Research Progress on Physiological, Biochemical, and Molecular Mechanisms of Potato in Response to Drought and High Temperature
Abstract
1. Introduction
2. Methodology of the Review
3. Drought and Its Impact on Potato
3.1. Drought’s Effect on Potato Yield and Quality
3.2. Drought Stress and Potato Growth
3.3. Impact of Drought Stress on Physiological and Biochemical Characteristics of Potato
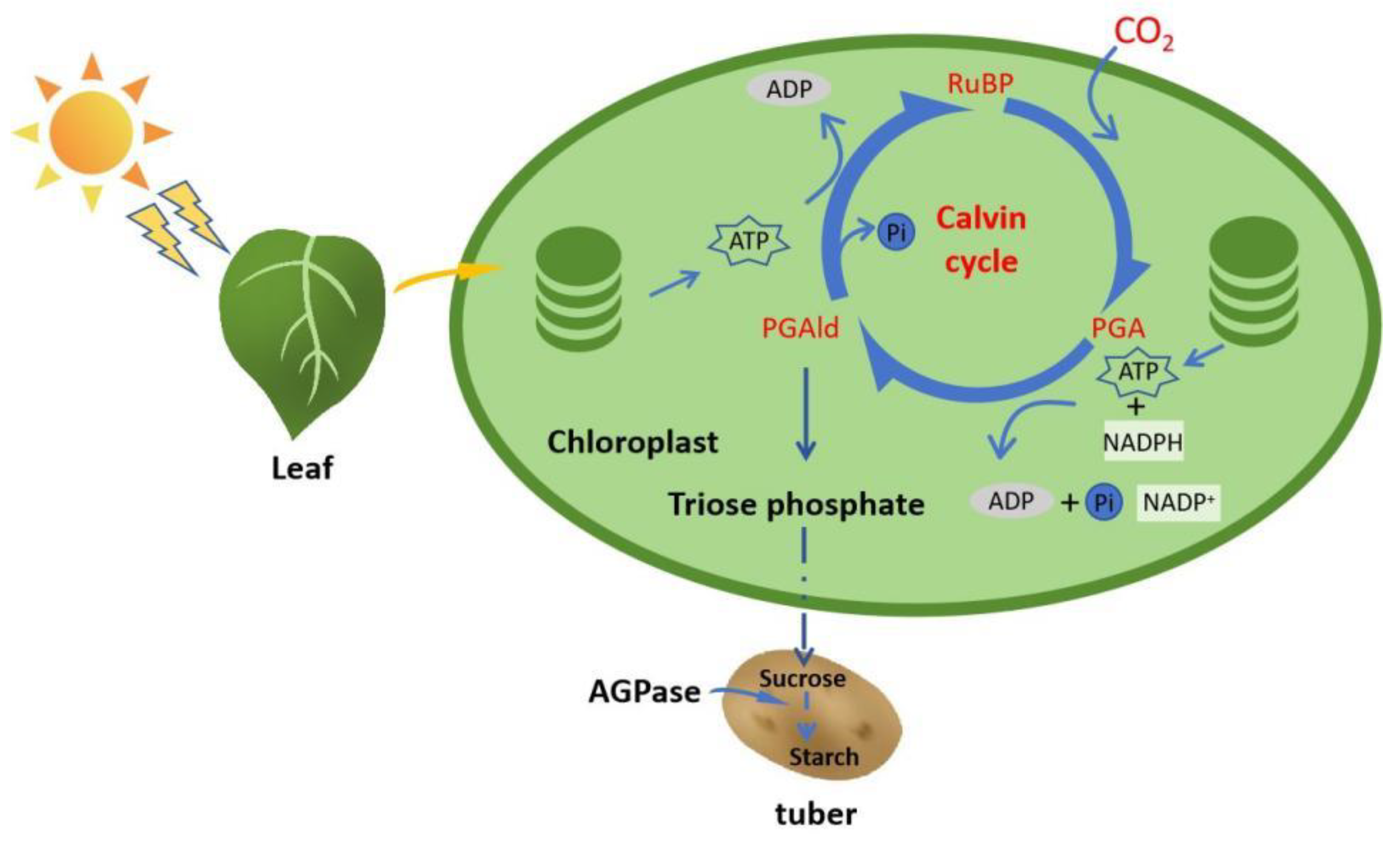
3.3.1. Influence of Drought Stress on Photosynthetic Properties of Potato
3.3.2. Effects of Drought Stress on Cellular Structure and Membrane Stability in Potato
3.3.3. Influence of Drought Stress on Osmoregulatory Compounds in Potato
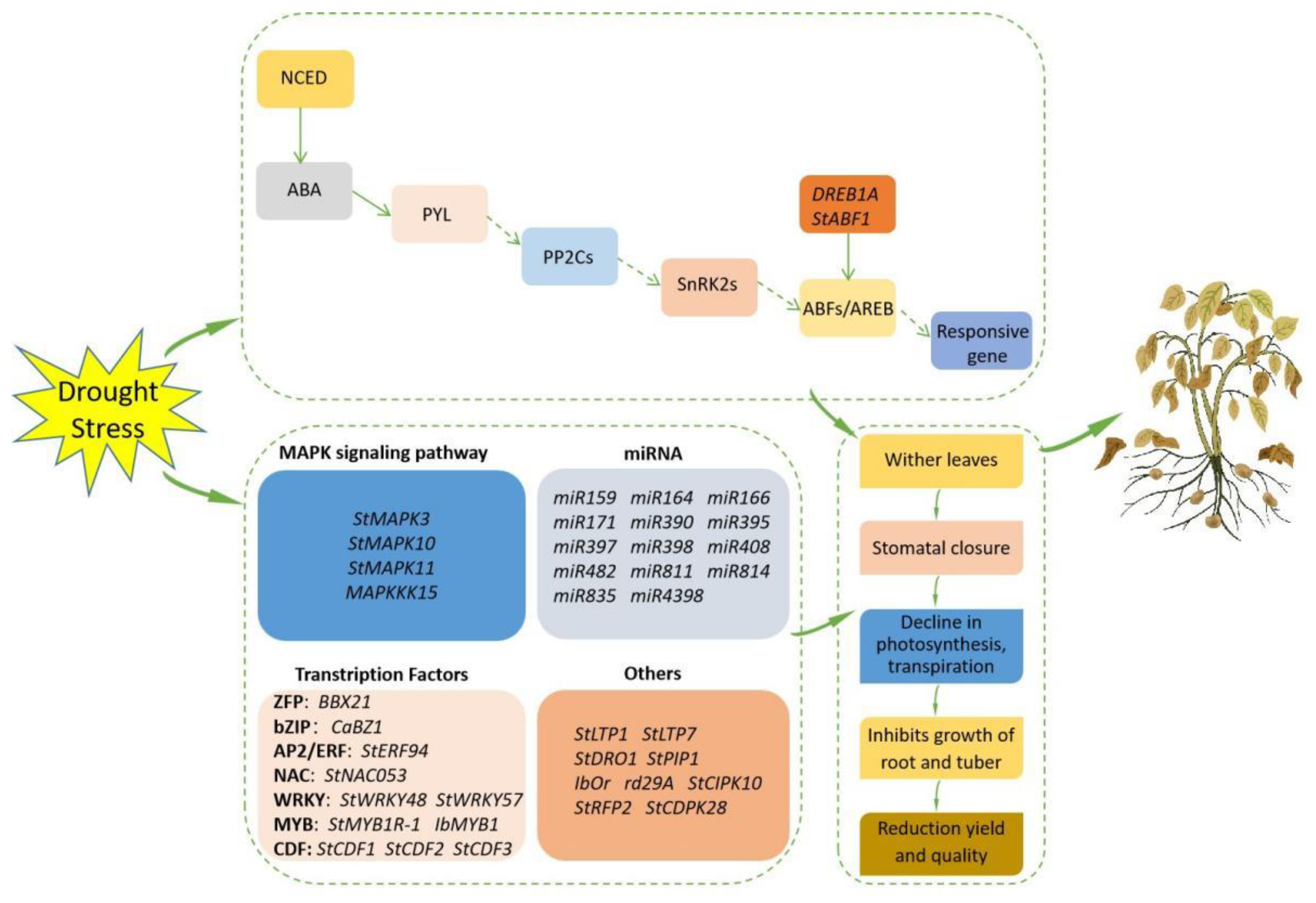
3.4. Molecular Mechanisms of Potato Response to Drought Stress
3.4.1. The Role of Abscisic Acid Signaling in Potato’s Drought Response
3.4.2. MAPK Signaling Pathway in Potato’s Response to Drought
3.4.3. Transcription Factors in Potato’s Drought Response
3.4.4. miRNA in Potato Drought Stress Response
3.4.5. Other Regulatory Factors in Potato Drought Stress Response
4. Effect of High-Temperature Stress on Potato
4.1. Effect of Heat Stress on Potato Yield and Quality
4.2. Effect of Heat Stress on Potato Growth and Development
4.3. Effect of Heat Stress on Physiological and Biochemical Properties of Potato
4.3.1. Photosynthetic Characteristics
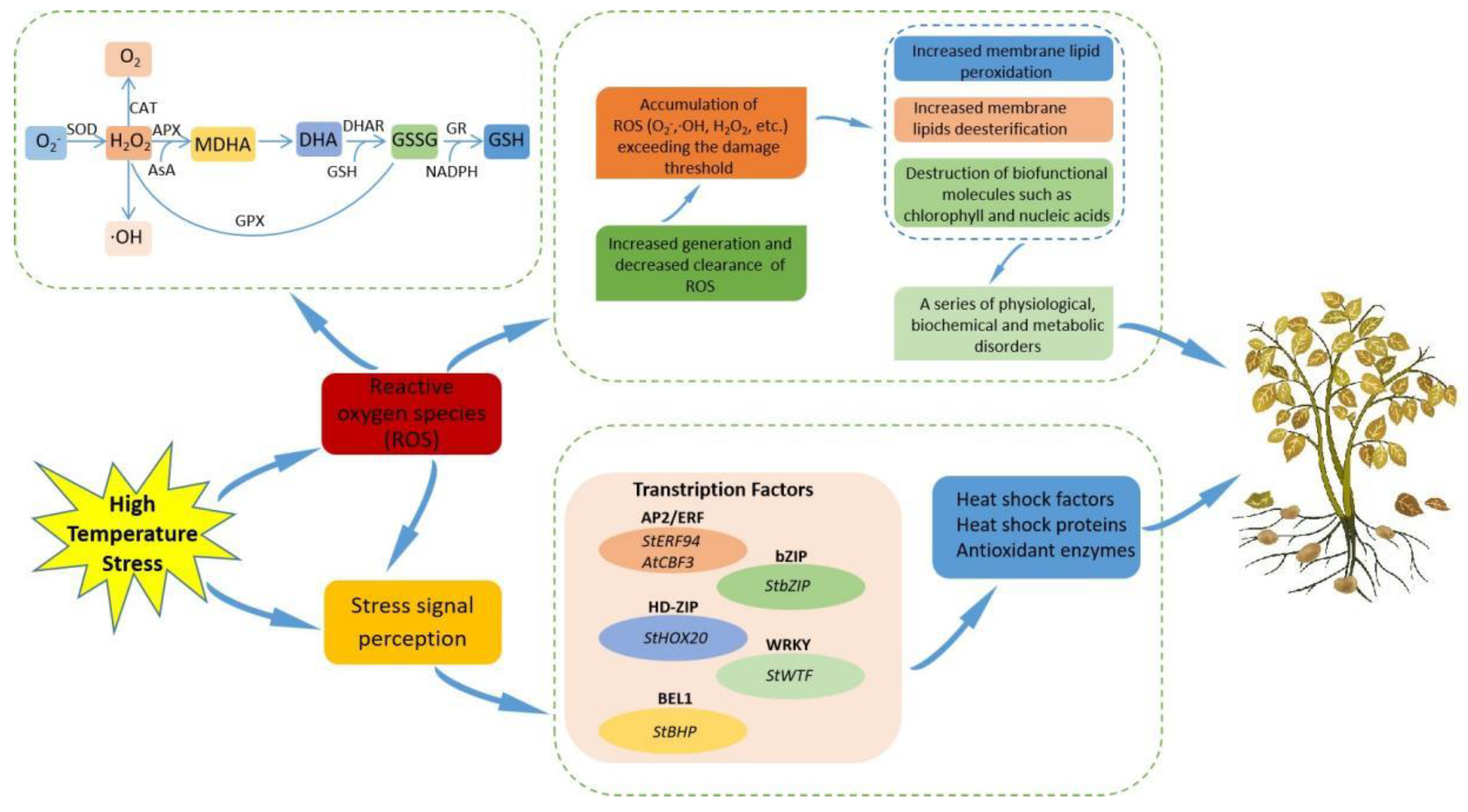
4.3.2. Membrane Stability and Antioxidant Capacity
4.4. Molecular Mechanism of Potato Response to Heat Stress
4.4.1. Heat Shock Proteins and Heat Shock Factors
4.4.2. Transcription Factors
5. Adaptation Strategies for Drought and High Temperature
5.1. Screening and Evaluation of Drought-Resistant and Heat-Tolerant Resources
5.2. Breeding Techniques to Deal with Drought and High Temperature
5.3. Cultivation Techniques for Coping with Drought and High Temperature
6. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability; Working Group II Contribution to the IPCC Sixth Assessment Report; IPCC: Geneva, Switzerland, 2022.
- IPCC. AR6Synthesis Report: Climate Change 2023; IPCC: Geneva, Switzerland, 2023.
- Fang, G.; Yang, S.; Ruan, B.; Liu, C.; Zhang, A.; Jiang, H.; Ding, S.; Tian, B.; Zhang, Y.; Jahan, N.; et al. Isolation of TSCD11 Gene for Early Chloroplast Development under High Temperature in Rice. Rice 2020, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2014: Synthesis Report; Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A.; IPCC: Geneva, Switzerland, 2014; 151p.
- Dahal, K.; Li, X.Q.; Tai, H.; Creelman, A.; Bizimungu, B. Improving potato stress tolerance and tuber yield under a climate change scenario—A current overview. Front. Plant Sci. 2019, 10, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Atwoli, L.; Erhabor, G.E.; Gbakima, A.A.; Haileamlak, A.; Ntumba, J.K.; Kigera, J.; Laybourn-Langton, L.; Mash, B.; Muhia, J.; Mulaudzi, F.M.; et al. COP27 Climate Change Conference: Urgent action needed for Africa and the world. East. Mediterr. Health J. 2022, 28, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Agricultural drought in a future climate: Results from 15 gobal claimte models participating in the IPCC 4th assessment. Clim. Dyn. 2005, 25, 739–753. [Google Scholar] [CrossRef]
- Vandegeer, R.; Miller, R.E.; Bain, M.; Gleadow, R.M.; Cavagnaro, T.R. Drought adversely affffects tuber development and nutritional quality of the staple crop cassava (Manihot esculenta Crantz). Funct. Plant Biol. 2012, 40, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Boote, K.J.; Kimball, B.A.; Ziska, L.H.; Izaurralde, R.C.; Ort, D.; Thomson, A.M.; Wolfe, D. Climate Impacts on Agriculture: Implications for Crop Production. Agron. J. 2011, 103, 351–370. [Google Scholar] [CrossRef]
- Elad, Y.; Pertot, I. Climate change impacts on plant pathogens and plant diseases. J. Crop Improv. 2014, 28, 99–139. [Google Scholar] [CrossRef]
- Vera, R. Impacts of Global Warming and Rising Atmospheric CO2 Concentrations on Physiology, Development and Productivity of Midwestern Crops. Ph.D. Dissertation, University of Illinois at Urbana, Champaign, IL, USA, 2014. [Google Scholar]
- Deryng, D.; Elliott, J.; Folberth, C.; Muller, C.; Pugh, T.A.M.; Boote, K.J.; Conway, D.; Ruane, A.C.; Gertenet, D.; Jones, J.W.; et al. Regional disparities in the beneficial effects of rising CO2 concentrations on crop water productivity. Nat. Clim. Chang. 2016, 6, 786–790. [Google Scholar] [CrossRef]
- Lobell, D.B.; Gourdji, S.M. The influence of climate change on global crop productivity. Plant Physiol. 2012, 160, 1686–1697. [Google Scholar] [CrossRef] [PubMed]
- United Nations Department of Economic and Social Affairs. World Population Prospects: The 2022 Revision, Highlights and Advance Tables. In Working Paper ESA/P/WP.220; United Nations Department of Economic and Social Affairs: New York, NY, USA, 2022. [Google Scholar]
- Wiebe, K. The state of food and agriculture, 2008. Biofuels: Prospects, risks and opportunities. J. Agric. Sci. 2008, 147, 503. [Google Scholar]
- Zhu, X.G.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Ann. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef] [PubMed]
- Halterman, D.; Guenthner, J.; Collinge, S.; Butler, N.; Douches, D. Biotech potatoes in the 21st century: 20 years since the first biotech potato. Am. J. Potato Res. 2016, 93, 1–20. [Google Scholar] [CrossRef]
- Umemoto, N.; Nakayasu, M.; Ohyama, K.; Yotsu-Yamashita, M.; Mizutani, M.; Seki, H.; Saito, K.; Muranaka, T. Two cytochrome p450 monooxygenases catalyze early hydroxylation steps in the potato steroid glycoalkaloid biosynthetic pathway. Plant Physiol. 2016, 171, 2458–2467. [Google Scholar] [CrossRef] [PubMed]
- Qu, D. Food and Agriculture Organization of the United Nations (FAO). 2022. Available online: http://www.fao.org/home/en/ (accessed on 30 May 2022).
- Haverkort, A.J.; Struik, P.C. Yield levels of potato crops: Recent achievements and future prospects. Field Crops Res. 2015, 182, 76–85. [Google Scholar] [CrossRef]
- Birch, P.R.J.; Bryan, G.; Fenton, B.; Gilroy, E.M.; Hein, I.; Jones, J.T.; Prashar, A.; Taylor, M.A.; Torrance, L.; Toth, L.K. Crops that feed the world 8: Potato: Are the trends of increased global production sustainable? Food Secur. 2012, 4, 477–508. [Google Scholar] [CrossRef]
- Jennings, S.A.; Koehler, A.K.; Nicklin, K.J.; Deva, C.; Sait, S.M.; Challinor, A.J. Global Potato Yields Increase under Climate Change with Adaptation and CO2 Fertilisation. Front. Sustain. Food Syst. 2020, 4, 519324. [Google Scholar] [CrossRef]
- Liu, J.M.; Wang, S.S.; Zheng, X.; Jin, N.; Wang, F.Z. Antimicrobial activity against phytopathogens and inhibitory activity on solanine in potatoes of the endophytic bacteria isolated from potato tubers. Front. Microbiol. 2020, 11, 570926. [Google Scholar] [CrossRef] [PubMed]
- MARA. Available online: https://www.gov.cn/lianbo/bumen/202309/content_6902997.htm (accessed on 8 September 2023).
- Dreesen, F.E.; Boeck, H.J.D.; Janssens, I.A.; Nijs, I. Summer heat and drought extremes trigger unexpected changes in productivity of a temperate annual/biannual plant community. Environ. Exp. Bot. 2012, 79, 21–30. [Google Scholar] [CrossRef]
- Suttle, J. Symposium introduction: Enhancing the nutritional value of potato tubers. Am. J. Potato Res. 2008, 85, 266. [Google Scholar] [CrossRef]
- Płaza, A.; Ceglarek, F. The influence of undersown crops and straw biomass on yield and nutritional value of potato tubers. J. Cent. Eur. Agric. 2010, 10, 397–403. [Google Scholar]
- Malschi, B.; Luca, E. Irrigated potato crop in traditional farms in Letca Area Salaj county. Agric. Agric. Pract. Sci. J. 2012. [Google Scholar]
- Simkin, A.J.; Faralli, M.; Ramamoorthy, S.; Lawson, T. Photosynthesis in non-foliar tissues: Implications for yield. Plant J. 2020, 101, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.H.; Tang, Y.J.; Blankenship, R.E. Carbon metabolic pathways in phototrophic bacteria and their broader evolutionary implications. Front. Microbiol. 2011, 2, 165. [Google Scholar] [CrossRef] [PubMed]
- Slattery, R.A.; Ort, D.R. Photosynthetic energy conversion efficiency: Setting a baseline for gauging future improvements in important food and biofuel crops. Plant Physiol. 2015, 168, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Xun, X.; Pan, S.; Cheng, S.; Bo, Z.; Visser, R. Genome sequence and analysis of tuber crop potato. Nature 2011, 475, 189–195. [Google Scholar]
- Lal, M.K.; Tiwari, R.K.; Kumar, A.; Dey, A.; Kumar, R.; Kumar, D.; Jaiswal, A.; Changan, S.S.; Raigond, P.; Dutt, S.; et al. Mechanistic Concept of Physiological, Biochemical, and Molecular Responses of the Potato Crop to Heat and Drought Stress. Plants 2022, 11, 2857. [Google Scholar] [CrossRef] [PubMed]
- Minhas, J.S. Potato: Production strategies under abiotic stress. In Improving Crop Resistance to Abiotic Stress; Tuteja, N., Gill, S.S., Tiburcio, A.F., Tuteja, R., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 1155–1167. [Google Scholar]
- Wang-Pruski, G.; Schofield, A. Potato: Improving crop productivity and abiotic stress tolerance. In Improving Crop Resistance to Abiotic Stress; Tuteja, N., Gill, S.S., Tiburcio, A.F., Tuteja, R., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 1121–1153. [Google Scholar]
- Bilgin, D.D.; Zavala, J.A.; Zhu, J.; Clough, S.J.; Ort, D.R.; DeLucia, E.H. Biotic stress globally downregulates photosynthesis genes. Plant Cell Environ. 2010, 33, 1597–1613. [Google Scholar] [CrossRef] [PubMed]
- Slattery, R.A.; Ainsworth, E.A.; Ort, D.R. A meta-analysis of responses of canopy photosynthetic conversion efficiency to environmental factors reveals major causes of yield gap. J. Exp. Bot. 2013, 64, 3723–3733. [Google Scholar] [CrossRef] [PubMed]
- Spooner, D.M.; McLean, K.; Ramsay, G.; Waugh, R.; Bryan, G.J. A single domestication for potato based on multilocus amplifified fragment length polymorphism genotyping. Proc. Natl. Acad. Sci. USA 2005, 102, 14694–14699. [Google Scholar] [CrossRef] [PubMed]
- Hoopes, G.; Meng, X.; Hamilton, J.P.; Achakkagari, S.R.; de Alves Freitas Guesdes, F.; Bolger, M.E.; Coombs, J.J.; Esselink, D.; Kaiser, N.R.; Kodde, L. Phased, chromosome-scale genome assemblies of tetraploid potato reveal a complex genome, transcriptome, and predicted proteome landscape underpinning genetic diversity. Mol. Plant 2022, 15, 520–536. [Google Scholar] [CrossRef] [PubMed]
- Teper-Bamnolker, P.; Dudai, N.; Fischer, R.; Belausov, E.; Zemach, H.; Shoseyov, O.; Eshel, D. Mint essential oil can induce or inhibit potato sprouting by differential alteration of apical meristem. Planta 2010, 232, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Zarzyńska, K.; Boguszewska-Mańkows, D.; Nosalewicz, A. Differences in size and architecture of the potato cultivars root system and their tolerance to drought stress. Plant Soil Environ. 2017, 64, 159–164. [Google Scholar] [CrossRef]
- Kumari, S. Influence of drip irrigation and mulch on leaf area maximization, water use efficiency and yield of potato (Solanum tuberosum L.). Agric. Sci. 2011, 4, 71–80. [Google Scholar] [CrossRef]
- Ahmad, P.; Wani, M.R.; Azooz, M.M.; Tran, L.S.P. Improvement of Crops in the Era of Climatic Changes; Springer: New York, NY, USA, 2014. [Google Scholar]
- Evers, D.; Lefèvre, I.; Legay, S.; Lamoureux, D.; Hausman, J.F.; Rosales, R.O.; Marca, L.R.; Hoffmann, L.; Bonierbale, M.; Schafleitner, R. Identification of drought-responsive compounds in potato through a combined transcriptomic and targeted metabolite approach. J. Exp. Bot. 2010, 61, 2327–2343. [Google Scholar] [CrossRef] [PubMed]
- Holden, N.; Brereton, A.; Fealy, R.; Sweeney, J. Possible change in Irish climate and its impact on barley and potato yields. Agric. For. Meteorol. 2003, 116, 181–196. [Google Scholar] [CrossRef]
- Abdrabbo, M.; Kotb, M.H. Sensitivity of potato yield to climate change. J. Appl. Sci. Res. 2010, 6, 751–755. [Google Scholar]
- Hijmans, R.J. The Effect of Climate Change on Global Potato Production. Am. J. Potato Res. 2003, 80, 271–279. [Google Scholar] [CrossRef]
- Anithakumari, A.M.; Dolstra, O.; Vosman, B.; Visser, R.G.F.; Linden, C.G.V.D. In vitro screening and qtl analysis for drought tolerance in diploid potato. Euphytica 2011, 181, 357–369. [Google Scholar] [CrossRef]
- Salinger, M.; Sivakumar, M.; Motha, R. Reducing vulnerability of agriculture and forestry to climate variability and change: Workshop summary and recommendations. Clim. Chang. 2005, 70, 341–362. [Google Scholar] [CrossRef]
- Cook, E.R.; Seager, R.; Cane, M.A. North American drought: Reconstructions, causes, and consequences. Earth Sci. Rev. 2007, 8, 93–134. [Google Scholar] [CrossRef]
- Djaman, K.; Irmak, S.; Koudahe, K.; Allen, S. Irrigation Management in Potato (Solanum tuberosum L.) Production: A Review. Sustainability 2021, 13, 1504. [Google Scholar] [CrossRef]
- Vishnoi, L.; Roy, S.; Murty, N.S.; Nain, A.S. Study on water requirement of Potato (Solanum tuberosum L.) using CROPWAT MODEL for Tarai Region of Uttarakhand. J. Agrometeorol. 2012, 14, 180–185. [Google Scholar]
- SruthyT, K.; Arjun, P.T. Water Requirement of Major Tuber Crops: A Review. Int. J. Environ. Clim. Chang. 2023, 13, 1482–1487. [Google Scholar] [CrossRef]
- Ferreira, T.C.; Gonçalves, D.A. Crop-yield/water-use production functions of potatoes (Solanum tuberosum L.) grown under differential nitrogen and irrigation treatments in a hot, dry climate. Agric. Water Manag. 2007, 90, 45–55. [Google Scholar] [CrossRef]
- Badra, M.A.; El-Tohamy, W.A.; Salman, S.R.; Gruda, N. Yield and water use relationships of potato under different timing and severity of water stress. Agric. Water Manag. 2022, 271, 107793. [Google Scholar] [CrossRef]
- Schafleitner, R.; Gutierrez Rosales, R.O.; Gaudin, A.; Alvarado Aliaga, C.A.; Martinez, G.N.; Tincopa Marca, L.R.; Bolivar, L.A.; Delgado, F.M.; Simon, R.; Bonierbale, M. Capturing candidate drought tolerance traits in two native andean potato clones by transcription profiling of field grown plants under water stress. Plant Physiol. Biochem. 2007, 45, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Deng, H.; Wang, Y.; Li, X.; Zhang, H. Potato growth, photosynthesis, yield, and quality response to regulated deficit drip irrigation under film mulching in a cold and arid environment. Sci. Rep. 2021, 11, 15888. [Google Scholar] [CrossRef] [PubMed]
- Obidiegwu, J.; Bryan, G.; Jones, G.; Prashar, A. Coping with drought: Stress and adaptive responses in potato and perspectives for improvement. Front. Plant Sci. 2015, 6, 542. [Google Scholar] [CrossRef] [PubMed]
- Gervais, T.; Creelman, A.; Li, X.Q.; Bizimungu, B.; De Koeyer, D.; Dahal, K. Potato response to drought stress: Physiological and growth basis. Front. Plant Sci. 2021, 12, 698060. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011, 60, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Dahal, K.; Wang, J.; Martyn, G.; Rahimy, F.; Vanlerberghe, G.C. Mitochondrial alternative oxidase maintains respiration and preserves photosynthetic capacity during moderate drought in Nicotiana tabacum. Plant Physiol. 2014, 166, 1560–1574. [Google Scholar] [CrossRef] [PubMed]
- Salih, S.A.; Abdulrahman, F.A.; Mahmood, Y.A. The effect of diferent irrigation interval on tuber yield and quality of potato (Solanum tuberosum L.). Kurdistan J. Appl. Res. 2018, 3, 21–37. [Google Scholar] [CrossRef]
- Hossain, A. Tuber Yield, Tuber Quality and Plant Water Status of Potato under Drought and Well Watered Condition. Glob. J. Sci. Front. Res. D Agric. Vet. 2014, 14, 101–107. [Google Scholar]
- Mthembu, S.G.; Magwaza, L.S.; Mashilo, J.; Mditshwa, A.; Odindo, A. Drought tolerance assessment of potato (Solanum tuberosum L.) genotypes at different growth stages, based on morphological and physiological traits. Agric. Water Manag. 2022, 261, 107361. [Google Scholar]
- Deblonde, P.M.K.; Ledent, J.F. Effects of moderate drought conditions on green leaf number, stem height, leaf length and tuber yield of potato cultivars. Eur. J. Agron. 2001, 14, 31–41. [Google Scholar] [CrossRef]
- Lahlou, O.; Ouattar, S.; Ledent, J.F. The effect of drought and cultivar on growth parameters, yield and yield components of potato. Agronomy 2003, 23, 257–268. [Google Scholar] [CrossRef]
- Shock, C.C.; Feiberg, E.B.G. Deficit Irrigation of Potato.Water Rep (FAO); Oregon State University: Corvallis, OR, USA, 2002. [Google Scholar]
- Liu, F.; Jensen, C.R.; Shahanzari, A.; Andersen, M.N.; Jacobsen, S.E. ABA regulated stomatal control and photosynthetic water use efficiency of potato (Solanum tuberosum L.) during progressive soil drying. Plant Sci. 2005, 168, 831–836. [Google Scholar] [CrossRef]
- Liu, F.; Andersen, M.N.; Shahnazari, A.; Jacobsen, S.E.; Jensen, C.R. Improved Photosynthetic Water-Use Efficiency of Potato Due to Partial Stomatal Closure Induced by ABA Signalling at Mild Soil Water Deficits; Tsinghua University Press: Beijing, China, 2005. [Google Scholar]
- Chang, D.C.; Jin, Y.I.; Nam, J.H.; Cheon, C.G.; Cho, J.H.; Kim, S.J.; Yu, H.S. Early drought effect on canopy development and tuber growth of potato cultivars with different maturities. Field Crops Res. 2018, 215, 156–162. [Google Scholar] [CrossRef]
- Faradonbeh, H.R.B.; Bistgani, Z.E.; Barker, A.V. Tuber yield and physiological characteristics of potato under irrigation and fertilizer application. Commun. Soil. Sci. Plant Analysis 2022, 53, 1432–1443. [Google Scholar] [CrossRef]
- Shock, C.C.; Wang, F.X.; Flock, R.; Eldredge, E.; Pereira, A. Successful Potato Irrigation Scheduling; Oregon State University: Corvallis, OR, USA, 2006; EM 8911. [Google Scholar]
- Shock, C.C.; Stieber, T.D.; Zalewski, J.C.; Eldredge, E.P.; Lewis, M.D. Potato tuber stem-end fry color determination. Am. Potato J. 1994, 71, 77–88. [Google Scholar] [CrossRef]
- Zhu, X.; Richael, C.; Chamberlain, P.; Busse, J.S.; Bussan, A.J.; Jiang, J.; Bethke, P.C. Vacuolar invertase gene silencing in potato (Solanum tuberosum L.) improves processing quality by decreasing the frequency of sugar-end defects. PLoS ONE 2014, 9, e93381. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, G.; Murphy, A.; De Koeyer, D.; Tai, H.; Bizimungu, B.; Si, H.; Li, X.Q. Difffferences between the bud end and stem end of potatoes in dry matter content, starch granule size, and carbohydrate metabolic gene expression at the growing and sprouting stages. J. Agric. Food Chem. 2016, 64, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Qadir, G.; Ishtiaq, M.; Ali, I. Effect of earthing- up at different stages of growth on yield of potato cultivar Cardinal under the soil and climatic conditions of Peshawar [Pakistan]. Sarhad. J. Agric. 1999, 15, 423–425. [Google Scholar]
- Muñiz García, M.N.; Cortelezzi, J.I.; Fumagalli, M.; Capiati, D.A. Expression of the Arabidopsis ABF4 gene in potato increases tuber yield, improves tuber quality and enhances salt and drought tolerance. Plant Mol. Biol. 2018, 98, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Boguszewska-Mańkowska, D.; Zarzyńska, K.; Nosalewicz, A. Drought differentially affects root system size and architecture of potato cultivars with differing drought tolerance. Am. J. Potato Res. 2020, 97, 54–62. [Google Scholar] [CrossRef]
- Zinta, R.; Tiwari, J.K.; Buckseth, T.; Thakur, K.; Goutam, U.; Kumar, D.; Challam, C.; Bhatia, N.; Poonia, A.K.; Naik, S.; et al. Root system architecture for abiotic stress tolerance in potato: Lessons from plants. Front. Plant Sci. 2022, 13, 926214. [Google Scholar] [CrossRef] [PubMed]
- Stalham, M.A.; Allen, E.J. Water uptake in the potato (Solanum tuberosum) crop. J. Agric. Sci. 2004, 142, 373–393. [Google Scholar] [CrossRef]
- Weisz, R.; Kaminski, J.; Smilowitz, Z. Water deficit effects on potato leaf growth and transpiration: Utilizing fraction extractable soil water for comparison with other crops. Am. Potato J. 1994, 71, 829–840. [Google Scholar] [CrossRef]
- Jefferies, R.A.; Mackerron, D.K.L. Responses of potato genotypes to drought. II. Leaf area index, growth and yield. Ann. Appl. Biol. 1993, 122, 105–112. [Google Scholar] [CrossRef]
- Kesiime, V.E.; Tusiime, G.; Kashaija, I.N.; Edema, R.; Gibson, P.; Namugga, P.; Kakuhenzire, R. Characterization and evaluation of potato genotypes (Solanum tuberosum L.) for tolerance to drought in Uganda. Am. J. Potato Res. 2016, 93, 543–551. [Google Scholar] [CrossRef]
- Romero, A.P.; Alarcón, A.; Valbuena, R.I.; Galeano, C.H. Physiological assessment of water stress in potato using spectral information. Front. Plant Sci. 2017, 8, 1608–1620. [Google Scholar] [CrossRef] [PubMed]
- Deblonde, P.M.K.; Haverkort, A.J.; Ledent, J.F. Responses of early and late potato cultivars to moderate drought conditions: Agronomic parameters and carbon isotope discrimination. Eur. J. Agron. 1999, 11, 91–105. [Google Scholar] [CrossRef]
- Rolando, J.L.; Ramírez, D.A.; Yactayo, W.; Monneveux, P.; Quiroz, R. Leaf greenness as a drought tolerance related trait in potato (Solanum tuberosum L.). Environ. Exp. Bot. 2015, 110, 27–35. [Google Scholar] [CrossRef]
- Hill, D.; Nelson, D.; Hammond, J.; Bell, L. Morphophysiology of potato (Solanum tuberosum) in response to drought stress: Paving the way forward. Front. Plant Sci. 2021, 11, 597554. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ocampo, G.; Ploschuk, E.L.; Mantese, A.; Crocco, C.D.; Botto, J.F. BBX21 reduces abscisic acid sensitivity, mesophyll conductance and chloroplast electron transport capacity to increase photosynthesis and water use efficiency in potato plants cultivated under moderated drought. Plant J. 2021, 108, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Nadal, M.; Roig-Oliver, M.; Bota, J.; Flexas, J. Leaf age- dependent elastic adjustment and photosynthetic performance under drought stress in Arbutus unedo seedlings. Flora 2020, 271, 151662. [Google Scholar] [CrossRef]
- Demirel, U.; Morris, W.L.; Ducreux, L.J.M.; Yavuz, C.; Asim, A.; Tindas, I.; Campbell, R.; Morris, J.A.; Verrall, S.R.; Hedley, P.E.; et al. Physiological, biochemical, and transcriptional responses to single and combined abiotic stress in stress-tolerant and stress- sensitive potato genotypes. Front Plant Sci. 2020, 11, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Hussain, S.; Rao, M.J.; Anjum, M.A.; Ejaz, S.; Zakir, I.; Ali, M.A.; Ahmad, N.; Ahmad, S. Oxidative Stress and Antioxidant Defense in Plants under Drought Conditions. Plant Abiotic. Stress Toler. 2019, 207–219. [Google Scholar] [CrossRef]
- Ristic, Z.; Williams, G.; Yang, G.; Martin, B.; Fullerton, S. Dehydration, damage to cellular membranes, and heat-shock proteins in maize hybrids from different climates. J. Plant Physiol. 1996, 149, 424. [Google Scholar] [CrossRef]
- Augustine, S.M. Function of Heat-Shock Proteins in Drought Tolerance Regulation of Plants. Drought Stress Toler. Plants 2016, 1, 163–185. [Google Scholar]
- Ozturk, M.; Unal, B.T.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Rodriguez, H.G.; Roberts, J.; Jordan, W.R.; Drew, M.C. Growth, Water Relations, and Accumulation of Organic and Inorganic Solutes in Roots of Maize Seedlings during Salt Stress. Plant Physiol. 1997, 113, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Rudack, K.; Seddig, S.; Sprenger, H.; Khl, K.; Uptmoor, R.; Ordon, F. Drought stress—induced changes in starch yield and physiological traits in potato. J. Agron. Crop Sci. 2017, 203, 494–505. [Google Scholar] [CrossRef]
- Sakamoto, A.; Murata, N. The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant Cell Environ. 2002, 25, 163–171. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B. Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci. 2000, 40, 503–510. [Google Scholar] [CrossRef]
- Zhang, L.L. Drought-Resistant Gene Expression and Proteome Analysis of Potato under Drought Stress. Ph.D. Dissertation, Shenyang Agricultural University, Shenyang, China, 2015. [Google Scholar]
- Bhargava, S.; Sawant, K. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- Fincher, G.B. Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annu. Rev. Plant Biol. 1989, 40, 305–346. [Google Scholar] [CrossRef]
- Daie, J. Annual review of plant physiology and plant molecular biology. Soil Sci. 1992, 154, 508. [Google Scholar] [CrossRef]
- Robertson, J.M.; Pharis, R.P.; Huang, Y.Y.; Reid, D.M.; Yeung, E.C. Drought induced increases in abscisic acid levels in the root apex of sunflower. Plant Physiol. 1985, 79, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Saez, A.; Robert, N.; Maktabi, M.H.; Schroeder, J.I.; Serrano, R.; Rodriguez, P.L. Enhancement of abscisic acid sensitivity and reduction of water consumption in Arabidopsis by combined inactivation of the protein phosphatase type 2C ABI1 and HAB1. Plant Physiol. 2006, 141, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Robinet, C.; Mane, S.P.; Ulanov, A.V.; Watkinson, J.I.; Stromberg, V.K.; De Koeyer, D.; Schafleitner, R.; Willmot, D.B.; Bonierbale, M.; Bohnert, H.J.; et al. Physiological and molecular adaptations to drought in Andean potato genotypes. J. Exp. Bot. 2008, 59, 2109–2123. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ding, Y.; Yang, Y.; Song, C.; Gong, Z. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 2021, 63, 53–78. [Google Scholar] [CrossRef] [PubMed]
- Schafleitner, R.; Gutierrez, R.; Espino, R.; Gaudin, A.; Pérez, J.; Martínez, M.; Domínguez, A.; Tincopa, L.; Alvarado, C.; Numberto, G.; et al. Field screening for variation of drought tolerance in Solanum tuberosum L. by agronomical, physiological and genetic analysis. Potato Res. 2007, 50, 71–85. [Google Scholar] [CrossRef]
- Watanabe, K.N.; Kikuchi, A.; Shimazaki, T.; Asahina, M. Salt and drought stress tolerances in transgenic potatoes and wild species. Potato Res. 2011, 54, 319–324. [Google Scholar] [CrossRef]
- Muñiz García, M.N.; Giammaria, V.; Grandellis, C.; Téllez-Iñón, M.T.; Ulloa, R.M.; Capiati, D.A. Characterization of StABF1, a stress- responsive bZIP transcription factor from Solanum tuberosum L. that is phosphorylated by StCDPK2 in vitro. Planta 2012, 235, 761–778. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, N.; Liu, X.; Wang, S.; Li, S.; Yang, J.; Wang, F.; Si, H. StMAPK3 controls oxidase activity, photosynthesis and stomatal aperture under salinity and osmosis stress in potato. Plant Physiol. Biochem. 2020, 156, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, N.; Liu, X.; Li, S.; Yang, J.; Hong, X.; Wang, F.; Si, H. Mitogen-activated protein kinase 11 (MAPK11) maintains growth and photosynthesis of potato plant under drought condition. Plant Cell Rep. 2021, 40, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Pieczynski, M.; Wyrzykowska, A.; Milanowska, K.; Boguszewska-Mankowska, D.; Zagdanska, B.; Karlowski, W.; Jarmolowski, A.; Szweykowska-Kulinska, Z. Genomewide identification of genes involved in the potato response to drought indicates functional evolutionary conservation with Arabidopsis plants. Plant Biotechnol. J. 2018, 16, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Zhu, X.; Li, S.G.; Tang, X.; Si, H.J. A novel potato microRNA stu-miR856 regulates mitogen-activated protein kinase genes contributing to drought tolerance. Biol. Plantarum. 2019, 63, 618–626. [Google Scholar]
- Tetsuo, M.; Masaki, I. Plant Transcription Factors. Plant Cell Physiol. 1995, 36, 1405–1420. [Google Scholar]
- Joshi, R.; Wani, S.H.; Singh, B.; Bohra, A.; Dar, Z.A.; Lone, A.A.; Pareek, A.; Singla-Pareek, S.L. Transcription Factors and Plants Response to Drought Stress: Current Understanding and Future Directions. Front. Plant Sci. 2016, 7, 1029. [Google Scholar] [CrossRef] [PubMed]
- Charfeddine, M.; Chiab, N.; Charfeddine, S.; Ferjani, A.; Gargouri-Bouzid, R. Heat, drought, and combined stress effect on transgenic potato plants overexpressing the StERF94 transcription factor. J. Plant Res. 2023, 136, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, C.; Li, Z.; Sun, J.; Deng, Z.; Wen, L.; Li, X.; Guo, Y. Potato NAC Transcription Factor StNAC053 Enhances Salt and Drought Tolerance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2021, 22, 2568. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, N.; Bai, J.; Duan, X.; Zhang, L.; Liu, S.; Tang, X.; Jin, X.; Li, S.; Si, H. Stu-miR827-Targeted StWRKY48 Transcription Factor Negatively Regulates Drought Tolerance of Potato by Increasing Leaf Stomatal Density. Int. J. Mol. Sci. 2022, 23, 14805. [Google Scholar] [CrossRef]
- Shin, D.; Moon, S.J.; Han, S.; Kim, B.G.; Park, S.R.; Lee, S.K.; Yoon, H.J.; Lee, H.E.; Kwon, H.B.; Baek, D.; et al. Expression of StMYB1R-1, a novel potato single MYB-like domain transcription factor, increases drought tolerance. Plant Physiol. 2011, 155, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Functional Identification of Potato StWRKY57 Gene in Response to Drought Stress. Master’s Dissertation, Gansu Agricultural University, Lanzhou, China, 2019. [Google Scholar]
- Cheng, Y.J.; Kim, M.D.; Deng, X.P.; Kwak, S.S.; Chen, W. Enhanced salt stress tolerance in transgenic potato plants expressing IbMYB1, a sweet potato transcription factor. J. Microbiol. Biotechnol. 2013, 23, 1737–1746. [Google Scholar] [CrossRef]
- Moon, S.J.; Han, S.Y.; Kim, D.Y.; Yoon, I.S.; Shin, D.; Byun, M.O.; Kwon, H.B.; Kim, B.G. Ectopic expression of a hot pepper bZIP-like transcription factor in potato enhances drought tolerance without decreasing tuber yield. Plant Mol. Biol. 2015, 89, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Kloosterman, B.; Abelenda, J.A.; Gomez Mdel, M.; Oortwijn, M.; de Boer, J.M.; Kowitwanich, K.; Horvath, B.M.; van Eck, H.J.; Smaczniak, C.; Prat, S.; et al. Naturally occurring allele diversity allows potato cultivation in northern latitudes. Nature 2013, 495, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.L.; Shi, L.; Bergonzi, S.B.; Oortwijn, M.; Franco-Zorrilla, J.M.; Solano-Tavira, R.; Visser, R.G.F.; Abelenda, J.A.; Bachem, C.W.B. Potato CYCLING DOF FACTOR 1 and its lncRNA counterpart StFLORE link tuber development and drought response. Plant J. 2021, 105, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, Z.; Ai, Q.; Li, X.; Yang, J.; Zhang, N.; Si, H. DNA-Binding with One Finger (Dof) Transcription Factor Gene Family Study Reveals Differential Stress-Responsive Transcription Factors in Contrasting Drought Tolerance Potato Species. Int. J. Mol Sci. 2024, 25, 3488. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, N.; Mi, X.; Wu, L.; Ma, R.; Zhu, X.; Yao, L.; Jin, X.; Si, H.; Wang, D. Identification of miR159s and their target genes and expression analysis under drought stress in potato. Comput. Biol. Chem. 2014, 53, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Öztürk Gökçe, Z.N.; Aksoy, E.; Bakhsh, A.; Demirel, U.; Çalışkan, S.; Çalışkan, M.E. Combined drought and heat stresses trigger different sets of miRNAs in contrasting potato cultivars. Funct. Integr. Genom. 2021, 21, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.W.; Shin, S.J.; Park, S.C.; Jeong, M.J.; Kwon, H.B. Identification of miR172 family members and their putative targets responding to drought stress in Solanum tuberosum. Genes Genom. 2011, 33, 105–110. [Google Scholar] [CrossRef]
- Pieczynski, M.; Marczewski, W.; Hennig, J.; Dolata, J.; Bielewicz, D.; Piontek, P.; Wyrzykowska, A.; Krusiewicz, D.; Strzelczyk-Zyta, D.; Konopka-Postupolska, D.; et al. Down-regulation of CBP 80 gene expression as a strategy to engineer a drought-tolerant potato. Plant Biotechnol. J. 2013, 11, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Marshall, D.; Bryan, G.J.; Hornyik, C. Identification and characterization of miRNA transcriptome in potato by high-throughput sequencing. PLoS ONE 2013, 8, e57233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, J.; Wang, Z.; Wen, Y.; Wang, J.; He, W.; Liu, B.; Si, H.; Wang, D. Identification of novel and conserved microRNAs related to drought stress in potato by deep sequencing. PLoS ONE 2014, 9, e95489. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Song, J.; Lin, T.; Yin, Y.; Mu, J.; Liu, S.; Wang, Y.; Kong, D.; Zhang, Z. Identification of potato Lipid transfer protein gene family and expression verification of drought genes StLTP1 and StLTP7. Plant Direct. 2023, 7, e491. [Google Scholar] [CrossRef] [PubMed]
- Kitomi, Y.; Hanzawa, E.; Kuya, N.; Inoue, H.; Hara, N.; Kawai, S.; Kanno, N.; Endo, M.; Sugimoto, K.; Yamazaki, T.; et al. Root angle modifications by the DRO1 homolog improve rice yields in saline paddy fields. Proc. Natl. Acad. Sci. USA 2020, 117, 21242–21250. [Google Scholar] [CrossRef]
- Sun, C.; Liang, W.; Yan, K.; Xu, D.; Qin, T.; Fiaz, S.; Kear, P.; Bi, Z.; Liu, Y.; Liu, Z.; et al. Expression of Potato StDRO1 in Arabidopsis Alters Root Architecture and Drought Tolerance. Front. Plant Sci. 2022, 13, 836063. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Feng, S.; Yang, J.; Li, D.; Zhang, J. Roles of Plasmalemma Aquaporin Gene StPIP1 in Enhancing Drought Tolerance in Potato. Front. Plant Sci. 2017, 8, 616. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.S.; Han, E.H.; Kwak, S.S.; Cho, J.H.; Im, J.S.; Hong, S.Y.; Sohn, H.B.; Kim, Y.H.; Lee, S.W. Expressing the sweet potato orange gene in transgenic potato improves drought tolerance and marketable tuber production. C R. Biol. 2016, 339, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Si, H.J.; Wen, G.; Du, H.H.; Liu, B.L.; Wang, D. Enhanced drought and salinity tolerance in transgenic potato plants with a BADH gene from spinach. Plant Biotechnol. Rep. 2011, 5, 71–77. [Google Scholar] [CrossRef]
- Ma, R.; Liu, W.; Li, S.; Zhu, X.; Yang, J.; Zhang, N.; Si, H. Genome-Wide Identification, Characterization and Expression Analysis of the CIPK Gene Family in Potato (Solanum tuberosum L.) and the Role of StCIPK10 in Response to Drought and Osmotic Stress. Int. J. Mol. Sci. 2021, 22, 13535. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Tang, X.; Liu, W.; Fu, X.; Luo, H.; Ghimire, S.; Zhang, N.; Si, H. A potato RING-finger protein gene StRFP2 is involved in drought tolerance. Plant Physiol. Biochem. 2020, 146, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, F.; Li, S.; Feng, Y.; Yang, J.; Zhang, N.; Si, H. Calcium-Dependent Protein Kinase 28 Maintains Potato Photosynthesis and Its Tolerance under Water Deficiency and Osmotic Stress. Int. J. Mol. Sci. 2022, 23, 8795. [Google Scholar] [CrossRef] [PubMed]
- Herman, D.J.; Knowles, L.O.; Knowles, N.R. Heat stress affects carbohydrate metabolism during cold-induced sweetening of potato (Solanum tuberosum L.). Planta 2017, 245, 563–582. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Xv, K.; Meng, Q.; Li, G.; Yang, X. Potato plants ectopically expressing Arabidopsis thaliana CBF3 exhibit enhanced tolerance to high-temperature stress. Plant Cell Environ. 2015, 38, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.Y.; Kaplan, F.; Lee, K.J.; Guy, C.L. Acquired tolerance to temperature extremes. Trends Plant Sci. 2003, 8, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Vreugdenhil, D. Responses of the potato plant to temperature. In Potato Biology and Biotechnology: Advances and Perspectives; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Dam, J.V.; Kooman, P.L.; Struik, P.C. Effects of temperature and photoperiod on early growth and final number of tubers in potato (Solanum tuberosum L.). Potato Res. 1996, 39, 51–62. [Google Scholar]
- Haverkort, A.J.; Verhagen, A. Climate change and its repercussions for the potato supply chain. Potato Res. 2008, 51, 223–237. [Google Scholar] [CrossRef]
- Wolf, S.; Marani, A.; Rudich, J. Effect of temperature on carbohydrate metabolism in potato plants. J. Exp. Bot. 1991, 42, 619–625. [Google Scholar] [CrossRef]
- Hancock, R.D.; Morris, W.L.; Ducreux, L.J.; Morris, J.A.; Usman, M.; Verrall, S.R.; Fuller, J.; Simpson, C.G.; Zhang, R.; Hedley, P.E.; et al. Physiological, biochemical and molecular responses of the potato (Solanum tuberosum L.) plant to moderately elevated temperature. Plant Cell Environ. 2014, 37, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Obiero, C.O.; Milroy, S.P.; Bell, R.W. Importance of Whole Plant Dry Matter Dynamics for Potato (Solanum tuberosum L.) Tuber Yield Response to an Episode of High Temperature. Environ. Exp. Bot. 2019, 162, 560–571. [Google Scholar] [CrossRef]
- Chen, C.T.; Setter, T.L. Role of tuber developmental processes in response of potato to high temperature and elevated CO2. Plants 2021, 10, 871. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.U.; Seo, B.S.; Choi, D.H.; Ban, H.Y.; Lee, B.W. Impact of high temperatures on the marketable tuber yield and related traits of potato. Eur. J. Agron. 2017, 89, 46–52. [Google Scholar] [CrossRef]
- Zhang, G.; Tang, R.; Niu, S.; Si, H.; Yang, Q.; Bizimungu, B.; Regan, S.; Li, X. Effects of earliness on heat stress tolerance in fifty potato cultivars. Am. J. Potato Res. 2020, 97, 23–32. [Google Scholar] [CrossRef]
- Busse, J.S.; Wiberley-Bradford, A.E.; Bethke, P.C. Transient heat stress during tuber development alters post-harvest carbohydrate composition and decreases processing quality of chipping potatoes. J. Sci. Food Agric. 2019, 99, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Ginzberg, I.; Barel, G.; Ophir, R.; Tzin, E.; Tanami, Z.; Muddarangappa, T.; de Jong, W.; Fogelman, E. Transcriptomic profiling of heat-stress response in potato periderm. J. Exp. Bot. 2009, 60, 4411–4421. [Google Scholar] [CrossRef]
- Obiero, C.O.; Bell, R.W.; Milroy, S.P. Photosynthetic and respiratory response of potato leaves of different ages during and after an episode of high temperature. J. Agron. Crop Sci. 2020, 206, 352–362. [Google Scholar] [CrossRef]
- Struik, P.C.; Geertsema, J.; Custers, H.M. Effects of shoot, root and stolon temperature on the development of the potato (Solanum tuberosum L.) plant. II. Development of stolons. Potato Res. 1989, 32, 143–149. [Google Scholar] [CrossRef]
- Andrea, Á.V.; Muriel, Q.; Stanley, L.; Pablo, M.J.; Carolina, L.X. Tuber yield and quality responses of potato to moderate temperature increase during tuber bulking under two water availability scenarios. Field Crops Res. 2020, 251, 107786. [Google Scholar]
- Borah, M. Growth of the potato as influenced by temperature. Indian J. Plant Physiol. 1962, 5, 53–72. [Google Scholar]
- Ewing, E.E. Heat stress and the tuberization stimulus. Am. Pot. J. 1981, 58, 31–49. [Google Scholar] [CrossRef]
- Navarro, C.; Abelenda, J.A.; Cuéllar, C.; Tamaki, S.; Shimamoto, K.; Prat, S. Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature 2011, 478, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.U.; Lee, B.W. Differential mechanisms of potato yield loss induced by high day and night temperatures during tuber initiation and bulking: Photosynthesis and tuber growth. Front. Plant Sci. 2019, 10, 300. [Google Scholar] [CrossRef] [PubMed]
- Hastilestari, B.R.; Lorenz, J.; Reid, S.; Hofmann, J.; Pscheidt, D.; Sonnewald, U.; Sonnewald, S. Deciphering source and sink responses of potato plants (Solanum tuberosum L.) to elevated temperatures. Plant Cell Environ. 2018, 41, 2600–2616. [Google Scholar] [CrossRef] [PubMed]
- Kooman, P.L.; Haverkort, A.J. Modelling development and growth of the potato crop influenced by temperature and daylength: LINTUL-POTATO. In Potato Ecology And Modelling of Crops under Conditions Limiting Growth; Springer: Dordrecht, The Netherlands, 1995; pp. 41–59. [Google Scholar]
- Fogelman, E.; Oren-Shamir, M.; Hirschberg, J.; Mandolino, G.; Parisi, B.; Ovadia, R.; Tanami, Z.; Faigenboim, A.; Ginzberg, I. Nutritional value of potato (Solanum tuberosum) in hot climates: Anthocyanins, carotenoids, and steroidal glycoalkaloids. Planta 2019, 249, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Pollastri, S.; Jorba, I.; Hawkins, T.J.; Llusià, J.; Michelozzi, M.; Navajas, D.; Peñuelas, J.; Hussey, P.J.; Knight, M.R.; Loreto, F. Leaves of isoprene-emitting tobacco plants maintain PSII stability at high temperatures. New Phytol. 2019, 223, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Burton, D. Physiological responses of melanophores and xanthophores of hypophysectomized and spinal winter flounder, Pseudopleuronectes americanus Walbaum. Proc. R. Soc. B-Biol. Sci. 1981, 213, 217–231. [Google Scholar]
- Reynolds, M.P.; Ewing, E.E.; Owens, T.G. Photosynthesis at high temperature in tuber-bearing Solanum species: A comparison between accessions of contrasting heat tolerance. Plant Physiol. 1990, 93, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Winkler, L.; Buanga, N.F.; Goetze, E. Gas-liquid chromatographic analysis of cardiolipin from fetal and maternal liver of the rat. Biochim. Biophys. Acta 1971, 231, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Jin, X.; Li, J.P.; Ma, G.F.; Cao, N.; Li, Q. Effects of short-term high temperature stress on the photosynthesis of potato in different growth stages. Agric. Sci. Tech. 2011, 12, 317–342. [Google Scholar]
- Mittler, R.; Blumwald, E. Genetic engineering for modern agriculture: Challenges and perspectives. Annu. Rev. Plant Biol. 2010, 61, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y. Effects of High Temperature on the Formation of Starch and Yield of Potato. Master’s Dissertation, Ningxia University, Yinchuan, China, 2017. [Google Scholar]
- Granda, E.; Scoffoni, C.; Rubio-Casal, A.E.; Sack, L.; Valladares, F. Leaf and stem physiological responses to summer and winter extremes of woody species across temperate ecosystems. Oikos 2014, 123, 1281–1290. [Google Scholar] [CrossRef]
- Yang, C.W.; Peng, C.L.; Duan, J.; Lin, G.Z.; Chen, Y.Z. Responses of chlorophyll fluorescence and carotenoids biosynthesis to high light stress in rice seedling leaves at different leaf position. Acta Bot. Sin. 2002, 44, 1303. [Google Scholar]
- Heraud, P.; Beardall, J. Changes in chlorophyll fluorescence during exposure of Dunaliella tertiolecta to UV radiation indicate a dynamic interaction between damage and repair processes. Photosynth. Res. 2004, 63, 123–134. [Google Scholar] [CrossRef]
- Zhu, X.; Duan, H.; Jin, H.; Chen, S.; Chen, Z.; Shao, S.; Tang, J.; Zhang, Y. Heat responsive gene StGATA2 functions in plant growth, photosynthesis and antioxidant defense under heat stress conditions. Front. Plant Sci. 2023, 14, 1227526. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant Responses of Wheat Plants under Stress. Genet. Mol. Biol. 2016, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sairam, R.K.; Tyagi, A. Physiology and Molecular Biology of Salinity Stress. Toler. Plants Curr. Sci. 2004, 86, 407–421. [Google Scholar]
- Xu, S.; Li, J.; Zhang, X.; Wei, H.; Cui, L. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ. Exp. Bot. 2006, 56, 274–285. [Google Scholar] [CrossRef]
- Pospíšil, P. Production of Reactive Oxygen Species by Photosystem II as a Response to Light and Temperature Stress. Front. Plant Sci. 2016, 7, 1950. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Liu, J.H.; Lu, J.P.; Zhai, Y.F.; Wang, H.; Gong, Z.H.; Wang, S.B.; Lu, M.H. Genome-wide analysis of the CaHsp20 gene family in pepper: Comprehensive sequence and expression profile analysis under heat stress. Front. Plant Sci. 2015, 6, 806. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.B.; Shao, Y.M.; Miao, S.; Wang, L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell Mol. Life Sci. 2006, 63, 2560–2570. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.J.; Zimmerman, J.L. Introduction of the carrot HSP17.7 into potato (Solanum tuberosum L.) enhances cellular membrane stability and tuberization in vitro. Plant Cell Environ. 2006, 29, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, D.; Wang, R.; Kong, N.; Zhang, C.; Yang, C.; Wu, W.; Ma, H.; Chen, Q. Genome wide analysis of the potato Hsp20 gene family: Identification, genomic organization and expression profiles in response to heat stress. BMC Genom. 2018, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.; Shah, S.H.; Ali, G.S.; Munir, I.; Khan, R.S.; Iqbal, A.; Ahmed, N.; Jan, A. Introduction of Arabidopsis’s heat shock factor HsfA1d mitigates adverse effects of heat stress on potato (Solanum tuberosum L.) plant. Cell Stress Chaperon 2020, 25, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Zhu, W.; Song, X.; Lin, X.; Cai, J.; Wang, M.; Yang, Q. Genome-Wide Identification and Function Analyses of Heat Shock Transcription Factors in Potato. Front. Plant Sci. 2016, 7, 490. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Y.; Ye, M.; Wang, D.; Chen, Q. Evolutionary history of the heat shock protein 90 (Hsp90) family of 43 plants and characterization of Hsp90s in Solanum tuberosum. Mol. Biol. Rep. 2020, 47, 6679–6691. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, P.; Sharma, D.; Verma, S.K.; Halterman, D.; Kumar, A. Genome-wide identification and expression profiling of basic leucine zipper transcription factors following abiotic stresses in potato (Solanum tuberosum L.). PLoS ONE 2021, 16, e0247864. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Dong, J.; Cao, M.; Gao, X.; Wang, D.; Liu, B.; Chen, Q. Genome-wide identification and characterization of HD-ZIP genes in potato. Gene 2019, 697, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Kiranmai, K.; Lokanadha Rao, G.; Pandurangaiah, M.; Nareshkumar, A.; Amaranatha Reddy, V.; Lokesh, U.; Venkatesh, B.; Anthony Johnson, A.M.; Sudhakar, C. A novel WRKY transcription factor, MuWRKY3 (Macrotyloma uniflorum Lam. Verdc.) enhances drought stress tolerance in transgenic groundnut (Arachis hypogaea L.) plants. Front. Plant Sci. 2018, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Rosin, F.M.; Hart, J.K.; Horner, H.T.; Davies, P.J.; Hannapel, D.J. Overexpression of a knotted-like homeobox gene of potato alters vegetative development by decreasing gibberellin accumulation. Plant Physiol. 2003, 132, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Tomar, M.S.S.; Singh, B.; Bhardwaj, V.; Sood, S.; Singh, B.; Salaria, N.; Thakur, K.; Kumar, A.; Sharma, N.; Goutam, U. Validation of molecular response of tuberization in response to elevated temperature by using a transient Virus Induced Gene Silencing (VIGS) in potato. Funct. Integr. Genom. 2021, 21, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Yu, B. Genetic Diversity Analysis of Phenotypic Traits and Comprehensive Assessment of Tuber Quality in Introduced Potato Germplasm Resources. Ph.D. Dissertation, Gansu Agricultural University, Lanzhou, China, 2017. [Google Scholar]
- Qin, J.; Zhang, T.; Meng, L.; Xu, J.; Meng, M.; Jin, L. Evaluation of Drought Tolerance in Exotic Potato Germplasm. J. Plant Genet. Resour. 2019, 20, 574–582. [Google Scholar]
- Ren, J.; Yang, M.; Wang, Y.; Huang, Y.; Li, R.; Hu, J.; Xiao, G. Response of Potato Antioxidant Defense System to Drought Stress Based on Main Planting Varieties in Yunnan. Southwest China J. Agric. Sci. 2020, 33, 1158–1164. [Google Scholar]
- Guo, X.; Zhang, G.; Li, K.; Guo, H. Evaluation of Heat Tolerance of Different Potato Varieties. J. Yunnan Agric. Univ. 2020, 35, 196–205. [Google Scholar]
- Trapero-Mozos, A.; Ducreux, L.J.M.; Bita, C.E.; Morris, W.; Wiese, C.; Morris, J.A.; Paterson, C.; Hedley, P.E.; Hancock, R.D.; Taylor, M. A reversible light and genotype-dependent acquired thermotolerance response protects the potato plant from damage due to excessive temperature. Planta 2018, 247, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.J.B.; Zotarelli, L.; Tormena, C.A.; Rens, L.R.; Rowland, D.L. Effects of water table management on least limiting water range and potato root growth. Agric. Water Manag. 2017, 186, 1–11. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Plauborg, F.; Kristensen, K.; Andersen, M.N. Dry matter production, radiation interception and radiation use efficiency of potato in response to temperature and nitrogen application regimes. Agric. For. Meteorol. 2017, 232, 595–605. [Google Scholar] [CrossRef]
- Paul, S.; Farooq, M.; Bhattacharya, S.S.; Gogoi, N. Management strategies for sustainable yield of potato crop under high temperature. Arch. Agron. Soil. Sci. 2017, 63, 276–287. [Google Scholar] [CrossRef]
| Effect of Drought and High Temperature in Potato | ||||
|---|---|---|---|---|
| Stress | Yield and Quality | Growth and Development | Physiology and Metabolism | Molecular Response Mechanism |
| Drought | Reduced tuber yield Reduced the number of tubers per plant Reduced yield per plant Reduced yield per unit area Reduced setting rate and harvest index Induced defects in potato tubers such as tuber hollowing, tuber rupture, internal brown spot, malformation and secondary growth Increased the content of α-solanine and α-chaconine alkaloids Suppressed starch content of tubers Affected the tuber market grade, tuber-specific gravity and tuber processing quality Induced sugar end defects | Changed root length and reduced root diameter size Inhibited leaf growth Reduced leaf area and the number of green leaves | Increased leaf SPAD value Reduced the tuber dry matter content Reduced leaf net photosynthetic rate (Pn) Reduced transpiration rate (Tr) Reduced stomatal conductance (Gs) Reduced leaf area index Changed intercellular CO2 concentration (Ci) Reduced carbon accumulation and carbon reactivation Damaged cell membrane Increased malondialdehyde (MDA) and proline (Pro) content Enhanced permeability of the cell membrane Increased the extent of membrane lipid peroxidation Changed POD SOD and CAT | Abscisic acid signaling pathway MAPK signaling pathway Transcription factors miRNA Other regulatory factors |
| High temperature | Reduced tuber yield Reduced tuber diameter Reduces the weight of individual tubers Reduced the number of potato tubers Lowed potato quality, such as secondary growth, tuber hollowing, cracking and tuber deformities Increased the basal and apical tuber reducing sugars content Decreased the dry matter content Increased the severity of stem-end chip defects | Hindered the occurrence of stolons Reduced the number of stolons per plant Reduced commercial potato rate Reduced the number of potatoes set Delayed tuberization Increased plant height, Inhibited leaf growth Caused early senescence, and even death | Inhibited carbon transport and carbon dioxide fixation Reduced chlorophyll content Inhibited the rate of dry matter accumulation Inhibit the photosynthesis Reduced leaf net photosynthetic rate (Pn) Inhibited the CO2 fixation Increased Tr Decreased Fm and Fv/Fm Inhibited the electron transfer and light energy conversion efficiency of PSII reaction centers Disrupted the composition and structure of the plasma membrane Increased intracellular electrolyte extravasation Increased plasma membrane peroxidation Increased malondialdehyde (MDA) and Pro content Increased CAT, POD and SOD enzyme activities | Heat shock proteins and heat shock factors Transcription factors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, G.; Yang, S.; Ruan, B.; Ye, G.; He, M.; Su, W.; Zhou, Y.; Wang, J.; Yang, S. Research Progress on Physiological, Biochemical, and Molecular Mechanisms of Potato in Response to Drought and High Temperature. Horticulturae 2024, 10, 827. https://doi.org/10.3390/horticulturae10080827
Fang G, Yang S, Ruan B, Ye G, He M, Su W, Zhou Y, Wang J, Yang S. Research Progress on Physiological, Biochemical, and Molecular Mechanisms of Potato in Response to Drought and High Temperature. Horticulturae. 2024; 10(8):827. https://doi.org/10.3390/horticulturae10080827
Chicago/Turabian StyleFang, Guonan, Shengwei Yang, Banpu Ruan, Guangji Ye, Miaomiao He, Wang Su, Yun Zhou, Jian Wang, and Shenglong Yang. 2024. "Research Progress on Physiological, Biochemical, and Molecular Mechanisms of Potato in Response to Drought and High Temperature" Horticulturae 10, no. 8: 827. https://doi.org/10.3390/horticulturae10080827
APA StyleFang, G., Yang, S., Ruan, B., Ye, G., He, M., Su, W., Zhou, Y., Wang, J., & Yang, S. (2024). Research Progress on Physiological, Biochemical, and Molecular Mechanisms of Potato in Response to Drought and High Temperature. Horticulturae, 10(8), 827. https://doi.org/10.3390/horticulturae10080827






