 ± 0.4 cd | ||
| 5.0 | 0.0 | 75 ± 16.6 bc | 1.5 ± 0.5 cd | |
| 1.0 | 90 ± 10.0 ab | 2.0 ± 0.1 c | ||
| 5.0 | 80 ± 14.1 bc | 1.9 ± 0.3 c | ||
| 10.0 | 90 ± 10.0 ab | 1.8 ± 0.2 cd | ||
| Combination No. | PGR (mg/L) | GGB Diameter * (mm) | Sporophyte Regeneration * (%) | ||
|---|---|---|---|---|---|
| 6-BA | TDZ | NAA | |||
| 1 | 0.5 | 0.0 | 1.63 ± 0.4 cd | 0.0 ± 0.0 | |
| 2 | 1.0 | 1.74 ± 0.28 bcd | |||
| 3 | 5.0 | 2.45 ± 0.61 a | |||
| 4 | 10.0 | 1.91 ± 0.34 bc | |||
| 5 | 1.0 | 0.0 | 1.28 ± 0.14 e | ||
| 6 | 1.0 | 1.53 ± 0.33 de | |||
| 7 | 5.0 | 1.69 ± 0.37 bcd | |||
| 8 | 1.0 | 0.0 | 1.75 ± 0.27 bcd | ||
| 9 | 1.0 | 1.65 ± 0.12 cd | |||
| 10 | 5.0 | 1.0 | 2.07 ± 0.43 b | ||
| 11 | 10.0 | 1.59 ± 0.28 cde | |||
| PGRs (mg/L) | Sporophyte Regeneration * (%) | Plantlet * (ind.) | ||
|---|---|---|---|---|
| 6-BA | TDZ | NAA | ||
| 0 | 100 ± 0.0 a | 34.2 ± 7.3 a | ||
| 0 | 0.5 | 0.0 ± 0.0 b | 0.0 ± 0.0 b | |
| 1.0 | 0.0 ± 0.0 b | 0.0 ± 0.0 b | ||
| 0 | 0.0 ± 0.0 b | 0.0 ± 0.0 b | ||
| 0.5 | 0.5 | 0.0 ± 0.0 b | 0.0 ± 0.0 b | |
| 1.0 | 0.0 ± 0.0 b | 0.0 ± 0.0 b | ||
| 0 | 0.0 ± 0.0 b | 0.0 ± 0.0 b | ||
| 1 | 0.5 | 0.0 ± 0.0 b | 0.0 ± 0.0 b | |
| 1.0 | 0.0 ± 0.0 b | 0.0 ± 0.0 b | ||
| 0 | 0.0 ± 0.0 b | 0.0 ± 0.0 b | ||
| 0.5 | 0.5 | 0.0 ± 0.0 b | 0.0 ± 0.0 b | |
| 1.0 | 0.0 ± 0.0 b | 0.0 ± 0.0 b | ||
| 0 | 0.0 ± 0.0 b | 0.0 ± 0.0 b | ||
| 1 | 0.5 | 0.0 ± 0.0 b | 0.0 ± 0.0 b | |
| 1.0 | 0.0 ± 0.0 b | 0.0 ± 0.0 b | ||
| Survival Rate * (%) | Initial Weight * (g/Piece) | Final Weight * (g/Piece) | Increased Weight * (g/Piece) |
|---|---|---|---|
| 100.0 ± 0.0 | 0.86 ± 0.16 | 1.72 ± 0.37 | 0.86 ± 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwannamali, W.; Wang, K.-T.; Su, C.-C.; Kantha, P.; Tzean, Y.; Wu, T.-M. Optimizing Green Globular Body Induction for Micropropagation of Microsorum pteropus ‘Windeløv’. Horticulturae 2024, 10, 673. https://doi.org/10.3390/horticulturae10070673
Suwannamali W, Wang K-T, Su C-C, Kantha P, Tzean Y, Wu T-M. Optimizing Green Globular Body Induction for Micropropagation of Microsorum pteropus ‘Windeløv’. Horticulturae. 2024; 10(7):673. https://doi.org/10.3390/horticulturae10070673
Chicago/Turabian StyleSuwannamali, Wirawan, Kuang-Teng Wang, Chia-Chen Su, Phunsin Kantha, Yuh Tzean, and Tsung-Meng Wu. 2024. "Optimizing Green Globular Body Induction for Micropropagation of Microsorum pteropus ‘Windeløv’" Horticulturae 10, no. 7: 673. https://doi.org/10.3390/horticulturae10070673
APA StyleSuwannamali, W., Wang, K.-T., Su, C.-C., Kantha, P., Tzean, Y., & Wu, T.-M. (2024). Optimizing Green Globular Body Induction for Micropropagation of Microsorum pteropus ‘Windeløv’. Horticulturae, 10(7), 673. https://doi.org/10.3390/horticulturae10070673







