Strategies to Delay Ethylene-Mediated Ripening in Climacteric Fruits: Implications for Shelf Life Extension and Postharvest Quality
Abstract
:1. Introduction
1.1. Ethylene: A Ripening Plant Hormone
- Observing the effect of different ethylene removal methods on as many foods as possible;
- Discovering to what extent food quality is maintained through these methods;
- Establishing which of them are truly applicable on an industrial scale and refining these methods to make them more effective.
1.2. Scope and Structure of This Review
- Climacteric fruits and ethylene: This section will provide a comprehensive overview of climacteric fruits and their characteristic ripening process. It will define climacteric fruits, delineate the stages of their ripening process, and discuss the physiological changes associated with ripening. Additionally, it will highlight the pivotal role of ethylene as a master regulator of climacteric fruit ripening, emphasizing its multifaceted functions and importance in fruit development and maturation.
- Regulation of ethylene biosynthesis and signalling: This section will delve into the molecular mechanisms underlying ethylene biosynthesis, perception, and signal transduction in climacteric fruits. It will explore the regulatory pathways governing ethylene production, receptor-mediated signalling cascades, and downstream responses in fruit tissues. Additionally, it will discuss the environmental and hormonal factors that modulate ethylene biosynthesis and signalling pathways, providing insights into the complexity of ethylene regulation during fruit ripening.
- Physiological and molecular effects of ethylene on fruit ripening: This section will examine the specific molecular and physiological effects of ethylene on climacteric fruit ripening processes. It will elucidate how ethylene influences key ripening-related events, such as fruit softening, flavour development, colour changes, and aroma production. By integrating molecular biology, biochemistry, and physiology, this section will offer a comprehensive understanding of ethylene-mediated ripening processes at the cellular and tissue levels.
- Ethylene managing strategies: This section will be destined to go deep in different strategies to remove, inhibit, or reduce the effect that ethylene could have according to its interaction with climacteric and non-climacteric fruits.
- Practical implications and future directions: This final section will discuss the practical implications of ethylene biology for agricultural practices, postharvest management, and fruit quality enhancement. It will highlight potential strategies for manipulating ethylene levels, optimizing postharvest handling techniques, and improving fruit quality and shelf life. Additionally, it will identify emerging research trends and future directions in ethylene research, pointing towards new opportunities for innovation and advancement in this field.
2. Climacteric Fruits and Ethylene
- Pre-climacteric phase: At the beginning of fruit development, climacteric fruits are in the pre-climacteric phase. During this stage, ethylene production and respiration rates are relatively low. The fruits are typically firm, green, and physiologically immature. Although metabolic processes are occurring, they are not yet at levels indicative of ripening.
- Climacteric peak: As fruits reach maturity, they undergo a dramatic increase in ethylene biosynthesis and respiration, marking the climacteric peak. This peak is a pivotal event in the ripening process and triggers a cascade of biochemical and physiological changes. One of the most notable transformations is the conversion of starches into sugars, leading to increased sweetness. Additionally, the fruit softens as cell wall components break down, resulting in changes in texture and juiciness. Other changes include alterations in pigmentation, aroma development, and flavour enhancement.
- Climacteric phase: Following the climacteric peak, fruits enter the climacteric phase, characterized by sustained ethylene production and ongoing metabolic activity. Ripening processes initiated during the peak continue, albeit at a slower pace. This phase is crucial for the completion of ripening, as fruits continue to develop desirable sensory attributes and undergo structural modifications indicative of ripeness.
- Post-climacteric phase: Eventually, climacteric fruits enter the post-climacteric phase, marked by a decline in ethylene production and respiration rates. While fruits remain physiologically ripe during this phase, they may exhibit signs of senescence, such as loss in firmness, increased susceptibility to decay, and decline in sensory quality.
- System I of ethylene production is associated with vegetative tissues and fruits in the early stages of development and is characterized by low rates of ethylene production, auto-inhibitory character (a process by which ethylene induces and controls its own production), and absence of relevant peaks in the production of this phytohormone [29,30].
- On the other hand, system II is present in more advanced development processes, especially in climacteric fruits, and is characterized by high rates of ethylene emission, feedback (higher ethylene concentration in the environment implies higher production of the same), and a high peak of ethylene production at the onset of physiological maturity [29].

3. Regulation of Ethylene Biosynthesis and Signalling
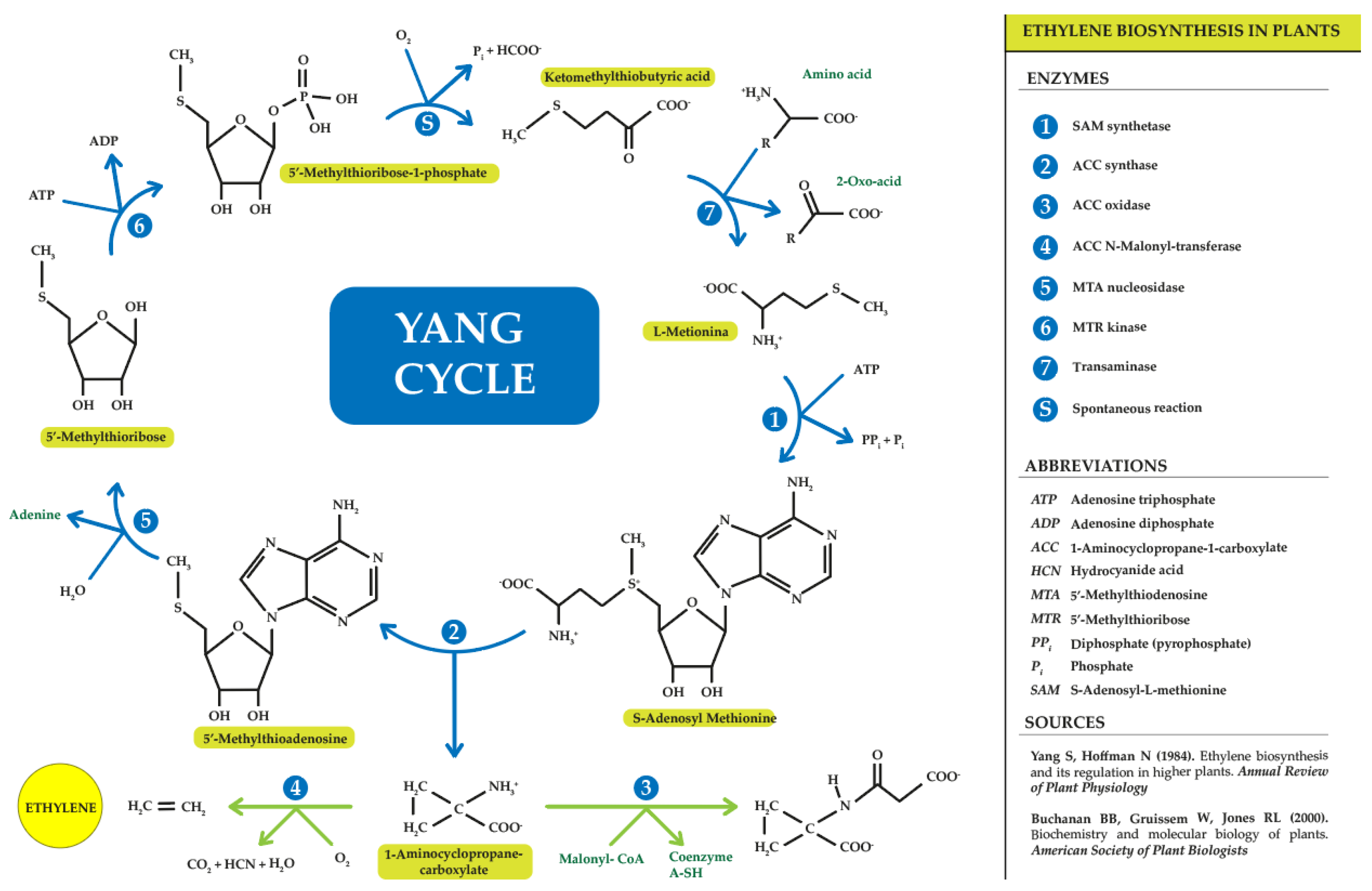
3.1. Biosynthesis of Ethylene in Climacteric Fruits and the Enzymes Involved
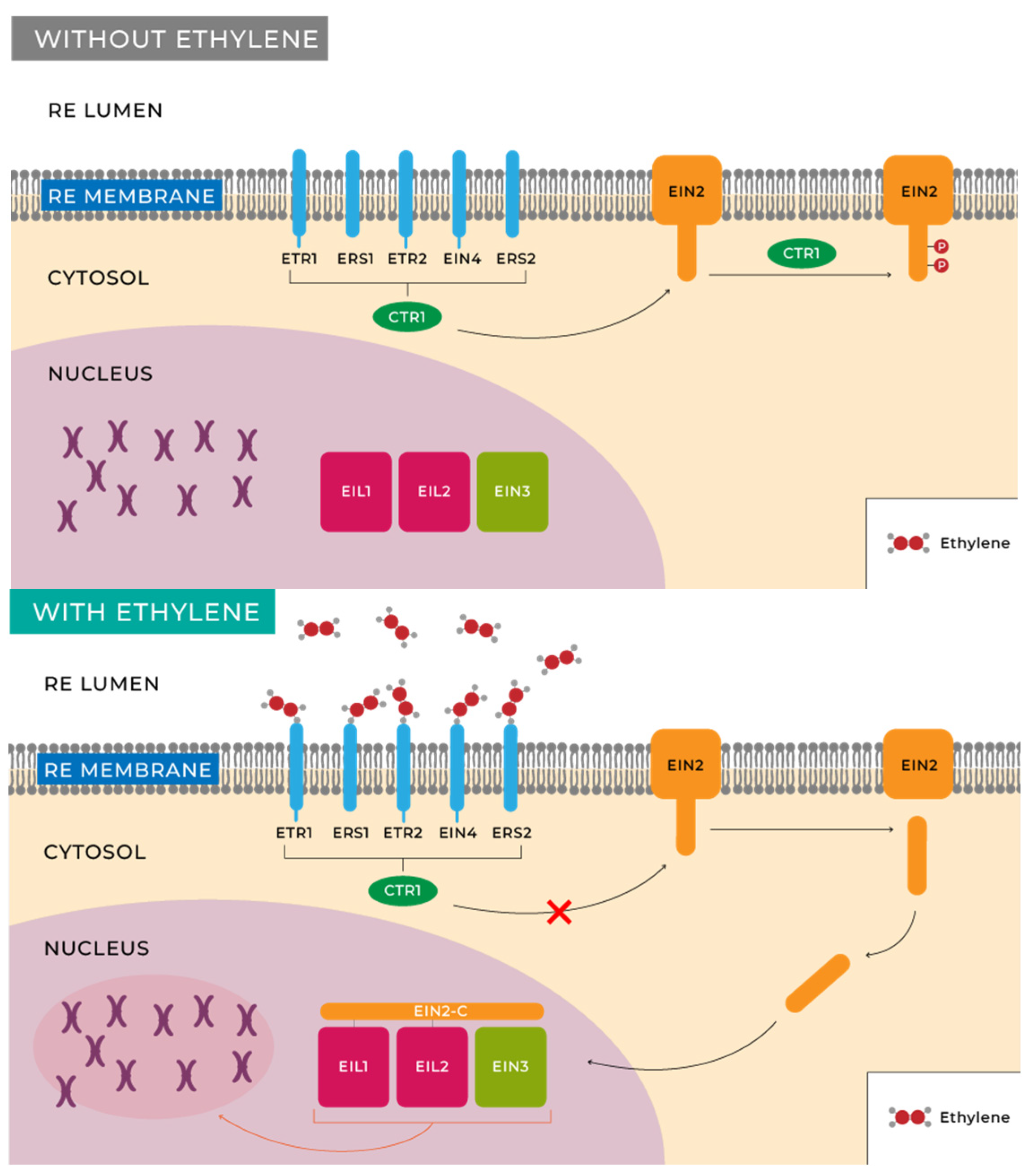
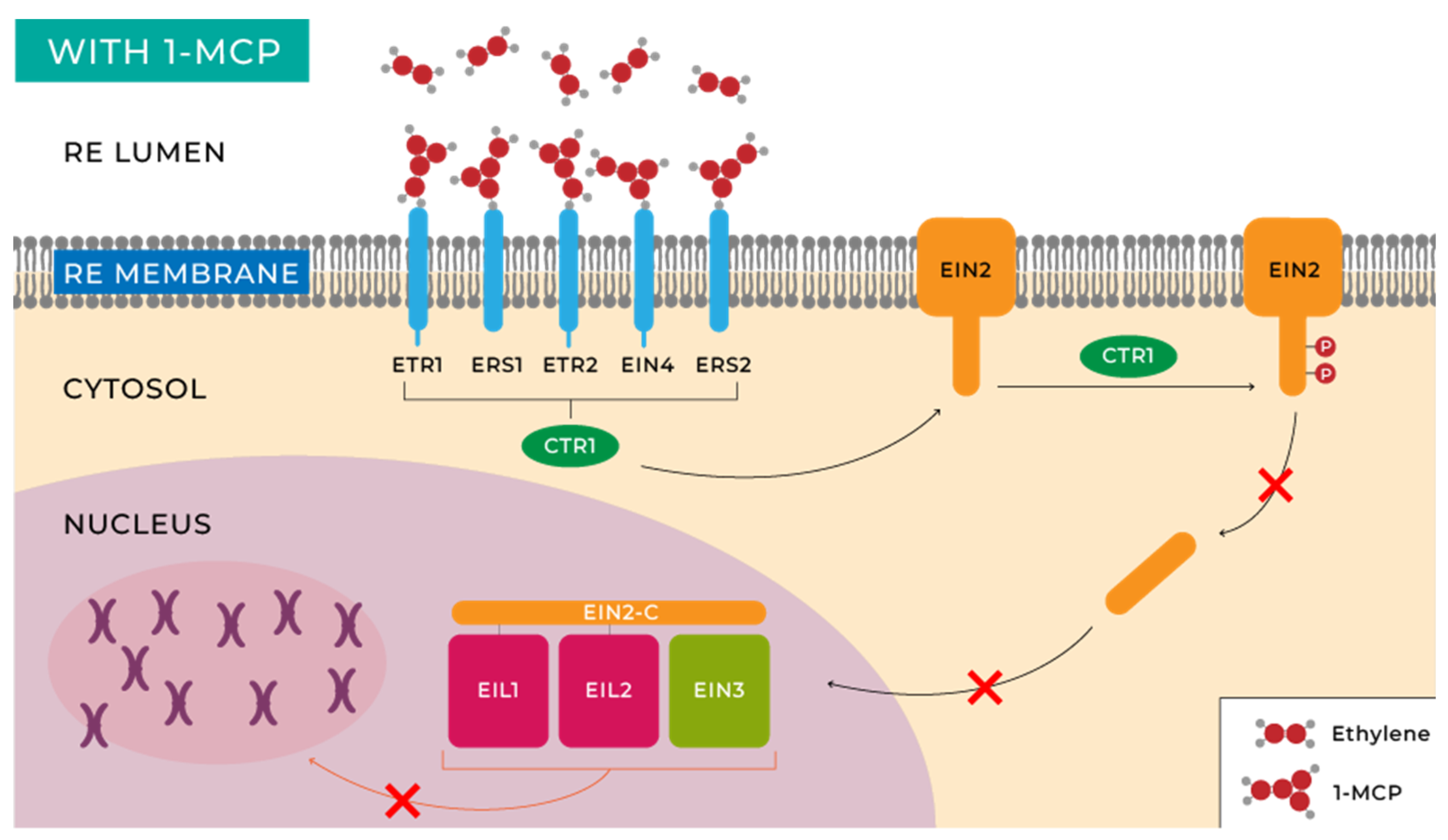
3.2. Ethylene Perception and Signalling
4. Physiological and Molecular Effects of Ethylene on Fruit Ripening
4.1. Effect of Ethylene Over Shelf Life, Quality and Disease Resistance
4.2. Effect of Ethylene on the Physical Characteristics of Fruits during Ripening
4.3. Effect of Ethylene on the Biochemical Characteristics of Fruits during Ripening
4.4. Effect of Ethylene on the Organoleptic Characteristics of Fruits during Ripening
5. Ethylene Managing Strategies
- Ethylene inhibitors:
- ○
- 1-MCP: 1-methylcyclopropene;
- ○
- SA: salicylic acid;
- ○
- AVG: aminoethoxyvinylglycine;
- ○
- AOA: amino-oxyacetic acid.
- Ethylene adsorbents:
- ○
- Zeolite;
- ○
- Activated carbon;
- ○
- Metal–organic frameworks;
- ○
- Silica gel.
- Ethylene scavengers by catalytic oxidation:
- ○
- KMnO4: potassium permanganate;
- ○
- UV radiation;
- ○
- TiO2: titanium dioxide;
- ○
- O3: ozone;
- ○
- Palladium;
- ○
- Cold plasma and other technologies.
5.1. Ethylene Inhibitors
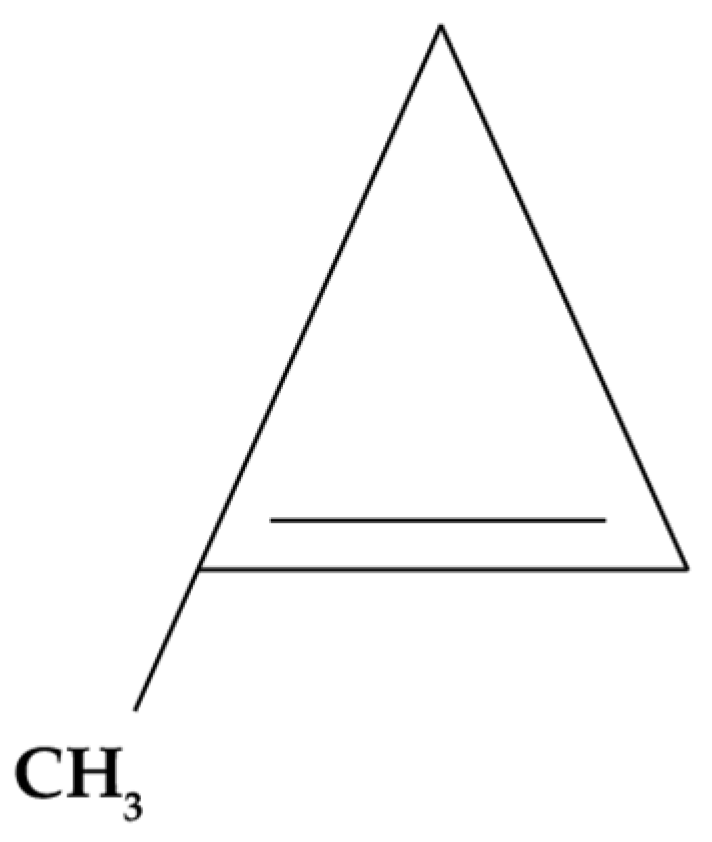
5.1.1. 1-Methylcyclopropene (1-MCP)
| Format | Concentration | Fruit | Conditions | Significative Results | Reference |
|---|---|---|---|---|---|
| Gas | 0.5 µL L−1 | ‘Raf’ Tomato | 10 °C, 90% RH, 7 days 20 °C, 90% RH, 4 days | It reduces both ethylene production and respiration rate and, in turn, delays weight, soluble solids content, and total acidity changes. | [165] |
| Gas | 1 µL L−1 | Pear cv: ‘Gorham’ ‘Gran Champion’ ‘La France’ ‘Gold La France’ | 20 °C, 25 days | Reduction in ethylene production, significant delay in the expression of genes related to the change in skin colour of pears, and preservation of chlorophyll and fruit firmness. | [166] |
| Gas Gas | 0.1 µL L−1 0.035 µL L−1 | ‘Unicorn’ Tomato | 10 °C, 85% RH, 15 days | The treatment using a higher concentration of 1-MCP showed a higher conservation of lycopene and weight. | [167] |
| Gas | 0.9 µL L−1 | ‘Hayward’ Kiwi | 20 °C, 95% RH, 20 days | Inhibition of ethylene production and respiration rate, delay of rot incidence, weight loss, increase in soluble solids content and total bacterial count. Improved preservation of firmness, chlorophyll, total acidity, ascorbic acid, and antioxidant capacity. | [168] |
| Micro-bubbles | 100–400 ppb | ‘Khai’ Banana | 25 °C, 85% RH, 12 days | Reduced respiration rate and ethylene production. Higher preservation of total chlorophyll content, colour, firmness, total soluble solids, antioxidant capacity, and total phenolic compounds. | [169] |
| Gas | 100 nL L−1 | ‘Gold’ and ‘Rainbow’ Papaya | 22 °C, 85% RH, 25 days 10 °C, 85% RH, 25 days | Fruits treated with high concentrations of 1-MCP showed increased firmness and delayed colour variation, meaning delayed ripening. The authors claim that commercial application could lead to a 30% reduction in papaya ripening. | [170] |
5.1.2. Salicylic Acid (SA)
5.1.3. Aminoethoxyvinylglycine (AVG)
5.1.4. Aminooxyacetic Acid (AOA)
5.2. Ethylene Adsorbents
5.2.1. Zeolite
5.2.2. Activated Carbon
5.2.3. Metal–Organic Frameworks
- Ethylene is only adsorbed on the surface or absorbed into the interior of these materials but cannot be decomposed.
- Desorption phenomena (the opposite process of adsorption/absorption) may occur, whereby a substance is released from or through a surface.
- Over time, the effectiveness of adsorption/absorption tends to decrease as these materials easily become saturated and require replacement.
5.3. Ethylene Removal by Catalytic Oxidation
5.3.1. Potassium Permanganate (KMnO4)
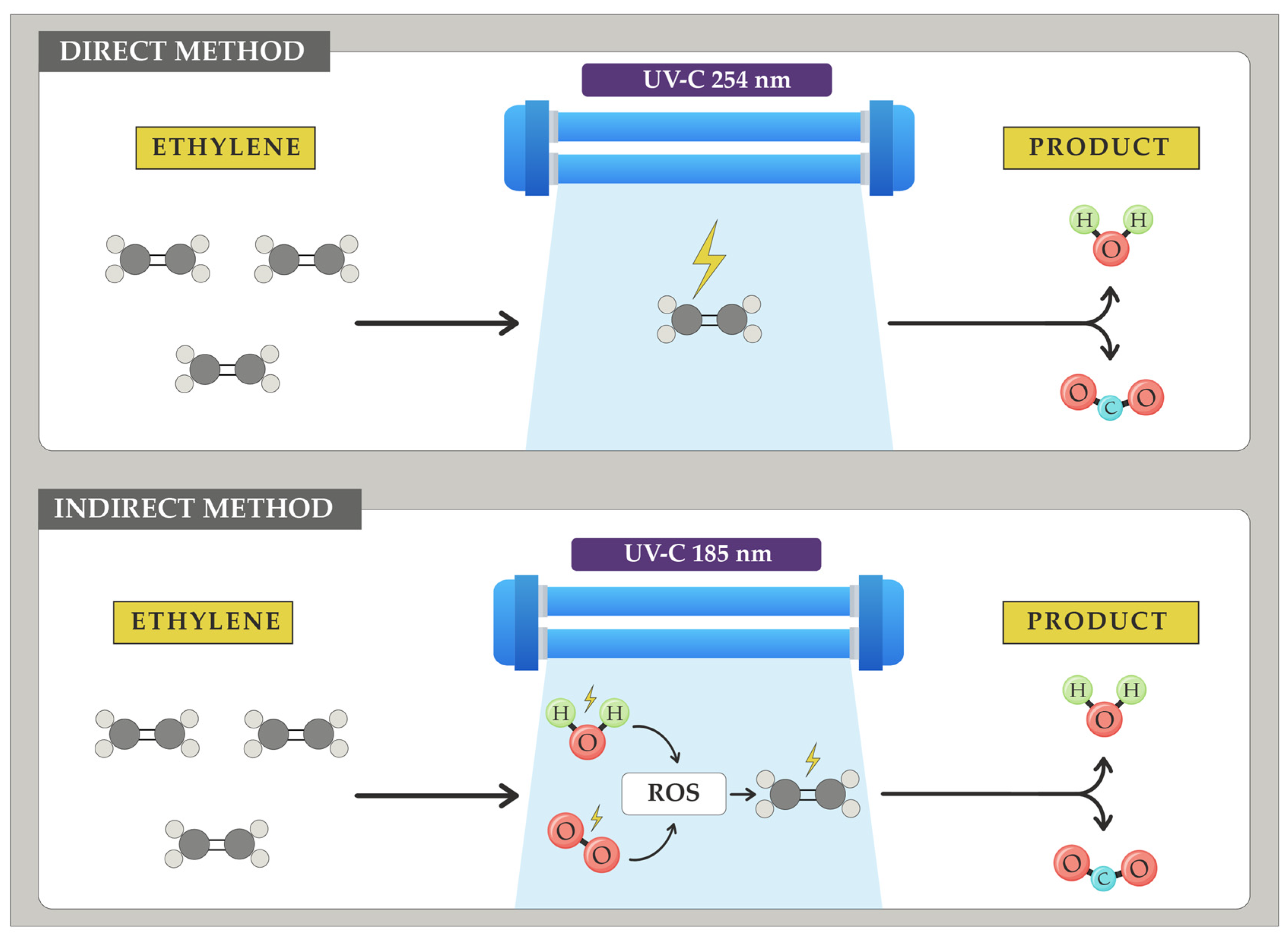
5.3.2. UV Radiation
- Relative humidity: As mentioned earlier, environmental water is the main source of certain ROS, crucial for the efficacy of this method. The lower the relative humidity, the lower the ethylene removal achievable with UV-C radiation [224].
- Oxygen concentration: Oxygen is the precursor of certain ROS and ozone that facilitate this process. A low concentration of this gas will hinder ethylene removal [225].
- Direct incidence on fruits: When UV-C radiation is directly targeted at fruits, it causes structural changes that negatively affect their quality [222].
5.3.3. Titanium Dioxide (TiO2)
- -
- Reaction 1: TiO2 + hν (UV energy/radiation) → TiO2 + e− + h+
- -
- Reaction 2: H2O + h+ → ·OH + H+
- -
- Reaction 3: ·OH + Organic compound (ethylene) → CO2 + H2O
- It is not a selective method, as seen in the summary of the reaction triggered by titanium dioxide and UV radiation. In addition to ethylene, it acts on other organic compounds such as aromatic compounds, which can affect the organoleptic quality of the fruits [232].
- It can increase the storage temperature, which may lead to fruit damage [233].
- If UV radiation, essential for this method to be effective, directly impacts the fruits, it can lead to negative consequences such as loss in aroma, rupture of cell membranes, and degradation of structures [234].
5.3.4. Ozone (O3)
- Handling is very challenging due to its easy decomposition into oxygen [159].
- If certain levels are exceeded, ozone shifts from offering significant improvements to being harmful as it can destroy tissues, leading to wounds that can promote ethylene production due to stress [219].
- Even though it is safe at low concentrations, ozone begins to have harmful effects on human health from 5 ppm onwards, including vision problems, sensation of asphyxia, headaches, pulmonary oedema, and coma when concentrations reach 50 ppm [239].
5.3.5. Palladium
5.3.6. Cold Plasma and Other Technologies
6. Practical Implications and Future Directions
6.1. Energy Consumption
6.2. Food, Microbiological, and Chemical Safety
6.3. Exploring Sustainable and Eco-Friendly Alternatives
6.4. Biocontrol Agents
6.5. Commercial and Industrial Applications
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministerio de Agricultura, Alimentación y Medio Ambiente. Estrategia Más Alimento, Menos Desperdicio. 2013, 60. Aprovecha los alimentos (alimentosdespana.es). Available online: https://www.alimentosdespana.es/es/estrategia-alimentos-espana/aprovecha-los-alimentos/#:~:text=Esta%20iniciativa%20del%20Ministerio%20de%20Agricultura%2C%20Pesca%20y,contribuir%20a%20la%20reducci%C3%B3n%20del%20desperdicio%20de%20alimentos (accessed on 25 June 2024).
- De Laurentiis, V.; Corrado, S.; Sala, S. Quantifying Household Waste of Fresh Fruit and Vegetables in the EU. Waste Manag. 2018, 77, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Informe Semanal RTVE. Comer Bien, Tirar Menos. 2023. Available online: https://www.rtve.es/play/videos/informe-semanal/comer-bien-tirar-menos/6832236/ (accessed on 25 June 2024).
- Yang, S.F.; Hoffman, N.E. Ethylene Biosynthesis and Its Regulation in Higher Plants. Annu. Rev. Plant Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Kader, A. Tecnología Postcosecha de Cultivos Hortofrutícolas; UCANR Publications: Irvine, CA, USA, 2011; Volume 311, ISBN 1601077440. [Google Scholar]
- Neljubow, D. Uber Die Horizontale Nutation Der Stengel von Pisum Sativum Und Einiger Anderen Planzen. Bot. Centralbl. Beih. 1901, 10, 128–139. [Google Scholar]
- Cousins, H.H., III. Agricultural Experiments: Citrus. Annu. Rep. Dep. Agric. Jam. 1910, 1–7. [Google Scholar]
- Doubt, S.L. The Response of Plants to Illuminating Gas. Bot. Gaz. 1917, 63, 209–224. [Google Scholar] [CrossRef]
- Denny, F.E. Effect of Ethylene Upon Respiration of Lemons. Bot. Gaz. 1924, 77, 322–329. [Google Scholar] [CrossRef]
- Gane, R. Production of Ethylene by Some Ripening Fruits. Nature 1934, 134, 1008. [Google Scholar] [CrossRef]
- Gane, R. The Formation of Ethylene by Plant Tissues, and Its Significance in the Ripening of Fruits. J. Pomol. Hortic. Sci. 1935, 13, 351–358. [Google Scholar] [CrossRef]
- Crocker, W.; Hitchcock, A.E.; Zimmerman, P.W. Similarities in the Effects of Ethylene and the Plant Auxins. Contrib. Boyce Thompson Inst. 1935, 7, 231–248. [Google Scholar]
- Forsyth, F.R.; Eaves, C.A.; Lightfoot, H.J. Storage Quality of McIntosh Appels as Affected Nby Removal of Ethylene from the Storage Atmosphere. Can. J. Plant Sci. 1969, 49, 567–572. [Google Scholar] [CrossRef]
- Southwick, F.W.; Smock, R.M. Lengthening the Storage Life of Apples By Removal of Volatile Materials From the Storage Atmosphere. Plant Physiol. 1943, 18, 716–717. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.J.; Wills, R.B.H.; Patterson, B.D. Removal by Ultra-Violet Lamp of Ethylene and Other Hydrocarbons Produced by Bananas. J. Sci. Food Agric. 1971, 22, 496–497. [Google Scholar] [CrossRef]
- Keller, N.; Ducamp, M.-N.; Robert, D.; Keller, V. Ethylene Removal and Fresh Product Storage: A Challenge at the Frontiers of Chemistry. Toward an Approach by Photocatalytic Oxidation. Chem. Rev. 2013, 113, 5029–5070. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Avalos-Belmontes, F.; Castillo-Campohermoso, M.A.; Contreras-Esquivel, J.C.; Artés-Hernández, F. Potassium Permanganate-Based Ethylene Scavengers for Fresh Horticultural Produce as an Active Packaging. Food Eng. Rev. 2019, 11, 159–183. [Google Scholar] [CrossRef]
- Awalgaonkar, G.; Beaudry, R.; Almenar, E. Ethylene-Removing Packaging: Basis for Development and Latest Advances. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3980–4007. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Nazar, R.; Khan, M.I.R.; Khan, N.A. Variation in Photosynthesis and Growth of Mustard Cultivars: Role of Ethylene Sensitivity. Sci. Hortic. 2012, 135, 1–6. [Google Scholar] [CrossRef]
- Giovannoni, J.J. Genetic Regulation of Fruit Development and Ripening. Plant Cell 2004, 16, S170–S180. [Google Scholar] [CrossRef] [PubMed]
- Carrari, F.; Fernie, A.R. Metabolic Regulation Underlying Tomato Fruit Development. J. Exp. Bot. 2006, 57, 1883–1897. [Google Scholar] [CrossRef] [PubMed]
- Cherian, S.; Figueroa, C.R.; Nair, H. “Movers and Shakers” in the Regulation of Fruit Ripening: A Cross-Dissection of Climacteric versus Non-Climacteric Fruit. J. Exp. Bot. 2014, 65, 4705–4722. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Pirrello, J.; Chervin, C.; Roustan, J.P.; Bouzayen, M. Ethylene Control of Fruit Ripening: Revisiting the Complex Network of Transcriptional Regulation. Plant Physiol. 2015, 169, 2380–2390. [Google Scholar] [CrossRef] [PubMed]
- Leng, P.; Yuan, B.; Guo, Y. The Role of Abscisic Acid in Fruit Ripening and Responses to Abiotic Stress. J. Exp. Bot. 2014, 65, 4577–4588. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Martínez-Madrid, M.C.; Romojaro, F. Ethylene Biosynthesis and Polyamine and ABA Levels in Cut Carnations Treated with Aminotriazole. J. Am. Soc. Hortic. Sci. JASHS 1999, 124, 81–85. [Google Scholar] [CrossRef]
- Symons, G.M.; Chua, Y.-J.; Ross, J.J.; Quittenden, L.J.; Davies, N.W.; Reid, J.B. Hormonal Changes during Non-Climacteric Ripening in Strawberry. J. Exp. Bot. 2012, 63, 4741–4750. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Li, M.; Liu, B.; Yan, M.; Yu, X.; Zi, H.; Liu, R.; Yamamuro, C. Interlinked Regulatory Loops of ABA Catabolism and Biosynthesis Coordinate Fruit Growth and Ripening in Woodland Strawberry. Proc. Natl. Acad. Sci. USA 2018, 115, E11542–E11550. [Google Scholar] [CrossRef] [PubMed]
- Forlani, S.; Masiero, S.; Mizzotti, C. Fruit Ripening: The Role of Hormones, Cell Wall Modifications, and Their Relationship with Pathogens. J. Exp. Bot. 2019, 70, 2993–3006. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Seidi, F.; Zhang, T.; Jin, Y.; Xiao, H. Ethylene Scavengers for the Preservation of Fruits and Vegetables: A Review. Food Chem. 2021, 337, 127750. [Google Scholar] [CrossRef] [PubMed]
- Lara, I.; Vendrell, M. Cold-Induced Ethylene Biosynthesis Is Differentially Regulated in Peel and Pulp Tissues of ‘Granny Smith’ Apple Fruit. Postharvest Biol. Technol. 2003, 29, 109–119. [Google Scholar] [CrossRef]
- Sdiri, S.; Navarro, P.; Monterde, A.; Benabda, J.; Salvador, A. New Degreening Treatments to Improve the Quality of Citrus Fruit Combining Different Periods with and without Ethylene Exposure. Postharvest Biol. Technol. 2012, 63, 25–32. [Google Scholar] [CrossRef]
- Mathooko, F.M.; Kubo, Y.; Inaba, A.; Nakamura, R. Characterization of the Regulation of Ethylene Biosynthesis in Tomato Fruit by Carbon Dioxide and Diazocyclopentadiene. Postharvest Biol. Technol. 1995, 5, 221–233. [Google Scholar] [CrossRef]
- Alberto, O.; Alzate, T. Hallazgos De La Biosíntesis Del Etileno En Frutas Climatéricas Y De Los Factores Que Afectan La Ruta Metabólica. Rev. Aliment. Hoy 2014, 22, 46–63. [Google Scholar]
- Asif, M.H.; Pathak, N.; Solomos, T.; Trivedi, P.K. Effect of Low Oxygen, Temperature and 1-Methylcyclopropene on the Expression of Genes Regulating Ethylene Biosynthesis and Perception during Ripening in Apple. S. Afr. J. Bot. 2009, 75, 137–144. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, Y.; Liu, C.; Chen, L.; Ma, J.; Sheng, J.; Shen, L. Inhibition of Nitric Oxide Synthesis Delayed Mature-Green Tomato Fruits Ripening Induced by Inhibition of Ethylene. Sci. Hortic. 2016, 211, 95–101. [Google Scholar] [CrossRef]
- Li, Y.; Golding, J.B.; Arcot, J.; Wills, R.B.H. Continuous Exposure to Ethylene in the Storage Environment Adversely Affects “Afourer” Mandarin Fruit Quality. Food Chem. 2018, 242, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-J.; Xie, X.-L.; Liu, S.-C.; Chen, K.-S.; Yin, X.-R. Auto- and Mutual-Regulation between Two CitERFs Contribute to Ethylene-Induced Citrus Fruit Degreening. Food Chem. 2019, 299, 125163. [Google Scholar] [CrossRef] [PubMed]
- Wills, R.B.H.; Golding, J.B. Reduction of Energy Usage in Postharvest Horticulture through Management of Ethylene. J. Sci. Food Agric. 2015, 95, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A Concise Guide to Active Agents for Active Food Packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Cara, B.; Giovannoni, J.J. Molecular Biology of Ethylene during Tomato Fruit Development and Maturation. Plant Sci. 2008, 175, 106–113. [Google Scholar] [CrossRef]
- Mansourbahmani, S.; Ghareyazie, B.; Zarinnia, V.; Kalatejari, S.; Mohammadi, R.S. Study on the Efficiency of Ethylene Scavengers on the Maintenance of Postharvest Quality of Tomato Fruit. J. Food Meas. Charact. 2018, 12, 691–701. [Google Scholar] [CrossRef]
- Emadpour, M.; Ghareyazie, B.; Kalaj, Y.R.; Entesari, M.; Bouzari, N. Effect of the Potassium Permanganate Coated Zeolite Nanoparticles on the Quality Characteristic and Shelf Life of Peach and Nectarine. Int. J. Agric. Technol. 2015, 11, 1411–1421. [Google Scholar]
- Gavin, C.; Barzallo, D.; Vera, H.; Lazo, R. Revisión Bibliográfica: Etileno En Poscosecha, Tecnologías Para Su Manejo y Control. Ecuadorian Sci. J. 2021, 5, 163–178. [Google Scholar] [CrossRef]
- Li, L.; Lichter, A.; Chalupowicz, D.; Gamrasni, D.; Goldberg, T.; Nerya, O.; Ben-Arie, R.; Porat, R. Effects of the Ethylene-Action Inhibitor 1-Methylcyclopropene on Postharvest Quality of Non-Climacteric Fruit Crops. Postharvest Biol. Technol. 2016, 111, 322–329. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Hu, Y.X.; Liu, J.; Wang, H.; Wei, J.H.; Sun, P.D.; Wu, L.F.; Zheng, H.J. Progress of Ethylene Action Mechanism and Its Application on Plant Type Formation in Crops. Saudi J. Biol. Sci. 2020, 27, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Serna-Escolano, V.; Gimenez, M.J.; Garcia-Pastor, M.E.; Dobon-Suarez, A.; Pardo-Pina, S.; Zapata, P.J. Effects of Degreening Treatment on Quality and Shelf-Life of Organic Lemons. Agronomy 2022, 12, 270. [Google Scholar] [CrossRef]
- Li, L.; Kaplunov, T.; Zutahy, Y.; Daus, A.; Porat, R.; Lichter, A. The Effects of 1-Methylcyclopropane and Ethylene on Postharvest Rachis Browning in Table Grapes. Postharvest Biol. Technol. 2015, 107, 16–22. [Google Scholar] [CrossRef]
- Özdemir, A.; Çandır, E. Impact of Hot Water and Modified Atmosphere Packaging Treatments on the Postharvest Quality of Pomegranate Fruit Cv. Hicaznar. Tarım Bilim. Derg. 2021, 27, 304–311. [Google Scholar] [CrossRef]
- Zhang, L.-H.; Zhang, Y.-H.; Li, L.-L.; Li, Y.-X. Effects of 1-MCP on peel browning of pomegranates. In Proceedings of the Acta Horticulturae, International Society for Horticultural Science (ISHS), Leuven, Belgium, 31 October 2008; pp. 275–282. [Google Scholar]
- Flores, F.; El Yahyaoui, F.; de Billerbeck, G.; Romojaro, F.; Latché, A.; Bouzayen, M.; Pech, J.; Ambid, C. Role of Ethylene in the Biosynthetic Pathway of Aliphatic Ester Aroma Volatiles in Charentais Cantaloupe Melons. J. Exp. Bot. 2002, 53, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Saltveit, M.E. Effect of Ethylene on Quality of Fresh Fruits and Vegetables. Postharvest Biol. Technol. 1999, 15, 279–292. [Google Scholar] [CrossRef]
- Abeles, F.B.; Morgan, P.W.; Saltveit, M.E. Chapter 3—The Biosynthesis of Ethylene, 2nd ed.; Abeles, F.B., Morgan, P.W., Saltveit, M.E.B.T.-E., Eds.; Academic Press: New York, NY, USA, 1992; pp. 26–55. ISBN 978-0-08-091628-6. [Google Scholar]
- Bleecker, A.B.; Kende, H. Ethylene: A Gaseous Signal Molecule in Plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, K.; Lee, Y.; Seo, J. Ethylene Scavenging Systems in Packaging of Fresh Produce: A Review. Food Rev. Int. 2021, 37, 155–176. [Google Scholar] [CrossRef]
- Bleecker, A.B.; Estelle, M.A.; Somerville, C.; Kende, H. Insensitivity to Ethylene Conferred by a Dominant Mutation in Arabidopsis Thaliana. Science 1988, 241, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, M.; Kunishi, A. Stimulation of Ethylene Production in Apple Tissue Slices by Methionine. Plant Physiol. 1966, 41, 376–382. [Google Scholar] [CrossRef]
- Bradford, K.J. Shang Fa Yang: Pioneer in Plant Ethylene Biochemistry. Plant Sci. 2008, 175, 2–7. [Google Scholar] [CrossRef]
- Zhang, T.C. Ethylene Biosynthesis and Signal Pathway Model. Chin. Bull. Bot. 2006, 23, 519–530. [Google Scholar]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants; American Society of Plant Biologists: Chichester, UK, 2000. [Google Scholar]
- Li, J.-F.; Qu, L.-H.; Li, N. Tyr152 Plays a Central Role in the Catalysis of 1-Aminocyclopropane-1-Carboxylate Synthase. J. Exp. Bot. 2005, 56, 2203–2210. [Google Scholar] [CrossRef]
- Ravanel, S.; Gakière, B.; Job, D.; Douce, R. The Specific Features of Methionine Biosynthesis and Metabolism in Plants. Proc. Natl. Acad. Sci. USA 1998, 95, 7805–7812. [Google Scholar] [CrossRef] [PubMed]
- Pretel, M.; Serrano, M.; Amoros, A.; Romojaro, F. Ripening and Ethylene Biosynthesis in Controlled Atmosphere Stored Apricots. Eur. Food Res. Technol. 1999, 209, 130–134. [Google Scholar] [CrossRef]
- Yamagami, T.; Tsuchisaka, A.; Yamada, K.; Haddon, W.F.; Harden, L.A.; Theologis, A. Biochemical Diversity among the 1-Amino-Cyclopropane-1-Carboxylate Synthase Isozymes Encoded by the Arabidopsis Gene Family. J. Biol. Chem. 2003, 278, 49102–49112. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, A.; Van Der Straeten, D. Ethylene Biosynthesis and Signaling: An Overview. In Plant Hormones; Litwack, G., Ed.; Vitamins & Hormones; Academic Press: Cambridge, MA, USA, 2005; Volume 72, pp. 399–430. [Google Scholar]
- Chung, M.-C.; Chou, S.-J.; Kuang, L.-Y.; Charng, Y.-Y.; Yang, S.F. Subcellular Localization of 1-Aminocyclopropane-1-Carboxylic Acid Oxidase in Apple Fruit. Plant Cell Physiol. 2002, 43, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Ono, E.; Mizutani, M. Evolution and Diversity of the 2-Oxoglutarate-Dependent Dioxygenase Superfamily in Plants. Plant J. Mol. Biol. 2014, 78, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Van de Poel, B.; Van Der Straeten, D. 1-Aminocyclopropane-1-Carboxylic Acid (ACC) in Plants: More than Just the Precursor of Ethylene! Front. Plant Sci. 2014, 5, 640. [Google Scholar] [CrossRef]
- Hudgins, J.W.; Ralph, S.G.; Franceschi, V.R.; Bohlmann, J. Ethylene in Induced Conifer Defense: CDNA Cloning, Protein Expression, and Cellular and Subcellular Localization of 1-Aminocyclopropane-1-Carboxylate Oxidase in Resin Duct and Phenolic Parenchyma Cells. Planta 2006, 224, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, J.; Vaughan-Hirsch, J.; van de Poel, B. The Regulation of Ethylene Biosynthesis: A Complex Multilevel Control Circuitry. New Phytol. 2021, 229, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Argueso, C.T.; Hansen, M.; Kieber, J.J. Regulation of Ethylene Biosynthesis. J. Plant Growth Regul. 2007, 26, 92–105. [Google Scholar] [CrossRef]
- Binder, B.M. Ethylene Signaling in Plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef] [PubMed]
- Javid, E.; Corpas Iguarán, E.; Alberto, O.; Alzate, T. Hallazgos de la biosíntesis del etileno en frutas climatéricas y de los factores que afectan la ruta metabólica. Aliment. Hoy 2014, 22, 46–63. [Google Scholar]
- Stearns, J.C.; Glick, B.R. Transgenic Plants with Altered Ethylene Biosynthesis or Perception. Biotechnol. Adv. 2003, 21, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Yoon, G.M.; Shemansky, J.M.; Lin, D.Y.; Ying, Z.I.; Chang, J.; Garrett, W.M.; Kessenbrock, M.; Groth, G.; Tucker, M.L.; et al. CTR1 Phosphorylates the Central Regulator EIN2 to Control Ethylene Hormone Signaling from the ER Membrane to the Nucleus in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 19486–19491. [Google Scholar] [CrossRef]
- Qiao, H.; Shen, Z.; Huang, S.C.; Schmitz, R.J.; Urich, M.A.; Briggs, S.P.; Ecker, J.R. Processing and Subcellular Trafficking of ER-Tethered EIN2 Control Response to Ethylene Gas. Science 2012, 338, 390–393. [Google Scholar] [CrossRef]
- Merchante, C.; Brumos, J.; Yun, J.; Hu, Q.; Spencer, K.R.; Enríquez, P.; Binder, B.M.; Heber, S.; Stepanova, A.N.; Alonso, J.M. Gene-Specific Translation Regulation Mediated by the Hormone-Signaling Molecule EIN2. Cell 2015, 163, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Binder, B.M.; Mortimore, L.A.; Stepanova, A.N.; Ecker, J.R.; Bleecker, A.B. Short-Term Growth Responses to Ethylene in Arabidopsis Seedlings Are EIN3/EIL1 Independent. Plant Physiol. 2004, 136, 2921–2927. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.N.; Zhong, S.; Weirauch, M.T.; Hon, G.; Pelizzola, M.; Li, H.; Huang, S.-S.C.; Schmitz, R.J.; Urich, M.A.; Kuo, D.; et al. Temporal Transcriptional Response to Ethylene Gas Drives Growth Hormone Cross-Regulation in Arabidopsis. eLife 2013, 2, e00675. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Stock, A.M. Biological Insights from Structures of Two-Component Proteins. Annu. Rev. Microbiol. 2009, 63, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, D.M.; Grossman, A.R. Similarity of a Chromatic Adaptation Sensor to Phytochrome and Ethylene Receptors. Science 1996, 273, 1409–1412. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Rujan, T.; Richly, E.; Hansen, A.; Cornelsen, S.; Lins, T.; Leister, D.; Stoebe, B.; Hasegawa, M.; Penny, D. Evolutionary Analysis of Arabidopsis, Cyanobacterial, and Chloroplast Genomes Reveals Plastid Phylogeny and Thousands of Cyanobacterial Genes in the Nucleus. Proc. Natl. Acad. Sci. USA 2002, 99, 12246–12251. [Google Scholar] [CrossRef] [PubMed]
- Mount, S.M.; Chang, C. Evidence for a Plastid Origin of Plant Ethylene Receptor Genes. Plant Physiol. 2002, 130, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Timmis, J.N.; Ayliffe, M.A.; Huang, C.Y.; Martin, W. Endosymbiotic Gene Transfer: Organelle Genomes Forge Eukaryotic Chromosomes. Nat. Rev. Genet. 2004, 5, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.E.; Shiu, S.-H.; Armitage, J.P. Two-Component Systems and Their Co-Option for Eukaryotic Signal Transduction. Curr. Biol. 2011, 21, R320–R330. [Google Scholar] [CrossRef] [PubMed]
- Hérivaux, A.; Dugé de Bernonville, T.; Roux, C.; Clastre, M.; Courdavault, V.; Gastebois, A.; Bouchara, J.-P.; James, T.Y.; Latgé, J.-P.; Martin, F.; et al. The Identification of Phytohormone Receptor Homologs in Early Diverging Fungi Suggests a Role for Plant Sensing in Land Colonization by Fungi. mBio 2017, 8, e01739-16. [Google Scholar] [CrossRef] [PubMed]
- Rivarola, M.; McClellan, C.A.; Resnick, J.S.; Chang, C. ETR1-Specific Mutations Distinguish ETR1 from Other Arabidopsis Ethylene Receptors as Revealed by Genetic Interaction with RTE1. Plant Physiol. 2009, 150, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, F.I.; Esch, J.J.; Hall, A.E.; Binder, B.M.; Schaller, G.E.; Bleecker, A.B. A Copper Cofactor for the Ethylene Receptor ETR1 from Arabidopsis. Science 1999, 283, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Lacey, R.F.; Binder, B.M. Ethylene Regulates the Physiology of the Cyanobacterium Synechocystis Sp. PCC 6803 via an Ethylene Receptor. Plant Physiol. 2016, 171, 2798–2809. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.J.; Lacey, R.F.; Binder Bickford, A.B.; Beshears, C.P.; Gilmartin, C.J.; Binder, B.M. Cyanobacteria Respond to Low Levels of Ethylene. Front. Plant Sci. 2019, 10, 950. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.E.; Bengochea, T.; Cairns, A.J.; Dodds, J.H.; Hall, M.A. Studies on Ethylene Binding by Cell-Free Preparations from Cotyledons of Phaseolus Vulgaris L.: Subcellular Localization. Plant Cell Environ. 1982, 5, 101–107. [Google Scholar] [CrossRef]
- Evans, D.E.; Dodds, J.H.; Lloyd, P.C.; Apgwynn, I.; Hall, M.A. A Study of the Subcellular Localisation of an Ethylene Binding Site in Developing Cotyledons of Phaseolus vulgaris L. by High Resolution Autoradiography. Planta 1982, 154, 48–52. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Randlett, M.D.; Findell, J.L.; Schaller, G.E. Localization of the Ethylene Receptor ETR1 to the Endoplasmic Reticulum of Arabidopsis. J. Biol. Chem. 2002, 277, 19861–19866. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Shakeel, S.N.; Bowers, J.; Zhao, X.-C.; Etheridge, N.; Schaller, G.E. Ligand-Induced Degradation of the Ethylene Receptor ETR2 through a Proteasome-Dependent Pathway in Arabidopsis. J. Biol. Chem. 2007, 282, 24752–24758. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Cui, M.-L.; Sun, H.-J.; Takada, K.; Mori, H.; Kamada, H.; Ezura, H. Subcellular Localization and Membrane Topology of the Melon Ethylene Receptor CmERS1. Plant Physiol. 2006, 141, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Lin, Z.; Grierson, D. Tomato Ethylene Receptor-CTR Interactions: Visualization of NEVER-RIPE Interactions with Multiple CTRs at the Endoplasmic Reticulum. J. Exp. Bot. 2008, 59, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.-H.; Rivarola, M.; Resnick, J.S.; Maggin, B.D.; Chang, C. Subcellular Co-Localization of Arabidopsis RTE1 and ETR1 Supports a Regulatory Role for RTE1 in ETR1 Ethylene Signaling. Plant J. Cell Mol. Biol. 2008, 53, 275–286. [Google Scholar] [CrossRef]
- Grefen, C.; Städele, K.; Růzicka, K.; Obrdlik, P.; Harter, K.; Horák, J. Subcellular Localization and in Vivo Interactions of the Arabidopsis Thaliana Ethylene Receptor Family Members. Mol. Plant 2008, 1, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Kwok, S.F.; Bleecker, A.B.; Meyerowitz, E.M. Arabidopsis Ethylene-Response Gene ETR1: Similarity of Product to Two-Component Regulators. Science 1993, 262, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Chang, C.; Sun, Q.; Meyerowitz, E.M. Ethylene Insensitivity Conferred by Arabidopsis ERS Gene. Science 1995, 269, 1712–1714. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Sakai, H.; Nourizadeh, S.; Chen, Q.G.; Bleecker, A.B.; Ecker, J.R.; Meyerowitz, E.M. EIN4 and ERS2 Are Members of the Putative Ethylene Receptor Gene Family in Arabidopsis. Plant Cell 1998, 10, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.E.; Bleecker, A.B. Ethylene-Binding Sites Generated in Yeast Expressing the Arabidopsis ETR1 Gene. Science 1995, 270, 1809–1811. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L.; Grierson, D. Ethylene Biosynthesis and Action in Tomato: A Model for Climacteric Fruit Ripening. J. Exp. Bot. 2002, 53, 2039–2055. [Google Scholar] [CrossRef] [PubMed]
- Kays, S.J.; Paull, R.E. Postharvest Biology; Exon Press: Athens, Greece, 2004; ISBN 1888186542/9781888186543. [Google Scholar]
- Tucker, G.; Yin, X.; Zhang, A.; Wang, M.; Zhu, Q.; Liu, X.; Xie, X.; Chen, K.; Grierson, D. Ethylene† and Fruit Softening. Food Qual. Saf. 2017, 1, 253–267. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Khajavi, M.Z.; Ahmadi, S.; Mortazavian, A.M.; Abdolshahi, A.; Rafiee, S.; Farhoodi, M. Novel Strategies to Control Ethylene in Fruit and Vegetables for Extending Their Shelf Life: A Review. Int. J. Environ. Sci. Technol. 2021, 19, 4599–4610. [Google Scholar] [CrossRef]
- Mope, C.; Adegoroye, A.; Oluwalade, T.A.; Adeyelu, A.A. Ethylene Management in Fresh Produce Transport. Asian J. Food Res. Nutr. 2024, 3, 60–71. [Google Scholar]
- Dong, T.; Zheng, T.; Fu, W.; Guan, L.; Jia, H.; Fang, J. The Effect of Ethylene on the Color Change and Resistance to Botrytis Cinerea Infection in ‘Kyoho’ Grape Fruits. Foods 2020, 9, 892. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, L.; Yang, Y.; Guo, X.; Chen, G.; Xiong, X.; Dong, D.; Li, G. Transcriptome Analysis Reveals That Exogenous Ethylene Activates Immune and Defense Responses in a High Late Blight Resistant Potato Genotype. Sci. Rep. 2020, 10, 21294. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Sun, P.; Li, Z.-Y.; Zhang, F.-J.; Wang, X.-F.; You, C.-X.; Zhang, C.-L.; Zhang, Z. Plant Disease Resistance Outputs Regulated by AP2/ERF Transcription Factor Family. Stress Biol. 2024, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Thirugnanasambantham, K.; Durairaj, S.; Saravanan, S.; Karikalan, K.; Muralidaran, S.; Islam, V.I.H. Role of Ethylene Response Transcription Factor (ERF) and Its Regulation in Response to Stress Encountered by Plants. Plant Mol. Biol. Report. 2015, 33, 347–357. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Etheridge, N.; Schaller, G.E. Ethylene Signal Transduction. Ann. Bot. 2005, 95, 901–915. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Hernández, M.H.; Artés-Hernández, F.; Ávalos-Belmontes, F.; Castillo-Campohermoso, M.A.; Contreras-Esquivel, J.C.; Ventura-Sobrevilla, J.M.; Martínez-Hernández, G.B. Current Scenario of Adsorbent Materials Used in Ethylene Scavenging Systems to Extend Fruit and Vegetable Postharvest Life. Food Bioprocess Technol. 2018, 11, 511–525. [Google Scholar] [CrossRef]
- Wu, B.; Guo, Q.; Wang, G.; Peng, X.; Wang, J.; Che, F. Effects of Different Postharvest Treatments on the Physiology and Quality of ‘Xiaobai’ Apricots at Room Temperature. J. Food Sci. Technol. 2015, 52, 2247–2255. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Salinas, R.; Acosta-Motos, J.R.; Pérez-López, A.J.; Noguera-Artiaga, L.; Núñez-Delicado, E.; Burló, F.; López-Miranda, S. Effect of Combination of KMnO4 Oxidation and UV-C Radiation on Postharvest Quality of Refrigerated Pears Cv. “Ercolini”. Horticulturae 2022, 8, 1078. [Google Scholar] [CrossRef]
- Alonso-Salinas, R.; Acosta-Motos, J.R.; Núñez-Delicado, E.; Gabaldón, J.A.; López-Miranda, S. Combined Effect of Potassium Permanganate and Ultraviolet Light as Ethylene Scavengers on Post-Harvest Quality of Peach at Optimal and Stressful Temperatures. Agronomy 2022, 12, 616. [Google Scholar] [CrossRef]
- Zhou, H.-W.; Dong, L.i.; Ben-Arie, R.; Lurie, S. The Role of Ethylene in the Prevention of Chilling Injury in Nectarines. J. Plant Physiol. 2001, 158, 55–61. [Google Scholar] [CrossRef]
- Fan, X.; Xi, Y.; Zhao, H.; Liu, B.; Cao, J.; Jiang, W. Improving Fresh Apricot (Prunus armeniaca L.) Quality and Antioxidant Capacity by Storage at near Freezing Temperature. Sci. Hortic. 2018, 231, 1–10. [Google Scholar] [CrossRef]
- Hayama, H.; Shimada, T.; Fujii, H.; Ito, A.; Kashimura, Y. Ethylene-Regulation of Fruit Softening and Softening-Related Genes in Peach. J. Exp. Bot. 2006, 57, 4071–4077. [Google Scholar] [CrossRef] [PubMed]
- Palou, L.; Crisosto, C.H.; Garner, D.; Basinal, L.M. Effect of Continuous Exposure to Exogenous Ethylene during Cold Storage on Postharvest Decay Development and Quality Attributes of Stone Fruits and Table Grapes. Postharvest Biol. Technol. 2003, 27, 243–254. [Google Scholar] [CrossRef]
- Álvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Avalos-Belmontes, F.; Miranda-Molina, F.D.; Artés-Hernández, F. Postharvest Quality Retention of Apricots by Using a Novel Sepiolite–Loaded Potassium Permanganate Ethylene Scavenger. Postharvest Biol. Technol. 2020, 160, 111061. [Google Scholar] [CrossRef]
- Pech, J.-C.; Purgatto, E.; Bouzayen, M.; Latché, A. Ethylene and Fruit Ripening. In Annual Plant Reviews Volume 44: The Plant Hormone Ethylene; Wiley: Hoboken, NJ, USA, 2018; pp. 275–304. ISBN 9781119312994. [Google Scholar]
- Pott, D.; Vallarino, J.; Osorio, S. Metabolite Changes during Postharvest Storage: Effects on Fruit Quality Traits. Metabolites 2020, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Yahia, E.; Serrano, M.; Valero, D.; Aguilar, G. Influence of Postharvest Technologies and Handling Practices on Phytochemicals in Fruits and Vegetables: Chemistry and Human Health. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 609–628. ISBN 9781119157946. [Google Scholar]
- Zhang, B.; Peng, B.; Zhang, C.; Song, Z.; Ma, R. Determination of Fruit Maturity and Its Prediction Model Based on the Pericarp Index of Absorbance Difference (IAD) for Peaches. PLoS ONE 2017, 12, e0177511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Shao, X.; Wei, Y.; Xu, F.; Wang, H. At-Harvest Fruit Maturity Affects Sucrose Metabolism during Cold Storage and Is Related to Chilling Injury in Peach. J. Food Sci. Technol. 2020, 57, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Beckles, D.M. Factors Affecting the Postharvest Soluble Solids and Sugar Content of Tomato (Solanum lycopersicum L.) Fruit. Postharvest Biol. Technol. 2012, 63, 129–140. [Google Scholar] [CrossRef]
- Brecht, J.; Yahia, E. Postharvest Physiology. In The Mango, 2nd Edition: Botany, Production and Uses; CABI: Wallingford, UK, 2009; pp. 484–528. ISBN 978-1-84593-489-7. [Google Scholar]
- Brizzolara, S.; Manganaris, G.; Fotopoulos, V.; Watkins, C.; Tonutti, P. Primary Metabolism in Fresh Fruits During Storage. Front. Plant Sci. 2020, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- McBey, D.; Nadathur, S. Flavors, Taste Preferences, and the Consumer: Taste Modulation and Influencing Change in Dietary Patterns for a Sustainable Earth. In Sustainable Protein Sources; Academic Press: Cambridge, MA, USA, 2024; pp. 629–647. ISBN 9780323916523. [Google Scholar]
- Hossain, M.A.; Karim, M.; Juthee, S. Postharvest Physiological and Biochemical Alterations in Fruits: A Review. Fundam. Appl. Agric. 2020, 5, 453–469. [Google Scholar] [CrossRef]
- Prasad, K.; Jacob, S.; Siddiqui, M. Fruit Maturity, Harvesting, and Quality Standards. In Preharvest Modulation of Postharvest Fruit and Vegetable Quality; Academic Press: Cambridge, MA, USA, 2017; pp. 41–69. [Google Scholar]
- Singh, D.; Sharma, R.; Kesharwani, A. Postharvest Losses of Horticultural Produce. In Postharvest Handling and Diseases of Horticultural Produce; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–24. ISBN 9781003045502. [Google Scholar]
- Mohammed, M.; Khalid Alqahtani, N.; Munir, M. Artificial Ripening Technologies for Dates. In New Discoveries in the Ripening Processes; BoD–Books on Demand: Norderstedt, Germany, 2023; ISBN 978-0-85014-126-9. [Google Scholar]
- Madani, B.; Mirshekari, A.; Yahia, E.; Golding, J. Sapota (Manilkara achras Forb.): Factors Influencing Fresh and Processed Fruit Quality. Hortic. Rev. 2018, 45, 105–142. [Google Scholar]
- Arabia, A.; Munné-Bosch, S.; Muñoz, P. Ascorbic Acid as a Master Redox Regulator of Fruit Ripening. Postharvest Biol. Technol. 2024, 207, 112614. [Google Scholar] [CrossRef]
- Ludwig-Ohm, S.; Dirksmeyer, W.; Klockgether, K. Approaches to Reduce Food Losses in German Fruit and Vegetable Production. Sustainability 2019, 11, 6576. [Google Scholar] [CrossRef]
- Gallagher, M.S.; Mahajan, P.V. The stability and shelf life of fruit and vegetables. In Food and Beverage Stability and Shelf-Life; Kilcast, D., Subramaniam, P., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2011; Chapter 22; pp. 641–656. [Google Scholar]
- Debebe, S. Post-Harvest Losses of Crops and Its Determinants in Ethiopia: Tobit Model Analysis. Agric. Food Secur. 2022, 11, 13. [Google Scholar] [CrossRef]
- Alonso-Salinas, R.; López-Miranda, S.; Pérez-López, A.J.; Noguera-Artiaga, L.; Carbonell-Barrachina, Á.A.; Núñez-Delicado, E.; Acosta-Motos, J.R. Novel Combination of Ethylene Oxidisers to Delay Losses on Postharvest Quality, Volatile Compounds and Sensorial Analysis of Tomato Fruit. LWT 2022, 170, 114054. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Negi, Y.S. Ethylene Scavengers for Active Packaging of Fresh Food Produce. Environ. Chem. Lett. 2020, 18, 269–284. [Google Scholar] [CrossRef]
- Zerbini, P. Role of Maturity for Improved Flavour. Fruit Veg. Flavour Recent Adv. Future Prospect. 2008, 180–199. [Google Scholar] [CrossRef]
- Cruz-Hernández, A.; Paredes-López, O. Fruit Quality: New Insights for Biotechnology. Crit. Rev. Food Sci. Nutr. 2012, 52, 272–289. [Google Scholar] [CrossRef] [PubMed]
- Klee, H.J.; Giovannoni, J.J. Genetics and Control of Tomato Fruit Ripening and Quality Attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, Q.; Li, J.; Gong, H.; Fan, X.; Yang, Y.; Liu, X.; Yin, X. Transcriptome Co-Expression Network Analysis Identifies Key Genes and Regulators of Ripening Kiwifruit Ester Biosynthesis. BMC Plant Biol. 2020, 20, 103. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Xu, Y.; Deng, Y.; Xie, Y.; Gao, Z.; Lang, Z.; Niu, Q. Key Transcription Factors Regulate Fruit Ripening and Metabolite Accumulation in Tomato. Plant Physiol. 2024, 195, 2256–2273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gao, F.; Chen, X.; Wu, W.; Wang, L.; Shi, J.; Huang, Y.; Shen, Y.; Wu, G.; Guo, J. Characterization of Key Aroma Compounds and Regulation Mechanism of Aroma Formation in Local Binzi (Malus pumila × Malus asiatica) Fruit. BMC Plant Biol. 2022, 22, 532. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Delrot, S.; Liang, Z. From Acidity to Sweetness: A Comprehensive Review of Carbon Accumulation in Grape Berries. Mol. Hortic. 2024, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, Z.; Peydayesh, M.; Zhang, B.; Jia, X.; Huang, Q. Ethylene Control in Fruit Quality Assurance: A Material Science Perspective. Aggregate 2024, e565. [Google Scholar] [CrossRef]
- Datta, H.S. Physiological Approaches for Regulation of Fruit Ripening: A Review. IJCS 2019, 7, 4587–4597. [Google Scholar]
- Abbas, F.; Zhou, Y.; O’Neill Rothenberg, D.; Alam, I.; Ke, Y.; Wang, H.-C. Aroma Components in Horticultural Crops: Chemical Diversity and Usage of Metabolic Engineering for Industrial Applications. Plants 2023, 12, 1748. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Liao, B.; Li, R.; Liu, Z. Changes in Taste and Volatile Compounds and Ethylene Production Determined the Eating Window of ‘Xuxiang’ and ‘Cuixiang’ Kiwifruit Cultivars. Postharvest Biol. Technol. 2022, 194, 112093. [Google Scholar] [CrossRef]
- Wendt, L.; Soldateli, F.; Both, V.; Thewes, F.; Ludwig, V.; Berghetti, M.; Ferrão, T.; Wagner, R.; Brackmann, A. Effects of Ethylene Control and Dynamic Controlled Atmosphere Storage on ‘Galaxy’ Apple Quality. J. Plant Growth Regul. 2022, 42, 701–718. [Google Scholar] [CrossRef]
- Alonso-Salinas, R.; López-Miranda, S.; González-Báidez, A.; Pérez-López, A.J.; Noguera-Artiaga, L.; Núñez-Delicado, E.; Carbonell-Barrachina, Á.; Acosta-Motos, J.R. Effect of Potassium Permanganate, Ultraviolet Radiation and Titanium Oxide as Ethylene Scavengers on Preservation of Postharvest Quality and Sensory Attributes of Broccoli Stored with Tomatoes. Foods 2023, 12, 2418. [Google Scholar] [CrossRef] [PubMed]
- Büchele, F.; Khera, K.; Thewes, F.; Kittemann, D.; Neuwald, D. Dynamic Control of Atmosphere and Temperature Based on Fruit CO2 Production: Practical Application in Apple Storage and Effects on Metabolism, Quality, and Volatile Profiles. Food Bioprocess Technol. 2023, 16, 2497–2510. [Google Scholar] [CrossRef]
- Zaitoon, A.; Luo, X.; Lim, L.-T. Triggered and Controlled Release of Active Gaseous/Volatile Compounds for Active Packaging Applications of Agri-Food Products: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 541–579. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, M.; Ayhan, Z. Combined Effects of Ethylene Scavenging-Active Packaging System and Modified Atmosphere to Reduce Postharvest Losses of Ethylene Sensitive Produce: Banana and Kiwifruit. Packag. Technol. Sci. 2023, 36, 951–967. [Google Scholar] [CrossRef]
- Pathak, N.; Caleb, O.J.; Geyer, M.; Herppich, W.B.; Rauh, C.; Mahajan, P. V Photocatalytic and Photochemical Oxidation of Ethylene: Potential for Storage of Fresh Produce—A Review. Food Bioprocess Technol. 2017, 10, 982–1001. [Google Scholar] [CrossRef]
- Pathak, N. Photocatalysis and Vacuum Ultraviolet Light Photolysis as Ethylene Removal Techniques for Potential Application in Fruit Storage. Ph.D. Thesis, Technische Universität Berlin, Berlin, Germany, 2019. [Google Scholar]
- Janjarasskul, T.; Suppakul, P. Active and Intelligent Packaging: The Indication of Quality and Safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 808–831. [Google Scholar] [CrossRef] [PubMed]
- Baswal, A.K.; Ramezanian, A. 1-Methylcyclopropene Potentials in Maintaining the Postharvest Quality of Fruits, Vegetables, and Ornamentals: A Review. J. Food Process. Preserv. 2021, 45, 15129. [Google Scholar] [CrossRef]
- Watkins, C. 1-Methylcyclopropene (1-MCP) Based Technologies for Storage and Shelf Life Extension. Int. J. Postharvest Technol. Innov. 2006, 1, 62–68. [Google Scholar] [CrossRef]
- Tsantili, E.; Gapper, N.E.; Arquiza, J.M.R.A.; Whitaker, B.D.; Watkins, C.B. Ethylene and α-Farnesene Metabolism in Green and Red Skin of Three Apple Cultivars in Response to 1-Methylcyclopropene (1-MCP) Treatment. J. Agric. Food Chem. 2007, 55, 5267–5276. [Google Scholar] [CrossRef] [PubMed]
- Yarılgaç, T.; Kadim, H.; Ozturk, B. Role of Maturity Stages and Modified-Atmosphere Packaging on the Quality Attributes of Cornelian Cherry Fruits (Cornus mas L.) throughout Shelf Life. J. Sci. Food Agric. 2019, 99, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Awalgaonkar, G.S. Development of active packaging trays with ethylene removing capacity. Master’s Thesis, Michigan State University, East Lansing, MI, USA, 2018. [Google Scholar]
- Guillén, F.; Castillo, S.; Zapata, P.J.; Martínez-Romero, D.; Serrano, M.; Valero, D. Efficacy of 1-MCP Treatment in Tomato Fruit. 1. Duration and Concentration of 1-MCP Treatment to Gain an Effective Delay of Postharvest Ripening. Postharvest Biol. Technol. 2007, 43, 23–27. [Google Scholar] [CrossRef]
- Charoenchongsuk, N.; Matsumoto, D.; Itai, A.; Murayama, H. Ripening Characteristics and Pigment Changes in Russeted Pear Fruit in Response to Ethylene and 1-MCP. Horticulturae 2018, 4, 22. [Google Scholar] [CrossRef]
- Taye, A.M.; Tilahun, S.; Seo, M.H.; Park, D.S.; Jeong, C.S. Effects of 1-MCP on Quality and Storability of Cherry Tomato (Solanum lycopersicum L.). Horticulturae 2019, 5, 29. [Google Scholar] [CrossRef]
- Xu, F.; Liu, S.; Liu, Y.; Xu, J.; Liu, T.; Dong, S. Effectiveness of Lysozyme Coatings and 1-MCP Treatments on Storage and Preservation of Kiwifruit. Food Chem. 2019, 288, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Pongprasert, N.; Srilaong, V.; Kaewsukseang, S. 1-MCP Micro-Bubbles Delaying Postharvest Ripening of “Khai” Banana. In Proceedings of the III Asia Pacific Symposium on Postharvest Research, Education and Extension: APS2014, Hochiminh City, Vietnam, 5 October 2018; Volume 1213, pp. 245–250. [Google Scholar]
- Manenoi, A.; Bayogan, E.R.V.; Thumdee, S.; Paull, R.E. Utility of 1-Methylcyclopropene as a Papaya Postharvest Treatment. Postharvest Biol. Technol. 2007, 44, 55–62. [Google Scholar] [CrossRef]
- Wang, J.; Allan, A.C.; Wang, W.Q.; Yin, X.R. The Effects of Salicylic Acid on Quality Control of Horticultural Commodities. New Zealand J. Crop Hortic. Sci. 2022, 50, 99–117. [Google Scholar] [CrossRef]
- Sayyari, M.; Babalar, M.; Kalantari, S.; Serrano, M.; Valero, D. Effect of Salicylic Acid Treatment on Reducing Chilling Injury in Stored Pomegranates. Postharvest Biol. Technol. 2009, 53, 152–154. [Google Scholar] [CrossRef]
- Babalar, M.; Asghari, M.; Talaei, A.; Khosroshahi, A. Effect of Pre- and Postharvest Salicylic Acid Treatment on Ethylene Production, Fungal Decay and Overall Quality of Selva Strawberry Fruit. Food Chem. 2007, 105, 449–453. [Google Scholar] [CrossRef]
- Asghari, M.; Aghdam, M.S. Impact of Salicylic Acid on Post-Harvest Physiology of Horticultural Crops. Trends Food Sci. Technol. 2010, 21, 502–509. [Google Scholar] [CrossRef]
- Leslie, C.A.; Romani, R.J. Inhibition of Ethylene Biosynthesis by Salicylic Acid. Plant Physiol. 1988, 88, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.K.; Dwivedi, U.N. Delayed Ripening of Banana Fruit by Salicylic Acid. Plant Sci. 2000, 158, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Elmenofy, H.M.; Okba, S.K.; Salama, A.M.; Alam-Eldein, S.M. Yield, Fruit Quality, and Storability of “Canino” Apricot in Response to Aminoethoxyvinylglycine, Salicylic Acid, and Chitosan. Plants 2021, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Bi, Y.; Li, Y.; Wang, Y.; Prusky, D.; Alkan, N. Preharvest Elicitors Spray Improves Antioxidant Activity, Alleviates Chilling Injury, and Maintains Quality in Harvested Fruit. Horticulturae 2022, 8, 1208. [Google Scholar] [CrossRef]
- Johnson, D.S.; Colgan, R. Low Ethylene Controlled Atmosphere Induces Adverse Effects on the Quality of ‘Cox’s Orange Pippin’ Apples Treated with Aminoethoxyvinylglycine during Fruit Development. Postharvest Biol. Technol. 2003, 27, 59–68. [Google Scholar] [CrossRef]
- Balaguera-López, H.; Salamanca-Gutiérrez, F.; García, J.; Herrera, A. Ethylene and Maturation Retardants in the Postharvest of Perishable Horticultural Products. A Review. Rev. Colomb. Cienc. Hortícolas 2014, 8, 302–313. [Google Scholar] [CrossRef]
- Capitani, G.; Tschopp, M.; Eliot, A.C.; Kirsch, J.F.; Grütter, M.G. Structure of ACC Synthase Inactivated by the Mechanism-Based Inhibitor l-Vinylglycine. FEBS Lett. 2005, 579, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Saltveit, M.E. Aminoethoxyvinylglycine (AVG) Reduces Ethylene and Protein Biosynthesis in Excised Discs of Mature-Green Tomato Pericarp Tissue. Postharvest Biol. Technol. 2005, 35, 183–190. [Google Scholar] [CrossRef]
- Ozturk, B.; Kucuker, E.; Yıldız, K.; Celik, S.M. Changes of Bioactive Compounds and Ethylene Production of Japanese Plums Treated with Pre-Harvest Aminoethoxyvinylglycine. Int. J. Food Prop. 2015, 18, 2165–2186. [Google Scholar] [CrossRef]
- Ozturk, B.; Uzun, S.; Karakaya, O. Combined Effects of Aminoethoxyvinylglycine and MAP on the Fruit Quality of Kiwifruit during Cold Storage and Shelf Life. Sci. Hortic. 2019, 251, 209–214. [Google Scholar] [CrossRef]
- Yuan, R.; Carbaugh, D.H. Effects of NAA, AVG, and 1-MCP on Ethylene Biosynthesis, Preharvest Fruit Drop, Fruit Maturity, and Quality of ‘Golden Supreme’ and ‘Golden Delicious’ Apples. HortScience Horts 2007, 42, 101–105. [Google Scholar] [CrossRef]
- Muñoz-Robredo, P.; Rubio, P.; Infante, R.; Campos-Vargas, R.; Manríquez, D.; González-Agüero, M.; Defilippi, B.G. Ethylene Biosynthesis in Apricot: Identification of a Ripening-Related 1-Aminocyclopropane-1-Carboxylic Acid Synthase (ACS) Gene. Postharvest Biol. Technol. 2012, 63, 85–90. [Google Scholar] [CrossRef]
- Xie, X.; Einhorn, T.; Wang, Y. Inhibition of Ethylene Biosynthesis and Associated Gene Expression by Aminoethoxyvinylglycine and 1-Methylcyclopropene and Their Consequences on Eating Quality and Internal Browning of ‘Starkrimson’ Pears. J. Am. Soc. Hortic. Sci. J. Amer. Soc. Hort. Sci. 2015, 140, 587–596. [Google Scholar] [CrossRef]
- Kim, Y.T.; Ha, S.T.T.; Chun, I.; In, B.C. Inhibition of Ethylene Binding and Biosynthesis Maintains Fruit Quality of “Formosa” Plums during Postharvest Storage. Hortic. Sci. Technol. 2021, 39, 368–378. [Google Scholar] [CrossRef]
- Win, N.M.; Yoo, J.; Lwin, H.P.; Lee, E.J.; Kang, I.K.; Lee, J. Effects of 1-Methylcyclopropene and Aminoethoxyvinylglycine Treatments on Fruit Quality and Antioxidant Metabolites in Cold-Stored “Sangjudungsi” Persimmons. Hortic. Environ. Biotechnol. 2021, 62, 891–905. [Google Scholar] [CrossRef]
- Salazar, N.A.; Molina-Corral, F.J.; Aguilar, G.; Otero, A.; Sepulveda, D.R.; Olivas, G. Volatile Production by ‘Golden Delicious’ Apples Is Affected by Preharvest Application of Aminoethoxyvinylglycine. Sci. Hortic. 2011, 130, 436–444. [Google Scholar] [CrossRef]
- Romani, R.; Labavitch, J.; Yamashita, T.; Hess, B.; Rae, H. Preharvest AVG Treatment of ‘Bartlett’ Pear Fruits: Effects on Ripening, Color Change, and Volatiles. J. Am. Soc. Hortic. Sci. 1983, 108, 1046–1049. [Google Scholar] [CrossRef]
- López, P.; Neisa, D.P.; Bacca, C.; Flórez, V.J. Evaluación de Preservantes Florales En La Poscosecha de Tres Variedades de Clavel Estándar. Agron. Colomb. 2008, 26, 116–126. [Google Scholar]
- Martínez-Romero, D.; Bailén, G.; Serrano, M.; Guillén, F.; Valverde, J.M.; Zapata, P.; Castillo, S.; Valero, D. Tools to Maintain Postharvest Fruit and Vegetable Quality through the Inhibition of Ethylene Action: A Review. Crit. Rev. Food Sci. Nutr. 2007, 47, 543–560. [Google Scholar] [CrossRef] [PubMed]
- Stabyl, G.; Basel, R.; Reid, M.; Dodge, L. Efficacies of Commercial Anti Ethylene Products for Fresh Cut Flowers. HortTechnology 1993, 3, 199–202. [Google Scholar] [CrossRef]
- Bulantseva, E.A.; Nguyen, T.T.; Ruzhitsky, A.O.; Protsenko, M.A.; Korableva, N.P. The Effect of Ethylene Biosynthesis Regulators on Metabolic Processes in the Banana Fruits in Various Physiological States. Appl. Biochem. Microbiol. 2009, 45, 93–96. [Google Scholar] [CrossRef]
- Lima, P.C.C.; Santos, M.N.D.; Guimaraes, M.E.D.; de Araujo, N.O.; Krause, M.R.; Finger, F.L. Ethylene and Its Inhibitors Affect the Quality of Processed Sweet Potatoes. Food Sci. Technol. 2021, 41, 825–832. [Google Scholar] [CrossRef]
- Kovaleva, L.V.; Zakharova, E.V.; Timofeeva, G.V.; Andreev, I.M.; Golivanov, Y.Y.; Bogoutdinova, L.R.; Baranova, E.N.; Khaliluev, M.R. Aminooxyacetic Acid (AOA), Inhibitor of 1-Aminocyclopropane-1-Carboxilic Acid (ACC) Synthesis, Suppresses Self-Incompatibility-Induced Programmed Cell Death in Self-Incompatible Petunia hybrida L. Pollen Tubes. Protoplasma 2020, 257, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Instituto Latinoamericano de la Comunicación Educativa ¿Que Es Una Zeolita? Available online: http://bibliotecadigital.ilce.edu.mx/sites/ciencia/volumen1/ciencia2/55/htm/sec_3.html (accessed on 24 May 2024).
- Patdhanagul, N.; Rangsriwatananon, K.; Siriwong, K.; Hengrasmee, S. Combined Modification of Zeolite NaY by Phenyl Trimethyl Ammonium Bromide and Potassium for Ethylene Gas Adsorption. Microporous Mesoporous Mater. 2012, 153, 30–34. [Google Scholar] [CrossRef]
- Coloma, A.; Rodríguez, F.; Bruna, J.; Guarda, A.; Galotto, M. Development of an Active Film with Natural Zeolite as Ethylene Scavenger. J. Chil. Chem. Soc. 2014, 59, 2409–2414. [Google Scholar] [CrossRef]
- Erdoğan, B.; Sakızcı, M.; Yörükoğulları, E. Characterization and Ethylene Adsorption of Natural and Modified Clinoptilolites. Appl. Surf. Sci. 2008, 254, 2450–2457. [Google Scholar] [CrossRef]
- Patdhanagul, N.; Srithanratana, T.; Rangsriwatananon, K.; Hengrasmee, S. Ethylene Adsorption on Cationic Surfactant Modified Zeolite NaY. Microporous Mesoporous Mater. 2010, 131, 97–102. [Google Scholar] [CrossRef]
- de Bruijn, J.; Gomez, A.; Loyola, C.; Melin, P.; Solar, V.; Abreu, N.; Azzolina-Jury, F.; Valdes, H. Use of a Copper- and Zinc-Modified Natural Zeolite to Improve Ethylene Removal and Postharvest Quality of Tomato Fruit. Crystals 2020, 10, 471. [Google Scholar] [CrossRef]
- Mariah, M.A.; Vonnie, J.M.; Erna, K.H.; Nur’Aqilah, N.M.; Huda, N.; Abdul Wahab, R.; Rovina, K. The Emergence and Impact of Ethylene Scavengers Techniques in Delaying the Ripening of Fruits and Vegetables. Membranes 2022, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Carbotecnia ¿Qué Es El Carbón Activado y Para Qué Sirve? Available online: https://www.carbotecnia.info/aprendizaje/carbon-activado/que-es-carbon-activado/ (accessed on 15 February 2024).
- Bailén, G.; Guillén, F.; Castillo, S.; Zapata, P.J.; Serrano, M.; Valero, D.; Martínez-Romero, D. Use of a Palladium Catalyst to Improve the Capacity of Activated Carbon to Absorb Ethylene, and Its Effect on Tomato Ripening. Span. J. Agric. Res. 2007, 5, 579–586. [Google Scholar] [CrossRef]
- Jaimun, R.; Sangsuwan, J. Efficacy of Chitosan-Coated Paper Incorporated with Vanillin and Ethylene Adsorbents on the Control of Anthracnose and the Quality of Nam Dok Mai Mango Fruit. Packag. Technol. Sci. 2019, 32, 383–394. [Google Scholar] [CrossRef]
- Kuppler, R.J.; Timmons, D.J.; Fang, Q.-R.; Li, J.-R.; Makal, T.A.; Young, M.D.; Yuan, D.; Zhao, D.; Zhuang, W.; Zhou, H.-C. Potential Applications of Metal-Organic Frameworks. Coord. Chem. Rev. 2009, 253, 3042–3066. [Google Scholar] [CrossRef]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Dhumal, S.; Abeli, P.; Beaudry, R.; Almenar, E. Metal-Organic Frameworks Have Utility in Adsorption and Release of Ethylene and 1-Methylcyclopropene in Fresh Produce Packaging. Postharvest Biol. Technol. 2017, 130, 48–55. [Google Scholar] [CrossRef]
- Shorter, A.J.; Scott, K.J.; Ward, G.; Best, D.J. Effect of Ethylene Absorption on the Storage of Granny Smith Apples Held in Polyethylene Bags. Postharvest Biol. Technol. 1992, 1, 189–194. [Google Scholar] [CrossRef]
- Álvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Avalos-Belmontes, F.; Rodríguez-Hernández, A.M.; Castillo-Campohermoso, M.A.; Artés-Hernández, F. An Innovative Ethylene Scrubber Made of Potassium Permanganate Loaded on a Protonated Montmorillonite: A Case Study on Blueberries. Food Bioprocess Technol. 2019, 12, 524–538. [Google Scholar] [CrossRef]
- Mujtaba, A.; Masud, T.; Butt, S.J.; Qazalbash, M.; Fareed, W.; Shahid, A. Potential Role of Calcium Chloride, Potassium Permanganate and Boric Acid on Quality Maintenance of Tomato Cv. Rio Grandi at Ambient Temperature. Int. J. Biosci. 2014, 5, 9–20. [Google Scholar]
- Scott, K.J.; Wills, R.B.H. Reduction of Brown Heart in Pears by Absorption of Ethylene from the Storage Atmosphere. Aust. J. Exp. Agric. 1974, 14, 266–268. [Google Scholar] [CrossRef]
- Illeperuma, C.K.; Nikapitiya, C. Extension of the Postharvest Life of `Pollock’ Avocado Using Modified Atmosphere Packaging. Fruits 2002, 57, 287–295. [Google Scholar] [CrossRef]
- García, J.; Herrera, A.; García, J. Conservación Del Fruto de Banano Bocadillo (Musa AA Simmonds) Con La Aplicación de Permanganato de Potasio (KMnO4). Rev. Colomb. De Cienc. Hortícolas 2012, 6, 161–171. [Google Scholar] [CrossRef]
- Çelik, S.; Bal, E. The Effects of Postharvest Treatments of Salicylic Acid and Potassium Permanganate on the Storage of Kiwifruit. Bulg. J. Agric. Sci. 2010, 16, 576–584. [Google Scholar]
- Giraldo, E.; Szczerbanik, M.J.; Scottpez, K.J.; Paton, J.E.; Best, D.J. Effects of Polyethylene Bags, Ethylene Absorbent and 1-Methylcyclopropene on the Storage of Japanese Pears. J. Hortic. Sci. Biotechnol. 2005, 80, 162–166. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, G.H.; Kim, S.-W. Ethylene Gas Decomposition Using ZSM-5/WO3-Pt-Nanorod Composites for Fruit Freshness. ACS Sustain. Chem. Eng. 2019, 7, 11250–11257. [Google Scholar] [CrossRef]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active Packaging from Chitosan-Titanium Dioxide Nanocomposite Film for Prolonging Storage Life of Tomato Fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Yu, Y.; Aisikaer, G.; Ying, T. Postharvest UV-C Irradiation Inhibits the Production of Ethylene and the Activity of Cell Wall-Degrading Enzymes during Softening of Tomato (Lycopersicon esculentum L.) Fruit. Postharvest Biol. Technol. 2013, 86, 337–345. [Google Scholar] [CrossRef]
- Mabusela, B.P.; Belay, Z.A.; Godongwana, B.; Pathak, N.; Mahajan, P.V.; Caleb, O.J. Advances in Vacuum Ultraviolet Photolysis in the Postharvest Management of Fruit and Vegetables Along the Value Chains: A Review. Food Bioprocess Technol. 2022, 15, 28–46. [Google Scholar] [CrossRef]
- Collazo Cordero, C.; Charles, F.; Aguiló-Aguayo, I.; Marín-Sáez, J.; Lafarga, T.; Abadias, M.; Vinas, I. Decontamination of Listeria Innocua from Fresh-Cut Broccoli Using UV-C Applied in Water or Peroxyacetic Acid, and Dry-Pulsed Light. Innov. Food Sci. Emerg. Technol. 2019, 52, 438–449. [Google Scholar] [CrossRef]
- Orhewere, A.; Ajayi, O.; Ajayi, A. Advances in the Development of a Tomato Postharvest Storage System: Towards Eradicating Postharvest Losses. J. Phys. Conf. Ser. 2019, 1378, 22064. [Google Scholar] [CrossRef]
- Pathak, N.; Caleb, O.J.; Rauh, C.; Mahajan, P. V Efficacy of Photocatalysis and Photolysis Systems for the Removal of Ethylene under Different Storage Conditions. Postharvest Biol. Technol. 2019, 147, 68–77. [Google Scholar] [CrossRef]
- Ibhadon, A.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Alves, M.J.D.; Nobias, M.C.; Soares, L.S.; Coelho, D.S.; Maraschin, M.; Basso, A.; Moreira, R.; Jose, H.J.; Monteiro, A.R. Physiological Changes in Green and Red Cherry Tomatoes after Photocatalytic Ethylene Degradation Using Continuous Air Flux. Food Sci. Technol. Int. 2023, 29, 3–12. [Google Scholar] [CrossRef]
- Li, W.H.; Liu, Z.Y.; Li, X.J.; Li, X.H. Quality Maintenance of 1-Methylcyclopropene Combined with Titanium Dioxide Photocatalytic Reaction on Postharvest Cherry Tomatoes. J. Food Process. Preserv. 2022, 46, e16500. [Google Scholar] [CrossRef]
- Fonseca, J.D.; Pabon, N.Y.L.; Nandi, L.G.; Valencia, G.A.; Moreira, R.; Monteiro, A.R. Gelatin-TiO2-Coated Expanded Polyethylene Foam Nets as Ethylene Scavengers for Fruit Postharvest Application. Postharvest Biol. Technol. 2021, 180, 111602. [Google Scholar] [CrossRef]
- Ghosh, A.; Saha, I.; Fujita, M.; Debnath, S.C.; Hazra, A.K.; Adak, M.K.; Hasanuzzaman, M. Photoactivated TiO2 Nanocomposite Delays the Postharvest Ripening Phenomenon through Ethylene Metabolism and Related Physiological Changes in Capsicum Fruit. Plants 2022, 11, 513. [Google Scholar] [CrossRef] [PubMed]
- de Chiara, M.L.V.; Pal, S.; Licciulli, A.; Amodio, M.L.; Colelli, G. Photocatalytic Degradation of Ethylene on Mesoporous TiO2/SiO2 Nanocomposites: Effects on the Ripening of Mature Green Tomatoes. Biosyst. Eng. 2015, 132, 61–70. [Google Scholar] [CrossRef]
- Chawengkijwanich, C.; Hayata, Y. Efficiency of TiO2 Photocatalytic Reaction on Delay of Fruit Ripening and Removal of Off-Flavors from the Fruit Storage Atmosphere. Trans. ASABE 2006, 49, 833–837. [Google Scholar] [CrossRef]
- Fonseca, J.D.; Alves, M.J.D.; Soares, L.S.; Moreira, R.; Valencia, G.A.; Monteiro, A.R. A Review on TiO2-Based Photocatalytic Systems Applied in Fruit Postharvest: Set-Ups and Perspectives. Food Res. Int. 2021, 144, 110378. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Wang, B.; Jin, W.F. Experimental Investigation on Decomposition of Ethylene by Ozone: Harmful Product, Food Safety, and Control Strategy. J. Food Process. Preserv. 2021, 45, e15861. [Google Scholar] [CrossRef]
- Liang, Y.Z.; Ji, L.L.; Chen, C.K.; Dong, C.H.; Wang, C.R. Effects of Ozone Treatment on the Storage Quality of Post-Harvest Tomato. Int. J. Food Eng. 2018, 14, 20180012. [Google Scholar] [CrossRef]
- Toti, M.; Carboni, C.; Botondi, R. Postharvest Gaseous Ozone Treatment Enhances Quality Parameters and Delays Softening in Cantaloupe Melon during Storage at 6 °C. J. Sci. Food Agric. 2018, 98, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.C.; Lin, Q.; Dong, C.H.; Ji, H.P.; Yu, J.Z.; Chen, C.K.; Zhu, Z.Q.; Ban, Z.J.; Zhang, N.; Bao, Y.Y. Effects of Ozone Concentration on the Postharvest Quality and Microbial Diversity of Muscat Hamburg Grapes. RSC Adv. 2020, 10, 9037–9045. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, X.S.; Huang, Z.C.; Qu, K.; Shi, W.B.; Peng, Z.M.; Zeng, L.M.; Xie, S.D.; Zhang, Y.H. Impacts of Synoptic Circulation on Surface Ozone Pollution in a Coastal Eco-City in Southeastern China during 2014–2019. J. Environ. Sci. 2023, 127, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Mok, Y.S.; Kim, S.G.; Han, J.; Nguyen, D.B.; Lee, H.W.; Jeon, H.; Kim, S.B. Removal of Dilute Ethylene Using Repetitive Cycles of Adsorption and Plasma-Catalytic Oxidation over Pd/ZSM-5 Catalyst. J. Phys. D Appl. Phys. 2020, 53, 334002. [Google Scholar] [CrossRef]
- Tzeng, J.H.; Weng, C.H.; Huang, J.W.; Shiesh, C.C.; Lin, Y.H.; Lin, Y.T. Application of Palladium-Modified Zeolite for Prolonging Post-Harvest Shelf Life of Banana. J. Sci. Food Agric. 2019, 99, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Yadav, S.K. Recent Advances in Cold Plasma Technology for Food Processing. Food Eng. Rev. 2022, 14, 555–578. [Google Scholar] [CrossRef]
- Gururani, P.; Bhatnagar, P.; Bisht, B.; Kumar, V.; Joshi, N.C.; Tomar, M.S.; Pathak, B. Cold Plasma Technology: Advanced and Sustainable Approach for Wastewater Treatment. Environ. Sci. Pollut. Res. 2021, 28, 65062–65082. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Kardam, S.K.; Kadam, A.A.; Singh, S.P.; Kumar, V.; Gaikwad, K.K. Ethylene Gas Scavenging Properties of Nano-Silica Isolated from Rice Straw and of Its PVA-Based Formulation Coated on Duplex Paperboard Made from Softwood and Rice Straw. Biomass Convers. Biorefinery 2023, 1–10. [Google Scholar] [CrossRef]
- Gundewadi, G.; Rudra, S.G.; Prasanna, R.; Banerjee, T.; Singh, S.K.; Dhakate, S.R.; Gupta, A.; Anand, A. Palladium Encapsulated Nanofibres for Scavenging Ethylene from Sapota Fruits. Front. Nutr. 2022, 9, 994813. [Google Scholar] [CrossRef] [PubMed]
- Rupérez, D.; Gracia-Vallés, N.; Clavero, E.; Silva, F.; Nerín, C. Mechanochemically Scaled-Up Alpha Cyclodextrin Nanosponges: Their Safety and Effectiveness as Ethylene Scavenger. Nanomaterials 2022, 12, 2900. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; Kathuria, A.; Gaikwad, K.K. Metal–Organic Frameworks for Active Food Packaging. A Review. Environ. Chem. Lett. 2022, 20, 1479–1495. [Google Scholar] [CrossRef] [PubMed]
- Büchele, F.; Hivare, K.; Khera, K.; Thewes, F.R.; Argenta, L.C.; Hoffmann, T.G.; Mahajan, P.V.; Prange, R.K.; Pareek, S.; Neuwald, D.A. Novel Energy-Saving Strategies in Apple Storage: A Review. Sustainability 2024, 16, 1052. [Google Scholar] [CrossRef]
- Abedrabboh, O.; Koç, M.; Biçer, Y. Sustainability Performance of Space-Cooling Technologies and Approaches. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 9017–9042. [Google Scholar] [CrossRef]
- Yenare, R.; Sonawane, C.; Sur, A.; Singh, B.; Panchal, H.; Kumar, A.; Sadasivuni, K.K.; Siddiqui, M.; Bhalerao, Y. A Comprehensive Review of Portable Cold Storage: Technologies, Applications, and Future Trends. Alex. Eng. J. 2024, 94, 23–33. [Google Scholar] [CrossRef]
- Marimuthu, S.; Saikumar, A.; Badwaik, L. Food Losses and Wastage within Food Supply Chain: A Critical Review of Its Generation, Impact, and Conversion Techniques. Waste Dispos. Sustain. Energy 2024, 1–16. [Google Scholar] [CrossRef]
- Onyeaka, H.; Ghosh, S.; Obileke, K.; Miri, T.; Odeyemi, O.; Nwaiwu, O.; Tamasiga, P. Preventing Chemical Contaminants in Food: Challenges and Prospects for Safe and Sustainable Food Production. Food Control 2023, 155, 110040. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, G.; Song, J.; Dai, J.; Shi, W.; Wang, L. Food Safety Practices of Food Handlers in China and Their Correlation with Self-Reported Foodborne Illness. J. Food Prot. 2024, 87, 100202. [Google Scholar] [CrossRef] [PubMed]
- Usman, A. Influence of Agricultural Practices on Food Safety in Nigeria. Int. J. Food Sci. 2024, 7, 43–54. [Google Scholar] [CrossRef]
- Kumar, N.; Devgan, K.; Kumar, A.; Kaur, P.; Mahajan, P. Active and Passive Modified Atmosphere Packaging. In Nonthermal Food Engineering Operations; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 225–259. ISBN 9781119776468. [Google Scholar]
- Nian, L.; Wang, M.; Sun, X.; Zeng, Y.; Xie, Y.; Cheng, S.; Cao, C. Biodegradable Active Packaging: Components, Preparation, and Applications in the Preservation of Postharvest Perishable Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2024, 64, 2304–2339. [Google Scholar] [CrossRef] [PubMed]
- Ozal, G.; Ilyasova, C.; Ilgiz, V. Post-Harvest Storage and Processing Technology in Russia: Reducing Yield Loss. Techno Agric. Stud. Res. 2024, 1, 28–40. [Google Scholar]
- Mavai, S.; Bains, A.; Sridhar, K.; Rashid, S.; Elossaily, G.M.; Ali, N.; Chawla, P.; Sharma, M. Formulation and Application of Poly Lactic Acid, Gum, and Cellulose-Based Ternary Bioplastic for Smart Food Packaging: A Review. Int. J. Biol. Macromol. 2024, 268, 131687. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Prasad, A.; Sonika; Katiyar, V. State of Art Review on Sustainable Biodegradable Polymers with a Market Overview for Sustainability Packaging. Mater. Today Sustain. 2024, 26, 100776. [Google Scholar] [CrossRef]
- Singh, A.; Itkor, P.; Lee, M.; Shin, J.; Lee, Y.S. Promoting Sustainable Packaging Applications in the Circular Economy by Exploring and Advancing Molded Pulp Materials for Food Products: A Review. Crit. Rev. Food Sci. Nutr. 2022, 63, 11010–11025. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.; Abdelkhalek, A. Packaging of the Future: Smart Technologies and Food Quality and Safety. In Intelligent Packaging; Academic Press: Cambridge, MA, USA, 2024; pp. 1–30. ISBN 9780443153884. [Google Scholar]
- Arijeniwa, F.; Akinsemolu, A.; Chukwugozie, D.; Onawo, G.; Ochulor, C.; Uju, N.; Kawino, D.; Onyeaka, H. Closing the Loop: A Framework for Tackling Single-Use Plastic Waste in the Food and Beverage Industry through Circular Economy—A Review. J. Environ. Manag. 2024, 359, 120816. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.; Ting, H.; Atanda, A. Enhancing Supply Chain Traceability through Blockchain and IoT Integration: A Comprehensive Review. Green Intell. Syst. Appl. 2024, 4, 11–28. [Google Scholar] [CrossRef]
- Ninama, N.; Gangal, L.; Khayum, A.; Harshitha, S.B.; Divya, C.; Sowmya, H.M.; Singh, A. Anushi Post-Harvest Biotechnology or Genetic Engineering Solutions: Extending Shelf Life and Reducing Food Waste. J. Adv. Biol. Biotechnol. 2024, 27, 1–26. [Google Scholar] [CrossRef]
- Shi, C.; Xiang, L.; Jiahu, G. Exploring the Frontier of Fruit Diseases Management: Advances in Nano-Based and Biocontrol Strategies and Underlying Action Mechanism. S. Afr. J. Bot. 2024, 166, 612–623. [Google Scholar] [CrossRef]
- Doerflinger, F.; Al Shoffe, Y.; Sutano, G.; Nock, J.F.; Watkins, C. Preharvest 1-Methylcyclopropene (1-MCP) Treatment Effects on Quality of Spot and Strip Picked ‘Gala’ Apples at Harvest and after Storage as Affected by Postharvest 1-MCP and Temperature Conditioning Treatments. Sci. Hortic. 2023, 325, 112682. [Google Scholar] [CrossRef]
- Zhu, M.; Li, J.; Liu, Y.; Wang, Q.; Fan, Z.; Zeng, J.; Yu, J. Preharvest Nano-Calcium Reduces the Table Grape Berry Abscission by Regulating Ethylene Production During Storage. J. Plant Growth Regul. 2023, 43, 1400–1409. [Google Scholar] [CrossRef]
- Opara, U.; Pathare, P. Mechanical Damage of Fresh Produce: An Overview. In Mechanical Damage in Fresh Horticultural Produce: Measurement, Analysis and Control; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–19. ISBN 978-981-99-7095-7. [Google Scholar]
- Baloyi, R.G.; Mafeo, T.P.; Mathaba, N. Effect of Pre- and Post-Harvest Factors on ‘Benny’ Valencia Fruit Rind Phenolics on Mitigation of Chilling and Non-Chilling Disorders during Cold Storage. J. Hortic. Postharvest Res. 2023, 6, 299–316. [Google Scholar] [CrossRef]
- Rattanakaran, J.; Saengrayap, R.; Prahsarn, C.; Kitazawa, H.; Chaiwong, S. Application of Room Cooling and Thermal Insulation Materials to Maintain Quality of Okra during Storage and Transportation. Horticulturae 2021, 7, 188. [Google Scholar] [CrossRef]
- Waldhans, C.; Albrecht, A.; Ibald, R.; Wollenweber, D.; Sy, S.-J.; Kreyenschmidt, J. Temperature Control and Data Exchange in Food Supply Chains: Current Situation and the Applicability of a Digitalized System of Time–Temperature-Indicators to Optimize Temperature Monitoring in Different Cold Chains. J. Packag. Technol. Res. 2024, 8, 79–93. [Google Scholar] [CrossRef]
- Bisht, A.; Singh, S. Postharvest Losses and Management of Horticultural Produce: A Review. J. Sci. Res. Rep. 2024, 30, 305–320. [Google Scholar] [CrossRef]
- Gupta, N.; Daruwalla, F. Examining the Effectiveness of Green Marketing Communication on Consumer Behavior towards Sustainable Purchases. Educ. Adm. Theory Pract. 2024, 30, 6861–6868. [Google Scholar] [CrossRef]
- Uda, S.; Basrowi, B. Environmental Education Using SARITHA-Apps to Enhance Environmentally Friendly Supply Chain Efficiency and Foster Environmental Knowledge towards Sustainability. Uncertain Supply Chain Manag. 2024, 12, 359–372. [Google Scholar] [CrossRef]




| Format | Concentration | Fruit | Conditions | Significative results | Reference |
|---|---|---|---|---|---|
| LDPE Sachet | Saturated and dried KMnO4 solution (50 mL) together with natural clays and activated carbon | ‘Pollock’ Avocado | 12 °C, 94% RH, 21 days | Reduction in CO2 and C2H4 concentrations in the packaging. Maintained good visual quality. Inhibited disease incidence and reduced chilling damage. Delayed weight and firmness losses. Soluble solid content and flesh colour were not significantly affected. | [215] |
| LDPE Sachet | 5 g KMnO4 and vermiculite mixture at doses of 1.5 and 1% w/w | ‘Musa’ Banana | 18 °C, 70–80% RH, 16 days | Delayed yellowing of the skin. Slower increase in CSS and decrease in AT. Reduced loss in firmness and weight. Minimal increase in the SSC/TA ratio. | [216] |
| Sachet | Mixture of natural clays and KMnO4 (9 g per kg of fruit) | ‘Hayward’ Kiwi | 0 °C, 85–95% RH, 200 days | Delayed firmness. Reduction in the increase in SSC and decrease in TA. Reduction in total chlorophyll and ascorbic acid degradation. | [217] |
| LDPE Sachet | 20 g KMnO4 together with aluminium oxide | ‘Nijisseiki’ Pear | 0 °C, 90% RH, 252 days | Delayed yellowing. Decrease in C2H4 levels and respiration rate. Reduced severity of disorders (browning of flesh and heart). | [218] |
| Sachet | 20% KMnO4 in bentonite zeolite (2:1 w/w) | ‘Valouro’ Tomato | 7 °C, 90% RH, 35 days | Reduction in C2H4. Delayed softening of the fruit. Minimization of the rate of decrease in phenol content and antioxidant activity. Decreased ascorbic content and increased lycopene content. Delayed water weight loss and reduced severity of rotting. | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramiro, A.-S.; Santiago, L.-M.; Antonio José, P.-L.; José Ramón, A.-M. Strategies to Delay Ethylene-Mediated Ripening in Climacteric Fruits: Implications for Shelf Life Extension and Postharvest Quality. Horticulturae 2024, 10, 840. https://doi.org/10.3390/horticulturae10080840
Ramiro A-S, Santiago L-M, Antonio José P-L, José Ramón A-M. Strategies to Delay Ethylene-Mediated Ripening in Climacteric Fruits: Implications for Shelf Life Extension and Postharvest Quality. Horticulturae. 2024; 10(8):840. https://doi.org/10.3390/horticulturae10080840
Chicago/Turabian StyleRamiro, Alonso-Salinas, López-Miranda Santiago, Pérez-López Antonio José, and Acosta-Motos José Ramón. 2024. "Strategies to Delay Ethylene-Mediated Ripening in Climacteric Fruits: Implications for Shelf Life Extension and Postharvest Quality" Horticulturae 10, no. 8: 840. https://doi.org/10.3390/horticulturae10080840






