Integrated Transcriptome and Metabolome to Elucidate the Mechanism of Aluminum-Induced Blue-Turning of Hydrangea Sepals
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Treatment
2.2. Flower Color Determination
2.3. Determination of Metal ion Content
2.4. Extraction and Determination of Anthocyanin
2.5. Metabolite Extraction and Analysis
2.6. Transcriptome Analysis
2.7. qRT-PCR
2.8. Data Analysis
3. Results
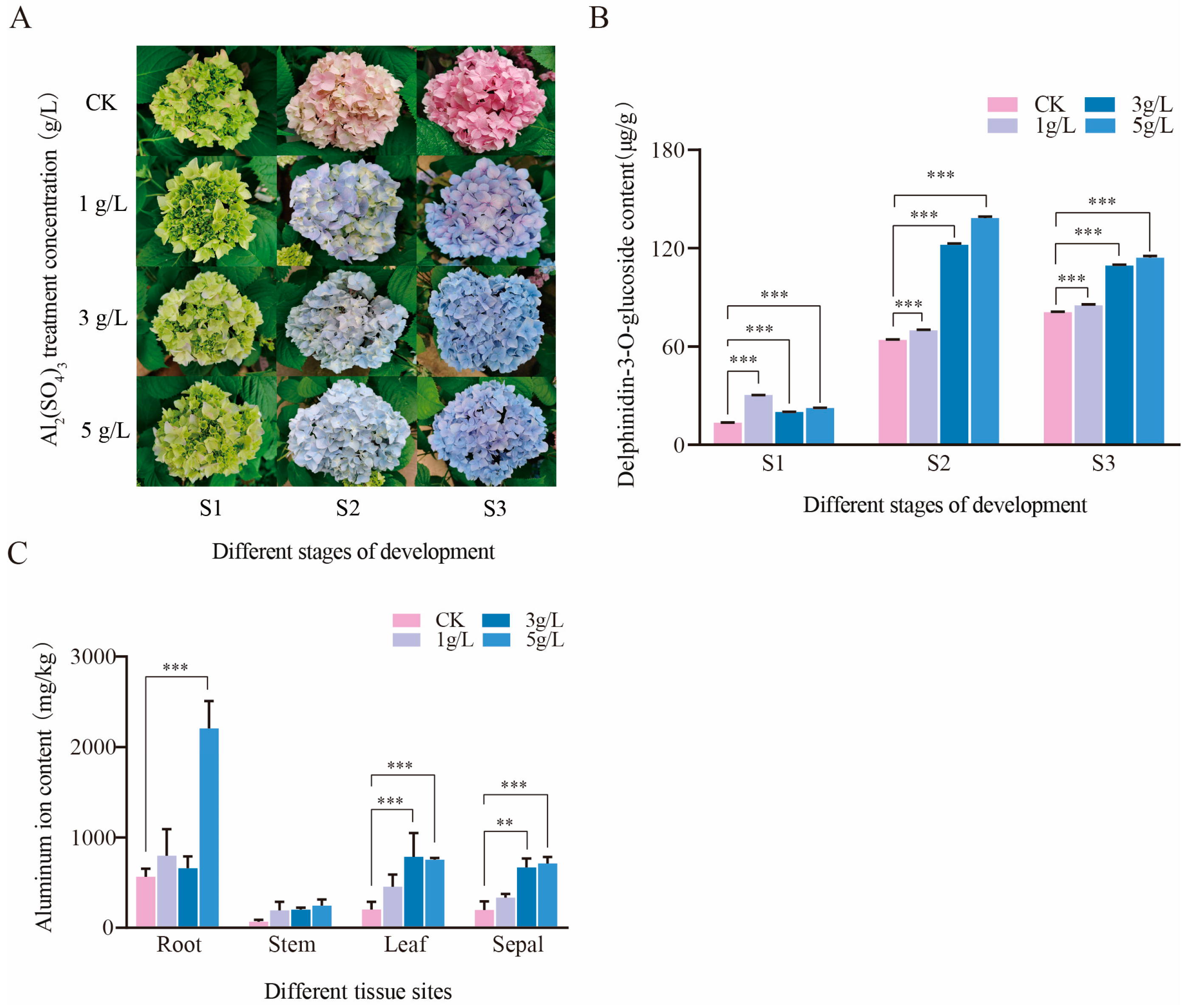
3.1. The Changes in Calyx Color and Metal Ion Content under Different Concentrations of Aluminum Treatment
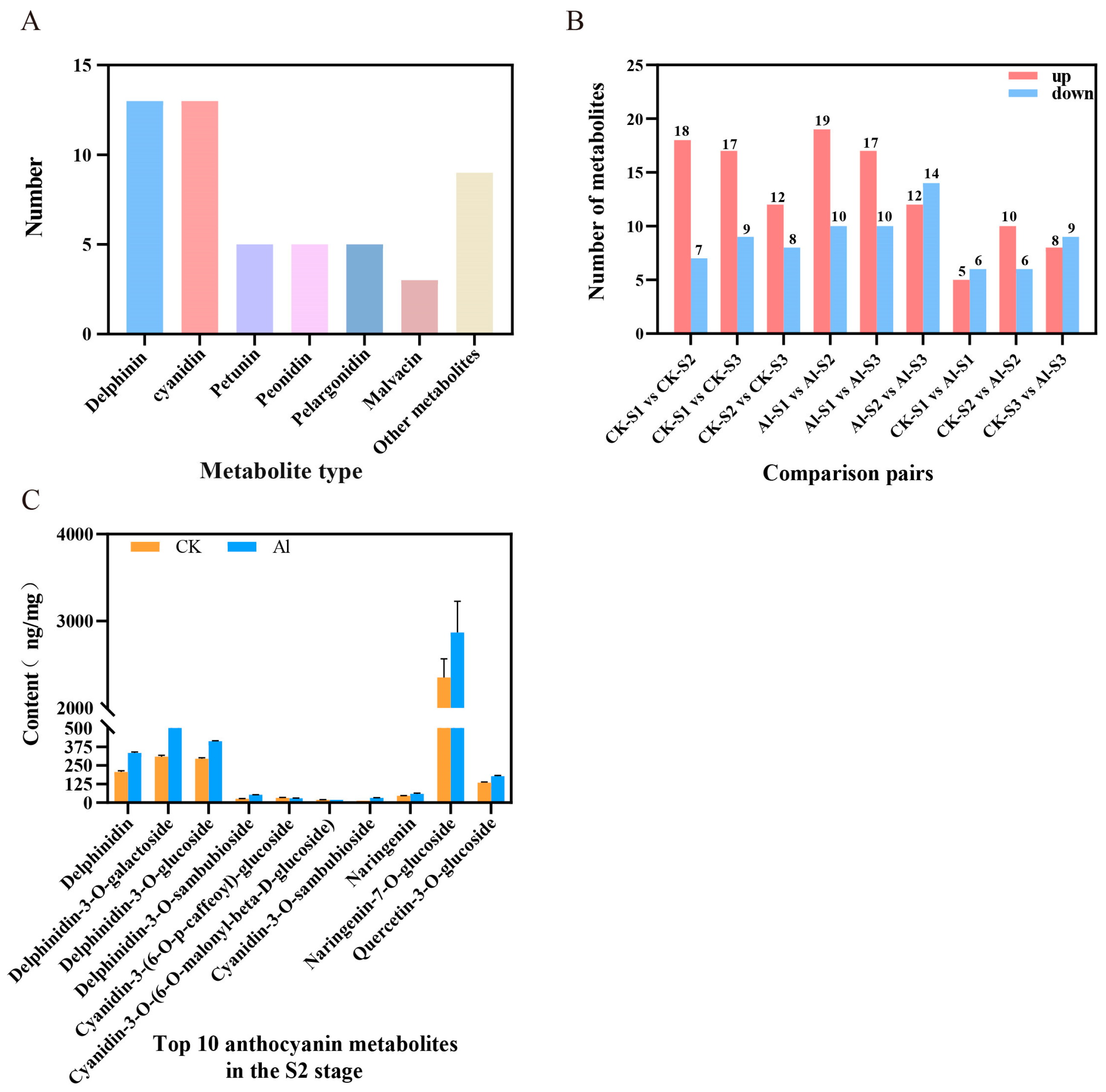
3.2. Metabolome Analysis of Anthocyanins
3.3. Calyx Transcriptome Analysis Data Statistics
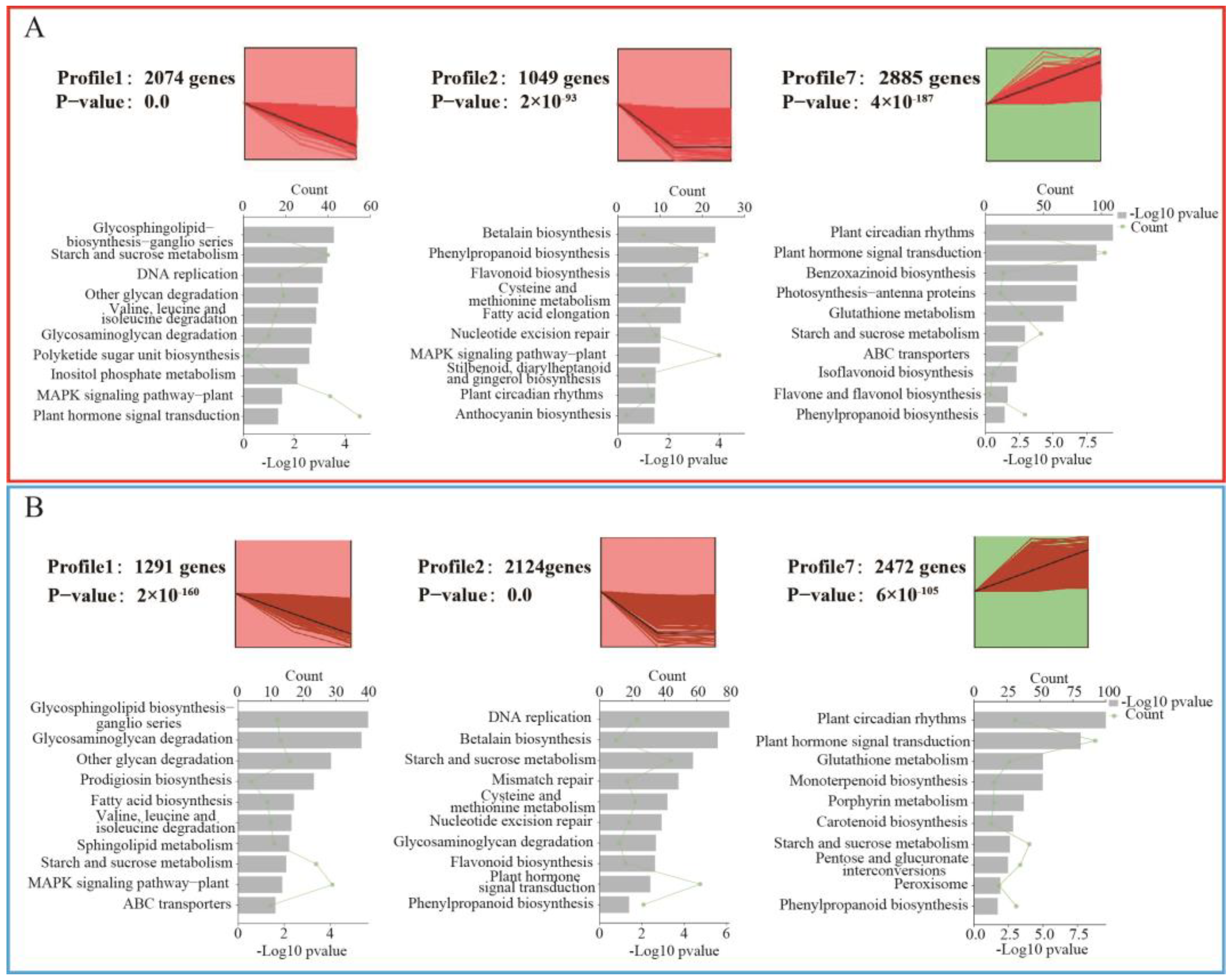
3.4. Changes in Gene Expression Profiles in Different Treatment Groups
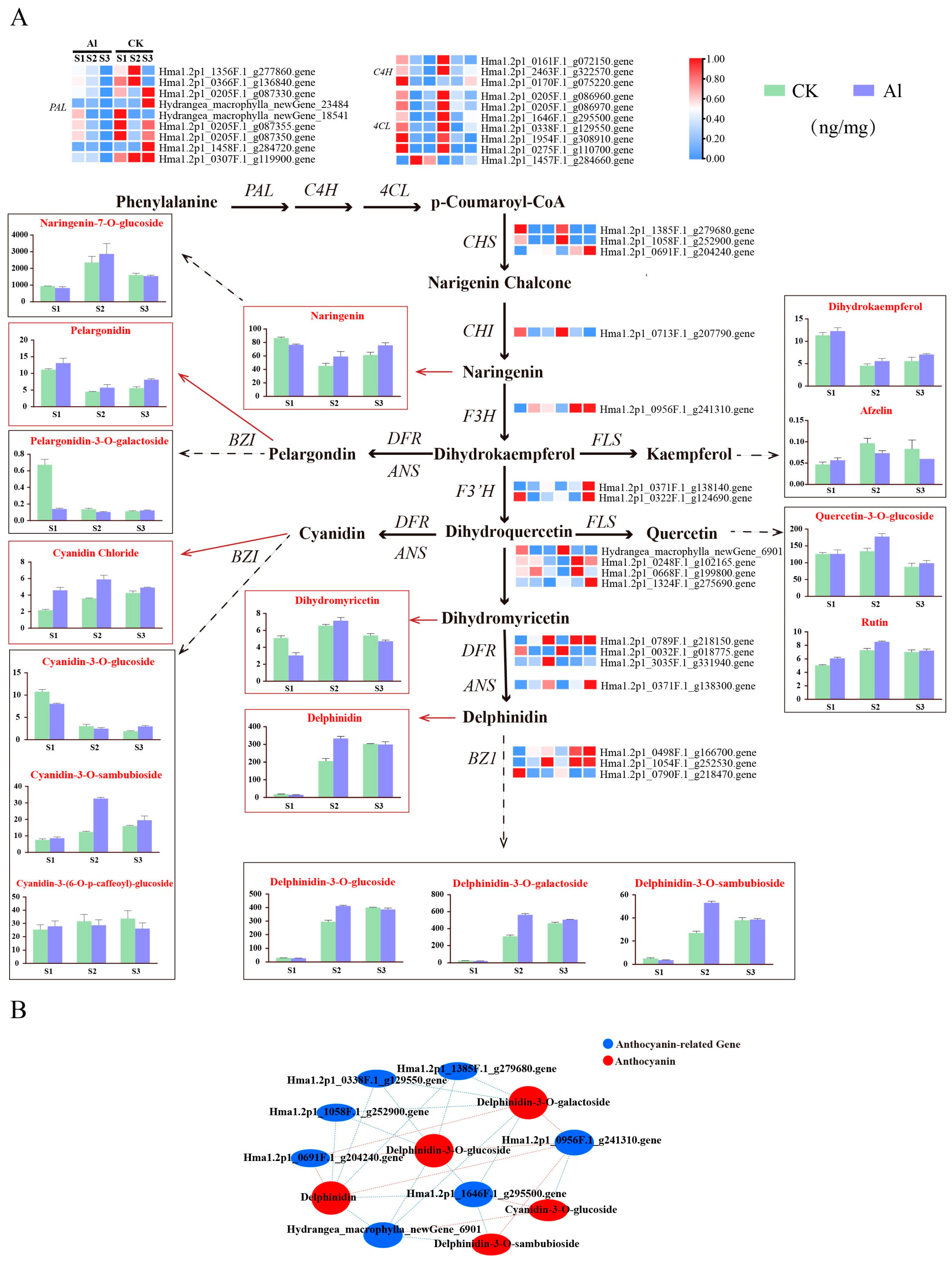
3.5. Association Analysis of Differential Genes and Metabolites in Anthocyanin Biosynthesis
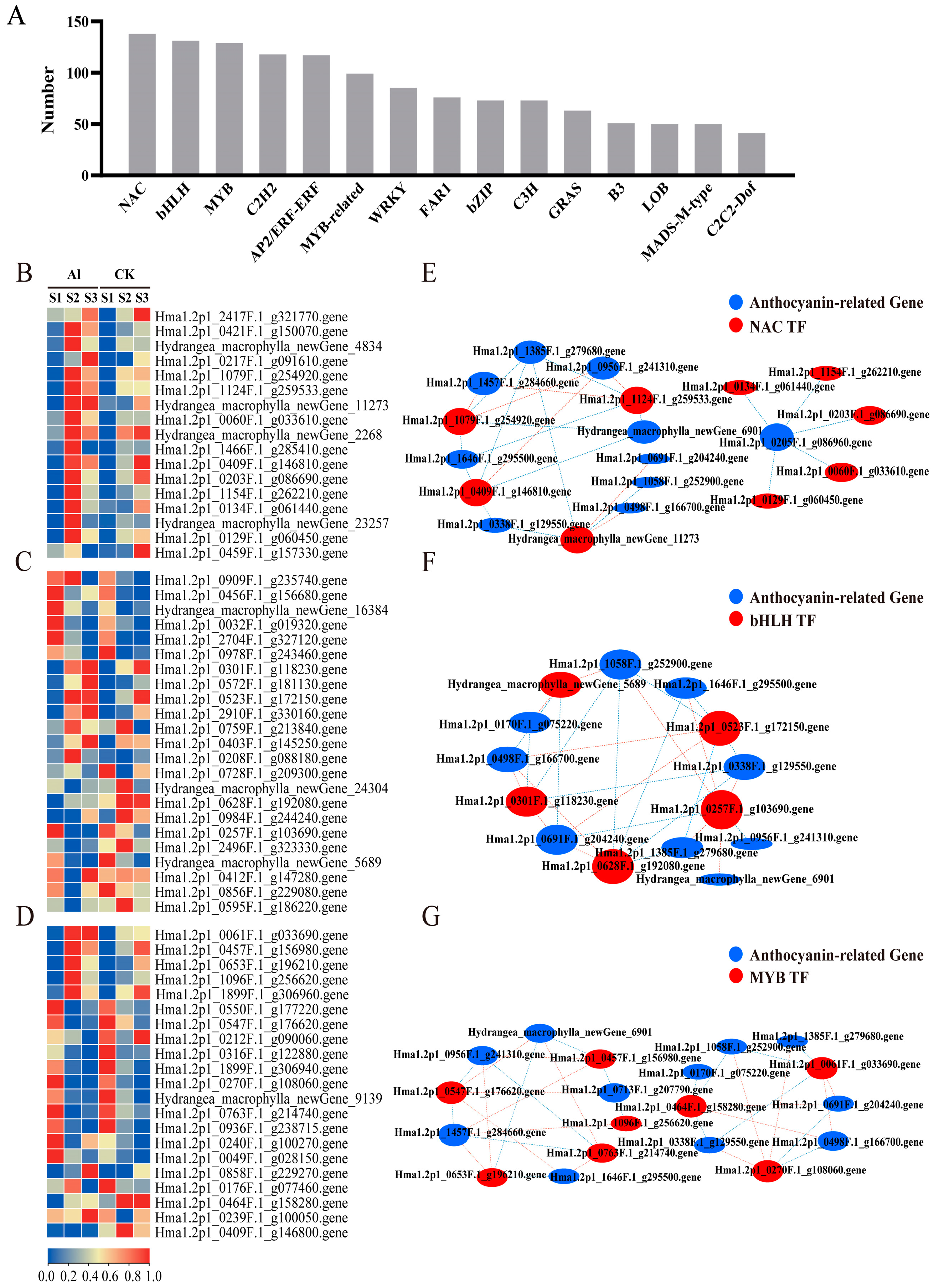
3.6. Expression Analysis of Transcription Factors Related to Anthocyanin Biosynthesis
3.7. Anthocyanin Transporter Gene Expression Analysis
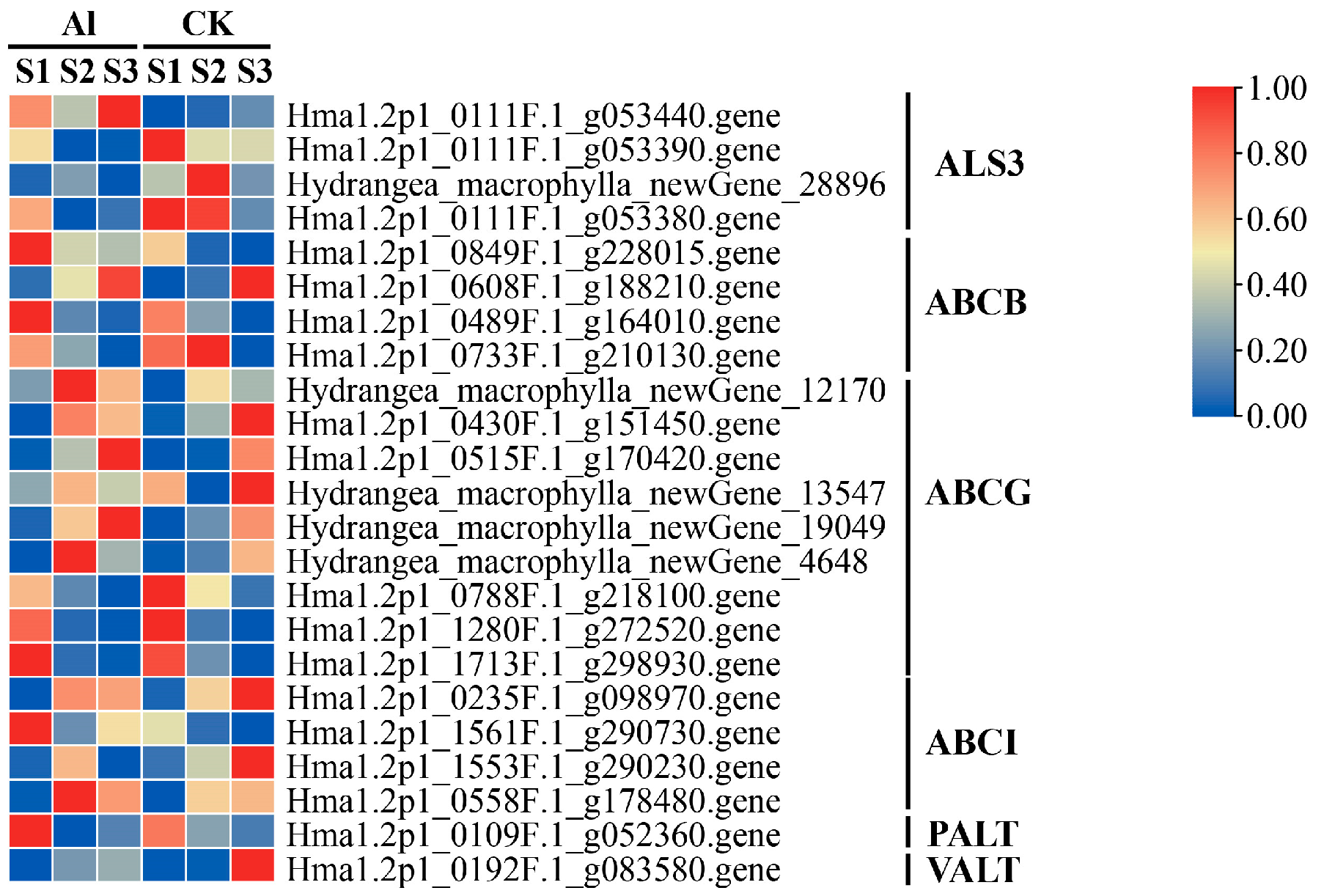
3.8. Expression Analysis of Aluminum Transport-Related Genes
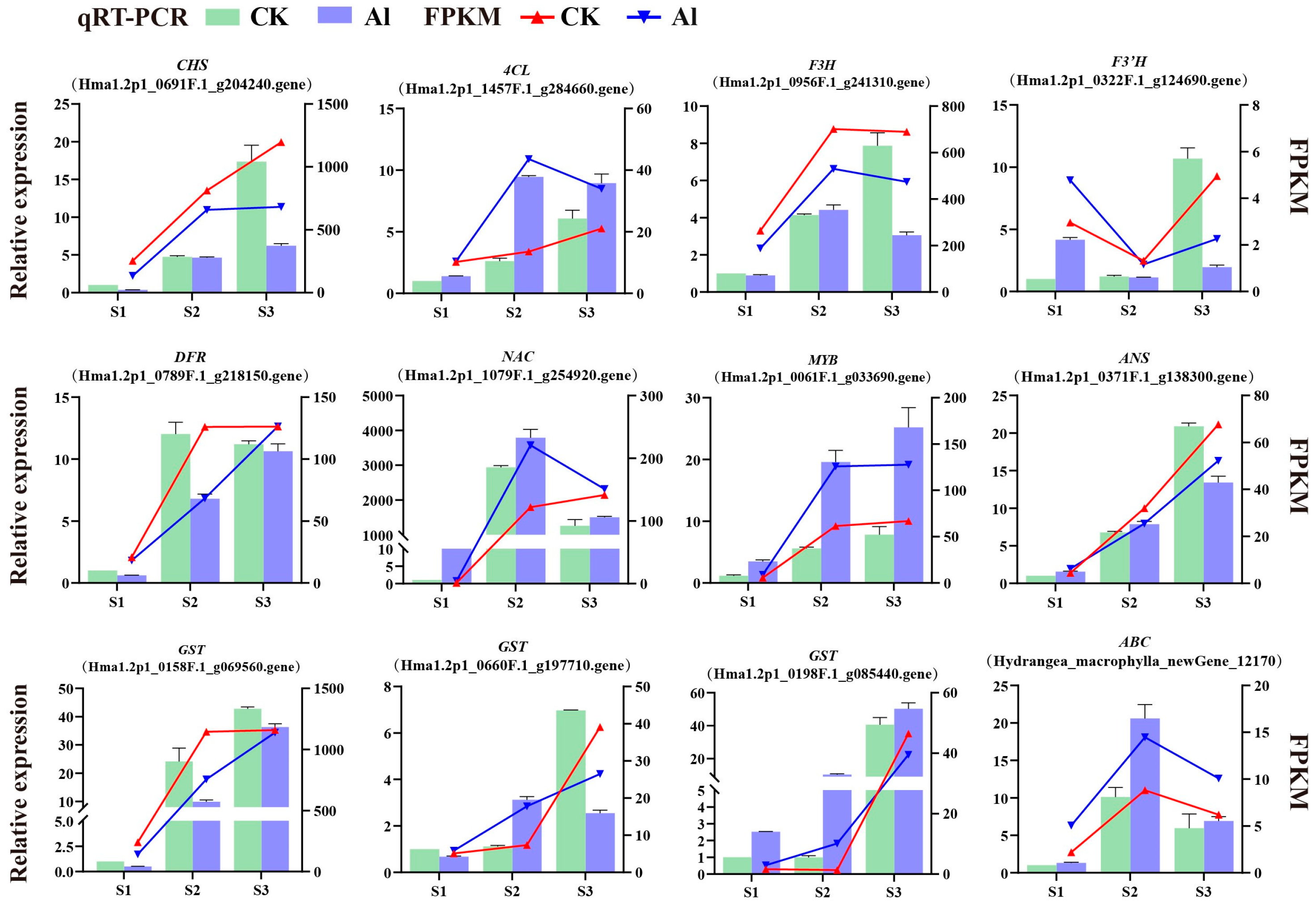
3.9. Real-Time PCR Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kodama, M.; Tanabe, Y.; Nakayama, M. Analyses of coloration-related components in Hydrangea sepals causing color variability according to soil conditions. Hortic. J. 2016, 85, 372–379. [Google Scholar] [CrossRef]
- Zhuang, W.; Liu, T.; Shu, X.; Qu, S.; Zhai, H.; Wang, T.; Zhang, F.; Wang, Z. Molecular regulation of anthocyanin glycoside biosynthesis and colour presentation in plants. Plant Physiol. J. 2018, 54, 1630–1644. [Google Scholar]
- Asen, S.; Siegelman, H.W.; Stuart, N.W. Anthocyanin and other phenolic compounds in red and blue sepals of Hydrangea macrophylla var. Merveille. Proc. Amer. Soc. Hort. Sci. 1957, 69, 561–569. [Google Scholar]
- Peng, J.; Xue, C.; Dong, X.; Zeng, C.; Wu, Y.; Cao, F. Gene cloning and analysis of the pattern of expression of the transcription factor HymMYB2 related to blue flower formation in Hydrangea macrophylla. Euphytica 2021, 217, 115. [Google Scholar] [CrossRef]
- Chen, D.; Li, M.; Peng, J.; Tian, Y.; Chao, S. Association between HmDFR gene expression and flower color of Hydrangea macrophylla. Plant Physiol. J. 2020, 56, 1641–1649. [Google Scholar]
- Zhao, J.; Dixon, R.A. The ‘ins’ and ‘outs’ of flavonoid transport. Trends Plant Sci. 2010, 15, 72–80. [Google Scholar] [CrossRef]
- Mueller, L.A.; Goodman, C.D.; Silady, R.A.; Walbot, V. AN9, a petunia glutathione S-transferase required for anthocyanin sequestration, is a flavonoid-binding protein. Plant Physiol. 2000, 123, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Marrs, K.A.; Alfenito, M.R.; Lloyd, A.M.; Walbot, V. A glutathione S-transferase involved in vacuolar transfer encoded by the maize gene Bronze-2. Nature 1995, 375, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, S.; Akita, Y.; Ishizaka, H.; Narumi, I.; Tanaka, A. Molecular characterization of an anthocyanin-related glutathione S-transferase gene in cyclamen. J. Plant Physiol. 2012, 169, 636–642. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Li, F.; Xiang, L.; Chen, K. The Isolation and Identification of Anthocyanin-Related GSTs in Chrysanthemum. Horticulturae 2021, 7, 231. [Google Scholar] [CrossRef]
- Cui, Y.; Fan, J.; Lu, C.; Ren, J.; Qi, F.; Huang, H.; Dai, S. ScGST3 and multiple R2R3-MYB transcription factors function in anthocyanin accumulation in Senecio cruentus. Plant Sci. 2021, 313, 111094. [Google Scholar] [CrossRef]
- Han, L.; Zou, H.; Zhou, L.; Wang, Y. Transcriptome-based identification and expression analysis of the glutathione S-transferase(GST)family in tree peony reveals a likely role in anthocyanin transport. Hortic. Plant J. 2022, 8, 787–802. [Google Scholar] [CrossRef]
- Alfenito, M.R.; Souer, E.; Goodman, C.D.; Buell, R.; Mol, J.; Koes, R.; Walbot, V. Functional complementation of anthocyanin sequestration in the vacuole by widely divergent glutathione S-transferases. Plant Cell 1998, 10, 1135–1149. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.P.; Li, Z.S.; Rea, P.A. AtMRP1 gene of Arabidopsis encodes a glutathione S-conjugate pump: Isolation and functional definition of a plant ATP-binding cassette transporter gene. Proc. Natl. Acad. Sci. USA 1997, 94, 8243–8248. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.P.; Li, Z.S.; Drozdowicz, Y.M.; Hortensteiner, S.; Martinoia, E.; Rea, P.A. AtMRP2, an Arabidopsis ATP binding cassette transporter able to transport glutathione S-conjugates and chlorophyll catabolites: Functional comparisons with Atmrp1. Plant Cell 1998, 10, 267–282. [Google Scholar] [CrossRef]
- Goodman, C.D.; Casati, P.; Walbot, V. A multidrug resistance–associated protein involved in anthocyanin transport in Zea mays. Plant Cell 2004, 16, 1812–1826. [Google Scholar] [CrossRef]
- Zhu, Q.; Xie, X.; Zhang, J.; Xiang, G.; Li, Y.T.; Wu, H. In Silico Analysis of a MRP Transporter Gene Reveals Its Possible Role in Anthocyanins or Flavonoids Transport in Oryze sativa. Am. J. Plant Sci. 2013, 4, 555–560. [Google Scholar] [CrossRef]
- Francisco, R.M.; Regalado, A.; Ageorges, A.; Burla, B.J.; Bassin, B.; Eisenach, C.; Zarrouk, O.; Vialet, S.; Marlin, T.; Chaves, M.M.; et al. ABCC1, an ATP binding cassette protein from grape berry, transports anthocyanidin 3-O-Glucosides. Plant Cell 2013, 25, 1840–1854. [Google Scholar] [CrossRef]
- Ito, D.; Shinkai, Y.; Kato, Y.; Kondo, T.; Yoshida, K. Chemical Studies on Different Color Development in Blue- and Red-Colored Sepal Cells of Hydrangea macrophylla. Biosci. Biotechnol. Biochem. 2009, 73, 1054–1059. [Google Scholar] [CrossRef]
- Ito, T.; Aoki, D.; Fukushima, K.; Yoshida, K. Direct mapping of hydrangea blue-complex in sepal tissues of Hydrangea macrophylla. Sci. Rep. 2019, 9, 5450. [Google Scholar] [CrossRef]
- Ito, T.; Oyama, K.-i.; Yoshida, K. Direct observation of hydrangea blue-complex composed of 3-O-glucosyldelphinidin, Al3+ and 5-O-acylquinic acid by ESI-mass spectrometry. Molecules 2018, 23, 1424. [Google Scholar] [CrossRef]
- Yoshida, K.; Oyama, K.-i.; Kondo, T. Insight into chemical mechanisms of sepal color development and variation in hydrangea. Proc. Jpn. Acad. Ser. B 2021, 97, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Negishi, T.; Oshima, K.; Hattori, M.; Kanai, M.; Mano, S.; Nishimura, M.; Yoshida, K. Tonoplast- and plasma membrane-localized aquaporin-family transporters in blue hydrangea sepals of aluminum hyperaccumulating plant. PLoS ONE 2012, 7, e43189. [Google Scholar] [CrossRef]
- Gong, Z.; He, Y.; Yang, J.; Song, Y.; Ye, Z.; Zhu, Z. Mechanism of exogenous Al2(SO4)3 on regulating the anthocyanin concen-tration in Hydrangea macrophylla petal. J. Plant Nutr. Fertil. 2017, 23, 821–826. [Google Scholar]
- Chen, H.; Wang, D.; Zhu, Y.; Li, W.; Chen, J.; Li, Y. Integrative transcriptomics and proteomics elucidate the regulatory mechanism of Hydrangea macrophylla flower-color changes induced by exogenous aluminum. Agronomy 2022, 12, 969. [Google Scholar] [CrossRef]
- Chen, S.; Qi, X.; Feng, J.; Chen, H.; Qin, Z.; Wang, H.; Deng, Y. Biochemistry and transcriptome analyses reveal key genes and pathways involved in high-aluminum stress response and tolerance in hydrangea sepals. Plant Physiol. Biochem. 2022, 185, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, J.; Gao, C.; Lu, J.; Du, G.; Sun, X. The taxonomic key of 63 herbaceous peony cultivar. Chin. Agric. Sci. Bull. 2018, 34, 51–59. [Google Scholar]
- Hu, Y.; Yuan, J.; Chen, H. Effect of aluminum stress on flower color of Hydrangea cultivar ‘Meihong Mother’. Mol. Plant Breed. 2021, 19, 6164–6171. [Google Scholar]
- Mestdagh, P.; Van Vlierberghe, P.; De Weer, A.; Muth, D.; Westermann, F.; Speleman, F.; Vandesompele, J. A novel and universal method for microRNA RT-qPCR data normalization. Genome Biol. 2009, 10, R64. [Google Scholar] [CrossRef]
- Oyama, K.; Yamada, T.; Ito, D.; Kondo, T.; Yoshida, K. Metal Complex Pigment Involved in the Blue Sepal Color Development of Hydrangea. J. Agric. Food Chem. 2015, 63, 7630–7635. [Google Scholar] [CrossRef]
- Nianiou-Obeidat, I.; Madesis, P.; Kissoudis, C.; Voulgari, G.; Chronopoulou, E.; Tsaftaris, A.; Labrou, N.E. Plant glutathione transferase-mediated stress tolerance: Functions and biotechnological applications. Plant Cell Rep. 2017, 36, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, W.; Ma, Y.; Han, E.; Han, L.; Sun, L.; Peng, Z.; Wang, B. Advances in the study of ABC transporter proteins in Arabidopsis thaliana. Plant Physiol. J. 2017, 53, 133–144. [Google Scholar]
- Schreiber, H.D.; Jones, A.H.; Lariviere, C.M.; Mayhew, K.M.; Cain, J.B. Role of aluminum in red-to-blue color changes in Hydrangea macrophylla sepals. Biometals 2011, 24, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.B.; Geisler, M.J.; Jones, C.A.; Williams, K.M.; Cancel, J.D. ALS3 encodes a phloem-localized ABC transporter-like protein that is required for aluminum tolerance in Arabidopsis. Plant J. 2005, 41, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.J.; Yokosho, K.; Yamaji, N.; Fujii-Kashino, M.; Ma, J.F. Functional characterization of two half-size ABC transporter genes in aluminium-accumulating buckwheat. New Phytol. 2017, 215, 1080–1089. [Google Scholar] [CrossRef]
- Huang, C.F.; Yamaji, N.; Chen, Z.; Ma, J.F. A tonoplast-localized half-size ABC transporter is required for internal detoxification of aluminum in rice. Plant J. 2012, 69, 857–867. [Google Scholar] [CrossRef]
- Yang, K.; Shan, X.; Shi, J.; Zhu, C.; Gao, Z. Identification and expression analysis of 4CL gene family in Phyllostachys edulis. J. Nucl. Agric. Sci. 2021, 35, 72–82. [Google Scholar]
- Dai, S.; Hong, Y. Molecular breeding for flower colors modification on ornamental plants based on the mechanism of antho-cyanins biosynthesis and coloration. Sci. Agric. Sin. 2016, 49, 529–542. [Google Scholar]
- Du, L.; Chen, K.; Liu, Y. Cloning and expression analysis of anthocyanidin-3-O-glucosytransferase gene in Grape hyacinth. Pratacultural Sci. 2017, 34, 10. [Google Scholar]
- Rahmati, R.; Hamid, R.; Ghorbanzadeh, Z.; Jacob, F.; Azadi, P.; Zeinalabedini, M.; Karimi Farsad, L.; Kazemi, M.; Ebrahimi, M.A.; Shahinnia, F. Comparative Transcriptome Analysis Unveils the Molecular Mechanism Underlying Sepal Colour Changes under Acidic pH Substratum in Hydrangea macrophylla. Int. J. Mol. Sci. 2022, 23, 15428. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ohmiya, A. Seeing is believing: Engineering anthocyanin and carotenoid biosynthetic pathways. Curr. Opin. Biotechnol. 2008, 19, 190–197. [Google Scholar] [CrossRef]
- Ramsay, N.A.; Glover, B.J. MYB–bHLH–WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 2005, 10, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Mao, L.; Zhou, Y.; Sun, Y.; Liu, Z.; Liang, C. Integrated transcriptome and metabolome analysis revealed the molecular mechanism of anthocyanin synthesis in purple leaf pepper (Capsicum annuum L.) under different light intensities. Horticulturae 2023, 9, 814. [Google Scholar] [CrossRef]
- Ke, Y.J.; Zheng, Q.D.; Yao, Y.H.; Ou, Y.; Chen, J.Y.; Wang, M.J.; Lai, H.P.; Yan, L.; Liu, Z.J.; Ai, Y. Genome-wide identification of the MYB gene family in cymbidium ensifolium and its expression analysis in different flower colors. Int. J. Mol. Sci. 2021, 22, 13245. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Huo, D.-A.; Hong, X.-X.; Guo, J.; Peng, T.; Liu, J.; Huang, X.-L.; Yan, H.-Q.; Weng, Q.-B.; Zhang, X.-C. The Salvia miltiorrhiza NAC transcription factor SmNAC1 enhances zinc content in transgenic Arabidopsis. Gene 2019, 688, 54–61. [Google Scholar] [CrossRef]
- Zhou, H.; Lin-Wang, K.; Wang, H.; Gu, C.; Dare, A.P.; Espley, R.V.; He, H.; Allan, A.C.; Han, Y. Molecular genetics of blood-fleshed peach reveals activation of anthocyanin biosynthesis by NAC transcription factors. Plant J. 2015, 82, 105–121. [Google Scholar] [CrossRef]
- Sun, Q.; Jiang, S.; Zhang, T.; Xu, H.; Fang, H.; Zhang, J.; Su, M.; Wang, Y.; Zhang, Z.; Wang, N.; et al. Apple NAC transcription factor MdNAC52 regulates biosynthesis of anthocyanin and proanthocyanidin through MdMYB9 and MdMYB11. Plant Sci. 2019, 289, 110286. [Google Scholar] [CrossRef]
- Kitamura, S.; Shikazono, N.; Tanaka, A. TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J. 2004, 37, 104–114. [Google Scholar] [CrossRef]
- Zhang, X.; Tao, L.; Qiao, S.; Du, B.; Guo, C. The role of glutathione transferase in plant resistance to abiotic stresses. China Biotechnol. 2017, 37, 92–98. [Google Scholar]
- Zhang, Y.; Zhang, F.; Wang, L.; Wei, K.; Cheng, H. Plant glutathione s-transferases: Roles in flavonoid accumulation. Plant Physiol. J. 2015, 51, 1815–1820. [Google Scholar]
- Estévez, I.H.; Hernández, M.R. Plant glutathione S-transferases: An overview. Plant Gene 2020, 23, 100233. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.; Huang, J.-R. Arabidopsis TT19 functions as a carrier to transport anthocyanin from the cytosol to tonoplasts. Mol. Plant 2012, 5, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Shibata, M.; Nishiyama, Y.; Springob, K.; Kitayama, M.; Shimada, N.; Aoki, T.; Ayabe, S.i.; Saito, K. Differential gene expression profiles of red and green forms of Perilla frutescens leading to comprehensive identification of anthocyanin biosynthetic genes. FEBS J. 2008, 275, 3494–3502. [Google Scholar] [CrossRef] [PubMed]
- Conn, S.; Curtin, C.; Bézier, A.; Franco, C.; Zhang, W. Purification, molecular cloning, and characterization of glutathione S-transferases (GSTs) from pigmented Vitis vinifera L. cell suspension cultures as putative anthocyanin transport proteins. J. Exp. Bot. 2008, 59, 3621–3634. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Nishizaki, Y.; Uchida, Y.; Wakamatsu, E.; Umemoto, N.; Momose, M.; Okamura, M.; Yoshida, H.; Yamaguchi, M.; Nakayama, M. Identification of the glutathione S-transferase gene responsible for flower color intensity in carnations. Plant Biotechnol. 2012, 29, 223–227. [Google Scholar] [CrossRef]
- Jin, X.; Hong, Y.; Huang, H.; Dai, S.; Zhu, Y. Isolation and expression analysis of GST gene encoding glutathione s-transferase from Senecio Cruentus. Acta Hortic. Sin. 2013, 40, 1129–1138. [Google Scholar]
- Shi, Q.; Zhou, L.; Li, Q.; Wang, Y. Isolation and expression analysis of GST gene encoding glutathione S-transferase of Paeonia delavayi var. lutea Wild Population in Yunnan. Sci. Silvae Sin. 2014, 50, 63–72. [Google Scholar]
- Park, J.; Song, W.Y.; Ko, D.; Eom, Y.; Hansen, T.H.; Schiller, M.; Lee, T.G.; Martinoia, E.; Lee, Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012, 69, 278–288. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Lei, P.; Zhu, T.; Zhang, H.; Jiang, H.; Chen, H. Integrated Transcriptome and Metabolome to Elucidate the Mechanism of Aluminum-Induced Blue-Turning of Hydrangea Sepals. Horticulturae 2024, 10, 745. https://doi.org/10.3390/horticulturae10070745
Li W, Lei P, Zhu T, Zhang H, Jiang H, Chen H. Integrated Transcriptome and Metabolome to Elucidate the Mechanism of Aluminum-Induced Blue-Turning of Hydrangea Sepals. Horticulturae. 2024; 10(7):745. https://doi.org/10.3390/horticulturae10070745
Chicago/Turabian StyleLi, Wenfang, Penghu Lei, Tingting Zhu, Huijun Zhang, Hui Jiang, and Haixia Chen. 2024. "Integrated Transcriptome and Metabolome to Elucidate the Mechanism of Aluminum-Induced Blue-Turning of Hydrangea Sepals" Horticulturae 10, no. 7: 745. https://doi.org/10.3390/horticulturae10070745
APA StyleLi, W., Lei, P., Zhu, T., Zhang, H., Jiang, H., & Chen, H. (2024). Integrated Transcriptome and Metabolome to Elucidate the Mechanism of Aluminum-Induced Blue-Turning of Hydrangea Sepals. Horticulturae, 10(7), 745. https://doi.org/10.3390/horticulturae10070745




