Abstract
Ferns are vascular plants with significant ornamental value, and in recent years, they have also been considered for the potential application in different economic sectors like nutrition, medicine and cosmetics. This study aimed to obtain in vitro fern gametophytes and sporophytes and evaluate their potential as secondary metabolites sources. For this study four fern species were used: Polypodium vulgare L. (PV), Asplenium trichomanes L. (AT), Athyrium filix-femina L. Roth (AF), and Osmunda regalis L. (OR). The polyphenols, flavonoids, tannins and terpenoids content and antioxidant activity were estimated by different colorimetric methods. For metabolite identification and their antioxidant activity, HP-TLC separations were used. Also, quantitative HPLC–DAD analysis was performed to estimate the content of certain metabolites. The results showed that in vitro generation of gametophytes registered higher growth rates in OR, PV, and AF, while the regeneration of sporophytes was better for PV, AT, and AF. The OR gametophyte produced the highest quantities in all analyzed metabolite classes and also had the highest antioxidant values. We report for the first time the presence of metabolites such as catechin, caffeic acid, gallic acid, ferulic acid, cinnamic acid, or rutin in OR. In this study, we demonstrated the possibility of producing potent antioxidant metabolites by in vitro cultures in ferns.
1. Introduction
In the last seven decades, significant progress has been made regarding the micropropagation of fern species with ornamental, medicinal, or conservative value. The specialized literature provides useful information on the types of explants, their sterilization, or the culture media used for the in vitro morphogenesis of the gametophyte and the sporophyte, adapted to the biology of different species [1]. Starting from spores, gametophytic, or sporophytic tissues, we can achieve the micropropagation of the species of interest and, through proteomic, transcriptomic, or other advanced analyses, explain the functions and evolution of these plants [2].
In recent years, a series of studies [3,4,5,6,7] have proposed the use of ferns not only for ornamental purposes but also as rich sources of polyphenols and antioxidants useful as food, nutraceuticals, pharmaceutics, and even cosmetics. The polyphenolic content and antioxidant activity in ferns proved to be superior to herbs (peppermint, thyme), medicinal plants, vegetables (spinach, rocket), and spices (turmeric), making them a valuable alternative food source [3]. For this reason, we considered it useful to develop in vitro multiplication methods for four fern species common to the European flora. Most studies conducted on Polypodium vulgare L. and Asplenium trichomanes L. investigated the pharmacological applications of these species, exploiting their recognized ethnobotanical properties, while Athyrium filix-femina L. Roth and Osmunda regalis L. are mostly recognized for their ornamental features.
The medicinal plant potential of P. vulgare (PV) stimulated a particular interest with the recommendation of the European Medicines Agency (EMA) to use the rhizome for the preparation of expectorant herbal medicine in cough, cold, and occasional constipation [8]. In fact, PV has been a remedy since ancient times, the rhizome infusion being used as an expectorant to treat cough and pertussis and diuretic in renal diseases, especially in chronic nephritis and pyelonephritis in traditional Polish medicine [9]. Additionally, it has been used as a therapeutic agent for kidney stone degradation [10], and the fronds were shown to have ethnoveterinary value in various ailments [11]. The rhizome of PV can also be used as a sweetener in the food industry, a quality attributed to its main saponin, osladin [12]. Some studies reported the phytochemical composition of the rhizome and fronds as follows: triterpenes [13], flavonoids derived from catechin [9,14], triterpenoids and saponins glycosides [15], phenylpropanoids [6], phloroglucine derivatives, volatile oils and phytoecdysteroids [16,17]. The PV extract showed multiple biological activities, such as antioxidant, antimicrobial [18], anti-inflamatory [19], wound healing properties [20] and anticholinesterase [21] and antityrosinase activities [6].
The traditional uses of A. trichomanes (AT) were reported for respiratory tract infection as anticatarrhal [22] and antitussive agent [23], but also for the treatment of alopecia and kidney stones [24]. Previous studies considered the fronds of Aspleniaceae genus a reservoir of flavonols as kaempferol and its glycosides [25], isoquercitrin and hyperoside [6], phenolic acids derived from cinnamic acid pathways [26], but also volatile compounds mostly with polyketide structure [27].
A. filix-femina (AF), known as the common lady fern, is a very decorative and polymorphic species, with many named varieties selected for their ornamental values and numerous cultivars developed for garden use [28]. The Native American Ethnobotany Database showed that stem or root tea had been used to relieve labor pains, stop breast pains, and induce milk flow in caked breasts. The dried powdered root has been applied externally to heal wounds, while cooked fiddleheads and rhizomes are used to treat internal ailments, such as pains and cancer of the womb. Additionally, the roots have been used as effective diuretic, antiparasitic, and anthelmintic agents [29]. However, the phytochemical composition of AF is poorly characterized. Currently, studies have described the volatile profiles, showing small amounts of isoprenoids, the main volatile compounds being 2-phenylethanal with lilac and hyacinth odor and 1-octen-3-ol with mushroom-like odor [30]. Soare et al. (2012) [31] reported a good antioxidant activity of the methanolic extract of AF leaves that was positively correlated with the total phenolic content, while another study showed that AF exhibited ABTS radical-scavenging activity [32]. The methanol extracts obtained from AF rhizome and leaves were found to display a strong antibacterial potential against E. coli, S. aureus, and B. megaterium [18].
O. regalis (OR), also known as the royal fern, is a very widespread species with unique aesthetic properties. Although OR has a cosmopolitan distribution and is mentioned as a Least Concern in IUCN Red List [33], the local populations might be critically endangered within particular areas, such as Norway, Switzerland, Croatia, Hungary, and Iran [34]. OR is extinct in the Romanian flora [35,36] and is found only in botanical gardens.
The rhizome of OR has been traditionally employed in Spain, mainly for the treatment of bone fractures, joint disorders, and rheumatic and arthritic pains [37], OR roots were used in the treatment of ulcers and mentioned as a cure for cancer [38]. A recent study investigated the effects of OR root extract on proliferation and invasion in head and neck cancers [39], thus demonstrating its potential use as an anticancer remedy.
OR phytochemical characterization data are scarce, the most studied taxon of this genus being Osmunda japonica Thunb with a rhizome composition consisting of diterpenoids derived from ent-kaurenic acid, anthraquinones, flavonoids, steroids [40], and acetone type phenolics derived from protocatechuic acid [41], but also lactones of the osmundalin type and its derivatives with demonstrated insecticidal effects [42].
Our goal was to establish in vitro gametophyte and sporophyte cultures producing valuable compounds in the following four species of ferns: Polypodium vulgare, Asplenium trichomanes, Athyrium filix-femina and Osmunda regalis. Because pteridophyte species are recognized for their rich polyphenolic content with strong antioxidant activity, the present study aimed to determine the polyphenols, flavonoids, tannins, and triterpenoids concentrations, of the four main classes of metabolites present in the methanolic extracts obtained from in vitro cultures, and to estimate the content of several phenolic acids commonly found in pteridophytes (gallic, caffeic, cinnamic, ferulic, syringic, and chlorogenic acids) and some phytotherapeutically important flavonoids (rutin, quercetin, catechin, and resveratrol stilbene), using HPLC-DAD analyses. To assess the antioxidant properties, different correlation tests between the contents of secondary metabolites and the antioxidant activities against four types of synthetic free radicals (DPPH radical, ABTS anion, Fe3+-TPTZ, and Cu2+-neocuproine ions) were used. Additionally, the antioxidant activity of phenolic compounds, gametophyte, and sporophyte extracts was determined using a HP-TLC separation combined with a DPPH developing method.
2. Materials and Methods
2.1. In Vitro Sporophyte and Gametophyte Cultures
For the initiation of in vitro cultures, spores collected from four species of ferns: Asplenium trichomanes L., Athyrium filix-femina L. Roth, Polypodium vulgare L. from the Valea Vâlsanului protected area, Argeș county (45°20’03.3” N; 024°44’21.0” E), and Osmunda regalis L. from the Dimitrie Brândză Botanical Garden from Bucharest were used.
Spores were sterilized in filter paper packs using 3% H2O2 or 6% CaCl2O2 solutions with Tween 20, with exposure times of 5, 7, and 10 min. Sterilized spores were inoculated on modified half-diluted MS nutritive medium (MS ½), pH 5.8 [43], supplemented with Gambourg’s vitamins [44] and 30 g/L sucrose.
The Petri dishes were kept in the growth room at a temperature of 22–24 ± 2 °C, with a photoperiod of 16 h light/8 h dark and a light intensity of 40.5 µmol/m2s.
After 3 weeks, the differentiation of prothalli was initiated. The gametophyte cultures were obtained by successive passage every two months on 40 mL MS ½ medium in the Erlenmeyer flasks in the same conditions provided by the growth chamber.
To obtain sporophytes in PV, AT, and AF, 1 g of the gametophyte previously obtained was ground in 1 mL MS ½ liquid medium. The mixture was then placed on solid MS ½ medium, and the culture dishes (Erlenmeyer flasks) were kept in the same conditions as previously described.
For OR sporophyte differentiation, 1 g of gametophyte culture was subcultivated on the modified MS ½ medium without ammonium nitrate and vitamins.
Biomass Production Evaluation
The gametophyte biomass production was evaluated by weighing with an analytical balance Radwag AS 220/C/2 (Radwag, Radom, Poland). The growth rate (GR) was calculated as the ratio between the weight of gametophyte after two months of cultivation on nutritive media and the initial weight of the inoculum. To determine the dry weight (DW), 1 g of each fresh gametophyte or sporophyte biomass (FW) was dried at 80 °C in an Ecocell oven (MMM Group, Munich, Germany) until weight variations were no longer recorded. Dry weight and water content were calculated as the percentage from FW. The regeneration rate was calculated as the average number of differentiated sporophytes per gametophyte inoculum.
2.2. Biochemical Analyses
In vitro obtained gametophytes and sporophytes were crushed using a mortar and pestle and mixed with methanol at a ratio of 1:10 (m/v). The samples were homogenized by strongly vortexing for 1 min and then shaked at 180 rpm using a Heidolph Unimax 1010 shaker (Heidolph InstrumentsGmbH, Schwabach, Germany) and incubated at room temperature in the dark for 72 h. The samples representing in homogeneous mixtures were separated at 11,000 G for 15 min, at 4 °C using a Universal 320R centrifuge (Hettich, Westphalia, Germany), and the supernatant was recovered after filtration and stored at 4 °C.
2.2.1. Determination of Total Phenolic Content (PC)
The total phenolic content of the extracts was evaluated by the classic method with the Folin–Ciocâlteu reagent [45]. The results were indicated as milligrams of gallic acid equivalents (GAE)/g of FW or DW, based on the calibration curve with gallic acid (concentrations: 10–100 g/mL, R2 = 0.9968).
2.2.2. Determination of Total Flavonoid Content (FC)
To estimate the total content of flavonoids, the method adapted by Cai et al. (2010) [46] was applied. Depending on the rutin calibration curve at a final concentration of 100–1000 g/mL, R2 = 0.9996, the results were expressed as mg rutin equivalents (RE)/g of FW or DW.
2.2.3. Determination of the Total Content of Triterpenoids (TT)
The total content of triterpenoids was measured following the method proposed by Ke et al. (2014) [47]. The total content of triterpenoids from the samples was expressed as milligram of ursolic acid equivalents (GAE)/g of FW or DW. The ursolic acid final concentration of 10–50 µg/mL (R2 = 0.9769) was prepared for the calibration curve.
2.2.4. Determination of Total Tannin Content (TC)
To determine the total content of tannins, a method adapted from Makkar et al. [48] was used. The concentration of remaining phenols in supernatant was estimated using the previously described method [45]. Subsequently, TC concentrations were determined as the difference between phenol concentrations prior to and subsequent to PVP precipitation. The results were expressed as milligrams of catechin equivalents (CE)/g of DW, based on the catechin calibration curve (final concentration: 60–140 µg/mL, R2 = 0.9976).
2.2.5. Assessment of Antioxidant Activity through the DPPH, FRAP, CUPRAC, and TEAC Assays
The DPPH (2,2-Diphenyl-1-picrylhydrazyl) assay proposed by Marxen et al. [49] was applied to evaluate the antioxidant activity. Results were expressed as mM of Trolox equivalents (TE)/g of DW, according to a Trolox standard curve prepared at a final concentration of 50–150 µg/mL (R2 = 0.9873).
The FRAP (Ferric-Reducing Antioxidant Power) assay was conducted according to Simirgiotis et al. [50]. Results were expressed as mM of Trolox equivalents (TE)/g of DW, in relation to a Trolox standard curve (final concentration: 0.2–1 mM, R2 = 0.9979).
The CUPRAC (Cupric-Reducing Antioxidant Power) and TEAC (Trolox Equivalent Antioxidant Assay) assays were applied following the protocols purported by Chamorro et al. (2019) [51].
For CUPRAC, the results were expressed as mM of Trolox equivalents (TE)/g of DW, depending on a Trolox standard curve with a final concentration of 0.25–2 mM (R2 = 0.9997).
For TEAC, the absorbance was measured at 734 nm against a reference sample, and results were expressed as mM of Trolox equivalents (TE)/g of DW, according to a Trolox standard curve (final concentration: 25–250 µg/mL, R2 = 0.9935).
All absorbance measurements were completed using a Spectronic Helios Gamma UV-VIS spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), in biological and technical triplicate. For reporting per gram of DW, the results were divided by the DW/FW ratio.2.2.6. Evaluation of Antioxidant Activity by HP-TLC
The filtered methanolic extracts were separated using HP-TLC system (CAMAG, Muttenz, Switzerland). Two mobile phases were used (A) ethyl acetate: acetic acid: formic acid: water (100:10:10:18, v/v) and (B) chloroform: ethyl acetate: acetone: formic acid (40:30:20:10, v/v), according to Jesionek et al. [52]. The separated phenolic acids and flavonoids were visualized under UV light (254 nm and 365 nm) and compared with standards. For the assessment of antioxidant activity, a 0.2% methanolic DPPH solution was sprayed onto the plate.
2.2.6. Quantitative Determinations Using the HPLC-DAD Analysis
A total of 500 mg of fresh gametophyte and sporophyte biomass was extracted with 10 mL of methanol: water 70:30 v/v for 30 min in an ultrasound bath at 40 °C. After cooling, the mixture was filtered into a volumetric flask and brought to 10 mL with the previously mentioned solvent.
For the qualitative and quantitative assessment of the ten analyzed phenolic compounds, an HPLC–DAD method derived from commonly used methods for plants and food supplements’ analysis was used [53,54]. For this, a SCL-40 HPLC system (Shimadzu, Kyoto, Japan) was used. The stationary phase was represented by a Nucleosil C18 column (250 mm × 4.6 mm, i.d. 5 µm) maintained at 25 °C. Three mobile phases, in gradient (A = purified water; mobile phase B = methanol; and mobile phase C = purified water: acetic acid = 96:4 v/v) were used to perform elution.
The following gradient elution program was used: 15% B and 85% C (0 min); 75% A and 25% B (15 min); 15% A and 85% B (20 min); 40% A and 60% B (40 min); 5% A and 95% B (45 min); 5% A and 95% B (55 min); 85% A and 15% B (60 min), and 85% A and 15% B (70 min). The flow rate program was 0.5 mL/min for the first 15 min and 0.8 mL/min for the next 55 min. The injection volume was 5 µL. Gallic acid, (+)-catechin, syringic acid, and cinnamic acid were detected at 280 nm, resveratrol at 303 nm, chlorogenic, caffeic and ferulic acids at 330 nm, rutin and quercetin at 360 nm, and the analysis was performed in triplicate. The results were expressed as g phenolic compound/g FW.
HPLC-DAD quantifications were carried out by calculating the response factor of the standard solution of known concentrations using the following formula:
where: A = concentration of considered compound (µg/g); Asa = peak area of the considered compound in the sample solution (mAU); pstd = standard purity (%); Cstd = concentration of the standard solution (µg/mL); D = dilution factor; Astd = peak area of the standard (mAU); and Csa = concentration of the sample solution (µg/mL).
A = (Asa × pstd × Cstd × D)/(Astd × Csa)
2.2.7. Chemicals
The purity of standards was suitable for HPLC analysis (gallic acid > 99%, ferulic acid > 99%, syringic acid > 95%, cinnamic acid > 99%, caffeic acid > 99%, (+)-catechin 98%, resveratrol > 99%, chlorogenic acid > 95%, quercetin > 95%, rutin > 94% and methanol ≥ 99.9%. The standards were purchased from Sigma-Aldrich (St. Louis, MO, USA), DPPH and Folin–Ciocâlteu reagent were purchased from Sigma-Aldrich (St. Louis, MO, USA), sodium acetate, glacial acetic acid, hydrochloric acid, ethanol, and all analytical grades, were purchased from the Chemical Company (Iasi, Romania). Ultrapure water with a conductivity 0.05 µS/cm a was used for the performed analyses.
2.2.8. Statistical Analysis
The one-way analysis of variance (ANOVA) was applied to highlight the statistical significance. The significant differences between the means were determined using the Tukey HSD test, and the strength and direction of the linear association between variables were assessed as Pearson’s correlation. Statistical analyses were performed using MS Excel. The standard deviation (±SD) is indicated by the error bars of the graphs, and the level of significance denoted as p-value is mentioned in the footnote of each figure.
3. Results
3.1. Gametophyte and Sporophyte Biomass Production
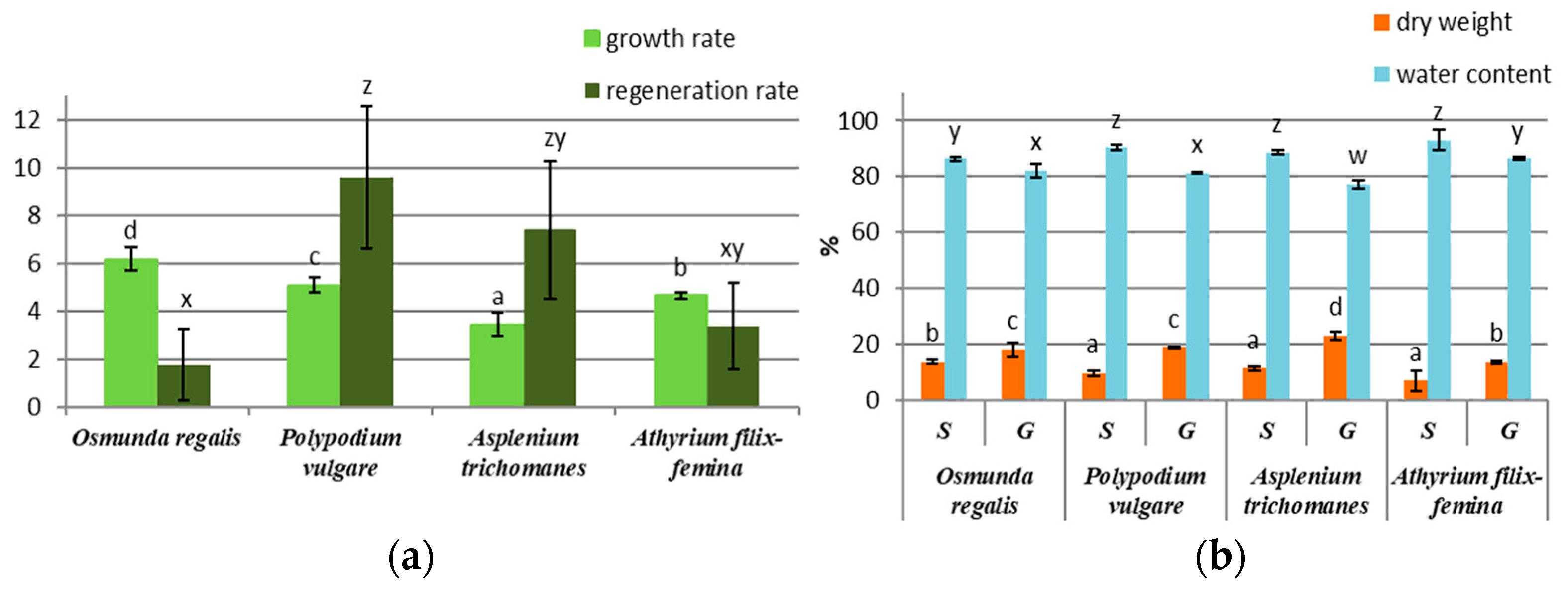
After 2 months of gametophyte cultivation on the MS ½ medium, a higher growth rate was recorded in the species OR, PV, and AF, the species AT having the lowest growth rate (Figure 1a). Regarding the sporophyte regeneration rate after three months, the species PV, AT, and AF had a higher number of sporophytes compared with OR (Figure 1a). In OR, the sporophyte differentiation was achieved only by ammonium nitrate and vitamin removal from the MS ½ medium. The percentage of water in the gametophytes of the four species was higher than that in the sporophytes, the difference being approximately 10% between the gametophytes and the sporophytes of each species (Figure 1b). In all four species, the percentage of dry mass was almost half in gametophytes compared to sporophytes (Figure 1b).
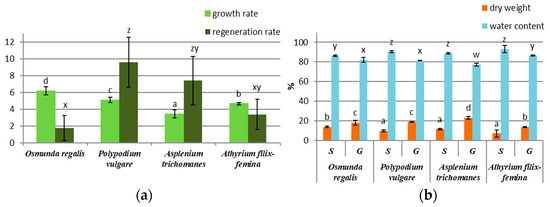
Figure 1.
In vitro culture growth parameter in four pteridophyte species: (a) growth rate of gametophytes and regeneration rate of sporophytes; (b) percentage of dry weight and water content of sporophytes (S) and gametophytes (G). The columns marked with different letters are significantly different (p < 0.05).
3.2. Determination of Polyphenols (PC), Flavonoids (FC), Tannins (TC), and Triterpenoids (TT) Contents
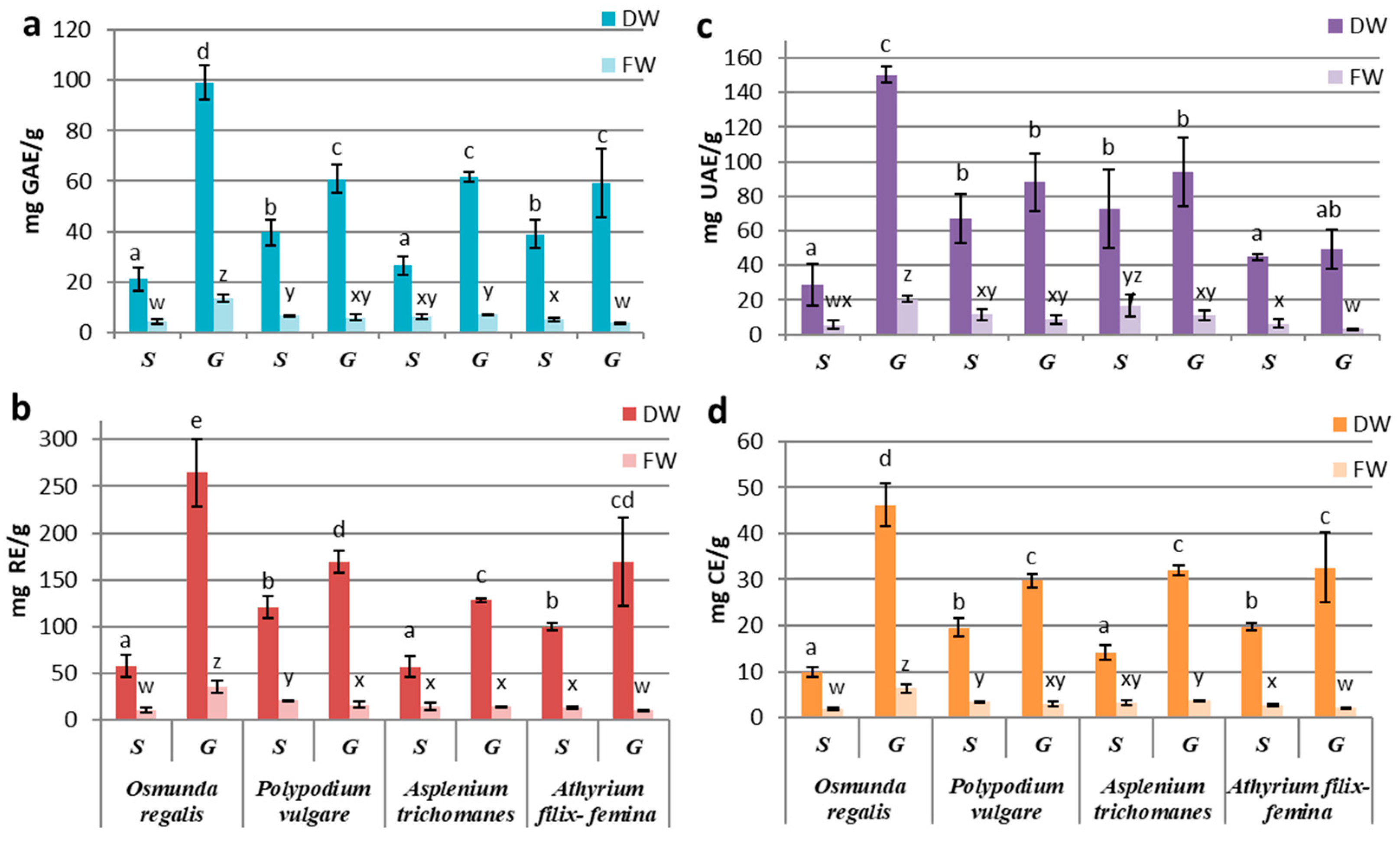
Our results related to fresh biomass obtained by in vitro culture showed a preponderance of secondary metabolite production in the sporophytes of PV and AT and in the gametophytic culture of OR and AT. The best-performing culture proved to be the OR gametophyte culture with the highest concentration of polyphenols (13.55 ± 1.51 mg GAE/g FW), flavonoids (36.29 ± 6.49 mg RE/g FW), triterpenes (20.55 ± 1.52 mg UAE/g FW) and tannins (6.32 ± 0.89 mg CE/g FW) (Figure 2a–d). In comparison, the AT gametophytes’ extracts showed the lowest values for all four classes of studied metabolites: total polyphenols (4.08 ± 0.98 mg GAE/g FW), flavonoids (11.12 ± 2.57 mg RE/g FW), tannins (1.88 ± 0.26 mg CE/g FW) and triterpenoids (5.57 ± 2.49 mg UAE/g FW) (Figure 2a–d).
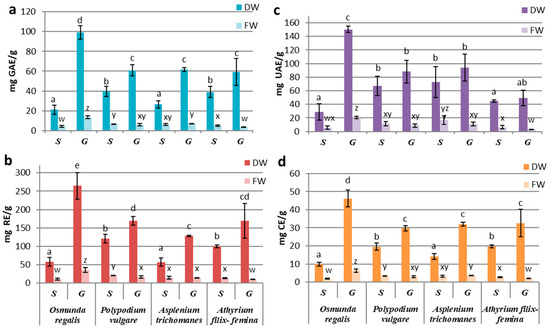
Figure 2.
Concentrations of compound classes: (a) PC, (b) FC, (c) TT, and (d) TC in sporophyte (S) and gametophyte (G) in vitro cultures in four pteridophyte species. The columns marked with different letters are significantly different (p < 0.05) in DW series (a–e) and FW series (w–z).
The differences between the extracts derived from sporophytes and gametophytes were significantly higher in the OR gametophytic culture (p ≤ 0.001) (Figure 2a–d). Likewise, significant differences were also seen in AF, where the sporophyte registered a significantly higher content of total polyphenols (p ≤ 0.05), flavonoids, and tannins (p ≤ 0.01) than the gametophyte (Figure 2a–d).
In the case of the pharmacologically valuable species, PV and AT, the differences in secondary metabolites’ productivity of the gametophyte and sporophyte were not significant (p ≥ 0.05) (Figure 2a–d). However, in PV, the content of total polyphenols (6.77 ± 0.11 GAE/g FW), flavonoids (20.72 ± 1.07 mg RE g FW), tannins (3.36 ± 0.16 mg CE/g FW) and triterpenoids (11.67 ± 3.25 mg UAE/g FW) was higher in the sporophyte extracts compared to gametophyte (Figure 2a–d). In contrast, in AT, the gametophyte had a higher content of polyphenols (7.05 ± 0.38 mg GAE/g FW) and tannins (3.64 ± 0.11 mg CE/g FW), while the sporophyte was richer in triterpenoids (16.87 ± 6.30 mg UAE/g FW; Figure 2a–d).
When reporting the concentrations of metabolites per gram of dry weight, the obtained values showed increased concentrations of metabolites in the gametophytic culture compared to the sporophytic one in all studied species. This increase is explained by the fact that the dry weight of the gametophyte culture was approximately two times less than the one registered in the sporophytic culture, with the exception of the sporophyte of OR (Figure 1b), so that the gametophytic culture accumulates more water than the sporophyte. The significantly increased values of polyphenols, flavonoids, and tannins were recorded in the gametophyte of all species (p ≤ 0.05) (Figure 2a,b,d). In the case of triterpenoids, although the concentrations in the gametophytes were higher than those in the sporophyte, the difference was significant (p ≤ 0.05) only for OR (Figure 2c). By reporting the results to dry weight, the highest metabolite concentrations were determined for the OR gametophytes and the lowest for the sporophytes in the same species (Figure 2).
By comparing the secondary metabolites productivities between species, significant differences were observed in gametophytes from OR and those from the other three species. Differences registered between sporophytes were statistically insignificant, with some exceptions. The content of polyphenols, flavonoids, and tannins from OR sporophytes was similar to those from AT, while the concentrations from PV sporophytes were alike with those in AF (Figure 2a,b,d). The differences between these two groups were statistically significant (p ≤ 0.05). For triterpenoids, significant differences between gametophytes and sporophytes were only registered at OR (Figure 2c).
Our results showed a high association between the variation patterns of polyphenols and tannins, followed by flavonoids–tannins and polyphenols–flavonoids. The weakest correlations were observed with triterpenoids.
3.3. Antioxidant Activities Evaluation by DPPH, FRAP, CUPRAC, and TEAC Methods and Correlations with the Contents of Metabolites
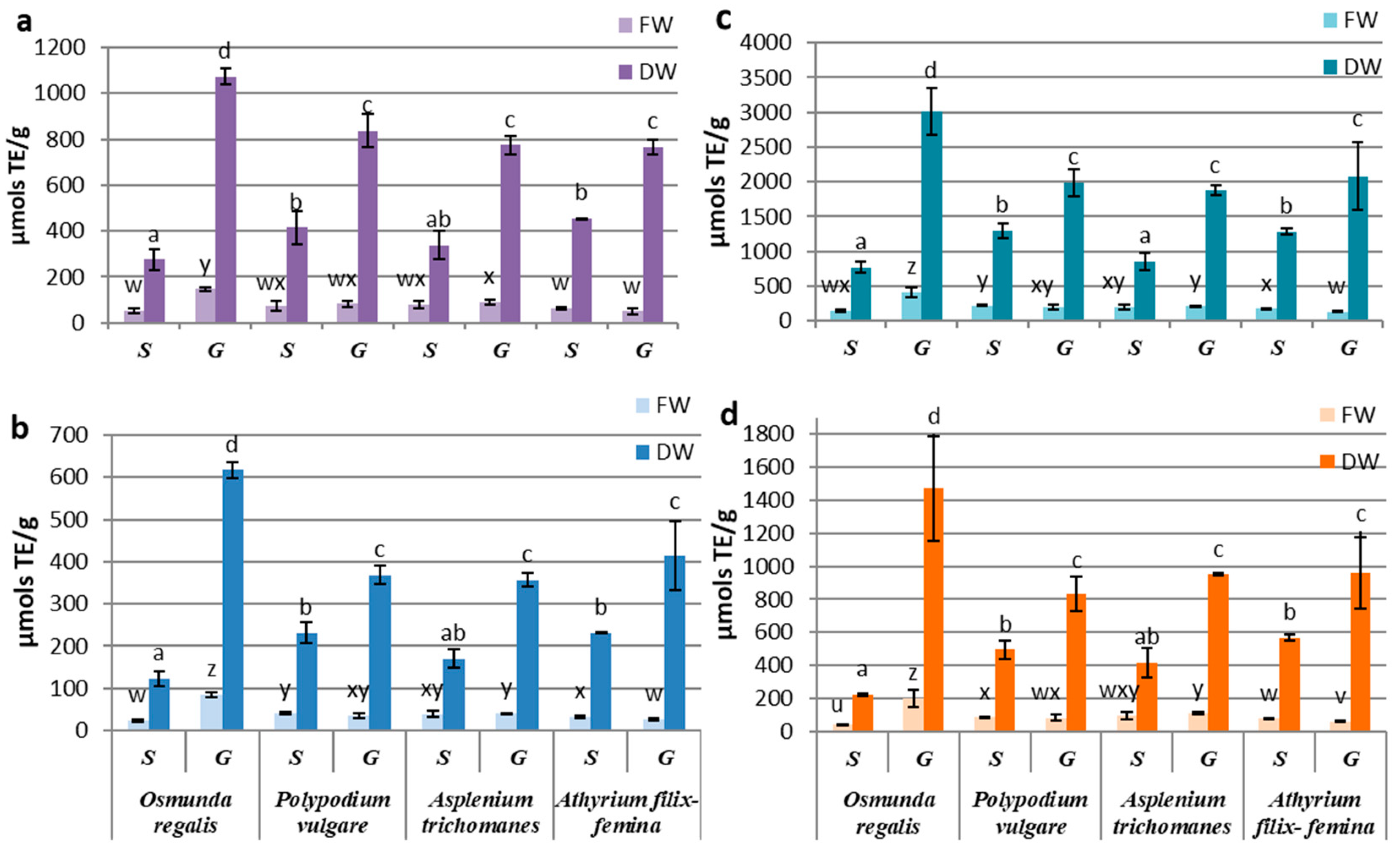
The antioxidant activity determinations of the extracts from the in vitro cultures of the four pteridophytes species by using different synthetic radicals, showed a higher affinity of the contained antioxidant compounds for the Cu2+-neocuproine ion, followed by that for the Fe3+-TPTZ anion and the DPPH radical, the lowest affinity being manifested for the ABTS radical. By reference to the fresh biomass, significant differences between the sporophyte and the gametophyte were recorded only for OR and AF, with the former favoring the gametophyte and the latter favoring the sporophyte. If we refer to the antioxidant activity reported to dry weight, the gametophyte cultures were significantly superior to the sporophyte for all four pteridophyte species (Figure 3a–d). The significantly highest activity of the OR gametophyte was noted, while the differences between the gametophytes of the other three species were not statistically significant. For sporophytes, the lowest antioxidant activity was determined for OR, and the best performance was recorded for AT, independent of the free radical used (Figure 3a–d).
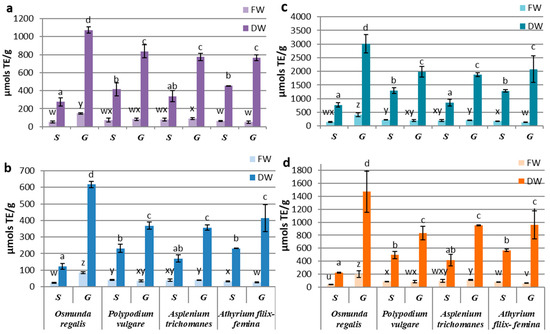
Figure 3.
Antioxidant activity of sporophyte (S) and gametophyte (G) produced by in vitro cultures at four pteridophyte species expressed as mM TE (µmols TE/g DW-FW) using (a) DPPH, (b) TEAC, (c) CUPRAC, or (d) FRAP method. The columns marked with different letters are significantly different (p < 0.05) in DW series (a–d) and FW series (u–z).
In the gametophyte cultures, the differences between the antioxidant activities determined for the studied species suggested a high affinity of the OR extract for the Cu2+-neocuproine ion, of PV for the DPPH radical and of AT for the Fe3+-TPTZ anion (Figure 3). Although minor variations regarding the affinity for certain substrates were observed, variation patterns of the antioxidant activities determined by all four methods were quite similar. Considering the similarities between the variation patterns of polyphenols, flavonoids, and tannins, correlation tests were carried out on metabolite concentrations and antioxidant activities assayed by four methods.
The best correlations between secondary metabolites content and antioxidant activities were obtained with TEAC and CUPRAC methods and while weaker associations were seen with FRAP and DPPH assays (Supplementary Materials Table S1). The Pearson’s correlation tests showed a low influence of triterpenoid content over the antioxidant activities in gametophytes and insignificant in sporophytes. The polyphenols and tannins of gametophytes contents were highly correlated with the antioxidant activities determined by using CUPRAC, TEAC, and FRAP methods, with R2 varing between 0.941 and 0.977 (p < 0.001). The correlation coefficients for flavonoids achieved the highest values with the CUPRAC and TEAC methods. In sporophytes, close correlations were registered for polyphenols, flavonoids, tannins, and TEAC antioxidant activity (R2 = 0.941–0.989) and between tannins and FRAP antioxidant activity (R2 = 0.939). The significant statistical correlations between the content of certain classes of metabolites and the antioxidant activity assayed by different methods were also obtained punctually on certain samples (Supplementary Materials Table S2).
3.4. HP-TLC Analyses
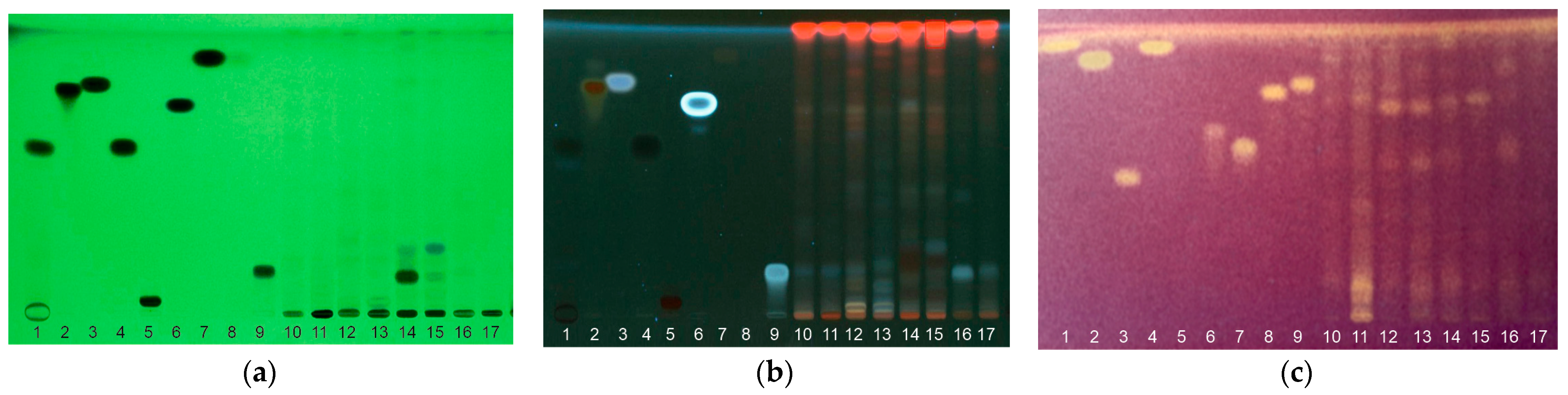
HP-TLC chromatographic separation, combined with the DPPH developing method, was used to reveal the compounds’ antioxidant activity from the gametophyte and sporophyte extracts of the four studied species. Two elution systems for phenolic acids (A) and glycosylated flavonoids (B) were tested. Several standards for the identification of phenolic acids such as caffeic, chlorogenic, gallic, benzoic, and cinnamic acid, as well as flavonoids such as quercetin, rutin, myricetin 3-O-glucoside, catechin, epicatechin, and tannins such as proanthocyanidin B1 and B2 were used.
Visualization at 254 nm (Figure 4a) highlighted the presence of bands corresponding to chlorogenic acid in the gametophyte and sporophyte of AT, AF, and OR, with intense expression in the sporophyte of AT and the gametophyte of OR. In the case of separating the extracts from the gametophyte and the sporophyte of PV, bands similar to myricetin 3-O-glucoside were observed. In AT, caffeic acid and resveratrol were highlighted, while in OR gallic acid, both in the sporophyte and the gametophyte, but also proanthocyanidin B1 in the gametophyte.

Figure 4.
HP-TLC of gametophyte (g) and sporophyte (s) methanolic extracts at four pteridophyte species migrated elution system A and visualized under UV light 254 nm (a), 365 nm (b) with lanes (1—catechin; 2—quercetin; 3—resveratrol; 4—gallic acid; 5—rutin; 6—caffeic acid; 7—trans cinnamic acid; 8—benzoic acid; 9—chlorogenic acid; 10—ORs; 11—ORg; 12—PVs; 13—PVg; 14—ATs; 15—ATg; 16—AFs; 17—AFg) and eluted with B system and visualized with DPPH reagent for antioxidant activity testing (c) with lanes (1—resveratrol; 2—gallic acid; 3—rutin; 4—caffeic acid; 5—trans cinnamic acid; 6—myricetin-3-O-glucoside; 7—chlorogenic acid; 8—Proanthocyanidin B1; 9—Proanthocyanidin B2; 10—ORs; 11—ORg; 12—PVs; 13—PVg; 14—ATs; 15—ATg; 16—AFs; 17—AFg.
Visualization at 365 nm (Figure 4b) revealed the existence of bands with Rfs similar to chlorogenic acid in all species, with weaker expression in AT and strong in the gametophyte of AF. Bands with Rfs similar to caffeic acid and quercetin were identified in the sporophyte and gametophyte of OR and AT. In PV, caffeic acid was determined in the gametophyte, while quercetin was present in the sporophyte. The presence of resveratrol was assumed in AT, and cinnamic acid was present in all samples, having a more pronounced expression in the gametophytes of OR and PV.
By developing the TLC plates with DPPH (Figure 4c), several spots with antioxidant activity were highlighted for the samples and for all the standards used except cinnamic and benzoic acid. Only a few spots with antioxidant activity could be attributed to compounds such as caffeic, gallic, chlorogenic acid, and proanthocyanidins. The antioxidant activity of caffeic acid was highlighted in all samples except the AT gametophyte. Gallic acids’ antioxidant activity was determined in the sporophyte and gametophyte of OR, the sporophyte of PV, and the gametophyte of AF. Chlorogenic acid showed weak antioxidant activity of in all samples with an emphasis on the AF sporophyte. Spots of antioxidant activity were also determined in the sporophyte of PV for proanthocyanidin B1 and in OR for proanthocyanidin B2. The rest of the compounds whose bands were visible in UV could not be highlighted by developing with DPPH due to the very small amounts found in our extracts.
3.5. Quantitative HPLC–UV–VIS–DAD Determinations of Some Known Phytoterapeutic Chemicals
The HPLC-DAD analyses followed the quantitative determination of 10 phenolic compounds with known pharmaceutical applications, including five phenolic acids (gallic, syringic, caffeic, ferulic, and chlorogenic) and one non-phenolic acid (trans cinnamic acid), resveratrol (a stilbene such as often used in nutraceutics and cosmetic industry and three flavonoids, an aglycone (quercetin), a glycosylated flavonoid (rutin), and a flavanol (catechin) through the consensus of which tannins are formed.
These compounds were quantified only for three species, namely PV, OR, and AT (Table 1), while for AF, we could only determine traces, and the quantification could not be achieved.

Table 1.
Content (µg/g) of 10 polyphenolic phytochemicals in gametophyte (G) and sporophyte (S) from in vitro cultures.
The syringic acid was identified only in AT, while resveratrol, quercetin, and chlorogenic acid were not present in OR. Caffeic acid was absent in AT samples.
Also, differences in the synthesis of some compounds between gametophyte and sporophyte were observed, thus the gametophyte or sporophyte in PV and AT could be identified based on the synthesis of certain metabolites. In PV, the sporophyte did not synthesize caffeic acid, while the presence of chlorogenic acid was not determined in the gametophyte. Catechin was synthesized only in sporophytes of AT, while resveratrol and cinnamic acid were found only in the gametophyte.
The two types of cultures could also be differentiated based on the amount of synthesized metabolites. Significant differences between sporophyte and gametophyte were determined in PV in favor of the sporophyte. In this species, the concentrations of gallic acid, catechin, resveratrol, and rutin were significantly higher in the sporophyte compared to the gametophyte. In OR, significantly increased concentrations were registered for gallic acid, catechin, and caffeic acid, also at the level of the sporophyte, although our determinations by classes of metabolites showed significantly increased concentrations only in the gametophyte. The metabolites we monitored are probably not part of the majority present in this species. In AT, the quantitative determinations showed the presence of significantly higher concentrations of gallic acid and rutin in the gametophyte and quercetin in the sporophyte. Also, catechin wass characteristic only for the sporophyte, while cinnamic acid and resveratrol were only seen in the gametophyte.
4. Discussion
In the case of pteridophytes, the results of in vitro culture techniques are differentiated according to the reactivity of each species and by the culture conditions (inoculum type, nutrient medium, physical factors), aspects that have been highlighted in specialized papers [1]. In vitro cultures of pteridophytes generally require poor nutritive media compared to in vitro cultures of higher plants; therefore, the culture media used for higher plants were adapted by dilution. From our previous experience with P. vulgare, A. trichomanes, and A. filix-femina the MS ½ culture medium gave the best results [55]. O. regalis proved to be a recalcitrant species to multiplication by apogamy. After long sub-passages on different culture media, sporophytes could only be obtained by removing ammonium nitrate and vitamins from the culture media, according to Makowski et al. [34].
Our results showed the in vitro production of gametophytes and sporophytes biomass characterized by polyphenols, flavonoids, triterpenes, and tannins synthesis and appreciable antioxidant activity, which was highly correlated with the content of these metabolites. Our data showed better productivity of these metabolites in the gametophytic culture compared to the sporophytes one. Moreover, the productivity in terms of the biomass obtained for the gametophyte was superior to that of the sporophytes, given that initiating apogamy in vitro is a difficult process. Apogamy, the sporophyte development without fertilization, is a very lengthy process that starts after several months of cultivation using various techniques of grinding, nutrient-depleted media, or submerged cultures. The species with the highest gametophyte productivity was OR, followed by PV, AT, and AF. In the case of flavonoid content, the hierarchy is modified in the sense that OR was followed by PV, AT, and AT. The lowest flavonoid content was recorded in AT, although data from the literature showed that in the methanolic extracts from the fronds, the flavonoids represented 92.82% of the total phytochemicals determined. The metabolism of the in vitro gametophyte is probably modified compared to that of in situ fronds.
Compared to the existing data in the literature, the gametophyte of OR synthesized 99.32 ± 11.08 mg GAE/g DW polyphenols compared to the leaves of young plants cultivated for horticultural purposes (94.53 ± 8.5 mg GAE/g DW) [3] or those from botanical gardens (123.7 ±15.9 mg GAE/g DW), but also higher than those from pots (66 ± 15.9 mg GAE/g DW) [4]. Instead, the concentration of synthesized flavonoids of 265.92 ± 47.56 mg RE/g DW was more than twice as high as that reported in the leaves of young plants in spring (96.89 ± 20.5 mg RE/g DW) [3].
In PV, the amount of polyphenols synthesized in the gametophyte obtained in vitro (61.21 ± 11.65 mg GAE/g DW) was clearly lower than the concentrations reported in leaf extracts [3,6], being even more than three times lower than the leaves of young plants [4]. At the same time, the concentration of flavonoids (169.71 ± 29.61 mg RE/g DW) was higher than those from the leaves of horticultural plants (90.92 ± 16.1 mg RE/g DW) or from botanical gardens (64.27 ± 18.5 mg RE/g DW) determined by Langhansova et al. [3].
The same differentiation between the results in the literature and those reported by us was also observed between the gametophyte obtained in vitro and the leaves of plants from nature in AT [6] or botanical gardens in AT and AF [3]. In AT, the concentration of polyphenols was lower (64.33 ± 15.71 compared to 86.80 ± 8.6 or 100.4 ± 0.7 mg RE/g DW), while that of flavonoids was higher (129.77 ± 3.46 compared to 96.68 ± 26.1 mg RE/g DW) than those reported in the literature. Almost four times lower concentrations of polyphenols were determined in the AT gametophyte [3,4] compared to the leaves of horticultural plants (205.24 ± 31.6 mg GAE/g DW) or fiddleheads (234.5 ± 4.03 mg GAE/g DW), but also higher flavonoid concentrations from 152.17 ± 4.52 to 114.68 ± 23.5 mg RE/g DW in favor of the gametophyte. Compared to fresh biomass, the sporophyte and gametophyte of AT obtained by in vitro culture had concentrations of 5.13 ± 0.77 and 3.73 ± 0.28 mg GAE/g FW, respectively, while the native plants from Valea Vâlsanului of 9.15 ± 0.25 mg GAE/g FW [31]. However, taking into account that the tissues regenerated in vitro tend to accumulate more water than those in situ, it is possible for the in vitro culture to be more performant in terms of metabolite synthesis
Regarding the concentrations of the other two analyzed metabolite classes, triterpenoids, and tannins, we did not find comparable quantitative data in the literature. However, a series of triterpenoid constituents were isolated and identified in PV [15]. These types of compounds, such as triterpenoid hydrocarbons or triterpenoid alcohols of the cycloartane group, could be involved in the anti-tyrosinase activity of methanolic extracts of PV [6]. Also, they could have photoprotective activity, as in the case of a standardized aqueous extract from Polypodium leucotomos fronds, one of the most popular systemic and local photoprotective agents used in the cosmetic industry marketed under the trade name Fernblock (Cantabria Labs, Santander, Spain). Regarding tannins, compounds of the flavan-3-ol type (flavanols), such as epicatechin and procyanidin B1 and A2 have been identified in the methanolic extracts of AT leaves [7], while epicatechin and catechin are major constituents of the methanolic extract obtained by macerating PV fronds [5].
The potent antioxidant activities of our extracts were highlighted by several methods that use free radicals of synthetic origin and expressed in Trolox equivalents. A strong antioxidant activity was determined, especially in the case of gametophyte extracts, similar to or greater than vegetables, herbs, or spices known for their antioxidant qualities [1]. The highest antioxidant activity was recorded for the gametophyte of OR followed by PV, AT, and AF. Most of the reports of the antioxidant activity in the four studied species were made using the ORAC fluorescent radical method, expressed in Trolox equivalents [3,4,6,31]). As our results demonstrated, due to the antioxidant compounds’ affinity for different substrates, the free radicals neutralized can vary, so the methods are not comparable. However, the correlations between the four methods for determining the antioxidant activity were high, which suggests differentiation patterns between similar samples for all tested methods. The results reported from the literature, where the ORAC method were used, did not showed the same ranking between species, the highest values being recorded for PV, followed by AF, OR, and AT [3,4].
AF exhibited the strongest ABTS radical scavenging activity (29.85 ± 1.39 µmol Trolox/g plant), cytotoxicity, antioxidant activity, and phenolic content from eight fern species originating in northern Iran [32].
Most articles incriminate the presence of phenolic acids and flavonoids with hydroxyl groups on the B ring in extracts with strong antioxidant activity [56]. Likewise, the content of terpenoids in ferns may also be responsible for the results regarding the antioxidant activities [57]. In gametophytes, we reported the high correlations between polyphenols, tannins, flavonoids, and antioxidant activity with R2 varying between 0.820–0.977 (p < 0.001), while in sporophytes polyphenols and tannins strongly influenced the antioxidant activities (R2 = 0.847–0.989 with p < 0.001). These findings suggest that metabolites from these classes were primarily responsible for the antioxidant activity of the studied fern extracts. Similarly, high correlations with r = 0.8251 were reported between polyphenols and antioxidant activity in PV fronds [6].
HP-TLC separations with specific mobile phases of phenolic acids and glycosylated flavonoids combined with DPPH development showed the presence of several compounds with antioxidant activity, including gallic, chlorogenic or caffeic acid in the extracts from the gametophytes or saprophytes of the four species. Quantitative UPLC-DAD determinations of these phenolic acids confirmed the presence of gallic acid in three of the studied species, with higher values in the gametophyte of AT (126.95 ± 0.51 µg/g) and in the sporophytes of PV (83.96 ± 0.60 µg/g) and OR (110.14 ± 0.14 µg/g). Moreover, on the HP-TLC/DPPH plates the antioxidant activity was visualized in the samples with these higher concentrations. Citations from the literature confirm gallic acids ’s presence in AT fronds [7] in a low concentration (5.36 mg/kg extract) and much higher (1791.3 mg/kg extract) in fronds of PV [5]. Caffeic acid could not be quantified in the sporophyte and gametophyte extracts of AT. Its absence was confirmed by the lack of bands with antioxidant activity on the HP-TLC/DPPH plates, although caffeic acid analogous bands were visualized in UV. However, the phytochemical characterization of the methanolic extracts from the fronds demonstrates its presence in AT [7]. Chlorogenic acid was determined neither in the samples of OR, nor in the gametophyte of PV, and its concentration registered low values in all of the other samples. A weak antioxidant activity was also observed on the TLC plates. Contrary to our results, other studies considered AT leaves reservoirs of cinnamic acids, especially chlorogenic acid [7,58], and PV fronds rich in phenylpropanoid compounds derived from quinic acids, such as chlorogenic acid (5-O-caffeoylqiunic acid) and its substituted caffeoyl derivatives. Moreover, the major compound of PV fronds extract collected from nature was isochlorogenic acid (3-O-caffeoylqiunic acid).
Among the other three phenolic acids we monitored, syringic acid was not determined in OR and PV but only in AT samples. However, its presence was not confirmed in the samples from AT leaves [7]. Ferulic acid was determined in higher concentrations in PV and lower in OR and AT, while cinnamic acid was identified in all analyzed samples except the AT sporophyte. Their presence was also detected in extracts from AT leaves but in very low concentrations [7]. HP-TLC analyses showed the presence of cinnamic acid but without antioxidant activity.
Among the flavonoids, rutin was identified by HPLC-DAD analyses in all samples with increased values in the PV sporophyte (646.60 µg/g extract) and the AT gametophyte (247.23 µg/g extract), although the HP-TLC analyzes did not highlight its presence in our extracts. Compared to the values reported in the literature of 422.7 mg/kg extract in the leaves of PV [5] and only 0.98 mg/kg extract in those of AT [7], the in vitro cultures of sporophyte and gametophyte, respectively, could represent efficient systems for the production of this metabolite.
Quercetin was determined in AT and PV but not in the OR extracts. The highest concentration was obtained in the AT sporophyte (128.03 µg/g extract), while the concentration observed in the leaves was 26.05 µg/g extract [7]. However, it seems that its glycosylated derivatives hyperoside (quercetin-3-O-galactoside) and quercitrin (quercetin-3-O-rhamnoside) represent the majority of the compounds of this extract. Older studies considered the AT leaves rich reservoirs of flavonols and mentioned the presence of kaempferol and its glycosylated derivatives [25], while recent studies demonstrated the presence of hyperoside in a concentration of 91.3 µg/g extract in PV leaves [5].
With respect to the tannins, our quantitative analyses only followed the monitorization of catechin concentrations, but the TLC separations also indicated the presence of procyanidins B1 and B2 with antioxidant activity in the OR gametophyte and PV sporophyte. The highest catechin concentrations were determined in the gametophyte (2019.56 µg/g extract) and the sporophyte of PV (3504.51 µg/g extract), these results being comparable to those obtained in the leaf (3879.8 µg/g extract) [5]. In AT, catechin was found only in the sporophytes, while in other studies, it was not detected in the leaves, although epicatechin and procyanidins B2 and B1 were reported [7]. Catechin was also determined in the OR sporophyte and gametophyte, but having no data to refer to, we can claim that all these compounds were detected in this species for the first time in our study.
Resveratrol could be quantified not only in PV with a higher concentration in the sporophyte (58.34 µg/g) but also in the gametophyte of AT (34.13 µg/g), although it was not detected at the leaf level [7].
5. Conclusions
In vitro cultures of the four fern species studied can represent a viable alternative for useful metabolite` production, especially the gametophytic cultures that are proven to be important sources of secondary metabolites due to their high multiplication rates.
Our studies have shown that OR gametophytes can represent a highly-performance system that produces metabolites with potent antioxidant activity. The synthesis of valuable compounds such as catechin, caffeic acid, gallic acid, ferulic acid, cinnamic acid, or rutin was reported for the first time in this species. Further, more complex methods, such as HPLC-MS-MS, could be employed to confirm their identity.
The sporophyte cultures can be used to produce certain compounds, but the apo-gamic multiplication should be optimized to obtain higher regeneration rates in a shorter time.
Our results confirm the rich phenolic content of fern extracts with strong antioxidant activity, such as caffeic, chlorogenic, or gallic acid, and demonstrate the possibility of their production by in vitro cultures of P. vulgare, O. regalis, A. trichomanes and A. filix-femina.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae10080799/s1. Table S1: Pearson’s correlation coefficient for the content of secondary metabolites and antioxidant activities in methanolic extracts (expressed as R2) from in vitro cultures of all four pteridophyte species; Table S2: Pearson’s correlation coefficient for the four metabolite classes and antioxidant activities in O. regalis, P. vulgare, A. trichomanes, and A. filix-femina gametophytes and sporophytes.
Author Contributions
Conceptualization, F.A., E.M.M. and L.C.S.; methodology, E.M.M., F.A., F.E.H., A.-G.C. and A.F.; software, A.-G.C. and E.M.M.; validation, F.A., A.-G.C., F.E.H., D.I.P. and L.C.S.; formal analysis, E.M.M., F.E.H., A.-G.C. and A.F.; investigation, E.M.M., F.A., F.E.H. and A.F.; resources, E.M.M., D.I.P., N.A.Ș. and L.C.S.; data curation, E.M.M., F.A, F.E.H., A.-G.C., A.F. and O.A.L.; writing-original draft preparation, E.M.M.; writing-review and editing, F.E.H., A.-G.C. and L.C.S.; visualization, E.M.M. and A.-G.C.; supervision, L.C.S.; project administration, E.M.M.; funding acquisition, E.M.M., D.I.P., and L.C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Romanian Academy for Institute of Biology Bucharest of Romanian Academy, project number RO1567-IBB06/2023. Romanian Ministry of Research, Innovation and Digitization through NUCLEU Program-Financing Contract No. 20N/05.01.2023, Project PN 23 15 03 01—The implementation of integrated isotopic-chemical-nuclear analytical methodologies for the authentication of traditional Romanian food products—IsoPRod, Establishment and operationalization of a Competence Center for Soil Health and Food Safety—CeSoH, Contract No.: 760005/2022, Code 2, financed through PNRR-III-C9-2022—I5 (PNRR-National Recovery and Resilience Plan, C9 Support for the private sector, research, development, and innovation, I5 Establishment and operationalization of Competence Centers), Project “Improving soil conservation and resilience by boosting biodiversity and functional security of organic food products”, financed by European Union through Romanian Ministry of Research, Innovation and Digitization.
Data Availability Statement
All data used and obtained during this study are included in this research paper as Figures, Tables and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
References
- Fernández, H.; Revilla, M.A. In vitro culture of ornamental ferns. Plant Cell Tissue Org. Cult. 2003, 73, 1–13. [Google Scholar] [CrossRef]
- Ojosnegros, S.; Alvarez, J.M.; Grossmann, J.; Gagliardini, V.; Quintanilla, L.G.; Grossniklaus, U.; Fernández, H. Proteome and Interactome Linked to Metabolism, Genetic Information Processing, and Abiotic Stress in Gametophytes of Two Woodferns. Int. J. Mol. Sci. 2023, 24, 12429. [Google Scholar] [CrossRef]
- Langhansová, L.; Pumprová, K.; Haisel, D.; Ekrt, L.; Pavicic, A.; Zajíčková, M.; Vaněk, T.; Dvořáková, M. European ferns as rich sources of antioxidants in the human diet. Food Chem. 2021, 356, 129637. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, M.; Pumprova, K.; Antoninova, Z.; Rezek, J.; Haisel, D.; Ekrt, L.; Vanek, T.; Langhansova, L. Nutritional and Antioxidant Potential of Fiddleheads from European Ferns. Foods 2021, 10, 460. [Google Scholar] [CrossRef] [PubMed]
- Farràs, A.; Mitjans, M.; Maggi, F.; Caprioli, G.; Vinardell, M.P.; López, V. Polypodium vulgare L. (Polypodiaceae) as a Source of Bioactive Compounds: Polyphenolic Profile, Cytotoxicity and Cytoprotective Properties in Different Cell Lines. Front. Pharmacol. 2021, 12, 727528. [Google Scholar] [CrossRef] [PubMed]
- Farràs, A.; Cásedas, G.; Les, F.; Terrado, E.M.; Mitjans, M.; López, V. Evaluation of Anti-Tyrosinase and Antioxidant Properties of Four Fern Species for Potential Cosmetic Applications. Forests 2019, 10, 179. [Google Scholar] [CrossRef]
- Farràs, A.; Mitjans, M.; Maggi, F.; Caprioli, G.; Vinardell, M.P.; López, V. Exploring wild Aspleniaceae ferns as safety sources of polyphenols: The case of Asplenium trichomanes L. and Ceterach officinarum Willd. Front. Nutr. 2022, 12, 994215. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Assessment Report on Polypodium vulgare L.; Rizoma European Medicines Agency: London, UK, 2008; p. 22. [Google Scholar]
- Gleńsk, M.; Dudek, M.K.; Ciach, M.; Wlodarczyk, M. Isolation and structural determination of flavan-3-ol derivatives from the Polypodium vulgare L. rhizomes water extract. Nat. Prod. Res. 2019, in press. [Google Scholar] [CrossRef]
- Leporatti, M.L.; Corradi, L. Ethnopharmacobotanical remarks on the province of Chieti town (Abruzzo, central Italy). J. Ethnopharmacol. 2001, 74, 17–40. [Google Scholar] [CrossRef] [PubMed]
- Bonet, M.A.; Vallès, J. Ethnobotany of Montseny Biosphere reserve (Catalonia, Iberian Peninsula): Plants Used in Veterinary Medicine. J. Ethnopharmacol. 2007, 110, 30–47. [Google Scholar] [CrossRef]
- Jizba, J.; Dolejš, L.; Herout, V.; Šorm, F. The structure of osladin—The sweet principle of the rhizomes of Polypodium vulgare L. Tetrahedron Lett. 1971, 12, 1329–1332. [Google Scholar] [CrossRef]
- Prakash, C.V.S.; Prakash, I. Isolation and structural characterization of lupine triterpenes from Polypodium Vulgare. Res. J. Pharm. Sci. 2012, 1, 23–27. Available online: http://www.isca.in/IJPS/Archive/v1i1/5.ISCA-RJPcS-2012-009.pdf (accessed on 15 June 2024).
- Jizba, J.; Herout, V. Plant substances. XXVI. Isolation of constituents of common polypody rhizomes (Polypodium vulgare L.). Collect. Czech. Chem. Commun. 1967, 32, 2867–2874. [Google Scholar] [CrossRef]
- Arai, Y.; Yamaide, M.; Yamazaki, S.; Ageta, H. Fern constituents–Triterpenoids isolated from Polypodium-vulgare, Polypodium-fauriei and Polypodium-virginianum. Phytochemistry 1991, 30, 3369–3377. [Google Scholar] [CrossRef]
- Messeguer, J.; Mele, E.; Reixach, N.; Irurre-Santilari, J.; Casas, J. Polypodium vulgare L.(Wood Fern): In vitro cultures and the production of phytoecdysteroids. In Biotechnology in Agriculture and Forestry, Medicinal and Aromatic Plants X; Bajaj, Y.P.S., Ed.; Springer: Berlin, Germany, 1998; Volume 41, pp. 333–348. [Google Scholar]
- Simon, A.; Vanyolos, A.; Beni, Z.; Dekany, M.; Toth, G.; Bathori, M. Ecdysteroids from Polypodium vulgare L. Steroids 2011, 76, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.B.; Kordi, F.M.; Ahmadi, A.A.; Bahadori, S.; Valizadeh, H. Antibacterial evaluation and preliminary phytochemical screening of selected ferns from Iran. Res. J. Pharmacogn. 2015, 2, 53–59. [Google Scholar]
- Sofiane, G.; Wafa, N.; Ouarda, D. Antioxidant, antimicrobial and anti-inflammatory activities of flavonoids and tannins extracted from Polypodium vulgare L. Asian. J. Biochem. Pharm. Res. 2015, 5, 114–122. [Google Scholar]
- Batur, S.; Ayla, S.; Sakul, A.A.; Okur, M.E.; Karadag, A.E.; Daylan, B.; Ozdemir, E.M.; Kepil, N.; Gunal, M.Y. An Alternative Approach Wound Healing Field with Polypodium Vulgare. Medeni Med. J. 2020, 35, 315–323. [Google Scholar] [CrossRef]
- Saeedi, M.; Babaie, K.; Karimpour-Razkenari, E.; Vazirian, M.; Akbarzadeh, T.; Khanavi, M.; Hajimahmoodi, M.; Shams Ardekani, M.R. In Vitro Cholinesterase Inhibitory Activity of Some Plants Used in Iranian Traditional Medicine. Nat. Prod. Res. 2017, 31, 2690–2694. [Google Scholar] [CrossRef]
- Rigat, M.; Vallès, J.; Iglésias, J.; Garnatje, T. Traditional and alternative natural therapeutic products used in the treatment of respiratory tract infectious diseases in the eastern Catalan Pyrenees (Iberian Peninsula). J. Ethnopharmacol. 2013, 148, 411–422. [Google Scholar] [CrossRef]
- Bonet, M.À.; Agelet, A.; Vallès, J.; Villar Pérez, L. Contribution à la connaissance ethnobotanique des ptéridophytes dans les Pyrénées. Bocconea 2001, 13, 605–612. Available online: http://www.herbmedit.org/bocconea/13-605.pdf (accessed on 15 June 2024).
- Fiorin, E.; Sáez, L.; Malgosa, A. Ferns as healing plants in medieval Mallorca, Spain? Evidence from human dental calculus. Int. J. Osteoarchaeol. 2019, 29, 82–90. [Google Scholar] [CrossRef]
- Imperato, F. Two novel flavonol glycosides from the fern Cheilanthes fragrans. Tetrahedron 1989, 45, 215–218. [Google Scholar] [CrossRef]
- Durdevic, L.; Mitrovic, M.; Pavlovic, P.; Bojovic, S.; Jaric, S.; Oberan, L.; Gajic, G.; Kostic, O. Total phenolics and phenolic acids content in leaves, rhizomes and rhizosphere soil under Ceterach officinarum D.C., Asplenium trichomanes L., and A. adiantum-nigrum L. in The Gorge of Sicevo (Serbia). Ekológia 2007, 26, 164–173. [Google Scholar]
- Froissard, D.; Fons, F.; Bessière, J.M.; Buatois, B.; Rapior, S. Volatiles of French ferns and “fougère” scent in perfumery. Nat. Prod. Commun. 2011, 6, 1723–1726. [Google Scholar] [CrossRef] [PubMed]
- Practical Plants. Available online: https://practicalplants.org/wiki/athyrium_filix-femina (accessed on 15 June 2024).
- Native American Ethnobotany Database. Available online: http://naeb.brit.org/uses/search/?string=Athyrium+filix-femina (accessed on 15 June 2024).
- Fons, F.; Froissard, D.; Bessière, J.-M.; Buatois, B.; Rapior, S. Biodiversity of Volatile Organic Compounds from Five French Ferns. Nat. Prod. Commun. 2010, 5, 1934578X1000501028. [Google Scholar] [CrossRef]
- Soare, L.C.; Ferdes, M.; Stefanov, S.; Denkova, Z.; Nicolova, R.; Denev, P.; Bejan, C.; Paunescu, A. Antioxidant activity, polyphenols content and antimicrobial activity of several native pteridophytes of Romania. Not. Bot. Hort. Agrobot. Cluj Napoca 2012, 40, 53–57. Available online: https://www.notulaebotanicae.ro/index.php/nbha/article/view/6648/6816 (accessed on 15 June 2024). [CrossRef]
- Valizadeh, H.; Sonboli, A.; Kordi, F.M.; Dehghan, H.; Bahadori, M.B. Cytotoxicity, antioxidant activity and phenolic content of eight fern species, from north of Iran. Pharm. Sci. 2015, 21, 18–24. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species 2014: e.T164368A63306495. Available online: https://doi.org/10.2305/IUCN.UK.2014-2.RLTS.T164368A63306495.en (accessed on 19 June 2024).
- Makowski, D.; Tomiczak, K.; Rybczyński, J.J.; Mikuła, A. Integration of tissue culture and cryopreservation methods for propagation and conservation of the fern Osmunda regalis L. Acta Physiol. Plant. 2016, 38, 19. [Google Scholar] [CrossRef]
- Boșcaiu, N.; Coldea, G.; Horeanu, C. The Red list of extinct, endangered, vulnerable and rare vascular plants from Romania’s flora. Ocrot. Nat. Med. Înconj. 1994, 1, 45–56. [Google Scholar]
- Dihoru, G.; Dihoru, A. Rare, endangered and endemic plants from Romania’s flora—Red List. Acta Bot. Horti Bucharest 1993–1994, 23, 173–197. Available online: https://ahbb.unibuc.ro/wp-content/uploads/2020/03/ABHB-1993-1994-Page-177-201.pdf (accessed on 15 June 2024).
- Molina, M.; Reyes-García, V.; Pardo-de-Santayana, M. Local Knowledge and Management of the Royal Fern (Osmunda regalis L.) in Northern Spain: Implications for Biodiversity Conservation. Am. Fern J. 2009, 99, 45–55. [Google Scholar] [CrossRef]
- Hartwell, J.L. Plants Used against Cancer; Quartermain Publishing: Lawrence, MA, USA, 1982; p. 694. [Google Scholar]
- Schmidt, M.Q.; David, J.; Yoshida, E.J.; Scher, K.; Mita, A.; Shiao, S.L.; Ho, A.S.; Zumsteg, Z.S. Predictors of survival in head and neck mucosal melanoma. Oral Oncol. 2017, 73, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, K.; Li, B.; Tian, Y.; Liu, S.; Dong, J. Chemical constituents, cytotoxic and antioxidant activities of extract from the rhizomes of Osmunda japonica Thunb. Nat. Prod. Res. 2018, 34, 847–850. [Google Scholar] [CrossRef]
- Zhu, X.-X.; Li, Y.-J.; Yang, L.; Zhang, N.; Chen, Y.; Kmoníčková, E.; Weng, X.-G.; Yang, Q.; Zídek, Z. Divergent immunomodulatory effects of extracts and phenolic compounds from the fern Osmunda japonica Thunb. Chin. J. Integr. Med. 2013, 19, 761–770. [Google Scholar] [CrossRef]
- Numata, A.; Takahashi, C.; Fujiki, R.; Kitano, E.; Kitajima, A.; Takemura, T. Plant Constituents Biologically Active to Insects. VI. Antifeedants for Larvae of the Yellow Butterfly, Eurema Hecabe Mandarina, in Osmunda Japonica. (2). Chem. Pharm. Bull. 1990, 38, 2862–2865. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gamborg, O.; Miller, R.; Ojimi, K. Nutrient Requirements of Suspension Cultures of Soybean Root Cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Stankovic, M.S.; Niciforovic, N.; Mihailovic, V.; Topuzovic, M.; Solujic, S. Antioxidant activity, total phenolic content and flavonoid concentrations of different plant parts of Teucrium polium L. subsp. polium. Acta Soc. Bot. Pol. 2012, 81, 117–122. Available online: https://pbsociety.org.pl/journals/index.php/asbp/article/view/asbp.2012.010 (accessed on 15 June 2024). [CrossRef]
- Cai, W.; Gu, X.; Tang, J. Extraction, purification, and characterisation of the flavonoids from Opuntia milpa alta skin. Czech J. Food Sci. 2010, 28, 108–116. Available online: https://cjfs.agriculturejournals.cz/artkey/cjf-201002-0003_extraction-purification-and-characterisation-of-the-flavonoids-from-opuntia-milpa-alta-skin.php (accessed on 15 June 2024). [CrossRef]
- Ke, Z.C.; Zhu, Z.P.; Xu, Z.Y.; Fang, C.; Hu, S.Q. Response surface optimized extraction of total triterpene acids from Eriobotrya japonica (Thunb) Lindl (Loquat) leaf and evaluation of their in vitro antioxidant activities. Trop. J. Pharm. Res. 2014, 13, 787–792. Available online: https://www.ajol.info/index.php/tjpr/article/view/107603 (accessed on 15 June 2024). [CrossRef]
- Makkar, H.P.; Blümmel, M.; Borowy, N.K.; Becker, K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food Agric. 1993, 61, 161–165. Available online: https://scijournals.onlinelibrary.wiley.com/doi/10.1002/jsfa.2740610205 (accessed on 15 June 2024). [CrossRef]
- Marxen, K.; Vanselow, K.H.; Lippemeier, S.; Hintze, R.; Ruser, A.; Hansen, U.-P. Determination of DPPH Radical Oxidation Caused by Methanolic Extracts of Some Microalgal Species by Linear Regression Analysis of Spectrophotometric Measurements. Sensors 2007, 7, 2080–2095. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Bórquez, J.; Schmeda-Hirschmann, G. Antioxidant Capacity, Polyphenolic Content and Tandem HPLC–DAD–ESI/MS Profiling of Phenolic Compounds from the South American Berries Luma Apiculata and L. Chequén. Food Chem. 2013, 139, 289–299. [Google Scholar] [CrossRef]
- Chamorro, M.F.; Reiner, G.; Theoduloz, C.; Ladio, A.; Schmeda-Hirschmann, G.; Gómez-Alonso, S.; Jiménez-Aspee, F. Polyphenol Composition and (Bio)Activity of Berberis Species and Wild Strawberry from the Argentinean Patagonia. Molecules 2019, 24, 3331. [Google Scholar] [CrossRef] [PubMed]
- Jesionek, W.; Majer-Dziedzic, B.; Choma, I.M. Separation, Identification, and Investigation of Antioxidant Ability of Plant Extract Components Using TLC, LC-MS, and TLC-DPPH. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1147–1153. [Google Scholar] [CrossRef]
- Frum, A.; Dobrea, C.M.; Rus, L.L.; Virchea, L.I.; Morgovan, C.; Chis, A.A.; Arseniu, A.M.; Butuca, A.; Gligor, F.G.; Vicas, L.G.; et al. Valorization of Grape Pomace and Berries as a New and Sustainable Dietary Supplement: Development, Characterization, and Antioxidant Activity Testing. Nutrients 2022, 14, 3065. [Google Scholar] [CrossRef] [PubMed]
- Popescu, D.I.; Frum, A.; Dobrea, C.M.; Cristea, R.; Gligor, F.G.; Vicas, L.G.; Ionete, R.E.; Sutan, N.A.; Georgescu, C. Comparative Antioxidant and Antimicrobial Activities of Several Conifer Needles and Bark Extracts. Pharmaceutics 2024, 16, 52. [Google Scholar] [CrossRef]
- Aldea, F.; Banciu, C.; Brezeanu, A.; Helepciuc, F.E.; Soare, L.C. In vitro micropropagation of fern species (Pteridophyta) of biotechnological interest, for ex situ conservation. Olten. Stud. Comunicări Ştiinţele Nat. 2016, 32, 27–35. [Google Scholar]
- Roginsky, V.; Lissi, E. Review of methods to determine chain-breaking antioxidant activity in food. Food Chem. 2005, 92, 235–254. [Google Scholar] [CrossRef]
- Graßmann, J. Terpenoids as Plant Antioxidants. Vitam. Horm. 2005, 72, 505–535. [Google Scholar] [CrossRef] [PubMed]
- Tomou, E.M.; Skaltsa, H. Phytochemical investigation of the fern Asplenium ceterach (Aspleniaceae). Nat. Prod. Commun. 2018, 13, 849–850. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).