Transcriptomic Analysis Reveals Dynamic Changes in Glutathione and Ascorbic Acid Content in Mango Pulp across Growth and Development Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Experiment Site
2.2. Fruit Ripening Index
2.3. Lipid Peroxidation
2.4. Ion Leakage
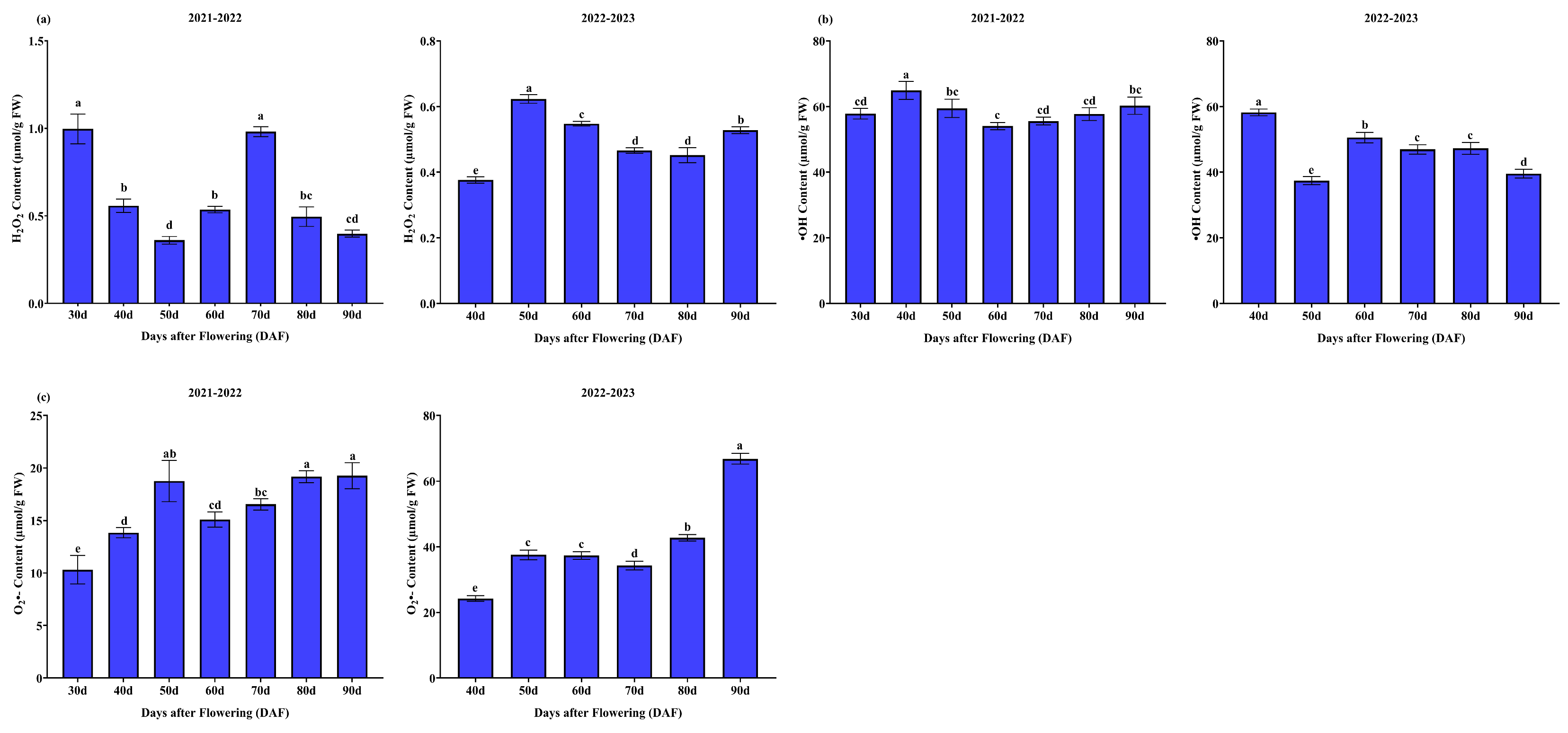
2.5. ROS Ascertainment
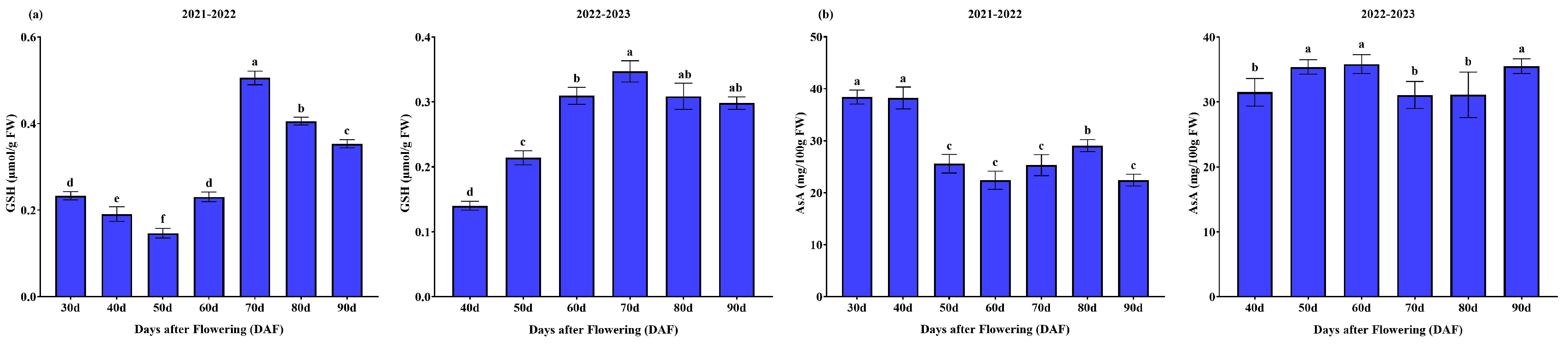
2.6. Glutathione Content
2.7. AsA Content
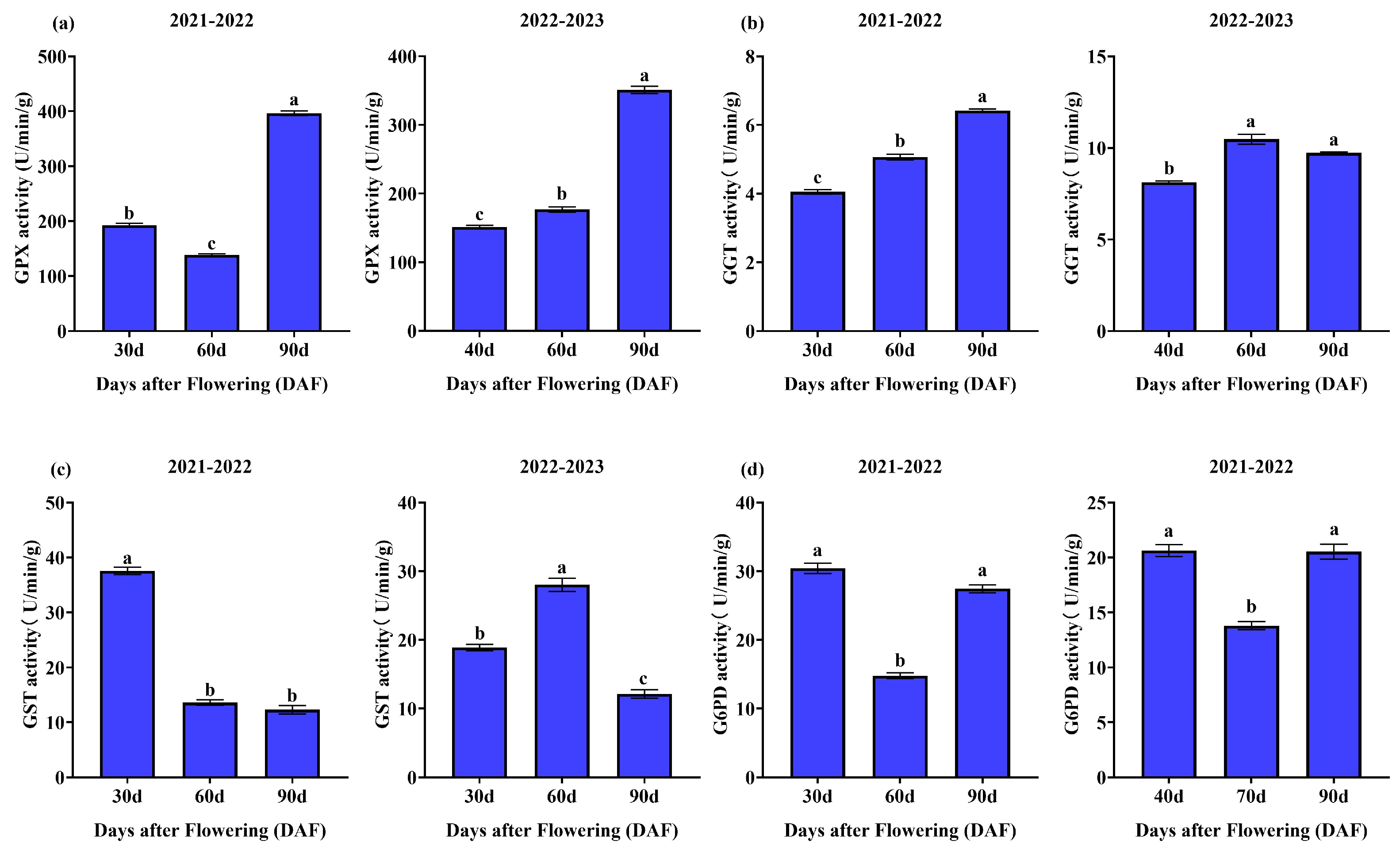
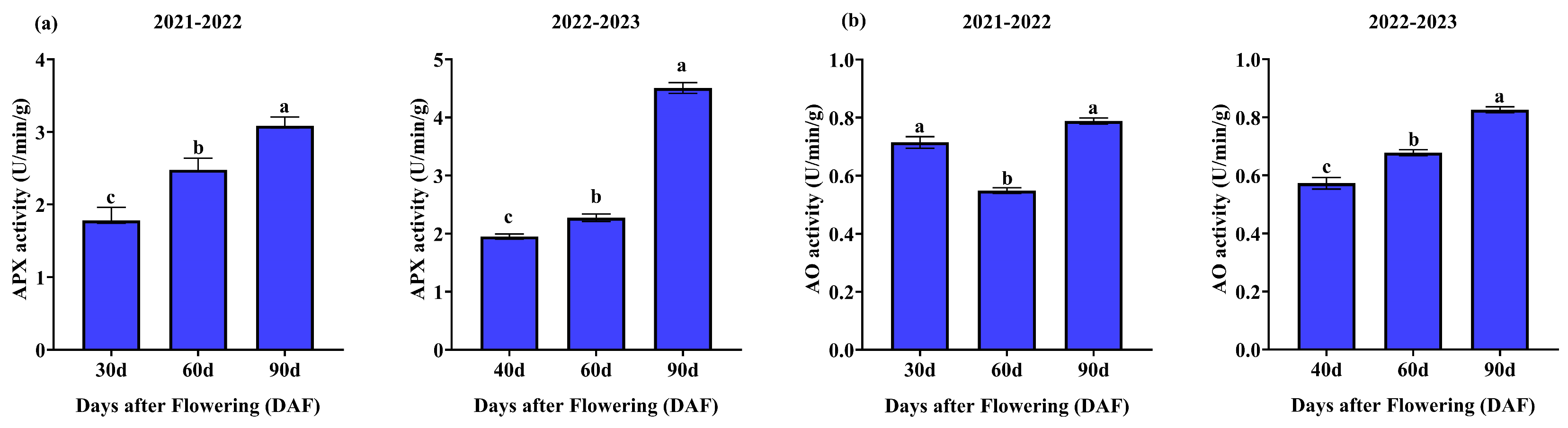
2.8. Measurement of Enzymatic Activity
2.9. Transcriptome Sequencing
2.10. qRT-PCR Analysis
2.11. Statistical Analysis
3. Results
3.1. Mango Pulp Physiochemical Responses
3.1.1. TSS, TA, and TSS/TA Ratio
3.1.2. MDA and Relative Conductivity
3.1.3. Reactive Oxygen Species
3.1.4. GSH and ASA Contents
3.1.5. Enzyme Activities
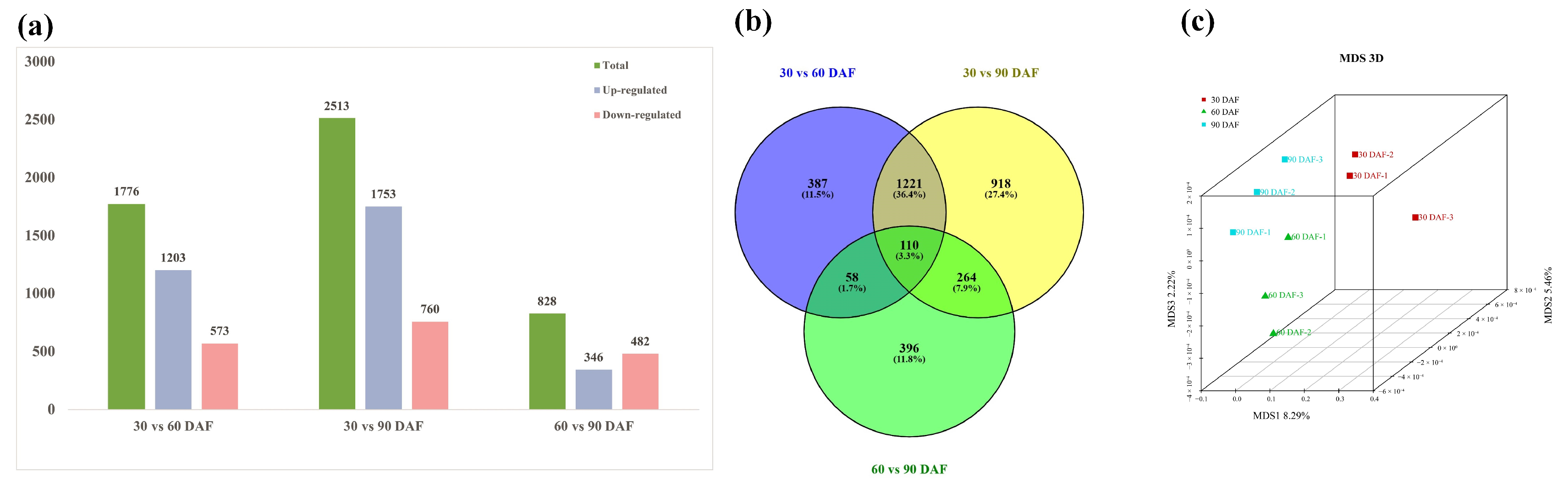
3.2. RNA Sequencing Analysis
3.3. GO and KEGG Pathway Enrichment Analysis of DEGs
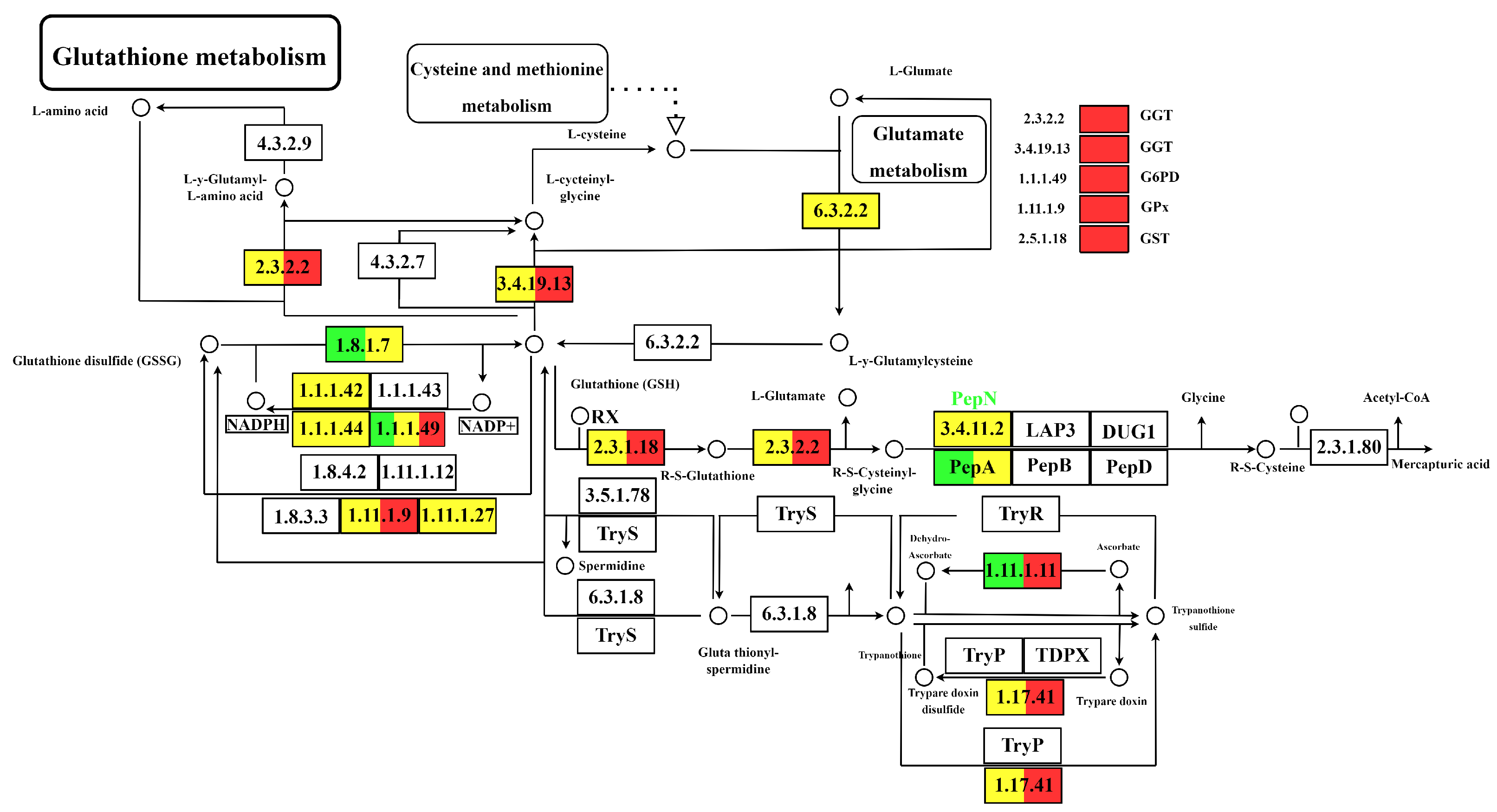
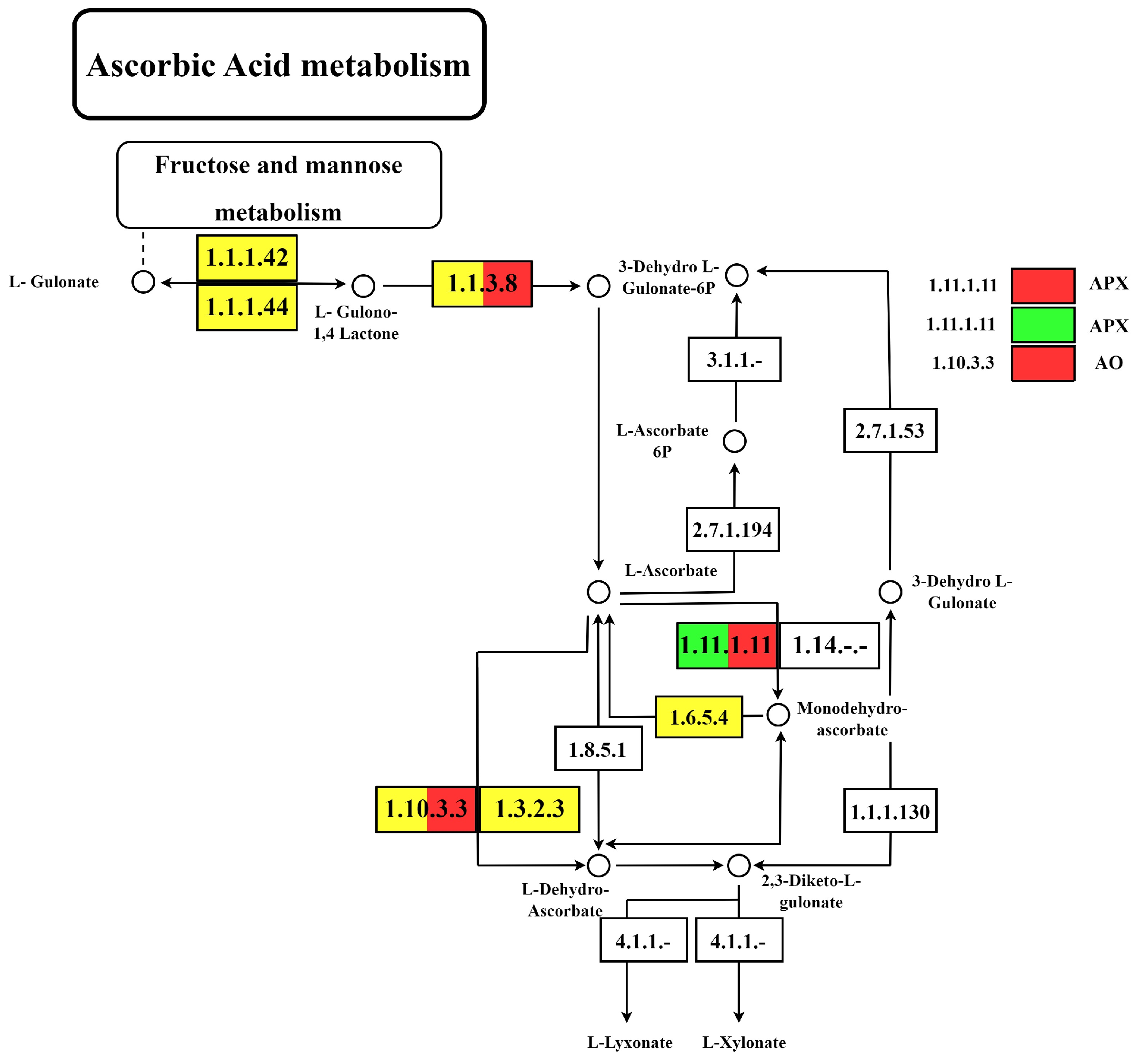
3.4. Identification and Analysis of DEGs Associated with GSH and AsA Metabolic Pathways
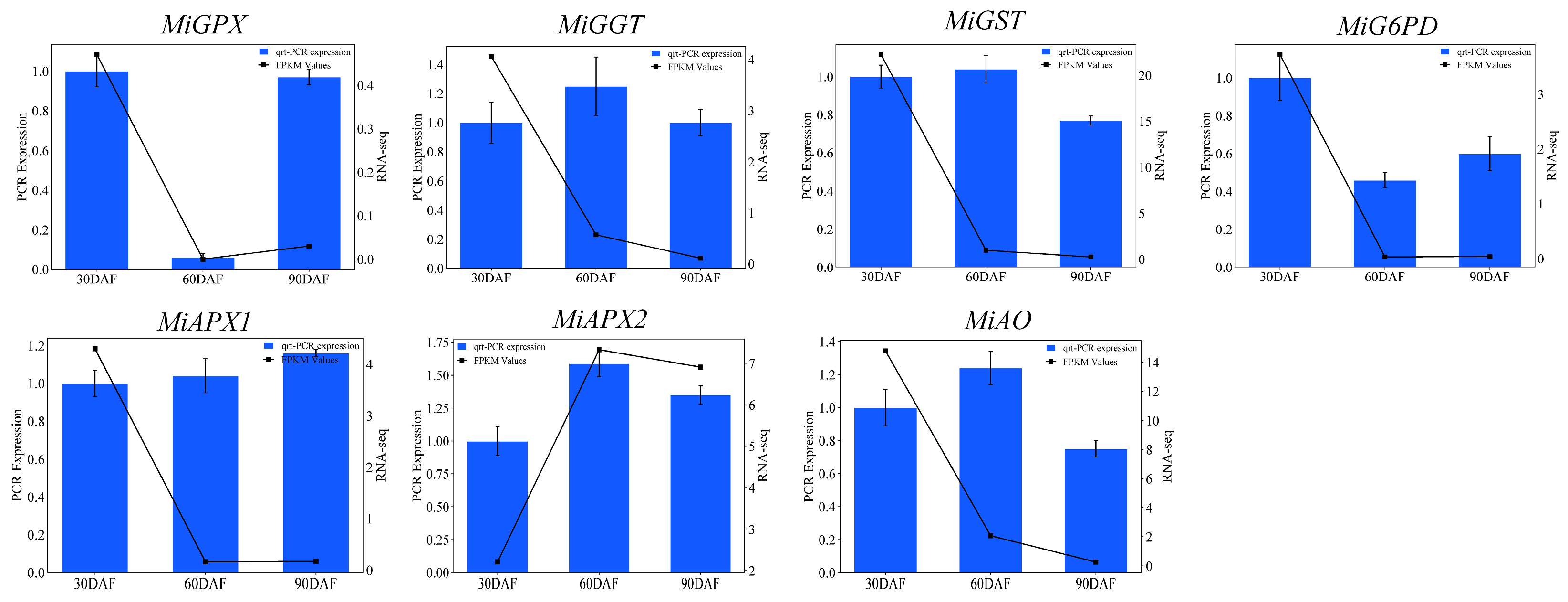
3.5. The qRT-PCR Analysis of Randomly Selected Genes
4. Discussion
4.1. TSS, TA, and TSS/TA Ratio
4.2. MDA, RC, and ROS
4.3. GSH-ASA Cycle and Other Functional Processes
4.4. Enzymatic Activities in Mango Pulp
4.5. Dynamic Changes in GSH and AsA Metabolic Pathways
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, P.; Luo, Y.; Huang, J.; Gao, S.; Zhu, G.; Dang, Z.; Gai, J.; Yang, M.; Zhu, M.; Zhang, H. The genome evolution and domestication of tropical fruit mango. Gen. Biol. 2020, 21, 60. [Google Scholar] [CrossRef] [PubMed]
- García-Mahecha, M.; Soto-Valdez, H.; Carvajal-Millan, E.; Madera-Santana, T.J.; Lomelí-Ramírez, M.G.; Colín-Chávez, C. Bioactive compounds in extracts from the agro-industrial waste of mango. Molecules 2023, 28, 458. [Google Scholar] [CrossRef] [PubMed]
- Tharanathan, R.; Yashoda, H.; Prabha, T. Mango (Mangifera indica L.), “The king of fruits”—An overview. Food Rev. Int. 2006, 22, 95–123. [Google Scholar] [CrossRef]
- Zhang, D.; Chong, W.; Li, X.-L. Yield gap and production constraints of mango (Mangifera indica) cropping systems in Tianyang County, China. J. Integr. Agric. 2019, 18, 1726–1736. [Google Scholar] [CrossRef]
- Sawangchote, P.; Grote, P.J.; Dilcher, D.L. Tertiary leaf fossils of Mangifera (Anacardiaceae) from Li Basin, Thailand as examples of the utility of leaf marginal venation characters. Am. J. Bot. 2009, 96, 2048–2061. [Google Scholar] [CrossRef] [PubMed]
- Jahurul, M.; Zaidul, I.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.-L.; Norulaini, N.; Sahena, F.; Omar, A.M. Mango (Mangifera indica L.) by-products and their valuable components: A review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Caravaca, A.M.; López-Cobo, A.; Verardo, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-DAD-q-TOF-MS as a powerful platform for the determination of phenolic and other polar compounds in the edible part of mango and its by-products (peel, seed, and seed husk). Electrophoresis 2016, 37, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Matharu, A.S.; Houghton, J.A.; Lucas-Torres, C.; Moreno, A. Acid-free microwave-assisted hydrothermal extraction of pectin and porous cellulose from mango peel waste–towards a zero waste mango biorefinery. Green Chem. 2016, 18, 5280–5287. [Google Scholar] [CrossRef]
- Saba, M.K.; Moradi, S. Sodium nitroprusside (SNP) spray to maintain fruit quality and alleviate postharvest chilling injury of peach fruit. Sci. Hortic. 2017, 216, 193–199. [Google Scholar] [CrossRef]
- Osorio, S.; Scossa, F.; Fernie, A.R. Molecular regulation of fruit ripening. Front. Plant Sci. 2013, 4, 198. [Google Scholar] [CrossRef]
- Kumar, V.; Irfan, M.; Ghosh, S.; Chakraborty, N.; Chakraborty, S.; Datta, A. Fruit ripening mutants reveal cell metabolism and redox state during ripening. Protoplasma 2016, 253, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Ge, C.; Luo, Y.; Mo, F.; Xiao, Y.-H.; Li, N.-Y.; Tang, H.-R. Effects of glutathione on the ripening quality of strawberry fruits. AIP Conf. Proc. 2019, 2079, 020013. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, G.; Yamawaki, K.; Ikoma, Y.; Matsumoto, H.; Yoshioka, T.; Ohta, S.; Kato, M. Regulation of ascorbic acid metabolism by blue LED light irradiation in citrus juice sacs. Plant Sci. 2015, 233, 134–142. [Google Scholar] [CrossRef]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Mata-Pérez, C.; Valderrama, R.; Padilla, M.N.; López-Jaramillo, J.; Luque, F.; Corpas, F.J.; Barroso, J.B. Differential molecular response of monodehydroascorbate reductase and glutathione reductase by nitration and S-nitrosylation. J. Exp. Bot. 2015, 66, 5983–5996. [Google Scholar] [CrossRef] [PubMed]
- Chongchatuporn, U.; Ketsa, S.; van Doorn, W.G. Chilling injury in mango (Mangifera indica) fruit peel: Relationship with ascorbic acid concentrations and antioxidant enzyme activities. Postharvest Biol. Technol. 2013, 86, 409–417. [Google Scholar] [CrossRef]
- Camejo, D.; Marti, M.C.; Roman, P.; Ortiz, A.; Jimenez, A. Antioxidant system and protein pattern in peach fruits at two maturation stages. J. Agric. Food Chem. 2010, 58, 11140–11147. [Google Scholar] [CrossRef] [PubMed]
- Meitha, K.; Pramesti, Y.; Suhandono, S. Reactive oxygen species and Antioxidants in postharvest vegetables and fruits. Int. J. Food Sci. 2020, 2020, 8817778. [Google Scholar] [CrossRef] [PubMed]
- Huan, C.; Jiang, L.; An, X.; Kang, R.; Yu, M.; Ma, R.; Yu, Z. Potential role of glutathione peroxidase gene family in peach fruit ripening under combined postharvest treatment with heat and 1-MCP. Postharvest Biol. Technol. 2016, 111, 175–184. [Google Scholar] [CrossRef]
- Yao, M.; Ge, W.; Zhou, Q.; Zhou, X.; Luo, M.; Zhao, Y.; Wei, B.; Ji, S. Exogenous glutathione alleviates chilling injury in postharvest bell pepper by modulating the ascorbate-glutathione (AsA-GSH) cycle. Food Chem. 2021, 352, 129458. [Google Scholar] [CrossRef]
- Wang, L.; Ma, M.; Zhang, S.; Wu, Z.; Li, J.; Luo, W.; Guo, L.; Lin, W.; Zhang, S. Characterization of genes involved in pear ascorbic acid metabolism and their response to bagging treatment during ‘Yali’fruit development. Sci. Hortic. 2021, 285, 110178. [Google Scholar] [CrossRef]
- Palma, J.M.; Terán, F.; Contreras-Ruiz, A.; Rodríguez-Ruiz, M.; Corpas, F.J. Antioxidant profile of pepper (Capsicum annuum L.) fruits containing diverse levels of capsaicinoids. Antioxidants 2020, 9, 878. [Google Scholar] [CrossRef] [PubMed]
- Vogelsang, L.; Dietz, K.-J. Plant thiol peroxidases as redox sensors and signal transducers in abiotic stress acclimation. Free Radic. Biol. Med. 2022, 193, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Gaber, A.; Ogata, T.; Maruta, T.; Yoshimura, K.; Tamoi, M.; Shigeoka, S. The involvement of Arabidopsis glutathione peroxidase 8 in the suppression of oxidative damage in the nucleus and cytosol. Plant Cell Physiol. 2012, 53, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Esaka, M. Expansion of transgenic tobacco protoplasts expressing pumpkin ascorbate oxidase is more rapid than that of wild-type protoplasts. Planta 2000, 210, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Potters, G.; Horemans, N.; Caubergs, R.J.; Asard, H. Ascorbate and dehydroascorbate influence cell cycle progression in a tobacco cell suspension. Plant Physiol. 2000, 124, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Marioni, J.C.; Mason, C.E.; Mane, S.M.; Stephens, M.; Gilad, Y. RNA-seq: An assessment of technical reproducibility and comparison with gene expression arrays. Genome Res. 2008, 18, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, L.; Zhang, H.; Zhu, B.; Lv, G.; Xiao, J. Transcriptome analysis and identification of genes associated with floral transition and fruit development in rabbiteye blueberry (Vaccinium ashei). PLoS ONE 2021, 16, e0259119. [Google Scholar] [CrossRef]
- Xie, M.; Huang, Y.; Zhang, Y.; Wang, X.; Yang, H.; Yu, O.; Dai, W.; Fang, C. Transcriptome profiling of fruit development and maturation in Chinese white pear (Pyrus bretschneideri Rehd). BMC Genom. 2013, 14, 823. [Google Scholar] [CrossRef]
- García-Gómez, B.E.; Ruiz, D.; Salazar, J.A.; Rubio, M.; Martínez-García, P.J.; Martínez-Gómez, P. Analysis of metabolites and gene expression changes relative to apricot (Prunus armeniaca L.) fruit quality during development and ripening. Front. Plant Sci. 2020, 11, 1269. [Google Scholar] [CrossRef]
- Su, L.; Zhang, T.; Wu, M.; Zhong, Y.; Cheng, Z. Transcriptome and Metabolome Reveal Sugar and Organic Acid Accumulation in Rosa roxburghii Fruit. Plants 2023, 12, 3036. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Uddin, M.K.; Quddus, M.A.; Samad, M.Y.A.; Hossain, M.M.; Haque, A.N.A. Impact of foliar application of zinc and zinc oxide nanoparticles on growth, yield, nutrient uptake and quality of tomato. Horticulturae 2023, 9, 162. [Google Scholar] [CrossRef]
- Ding, Y.; Sheng, J.; Li, S.; Nie, Y.; Zhao, J.; Zhu, Z.; Wang, Z.; Tang, X. The role of gibberellins in the mitigation of chilling injury in cherry tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2015, 101, 88–95. [Google Scholar] [CrossRef]
- Khaliq, G.; Mohamed, M.T.M.; Ghazali, H.M.; Ding, P.; Ali, A. Influence of gum arabic coating enriched with calcium chloride on physiological, biochemical and quality responses of mango (Mangifera indica L.) fruit stored under low temperature stress. Postharvest Biol. Technol. 2016, 111, 362–369. [Google Scholar] [CrossRef]
- Rao, B.; Deshpande, V. Experimental biochemistry. Tunbridge Wells Kent Anshan. 2006. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, L.; Zhu, L.; Cao, Y.; Dou, Z.; Yu, Q. HDAC5 promotes intestinal sepsis via the Ghrelin/E2F1/NF-κB axis. FASEB J. 2021, 35, e21368. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, S.; Salem, M.; Jahed, K.R.; Artlip, T.S.; Sherif, S.M. From endodormancy to ecodormancy: The transcriptional landscape of apple floral buds. Front. Plant Sci. 2023, 14, 1194244. [Google Scholar] [CrossRef]
- Gharezi, M.; Joshi, N.; Sadeghian, E. Effect of postharvest treatment on stored cherry tomatoes. J. Nutr. Food Sci. 2012, 2, 1–10. [Google Scholar]
- Nawaz, R.; Abbasi, N.; Hafiz, I.A.; Khalid, A. Impact of climate variables on fruit internal quality of Kinnow mandarin (Citrus nobilis Lour × Citrus deliciosa Tenora) in ripening phase grown under varying environmental conditions. Sci. Hortic. 2020, 265, 109235. [Google Scholar] [CrossRef]
- Lebaka, V.R.; Wee, Y.-J.; Ye, W.; Korivi, M. Nutritional composition and bioactive compounds in three different parts of mango fruit. Int. J. Environ. Res. Public Health 2021, 18, 741. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Anjum, M.A.; Nawaz, A.; Shah, H.M.S. Modified atmosphere packaging delays enzymatic browning and maintains quality of harvested litchi fruit during low temperature storage. Sci. Hortic. 2019, 254, 14–20. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L. Analytical methods for determination of sugars and sweetness of horticultural products—A review. Sci. Hortic. 2015, 184, 179–192. [Google Scholar] [CrossRef]
- Hodges, D.M.; Lester, G.E.; Munro, K.D.; Toivonen, P.M. Oxidative stress: Importance for postharvest quality. Hortic. Sci. 2004, 39, 924–929. [Google Scholar] [CrossRef]
- Luo, P.; He, J.J.; Yao, Y.L.; Mo, Y.W. Effect of chilling stress on leaf antioxidative abilities of rubble trees with different chilling tolerance. Acta Bot. Boreal.-Occident. Sin. 2014, 34, 311–317. [Google Scholar]
- Ren, J.; Zhao, S.; Su, Y.; Qi, G.; Li, B. Effects of low temperature stress in spring on antioxidase indexes of walnuts. J. Northwest A F Univ.-Nat. Sci. Ed. 2016, 44, 75–81. [Google Scholar]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Prášil, I.; Zámečník, J. The use of a conductivity measurement method for assessing freezing injury: I. Influence of leakage time, segment number, size and shape in a sample on evaluation of the degree of injury. Environ. Exp. Bot. 1998, 40, 1–10. [Google Scholar] [CrossRef]
- Mondal, K.; Malhotra, S.P.; Jain, V.; Singh, R. Oxidative stress and antioxidant systems in Guava (Psidium guajava L.) fruits during ripening. Physiol. Mol. Biol. Plants 2009, 15, 327–334. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zhang, J. Advances in the research on the AsA-GSH cycle in horticultural crops. Front. Agric. China 2010, 4, 84–90. [Google Scholar] [CrossRef]
- Jin, Y.-H.; Tao, D.-L.; Hao, Z.-Q.; Ye, J.; Du, Y.-J.; Liu, H.-L.; Zhou, Y.-B. Environmental stresses and redox status of ascorbate. J. Integr. Plant Biol. 2003, 45, 795. [Google Scholar]
- Pan, Y.; Jiang, Y.; Huang, Q.; Zhu, Y.; Nie, Y.; Yuan, R.; Zhang, Z. Abnormal chilling injury of postharvest papaya is associated with the antioxidant response. J. Food Biochem. 2022, 46, e14272. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Zhuo, Q.; Zhang, K.; Yan, J.; Tang, B.; Wei, X.; Lin, L.; Liu, K. Exogenous glutathione maintains the postharvest quality of mango fruit by modulating the ascorbate-glutathione cycle. PeerJ 2023, 11, e15902. [Google Scholar] [CrossRef]
- Mellidou, I.; Keulemans, J.; Kanellis, A.K.; Davey, M.W. Regulation of fruit ascorbic acid concentrations during ripening in high and low vitamin C tomato cultivars. BMC Plant Biol. 2012, 12, 239. [Google Scholar] [CrossRef]
- Fang, T.; Zhen, Q.; Liao, L.; Owiti, A.; Zhao, L.; Korban, S.S.; Han, Y. Variation of ascorbic acid concentration in fruits of cultivated and wild apples. Food Chem. 2017, 225, 132–137. [Google Scholar] [CrossRef]
- Singh, J.; Mirza, A. Influence of ascorbic acid application on quality and storage life of fruits. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 4319–4328. [Google Scholar] [CrossRef]
- Durán-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar signaling during fruit ripening. Front. Plant Sci. 2020, 11, 564917. [Google Scholar] [CrossRef]
- Decros, G.; Baldet, P.; Beauvoit, B.; Stevens, R.; Flandin, A.; Colombié, S.; Gibon, Y.; Pétriacq, P. Get the balance right: ROS homeostasis and redox signalling in fruit. Front. Plant Sci. 2019, 10, 1091. [Google Scholar] [CrossRef]
- Kaushal, S. Reactive oxygen species (ROS) and associated scavenging mechanisms during fruit ripening. Botany 2016, 66, 176–186. [Google Scholar]
- Jin, J.; Wang, W.; Fan, D.; Hao, Q.; Jia, W. Emerging Roles of Mitogen-Activated Protein Kinase Signaling Pathways in the Regulation of Fruit Ripening and Postharvest Quality. Int. J. Mol. Sci. 2024, 25, 2831. [Google Scholar] [CrossRef]
- Li, S.; Chen, K.; Grierson, D. Molecular and hormonal mechanisms regulating fleshy fruit ripening. Cells 2021, 10, 1136. [Google Scholar] [CrossRef]
- Wang, W.; Fan, D.; Hao, Q.; Jia, W. Signal transduction in non-climacteric fruit ripening. Hortic. Res. 2022, 9, uhac190. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.; Ji, H.; Sun, M.; Liu, T.; Wang, J.; Cao, H.; Zhu, Q. Ethylene biosynthesis and signal transduction during ripening and softening in non-climacteric fruits: An overview. Front. Plant Sci. 2024, 15, 1368692. [Google Scholar] [CrossRef]
- Patwardhan, R.; Sharma, D.; Checker, R.; Thoh, M.; Sandur, S. Spatio-temporal changes in glutathione and thioredoxin redox couples during ionizing radiation-induced oxidative stress regulate tumor radio-resistance. Free Radic. Res. 2015, 49, 1218–1232. [Google Scholar] [CrossRef]
- Sati, H.; Khandelwal, A.; Pareek, S. Effect of exogenous melatonin in fruit postharvest, crosstalk with hormones, and defense mechanism for oxidative stress management. Food Front. 2023, 4, 233–261. [Google Scholar] [CrossRef]
- Storozhenko, S.; Belles-Boix, E.; Babiychuk, E.; Hérouart, D.; Davey, M.W.; Slooten, L.; Van Montagu, M.; Inzé, D.; Kushnir, S. γ-Glutamyl transpeptidase in transgenic tobacco plants. Cellular localization, processing, and biochemical properties. Plant Physiol. 2002, 128, 1109–1119. [Google Scholar] [CrossRef]
- Martin, M.N.; Saladores, P.H.; Lambert, E.; Hudson, A.O.; Leustek, T. Localization of members of the γ-glutamyl transpeptidase family identifies sites of glutathione and glutathione S-conjugate hydrolysis. Plant Physiol. 2007, 144, 1715–1732. [Google Scholar] [CrossRef]
- Sorrequieta, A.; Ferraro, G.; Boggio, S.B.; Valle, E.M. Free amino acid production during tomato fruit ripening: A focus on l-glutamate. Amino Acids 2010, 38, 1523–1532. [Google Scholar] [CrossRef]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Palma, J.M.; Corpas, F.J. NADPH as a quality footprinting in horticultural crops marketability. Trends Food Sci. Technol. 2020, 103, 152–161. [Google Scholar] [CrossRef]
- Mittler, R.; Lam, E.; Shulaev, V.; Cohen, M. Signals controlling the expression of cytosolic ascorbate peroxidase during pathogen-induced programmed cell death in tobacco. Plant Mol. Biol. 1999, 39, 1025–1035. [Google Scholar] [CrossRef]
- Wang, L.; Li, R.; Shi, X.; Wei, L.; Li, W.; Shao, Y. Ripening patterns (off-tree and on-tree) affect physiology, quality, and ascorbic acid metabolism of mango fruit (cv. Guifei). Sci. Hortic. 2023, 315, 111971. [Google Scholar] [CrossRef]
- Huan, C.; Jiang, L.; An, X.; Yu, M.; Xu, Y.; Ma, R.; Yu, Z. Potential role of reactive oxygen species and antioxidant genes in the regulation of peach fruit development and ripening. Plant Physiol. Biochem. 2016, 104, 294–303. [Google Scholar] [CrossRef]
- Tolin, S.; Arrigoni, G.; Trentin, A.R.; Veljovic-Jovanovic, S.; Pivato, M.; Zechman, B.; Masi, A. Biochemical and quantitative proteomics investigations in Arabidopsis ggt1 mutant leaves reveal a role for the gamma-glutamyl cycle in plant’s adaptation to environment. Proteomics 2013, 13, 2031–2045. [Google Scholar] [CrossRef]
- Destro, T.; Prasad, D.; Martignago, D.; Lliso Bernet, I.; Trentin, A.R.; Renu, I.K.; Ferretti, M.; Masi, A. Compensatory expression and substrate inducibility of γ-glutamyl transferase GGT2 isoform in Arabidopsis thaliana. J. Exp. Bot. 2011, 62, 805–814. [Google Scholar] [CrossRef]
- Shi, H.-Y.; Li, Z.-H.; Zhang, Y.-X.; Chen, L.; Xiang, D.-Y.; Zhang, Y.-F. Two pear glutathione S-transferases genes are regulated during fruit development and involved in response to salicylic acid, auxin, and glucose signaling. PLoS ONE 2014, 9, e89926. [Google Scholar] [CrossRef]
- Pechanova, O.; Pechan, T.; Williams, W.P.; Luthe, D.S. Proteomic analysis of the maize rachis: Potential roles of constitutive and induced proteins in resistance to Aspergillus flavus infection and aflatoxin accumulation. Proteomics 2011, 11, 114–127. [Google Scholar] [CrossRef]
- Vaish, S.; Gupta, D.; Mehrotra, R.; Mehrotra, S.; Basantani, M.K. Glutathione S-transferase: A versatile protein family. 3 Biotech 2020, 10, 321. [Google Scholar] [CrossRef]
- Wei, M.; Ge, Y.; Li, C.; Han, X.; Qin, S.; Chen, Y.; Tang, Q.; Li, J. G6PDH regulated NADPH production and reactive oxygen species metabolism to enhance disease resistance against blue mold in apple fruit by acibenzolar-S-methyl. Postharvest Biol. Technol. 2019, 148, 228–235. [Google Scholar] [CrossRef]
- Bradfield, M.F.; Nicol, W. The pentose phosphate pathway leads to enhanced succinic acid flux in biofilms of wild-type Actinobacillus succinogenes. Appl. Microbiol. Biotechnol. 2016, 100, 9641–9652. [Google Scholar] [CrossRef]
- Guo, X.-H.; Yan, H.; Zhang, Y.; Yi, W.; Huang, S.-X.; Liu, Y.-S.; Wei, L. Kiwifruit (Actinidia chinensis ‘Hongyang’) cytosolic ascorbate peroxidases (AcAPX1 and AcAPX2) enhance salinity tolerance in Arabidopsis thaliana. J. Integrative Agric. 2022, 21, 1058–1070. [Google Scholar] [CrossRef]
- Alós, E.; Rodrigo, M.J.; Zacarías, L. Differential transcriptional regulation of L-ascorbic acid content in peel and pulp of citrus fruits during development and maturation. Planta 2014, 239, 1113–1128. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, J.; Li, X. Transcriptomic analysis reveals the metabolic mechanism of L-ascorbic acid in Ziziphus jujuba Mill. Front. Plant Sci. 2016, 7, 172973. [Google Scholar] [CrossRef]
- Abdelgawad, F.K.; El-Mogy, M.M.; IA Mohamed, M.; Garchery, C.G.; Stevens, R. Increasing ascorbic acid content and salinity tolerance of cherry tomato plants by suppressed expression of the ascorbate oxidase gene. Agronomy 2019, 9, 51. [Google Scholar] [CrossRef]
- Fotopoulos, V.; Sanmartin, M.; Kanellis, A.K. Effect of ascorbate oxidase over-expression on ascorbate recycling gene expression in response to agents imposing oxidative stress. J. Exp. Bot. 2006, 57, 3933–3943. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahir, H.; Sajjad, M.; Qian, M.; Zeeshan Ul Haq, M.; Tahir, A.; Chen, T.; Shaopu, S.; Farooq, M.A.; Ling, W.; Zhou, K. Transcriptomic Analysis Reveals Dynamic Changes in Glutathione and Ascorbic Acid Content in Mango Pulp across Growth and Development Stages. Horticulturae 2024, 10, 694. https://doi.org/10.3390/horticulturae10070694
Tahir H, Sajjad M, Qian M, Zeeshan Ul Haq M, Tahir A, Chen T, Shaopu S, Farooq MA, Ling W, Zhou K. Transcriptomic Analysis Reveals Dynamic Changes in Glutathione and Ascorbic Acid Content in Mango Pulp across Growth and Development Stages. Horticulturae. 2024; 10(7):694. https://doi.org/10.3390/horticulturae10070694
Chicago/Turabian StyleTahir, Hassam, Muhammad Sajjad, Minjie Qian, Muhammad Zeeshan Ul Haq, Ashar Tahir, Tiantian Chen, Shi Shaopu, Muhammad Aamir Farooq, Wei Ling, and Kaibing Zhou. 2024. "Transcriptomic Analysis Reveals Dynamic Changes in Glutathione and Ascorbic Acid Content in Mango Pulp across Growth and Development Stages" Horticulturae 10, no. 7: 694. https://doi.org/10.3390/horticulturae10070694




