Adding Phyto-LED Spectrum to White-LED Light Increases the Productivity of Lettuce Plants
Abstract
:Highlights
- The spectral characteristics of different types of white LEDs differ significantly.
- The Phyto-LED spectrum increases the productivity of lettuce plants
- The combination of white LEDs and Phyto-LEDs had the same effect on the productivity of lettuce plants as the Phyto-LEDs alone.
- The combination of white LEDs and Phyto-LEDs has a significantly greater effect on color rendering than does the use of sole Phyto-LED spectrum
- Phyto-LEDs provoke flowering of lettuce plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growing Conditions and Experimental Design
2.2. Spectral and Energy Characteristics of Light
2.3. Leaf Area and Gravimetric Indicators
2.4. Photosynthetic and Transpiration Rates
2.5. Chlorophyll Fluorescence and Total Chlorophyll Content
2.6. Microscopy
2.7. Statistics
3. Results
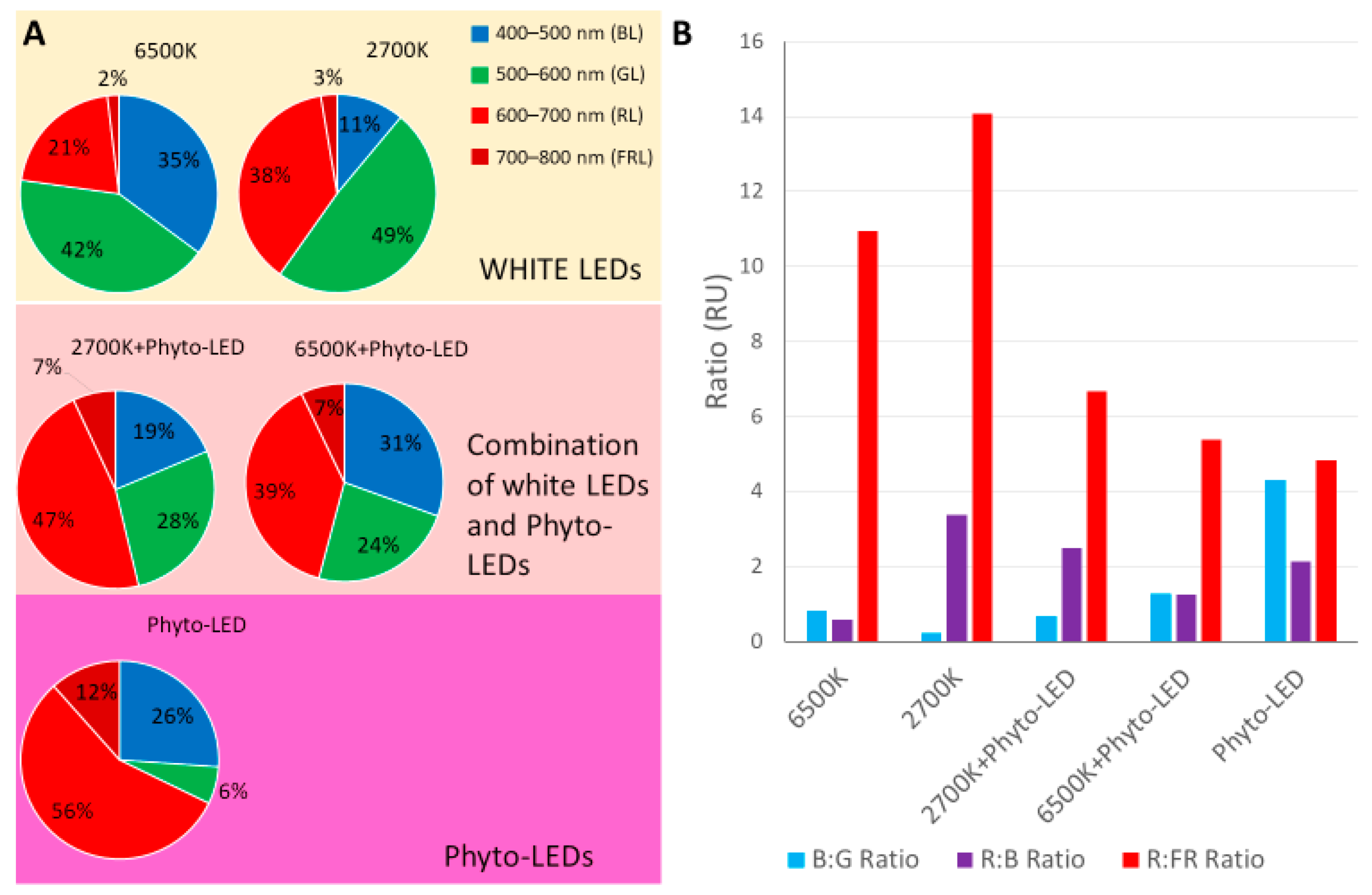
3.1. Spectral and Energy Characteristics of Light Sources
- (1)
- A decrease in the proportion of GL by 1.75 times for 6500 K and 2700 K
- (2)
- An increase in the proportion of RL in the spectrum by an average of 1.85 times for 6500 K and 1.23 times for 2700 K
- (3)
- An increase in the proportion of FRL in the spectrum by an average of 3.5 times for 6500 K and 2.3 times for 2700 K
3.2. Dry and Fresh Mass of Organs
3.3. CO2 Gas Exchange and Transpiration, Stomatal Conductance, Water Use Efficiency, Fluorescent Parameters and Chl Content
3.4. Morphometric Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bian, Z.-H.; Cheng, R.-F.; Yang, Q.-C.; Wang, J.; Lu, C. Continuous Light from Red, Blue, and Green Light-Emitting Diodes Reduces Nitrate Content and Enhances Phytochemical Concentrations and Antioxidant Capacity in Lettuce. J. Am. Soc. Hortic. Sci. 2016, 141, 186–195. [Google Scholar] [CrossRef]
- Lee, M.; Xu, J.; Wang, W.; Rajashekar, C.B. The Effect of Supplemental Blue, Red and Far-Red Light on the Growth and the Nutritional Quality of Red and Green Leaf Lettuce. Am. J. Plant Sci. 2019, 10, 2219–2235. [Google Scholar] [CrossRef]
- Sellaro, R.; Crepy, M.; Trupkin, S.A.; Karayekov, E.; Buchovsky, A.S.; Rossi, C.; Casal, J.J. Cryptochrome as a Sensor of the Blue/Green Ratio of Natural Radiation in Arabidopsis. Plant Physiol. 2010, 154, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, P.; Bugbee, B. Far-Red Fraction: An Improved Metric for Characterizing Phytochrome Effects on Morphology. J. Am. Soc. Hortic. Sci. 2021, 146, 3–13. [Google Scholar] [CrossRef]
- Seaton, D.D.; Smith, R.W.; Song, Y.H.; MacGregor, D.R.; Stewart, K.; Steel, G.; Foreman, J.; Penfield, S.; Imaizumi, T.; Millar, A.J.; et al. Linked Circadian Outputs Control Elongation Growth and Flowering in Response to Photoperiod and Temperature. Mol. Syst. Biol. 2015, 11, 776. [Google Scholar] [CrossRef]
- Srikanth, A.; Schmid, M. Regulation of Flowering Time: All Roads Lead to Rome. Cell. Mol. Life Sci. 2011, 68, 2013–2037. [Google Scholar] [CrossRef] [PubMed]
- Tarakanov, I.G.; Tovstyko, D.A.; Lomakin, M.P.; Shmakov, A.S.; Sleptsov, N.N.; Shmarev, A.N.; Litvinskiy, V.A.; Ivlev, A.A. Effects of Light Spectral Quality on Photosynthetic Activity, Biomass Production, and Carbon Isotope Fractionation in Lettuce, Lactuca Sativa L., Plants. Plants 2022, 11, 441. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Yanagi, T.; Kondo, S. Growth and morphogenesis of lettuce seedlings raised under different combinations of red and blue light. Acta Hortic. 1997, 435, 149–158. [Google Scholar] [CrossRef]
- Cope, K.R.; Snowden, M.C.; Bugbee, B. Photobiological Interactions of Blue Light and Photosynthetic Photon Flux: Effects of Monochromatic and Broad-Spectrum Light Sources. Photochem. Photobiol. 2014, 90, 574–584. [Google Scholar] [CrossRef]
- McCree, K.J. The Action Spectrum, Absorptance and Quantum Yield of Photosynthesis in Crop Plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Fang, S.; Lang, T.; Cai, M.; Han, T. Light Keys Open Locks of Plant Photoresponses: A Review of Phosphors for Plant Cultivation LEDs. J. Alloys Compd. 2022, 902, 163825. [Google Scholar] [CrossRef]
- Chen, J.; Guo, C.; Yang, Z.; Li, T.; Zhao, J. Li2SrSiO4:Ce3+, Pr3+ Phosphor with Blue, Red, and Near-Infrared Emissions Used for Plant Growth LED. J. Am. Ceram. Soc. 2016, 99, 218–225. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, N.; Guo, C.; Pan, F.; Zhou, X.; Suo, H.; Zhao, X.; Goldys, E.M. Site-Dependent Luminescence and Thermal Stability of Eu2+ Doped Fluorophosphate toward White LEDs for Plant Growth. ACS Appl. Mater. Interfaces 2016, 8, 20856–20864. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, N.; Ishikura, M.; Suzuki, H.; Yamori, W.; Goto, E. Continuous Irradiation with Alternating Red and Blue Light Enhances Plant Growth While Keeping Nutritional Quality in Lettuce. HortScience 2018, 53, 1804–1809. [Google Scholar] [CrossRef]
- Spalholz, H.; Perkins-Veazie, P.; Hernández, R. Impact of Sun-Simulated White Light and Varied Blue:Red Spectrums on the Growth, Morphology, Development, and Phytochemical Content of Green- and Red-Leaf Lettuce at Different Growth Stages. Sci. Hortic. 2020, 264, 109195. [Google Scholar] [CrossRef]
- Kim, H.-H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-Light Supplementation for Enhanced Lettuce Growth under Red- and Blue-Light-Emitting Diodes. HortScience 2004, 39, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.L.; Cho, K.M.; Lee, H.Y.; Cho, D.Y.; Lee, G.O.; Jang, S.N.; Lee, Y.; Kim, D.; Son, K.-H. Effects of White LED Lighting with Specific Shorter Blue and/or Green Wavelength on the Growth and Quality of Two Lettuce Cultivars in a Vertical Farming System. Agronomy 2021, 11, 2111. [Google Scholar] [CrossRef]
- Peng, H.Y.; Yam, F.K. Comparison of the Changes in Luminescence Properties Between Cool White and Warm White LEDs at Varying Temperatures. IEEE Trans. Compon. Packag. Manufact. Technol. 2018, 8, 2113–2121. [Google Scholar] [CrossRef]
- Touliatos, D.; Dodd, I.C.; McAinsh, M. Vertical Farming Increases Lettuce Yield per Unit Area Compared to Conventional Horizontal Hydroponics. Food Energy Secur. 2016, 5, 184–191. [Google Scholar] [CrossRef]
- Goltsev, V.N.; Kalaji, H.M.; Paunov, M.; Bąba, W.; Horaczek, T.; Mojski, J.; Kociel, H.; Allakhverdiev, S.I. Variable Chlorophyll Fluorescence and Its Use for Assessing Physiological Condition of Plant Photosynthetic Apparatus. Russ. J. Plant Physiol. 2016, 63, 869–893. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Han, T.; Vaganov, V.; Cao, S.; Li, Q.; Ling, L.; Cheng, X.; Peng, L.; Zhang, C.; Yakovlev, A.N.; Zhong, Y.; et al. Improving “Color Rendering” of LED Lighting for the Growth of Lettuce. Sci. Rep. 2017, 7, 45944. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Han, T.; Li, Q.; Peng, L.; Zhao, C.; Tang, Y.; Xu, J. Tunable Spectrum Resemblance of LED Lights for Improving the Photosynthetic Action of Chinese Cabbages. Life Sci. Space Res. 2020, 26, 28–33. [Google Scholar] [CrossRef]
- Zhou, Z.; Xia, M.; Zhong, Y.; Gai, S.; Huang, S.; Tian, Y.; Lu, X.; Zhou, N. Dy3+@Mn4+ Co-Doped Ca14Ga10−mAlmZn6O35 Far-Red Emitting Phosphors with High Brightness and Improved Luminescence and Energy Transfer Properties for Plant Growth LED Lights. J. Mater. Chem. C 2017, 5, 8201–8210. [Google Scholar] [CrossRef]
- Talbott, L.D.; Nikolova, G.; Ortiz, A.; Shmayevich, I.; Zeiger, E. Green Light Reversal of Blue-Light-Stimulated Stomatal Opening Is Found in a Diversity of Plant Species. Am. J. Bot. 2002, 89, 366–368. [Google Scholar] [CrossRef]
- Boccalandro, H.E.; Rugnone, M.L.; Moreno, J.E.; Ploschuk, E.L.; Serna, L.; Yanovsky, M.J.; Casal, J.J. Phytochrome B Enhances Photosynthesis at the Expense of Water-Use Efficiency in Arabidopsis. Plant Physiol. 2009, 150, 1083–1092. [Google Scholar] [CrossRef]
- Shibuya, T.; Endo, R.; Yuba, T.; Kitaya, Y. The Photosynthetic Parameters of Cucumber as Affected by Irradiances with Different Red:Far-Red Ratios. Biol. Plant 2015, 59, 198–200. [Google Scholar] [CrossRef]
- Kasperbauer, M.J.; Peaslee, D.E. Morphology and Photosynthetic Efficiency of Tobacco Leaves That Received End-of-Day Red and Far Red Light during Development 1. Plant Physiol. 1973, 52, 440–442. [Google Scholar] [CrossRef]
- Jiang, C.; Johkan, M.; Maruo, T.; Hohjo, M.; Tsukagoshi, S.; Ebihara, M.; Nakaminami, A. Effect of Supplemental Far-Red Light with Blue and Red LED Lamps on Leaf Photosynthesis, Stomatal Regulation and Plant Development of Protected Cultivated Tomato. Acta Hortic. 2018, 1227, 533–540. [Google Scholar] [CrossRef]
- Meng, Q.; Boldt, J.; Runkle, E.S. Blue Radiation Interacts with Green Radiation to Influence Growth and Predominantly Controls Quality Attributes of Lettuce. J. Am. Soc. Hortic. Sci. 2020, 145, 75–87. [Google Scholar] [CrossRef]
- Son, K.-H.; Oh, M.-M. Growth, Photosynthetic and Antioxidant Parameters of Two Lettuce Cultivars as Affected by Red, Green, and Blue Light-Emitting Diodes. Hortic. Environ. Biotechnol. 2015, 56, 639–653. [Google Scholar] [CrossRef]
- Li, L.; Tong, Y.; Lu, J.; Li, Y.; Liu, X.; Cheng, R. Morphology, Photosynthetic Traits, and Nutritional Quality of Lettuce Plants as Affected by Green Light Substituting Proportion of Blue and Red Light. Front. Plant Sci. 2021, 12, 627311. [Google Scholar] [CrossRef] [PubMed]
- Beatrice, P.; Terzaghi, M.; Chiatante, D.; Scippa, G.S.; Montagnoli, A. Morpho-Physiological Responses of Arabidopsis thaliana L. to the LED-Sourced CoeLux® System. Plants 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.G.; Mickens, M.A.; Aronne, G.; Gómez, C. Spectral Effects of Blue and Red Light on Growth, Anatomy, and Physiology of Lettuce. Physiol. Plant. 2021, 172, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Snowden, M.C.; Cope, K.R.; Bugbee, B. Sensitivity of Seven Diverse Species to Blue and Green Light: Interactions with Photon Flux. PLoS ONE 2016, 11, e0163121. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.H.; Park, J.S.; Park, K.S.; Son, J.E. Leaf Photosynthetic Rate, Growth, and Morphology of Lettuce under Different Fractions of Red, Blue, and Green Light from Light-Emitting Diodes (LEDs). Hortic. Environ. Biotechnol. 2016, 57, 573–579. [Google Scholar] [CrossRef]
- Li, J.; Li, G.; Wang, H.; Wang Deng, X. Phytochrome Signaling Mechanisms. Arabidopsis Book 2011, 9, e0148. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, X.; Liu, Y.; Fan, S.; Ma, Q. Progress of Research on the Regulatory Pathway of the Plant Shade-Avoidance Syndrome. Front. Plant Sci. 2020, 11, 439. [Google Scholar] [CrossRef]
- Legendre, R.; van Iersel, M.W. Supplemental Far-Red Light Stimulates Lettuce Growth: Disentangling Morphological and Physiological Effects. Plants 2021, 10, 166. [Google Scholar] [CrossRef]
- Liu, J.; van Iersel, M.W. Far-Red Light Effects on Lettuce Growth and Morphology in Indoor Production Are Cultivar Specific. Plants 2022, 11, 2714. [Google Scholar] [CrossRef]
- Zhang, T.; Maruhnich, S.A.; Folta, K.M. Green Light Induces Shade Avoidance Symptoms. Plant Physiol. 2011, 157, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A. Shade Avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Samuolienė, G.; Viršilė, A.; Haimi, P.; Miliauskienė, J. Photoresponse to Different Lighting Strategies during Red Leaf Lettuce Growth. J. Photochem. Photobiol. B Biol. 2020, 202, 111726. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Resource Use Efficiency of Indoor Lettuce (Lactuca Sativa L.) Cultivation as Affected by Red:Blue Ratio Provided by LED Lighting. Sci. Rep. 2019, 9, 14127. [Google Scholar] [CrossRef] [PubMed]
- Basahi, J.M.; Ismail, I.M.; Hassan, I.A. Effects of Enhanced UV-B Radiation and Drought Stress on Photosynthetic Performance of Lettuce (Lactuca Sativa L. Romaine) Plants. Annu. Res. Rev. Biol. 2014, 4, 1739–1756. [Google Scholar] [CrossRef]
- Kim, H.-H.; Goins, G.D.; Wheeler, R.; Sager, J.C. Stomatal Conductance of Lettuce Grown Under or Exposed to Different Light Qualities. Ann. Bot. 2004, 94, 691–697. [Google Scholar] [CrossRef]
- Fukuda, M.; Matsuo, S.; Kikuchi, K.; Kawazu, Y.; Fujiyama, R.; Honda, I. Isolation and Functional Characterization of the FLOWERING LOCUS T Homolog, the LsFT Gene, in Lettuce. J. Plant Physiol. 2011, 168, 1602–1607. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Regulation of Flowering by Green Light Depends on Its Photon Flux Density and Involves Cryptochromes. Physiol. Plant. 2019, 166, 762–771. [Google Scholar] [CrossRef]
- Ohto, M.; Onai, K.; Furukawa, Y.; Aoki, E.; Araki, T.; Nakamura, K. Effects of Sugar on Vegetative Development and Floral Transition in Arabidopsis. Plant Physiol. 2001, 127, 252–261. [Google Scholar] [CrossRef]




| Light (BL, GL, RL, FRL) | FW Leaves, g | DW Leaves, g | FW Roots, g | DW Roots, g |
|---|---|---|---|---|
| 6500 K (35%, 42%, 21%, 2%) | 50.2 ± 0.8 d | 2.2 ± 0.1 c | 9.42 ± 0.7 a | 0.325 ± 0.043 a |
| 2700 K (11%, 49%, 38%, 3%) | 54.4 ± 0.9 c | 2.7 ± 0.2 b | 8.47 ± 1.24 a | 0.292 ± 0.035 a |
| 2700 K + Phyto-LED (19%, 28%, 47%, 7%) | 70.5 ± 2.1 b | 3.4 ± 0.2 a | 9.59 ± 1.58 a | 0.310 ± 0.016 a |
| 6500 K + Phyto-LED (31%, 24%, 39%, 7%) | 74.0 ± 1.5 ab | 3.3 ± 0.2 a | 8.38 ± 0.75 a | 0.285 ± 0.028 a |
| Phyto-LED (26%, 6%, 56%, 12%) | 75.3 ± 2.1 a | 3.2 ± 0.2 a | 8.39 ± 1.38 a | 0.295 ± 0.028 a |
| Light (BL, GL, RL, FRL) | Tr, mmol H2O/m2 s | gs, mmol/m2 s | WUE, µmol CO2/mmol H2O | Pn, µmol CO2/m2 s | Chl a+b, mg/g FW | Fv/Fm |
|---|---|---|---|---|---|---|
| 6500 K (35%, 42%, 21%, 2%) | 1.58 ± 0.15 c | 0.149 ±0.013 b | 2.52 ± 0.40 a | 3.90 ± 0.26 a | 2.57 ± 0.4 a | 0.840 ± 0.018 a |
| 2700 K (11%, 49%, 38%, 3%) | 1.75 ± 0.08 c | 0.122 ±0.007 c | 2.81 ± 0.18 a | 4.27 ± 0.50 a | 2.51 ± 0.3 a | 0.838 ± 0.014 a |
| 2700 K + Phyto-LED (19%, 28%, 47%, 7%) | 2.39 ± 0.16 b | 0.184 ±0.007 a | 1.60 ± 0.05 b | 3.75 ± 0.26 a | 2.49 ± 0.3 a | 0.840 ± 0.022 a |
| 6500 K + Phyto-LED (31%, 24%, 39%, 7%) | 2.7 ± 0.22 ab | 0.193 ±0.012 a | 1.64 ± 0.07 b | 4.1 ± 0.30 a | 2.54 ± 0.4 a | 0.827 ± 0.025 a |
| Phyto-LED (26%, 6%, 56%, 12%) | 2.81 ± 0.21 a | 0.179 ±0.009 a | 1.58 ± 0.04 b | 4.2 ± 0.33 a | 2.52 ± 0.3 a | 0.844 ± 0.013 a |
| Light (BL, GL, RL, FRL) | Number of Leaves per Plant | Length of the Largest Leaf, cm | Width of the Largest Leaf, cm | Total Leaf Area per Plant, cm2 | Number of Leaves per Plant | Stem Length, cm | Flowering Plants, % |
|---|---|---|---|---|---|---|---|
| 30th Day of Experiment | 60th Day of Experiment | ||||||
| 6500 K (35%, 42%, 21%, 2%) | 13.0 ± 1 ab | 13.8 ± 1.0 c | 13.1 ±2 a | 806 ± 25 c | 52 ± 1.52 d | 17.3 ± 1.5 c | 0 |
| 2700 K (11%, 49%, 38%, 3%) | 14.3 ± 0.5 ab | 14.7 ± 1.6 bc | 13.4 ± 1 a | 925 ± 27 b | 54.0 ± 1.00 c | 18.3 ± 2.5.c | 0 |
| 2700 K + Phyto-LED (19%, 28%, 47%, 7%) | 15 ± 0.6 ab | 16.3 ± 1.6 ab | 15.7 ± 2 a | 1190 ± 33 a | 51.3 ± 1.08 d | 26 ± 1.15 b | 15 c |
| 6500 K + Phyto-LED (31%, 24%, 39%, 7%) | 14 ± 0.8 ab | 17.6 ± 1.3 ab | 16.0 ± 2.8 a | 1180 ± 30 a | 63.3 ± 2.51 a | 24.3 ± 1.5 b | 60 b |
| Phyto-LED (26%, 6%, 56%, 12%) | 15.8 ± 0.5 a | 18.2 ± 1.8 a | 17.0 ± 2.4 a | 1210 ± 28 a | 59.3 ± 1.15 b | 28 ± 1 a | 100 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vereshchagin, M.; Pashkovskiy, P.; Tarakanov, I. Adding Phyto-LED Spectrum to White-LED Light Increases the Productivity of Lettuce Plants. Horticulturae 2024, 10, 795. https://doi.org/10.3390/horticulturae10080795
Vereshchagin M, Pashkovskiy P, Tarakanov I. Adding Phyto-LED Spectrum to White-LED Light Increases the Productivity of Lettuce Plants. Horticulturae. 2024; 10(8):795. https://doi.org/10.3390/horticulturae10080795
Chicago/Turabian StyleVereshchagin, Mikhail, Pavel Pashkovskiy, and Ivan Tarakanov. 2024. "Adding Phyto-LED Spectrum to White-LED Light Increases the Productivity of Lettuce Plants" Horticulturae 10, no. 8: 795. https://doi.org/10.3390/horticulturae10080795
APA StyleVereshchagin, M., Pashkovskiy, P., & Tarakanov, I. (2024). Adding Phyto-LED Spectrum to White-LED Light Increases the Productivity of Lettuce Plants. Horticulturae, 10(8), 795. https://doi.org/10.3390/horticulturae10080795






