The Shelf Life of Yellow Passion Fruit with an Edible Biocomposite Coating Based on Chitosan, Graphene Oxide Nanoparticles, and Beeswax
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Film Solutions
2.3. Film Characterization
2.4. Preparation of Fruits
2.5. Treatments and Edible Coating Application
2.6. Physiological and Quality Parameters
2.7. Antioxidant Compounds
2.8. Sensory Analysis
2.9. Statistical Analysis
3. Results
3.1. Barrier and Optical Properties of Polymeric Films
3.2. Application and Postharvest Evaluation of Edible Coatings on Passion Fruit
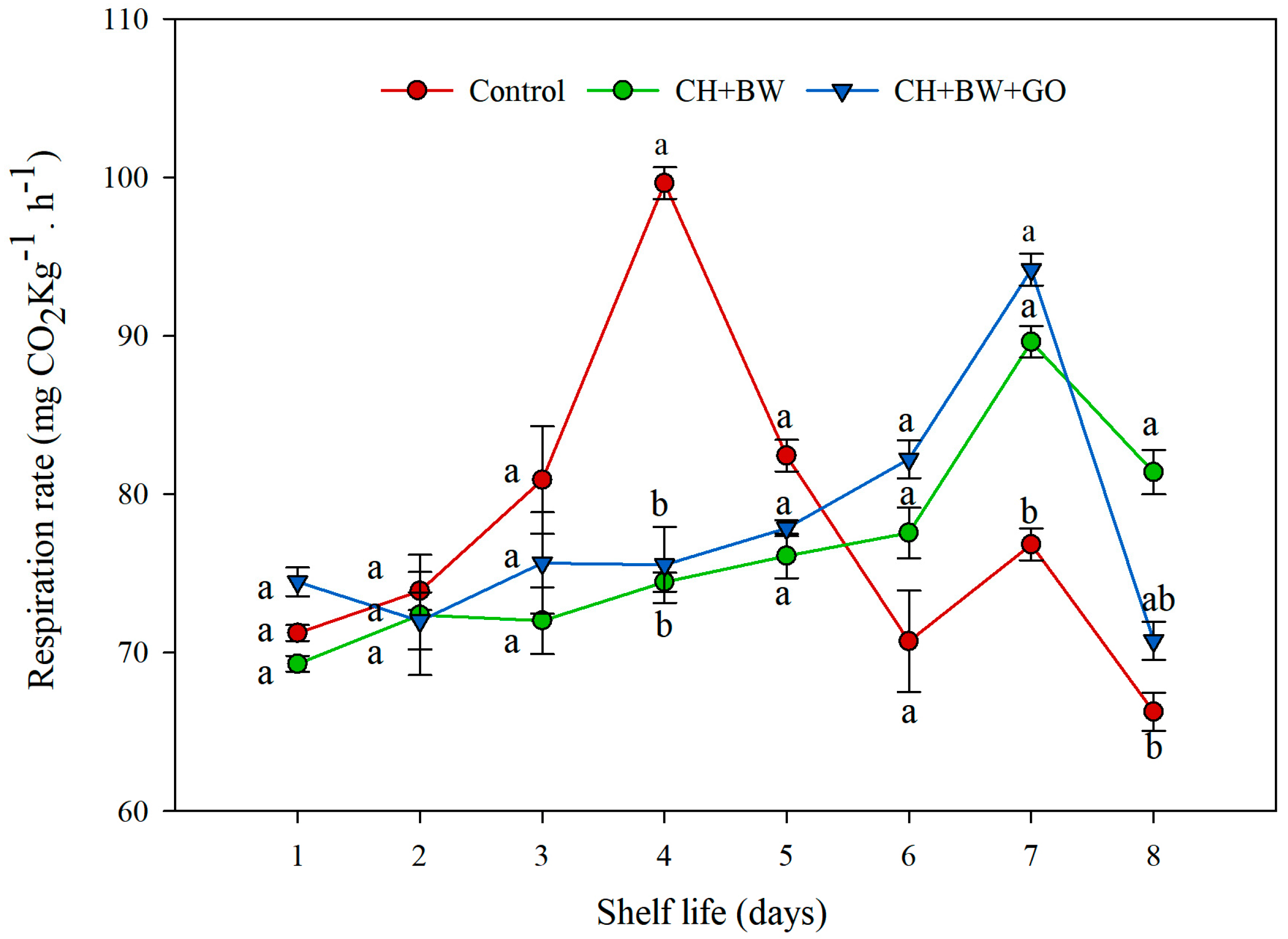
3.2.1. Respiration Rate
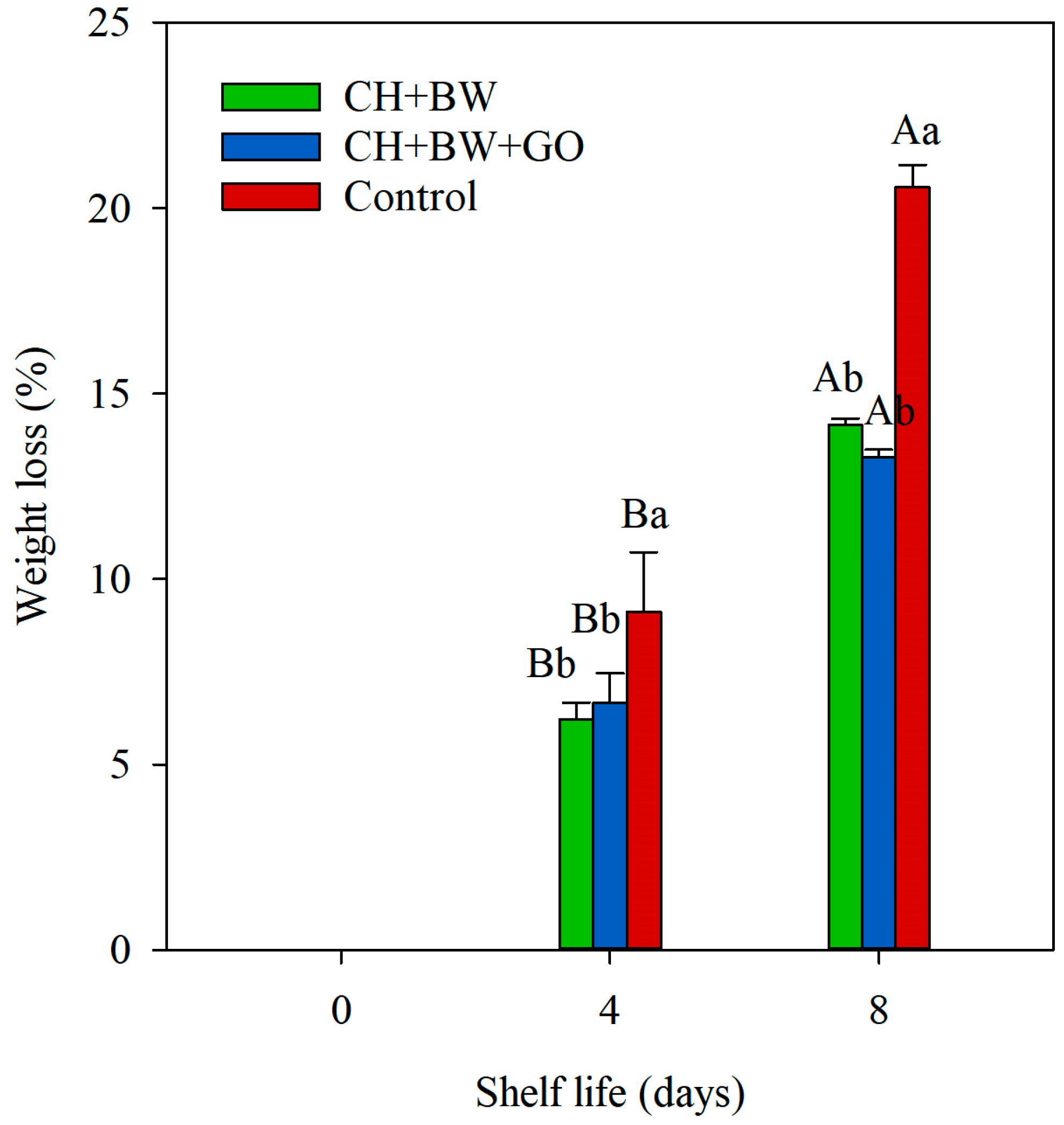
3.2.2. Weight Loss
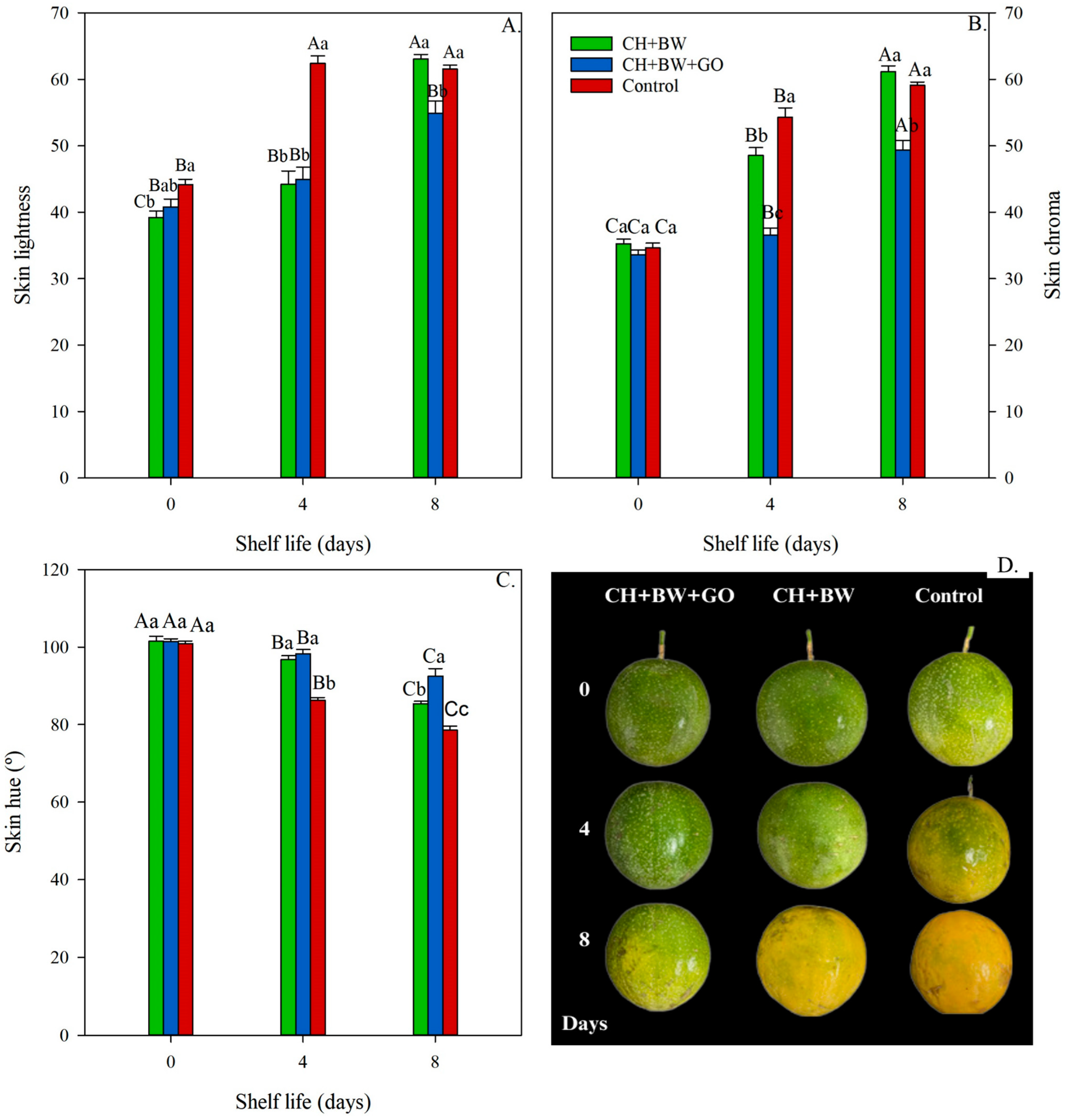
3.2.3. Skin Color
3.2.4. Soluble Solids Content (SS), Titratable Acidity (TA), and Ratio (SS/TA)
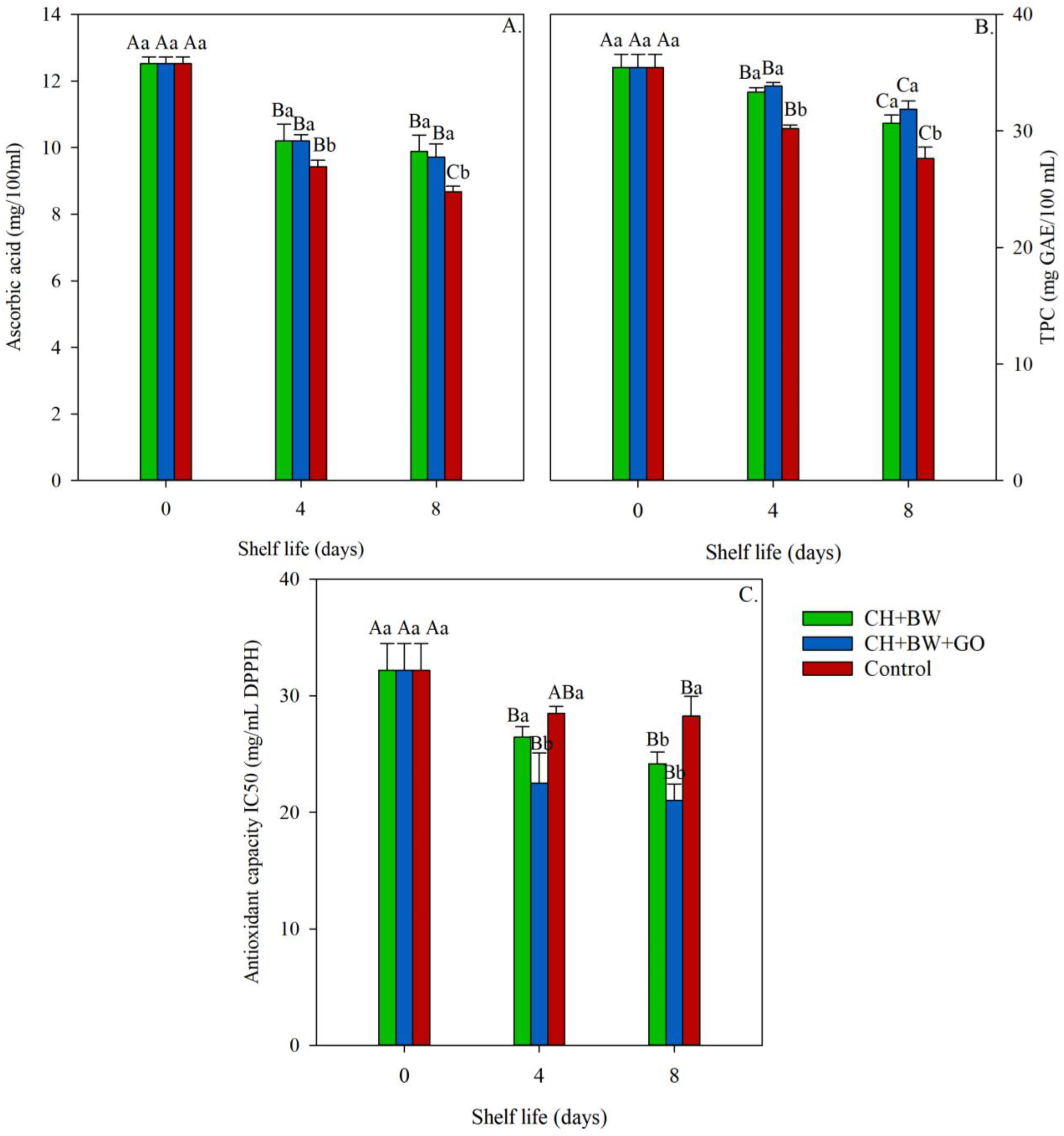
3.2.5. Ascorbic Acid Content, Total Phenolic Compound Content, and Antioxidant Capacity (IC50)
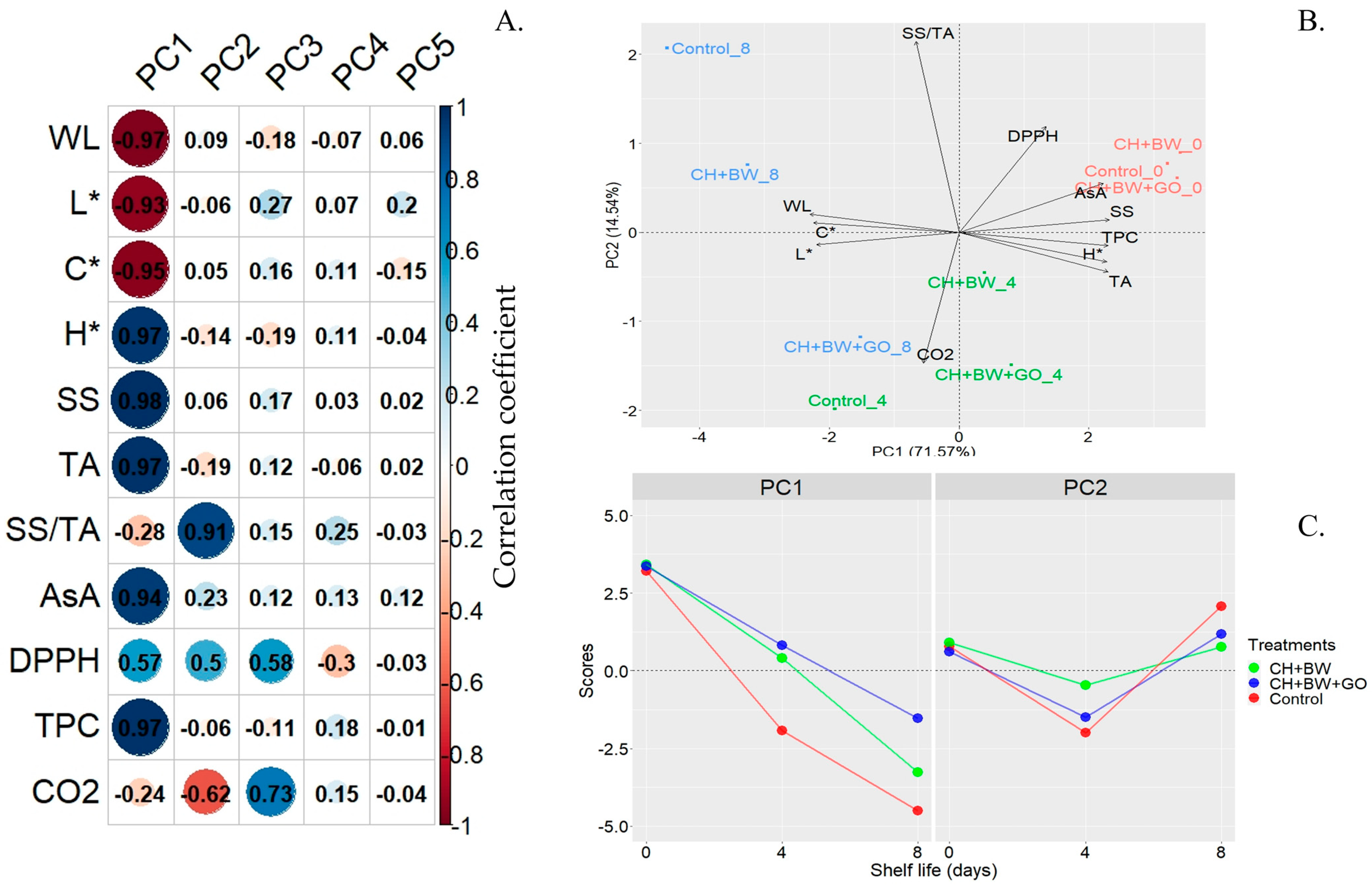
3.2.6. Principal Component Analysis
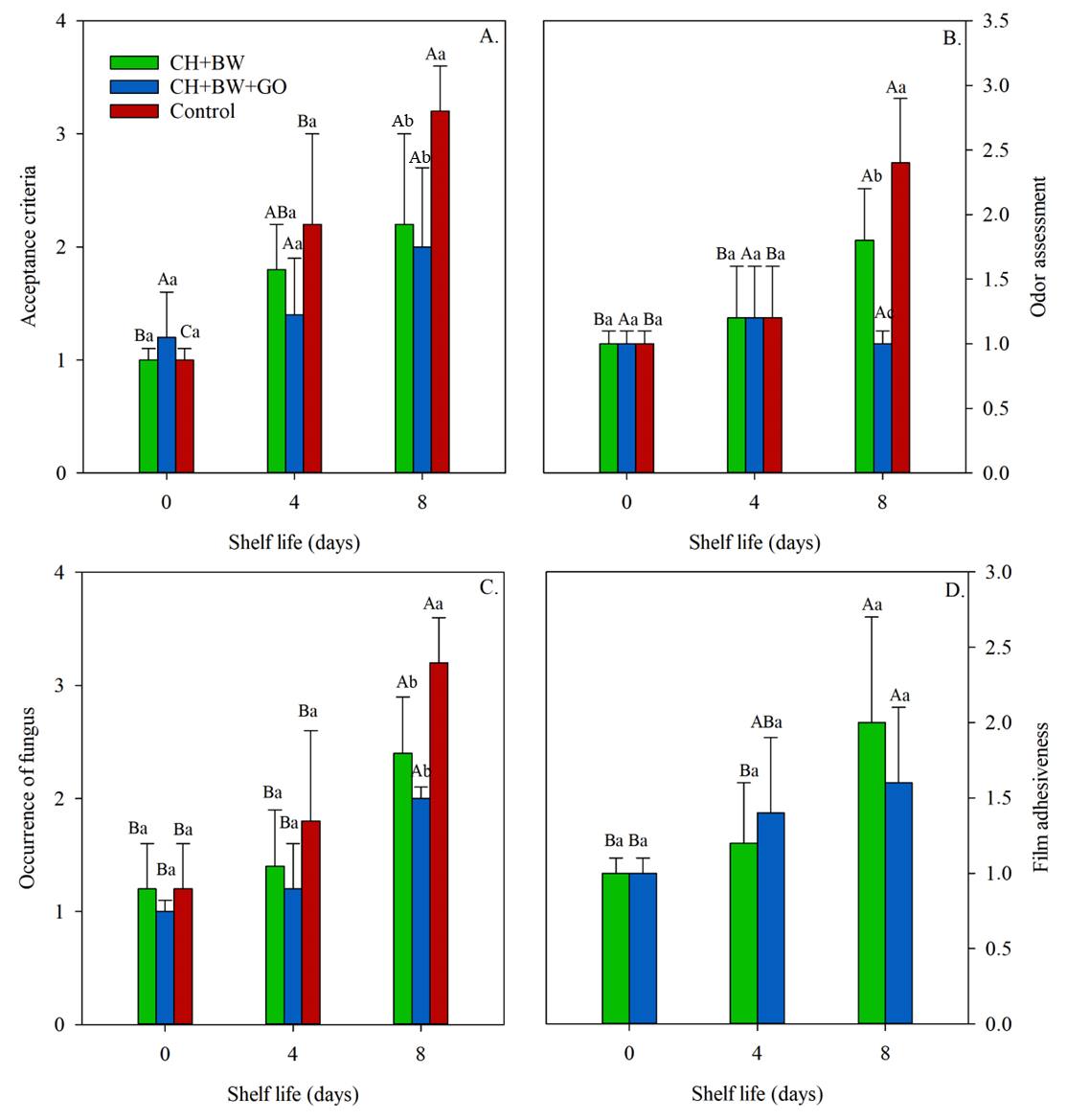
3.2.7. Sensory Analysis
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, J.; Zhong, B.; Subbiah, V.; Barrow, C.J.; Dunshea, F.R.D.; Suléria, H.A.R. LC-ESI-QTOF-MS/MS Profiling and Antioxidant Activity of Phenolics from Custard Apple Fruit and By-Products. Separations 2021, 8, 62. [Google Scholar] [CrossRef]
- Wang, W.; Gao, Y.T.; Wei, J.W.; Chen, Y.F.; Liu, Q.L.; Liu, H.M. Optimization of ultrasonic cellulase-assisted extraction and antioxidant activity of natural polyphenols from passion fruit. Molecules 2021, 9, 2494. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, L.C.R.; Facco, E.M.P.; Salvador, M.; Flôres, S.H.; Rios, A.d.O. Antioxidant potential and physicochemical characterization of yellow, purple, and orange passion fruit. J. Food Sci. Technol. 2018, 55, 2679–2691. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, P.; Yang, Q.; Shen, Y.; Deng, J.; Li, L.; Zhao, D. Comparative studies on anxiolytic activities and flavonoid compositions of Passiflora edulis ‘edulis’ and Passiflora edulis ‘flavicarpa’. J. Ethnopharmacol. 2011, 133, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Junior, J.R.; Corrêa-Filho, L.C.; Pereira, V.O.; Barbosa, H.T.G.; Soares, A.G.; Tonon, R.V.; Cabral, L.M.C. Application of rosin resin and zinc oxide nanocomposites to chitosan coatings for extending the shelf life of passion fruit s. Sustain. Food Technol. 2024, 2, 415–425. [Google Scholar] [CrossRef]
- Faleiro, F.G. Maracujá: Fruta nativa do Brasil para o mundo. Anuário Camp. Negóc. Hortif. 2022, 11, 79–81. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística, IBGE. Produção de Maracujá no Brasil. 2022. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/maracuja/brm (accessed on 10 April 2024).
- Fischer, G.; Melgarejo, L.M.; Cutler, J. Preharvest factors that influence the quality of passion fruit: A review. Agron. Colomb. 2018, 36, 217–226. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhou, L.; Yu, K.; Jiang, F.; Xu, J.; Zou, L.; Du, L.; Liu, W. Effects of Microporous Packaging Combined with Chitosan Coating on the Quality and Physiological Metabolism of Passion fruit after Harvest. Food Bioprocess Technol. 2022, 15, 1836–1850. [Google Scholar] [CrossRef]
- Villacís-chiriboga, J.; Elst, K.; Camp, J.V.; Vera, E.; Rules, J. Valorization of byproducts from tropical fruits: Extraction methodologies, applications, environmental, and economic assessment: A review (Part 1: General overview of the byproducts, traditional biorefinery practices, and possible applications). Compr. Rev. Food Sci. Food Saf. 2020, 19, 405–447. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhong, Y.; Lia, L.; Jiangb, K.; Gao, J.; Zhong, K.; Pan, M.; Yan, B. A multifunctional chitosan-derived conformal coating for the preservation of passion fruit. LWT 2022, 163, 113584. [Google Scholar] [CrossRef]
- Jung, S.; Cui, Y.; Barnes, M.; Satam, C.; Zhang, S.; Chowdhury, R.A.; Adumbumkulath, A.; Sahin, O.; Miller, C.; Sajadi, S.M.; et al. Multifunctional bio-nanocomposite coatings for perishable fruits. Adv. Mater. 2020, 32, 1908291. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.R.L.; Santos, F.K.G.; Leite, R.H.L.; Aroucha, E.M.M.A.; Silva, K.N.O. Use of biopolymeric coating hydrophobized with beeswax in postharvest conservation of guavas. Food Chem. 2018, 259, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Riva, S.C.; Opara, U.O.; Fawole, O.A. Recent developments on postharvest application of edible coatings on stone fruit: A review. Sci. Hortic. 2020, 262, 109074. [Google Scholar] [CrossRef]
- Parvin, N.; Rahman, A.; Roy, J.; Rashid, H.; Paulo, N.C.; Mahamud, M.A.; Imran, S.; Sakil, A.; Uddin, F.M.J.; Molla, D.E.; et al. Chitosan coating improves postharvest shelf life of mango (Mangifera indica L.). Horticulturae 2023, 9, 64. [Google Scholar] [CrossRef]
- Vilvert, J.C.; Freitas, S.T.; Ferreira, M.A.R.; Leite, R.H.L.; Santos, F.K.G.; Costa, C.S.R.; Aroucha, E.M.M. Chitosan and graphene oxide-based biodegradable bags: An eco-friendly and effective packaging alternative to maintain postharvest quality of ‘Palmer’mango. LWT 2022, 154, 112741. [Google Scholar] [CrossRef]
- Paiva, C.A.; Vilvert, J.C.; Menezes, F.L.G.; Leite, R.H.L.; Santos, F.K.G.; Medeiros, J.F.; Aroucha, E.M.M. Extended shelf life of melons using chitosan and graphene oxide based biodegradable bags. J. Food Process. Preserv. 2020, 44, e14871. [Google Scholar] [CrossRef]
- Xavier, T.D.N.; Oliveira, V.R.L.D.; Leite, R.H.D.L.; Aroucha, E.M.M.; Santos, F.K.G.D. Characterization of biopolymeric films based on cassava starch, chitosan and carnauba wax. Matéria 2020, 25, 12866. [Google Scholar]
- Kwanele, A.N.; Olaniyi, A.F. Effects of chitosan coatings fused with medicinal plant extracts on postharvest quality and storage stability of purple passion fruit (Passiflora edulis var. Ester). Food Qual. Saf. 2022, 6, fyac016. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Mousavi, Z.; McClements, D.J. Beeswax: A review on the recent progress in the development of superhydrophobic films/coatings and their applications in fruits preservation. Food Chem. 2023, 424, 136404. [Google Scholar] [CrossRef]
- Aroucha, E.M.M.; Anastasiadi, M.; Collings, E.; Araujo, N.; Terry, L. Shelf life of green asparagus using cassava and chitosan blend coating. Braz. J. Food Technol. 2024, 27, e2022138. [Google Scholar] [CrossRef]
- Wang, W.; Qu, W.; Zhang, C.; Fan, Q.; Xue, W.; Wang, J.; Yu, M.; Wu, H.; Zhu, J. Graphene-Oxide-Infused Marine Biopolymers: Layer-by-Layer Assembled Nanocomposite Packaging for Prolonged Fresh-Cut Apple Preservation. ACS Sustain. Chem. Eng. 2024, 12, 3012–3024. [Google Scholar] [CrossRef]
- Sharma, S.; Biswal, B.K.; Kumari, D.; Bindra, P.; Kumar, S.; Stobdan, T.; Shanmugam, V. Ecofriendly Fruit Switches: Graphene Oxide-Based Wrapper for Programmed Fruit Preservative Delivery to Extend Shelf Life. ACS Appl. Mater. Interfaces 2018, 10, 18478–18488. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, J.; Lu, K.; Wang, Z.; Yin, L.; Zheng, H.; Wang, X.; Mao, L.; Xing, B. Biodegradation of graphene oxide by insects (Tenebrio molitor Larvae): Role of the gut microbiome and enzymes. Environ. Sci. Technol. 2022, 56, 16737–16747. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Yan, X.; Xiang, D.; Linjie, L.; Zhao, Y.; Huahong, P.; Gong, W.; Yang, R.L.; Xiang, Z.; Li, G. Effect of 1-Methylcyclopropene combined with chitosan-coated film on storage quality of passion fruit. Sustain. Chem. Pharm. 2022, 27, 100679. [Google Scholar] [CrossRef]
- Chen, F.P.; Xu, X.Y.; Luo, Z.; Chen, Y.; Xu, Y.; Xiao, G. Effect of high O2 atmosphere packaging on postharvest quality of purple passion fruit (Passiflora edulis Sims). J. Food Process. Preserv. 2018, 42, e13749. [Google Scholar] [CrossRef]
- Pereira, G.V.S.; Neves, E.M.P.X.; Albuquerque, G.A.; Rêgo, J.A.R.; Cardoso, D.N.P.; Brasil, D.S.B.; Joele, M.R.S.P. Effect of the Mixture of Polymers on the Rheological and Technological Properties of Composite Films of Acoupa Weakfish (Cynoscion acoupa) and Cassava Starch (Manihot esculenta C.). Food Bioprocess Technol. 2021, 14, 1199–1215. [Google Scholar] [CrossRef]
- de Menezes, F.L.G. Avaliação de Filmes Biopoliméricos com Incorporação de Nanopartículas de tio2 e sua Aplicação na Conservação Pós-Colheita do Mamão. Ph.D. Thesis, Universidade Federal Rural do Semiarido, Mossoró, Brazil, 2021. [Google Scholar]
- Isermeyer, H. Eine einfache Methode zur Bestimmung der Bodenatmung und der Karbonate im Boden. Z. Pflanzenernährung Düngung Bodenkd. 1952, 56, 26–38. [Google Scholar] [CrossRef]
- Benassi, M.T.; Antunes, A.J.A. Comparison of meta-phosphoric and oxalic acids as extractant solutions for the determination of vitamin C in selected vegetables. Arq. Biol. Tecnol. 1988, 31, 507–513. [Google Scholar]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A Guide for its Bootstrap procedures in multiple comparisons. Rev. Bras. Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Xiong, R.; Grant, A.M.; Ma, R.; Zhang, S.; Tsukruk, V.V. Naturally, derived biopolymer nanocomposites: Interfacial design, properties and emerging applications. Mater. Sci. Eng. 2018, 125, 1–41. [Google Scholar] [CrossRef]
- Han Lyn, F.; Chin Peng, T.; Ruzniza, M.Z.; Nur Hanani, Z.A. Effect of oxidation degrees of graphene oxide (GO) on the structure and physical properties of chitosan/GO composite films. Food Packag. Shelf Life 2019, 21, 100373. [Google Scholar] [CrossRef]
- Vilvert, J.C.; de Freitas, S.T.; Ferreira, M.A.R.; Costa, C.d.S.R.; Leite, R.H.d.L.; dos Santos, F.K.G.; Aroucha, E.M.M. Preservation of Quality and Bioactive Compounds in Mangoes Using Chitosan-Graphene-Oxide-Based Biodegradable Packaging. Horticulturae 2023, 9, 1145. [Google Scholar] [CrossRef]
- Han Lyn, F.; Tan, Q.P.; Zawawi, R.M.; Hanani, Z.A. Enhancing the mechanical and barrier properties of chitosan/graphene oxide composite films using trisodium citrate and sodium tripolyphosphate crosslinkers. J. Appl. Polym. Sci. 2021, 138, 50618. [Google Scholar] [CrossRef]
- De Menezes, F.L.G.; Leite, R.H.L.; Santos, F.K.G.; Aria, A.I.; Aroucha, E.M.M. TiO2 incorporated into a blend of biopolymeric matrices improves film properties and affects the postharvest conservation of papaya fruits under UV light. Food Chem. 2024, 433, 137387. [Google Scholar] [CrossRef] [PubMed]
- Dos Passos Braga, S.; Magnani, M.; Madruga, M.S.; Galvão, M.S.; Medeiros, L.L.; Batista, A.U.D.; Dias, R.T.A.; Fernandes, L.R.; Medeiros, E.S.; Souza, E.L. Characterization of edible coatings formulated with chitosan and Mentha essential oils and their use to preserve papaya (Carica papaya L.). Innov. Food Sci. Emerg. Technol. 2020, 65, 102472. [Google Scholar] [CrossRef]
- Chen, J.T.; Fu, Y.J.; An, Q.F.; Lo, S.C.; Zhong, Y.Z.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Enhancing polymer/graphene oxide gas barrier film properties by introducing new crystals. Carbon 2014, 75, 443–451. [Google Scholar] [CrossRef]
- Pott, D.M.; Vallarino, J.G.; Osorio, S. Metabolite changes during postharvest storage: Effects on fruit quality traits. Metabolites 2020, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Development and application of rice starch based edible coating to improve the postharvest storage potential and quality of plum fruit (Prunus salicina). Sci. Hortic. 2018, 237, 59–66. [Google Scholar] [CrossRef]
- Zhang, R.; Lan, W.T.; Ding, J.; Ahmed, S.; Qin, W.; He, L. Efect of PLA/PBAT antibacterial flm on storage quality of passion fruit during the shelf life. Molecules 2019, 24, 3378. [Google Scholar] [CrossRef]
- Rinaldi, M.M.; Costa, A.M.; Faleiro, F.G.; Junqueira, N.T.V. Postharvest conservation of Passiflora setacea DC. fruits submitted to different sanitizers and storage temperatures. Braz. J. Food Technol. 2017, 20, 1–12. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Boonyarithongchai, P.; Buanong, M.; Supapvanich, S.; WongsAree, C. Postharvest hot water treatment followed by chitosan-and κ-carrageenan-based composite coating induces the disease resistance and preserves the quality in dragon fruit (Hylocereus undatus). Int. J. Fruit Sci. 2020, 20, S2030–S2044. [Google Scholar] [CrossRef]
- Cai, M.; Zhong, H.Z.; Ma, Q.H.; Yang, K.; Sun, P.L. Physicochemical and microbial quality of Agaricus bisporus packaged in nano-SiO2/TiO2 loaded polyvinyl alcohol films. Food Control 2022, 131, 108452. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Stability of total phenolic concentration and antioxidant capacity of extracts from pomegranate coproducts subjected to in vitro digestion. BMC Complement. Altern. Med. 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wang, Q.; Wang, Y.; Qin, L.; Shi, Y.; Wang, X.; Wang, R. NtDREB-1BL1 Enhances Carotenoid Biosynthesis by Regulating Phytoene Synthase in Nicotiana tabacum. Genes 2022, 13, 1134. [Google Scholar] [CrossRef] [PubMed]
- Mellidou, I.; Georgiadou, E.C.; Kaloudas, D.; Kalaitzis, P.; Fotopoulos, V.; Kanellis, A.K. Vitamins. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E., Ed.; Academic Press: New York, NY, USA, 2019; pp. 359–384. [Google Scholar]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed Editora: Porto Alegre, Brazil, 2017. [Google Scholar]
- Ren, Z.; Liu, Y.; Huang, J.; An, L.; Zhang, Y.; Yang, W.; Lei, T. Low oxygen concentration alleviates banana peel browning by inhibiting membrane lipid oxidation and polyphenol oxidase activity. Int. J. Food Sci. Technol. 2024, 59, 3350–3359. [Google Scholar] [CrossRef]
- Ali, S.; Akbar Anjum, M.; Nawaz, A.; Naz, S.; Ejaz, S.; Shahzad Saleem, M.; Ul Hasan, M. Effect of gum arabic coating on antioxidative enzyme activities and quality of apricot (Prunus armeniaca L.) fruit during ambient storage. J. Food Biochem. 2021, 45, e13656. [Google Scholar] [CrossRef] [PubMed]
- Baswal, A.K.; Dhaliwal, H.S.; Singh, Z.; Mahajan, B.V.C.; Kalia, A.; Gill, K.S. Influence of carboxy methylcellulose, chitosan and beeswax coatings on cold storage life and quality of Kinnow mandarin fruit. Sci. Hortic. 2020, 260, 108887. [Google Scholar] [CrossRef]
- Almeida, C.L.; Figueiredo, L.R.; Ribeiro, D.V.; Santos, A.M.; Souza, E.L.; Oliveira, K.A.; Oliveira, J.E.; Medeiros, E.S. Antifungal edible coatings for fruits based on zein and chitosan nanowhiskers. J. Food Sci. 2024, 89, 404–418. [Google Scholar] [CrossRef]
- Zárate-Moreno, J.C.; Escobar-Sierra, D.M.; Ríos-Estepa, R. Development and Evaluation of Chitosan-Based Food Coatings for Exotic Fruit Preservation. BioTech 2023, 12, 20. [Google Scholar] [CrossRef]
- Vanden braber, N.L.; Di giorgio, L.; Aminahuel, C.A.; Díaz vergara, L.I.; Martin costa, A.O.; Montenegro, M.A.; Mauri, A.N. Antifungal whey protein films activated with low quantities of water-soluble chitosan. Food Hydrocol. 2021, 110, 106156. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef] [PubMed]

| Films | Solubility (%) | WVP (g.mm/(h.kPa.m2)) | a* | b* | L* | Opacity (%) |
|---|---|---|---|---|---|---|
CH + BW  | 16.83 ± 1.40 ab | 15.55 ± 1.70 b | 5.50 ± 0.10 a | 17.70 ± 1.00 b | 78.30 ± 0.30 a | 48.02 ± 1.10 ab |
CH + BW + GO  | 12.85 ± 1.50 b | 13.30 ± 1.00 c | 4.60 ± 0.10 b | 18.73 ± 0.10 ab | 71.43 ± 2.30 b | 49.37 ± 1.50 a |
CH  | 19.79 ± 1.30 a | 19.05 ± 1.00 a | 5.36 ± 0.06 a | 21.0 ± 0.50 a | 76.93 ± 0.30 a | 45.27 ± 1.40 b |
| Variables | Treatments | Shelf Life (Days) | ||
|---|---|---|---|---|
| 0 | 4 | 8 | ||
| SS (°Brix) | ||||
| CH + BW | 15.50 ± 1.10 aA | 12.92 ± 0.90 aB | 10.96 ± 0.10 aC | |
| CH + BW + GO | 15.50 ± 1.10 aA | 12.97 ± 0.07 aB | 11.32 ± 0.20 aC | |
| Control | 15.50 ± 1.10 aA | 12.05 ± 1.60 bB | 9.70 ± 0.30 bC | |
| TA (% citric acid) | ||||
| CH + BW | 5.32 ± 0.30 aA | 4.67 ± 0.14 abB | 3.54 ± 0.14 bC | |
| CH + BW + GO | 5.32 ± 0.30 aA | 4.75 ± 0.14 aB | 4.18 ± 0.02 aC | |
| Control | 5.32 ± 0.30 aA | 4.46 ± 0.08 bB | 3.17 ± 0.06 bC | |
| Ratio (SS/TA) | ||||
| CH + BW | 2.90 ± 0.10 aB | 2.72 ± 0.10 aB | 3.12 ± 0.30 aA | |
| CH + BW + GO | 2.90 ± 0.10 aA | 2.77 ± 0.10 aA | 2.70 ± 0.10 bA | |
| Control | 2.90 ± 0.10 aAB | 2.72 ± 0.40 aB | 3.05 ± 0.20 aA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, W.A.O.; Aroucha, E.M.M.; de Araújo, N.O.; Santos, F.K.G.d.; de Medeiros, J.F.; de Sousa, A.L.V.; de Oliveira Queiroz, L.P.; de Lima Leite, R.H. The Shelf Life of Yellow Passion Fruit with an Edible Biocomposite Coating Based on Chitosan, Graphene Oxide Nanoparticles, and Beeswax. Horticulturae 2024, 10, 756. https://doi.org/10.3390/horticulturae10070756
da Silva WAO, Aroucha EMM, de Araújo NO, Santos FKGd, de Medeiros JF, de Sousa ALV, de Oliveira Queiroz LP, de Lima Leite RH. The Shelf Life of Yellow Passion Fruit with an Edible Biocomposite Coating Based on Chitosan, Graphene Oxide Nanoparticles, and Beeswax. Horticulturae. 2024; 10(7):756. https://doi.org/10.3390/horticulturae10070756
Chicago/Turabian Styleda Silva, Wedson Aleff Oliveira, Edna Maria Mendes Aroucha, Nícolas Oliveira de Araújo, Francisco Klebson Gomes dos Santos, José Francismar de Medeiros, Arthur Lira Vasconcelos de Sousa, Luiz Paulo de Oliveira Queiroz, and Ricardo Henrique de Lima Leite. 2024. "The Shelf Life of Yellow Passion Fruit with an Edible Biocomposite Coating Based on Chitosan, Graphene Oxide Nanoparticles, and Beeswax" Horticulturae 10, no. 7: 756. https://doi.org/10.3390/horticulturae10070756









