Elicitors and Biostimulants to Mitigate Water Stress in Vegetables
Abstract
:1. Introduction
2. Vegetable Responses to Water Stress
2.1. Germination and Growth Responses to Water Stress
2.2. Physiological Responses to Water Stress
2.3. Biochemical Responses to Water Stress
2.4. Molecular Responses to Water Stress
3. Management Strategies
3.1. Agronomic Approaches for Water Stress Management
3.1.1. Tillage
3.1.2. Mulching
3.1.3. Intercropping
3.1.4. Nutrient Handling
3.1.5. Deficit Irrigation
3.1.6. Phytohormones and Osmoprotectants
3.2. Plant Breeding Approaches
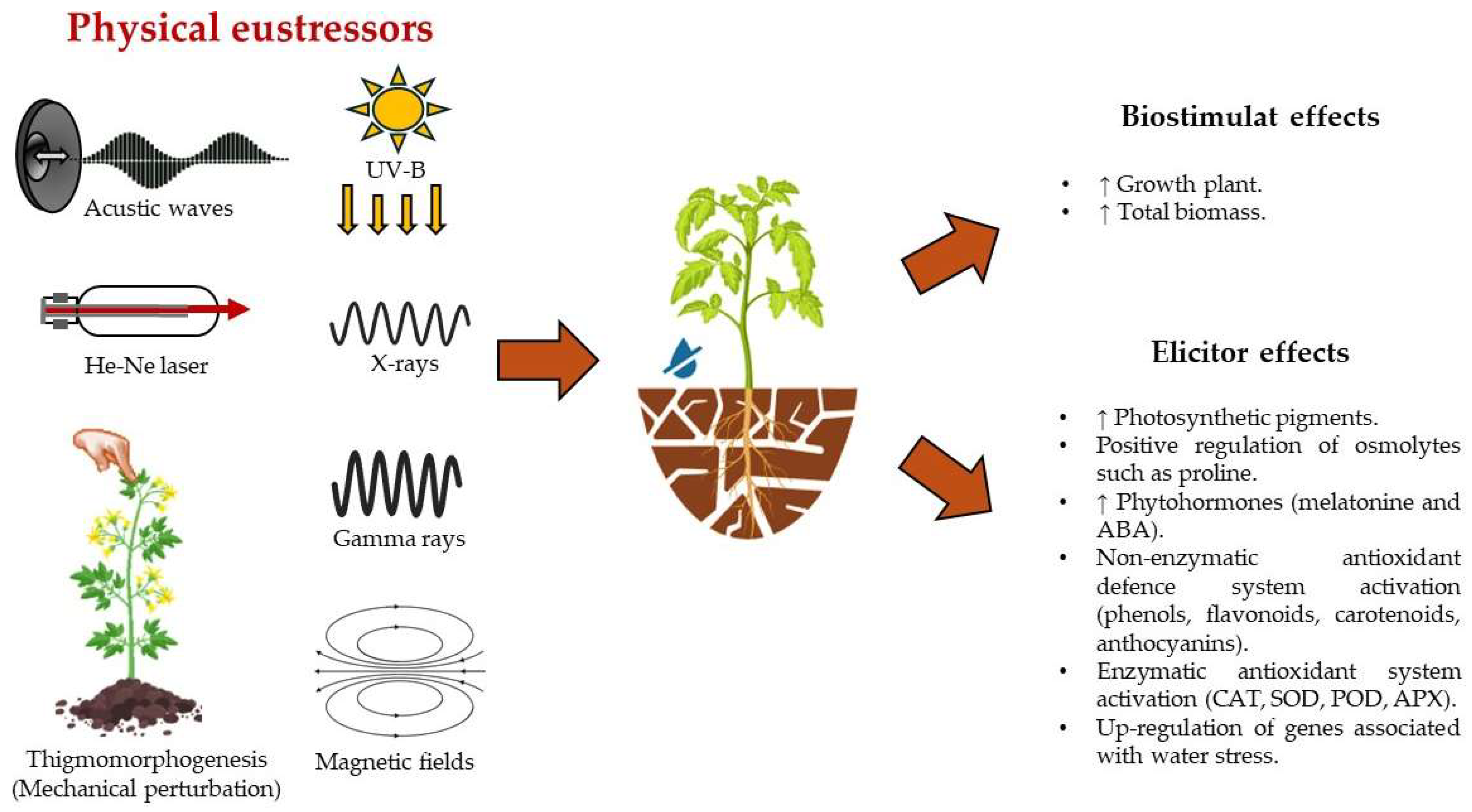
4. Trends in the Use of Physical Eustressors (Biostimulants/Elicitors) to Promote Drought Tolerance
5. Combination between Agronomic Approaches and Physical Stressors to Alleviate the Effects of Water Stress and Develop Tolerance to Drought
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asaduzzaman, M.; Asao, T. (Eds.) Introductory Chapter: Quality Vegetable Production and Human Health Benefits; InTech: Rijeka, Croatia, 2018; p. Ch. 1. ISBN 978-1-78923-507-4. [Google Scholar]
- Muhammad, H.M.D.; Naz, S.; Lal, M.K.; Tiwari, R.K.; Ahmad, R.; Nawaz, M.A.; Das, R.; Altaf, M.A. Melatonin in Business with Abiotic Stresses in Vegetable Crops. Sci. Hortic. 2024, 324, 112594. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Lal, P.; Ahmad, R.; Zulfiqar, F.; Kumar, A.; Hayat, F.; Kumar, R.; Lal, M.K.; Naz, S.; et al. Current Understanding of Boosting Power of Salicylic Acid for Abiotic Stress Tolerance in Horticultural Crops. S. Afr. J. Bot. 2023, 163, 285–293. [Google Scholar] [CrossRef]
- Parkash, V.; Singh, S. A Review on Potential Plant-Based Water Stress Indicators for Vegetable Crops. Sustainability 2020, 12, 3945. [Google Scholar] [CrossRef]
- Khalid, M.F.; Huda, S.; Yong, M.; Li, L.; Li, L.; Chen, Z.-H.; Ahmed, T. Alleviation of Drought and Salt Stress in Vegetables: Crop Responses and Mitigation Strategies. Plant Growth Regul. 2022, 99, 177–194. [Google Scholar] [CrossRef]
- Abbas, K.; Li, J.; Gong, B.; Lu, Y.; Wu, X.; Lü, G.; Gao, H. Drought Stress Tolerance in Vegetables: The Functional Role of Structural Features, Key Gene Pathways, and Exogenous Hormones. Int. J. Mol. Sci. 2023, 24, 13876. [Google Scholar] [CrossRef]
- Luo, Q.; Xie, H.; Chen, Z.; Ma, Y.; Yang, H.; Yang, B.; Ma, Y. Morphology, Photosynthetic Physiology and Biochemistry of Nine Herbaceous Plants under Water Stress. Front. Plant Sci. 2023, 14, 1147208. [Google Scholar] [CrossRef]
- Khan, A.; Pan, X.; Najeeb, U.; Tan, D.K.Y.; Fahad, S.; Zahoor, R.; Luo, H. Coping with Drought: Stress and Adaptive Mechanisms, and Management through Cultural and Molecular Alternatives in Cotton as Vital Constituents for Plant Stress Resilience and Fitness. Biol. Res. 2018, 51, 47. [Google Scholar] [CrossRef]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant Survival under Drought Stress: Implications, Adaptive Responses, and Integrated Rhizosphere Management Strategy for Stress Mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef] [PubMed]
- Mañas, F.M.D.S.O. Agua y Agronomía; Mundi-Prensa Libros: Madrid, Spain, 2005; ISBN 9788484762461. [Google Scholar]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I. Drought Stress Tolerance in Plants: Interplay of Molecular, Biochemical and Physiological Responses in Important Development Stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Saruyama, N.; Sakakura, Y.; Asano, T.; Nishiuchi, T.; Sasamoto, H.; Kodama, H. Quantification of Metabolic Activity of Cultured Plant Cells by Vital Staining with Fluorescein Diacetate. Anal. Biochem. 2013, 441, 58–62. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Q.; Zhang, B. Evaluation and Selection of Reliable Reference Genes for Gene Expression under Abiotic Stress in Cotton (Gossypium hirsutum L.). Gene 2013, 530, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Taromi Aliabadi, B.; Hassandokht, M.R.; Etesami, H.; Alikhani, H.A.; Dehghanisanij, H. Effect of Mulching on Some Characteristics of Tomato (Lycopersicum esculentum Mill.) under Deficit Irrigation. mdrsjrns 2019, 21, 927–941. [Google Scholar]
- Menezes, C.S.L.; Rezende, R.; Terassi, D.D.S.; Hachmann, T.L.; Saath, R. Lettuce and Radish Grown in Single Crop and Intercropping Systems under Different Irrigation Water Depths in a Protected Environment. Rev. Caatinga 2022, 35, 658–666. [Google Scholar] [CrossRef]
- Kumari, L.; Hasan, M.; Randhe, R.; Singh, D.K.; Singh, A.; Alam, W. Effects of Mulching and Irrigation Levels on Greenhouse Capsicum (Capsicum annuum). Indian J. Agric. Sci. 2021, 91, 833–839. [Google Scholar] [CrossRef]
- Sakr, M.T.; Ibrahim, H.M.; ElAwady, A.E.; AboELMakarm, A.A. Growth, Yield and Biochemical Constituents as Well as Post-Harvest Quality of Water-Stressed Broccoli (Brassica oleraceae L. Var. Italica) as Affected by Certain Biomodulators. Sci. Hortic. 2021, 275, 109605. [Google Scholar] [CrossRef]
- Akram, N.A.; Bashir, R.; Ashraf, G.; Bashir, S.; Ashraf, M.; Alyemeni, M.N.; Bajguz, A.; Ahmad, P. Exogenous α-Tocopherol Regulates the Growth and Metabolism of Eggplant (Solanum melongena L.) under Drought Stress. Plants 2023, 12, 237. [Google Scholar] [CrossRef]
- Abdelghany, A.E.; Dou, Z.; Alashram, M.G.; Eltohamy, K.M.; Elrys, A.S.; Liu, X.; Wu, Y.; Cheng, M.; Fan, J.; Zhang, F. The Joint Application of Biochar and Nitrogen Enhances Fruit Yield, Quality and Water-Nitrogen Productivity of Water-Stressed Greenhouse Tomato under Drip Fertigation. Agric. Water Manag. 2023, 290, 108605. [Google Scholar] [CrossRef]
- Bahar, A.A.; Faried, H.N.; Razzaq, K.; Ullah, S.; Akhtar, G.; Amin, M.; Bashir, M.; Ahmed, N.; Wattoo, F.M.; Ahmar, S.; et al. Potassium-Induced Drought Tolerance of Potato by Improving Morpho-Physiological and Biochemical Attributes. Agronomy 2021, 11, 2573. [Google Scholar] [CrossRef]
- Kaya, C.; Shabala, S. Melatonin Improves Drought Stress Tolerance of Pepper (Capsicum annuum) Plants via Upregulating Nitrogen Metabolism. Funct. Plant Biol. 2023, 51. [Google Scholar] [CrossRef]
- Tlig, W.; Mokh, F.; Autovino, D.; Iovino, M.; Nagaz, K. Carrot Productivity and Its Physiological Response to Irrigation Methods and Regimes in Arid Regions. Water Supply 2023, 23, 5093–5105. [Google Scholar] [CrossRef]
- Rodan, M.A.; Hassandokht, M.R.; Sadeghzadeh-Ahari, D.; Mousavi, A. Mitigation of Drought Stress in Eggplant by Date Straw and Plastic Mulches. J. Saudi Soc. Agric. Sci. 2020, 19, 492–498. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Rady, M.O.A.; Marey, R.A.; Abd El-Mageed, T.A. Exogenously Applied Proline Enhances Growth and Productivity of Drought Stressed Onion by Improving Photosynthetic Efficiency, Water Use Efficiency and up-Regulating Osmoprotectants. Sci. Hortic. 2020, 272, 109580. [Google Scholar] [CrossRef]
- Parkash, V.; Singh, S.; Deb, S.K.; Ritchie, G.L.; Wallace, R.W. Effect of Deficit Irrigation on Physiology, Plant Growth, and Fruit Yield of Cucumber Cultivars. Plant Stress 2021, 1, 100004. [Google Scholar] [CrossRef]
- Léllis, B.C.; Martínez-Romero, A.; Schwartz, R.C.; Pardo, J.J.; Tarjuelo, J.M.; Domínguez, A. Effect of the Optimized Regulated Deficit Irrigation Methodology on Water Use in Garlic. Agric. Water Manag. 2022, 260, 107280. [Google Scholar] [CrossRef]
- Sakya, A.T.; Sulistyaningsih, E.; Purwanto, B.H.; Indradewa, D. Application ZnSO4 on Tomato Growth under Drought Stress Conditions. IOP Conf. Ser. Earth Environ. Sci. 2021, 637, 12077. [Google Scholar] [CrossRef]
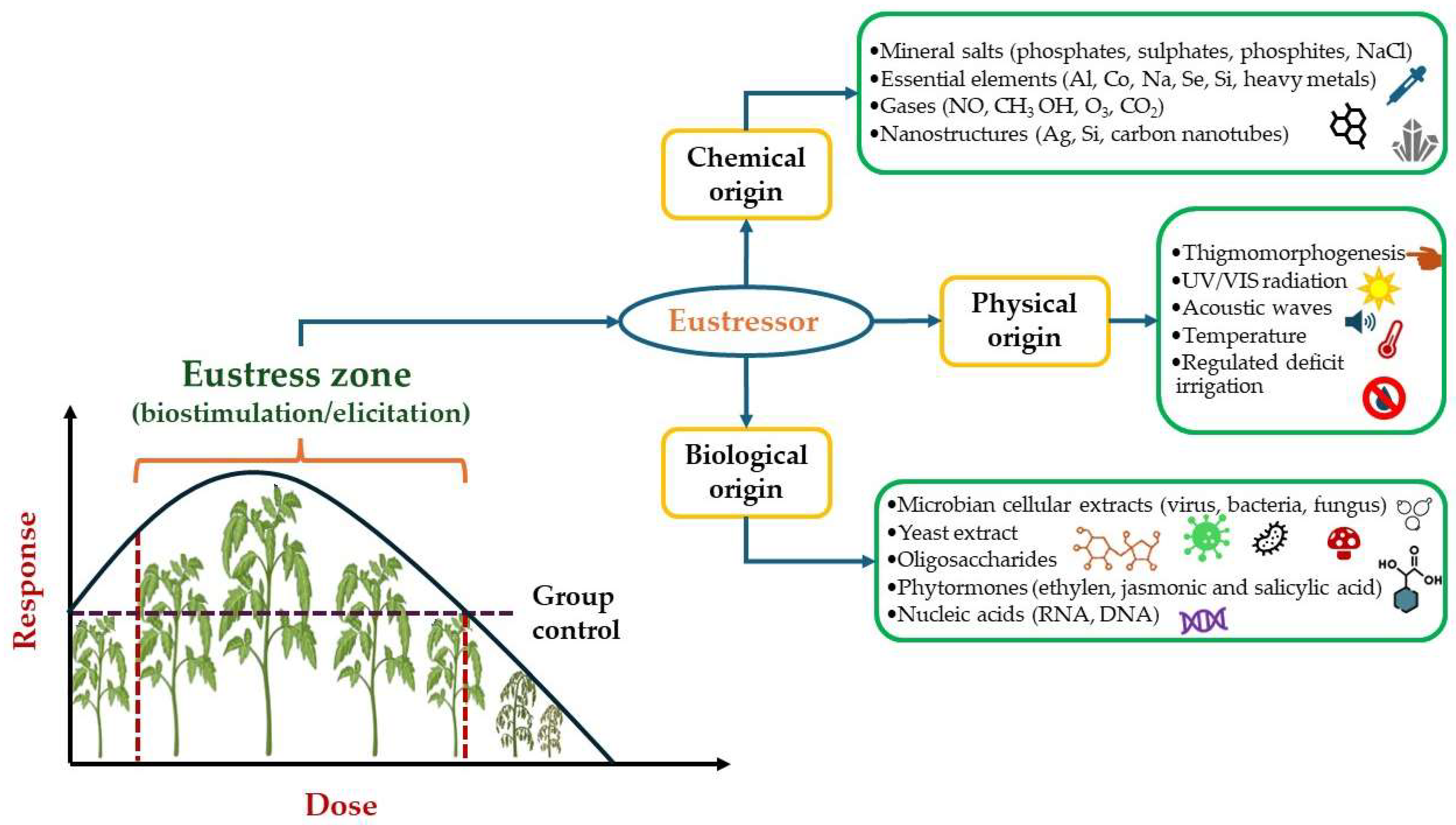
- Godínez-Mendoza, P.L.; Rico-Chávez, A.K.; Ferrusquía-Jimenez, N.I.; Carbajal-Valenzuela, I.A.; Villagómez-Aranda, A.L.; Torres-Pacheco, I.; Guevara-González, R.G. Science of the Total Environment Plant Hormesis: Revising of the Concepts of Biostimulation, Elicitation and Their Application in a Sustainable Agricultural Production. Sci. Total Environ. 2023, 894, 164883. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Hernandez, C.; Feregrino-Perez, A.A.; Perez-Ramirez, I.; Ocampo-Velazquez, R.V.; Rico-García, E.; Torres-Pacheco, I.; Guevara-Gonzalez, R.G. Controlled Elicitation Increases Steviol Glycosides (SGs) Content and Gene Expression-Associated to Biosynthesis of SGs in Stevia Rebaudiana B. Cv. Morita II. Ind. Crops Prod. 2019, 139, 111479. [Google Scholar] [CrossRef]
- Vázquez-Hernández, M.C.; Parola-Contreras, I.; Montoya-Gómez, L.M.; Torres-Pacheco, I.; Schwarz, D.; Guevara-González, R.G. Eustressors: Chemical and Physical Stress Factors Used to Enhance Vegetables Production. Sci. Hortic. 2019, 250, 223–229. [Google Scholar] [CrossRef]
- Mardaninejad, S.; Tabatabaei, S.H.; Pessarakli, M.; Zareabyaneh, H. Physiological Responses of Pepper Plant (Capsicum annuum L.) to Drought Physiological Responses of Pepper Plant (Capsicum annuum L.) to Drought Stress. J. Plant Nutr. 2017, 40, 1453–1464. [Google Scholar] [CrossRef]
- Wang, J.Q.; Li, H.; Liu, Q.; Xiang, D. Effects of Drought Stress on Root Development and Physiological Characteristics of Sweet Potato at Seedling Stage. Ying Yong Sheng Tai Xue Bao-The J. Appl. Ecol. 2019, 30, 3155–3163. [Google Scholar] [CrossRef]
- Khodabin, G.; Tahmasebi-Sarvestani, Z.; Rad, A.H.S.; Modarres-Sanavy, S.A.M. Effect of Drought Stress on Certain Morphological and Physiological Characteristics of a Resistant and a Sensitive Canola Cultivar. Chem. Biodivers. 2020, 17, e1900399. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Cardoso, V.J.M. Germinação. In Fisiologia Vegetal; Kerbauy, G.B., Ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2012; pp. 386–408. [Google Scholar]
- Kumar, R.; Berwal, M.K.; Saroj, P.L. Morphological, Physiological, Biochemical and Molecular Facet of Drought Stress in Horticultural Crops. Int. J. Bio-Resour. Stress Manag. 2019, 10, 545–560. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, R.; Ashraf, M.Y.; Ashraf, M.; Waraich, E.A. Sunflower (Helianthus annuus L.) Response to Drought Stress at Germination and Seedling Growth Stages. Pak. J. Bot. 2009, 41, 647–654. [Google Scholar]
- Mut, Z.; Akay, H. Effect of Seed Size and Drought Stress on Germination and Seedling Growth of Naked Oat (Avena sativa L.). Bulg. J. Agric. Sci. 2010, 16, 459–467. [Google Scholar]
- Sun, Y.D.; Du, X.H.; Zhang, W.J.; Sun, L.; Li, R. Seed Germination and Physiological Characteristics of Amaranthus mangostanus L. under Drought Stress. Adv. Mater. Res. 2011, 183–185, 1071–1074. [Google Scholar] [CrossRef]
- Rahimi, A. Seed Priming Improves the Germination Performance of Cumin (Cuminum syminum L.) under Temperature and Water Stress. Ind. Crops Prod. 2013, 42, 454–460. [Google Scholar] [CrossRef]
- Sharp, R.E.; Poroyko, V.; Hejlek, L.G.; Spollen, W.G.; Springer, G.K.; Bohnert, H.J.; Nguyen, H.T. Root Growth Maintenance during Water Deficits: Physiology to Functional Genomics. J. Exp. Bot. 2004, 55, 2343–2351. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Analysis, A.E.; Schwarz, D.; Crops, O.; Franken, P. Effects of Drought on Nutrient Uptake and Assimilation in Vegetable Crops. In Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 171–195. ISBN 9783642326530. [Google Scholar]
- Chun, H.C.; Lee, S.; Choi, Y.D.; Gong, D.H.; Jung, K.Y. Effects of Drought Stress on Root Morphology and Spatial Distribution of Soybean and Adzuki Bean. J. Integr. Agric. 2021, 20, 2639–2651. [Google Scholar] [CrossRef]
- Kim, Y.; Chung, Y.S.; Lee, E.; Tripathi, P.; Heo, S.; Kim, K.H. Root Response to Drought Stress in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2020, 21, 1513. [Google Scholar] [CrossRef]
- Kavar, T.; Maras, M.; Kidric, M.; Sustar-Vozlic, J.; Meglic, V. Identification of Genes Involved in the Response of Leaves of Phaseolus Vulgaris to Drought Stress. Mol. Breed. 2008, 21, 159–172. [Google Scholar] [CrossRef]
- Ghosh, D.; Xu, J. Abiotic Stress Responses in Plant Roots: A Proteomics Perspective. Front. Plant Sci. 2014, 5, 6. [Google Scholar] [CrossRef]
- Sadras, V.O.; Villalobos, F.J.; Orgaz, F.; Fereres, E. Effects of Water Stress on Crop Production. In Principles of Agronomy for Sustainable Agriculture; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2016; pp. 189–204. ISBN 9783319461168. [Google Scholar]
- Carvalho, P.; Foulkes, M.J. Roots and Uptake of Water and Nutrients. In Encyclopedia of Sustainability Science and Technology; Springer: New York, NY, USA, 2018; pp. 1–24. ISBN 9781493924936. [Google Scholar]
- Munns, R. Plant Adaptations to Salt and Water Stress. Differences and Commonalities, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; Volume 57, ISBN 9780123876928. [Google Scholar]
- Durigon, A.; Evers, J.; Metselaar, K.; de Jong van Lier, Q. Water Stress Permanently Alters Shoot Architecture in Common Bean Plants. Agronomy 2019, 9, 160. [Google Scholar] [CrossRef]
- Mosenda, E.; Chemining, G.; Ambuko, J.; Owino, W. Effect of Water Stress on Growth and Yield Components of Selected Spider Plant Accessions. J. Med. Act. Plants 2020, 9, 81–97. [Google Scholar] [CrossRef]
- Sibomana, I.; Aguyoh, J.; Opiyo, A.; Imani, C. Water Stress Affects Growth and Yield of Container Grown Tomato (Lycopersicon Esculentum Mill) Plants. Glob. J. Bio-Sci. Biotechnol. 2020, 2, 461–466. [Google Scholar]
- Maseko, I.; Ncube, B.; Mabhaudhi, T.; Tesfay, S.; Chimonyo, V.G.P.; Araya, H.T.; Fessehazion, M.; Du Plooy, C.P. Moisture Stress on Physiology and Yield of Some Indigenous Leafy Vegetables under Field Conditions. S. Afr. J. Bot. 2019, 126, 85–91. [Google Scholar] [CrossRef]
- Kaysar, M.S.; Sarker, U.K.; Monira, S.; Hossain, M.A.; Mokarroma, N.; Somaddar, U.; Saha, G.; Hossain, S.S.F.; Chaki, A.K.; Uddin, M.R. Water Stress Induced Changes in Root Traits and Yield of Irrigated Rice under Subtropical Condition. Water 2023, 15, 618. [Google Scholar] [CrossRef]
- Singh, M.; Saini, R.K.; Singh, S. Potential of Integrating Biochar and Deficit Irrigation Strategies for Sustaining Vegetable Production in Water-Limited Regions: A Review. HortScience 2019, 54, 1872–1878. [Google Scholar] [CrossRef]
- Comparini, D.; Masi, E.; Pandolfi, C.; Sabbatini, L.; Dolfi, M.; Morosi, S.; Mancuso, S. Stem Electrical Properties Associated with Water Stress Conditions in Olive Tree. Agric. Water Manag. 2020, 234, 106109. [Google Scholar] [CrossRef]
- Yahia, E.M.; Carrillo-lópez, A.; Barrera, G.M. Photosynthesis. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 47–72. ISBN 9780128132784. [Google Scholar]
- Keller, M. Photosynthesis and Respiration. In The Science of Grapevines; Academic Press: Cambridge, MA, USA, 2020; pp. 129–148. ISBN 9780128163658. [Google Scholar]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of Drought Stress on Photosynthesis and Photosynthetic Electron Transport Chain in Young Apple Tree Leaves. Biol. Open 2018, 7, bio035279. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Kubota, C. Growth, Development, Transpiration, and Translocation as Affected by Abiotic Environmental Factors; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128166918. [Google Scholar]
- Díaz-Pérez, J.C. Transpiration; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128132784. [Google Scholar]
- Lambers, H.; Oliveira, R.S. Plant Water Relations. In Plant Physiological Ecology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 187–263. ISBN 978-1-4757-2857-6. [Google Scholar]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A Review on Drought Stress in Plants: Implications, Mitigation and the Role of Plant Growth Promoting Rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Liu, B.; Liang, J.; Tang, G.; Wang, X.; Liu, F.; Zhao, D. Drought Stress Affects on Growth, Water Use Efficiency, Gas Exchange and Chlorophyll Fluorescence of Juglans Rootstocks. Sci. Hortic. 2019, 250, 230–235. [Google Scholar] [CrossRef]
- Yan, M.; Yao, Y.; Mou, K.; Dan, Y.; Li, W.; Wang, C.; Liao, W. The Involvement of Abscisic Acid in Hydrogen Gas-Enhanced Drought Resistance in Tomato Seedlings. Sci. Hortic. 2022, 292, 110631. [Google Scholar] [CrossRef]
- Jaime, R.; Serichol, C.; Alcántara, J.M.; Rey, P.J. Differences in Gas Exchange Contribute to Habitat Differentiation in Iberian Columbines from Contrasting Light and Water Environments. Plant Biol. 2014, 16, 354–364. [Google Scholar] [CrossRef]
- Toscano, S.; Scuderi, D.; Giuffrida, F.; Romano, D. Responses of Mediterranean Ornamental Shrubs to Drought Stress and Recovery. Sci. Hortic. 2014, 178, 145–153. [Google Scholar] [CrossRef]
- Giménez, C.; Gallardo, M.; Thompson, R. Plant–Water Relations. Ref. Modul. Earth Syst. Environ. Sci. 2013, 231–238. [Google Scholar] [CrossRef]
- Bao, Y.; Liu, T.; Duan, L.; Tong, X.; Ji, H.; Zhang, L.; Singh, V.P. A Comparative Study of Three Stomatal Conductance Models for Estimating Evapotranspiration in a Dune Ecosystem in a Semi-Arid Region. Sci. Total Environ. 2022, 802, 149937. [Google Scholar] [CrossRef]
- Goto, K.; Yabuta, S.; Ssenyonga, P.; Tamaru, S.; Sakagami, J.-I. Response of Leaf Water Potential, Stomatal Conductance and Chlorophyll Content under Different Levels of Soil Water, Air Vapor Pressure Deficit and Solar Radiation in Chili Pepper (Capsicum Chinense). Sci. Hortic. 2021, 281, 109943. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, G.; He, Q.; Zhou, L.; Ji, Y.; Lv, X. Capability of Leaf Water Content and Its Threshold Values in Reflection of Soil–Plant Water Status in Maize during Prolonged Drought. Ecol. Indic. 2021, 124, 107395. [Google Scholar] [CrossRef]
- Jin, X.; Shi, C.; Yu, C.Y.; Yamada, T.; Sacks, E.J. Determination of Leaf Water Content by Visible and Near-Infrared Spectrometry and Multivariate Calibration in Miscanthus. Front. Plant Sci. 2017, 8, 721. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Y. Drought Effects on Leaf Canopy Temperature and Leaf Senescence in Barley. Iraqi J. Agric. Sci. 2020, 51, 1684–1693. [Google Scholar] [CrossRef]
- Nemeskéri, E.; Helyes, L. Physiological Responses of Selected Vegetable Crop Species to Water Stress. Agronomy 2019, 9, 447. [Google Scholar] [CrossRef]
- Helyes, L.; Bőcs, A.; Pék, Z. Effect of Water Supply on Canopy Temperature, Stomatal Conductance and Yield Quantity of Processing Tomato (Lycopersicon esculentum Mill.). Int. J. Hortic. Sci. 2010, 16, 13–15. [Google Scholar] [CrossRef]
- Biriah, N.; Chemining’wa, G.; Olubayo, F.; Saha, H. Effect of Drought Stress on Canopy Temperature, Growth and Yield Performance of Cowpea Varieties. Int. J. Plant Soil Sci. 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Hessini, K.; Martínez, J.P.; Gandour, M.; Albouchi, A.; Soltani, A.; Abdelly, C. Effect of Water Stress on Growth, Osmotic Adjustment, Cell Wall Elasticity and Water-Use Efficiency in Spartina alterniflora. Environ. Exp. Bot. 2009, 67, 312–319. [Google Scholar] [CrossRef]
- Hessini, K.; Issaoui, K.; Ferchichi, S.; Saif, T.; Abdelly, C.; Siddique, K.H.M.; Cruz, C. Interactive Effects of Salinity and Nitrogen Forms on Plant Growth, Photosynthesis and Osmotic Adjustment in Maize. Plant Physiol. Biochem. 2019, 139, 171–178. [Google Scholar] [CrossRef]
- Girma, F.S.; Krieg, D.R. Osmotic Adjustment in Sorghum: I. Mechanisms of Diurnal Osmotic Potential Changes. Plant Physiol. 1992, 99, 577–582. [Google Scholar] [CrossRef]
- Mahmood, T.; Abdullah, M.; Ahmar, S.; Yasir, M.; Iqbal, M.S.; Yasir, M.; Ur Rehman, S.; Ahmed, S.; Rana, R.M.; Ghafoor, A.; et al. Incredible Role of Osmotic Adjustment in Grain Yield Sustainability under Water Scarcity Conditions in Wheat (Triticum aestivum L.). Plants 2020, 9, 1208. [Google Scholar] [CrossRef]
- Ors, S.; Ekinci, M.; Yildirim, E.; Sahin, U.; Turan, M.; Dursun, A. Interactive Effects of Salinity and Drought Stress on Photosynthetic Characteristics and Physiology of Tomato (Lycopersicon esculentum L.) Seedlings. S. Afr. J. Bot. 2021, 137, 335–339. [Google Scholar] [CrossRef]
- Dalal, A.; Bourstein, R.; Haish, N.; Shenhar, I.; Wallach, R.; Moshelion, M. Dynamic Physiological Phenotyping of Drought-Stressed Pepper Plants Treated with “Productivity-Enhancing” and “Survivability-Enhancing” Biostimulants. Front. Plant Sci. 2019, 10, 905. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Ekinci, M.; Ors, S.; Turan, M.; Yildiz, S.; Yildirim, E. Effects of Individual and Combined Effects of Salinity and Drought on Physiological, Nutritional and Biochemical Properties of Cabbage (Brassica oleracea Var. Capitata). Sci. Hortic. 2018, 240, 196–204. [Google Scholar] [CrossRef]
- Razzaq, M.; Akram, N.A.; Ashraf, M.; Naz, H.; Al-Qurainy, F. Interactive Effect of Drought and Nitrogen on Growth, Some Key Physiological Attributes and Oxidative Defense System in Carrot (Daucus carota L.) Plants. Sci. Hortic. 2017, 225, 373–379. [Google Scholar] [CrossRef]
- Yadav, B.; Jogawat, A.; Rahman, M.S.; Narayan, O.P. Secondary Metabolites in the Drought Stress Tolerance of Crop Plants: A Review. Gene Rep. 2021, 23, 101040. [Google Scholar] [CrossRef]
- Jogawat, A. Osmolytes and Their Role in Abiotic Stress Tolerance in Plants. In Molecular Plant Abiotic Stress; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 91–104. [Google Scholar]
- Hasanuzzaman, M.; Anee, T.I.; Bhuiyan, T.F.; Nahar, K.; Fujita, M. Emerging Role of Osmolytes in Enhancing Abiotic Stress Tolerance in Rice. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J.K., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 677–708. ISBN 978-0-12-814332-2. [Google Scholar]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, Distribution and Roles of Osmoprotective Compounds Accumulated in Halophytes under Abiotic Stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Hasanuzzaman, M.; Fujita, M. Roles of Osmolytes in Plant Adaptation to Drought and Salinity BT—Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologie; Iqbal, N., Nazar, R., A. Khan, N., Eds.; Springer: New Delhi, India, 2016; pp. 37–68. ISBN 978-81-322-2616-1. [Google Scholar]
- Llauradó Maury, G.; Méndez Rodríguez, D.; Hendrix, S.; Escalona Arranz, J.C.; Fung Boix, Y.; Pacheco, A.O.; García Díaz, J.; Morris-Quevedo, H.J.; Ferrer Dubois, A.; Aleman, E.I.; et al. Antioxidants in Plants: A Valorization Potential Emphasizing the Need for the Conservation of Plant Biodiversity in Cuba. Antioxidants 2020, 9, 1048. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Catalase, Superoxide Dismutase and Ascorbate-Glutathione Cycle Enzymes Confer Drought Tolerance of Amaranthus Tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S.K. Reactive Oxygen Species Signaling in Plants under Abiotic Stress. Plant Signal. Behav. 2013, 8, e23681. [Google Scholar] [CrossRef]
- Li, R.; Jia, Z.; Trush, M.A. Defining ROS in Biology and Medicine. React. Oxyg. Species 2016, 1, 9–21. [Google Scholar] [CrossRef]
- Sandalio, L.M.; Rodríguez-Serrano, M.; Romero-Puertas, M.C.; del Río, L.A. Role of Peroxisomes as a Source of Reactive Oxygen Species (ROS) Signaling Molecules. In Subcellular Biochemtistry; del Río, L.A., Ed.; Springer: Granada, Spain, 2013; pp. 231–255. [Google Scholar] [CrossRef]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic Stress: Interplay between ROS, Hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Pacheco, J.; Plazas, M.; Pettinari, I.; Landa-Faz, A.; González-Orenga, S.; Boscaiu, M.; Soler, S.; Prohens, J.; Vicente, O.; Gramazio, P. Moderate and Severe Water Stress Effects on Morphological and Biochemical Traits in a Set of Pepino (Solanum muricatum) Cultivars. Sci. Hortic. 2021, 284, 110143. [Google Scholar] [CrossRef]
- Nxele, X.; Klein, A.; Ndimba, B.K. Drought and Salinity Stress Alters ROS Accumulation, Water Retention, and Osmolyte Content in Sorghum Plants. S. Afr. J. Bot. 2017, 108, 261–266. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Huihui, Z.; Xin, L.; Yupeng, G.; Mabo, L.; Yue, W.; Meijun, A.; Yuehui, Z.; Guanjun, L.; Nan, X.; Guangyu, S. Physiological and Proteomic Responses of Reactive Oxygen Species Metabolism and Antioxidant Machinery in Mulberry (Morus alba L.) Seedling Leaves to NaCl and NaHCO3 Stress. Ecotoxicol. Environ. Saf. 2020, 193, 110259. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; Gill, R.; Nehra, A.; Sharma, K.K.; Hasanuzzaman, M.; Prasad, R.; Tuteja, N.; Gill, S.S. Reactive Oxygen Species (ROS) Management in Engineered Plants for Abiotic Stress Tolerance. In Advancement in Crop Improvement Techniques; Tuteja, N., Tuteja, R., Passricha, N., Saifi, S.K., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 241–262. ISBN 978-0-12-818581-0. [Google Scholar]
- Liu, S.; Yang, R. Regulations of Reactive Oxygen Species in Plants Abiotic Stress: An Integrated Overview. In Plant Life Under Changing Environment; Tripathi, D.K., Pratap Singh, V., Chauhan, D.K., Sharma, S., Prasad, S.M., Dubey, N.K., Ramawat, N., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 323–353. ISBN 978-0-12-818204-8. [Google Scholar]
- Mahmood, T.; Rana, R.M.; Ahmar, S.; Saeed, S.; Gulzar, A.; Khan, M.A.; Wattoo, F.M.; Wang, X.; Branca, F.; Mora-Poblete, F.; et al. Effect of Drought Stress on Capsaicin and Antioxidant Contents in Pepper Genotypes at Reproductive Stage. Plants 2021, 10, 1286. [Google Scholar] [CrossRef] [PubMed]
- Paim, B.T.; Crizel, R.L.; Tatiane, S.J.; Rodrigues, V.R.; Rombaldi, C.V.; Galli, V. Mild Drought Stress Has Potential to Improve Lettuce Yield and Quality. Sci. Hortic. 2020, 272, 109578. [Google Scholar] [CrossRef]
- Jaswanthi, N.; Krishna, M.S.R.; Sahitya, U.L.; Suneetha, P. Apoplast Proteomic Analysis Reveals Drought Stress-Responsive Protein Datasets in Chilli (Capsicum annuum L.). Data Br. 2019, 25, 104041. [Google Scholar] [CrossRef]
- Razi, K.; Muneer, S. Drought Stress-Induced Physiological Mechanisms, Signaling Pathways and Molecular Response of Chloroplasts in Common Vegetable Crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef]
- Xu, T.; Niu, J.; Jiang, Z. Sensing Mechanisms: Calcium Signaling Mediated Abiotic Stress in Plants. Front. Plant Sci. 2022, 13, 925863. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bheri, M.; Bisht, D.; Pandey, G.K. Calcium Signaling and Transport Machinery: Potential for Development of Stress Tolerance in Plants. Curr. Plant Biol. 2022, 29, 100235. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of Plants to Water Stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Brunetti, C.; Gori, A.; Marino, G.; Latini, P.; Sobolev, A.P.; Nardini, A.; Haworth, M.; Giovannelli, A.; Capitani, D.; Loreto, F.; et al. Dynamic Changes in ABA Content in Water-Stressed Populus nigra: Effects on Carbon Fixation and Soluble Carbohydrates. Ann. Bot. 2019, 124, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Todaka, D.; Takahashi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Chapter Three—ABA-Responsive Gene Expression in Response to Drought Stress: Cellular Regulation and Long-Distance Signaling. In Abscisic Acid in Plants; Seo, M., Marion-Poll, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 92, pp. 83–113. ISBN 0065-2296. [Google Scholar]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of Abscisic Acid-Mediated Drought Stress Responses in Plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Saradadevi, R.; Palta, J.A.; Siddique, K.H.M. ABA-Mediated Stomatal Response in Regulating Water Use during the Development of Terminal Drought in Wheat. Front. Plant Sci. 2017, 8, 1251. [Google Scholar] [CrossRef]
- Ye, N.; Zhu, G.; Liu, Y.; Li, Y.; Zhang, J. ABA Controls H2O2 Accumulation Through the Induction of OsCATB in Rice Leaves Under Water Stress. Plant Cell Physiol. 2011, 52, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Urano, K.; Maruyama, K.; Jikumaru, Y.; Kamiya, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Analysis of Plant Hormone Profiles in Response to Moderate Dehydration Stress. Plant J. 2017, 90, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Urano, K.; Yoshiwara, K.; Morishita, Y.; Sakurai, N.; Suzuki, H.; Kojima, M.; Sakakibara, H.; Shibata, D.; Saito, K.; et al. Integrated Analysis of the Effects of Cold and Dehydration on Rice Metabolites, Phytohormones, and Gene Transcripts. Plant Physiol. 2014, 164, 1759–1771. [Google Scholar] [CrossRef]
- Shi, X.; Bao, J.; Lu, X.; Ma, L.; Zhao, Y.; Lan, S.; Cao, J.; Ma, S.; Li, S. The Mechanism of Ca2+ Signal Transduction in Plants Responding to Abiotic Stresses. Environ. Exp. Bot. 2023, 216, 105514. [Google Scholar] [CrossRef]
- Mak, M.; Babla, M.; Xu, S.-C.; O’Carrigan, A.; Liu, X.-H.; Gong, Y.-M.; Holford, P.; Chen, Z.-H. Leaf Mesophyll K+, H+ and Ca2+ Fluxes Are Involved in Drought-Induced Decrease in Photosynthesis and Stomatal Closure in Soybean. Environ. Exp. Bot. 2014, 98, 1–12. [Google Scholar] [CrossRef]
- Li, L.; Xing, J.; Ma, H.; Liu, F.; Wang, Y. In Situ Determination of Guard Cell Ion Flux Underpins the Mechanism of ABA-Mediated Stomatal Closure in Barley Plants Exposed to PEG-Induced Drought Stress. Environ. Exp. Bot. 2021, 187, 104468. [Google Scholar] [CrossRef]
- Wang, P.; Qi, S.; Wang, X.; Dou, L.; Jia, M.-A.; Mao, T.; Guo, Y.; Wang, X. The OPEN STOMATA1-SPIRAL1 Module Regulates Microtubule Stability during Abscisic Acid-Induced Stomatal Closure in Arabidopsis. Plant Cell 2023, 35, 260–278. [Google Scholar] [CrossRef] [PubMed]
- Chong, L.; Xu, R.; Ku, L.; Zhu, Y. Beyond Stress Response: OST1 Opening Doors for Plants to Grow. Stress Biol. 2022, 2, 44. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Feng, Z.; Ding, Y.; Qi, Y.; Jiang, S.; Li, Z.; Wang, Y.; Qi, J.; Song, C.; Yang, S.; et al. RAF22, ABI1 and OST1 Form a Dynamic Interactive Network That Optimizes Plant Growth and Responses to Drought Stress in Arabidopsis. Mol. Plant 2022, 15, 1192–1210. [Google Scholar] [CrossRef]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.K.N.; Ghafoor, A.; Du, X. Insights into Drought Stress Signaling in Plants and the Molecular Genetic Basis of Cotton Drought Tolerance. Cells 2019, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, C.; Chen, H.Y.H.; Ruan, H. Response of Plants to Water Stress: A Meta-Analysis. Front. Plant Sci. 2020, 11, 978. [Google Scholar] [CrossRef]
- Zakhidov, E.; Nematov, S.; Kuvondikov, V. Monitoring of the Drought Tolerance of Various Cotton Genotypes Using Chlorophyll Fluorescence. In Applied Photosynthesis—New Progress; Najafpour, M.M., Ed.; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar]
- Acosta-García, G.; Chapa-Oliver, A.M.; Millán-Almaraz, J.R.; Guevara-González, R.G.; Cortez-Baheza, E.; Rangel-Cano, R.M.; Ramírez-Pimentel, J.G.; Cruz-Hernandez, A.; Guevara-Olvera, L.; Aguilera-Bibian, J.E.; et al. CaLEA 73 Gene from Capsicum annuum L. Enhances Drought and Osmotic Tolerance Modulating Transpiration Rate in Transgenic Arabidopsis Thaliana. Can. J. Plant Sci. 2015, 95, 227–235. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Li, T.; Ni, C.; Han, L.; Du, P.; Xiao, K. TaPYL4, an ABA Receptor Gene of Wheat, Positively Regulates Plant Drought Adaptation through Modulating the Osmotic Stress-Associated Processes. BMC Plant Biol. 2022, 22, 423. [Google Scholar] [CrossRef]
- An, J.; Li, Q.; Yang, J.; Zhang, G.; Zhao, Z.; Wu, Y.; Wang, Y.; Wang, W. Wheat F-Box Protein TaFBA1 Positively Regulates Plant Drought Tolerance but Negatively Regulates Stomatal Closure. Front. Plant Sci. 2019, 10, 1242. [Google Scholar] [CrossRef]
- Thomas, A.; Beena, R.; Laksmi, G.; Soni, K.B.; Alex, S.; Viji, M.M. Changes in Sucrose Metabolic Enzymes to Water Stress in Contrasting Rice Genotypes. Plant Stress 2022, 5, 100088. [Google Scholar] [CrossRef]
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in Plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.X.; Wang, S.B.; Zhang, H.X.; Xiao, H.J.; Jin, J.H.; Ji, J.J.; Jing, H.; Chen, R.G.; Arisha, M.H.; Gong, Z.H. Cloning and Expression Analysis of CaPIP1-1 Gene in Pepper (Capsicum annuum L.). Gene 2015, 563, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Zia, S.; Romano, G.; Spreer, W.; Sanchez, C.; Cairns, J.; Araus, J.L.; Müller, J. Infrared Thermal Imaging as a Rapid Tool for Identifying Water-Stress Tolerant Maize Genotypes of Different Phenology. J. Agron. Crop Sci. 2013, 199, 75–84. [Google Scholar] [CrossRef]
- Blanco, H.; Lal, R. Soil Water Management. In Soil Conservation and Management; Springer Nature: Cham, Switzerland, 2023; pp. 417–438. ISBN 3031303415. [Google Scholar]
- Majcher, J.; Kafarski, M.; Wilczek, A.; Szypłowska, A.; Lewandowski, A.; Woszczyk, A.; Skierucha, W. Application of a Dagger Probe for Soil Dielectric Permittivity Measurement by TDR. Measurement 2021, 178, 109368. [Google Scholar] [CrossRef]
- Ihuoma, S.O.; Madramootoo, C.A. Recent Advances in Crop Water Stress Detection. Comput. Electron. Agric. 2017, 141, 267–275. [Google Scholar] [CrossRef]
- Millán, S.; Campillo Torres, C.; Vivas, A.; Moñino, M.; Prieto, M. Evaluation of Soil Water Content Measurements with Capacitance Probes to Support Irrigation Scheduling in a ‘Red Beaut’ Japanese Plum Orchard. Agronomy 2020, 10, 1757. [Google Scholar] [CrossRef]
- Contreras Medina, L.M.; Macías Bobadilla, I.; Melo, D.; Caicedo López, L.H.; Sáenz de la O, D.; Aguirre Becerra, H.; García Servín, M.; Vázquez Hernández, C.; Ortega Torres, A.E.; Rico García, E. Ejemplos de Aplicación de Factores de Estrés Físicos En La Agricultura. In Manejo del Estrés Vegetal como una Estrategia para una Agricultura Sostenible; Guevara-González, R.G., Torres-Pacheco, I., Eds.; Universidad Almería: Almería, Spain, 2022; pp. 181–288. ISBN 978-84-1351-153-5. [Google Scholar]
- Elnmer, A.; Khadr, M.; Tawfik, A. Using Remote Sensing Techniques for Estimating Water Stress Index for Central of Nile Delta. IOP Conf. Ser. Earth Environ. Sci. 2018, 151, 12026. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, L.; Lipan, L.; Andreu, L.; Martín-Palomo, M.J.; Carbonell-Barrachina, Á.A.; Hernández, F.; Sendra, E. Effect of Regulated Deficit Irrigation on the Quality of Raw and Table Olives. Agric. Water Manag. 2019, 221, 415–421. [Google Scholar] [CrossRef]
- Liu, N.; Deng, Z.; Wang, H.; Luo, Z.; Gutiérrez-Jurado, H.A.; He, X.; Guan, H. Thermal Remote Sensing of Plant Water Stress in Natural Ecosystems. For. Ecol. Manag. 2020, 476, 118433. [Google Scholar] [CrossRef]
- Pace, G.; Gutiérrez-Cánovas, C.; Henriques, R.; Boeing, F.; Cássio, F.; Pascoal, C. Remote Sensing Depicts Riparian Vegetation Responses to Water Stress in a Humid Atlantic Region. Sci. Total Environ. 2021, 772, 145526. [Google Scholar] [CrossRef]
- Rakshit, A.; Singh, H.; Singh, A.; Singh, U.; Fraceto, L. New Frontiers in Stress Management for Durable Agriculture; Springer: Singapore, 2020; ISBN 978-981-15-1322-0. [Google Scholar]
- Ghadirnezhad Shiade, S.R.; Fathi, A.; Taghavi Ghasemkheili, F.; Amiri, E.; Pessarakli, M. Plants’ Responses under Drought Stress Conditions: Effects of Strategic Management Approaches—A Review. J. Plant Nutr. 2023, 46, 2198–2230. [Google Scholar] [CrossRef]
- Hossain, M.A.; Kumar, V.; Burritt, D.; Fujita, M.; Mäkelä, P. Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants Recent Advances and Future Perspectives: Recent Advances and Future Perspectives; Spring: Cham, Switzerland, 2019; ISBN 978-3-030-27422-1. [Google Scholar]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and Drought Stresses in Crops and Approaches for Their Mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Busari, M.A.; Kukal, S.S.; Kaur, A.; Bhatt, R.; Dulazi, A.A. Conservation Tillage Impacts on Soil, Crop and the Environment. Int. Soil Water Conserv. Res. 2015, 3, 119–129. [Google Scholar] [CrossRef]
- Carter, M.R. Conservation Tillage. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Oxford, UK, 2005; pp. 306–311. ISBN 978-0-12-348530-4. [Google Scholar]
- Lobb, D.A. Soil Movement by Tillage and Other Agricultural Activities. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Academic Press: Oxford, UK, 2008; pp. 3295–3303. ISBN 978-0-08-045405-4. [Google Scholar]
- Álvaro-Fuentes, J.; López, M.V.; Cantero-Martinez, C.; Arrúe, J.L. Tillage Effects on Soil Organic Carbon Fractions in Mediterranean Dryland Agroecosystems. Soil Sci. Soc. Am. J. 2008, 72, 541–547. [Google Scholar] [CrossRef]
- Miransari, M. Soybean Tillage Stress; Academic Press: San Diego, CA, USA, 2016; ISBN 978-0-12-801535-3. [Google Scholar]
- Githongo, M.W.; Kiboi, M.N.; Ngetich, F.K.; Musafiri, C.M.; Muriuki, A.; Fliessbach, A. The Effect of Minimum Tillage and Animal Manure on Maize Yields and Soil Organic Carbon in Sub-Saharan Africa: A Meta-analysis. Environ. Chall. 2021, 5, 100340. [Google Scholar] [CrossRef]
- Nath, A.J.; Lal, R. Effects of Tillage Practices and Land Use Management on Soil Aggregates and Soil Organic Carbon in the North Appalachian Region, USA. Pedosphere 2017, 27, 172–176. [Google Scholar] [CrossRef]
- Zoz, T.; de Castro Seron, C.; da Silva Oliveira, C.E.; Dutra Zanotto, M.; Maior Bono, J.A.; Barreto Aguiar, E.; Wilson Witt, T. Growth of Dwarf Castor Hybrids at Different Soil Bulk Densities. Ind. Crops Prod. 2021, 159, 113069. [Google Scholar] [CrossRef]
- Melander, B.; Munier-Jolain, N.; Charles, R.; Wirth, J.; Schwarz, J.; van Der Weide, R.; Bonin, L.; Jensen, P.K.; Kudsk, P. European Perspectives on the Adoption of Nonchemical Weed Management in Reduced-Tillage Systems for Arable Crops. Weed Technol. 2013, 27, 231–240. [Google Scholar] [CrossRef]
- He, Y.; Lin, L.; Che, J. Maize Root Morphology Responses to Soil Penetration Resistance Related to Tillage and Drought in a Clayey Soil. J. Agric. Sci. 2017, 155, 1137–1149. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.K.; Halder, S.; Mondal, K.; Singh, K.C.; Nandi, R.; Ghosh, P.K. Response of Lentil (Lens culinaries) to Post-Rice Residual Soil Moisture Under Contrasting Tillage Practices. Agric. Res. 2018, 7, 463–479. [Google Scholar] [CrossRef]
- Madejón, P.; Fernández-Boy, E.; Morales-Salmerón, L.; Navarro-Fernández, C.M.; Madejón, E.; Domínguez, M.T. Could Conservation Tillage Increase the Resistance to Drought in Mediterranean Faba Bean Crops? Agric. Ecosyst. Environ. 2023, 349, 108449. [Google Scholar] [CrossRef]
- Frederick, J.; Camp, C.; Bauer, P. Drought-Stress Effects on Branch and Mainstem Seed Yield and Yield Components of Determinate Soybean. Crop Ecol. Prod. Manag. 2001, 41, 759–763. [Google Scholar] [CrossRef]
- de Moraes, M.T.; Debiasi, H.; Franchini, J.C.; de Andrade Bonetti, J.; Levien, R.; Schnepf, A.; Leitner, D. Mechanical and Hydric Stress Effects on Maize Root System Development at Different Soil Compaction Levels. Front. Plant Sci. 2019, 10, 1358. [Google Scholar] [CrossRef]
- Ali, M.A.; Ilyas, F.; Danish, S.; Mustafa, G.; Ahmed, N.; Hussain, S.; Arshad, M.; Ahmad, S. Soil Management and Tillage Practices for Growing Cotton Crop BT—Cotton Production and Uses: Agronomy, Crop Protection, and Postharvest Technologies. In Cotton Production and Uses; Ahmad, S., Hasanuzzaman, M., Eds.; Springer: Singapore, 2020; pp. 9–30. ISBN 978-981-15-1472-2. [Google Scholar]
- Yan, S.; Wu, Y.; Fan, J.; Zhang, F.; Paw, U.K.T.; Zheng, J.; Qiang, S.; Guo, J.; Zou, H.; Xiang, Y.; et al. A Sustainable Strategy of Managing Irrigation Based on Water Productivity and Residual Soil Nitrate in a No-Tillage Maize System. J. Clean. Prod. 2020, 262, 121279. [Google Scholar] [CrossRef]
- Zheng, C.; Yu, Z.; Shi, Y.; Cui, S.; Wang, D.; Zhang, Y.; Zhao, J. Effects of Tillage Practices on Water Consumption, Water Use Efficiency and Grain Yield in Wheat Field. J. Integr. Agric. 2014, 13, 2378–2388. [Google Scholar] [CrossRef]
- Boydston, R.; Porter, L.; Chaves Cordoba, B.; Khot, L.; Miklas, P. The Impact of Tillage on Pinto Bean Cultivar Response to Drought Induced by Deficit Irrigation. Soil Tillage Res. 2018, 180, 63–72. [Google Scholar] [CrossRef]
- Bhardwaj, R.L. Effect of Mulching on Crop Production under Rainfed Condition—A Review. Agric. Rev. 2013, 34, 188–197. [Google Scholar] [CrossRef]
- Behzadnejad, J.; Tahmasebi-Sarvestani, Z.; Aein, A.; Mokhtassi-Bidgoli, A. Wheat Straw Mulching Helps Improve Yield in Sesame (Sesamum indicum L.) Under Drought Stress. Int. J. Plant Prod. 2020, 14, 389–400. [Google Scholar] [CrossRef]
- Muharam Al-Bayati, H.J.; Hamdoon, D.N. Response of Eggplant Solanum melongena L. To Soil Mulching, Organic and Inorganic Fertilizers on Vegetative Growth Traits and Yield Grown under Unheated Plastic House. IOP Conf. Ser. Earth Environ. Sci. 2019, 388, 12075. [Google Scholar] [CrossRef]
- Asseng, S.; Zhu, Y.; Basso, B.; Wilson, T.; Cammarano, D. Simulation Modeling: Applications in Cropping Systems. Encycl. Agric. Food Syst. 2014, 5, 102–112. [Google Scholar] [CrossRef]
- Singh, L.; Beg, M.; Akther, S.; Qayoom, S.; Lone, B.; Singh, P.; Singh, P. Efficient Techniques to Increase Water Use Efficiency under Rainfed Eco-Systems. J. AgriSearch 2014, 1, 193–200. [Google Scholar]
- Zhang, F.-Y.; Wu, P.-T.; Zhao, X.-N.; Cheng, X.-F. Water-saving mechanisms of intercropping system in improving cropland water use efficiency. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol. 2012, 23, 1400–1406. [Google Scholar]
- Hu, F.; Feng, F.; Zhao, C.; Chai, Q.; Yu, A.; Yin, W.; Gan, Y. Integration of Wheat-Maize Intercropping with Conservation Practices Reduces CO2 Emissions and Enhances Water Use in Dry Areas. Soil Tillage Res. 2017, 169, 44–53. [Google Scholar] [CrossRef]
- Sharma, N.K.; Singh, R.J.; Mandal, D.; Kumar, A.; Alam, N.M.; Keesstra, S. Increasing Farmer’s Income and Reducing Soil Erosion Using Intercropping in Rainfed Maize-Wheat Rotation of Himalaya, India. Agric. Ecosyst. Environ. 2017, 247, 43–53. [Google Scholar] [CrossRef]
- Meißner, A.; Granzow, S.; Wemheuer, F.; Pfeiffer, B. The Cropping System Matters—Contrasting Responses of Winter Faba Bean (Vicia faba L.) Genotypes to Drought Stress. J. Plant Physiol. 2021, 263, 153463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; He, N.; Wang, J.; Siddique, K.H.M.; Gao, X.; Zhao, X. Plasticity of Root Traits in a Seedling Apple Intercropping System Driven by Drought Stress on the Loess Plateau of China. Plant Soil 2022, 480, 541–560. [Google Scholar] [CrossRef]
- Pourali, S.; Aghayari, F.; Ardakani, M.R.; Paknejad, F.; Golzardi, F. Benefits from Intercropped Forage Sorghum–Red Clover Under Drought Stress Conditions. Gesunde Pflanz. 2023, 75, 1769–1780. [Google Scholar] [CrossRef]
- Assadi, N.M.; Bijanzadeh, E. Influence of Relay Intercropping of Barley with Chickpea on Biochemical Characteristics and Yield under Water Stress. PLoS ONE 2023, 18, e0273272. [Google Scholar] [CrossRef]
- Li, S.-X.; Wang, Z.-H.; Malhi, S.S.; Li, S.-Q.; Gao, Y.-J.; Tian, X.-H. Nutrient and Water Management Effects on Crop Production, and Nutrient and Water Use Efficiency in Dryland Areas of China. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2009; Volume 102, pp. 223–265. ISBN 0065-2113. [Google Scholar]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Mirbahar, G.; Ahmad, F.; ud din, S. Nutrient Management in Cotton Under Water Stress Environments. In Proceedings of the The First International Conference on Science, Industry and Trade of Cotton, Gorgan, Iran, 2–4 October 2012. [Google Scholar]
- Mamnabi, S.; Nasrollahzadeh, S.; Ghassemi-Golezani, K.; Raei, Y. Improving Yield-Related Physiological Characteristics of Spring Rapeseed by Integrated Fertilizer Management under Water Deficit Conditions. Saudi J. Biol. Sci. 2020, 27, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Yang, H.; Duan, L.; Shi, J.; Guo, L. Potassium Fertilizer Improves Drought Stress Alleviation Potential in Sesame by Enhancing Photosynthesis and Hormonal Regulation. Plant Physiol. Biochem. 2023, 200, 107744. [Google Scholar] [CrossRef] [PubMed]
- Lipiec, J.; Doussan, C.; Nosalewicz, A.; Kondracka, K. Effect of Drought and Heat Stresses on Plant Growth and Yield: A Review. Int. Agrophysics 2013, 27, 463–477. [Google Scholar] [CrossRef]
- Pérez-López, D.; Memmi, H.; del Carmen Gijón-López, M.; Moreno, M.M.; Couceiro, J.F.; Centeno, A.; Martín-Palomo, M.J.; Corell, M.; Noguera-Artiaga, L.; Galindo, A.; et al. Irrigation of Pistachios: Strategies to Confront Water Scarcity. In Water Scarcity and Sustainable Agriculture in Semiarid Environment: Tools, Strategies, and Challenges for Woody Crops; García Tejero, I.F., Durán Zuazo, V.H., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 247–269. ISBN 978-0-12-813164-0. [Google Scholar]
- Abrisqueta, I.; Ayars, J.E. Effect of Alternative Irrigation Strategies on Yield and Quality of Fiesta Raisin Grapes Grown in California. Water 2018, 10, 583. [Google Scholar] [CrossRef]
- Chai, Q.; Gan, Y.; Turner, N.C.; Zhang, R.-Z.; Yang, C.; Niu, Y.; Siddique, K.H.M. Water-Saving Innovations in Chinese Agriculture. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 126, pp. 149–201. ISBN 0065-2113. [Google Scholar]
- Sharma, S.; Leskovar, D.; Crosby, K. Genotypic Differences in Leaf Gas Exchange and Growth Responses to Deficit Irrigation in Reticulatus and Inodorus Melons (Cucumis melo L.). Photosynthetica 2018, 57, 237–247. [Google Scholar] [CrossRef]
- Coyago-Cruz, E.; Meléndez-Martínez, A.J.; Moriana, A.; Girón, I.F.; Martín-Palomo, M.J.; Galindo, A.; Pérez-López, D.; Torrecillas, A.; Beltrán-Sinchiguano, E.; Corell, M. Yield Response to Regulated Deficit Irrigation of Greenhouse Cherry Tomatoes. Agric. Water Manag. 2019, 213, 212–221. [Google Scholar] [CrossRef]
- Khapte, P.; Kumar, P.; Burman, U.; Kumar, P. Deficit Irrigation in Tomato: Agronomical and Physio-Biochemical Implications. Sci. Hortic. 2019, 248, 256–264. [Google Scholar] [CrossRef]
- Vélez-Sánchez, J.E.; Balaguera-López, H.E.; Alvarez-Herrera, J.G. Effect of Regulated Deficit Irrigation (RDI) on the Production and Quality of Pear Triunfo de Viena Variety under Tropical Conditions. Sci. Hortic. 2021, 278, 109880. [Google Scholar] [CrossRef]
- Kovalenko, Y.; Tindjau, R.; Madilao, L.L.; Castellarin, S.D. Regulated Deficit Irrigation Strategies Affect the Terpene Accumulation in Gewürztraminer (Vitis vinifera L.) Grapes Grown in the Okanagan Valley. Food Chem. 2021, 341, 128172. [Google Scholar] [CrossRef]
- Durán, V.; Rodriguez, C.; Tarifa, D. Impact of Sustained-Deficit Irrigation on Tree Growth, Mineral Nutrition, Fruit Yield and Quality of Mango in Spain. Fruits 2011, 66, 257–268. [Google Scholar] [CrossRef]
- Mena, P.; Galindo, A.; Collado, J.; Ondoño, S.; Cristina, G.-V.; Ferreres, F.; Torrecillas, A. Sustained Deficit Irrigation Affects the Colour and Phytochemical Characteristics of Pomegranate Juice. J. Sci. Food Agric. 2013, 93, 1922–1927. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Gan, Y.; Zhao, C.; Xu, H.-L.; Waskom, R.M.; Niu, Y.; Siddique, K.H.M. Regulated Deficit Irrigation for Crop Production under Drought Stress. A Review. Agron. Sustain. Dev. 2015, 36, 3. [Google Scholar] [CrossRef]
- Schaible, G.D.; Aillery, M.P. Challenges for US Irrigated Agriculture in the Face of Emerging Demands and Climate Change. In Competition for Water Resources. Experiences and Management Approaches in the US and Europe; Ziolkowska, J.R., Peterson, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 44–79. ISBN 978-0-12-803237-4. [Google Scholar]
- Ben-Gal, A.; Ron, Y.; Yermiyahu, U.; Zipori, I.; Naoum, S.; Dag, A. Evaluation of Regulated Deficit Irrigation Strategies for Oil Olives: A Case Study for Two Modern Israeli Cultivars. Agric. Water Manag. 2021, 245, 106577. [Google Scholar] [CrossRef]
- Acevedo-Opazo, C.; Ortega-Farias, S.; Fuentes, S. Effects of Grapevine (Vitis vinifera L.) Water Status on Water Consumption, Vegetative Growth and Grape Quality: An Irrigation Scheduling Application to Achieve Regulated Deficit Irrigation. Agric. Water Manag. 2010, 97, 956–964. [Google Scholar] [CrossRef]
- Jackson, R. Viticulture. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–14. ISBN 978-0-08-100596-5. [Google Scholar]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and Drought Stresses in Crop Plants: Implications, Cross Talk, and Potential Management Opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones Enhanced Drought Tolerance in Plants: A Coping Strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef] [PubMed]
- Chhaya; Yadav, B.; Jogawat, A.; Gnanasekaran, P.; Kumari, P.; Lakra, N.; Lal, S.K.; Pawar, J.; Narayan, O.P. An Overview of Recent Advancement in Phytohormones-Mediated Stress Management and Drought Tolerance in Crop Plants. Plant Gene 2020, 25, 100264. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones Regulate Accumulation of Osmolytes under Abiotic Stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A. Phytohormones and Beneficial Microbes: Essential Components for Plants to Balance Stress and Fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Ramachandran, M.; Arulbalachandran, D.; Dilipan, E.; Ramya, S. Comparative Analysis of Abscisic Acid Recovery on Two Varieties of Rice (Oryza sativa L.) under Drought Condition. Biocatal. Agric. Biotechnol. 2021, 33, 102006. [Google Scholar] [CrossRef]
- Sharma, L.; Dalal, M.; Verma, R.K.; Kumar, S.V.V.; Yadav, S.K.; Pushkar, S.; Kushwaha, S.R.; Bhowmik, A.; Chinnusamy, V. Auxin Protects Spikelet Fertility and Grain Yield under Drought and Heat Stresses in Rice. Environ. Exp. Bot. 2018, 150, 9–24. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Ahmed, N.; Zhang, Y.; Li, K.; Zhou, Y.; Zhang, M.; Li, Z. Exogenous Application of Glycine Betaine Improved Water Use Efficiency in Winter Wheat (Triticum aestivum L.) via Modulating Photosynthetic Efficiency and Antioxidative Capacity under Conventional and Limited Irrigation Conditions. Crop J. 2019, 7, 635–650. [Google Scholar] [CrossRef]
- Ibrahim, H.A.; Abdellatif, Y.M.R. Effect of Maltose and Trehalose on Growth, Yield and Some Biochemical Components of Wheat Plant under Water Stress. Ann. Agric. Sci. 2016, 61, 267–274. [Google Scholar] [CrossRef]
- Ali, Q.; Ashraf, M. Induction of Drought Tolerance in Maize (Zea mays L.) Due to Exogenous Application of Trehalose: Growth, Photosynthesis, Water Relations and Oxidative Defence Mechanism. J. Agron. Crop Sci. 2011, 197, 258–271. [Google Scholar] [CrossRef]
- Kathuria, H.; Giri, J.; Nataraja, K.N.; Murata, N.; Udayakumar, M.; Tyagi, A.K. Glycinebetaine-Induced Water-Stress Tolerance in CodA-Expressing Transgenic Indica Rice Is Associated with up-Regulation of Several Stress Responsive Genes. Plant Biotechnol. J. 2009, 7, 512–526. [Google Scholar] [CrossRef]
- Sharma, S.; Chen, C.; Khatri, K.; Rathore, M.S.; Pandey, S.P. Gracilaria Dura Extract Confers Drought Tolerance in Wheat by Modulating Abscisic Acid Homeostasis. Plant Physiol. Biochem. 2019, 136, 143–154. [Google Scholar] [CrossRef]
- Pourghasemian, N.; Moradi, R.; Naghizadeh, M.; Landberg, T. Mitigating Drought Stress in Sesame by Foliar Application of Salicylic Acid, Beeswax Waste and Licorice Extract. Agric. Water Manag. 2020, 231, 105997. [Google Scholar] [CrossRef]
- Du, C.; Li, L.; Xie, J.; Effah, Z.; Luo, Z.; Wang, L. Long-Term Conservation Tillage Increases Yield and Water Use Efficiency of Spring Wheat (Triticum aestivum L.) by Regulating Substances Related to Stress on the Semi-Arid Loess Plateau of China. Agronomy 2023, 13, 1301. [Google Scholar] [CrossRef]
- de Vries, D.R.B. Rootstock. In Rootstock Breeding; Roberts, A.V., Ed.; Elsevier: Oxford, UK, 2003; pp. 639–645. ISBN 978-0-12-227620-0. [Google Scholar]
- Gudin, S. Overview. In Breeding; Roberts, A.V., Ed.; Elsevier: Oxford, UK, 2003; pp. 25–30. ISBN 978-0-12-227620-0. [Google Scholar]
- Orton, T.J. Mass Selection and the Basic Plant Breeding Algorithm. In Horticultural Plant Breeding; Orton, T.J., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 85–95. ISBN 978-0-12-815396-3. [Google Scholar]
- Ranjith, P.; Rao, M. Breeding for Drought Resistance. In Plant Breeding—Current and Future Views; IntechOpen: Rijeka, Croatia, 2021; ISBN 978-1-83968-309-1. [Google Scholar]
- Putnik-Delic, M.; Maksimovic, I.; Nagl, N.; Lalic, B. Sugar Beet Tolerance to Drought: Physiological and Molecular Aspects. In Plant, Abiotic Stress and Responses to Climate Change; IntechOpen: Rijeka, Croatia, 2018; ISBN 978-1-78923-122-9. [Google Scholar]
- Rosero, A.; Granda, L.; Berdugo-Cely, J.A.; Šamajová, O.; Šamaj, J.; Cerkal, R. A Dual Strategy of Breeding for Drought Tolerance and Introducing Drought-Tolerant, Underutilized Crops into Production Systems to Enhance Their Resilience to Water Deficiency. Plants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Liu, R.; Wu, F.; Yi, X.; Lin, Y.; Wang, Z.; Liu, S.; Deng, M.; Ma, J.; Wei, Y.; Zheng, Y.; et al. Quantitative Trait Loci Analysis for Root Traits in Synthetic Hexaploid Wheat under Drought Stress Conditions. J. Integr. Agric. 2020, 19, 1947–1960. [Google Scholar] [CrossRef]
- Miles, C.; Wayne, M. Quantitative Trait Locus (QTL) Analysis. Nat. Educ. 2008, 1, 208. [Google Scholar]
- Cattivell, L.; Baldi, P.; Crosatti, C.; Di Fonzo, N.; Faccioli, P.; Grossi, M.; Mastrangelo, A.M.; Pecchioni, N.; Stanca, A.M. Chromosome Regions and Stress-Related Sequences Involved in Resistance to Abiotic Stress in Triticeae. Plant Mol. Biol. 2002, 48, 649–665. [Google Scholar] [CrossRef]
- Shahzad, A.; Qian, M.; Sun, B.; Mahmood, U.; Li, S.; Fan, Y.; Chang, W.; Dai, L.; Zhu, H.; Li, J.; et al. Genome-Wide Association Study Identifies Novel Loci and Candidate Genes for Drought Stress Tolerance in Rapeseed. Oil Crop Sci. 2021, 6, 12–22. [Google Scholar] [CrossRef]
- Mkhabela, S.S.; Shimelis, H.; Gerrano, A.S.; Mashilo, J. Phenotypic and Genotypic Divergence in Okra [Abelmoschus esculentus (L.) Moench] and Implications for Drought Tolerance Breeding: A Review. S. Afr. J. Bot. 2021, 145, 56–64. [Google Scholar] [CrossRef]
- Cattivelli, L.; Rizza, F.; Badeck, F.-W.; Mazzucotelli, E.; Mastrangelo, A.M.; Francia, E.; Marè, C.; Tondelli, A.; Stanca, A.M. Drought Tolerance Improvement in Crop Plants: An Integrated View from Breeding to Genomics. Field Crop. Res. 2008, 105, 1–14. [Google Scholar] [CrossRef]
- Devi, E.L.; Devi, C.P.; Kumar, S.; Sharma, S.K.; Beemrote, A.; Chongtham, S.K.; Singh, C.H.; Tania, C.; Singh, T.B.; Ningombam, A.; et al. Marker Assisted Selection (MAS) towards Generating Stress Tolerant Crop Plants. Plant Gene 2017, 11, 205–218. [Google Scholar] [CrossRef]
- Berni, R.; Thiry, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Eustress and Plants: A Synthesis with Prospects for Cannabis Sativa Cultivation. Horticulturae 2024, 10, 127. [Google Scholar] [CrossRef]
- Romero-Galindo, R.; Hernandez-Aguilar, C.; Dominguez-Pacheco, A.; Godina-Nava, J.J.; Ivanov Tsonchev, R. Biophysical Methods Used to Generate Tolerance to Drought Stress in Seeds and Plants: A Review. Int. Agrophysics 2021, 35, 389–410. [Google Scholar] [CrossRef]
- Shoaib, N.; Pan, K.; Mughal, N.; Raza, A.; Liu, L.; Zhang, J.; Wu, X.; Sun, X.; Zhang, L.; Pan, Z. Potential of UV-B Radiation in Drought Stress Resilience: A Multidimensional Approach to Plant Adaptation and Future Implications. Plant. Cell Environ. 2024, 47, 387–407. [Google Scholar] [CrossRef]
- Luo, D.; Li, J.; Luo, J.; Ma, Y.; Wang, Y.; Liu, W.; Rodriguez, L.G.; Yao, Y. Responses to Solar UV-B Exclusion and Drought Stress in Two Cultivars of Chestnut Rose with Different Leaf Thickness. Forests 2023, 14, 50. [Google Scholar] [CrossRef]
- Veselá, B.; Holub, P.; Urban, O.; Surá, K.; Hodaňová, P.; Oravec, M.; Divinová, R.; Jansen, M.A.K.; Klem, K. UV Radiation and Drought Interact Differently in Grass and Forb Species of a Mountain Grassland. Plant Sci. 2022, 325, 111488. [Google Scholar] [CrossRef] [PubMed]
- Mshenskaya, N.S.; Grinberg, M.A.; Kalyasova, E.A.; Vodeneev, V.A.; Ilin, N.V.; Slyunyaev, N.N.; Mareev, E.A.; Sinitsyna, Y.V. The Effect of an Extremely Low-Frequency Electromagnetic Field on the Drought Sensitivity of Wheat Plants. Plants 2023, 12, 826. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R. Magnetic Field Regulates Plant Functions, Growth and Enhances Tolerance against Environmental Stresses. Physiol. Mol. Biol. Plants 2019, 25, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Yakupoğlu, G. Effects of Magnetic Field and Ultrasound Applications on Endogenous Melatonin Content and Drought Stress Tolerance of Pepper Seedlings. Horticulturae 2023, 9, 704. [Google Scholar] [CrossRef]
- Aslam, H.; Ahmad, M.S.; Alvi, A.K.; Rani, W.; Athar, H.-R.; Al-Ashkar, I.; Almutairi, K.F.; Ullah, N.; Ayman, E.-S. He–Ne Laser Priming Enhances Drought Tolerance in Wheat through Differential Modification of Photosynthetic Pigments and Antioxidative Enzymes. Agronomy 2022, 12, 2376. [Google Scholar] [CrossRef]
- Qiu, Z.; Yuan, M.; He, Y.; Li, Y.; Zhang, L. Physiological and Transcriptome Analysis of He-Ne Laser Pretreated Wheat Seedlings in Response to Drought Stress. Sci. Rep. 2017, 7, 6108. [Google Scholar] [CrossRef]
- Mahmood, S.; Afzal, B.; Perveen, S.; Wahid, A.; Azeem, M.; Iqbal, N. He-Ne Laser Seed Treatment Improves the Nutraceutical Metabolic Pool of Sunflowers and Provides Better Tolerance Against Water Deficit. Front. Plant Sci. 2021, 12, 579429. [Google Scholar] [CrossRef]
- Katiyar, P.; Pandey, N.; Keshavkant, S. Gamma Radiation: A Potential Tool for Abiotic Stress Mitigation and Management of Agroecosystem. Plant Stress 2022, 5, 100089. [Google Scholar] [CrossRef]
- Suhesti, S.; Khumaida, N.; Wattimena, G.A.; Syukur, M.; Husni, A.; Hadipoentyanti, E. Gamma Irradiation and In Vitro Selection Could Increase Drought Tolerance in Sugarcane. Int. J. Sci. Basic Appl. Res. 2015, 23, 370–380. [Google Scholar]
- El-Sallami, I.H.; Abdul-Hafeez, E.Y.; Mostafa, G.G.; Gad, M.S. Enhancement of Drought Tolerance in Salvia Coccinea Plants by Irradiation with Gamma and Laser Pre-Treatments. Assiut J. Agric. Sci. 2019, 50, 68–92. [Google Scholar] [CrossRef]
- Suharjo, U.K.J.; Pamekas, T. Improving Potato Crop Tolerance to Drought Stress at Medium Elevation by Gamma Rays Irradiation. In Proceedings of the 3rd International Conference of Bio-Based Economy for Application and Utility, Padang, Indonesia, 10 November 2021; AIP Conference Proceedings. AIP Publishing: Melville, NY, USA, 2023; Volume 2730. [Google Scholar]
- Fernandez-Jaramillo, A.A.; Duarte-Galvan, C.; Garcia-Mier, L.; Jimenez-Garcia, S.N.; Contreras-Medina, L.M. Effects of Acoustic Waves on Plants: An Agricultural, Ecological, Molecular and Biochemical Perspective. Sci. Hortic. 2018, 235, 340–348. [Google Scholar] [CrossRef]
- López-Ribera, I.; Vicient, C.M. Drought Tolerance Induced by Sound in Arabidopsis Plants. Plant Signal. Behav. 2017, 2324, e1368938. [Google Scholar] [CrossRef] [PubMed]
- Caicedo-Lopez, L.H.; Guevara-Gonzalez, R.G.; Andrade, J.E.; Esquivel-Delgado, A.; Perez-Matzumoto, A.E.; Torres-Pacheco, I.; Contreras-Medina, L.M. Effect of Hydric Stress-Related Acoustic Emission on Transcriptional and Biochemical Changes Associated with a Water Deficit in Capsicum annuum L. Plant Physiol. Biochem. 2021, 165, 251–264. [Google Scholar] [CrossRef]
- Caicedo-Lopez, L.H.; Contreras-Medina, L.M.; Guevara-Gonzalez, R.G.; Perez-Matzumoto, A.E.; Ruiz-Rueda, A. Effects of Hydric Stress on Vibrational Frequency Patterns of Capsicum annuum Plants. Plant Signal. Behav. 2020, 15, 1770489. [Google Scholar] [CrossRef]
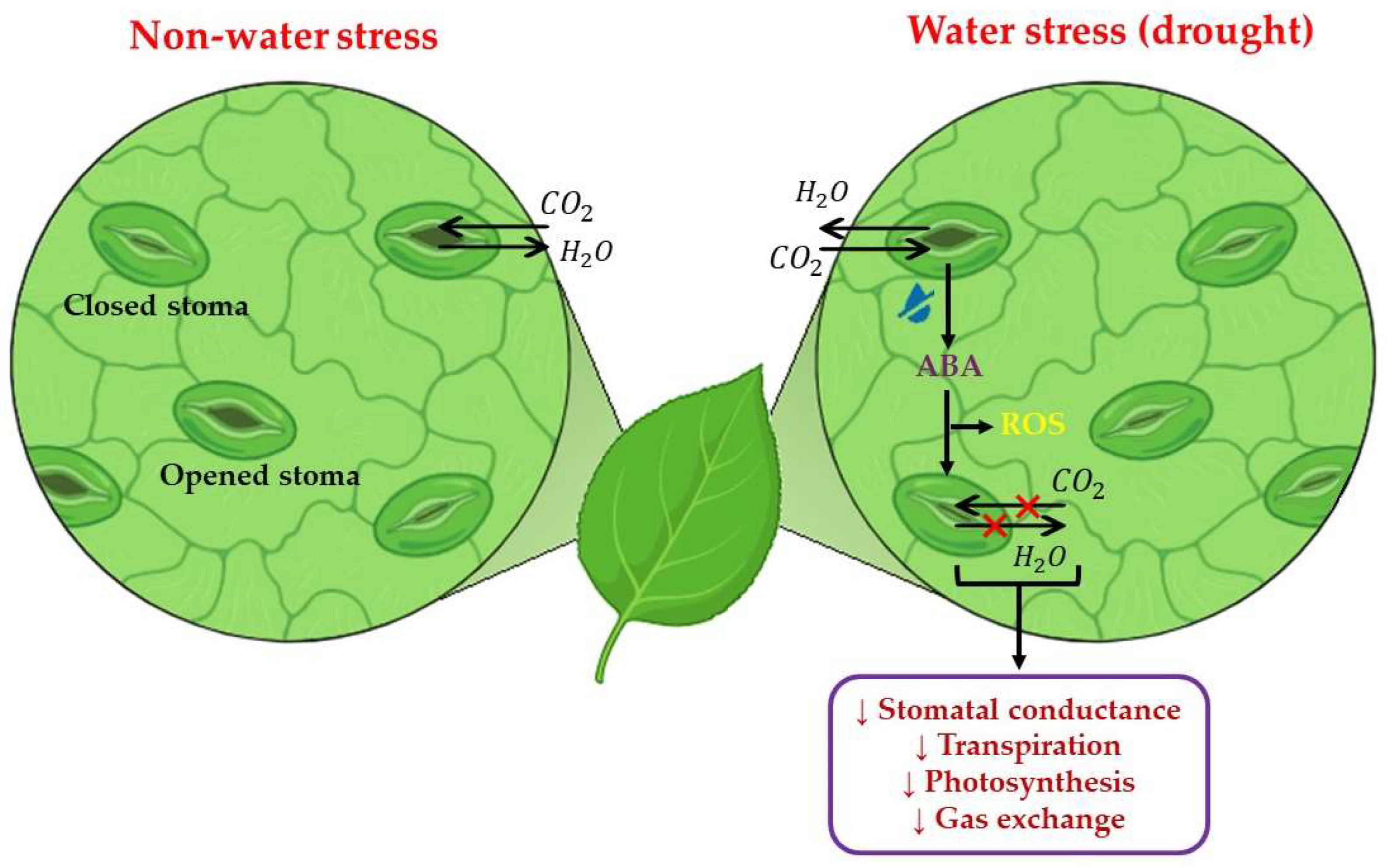

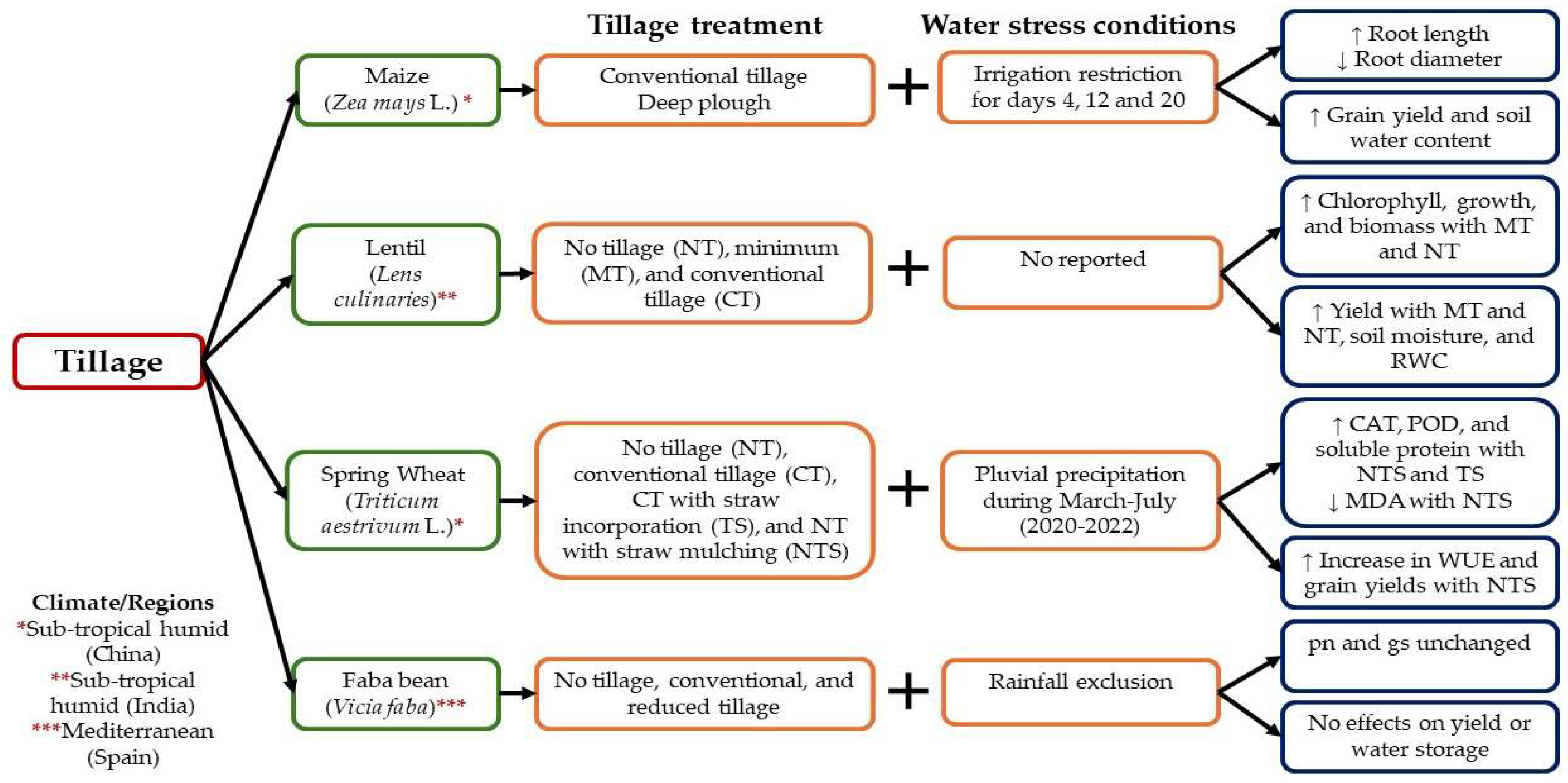
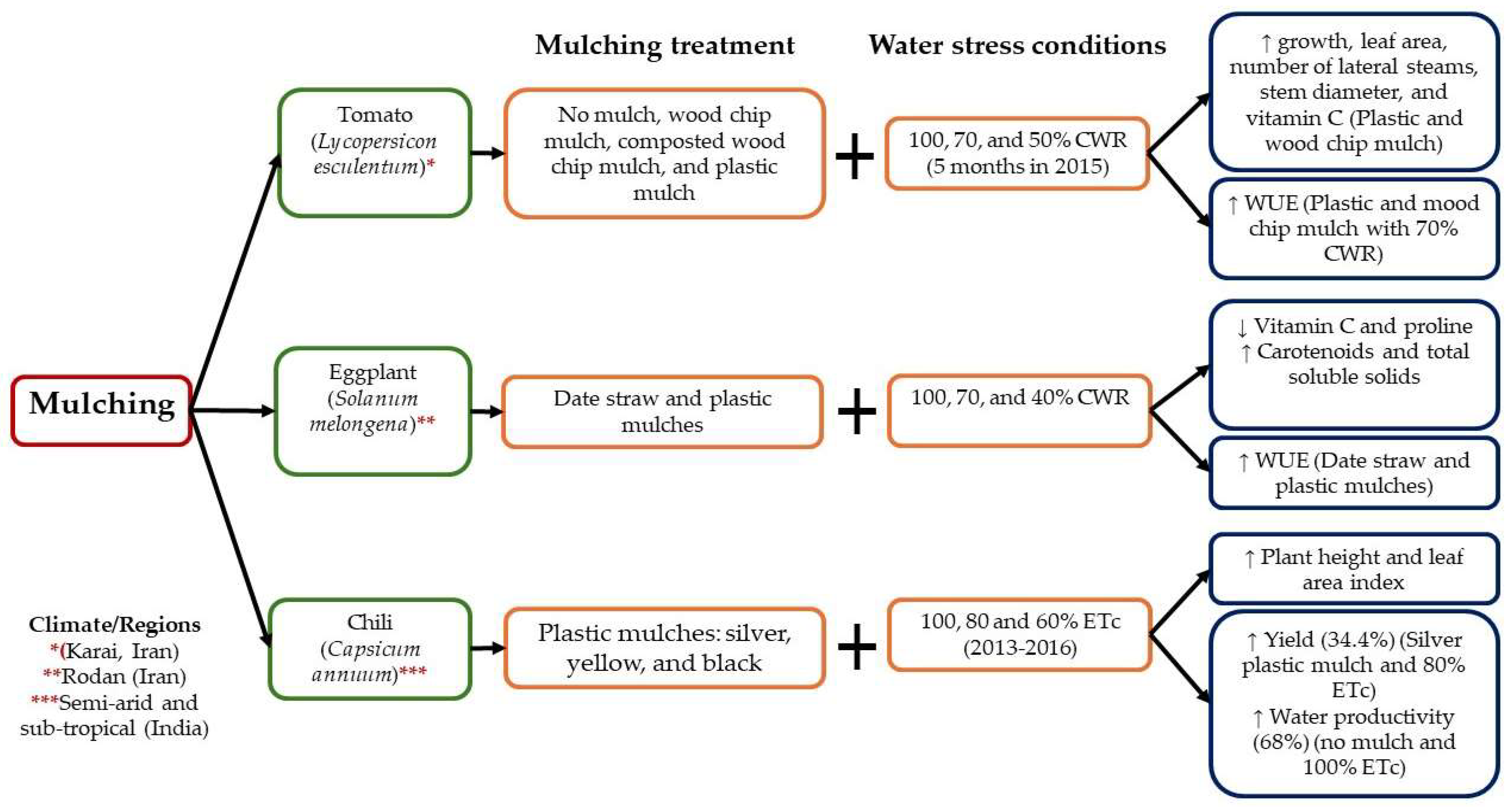

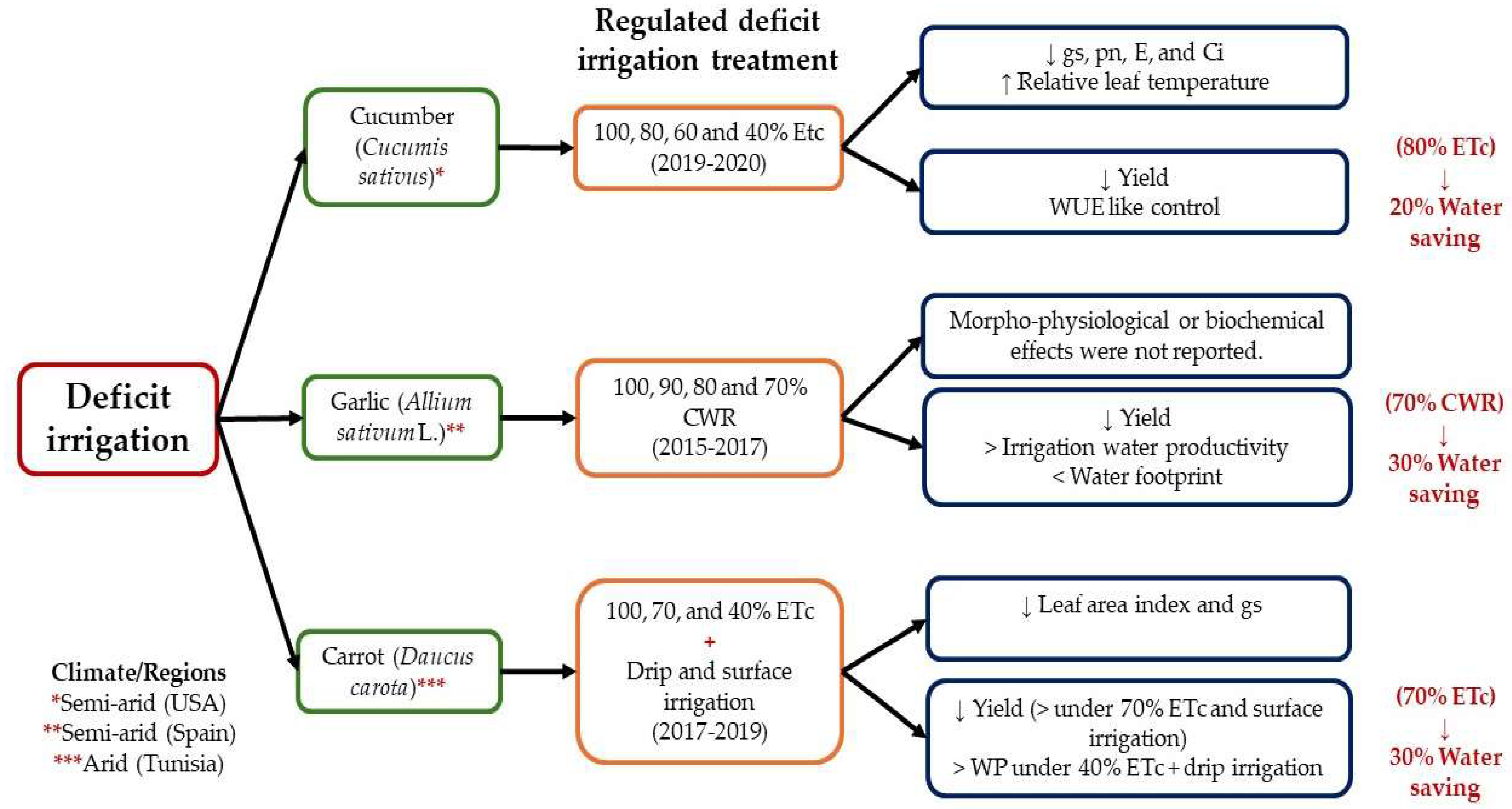

| Vegetable Crop | Water Stress Condition | Phenological Stage of Application of Water Stress | Frequency of Measurement of Responses to Water Stress | Plant Responses | References |
|---|---|---|---|---|---|
| Tomato (Lycopersicum Esculentum L.) | 100, 75, and 50% FC. | Growing period | N.R. | ↓ pn, gs, and CRV. | [83] |
| Cucumber (Cucumis sativus) | 100, 80, 60, 40% ETc. | Complete cycle (2019–2020) | 36, 48, 62, and 76 DATs (2019), and 30, 46, 58, and 71 DATs (2020). | ↓ gs, E, Pn, Ci, and leaf area. ↑ stomatal limitations, WUE, and leaf temperature. Significant effects at 40–60% ETc. | [25] |
| Chili (Capsicum annuum L.) | Irrigation suppression (17 d). recovery irrigation (5 d). | From 13 DAS to fruit setting (before reaching total fruit weight potential) on the 30th day | Daily—36 DASs | ↓ Tr and VWC; ↑ WUE. | [84] |
| Leafy vegetables (Amaranthus cruentus L., Corchorus olitorius L., Vigna unguiculata L. Walp., Beta vulgaris L.) | 30, 60, and 100% ETc. | Growth stage | Once per season (2015/2016 and 2016/2017) | ↓ Chlorophyll content index, significant effects at 30% ETc. | [53] |
| Cabbage (Brassica oleracea) | 100, 80, and 60% FC (irrigation every 3rd day). | Growth stage | Once—last week before the harvest | ↓ Chlorophyll, SPAD, LRWC, gs, Ci, and Tr. | [85] |
| Carrot (Daucus carota L.) | 100 and 50% FC (5 weeks). | 4 weeks after germination | N.R. | ↓ Total chlorophyll | [86] |
| Vegetable Crop | Water Stress Condition | Phenological Stage of Application of Water Stress | Frequency of Measurement of Responses to Water Stress | Plant Responses | References |
|---|---|---|---|---|---|
| Hot pepper (Pusajuala and Ghotki) and bell pepper (Green Wonder and PPE-311) genotypes | 35 and 65% FC. | Early floral bud stage and pod formation stage | Once—fruits harvested 45 d after flowering | ↑ Proline ↑ CAT, APX, and GXP activity. ↓ capsaicin. | [105] |
| Cucumber (Solanum muricatum) | Irrigation: 300 mL (control), 100 mL (moderate water stress) 3 times/week; Without irrigation (severe water stress) for 19 days. | 3 weeks after plants reached the phenological stage. (9 ≤ leaves on the main unfurled shoot) | Once, at the end of treatments | ↑ Proline, Na+, and K+ ions; ↓ photosynthetic pigments, flavonoids, and MDA. | [99] |
| Lettuce (Lactuca sativa L.) | 100, 90, and 80% of soil saturation, and water restriction. | The water supply was stopped 4 d before the harvest | During storage time (days 0, 3, and 7) | ↑ Total phenols, total flavonoids, and antioxidant activity at 80% of soil saturation; ↓ carotenoids and total phenolic compounds at 90% of soil saturation. | [106] |
| Chili pepper (Capsicum annuum L.) | 100 and 40% FC, for one week. | Seedling (one week’s growth) | Once, at the end of treatments | ↑ Phenolic content; ↑ PAL and POD activities; ↓ CAT activity. | [107] |
| Cabbage (Brassica oleracea var. capitata cv. Yalova 1) | 100, 80 and 60% FC. | Growth period | Once, one week before the harvest | ↑ Proline, sucrose, MDA, and H2O2 with drought severity; ↓ SOD, CAT, and POD. | [85] |
| Carrot (Daucus carota L.) | 100 and 50% FC. | After 4 weeks of germination, for 5 weeks | Not reported | ↑ Proline, glycine betaine, H2O2, ascorbic acid, total phenols, total soluble proteins, and MDA; ↑ CAT, SOD, POD. | [86] |
| Improved Drought Response | Management Strategy | Plant/References |
|---|---|---|
| Morphological traits (root length and diameter, leaf area, plant height, stem diameter) | Tillage | Maize [157] Lentil [158] Tomato [14,27] Chili [16] Lettuce [15] Broccoli [17] Potato [20,241] |
| Mulching | ||
| Intercropping | ||
| Nutrient handling | ||
| Exogenous hormones and osmoprotectants | ||
| Gamma rays | ||
| Chlorophyll | Tillage | Lentil [158] Eggplant [18] Wheat [235] |
| Exogenous osmoprotectants | ||
| He-Ne laser | ||
| Osmolytes (proline, glycine betaine) | Exogenous hormones and osmoprotectants | Chili [21] Eggplant [18] Chestnut rose [230] Sunflower [237] Jerico flower [240] |
| UV-B | ||
| He-Ne laser | ||
| Gamma rays | ||
| Antioxidant enzymes (CAT, POD, SOD, APX) | Tillage | Wheat [213] Potato [20] Eggplant [18] Chestnut rose [230] Chili [232] Sunflower [237] Jerico flower [240] |
| Nutrient handling | ||
| UV-B | ||
| Magnetic fields | ||
| Gamma rays | ||
| He-Ne laser | ||
| Exogenous hormones | ||
| Antioxidant compounds (ascorbic acid, carotenoids, anthocyanins, phenolic compounds, capsaicin) | Mulching | Tomato [14] Eggplant [18,23] Chestnut rose [230] Wheat [235] Sunflower [237] Chili [245] |
| Exogenous hormones | ||
| UV-B | ||
| He-Ne laser | ||
| Sound vibrations | ||
| Secondary metabolites (MDA, soluble sugars, and protein) | Mulching | Eggplant [23] Chili [21] Wheat [236] |
| Exogenous hormones | ||
| He-Ne laser | ||
| Up or downregulation genes, and expression of genes associated with drought stress | He-Ne laser | Wheat [236] Arabidopsis [243] Chili [245] |
| Sound vibrations | ||
| Biomass and yield increase | Tillage | Maize [157] Lentil [158] Lettuce [15] Chestnut rose [230] Sunflower [237] Wheat [236] Chili [16] Jerico flower [240] |
| Intercropping | ||
| UV-B | ||
| He-Ne laser | ||
| Mulching |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo-Sabogal, D.V.; Contreras-Medina, L.M. Elicitors and Biostimulants to Mitigate Water Stress in Vegetables. Horticulturae 2024, 10, 837. https://doi.org/10.3390/horticulturae10080837
Melo-Sabogal DV, Contreras-Medina LM. Elicitors and Biostimulants to Mitigate Water Stress in Vegetables. Horticulturae. 2024; 10(8):837. https://doi.org/10.3390/horticulturae10080837
Chicago/Turabian StyleMelo-Sabogal, Diana Victoria, and Luis Miguel Contreras-Medina. 2024. "Elicitors and Biostimulants to Mitigate Water Stress in Vegetables" Horticulturae 10, no. 8: 837. https://doi.org/10.3390/horticulturae10080837








