Optimization of Extraction Method of Bioactive Compounds from Elderberries (Sambucus nigra L.) and Testing Extract Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemical and Reagents
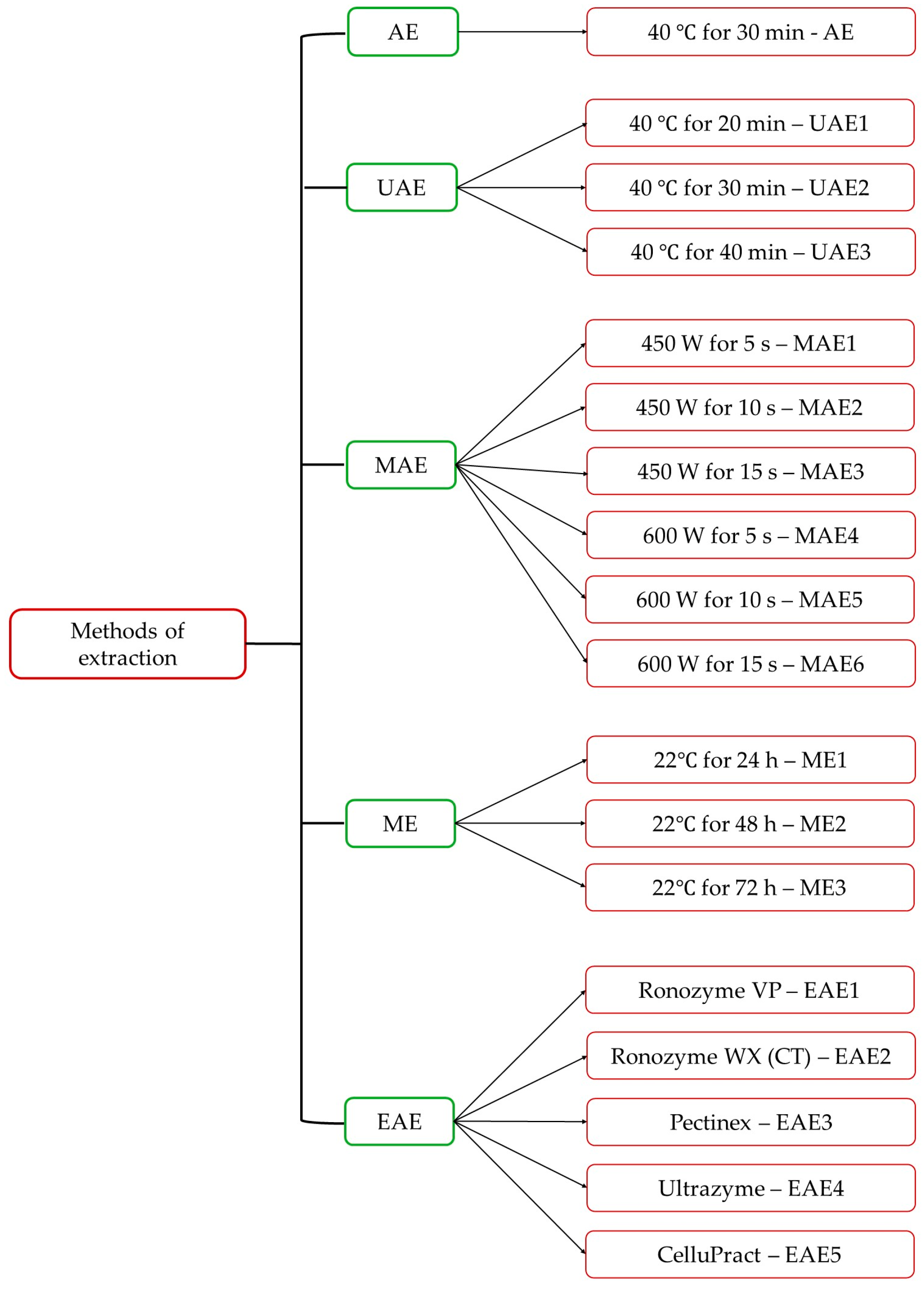
2.3. Extraction Methods
2.3.1. Extraction with Continuous Agitation (AE)
2.3.2. Extraction Assisted by Ultrasounds (UAE)
2.3.3. Extraction Assisted by Microwaves (MAE)
2.3.4. Maceration (ME)
2.3.5. Enzyme-Assisted Extraction (EAE)
2.4. Optimization of Extraction Parameters
2.5. Effects of Concentration, Lyophilization, and Storage on EDB Extracts
2.6. Chemical Profile
2.6.1. Total Phenolic Content (TPC)
2.6.2. Total Flavonoid Content (TFC)
2.6.3. Total Anthocyanin Content (TAC) and Monomeric Anthocyanin Content (MAC)
2.6.4. Individual Polyphenol Content Detected by High-Performance Liquid Chromatography (HPLC)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Profile of the Extracts Obtained by Different Extraction Methods
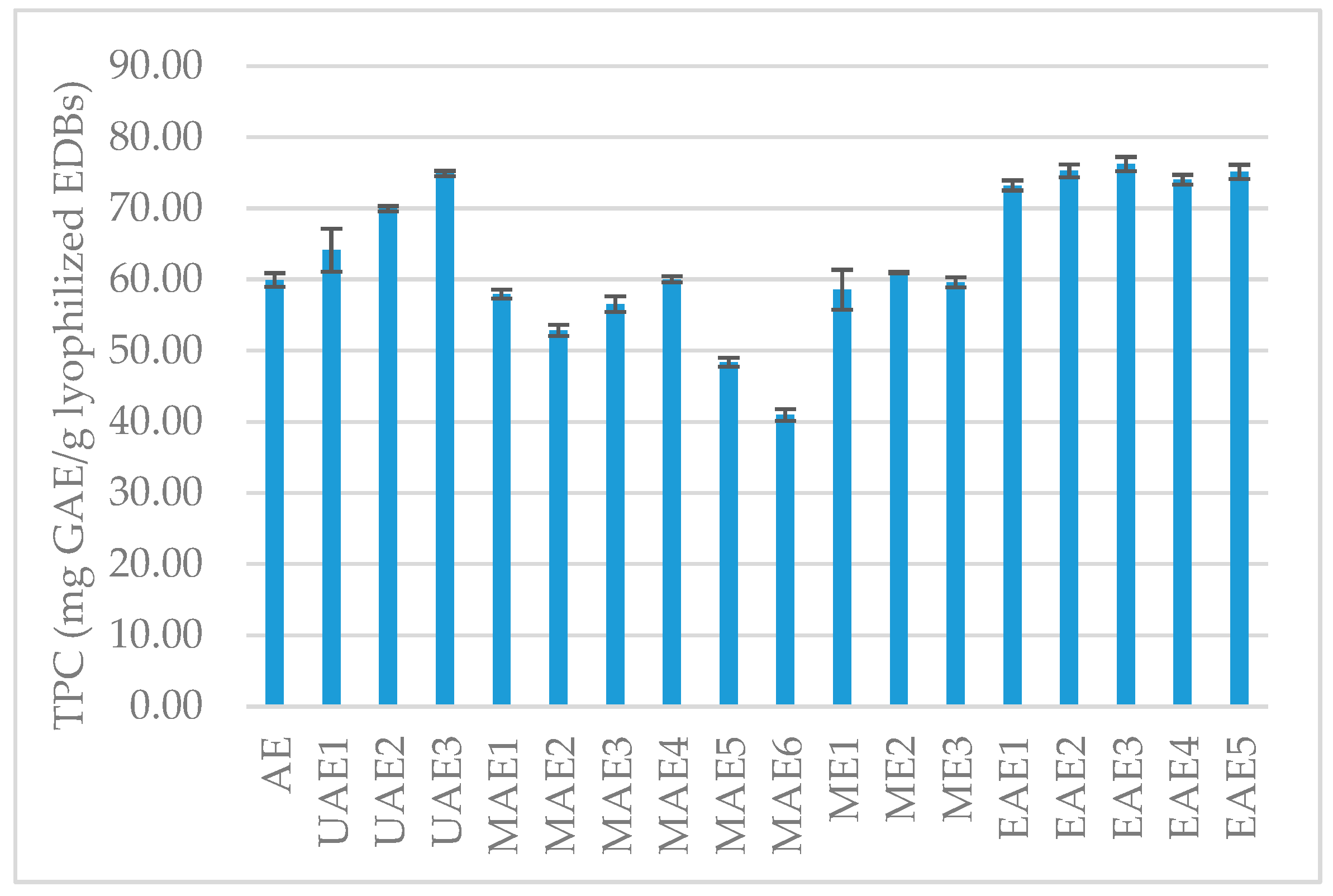
3.1.1. Total Phenolic Content
3.1.2. Total Flavonoid Content, Total and Monomeric Anthocyanin Content
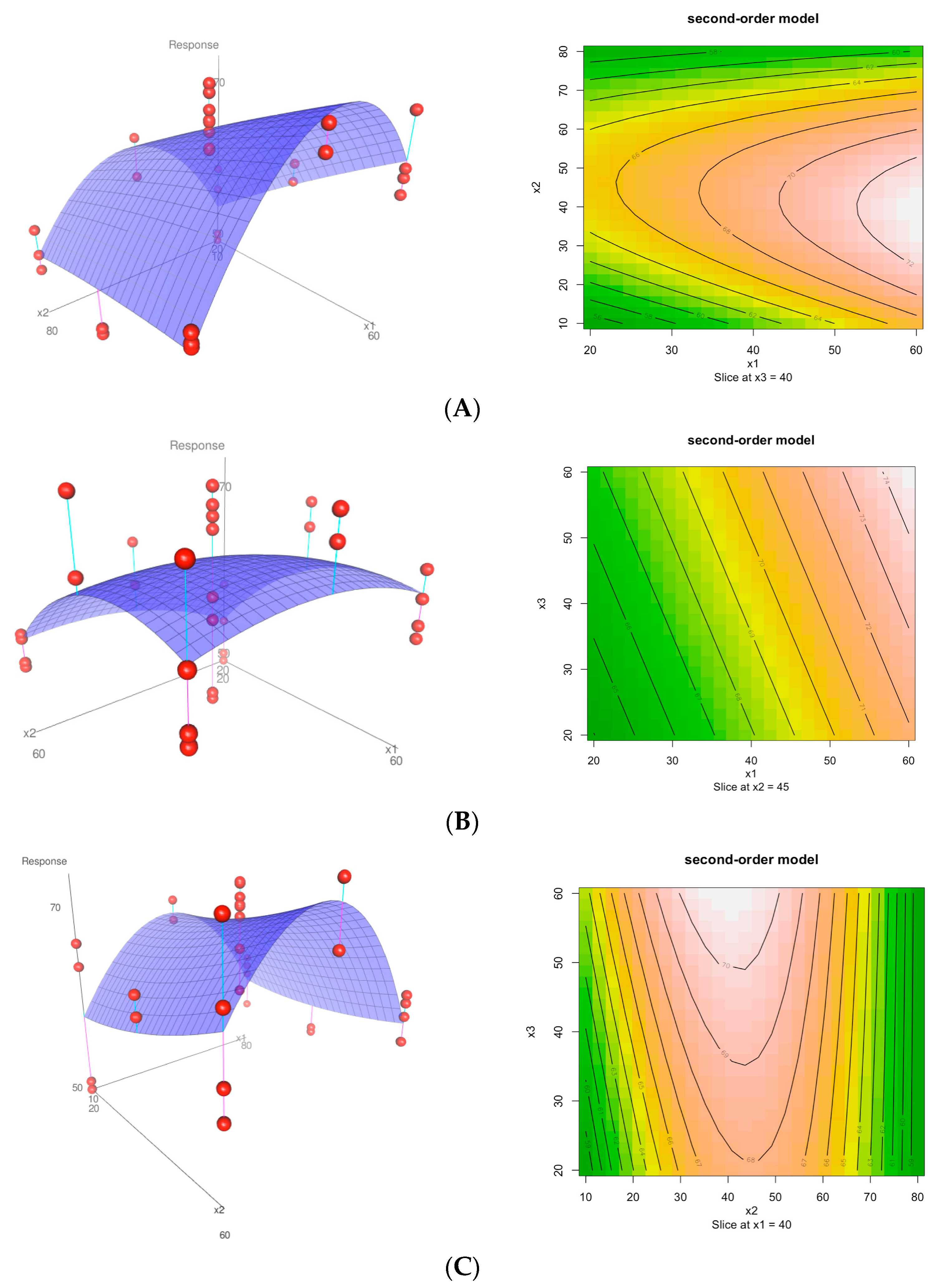
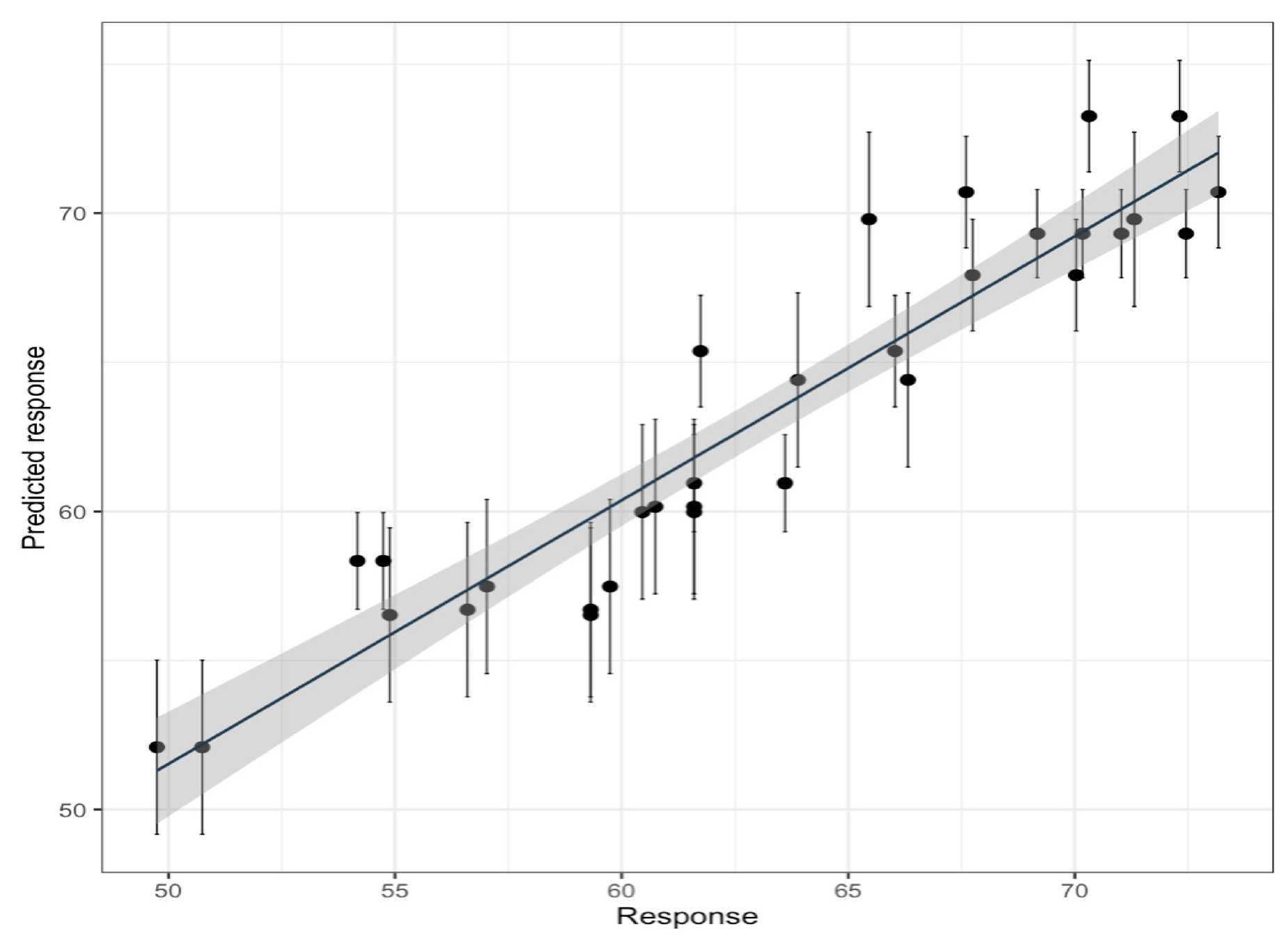
3.2. Optimization of the Extraction Parameters
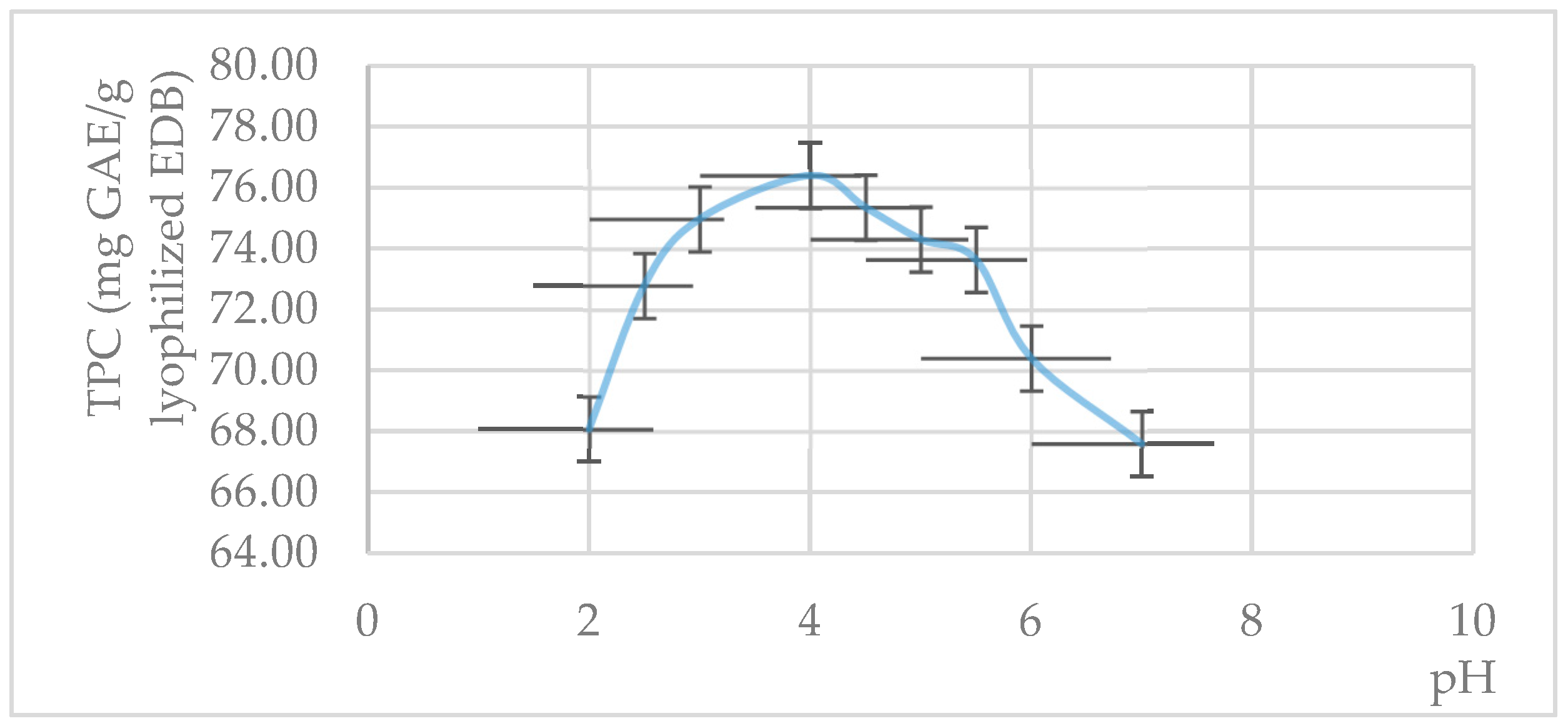
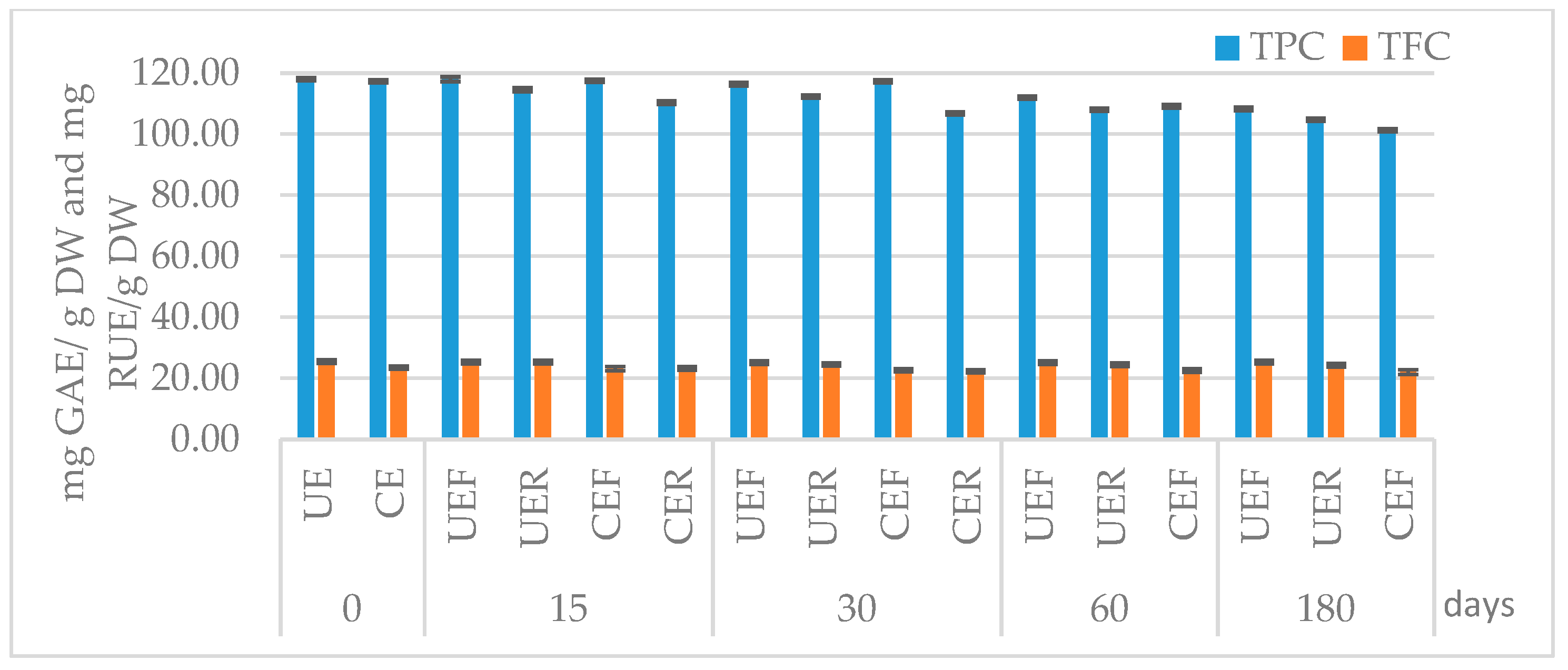
3.3. Effects of Concentration, Lyophilization, and Storage on Extract Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nichita, C.; Neagu, G.; Cucu, A.; Vulturescu, V.; Vifor, Ș.; Berteşteanu, G. Antioxidative properties of Plantago anceola L. extracts evaluated by chemiluminescence method. AgroLife Sci. J. 2016, 2, 95–102. [Google Scholar]
- Aguiar, J.; Estevinho, B.N.; Santos, L. Microencapsulation of natural antioxidants for food application—The specific case of coffee antioxidants—A review. Trends Food Sci. Technol. 2016, 58, 21–39. [Google Scholar] [CrossRef]
- Pap, N.; Fidelis, M.; Azevedo, L.; doCarmo, M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B.; et al. Berrypolyphenols and human health: Evidence of antioxidant, anti-inflammatory, microbiota modulation, and cell-protecting effects. Curr. Opin. Food Sci. 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Duthie, S.J. Berry phytochemicals, genomic stability and cancer: Evidence for chemoprotection at several stages in the carcinogenic process. Mol. Nutr. Food. Res. 2007, 51, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Varsta, M.; Popa, M.E. The influence of processing on active—Biologically compounds of some berries—A review. Sci. Bull. Ser. F Biotechnol. 2015, 19, 206–210. [Google Scholar]
- Curtis, P.J.; van der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M.; et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome—Results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Habanova, M.; Saraiva, J.A.; Holovicova, M.; Moreira, S.A.; Fidalgo, L.G.; Haban, M.; Gazo, J.; Schwarzova, M.; Chlebo, P.; Bronkowska, M. Effect of berries/apple mixed juice consumption on the positive modulation of human lipid profile. J. Funct. Foods 2019, 60, 103417. [Google Scholar] [CrossRef]
- Chaves, V.C.; Soares, M.S.P.; Spohr, L.; Teixeira, F.; Vieira, A.; Constantino, L.S.; Pizzol, F.D.; Lencina, C.L.; Spanevello, R.M.; Freitas, M.P.; et al. Blackberry extract improves behavioral and neurochemical dysfunctions in a ketamine-induced rat model of mania. Neurosci. Lett. 2020, 714, 134566. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.; Malik, A.; Ayyaz, U.; Shafique, H.; Rana, Z.; Hussain, Z. Efficient hepatoprotective activity of cranberry extract against CCl4-induced hepatotoxicity in Wistar albino rat model: Down regulation of liver enzymes and strong antioxidant activity. Asian Pac. J. Trop. Med. 2017, 10, 1054–1058. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-suarez, J.M.; Cordero, M.D.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Santos-Buelga, C.; Gonzalez-Paramas, A.M.; Astolfi, P.; Rubini, C.; et al. Strawberry consumption improves aging-associated impairments, mitochondrial biogenesis and functionality through the AMP-activated protein kinase signaling cascade. Food Chem. 2017, 234, 464–471. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, A.; Olkowicz, M.; Kowalska, K.; Rychlik, J.; Dembczynski, R.; Myszka, K.; Juzwa, W.; Białas, W.; Moyer, M.P. Gastrointestinal digested Sambucus nigra L. fruit extract protects in vitro cultured human colon cells against oxidative stress. Food Chem. 2016, 197, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Sidor, A.; Gramza-Michałowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food—A review. J. Funct. Foods 2014, 18, 941–958. [Google Scholar] [CrossRef]
- Gleńsk, M.; Glinski, J.A.; Wlodarczyk, M.; Stefanowicz, P. Determination of ursolic and oleanolic acid in Sambucus fruits. Chem. Biodiver. 2014, 11, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Krawitz, C.; Mraheil, M.A.; Stein, M.; Imirzalioglu, C.; Domann, E.; Pleschka, S.; Hain, T. Inhibitory activity of a standardized elderberry liquid extract against clinically-relevant human respiratory bacterial pathogens and influenza A and B viruses. BMC Complement. Altern. Med. 2011, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Boroduske, A.; Jekabsons, K.; Riekstina, U.; Muceniece, R.; Rostoks, N.; Nakurte, I. Wild Sambucus nigra L. from north-east edge of the species range: A valuable germplasm with inhibitory capacity against SARS-CoV2 S-protein RBD and hACE2 binding in vitro. Ind. Crops Prod. 2021, 165, 113438. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Baker, C.; Cherry, L.; Dunne, E. Black elderberry (Sambucus nigra) supplementation effectively treats upper respiratory symptoms: A meta-analysis of randomized, controlled clinical trials. Complement. Ther. Med. 2019, 42, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Harnett, J.; Oakes, K.; Carè, J.; Leach, M.; Brown, D.; Cramer, H.; Pinder, T.A.; Steel, A.; Anheyer, D. The effects of Sambucus nigra berry on acute respiratory viral infections: A rapid review of clinical studies. Adv. Integr. Med. 2020, 7, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Akduman, G.; Korkmaz, S.; Taşkın, T.; Güneş, E.F. Cytotoxicity of Sambucus nigra L. on Cancer Cell Line and In Vitro Antioxidant Properties. Clin. Exp. Health Sci. 2023, 13, 896–901. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: Causes and therapeutic strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Salvador, Â.C.; Król, E.; Lemos, V.C.; Santos, S.A.; Bento, F.P.; Costa, C.P.; Almeida, A.; Szczepankiewicz, D.; Kulczyński, B.; Krejpcio, Z.; et al. Effect of elderberry (Sambucus nigra L.) extract supplementation in STZ-induced diabetic rats fed with a high-fat diet. Int. J. Mol. Sci. 2017, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Opriș, R.; Tatomirn, C.; Olteanu, D.; Moldovan, R.; Moldovan, B.; David, L.; Nagy, A.; Decea, N.; Kiss, M.L.; Filip, G.A. The effect of Sambucus nigra L. extract and phytosinthesized gold nanoparticles on diabetic rats. Colloids Surf. B Biointerfaces 2017, 150, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.S.; Bode, R.F. A review of the antiviral properties of black elder (Sambucus nigra L.) products. Phytother. Res. 2017, 31, 533–554. [Google Scholar] [CrossRef] [PubMed]
- Zielinska-Wasielica, J.; Olejnik, A.; Kowalska, K.; Olkowicz, M.; Dembczynski, R. Elderberry (Sambucus nigra L.) fruit extract alleviates oxidative stress, insulin resistance, and inflammation in hypertrophied 3T3-L1 adipocytes and activated RAW 264.7 macrophages. Foods 2019, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. The higher the better? Differences in phenolics and cyanogenic glycosides in Sambucus nigra leaves, flowers and berries from different altitudes. J. Sci. Food Agric. 2016, 97, 2623–2632. [Google Scholar] [CrossRef] [PubMed]
- Senica, M.F.; Stampar, R.; Veberic, R.; Mikulic-Petkovsek, M. Processed elderberry (Sambucus nigra L.) products: A beneficial or harmful food alternative? LWT Food Sci. Technol. 2016, 72, 182–188. [Google Scholar] [CrossRef]
- Schmitzer, V.; Veberic, R.; Slatnar, A.; Stampar, F. Elderberry (Sambucus nigra L.) wine: A product rich in health promoting compounds. J. Agric. Food Chem. 2010, 58, 10143–10146. [Google Scholar] [CrossRef] [PubMed]
- Cais-Sokolińska, D.; Walkowiak-Tomczak, D. Consumer-perception, nutritional, and functional studies of a yogurt with restructured elderberry juice. J. Dairy Sci. 2021, 104, 1318–1335. [Google Scholar] [CrossRef]
- Najgebauer-Lejko, D.; Liszka, K.; Tabaszewska, M.; Domagała, J. Probiotic Yoghurts with Sea Buckthorn, Elderberry, and Sloe Fruit Purees. Molecules 2021, 26, 2345. [Google Scholar] [CrossRef]
- Jin, S.K.; Kim, G.D.; Jeong, J.Y. Evaluation of the Effect of Inhibiting Lipid Oxidation of Natural Plant Sources in a Meat Model System. J. Food Qual. 2021, 2021, 6636335. [Google Scholar] [CrossRef]
- Cordeiro, T.; Viegas, O.; Silva, M.; Martins, Z.E.; Fernandes, I.; Ferreira, I.M.L.P.V.O.; Pinho, O.; Mateus, N.; Calhau, C. Inhibitory effect of vinegars on the formation of polycyclic aromatic hydrocarbons in charcoal-grilled pork. Meat Sci. 2020, 167, 108083. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczyk, K.; Walkowiak-Tomczak, D.; Lysiak, G.P. Bioactive properties of Sambucus nigra L. As a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; He, X.-Q.; Wu, D.-T.; Li, H.-B.; Feng, Y.-B.; Zou, L.; Gan, R.-Y. Elderberry (Sambucus nigra L.): Bioactive Compounds, Health Functions, and Applications. J. Agric. Food Chem. 2022, 70, 4202–4220. [Google Scholar] [CrossRef] [PubMed]
- Stănciuc, N.; Oancea, A.M.; Aprodu, I.; Turturică, M.; Barbu, V.; Ioniţă, E.; Râpeanu, G.; Bahrim, G. Investigations on binding mechanism of bioactives from elderberry (Sambucus nigra L.) by whey proteins for efficient microencapsulation. J. Food Eng. 2018, 223, 197–207. [Google Scholar] [CrossRef]
- Silva, P.; Ferreira, S.; Nunes, F.M. Elderberry (Sambucus nigra L.) by-products a source of anthocyanins and antioxidant polyphenols. Ind. Crops Prod. 2017, 95, 227–234. [Google Scholar] [CrossRef]
- Hubbermann, E.M.; Heins, A.; Stöckmann, H.; Schwarz, K. Influence of acids, salt, sugars and hydrocolloids on the colour stability of anthocyanin rich black currant and elderberry concentrates. Eur. Food Res. Technol. 2006, 223, 83–90. [Google Scholar] [CrossRef]
- Przybylska-Balcerek, A.; Szablewski, T.; Szwajkowska-Michałek, L.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z.; Suchowilska, E.; Tomczyk, Ł.; Stuper-Szablewska, K. Sambucus nigra Extracts–Natural Antioxidants and Antimicrobial Compounds. Molecules 2021, 26, 2910. [Google Scholar] [CrossRef] [PubMed]
- Česlová, L.; Kalendová, P.; Dubnová, L.; Pernica, M.; Fischer, J. The Effect of Sample Pretreatment on the Anthocyanin Content in Czech Wild Elderberry (Sambucus nigra L.). Molecules 2023, 28, 6690. [Google Scholar] [CrossRef] [PubMed]
- Floares, D.; Cocan, I.; Alexa, E.; Poiana, M.-A.; Berbecea, A.; Boldea, M.V.; Negrea, M.; Obistioiu, D.; Radulov, I. Influence of Extraction Methods on the Phytochemical Profile of Sambucus nigra L. Agronomy 2023, 13, 3061. [Google Scholar] [CrossRef]
- Braga, M.E.M.; Seabra, I.J.; Ama, D.; De Sousa, H.C. Recent trends and perspectives for the extraction of natural products. In Natural Product Extraction: Principles and Applications; Rostagno, M.A., Prado, J.M., Eds.; RSC Publishing: Cambridge, UK, 2013; pp. 231–275. [Google Scholar]
- Seabra, I.J.; Braga, M.E.M.; Batista, M.T.; de Sousa, H.C. Effect of solvent (CO2/ethanol/H2O) on the fractionated enhanced solvent extraction of anthocyanins from elderberry pomace. J. Supercrit. Fluids 2010, 54, 145–152. [Google Scholar] [CrossRef]
- Seabra, I.J.; Braga, M.E.M.; Batista, M.T.P.; de Sousa, H.C. Fractioned High Pressure Extraction of Anthocyanins from Elderberry (Sambucus nigra L.) Pomace. Food Bioprocess Technol. 2010, 3, 674–683. [Google Scholar] [CrossRef]
- Salamon, I.; Mariychuk, R.; Grulova, D. Optimal Extraction of Pure Anthocyanins from Fruits of Sambucus nigra. Acta Hortic. 2015, 1061, 73–78. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). LWT 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Duymuş, H.G.; Göger, F.; Başer, K.H.C. In Vitro Antioxidant Properties and Anthocyanin Compositions of Elderberry Extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef]
- Mattson, M.L.; Corfield, R.; Bajda, L.; Pérez, O.E.; Schebor, C.; Salvatori, D.M. Potential bioactive ingredient from elderberry fruit: Process optimization for a maximum phenolic recovery, physicochemical characterization, and bioaccesibility. J. Berry Res. 2021, 11, 51–68. [Google Scholar] [CrossRef]
- Radványi, D.; Juhász, R.; Kun, S.Z.; Szabó-Nótin, B.; Barta, J. Preliminary study of extraction of biologically active compounds from elderberry (Sambucus nigra L.) pomace. Acta Aliment. 2013, 42, 63–72. [Google Scholar] [CrossRef]
- dos Santos Nascimento, L.B.; Gori, A.; Degano, I.; Mandoli, A.; Ferrini, F.; Brunetti, C. Comparison between Fermentation and Ultrasound-Assisted Extraction: Which Is the Most Efficient Method to Obtain Antioxidant Polyphenols from Sambucus nigra and Punica granatum Fruits? Horticulturae 2021, 7, 386. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Olech, M.; Oniszczuk, T.; Wojtunik-Kulesza, K.; Wójtowicz, A. Extraction methods, LC-ESI-MS/MS analysis of phenolic compounds and antiradical properties of functional food enriched with elderberry flowers or fruits. Arab. J. Chem. 2016, 12, 4719–4730. [Google Scholar] [CrossRef]
- Sandri, I.G.; Fontana, R.C.; Barfknecht, D.M.; Silveira, M.M. Clarification of fruit juices by fungal pectinases. LWT-Food Sci. Technol. 2011, 44, 2217–2222. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and others oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–153. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, natural colorants, and wines, by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Lenth, R.V. Response-Surface Methods in R, Using rsm. J. Stat. Softw. 2009, 32, 1–17. [Google Scholar] [CrossRef]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Tchabo, W.; Ma, Y.; Engmann, F.N.; Zhang, H. Ultrasound-assisted enzymatic extraction (UAEE) of phytochemical compounds from mulberry (Morus nigra) must and optimization study using response surface methodology. Ind. Crops Prod. 2015, 63, 214–225. [Google Scholar] [CrossRef]
- Suwal, S.; Marciniak, A. Technologies for the Extraction, Separation and Purification of polyphenols—A Review. Nepal J. Biotechnol. 2019, 6, 74–91. [Google Scholar] [CrossRef]
- Denery, J.R.; Dragull, K.; Tang, C.S.; Li, Q.X. Pressurized fluid extraction of carotenoids from Haematococcus pluvialis and Dunaliella salina and kavalactones from Piper methysticum. Anal. Chim. Acta 2004, 501, 175–181. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; García-Ayuso, L.E. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Silva, J.C.; França, P.R.L.; Melob, A.H.F.; Neves-Petersenc, M.T.; Convertie, A.; Porto, T.S. Optimized production of Aspergillus aculeatus URM4953 polygalacturonases for pectin hydrolysis in hog plum (Spondias mombin L.) juice. Process Biochem. 2019, 79, 18–27. [Google Scholar] [CrossRef]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127–266. [Google Scholar] [CrossRef] [PubMed]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Staniek, H.; Kidoń, M.; Łysiak, G.P. The content of selected minerals, bioactive compounds, and the antioxidant properties of the flowers and fruit of selected cultivars and wildly growing plants of Sambucus nigra L. Molecules 2020, 25, 876. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.C.; Galgano, F.; Grippo, A.; Condelli, N.; Di Cairano, M.; Tolve, R. Assay of Healthful Properties of Wild Blackberry and Elderberry Fruits Grown in Mediterranean Area. J. Food Meas. Charact. 2019, 13, 1591–1598. [Google Scholar] [CrossRef]
- Viapiana, A.; Wesolowski, M. The Phenolic Contents and Antioxidant Activities of Infusions of Sambucus nigra L. Plant Foods Hum. Nutr. 2017, 72, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Liazid, A.; Guerrero, R.F.; Cantos, E.; Palma, M.; Barroso, C.G. Microwave assisted extraction of anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Garofulić, I.E.; Dragović-Uzelac, V.; Režek, J.A.; Jukić, M. The effect of microwave assisted extraction on the isolation of anthocyanins and phenolic acids from sour cherry Marasca (Prunus cerasus var. Marasca). J. Food Eng. 2013, 117, 437–442. [Google Scholar] [CrossRef]
- Dangles, O.; Fenger, J.A. The Chemical Reactivity of Anthocyanins and its Consequences in Food Science and Nutrition. Molecules 2018, 23, 1970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.A.; Shen, H.; Fan, X.H.; Shen, Y.; Wang, X.; Song, Y. Changes of gallic acid mediated by ultrasound in a model extraction solution. Ultrason. Sonochem. 2015, 22, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, L.; Preda, D.; Constantinescu-Aruxandei, D.; Oancea, F.; Băbeanu, N. Optimization of ultrasound-assisted extraction of polyphenols from honeysuckle (Lonicera caprifolium). AgroLife Sci. J. 2021, 1, 47–55. [Google Scholar] [CrossRef]
- Liao, J.; Xue, H.; Li, J. Extraction of phenolics and anthocyanins from purple eggplant peels by multi-frequency ultrasound: Effects of different extraction factors and optimization using uniform design. Ultrason. Sonochem. 2022, 90, 106174. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- George, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of Thermal Processing on Anthocyanin Stability in Foods; Mechanisms and Kinetics of Degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004; p. 576. [Google Scholar] [CrossRef]
- Speroni, F.; Tullio, V.; Litterio, N.; Caccia, R.; Piccinini, R.; Rossetti, L.; Tullio, V. Inactivation of microorganisms in liquids: A review of factors influencing the antimicrobial effectiveness of ethanol. J. Water Health 2020, 18, 667–683. [Google Scholar]
- Salazar-Orbea, G.L.; García-Villalba, R.; Bernal, M.J.; Hernández-Jiménez, A.; Egea, J.A.; Tomás-Barberán, F.A.; Sánchez-Siles, L.M. Effect of Storage Conditions on the Stability of Polyphenols of Apple and Strawberry Purees Produced at Industrial Scale by Different Processing Techniques. J. Agric. Food. Chem. 2023, 71, 2541–2553. [Google Scholar] [CrossRef]
- Cătunescu, G.M.; Rotar, I.; Vidican, R.; Rotar, A.M. Effect of cold storage on antioxidants from minimally processed herbs. Sci. Bull. Ser. F Biotechnol. 2017, 2, 121–126. [Google Scholar]


| Factor | Range and Levels | |||
|---|---|---|---|---|
| Independent variables | Xi | −1 | 0 | 1 |
| Temperature (°C) | X1 | 20 | 40 | 60 |
| Concentration (% ethanol) | X2 | 10 | 45 | 80 |
| Time (min) | X3 | 20 | 40 | 60 |
| Extraction Method | Gallic Acid (mg/g) | Chlorogenic Acid (mg/g) | Caffeic Acid (mg/g) | Syringic Acid (mg/g) | 4-Coumaric Acid (mg/g) | Rutin (mg/g) |
|---|---|---|---|---|---|---|
| AE | 0.34 ± 0.03 | 0.52 ± 0.02 | ND | ND | 0.11 ± 0.04 | 1.98 ± 0.01 |
| UAE3 | 0.42 ± 0.09 | 0.59 ± 0.01 | ND | ND | 0.17 ± 0.03 | 2.43 ± 0.01 |
| MAE1 | 0.27 ± 0.09 | 0.49 ± 0.02 | ND | ND | 0.09 ± 0.03 | 2.17 ± 0.02 |
| ME2 | 0.21 ± 0.11 | 0.49 ± 0.02 | ND | ND | 0.15 ± 0.02 | 1.98 ± 0.02 |
| EAE3 | 0.68 ± 0.07 | 0.13 ± 0.01 | 0.20 ± 0.01 | 0.14 ± 0.07 | 0.13 ± 0.06 | 1.87 ± 0.03 |
| Run | Temperature (°C) X1 | Solvent (% Ethanol) X2 | Time (min) X3 | Response TPC (mg GAE/g) |
|---|---|---|---|---|
| 1 | 40 | 45 | 40 | 70.17 ± 0.13 |
| 2 | 60 | 10 | 60 | 65.46 ± 0.30 |
| 3 | 40 | 45 | 60 | 73.17 ± 0.15 |
| 4 | 60 | 80 | 60 | 61.60 ± 0.20 |
| 5 | 60 | 10 | 60 | 71.31 ± 0.08 |
| 6 | 40 | 10 | 40 | 61.60 ± 0.28 |
| 7 | 40 | 80 | 40 | 54.17 ± 0.08 |
| 8 | 20 | 10 | 20 | 49.74 ± 0.14 |
| 9 | 20 | 80 | 20 | 54.89 ± 0.13 |
| 10 | 60 | 10 | 20 | 66.31 ± 0.28 |
| 11 | 60 | 80 | 20 | 60.46 ± 0.25 |
| 12 | 40 | 10 | 40 | 63.60 ± 0.13 |
| 13 | 20 | 45 | 40 | 66.03 ± 0.17 |
| 14 | 40 | 45 | 40 | 69.17 ± 0.01 |
| 15 | 60 | 10 | 20 | 63.89 ± 0.25 |
| 16 | 20 | 80 | 20 | 59.31 ± 0.16 |
| 17 | 20 | 80 | 60 | 59.31 ± 0.09 |
| 18 | 20 | 10 | 20 | 50.74 ± 0.17 |
| 19 | 40 | 80 | 40 | 54.74 ± 0.06 |
| 20 | 60 | 45 | 40 | 72.31 ± 0.32 |
| 21 | 40 | 45 | 40 | 72.46 ± 0.10 |
| 22 | 20 | 10 | 60 | 57.03 ± 0.10 |
| 23 | 60 | 80 | 20 | 61.60 ± 0.35 |
| 24 | 20 | 80 | 60 | 56.60 ± 0.07 |
| 25 | 40 | 45 | 60 | 67.60 ± 0.16 |
| 26 | 40 | 45 | 20 | 67.74 ± 0.03 |
| 27 | 40 | 45 | 20 | 70.03 ± 0.11 |
| 28 | 20 | 45 | 40 | 61.74 ± 0.09 |
| 29 | 60 | 45 | 40 | 70.31 ± 0.05 |
| 30 | 20 | 10 | 60 | 59.74 ± 0.19 |
| 31 | 60 | 80 | 60 | 60.74 ± 0.12 |
| 32 | 40 | 45 | 40 | 71.03 ± 0.11 |
| Type of Extract | Gallic Acid (mg/g DW) | Chlorogenic Acid (mg/g DW) | Caffeic Acid (mg/g DW) | Syringic Acid (mg/g DW) | 4-Coumaric Acid (mg/g DW) | Rutin (mg/g DW) |
|---|---|---|---|---|---|---|
| After 1 day | ||||||
| UE | 0.24 ± 0.02 | 0.92 ± 0.02 | ND | ND | 0.15 ± 0.02 | 3.61 ± 0.02 |
| CE | <loQ | 0.92 ± 0.03 | ND | ND | 0.11 ± 0.02 | 3.55 ± 0.01 |
| After 15 days | ||||||
| UEF | <loQ | 0.92 ± 0.01 | ND | ND | 0.14 ± 0.02 | 3.56 ± 0.03 |
| UER | <loQ | 0.91 ± 0.00 | ND | ND | 0.16 ± 0.01 | 3.38 ± 0.02 |
| CEF | <loQ | 0.91 ± 0.01 | ND | ND | 0.08 ± 0.01 | 3.42 ± 0.01 |
| CER | <loQ | 0.85 ± 0.02 | ND | ND | 0.07 ± 0.01 | 3.34 ± 0.00 |
| After 180 days | ||||||
| UEF | <loQ | 0.86 ± 0.02 | ND | ND | 0.10 ± 0.01 | 3.02 ± 0.02 |
| UER | <loQ | 0.80 ± 0.01 | ND | ND | 0.14 ± 0.02 | 2.71 ± 0.02 |
| CEF | <loQ | 0.79 ± 0.03 | ND | ND | 0.04 ± 0.01 | 2.91 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascariu, O.-E.; Dias, L.G.; Israel-Roming, F. Optimization of Extraction Method of Bioactive Compounds from Elderberries (Sambucus nigra L.) and Testing Extract Stability. Horticulturae 2024, 10, 743. https://doi.org/10.3390/horticulturae10070743
Pascariu O-E, Dias LG, Israel-Roming F. Optimization of Extraction Method of Bioactive Compounds from Elderberries (Sambucus nigra L.) and Testing Extract Stability. Horticulturae. 2024; 10(7):743. https://doi.org/10.3390/horticulturae10070743
Chicago/Turabian StylePascariu, Oana-Elena, Luís Guimarães Dias, and Florentina Israel-Roming. 2024. "Optimization of Extraction Method of Bioactive Compounds from Elderberries (Sambucus nigra L.) and Testing Extract Stability" Horticulturae 10, no. 7: 743. https://doi.org/10.3390/horticulturae10070743
APA StylePascariu, O. -E., Dias, L. G., & Israel-Roming, F. (2024). Optimization of Extraction Method of Bioactive Compounds from Elderberries (Sambucus nigra L.) and Testing Extract Stability. Horticulturae, 10(7), 743. https://doi.org/10.3390/horticulturae10070743









