Physiological Responses of Hollyhock (Alcea rosea L.) to Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
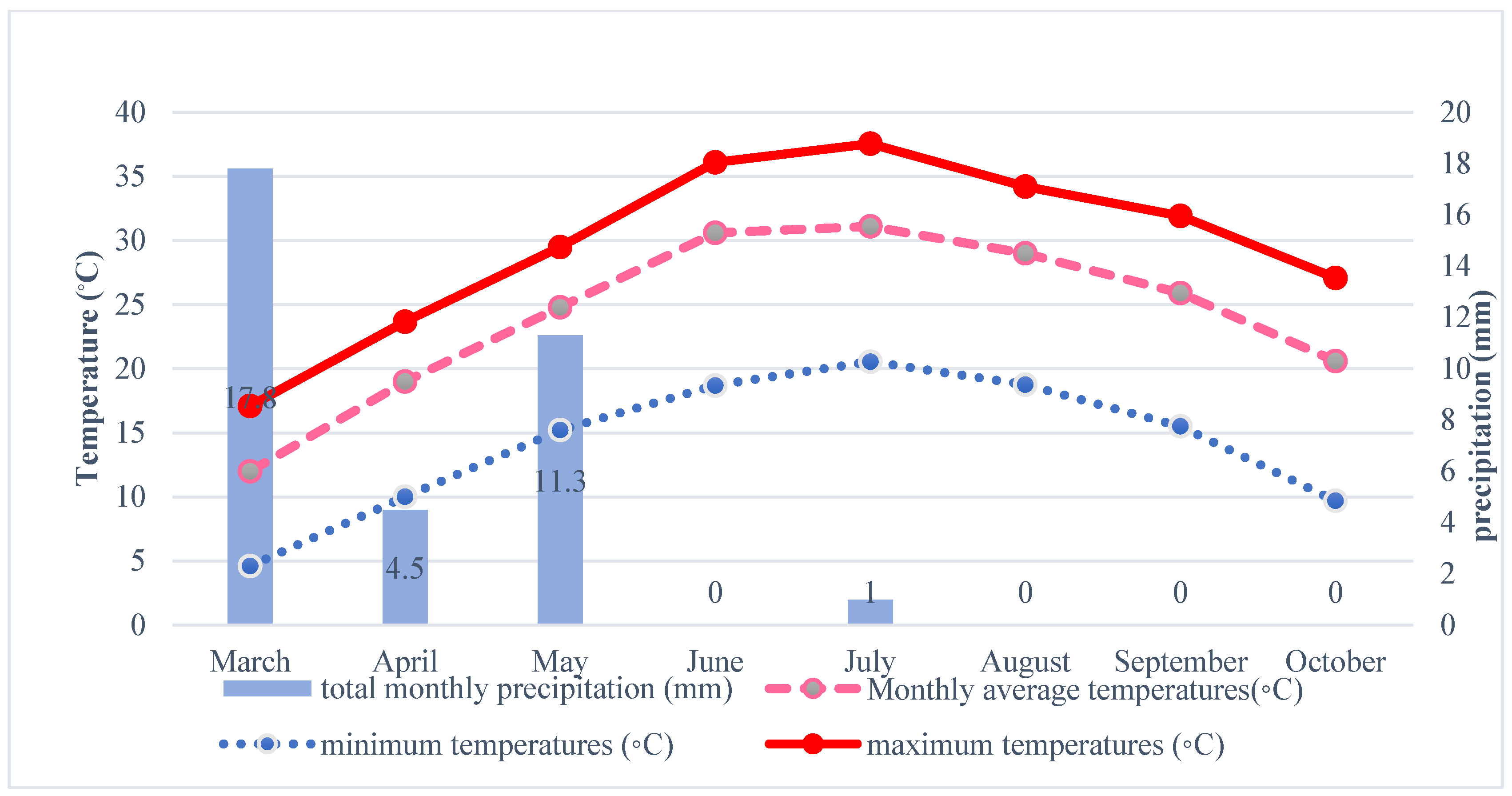
2.1. Plant Materials and Growth Conditions
2.2. Drought Treatments
2.3. Physiological Measurements
2.4. Statistical Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to Drought and Salt Stress in Plants: Unraveling the Signaling Networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Ashraf, U.; Zohaib, A.; Tanveer, M.; Naeem, M.; Ali, I.; Tabassum, T.; Nazir, U. Žemės Ūkio Augalų Reakcija į Sausros Sukurtą Stresą: Apžvalga. Zemdirbyste 2017, 104, 267–276. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, S.; Hasan, W.; Ul-Allah, S.; Tanveer, M.; Farooq, M.; Nawaz, A. Drought Stress in Sunflower: Physiological Effects and Its Management through Breeding and Agronomic Alternatives. Agric. Water Manag. 2018, 201, 152–166. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research Progress and Perspective on Drought Stress in Legumes: A Review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef] [PubMed]
- Bayati, P.; Karimmojeni, H.; Razmjoo, J. Changes in Essential Oil Yield and Fatty Acid Contents in Black Cumin (Nigella sativa L.) Genotypes in Response to Drought Stress. Ind. Crops Prod. 2020, 155, 112764. [Google Scholar] [CrossRef]
- Ghadyeh Zarrinabadi, I.; Razmjoo, J.; Abdali Mashhadi, A.; Karim Mojeni, H.; Boroomand, A. Physiological Response and Productivity of Pot Marigold (Calendula officinalis) Genotypes under Water Deficit. Ind. Crops Prod. 2019, 139, 111488. [Google Scholar] [CrossRef]
- Abdel-Farid, I.B.; Marghany, M.R.; Rowezek, M.M.; Sheded, M.G. Effect of Salinity Stress on Growth and Metabolomic Profiling of Cucumis sativus and Solanum lycopersicum. Plants 2020, 9, 1626. [Google Scholar] [CrossRef] [PubMed]
- Kleinwächter, M.; Selmar, D. New Insights Explain That Drought Stress Enhances the Quality of Spice and Medicinal Plants: Potential Applications. Agron. Sustain. Dev. 2015, 35, 121–131. [Google Scholar] [CrossRef]
- Laribi, B.; Bettaieb, I.; Kouki, K.; Sahli, A.; Mougou, A.; Marzouk, B. Water Deficit Effects on Caraway (Carum carvi L.) Growth, Essential Oil and Fatty Acid Composition. Ind. Crops Prod. 2009, 30, 372–379. [Google Scholar] [CrossRef]
- Alinian, S.; Razmjoo, J.; Zeinali, H. Flavonoids, Anthocynins, Phenolics and Essential Oil Produced in Cumin (Cuminum cyminum L.) Accessions under Different Irrigation Regimes. Ind. Crops Prod. 2016, 81, 49–55. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Influencing the Product Quality by Deliberately Applying Drought Stress during the Cultivation of Medicinal Plants. Ind. Crops Prod. 2013, 42, 558–566. [Google Scholar] [CrossRef]
- Mombeini, T.; Pourbadie, H.G.; Kamalinejad, M.; Mazloumi, S.; Dehpour, A.R. Anxiolytic-like and Sedative Effects of Alcea aucheri (Boiss.) Alef. Flower Extract in the Laboratory Rat. Iran. J. Pharm. Res. 2017, 16, 1495–1508. [Google Scholar] [PubMed]
- Sadeghi, A.; Razmjoo, J.; Karimmojeni, H.; Baldwin, T.C. Differential Responses of Hollyhock (Alcea rosea L.) Varieties to Salt Stress in Relation to Physiological and Biochemical Parameters. Sci. Rep. 2024, 14, 8105. [Google Scholar] [CrossRef]
- Javed, S.; Ashraf, M.; Meraj, M.; Bukhari, S.; Zovia, I. Salinity and Drought Induced Antioxidant Responses in Different Cultivars of Safflower (Carthamus tinctorius L.). Curr. Pharm. Biotechnol. 2014, 14, 814–819. [Google Scholar] [CrossRef]
- Hayano-Kanashiro, C.; Calderón-Vásquez, C.; Ibarra-Laclette, E.; Herrera-Estrella, L.; Simpson, J. Analysis of Gene Expression and Physiological Responses in Three Mexican Maize Landraces under Drought Stress and Recovery Irrigation. PLoS ONE 2009, 4, e7531. [Google Scholar] [CrossRef]
- Ying, Y.Q.; Song, L.L.; Jacobs, D.F.; Mei, L.; Liu, P.; Jin, S.H.; Wu, J.S. Physiological Response to Drought Stress in Camptotheca Acuminata Seedlings from Two Provenances. Front. Plant Sci. 2015, 6, 361. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Razmjoo, J.; Karimmojeni, H.; Baldwin, T.C.; Mastinu, A. Changes in Secondary Metabolite Production in Response to Salt Stress in Alcea rosea L. Horticulturae 2024, 10, 139. [Google Scholar] [CrossRef]
- Shao, H.B.; Chu, L.Y.; Jaleel, C.A.; Manivannan, P.; Panneerselvam, R.; Shao, M.A. Understanding Water Deficit Stress-Induced Changes in the Basic Metabolism of Higher Plants–Biotechnologically and Sustainably Improving Agriculture and the Ecoenvironment in Arid Regions of the Globe. Crit. Rev. Biotechnol. 2009, 29, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, L.; Shen, Q.; Yang, J.; Han, X.; Tian, F.; Wu, J. Effects of Water Stress on Photosynthesis, Yield, and Water Use Efficiency in Winter Wheat. Water 2020, 12, 2127. [Google Scholar] [CrossRef]
- Wasaya, A.; Manzoor, S.; Yasir, T.A.; Sarwar, N.; Mubeen, K.; Ismail, I.A.; Raza, A.; Rehman, A.; Hossain, A.; Sabagh, A.E.L. Evaluation of Fourteen Bread Wheat (Triticum aestivum L.) Genotypes by Observing Gas Exchange Parameters, Relative Water and Chlorophyll Content, and Yield Attributes under Drought Stress. Sustainability 2021, 13, 4799. [Google Scholar] [CrossRef]
- Bandurska, H. Drought Stress Responses: Coping Strategy and Resistance. Plants 2022, 11, 922. [Google Scholar] [CrossRef]
- Samieadel, S.; Eshghizadeh, H.R.; Nematpour, A.; Majidi, M.M. Wheat Cultivars Responses to Drought Stress and Atmospheric CO2 Concentration Variability. Cereal Res. Commun. 2023, 51, 1–19. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids Pigments of Photosynthetic Biomembranes. Chlorophylls Carotenoids Pigment. Photosynth. Biomembr. 1987, 148, 350–382. [Google Scholar]
- Valentovič, P.; Luxová, M.; Kolarovič, L.; Gašparíková, O. Effect of Osmotic Stress on Compatible Solutes Content, Membrane Stability and Water Relations in Two Maize Cultivars. Plant Soil Environ. 2006, 52, 184. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive Oxygen Species Homeostasis and Signalling during Drought and Salinity Stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Antolín, M.C.; Muro, I.; Sánchez-Díaz, M. Application of Sewage Sludge Improves Growth, Photosynthesis and Antioxidant Activities of Nodulated Alfalfa Plants under Drought Conditions. Environ. Exp. Bot. 2010, 68, 75–82. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Chaves, A.R.M.; Pinheiro, H.A.; Ducatti, C.; Loureiro, M.E. Drought Tolerance of Two Field-Grown Clones of Coffea canephora. Plant Sci. 2003, 164, 111–117. [Google Scholar] [CrossRef]
- Reynolds, S.G. The Gravimetric Method of Soil Moisture Determination Part I A Study of Equipment, and Methodological Problems. J. Hydrol. 1970, 11, 258–273. [Google Scholar] [CrossRef]
- Adi, S.; Ghobadi, M.E.; Mohammadi, G.R.; Jalali-Honarmand, S. Antioxidant Capacity, Photosynthetic Characteristics and Water Relations of Sunflower (Helianthus annuus L.) Cultivars in Response to Drought Stress. Ind. Crops Prod. 2013, 50, 29–38. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Blume, D.E.; McClure, J.W. Developmental Effects of Sandoz 6706 on Activities of Enzymes of Phenolic and General Metabolism in Barley Shoots Grown in the Dark or under Low or High Intensity Light. Plant Physiol. 1980, 65, 238–244. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of Catalases and Peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wardlaw, I.F.; Willenbrink, J. Mobilization of Fructan Reserves and Changes in Enzyme Activities in Wheat Stems Correlate with Water Stress during Kernel Filling. New Phytol. 2000, 148, 413–422. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought Stress and Reactive Oxygen Species. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Cazzonelli, C.I. Carotenoids in Nature: Insights from Plants and Beyond. Funct. Plant Biol. 2011, 38, 833–847. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Review Article Plant Drought Stress: Effects, Mechanisms and Management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Efeoǧlu, B.; Ekmekçi, Y.; Çiçek, N. Physiological Responses of Three Maize Cultivars to Drought Stress and Recovery. S. Afr. J. Bot. 2009, 75, 34–42. [Google Scholar] [CrossRef]
- Stout, D.G.; Kannangara, T.; Sympson, G.M. Drought Resistance of Sorghum biocolor. 2. Water Stress Effects on Growth. J. Plant Sci. 1978, 58, 225–233. [Google Scholar]
- Mamnoei, E.; Seyed Sharifi, R. Study the Effects of Water Deficit on Chlorophyll Fluorescence Indices and the Amount of Proline in Six Barley Genotypes and Its Relation with Canopy Temperature and Yield. Iran. J. Plant Biol. 2010, 2, 51–62. [Google Scholar]
- Slabbert, M.M.; Krüger, G.H.J. Antioxidant Enzyme Activity, Proline Accumulation, Leaf Area and Cell Membrane Stability in Water Stressed Amaranthus Leaves. S. Afr. J. Bot. 2014, 95, 123–128. [Google Scholar] [CrossRef]
- Delauney, A.J.; Verma, D.P.S. Proline Biosynthesis and Osmoregulation in Plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Plaut, Z.; Butow, B.J.; Blumenthal, C.S.; Wrigley, C.W. Transport of Dry Matter into Developing Wheat Kernels and Its Contribution to Grain Yield under Post-Anthesis Water Deficit and Elevated Temperature. Field Crops Res. 2004, 86, 185–198. [Google Scholar] [CrossRef]
- Kundu, P.B.; Paul, N.K. Effects of Water Stress on Chlorophyll, Proline and Sugar Accumulation in Rape (Brassica campestris L.). Bangladesh J. Bot. 1997, 26, 83–85. [Google Scholar]
- Khurana, E.; Singh, J.S. Influence of Seed Size on Seedling Growth of Albizia procera under Different Soil Water Levels. Ann. Bot. 2000, 86, 1185–1192. [Google Scholar] [CrossRef]
- Siddique, M.R.B.; Hamid, A.; Islam, M.S. Drought Stress Effects on Water Relations of Wheat. Bot. Bull. Acad. Sin. 2000, 41, 35–39. [Google Scholar]
- Blum, A.; Ebercon, A. Cell Membrane Stability as a Measure of Drought and Heat Tolerance in Wheat. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Premachandra, G.S.; Saneoka, H.; Kanaya, M.; Ogata, S. Cell Membrane Stability and Leaf Surface Wax Content as Affected by Increasing Water Deficits in Maize. J. Exp. Bot. 1991, 42, 167–171. [Google Scholar] [CrossRef]
- Guttieri, M.J.; Stark, J.C.; O’Brien, K.; Souza, E. Relative Sensitivity of Spring Wheat Grain Yield and Quality Parameters to Moisture Deficit. Crop Sci. 2001, 41, 327–335. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General Mechanisms of Drought Response and Their Application in Drought Resistance Improvement in Plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Vandeleur, R.K.; Mayo, G.; Shelden, M.C.; Gilliham, M.; Kaiser, B.N.; Tyerman, S.D. The Role of Plasma Membrane Intrinsic Protein Aquaporins in Water Transport through Roots: Diurnal and Drought Stress Responses Reveal Different Strategies between Isohydric and Anisohydric Cultivars of Grapevine. Plant Physiol. 2009, 149, 445–460. [Google Scholar] [CrossRef]
- Kocheva, K.; Lambrev, P.; Georgiev, G.; Goltsev, V.; Karabaliev, M. Evaluation of Chlorophyll Fluorescence and Membrane Injury in the Leaves of Barley Cultivars under Osmotic Stress. Bioelectrochemistry 2004, 63, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Habibi, G. Effect of Drought Stress and Selenium Spraying on Photosynthesis and Antioxidant Activity of Spring Barley/Učinek Sušnega Stresa in Škropljenja s Selenom Na Fotosintezo in Antioksidativno Aktivnost Jarega Ječmena. Acta Agric. Slov. 2013, 101, 31–39. [Google Scholar] [CrossRef]
- Esfandiari, E.; Enayati, V.; Abbasi, A. Biochemical and Physiological Changes in Response to Salinity in Two Durum Wheat (Triticum turgidum L.) Genotypes. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 165–170. [Google Scholar] [CrossRef]
- Saeidi, M.; Ardalani, S.; Jalali-Honarmand, S.; Ghobadi, M.E.; Abdoli, M. Antioxidant Enzyme Responses and Crop Yield of Wheat under Drought Stress and Re-Watering at Vegetative Growth Period. Iran. J. Plant Physiol. 2018, 8, 2257–2267. [Google Scholar]
- Kolarovič, L.; Valentovič, P.; Luxová, M.; Gašparíková, O. Changes in Antioxidants and Cell Damage in Heterotrophic Maize Seedlings Differing in Drought Sensitivity after Exposure to Short-Term Osmotic Stress. Plant Growth Regul. 2009, 59, 21–26. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M.B. Antioxidant Responses to Drought in Sunflower and Sorghum Seedlings. New Phytol. 1996, 132, 361–373. [Google Scholar] [CrossRef]
- Patel, P.K.; Hemantaranjan, A.; Sarma, B.K.; Singh, R. Growth and Antioxidant System under Drought Stress in Chickpea (Cicer Arietinum L.) as Sustained by Salicylic Acid. J. Stress Physiol. Biochem. 2011, 7, 130–144. [Google Scholar]
- Lima, A.L.S.; DaMatta, F.M.; Pinheiro, H.A.; Totola, M.R.; Loureiro, M.E. Photochemical Responses and Oxidative Stress in Two Clones of Coffea Canephora under Water Deficit Conditions. Environ. Exp. Bot. 2002, 47, 239–247. [Google Scholar] [CrossRef]
- Heuer, B. Osmoregulatory Role of Proline in Water and Salt-Stressed Plants. Handb. Plant Crop Stress. 1994, 19, 363–381. [Google Scholar]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate Peroxidase and Catalase Activities and Their Genetic Regulation in Plants Subjected to Drought and Salinity Stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef]
- Kamarudin, Z.S.; Yusop, M.R.; Muda Mohamed, M.T.; Ismail, M.R.; Harun, A.R. Growth Performance and Antioxidant Enzyme Activities of Advanced Mutant Rice Genotypes under Drought Stress Condition. Agronomy. 2018, 8, 279. [Google Scholar] [CrossRef]

| Variety | Geographic Origin | Petal Shape | Color | Latitude/Longitude | Altitude (amsl) |
|---|---|---|---|---|---|
| Shiraz 1 | Shiraz, Fars | Queeny | Black | 29.5926° N 52.5836° E | 1519 m |
| Shahin Shahr | Shahin Shahr, Isfahan | Ordinary | Dark pink | 32.8609° N 51.5533° E | 1595 m |
| Isfahan 2 | Isfahan | Ordinary | Pink and light purple | 32.6883° N 53.2019° E | 1571 m |
| Khomeini Shahr 2 | Khomeini Shahr, Isfahan | Ordinary | Red | 32.6883° N 51.5304° E | 1602 m |
| Isfahan 1 | Isfahan | Ordinary | Dark pink and crimson | 32.6883° N 53.2019° E | 1571 m |
| Mahallat | Mahallat, Markazi | Ordinary | Dark violet | 33.9115° N 50.4525° E | 1721 m |
| Shiraz 2 | Shiraz, Fars | Queeny | White | 29.5926° N 52.5836° E | 1519 m |
| Tabriz | Tabriz, Azarbaijan | Ordinary | Yellow | 38.0792° N 46.2887° E | 1345 m |
| Khomeini Shahr 1 | Khomeini Shahr, Isfahan | Ordinary | Dark purple | 32.6883° N 51.5304° E | 1602 m |
| Depth (cm) | 30 cm |
|---|---|
| Electrical conductivity (dS m−1) | 1.81 ds m−1 |
| pH | 7.5 |
| Organic carbon percentage (OC %) | 0.60% |
| Phosphorus (PPM) | 7.14 mg kg−1 |
| Potassium (PPM) | 44.2 mg kg−1 |
| Clay (%) | 38.5 |
| Silt (%) | 36 |
| Sand (%) | 25.5 |
| S.O.V | df | Chl. | Car | Fv/Fm | RWC | MSI |
|---|---|---|---|---|---|---|
| Irrigation regimes (I) | 2 | 0.009 ** | 0.58 ** | 0.077 ** | 2606.93 ** | 760.24 ** |
| Variety (V) | 8 | 0.006 ** | 18.57 ** | 0.004 ** | 1919.11 ** | 286.681 ** |
| I × V | 16 | 0.0004 ** | 6.88 ** | 0.002 * | 15.508 * | 663.35 ** |
| Error | 48 | 0.00003 | 0.67 | 0.0006 | 6.86 | 19.15 |
| Chl. (mg g−1 FW) | Car (mg g−1 FW) | Fv/Fm | RWC (%) | MSI (%) | |
|---|---|---|---|---|---|
| Irrigation regimes | |||||
| I1 | 0.064 c | 6.53 a | 0.68 a | 71 a | 69.34 a |
| I2 | 0.077 b | 6.56 a | 0.64 b | 60.18 b | 52.67 b |
| I3 | 0.1 a | 6.58 a | 0.55 c | 51.39 c | 48.25 c |
| LSD | 0.01 | 0.995 | 0.01 | 3.15 | 1.9 |
| Varieties | |||||
| Shiraz 1 | 0.064 a | 6.22 c | 0.67 a | 54.44 e | 68.73 b |
| Shahin Shahr | 0.065 a | 9.23 a | 0.623 b | 70.35 b | 56.3 c |
| Isfahan 2 | 0.054 b | 8.58 a | 0.619 bc | 66.26 c | 51.44 c |
| Khomeini Shahr 2 | 0.045 c | 4.95 e | 0.621 bc | 67.56 c | 47.34 cd |
| Isfahan 1 | 0.04 c | 6.76 bc | 0.620 bc | 78.15 a | 45.43 cd |
| Mahallat | 0.03 d | 4.61 e | 0.609 c | 59.22 d | 52.60 c |
| Shiraz 2 | 0.026 e | 5.8 d | 0.588 d | 28.54 f | 77.37 a |
| Tabriz | 0.02 f | 6.89 bc | 0.594 d | 70.36 b | 81.42 a |
| Khomeini Shahr 1 | 0.013 g | 5.52 c | 0.629 bc | 52.84 e | 29.57 e |
| LSD | 0.003 | 0.77 | 0.02 | 2.84 | 4.14 |
| Variety | Irrigation Regimes | Chl. (mg g−1 FW) | Car (mg g−1 FW) | Fv/Fm | RWC (%) | MSI (%) | |
|---|---|---|---|---|---|---|---|
| Shiraz 1 | I1 | 0.051 g | 5.46 f–h | 0.721 a | 61.96 f–h | 111.6 a | |
| Shahin Shahr | I1 | 0.095 d | 7.81 b–d | 0.703 ab | 79.96 b | 53.86 e–h | |
| Isfahan 2 | I1 | 0.084 d | 8.78 b | 0.667 b–d | 73.99 cd | 75.56 cd | |
| Khomeini Shahr 2 | I1 | 0.041 gh | 5.49 f–h | 0.695 a–c | 79.31 b | 48.73 e–i | |
| Isfahan 1 | I1 | 0.096 d | 5.17 f–h | 0.659 d | 92.16 a | 53.1 e–h | |
| Mahallat | I1 | 0.033 h | 6.11 e–g | 0.709 a | 67.18 e | 61.96 d–f | |
| Shiraz 2 | I1 | 0.05 g | 6.31 e–h | 0.619 e–g | 37.45 m | 88.56 bc | |
| Tabriz | I1 | 0.06 f | 9.10 b | 0.652 de | 80.64 b | 95.06 b | |
| Khomeini Shahr 1 | I1 | 0.055 f | 4.52 h–j | 0.717 a | 66.42 ef | 34.63 i–k | |
| Shiraz 1 | I2 | 0.063 f | 7.15 c–e | 0.697 b–d | 54.45 jk | 16.21 l | |
| Shahin Shahr | I2 | 0.118 c | 8.62 bc | 0.653 de | 70.15 de | 75.56 cd | |
| Isfahan 2 | I2 | 0.102 cd | 8.28 bc | 0.613 e–h | 66.82 e | 39.63 h–k | |
| Khomeini Shahr 2 | I2 | 0.047 g | 5.84 e–h | 0.644 d–f | 66.8 e | 59.13 ef | |
| Isfahan 1 | I2 | 0.1 d | 8.46 bc | 0.656 dc | 76.48 b | 46.4 e–i | |
| Mahallat | I2 | 0.051 g | 4.52 hij | 0.608 f–h | 59.07 h–j | 53.43 e–h | |
| Shiraz 2 | I2 | 0.073 ef | 5.61 f–h | 0.604 f–h | 27.39 n | 85.33 bc | |
| Tabriz | I2 | 0.076 ef | 4.38 h–j | 0.611 f–h | 68.95 c | 84.96 bc | |
| Khomeini Shahr 1 | I2 | 0.06 f | 6.15 e–g | 0.642 d–f | 51.49 kl | 29.43 jk | |
| Shiraz 1 | I3 | 0.1 c | 7.25 c–e | 0.594 gh | 46.92 l | 93.43 b | |
| Shahin Shahr | I3 | 0.18 a | 11.46 a | 0.513 j | 60.94 hi | 39.46 h–k | |
| Isfahan 2 | I3 | 0.13 b | 8.66 bc | 0.577 hi | 57.97 h–j | 39.13 h–k | |
| Khomeini Shahr 2 | I3 | 0.06 f | 3.52 ij | 0.525 j | 56.59 ij | 34.16 i–k | |
| Isfahan 1 | I3 | 0.11 d | 6.64 d–f | 0.545 ij | 65.81 e–g | 36.8 i–k | |
| Mahallat | I3 | 0.06 f | 3.2 j | 0.509 j | 51.41 kl | 42.4 de | |
| Shiraz 2 | I3 | 0.08 e | 5.49 f–h | 0.542 ij | 20.79 o | 58.32 e–g | |
| Tabriz | I3 | 0.11 d | 7.18 c–e | 0.521 j | 61.44 gh | 64.23 de | |
| Khomeini Shahr 1 | I3 | 0.06 f | 5.87 e–h | 0.528 j | 40.61 m | 24.66 k | |
| LSD | 0.01 | 1.5 | 0.03 | 4.8 | 16 | ||
| S.O.V | df | Proline | CAT | APX | GPX | SY |
|---|---|---|---|---|---|---|
| Irrigation regimes (I) | 2 | 0.23 ** | 0.74 ** | 7.146 ** | 4.99 ** | 1,202,581 ** |
| Variety (V) | 8 | 0.004 ** | 0.2 ** | 4.54 ** | 16.30 ** | 102,561 ** |
| I × V | 16 | 0.006 ** | 0.68 ** | 5.04 ** | 41.93 ** | 14,175 ** |
| Error | 48 | 0.0009 | 0.001 | 0.02 | 0.007 | 945 |
| Proline (μmol g−1 FW) | CAT (U mg−1 Protein) | APX (U mg−1 Protein) | GPX (U mg−1 Protein) | SY (g) | |
|---|---|---|---|---|---|
| Irrigation regimes | |||||
| I1 | 1.98 c | 0.24 c | 0.98 b | 1.15 a | 729 a |
| I2 | 3 b | 0.42 b | 0.41 c | 0.46 b | 456 b |
| I3 | 5.58 a | 0.57 a | 1.43 a | 0.35 c | 313 c |
| LSD | 0.4 | 0.02 | 0.1 | 0.06 | 46.7 |
| Variety | |||||
| Shiraz 1 | 3.1 h | 0.043 f | 1.36 c | 0.768 d | 376 f |
| Shahin Shahr | 3.3 e | 0.494 c | 0.385 d | 0.185 g | 563 b |
| Isfahan 2 | 3.16 g | 0.313 d | 1.54 b | 2.478 a | 659 a |
| Khomeini Shahr 2 | 3.4 d | 0.115 e | 0.056 e | 0.628 d | 577 b |
| Isfahan 1 | 0.54 i | 0.092 ef | 0.185 e | 0.241 f | 460 d |
| Mahallat | 3.2 f | 0.06 f | 2.182 a | 0.278 f | 554 b |
| Shiraz 2 | 4.44 a | 0.093 ef | 0.372 d | 0.562 e | 334 g |
| Tabriz | 3.46 c | 0.536 b | 1.49 bc | 0.959 c | 421 e |
| Khomeini Shahr 1 | 4.02 b | 0.947 a | 0.378 d | 1.217 b | 520 c |
| LSD | 0.02 | 0.04 | 0.13 | 0.08 | 29.13 |
| Variety | Irrigation Regimes | Proline (μmol g−1 FW) | CAT (U mg−1 Protein) | APX (U mg−1 Protein) | GPX (U mg−1 Protein) | Seed Yield (g) |
|---|---|---|---|---|---|---|
| Shiraz 1 | I1 | 2.14 ef | 0.017 m | 0.39 g–i | 1.49 c | 521 de |
| Shahin Shahr | I1 | 1.54 f | 0.257 gh | 0.099 j–l | 0.119 i–k | 857 b |
| Isfahan 2 | I1 | 2.06 ef | 0.182 h | 2.021 c | 4.62 a | 949 a |
| Khomeini Shahr 2 | I1 | 2.02 ef | 0.036 kl | 0.982 e | 1.07 e | 875 b |
| Isfahan 1 | I1 | 1.56 f | 0.082 i | 0.046 kl | 0.05 jk | 637 c |
| Mahallat | I1 | 2.38 ef | 0.029 l | 1.037 e | 0.30 gh | 846 b |
| Shiraz 2 | I1 | 2 ef | 0.038 kl | 0.09 j–l | 0.25 g–i | 486 ef |
| Tabriz | I1 | 1.7 f | 0.247 g | 0.569 fg | 0.58 f | 560 d |
| Khomeini Shahr 1 | I1 | 2.36 e | 0.465 f | 0.591 fg | 1.89 b | 827 b |
| Shiraz 1 | I2 | 2.28 ef | 0.033 l | 0.119 j–l | 0.51 f | 342 hi |
| Shahin Shahr | I2 | 2.9 e | 0.5 f | 1.109 e | 0.38 g | 497 e |
| Isfahan 2 | I2 | 3.04 d-f | 0.256 g | 0.207 j–l | 2.02 b | 635 c |
| Khomeini Shahr 2 | I2 | 1.56 f | 0.053 k | 0.212 h–l | 0.25 g–i | 511 de |
| Isfahan 1 | I2 | 4.18 cd | 0.092 i | 0.279 h–k | 0.35 g | 432 hi |
| Mahallat | I2 | 2.94 d | 0.052 k | 0.065 kl | 0.18 h–j | 495 e |
| Shiraz 2 | I2 | 4.5 d | 0.074 j | 0.717 f | 1.04 e | 309 ij |
| Tabriz | I2 | 3.04 d | 0.641 d | 0.589 fg | 1.04 e | 394 gh |
| Khomeini Shahr 1 | I2 | 2.12 e | 0.856 b | 0.45 g–i | 1.21 d | 493 e |
| Shiraz 1 | I3 | 3.34 cd | 0.078 j | 0.58 fg | 0.299 c | 264 jk |
| Shahin Shahr | I3 | 4.5 c | 0.725 c | 0.036 l | 0.05 jk | 334 hi |
| Isfahan 2 | I3 | 4.36 c | 0.503 e | 2.39 b | 0.79 e | 394 ij |
| Khomeini Shahr 2 | I3 | 6.6 b | 0.255 g | 0.50 f–h | 0.56 f | 346 hi |
| Isfahan 1 | I3 | 5.12 bc | 0.102 hi | 0.23 h–k | 0.32 gh | 312 ij |
| Mahallat | I3 | 4.3 c | 0.098 i | 5.44 a | 0.35 g | 320 ij |
| Shiraz 2 | I3 | 6.82 ab | 0.167 h | 0.31 h–j | 0.39 g | 209 k |
| Tabriz | I3 | 5.62 b | 0.72 c | 3.31 b | 1.25 d | 310 ij |
| Khomeini Shahr 1 | I3 | 7.56 a | 1.52 a | 0.09 j–l | 0.55 f | 329 i |
| LSD | 1.6 | 0.01 | 0.12 | 0.09 | 61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadeghi, A.; Karimmojeni, H.; Razmjoo, J.; Baldwin, T.C. Physiological Responses of Hollyhock (Alcea rosea L.) to Drought Stress. Horticulturae 2024, 10, 841. https://doi.org/10.3390/horticulturae10080841
Sadeghi A, Karimmojeni H, Razmjoo J, Baldwin TC. Physiological Responses of Hollyhock (Alcea rosea L.) to Drought Stress. Horticulturae. 2024; 10(8):841. https://doi.org/10.3390/horticulturae10080841
Chicago/Turabian StyleSadeghi, Arezoo, Hassan Karimmojeni, Jamshid Razmjoo, and Timothy C. Baldwin. 2024. "Physiological Responses of Hollyhock (Alcea rosea L.) to Drought Stress" Horticulturae 10, no. 8: 841. https://doi.org/10.3390/horticulturae10080841
APA StyleSadeghi, A., Karimmojeni, H., Razmjoo, J., & Baldwin, T. C. (2024). Physiological Responses of Hollyhock (Alcea rosea L.) to Drought Stress. Horticulturae, 10(8), 841. https://doi.org/10.3390/horticulturae10080841






