Jackfruit Genotypes in Southern Nayarit: A Comparative Study of Morphological, Physiological, Physicochemical, Phytochemical, and Molecular Assessments
Abstract
1. Introduction
2. Materials and Methods
2.1. Genotypes and Collection Site
2.2. Sampling of Fruits and Leaves
2.3. Morphological Analysis of Fruits and Leaves
2.4. Physiological Analysis (Respiration Rate, Ethylene Production, and Physiological Weight Loss)
2.5. Physicochemical Analysis
2.6. Phytochemical Analysis
2.6.1. Preparation of Phenolic Extract
2.6.2. Quantification of Total Soluble Phenols
2.6.3. Preparation of Carotenoid Extract
2.6.4. Quantification of Total Carotenoids
2.6.5. Evaluation of the Antioxidant Capacity of Total Soluble Phenols and Carotenoids
2.7. DNA Extraction, Concentration and Quality
2.7.1. Simple Sequence Repeat Markers (SSR)
2.7.2. Sequence-Related Amplified Polymorphism Markers (SRAPs)
2.7.3. Statistical Analysis
3. Results and Discussion
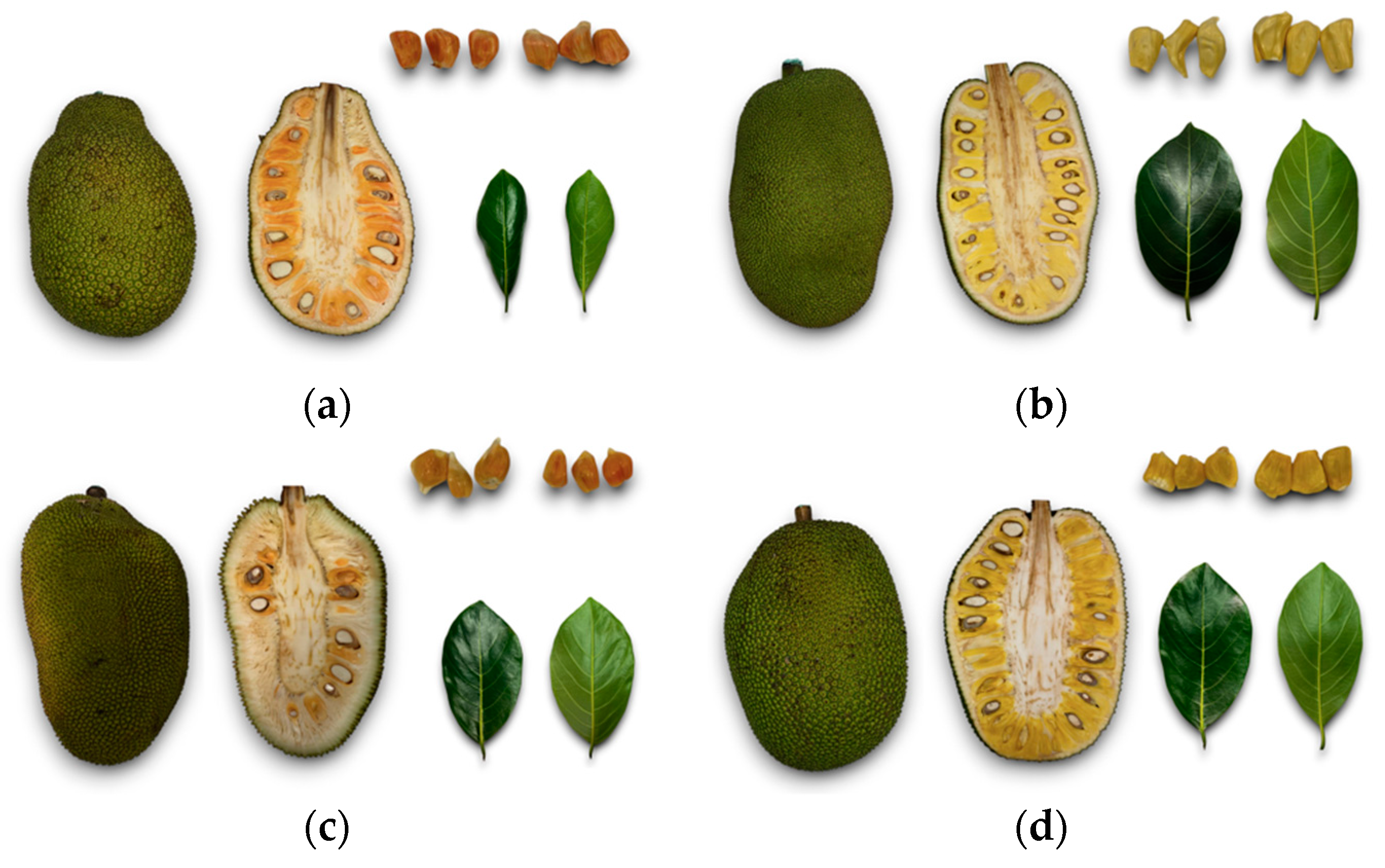
3.1. Morphological Analysis of Fruits and Leaves
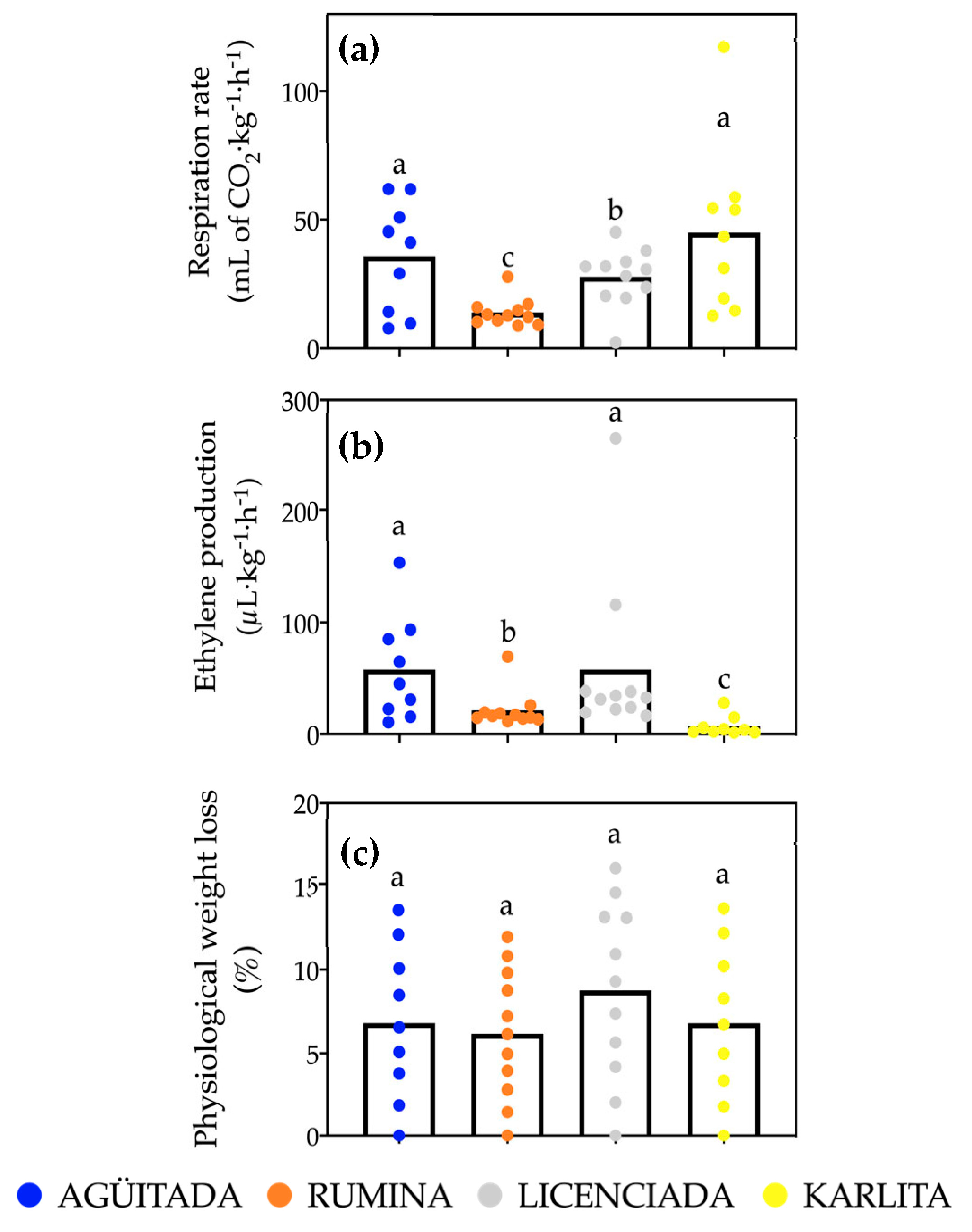
3.2. Physiological Analysis (Respiration Rate, Ethylene Production, and Physiological Weight Loss)
3.3. Physicochemical Analysis
3.4. Phytochemical Analysis
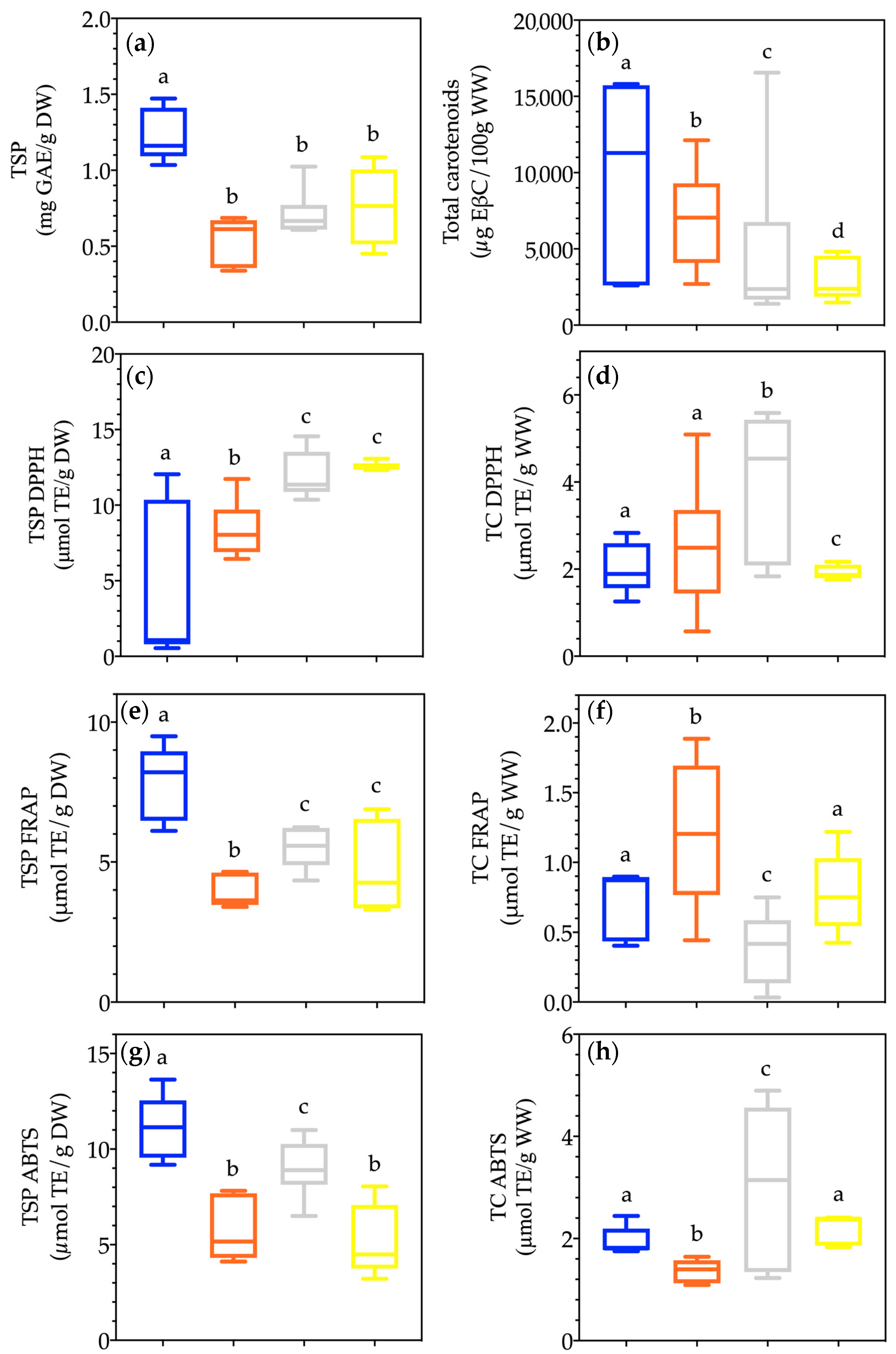
3.4.1. Quantification of Total Soluble Phenols and Total Carotenoids
3.4.2. Evaluation of Antioxidant Capacity
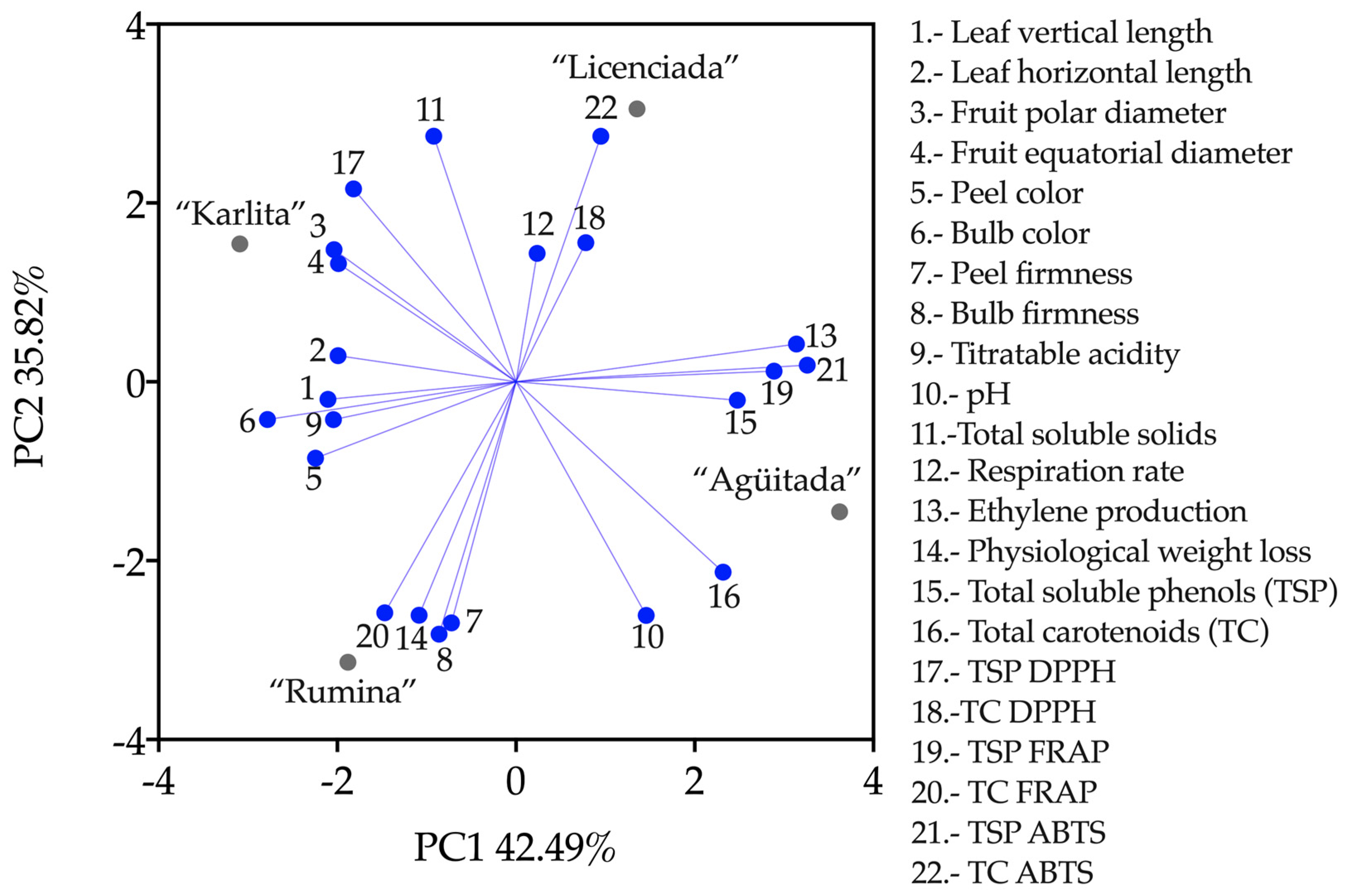
3.5. Principal Component Analysis
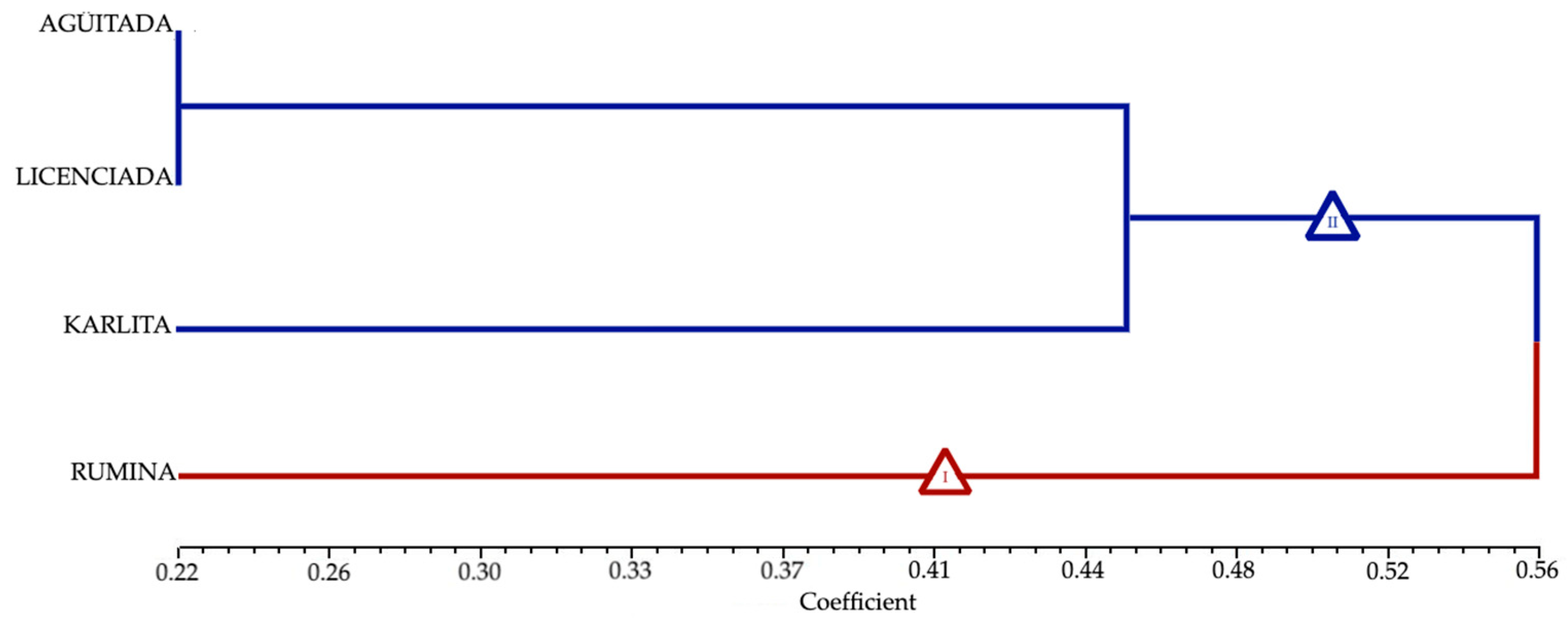
3.6. Molecular Markers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Somashekhar, M.; Nayeem, N.; Sonnad, B. A Review on Family Moraceae (Mulberry) with a Focus on Artocarpus Species. 2013. Available online: www.wjpps.com (accessed on 25 August 2024).
- Khan, A.U.; Ema, I.J.; Faruk, R.; Tarapder, S.A.; Khan, A.U.; Noreen, S.; Adnan, M. Review on Importance of Artocarpus heterophyllus L. (Jackfruit). J. Multidiscip. Appl. Nat. Sci. 2021, 1, 106–116. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; González-Aguilar, G.A.; Domínguez-Ávila, J.A.; Olmos-Cornejo, J.E.; Pérez-Larios, A.; Montalvo-González, E. Effects of Minimal Processing Technologies on Jackfruit (Artocarpus heterophyllus Lam.) Quality Parameters. Food Bioproc. Tech. 2018, 11, 1761–1774. [Google Scholar] [CrossRef]
- SIAP. Anuario Estadístico de la Producción Agrícola, Cierre de la Producción Agrícola. 2024. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 10 March 2024).
- De Oliveira, F.F.; Souto, A.G.L.; Cavalcante, L.; De Oliveira, F.F.; Nascimento, J.A.M.D.; Bezerra, F.T.C. Salt tolerance of Soft and Hard jackfruit varieties in the seedling stage. Emir. J. Food Agric. 2022, 34, 703–710. [Google Scholar] [CrossRef]
- Mijin, S.; Ding, P.; Saari, N.; Ramlee, S. Effects of pollination techniques and harvesting stage on the physico-chemical characteristics of jackfruit. Sci. Hortic. 2021, 285, 110199. [Google Scholar] [CrossRef]
- Luna, E.G.; Alejo, S.G.; Ramírez, G.; Arévalo, Y.G. La Yaca un Fruto de Exportación. Agro Product. 2013, 6, 65–70. [Google Scholar]
- Morelos-Flores, D.A.; Montalvo-González, E.; Chacón-López, M.A.; Santacruz-Varela, A.; Zamora-Gasga, V.M.; Torres-García, G.; de Lourdes García-Magaña, M. Comparative Study of Four Jackfruit Genotypes: Morphology, Physiology and Physicochemical Characterization. Horticulturae 2022, 8, 1010. [Google Scholar] [CrossRef]
- Villalobos, M.C. Jaca (Artocarpus heterophyllus Lam.) Centenario de Nayarit. In Enciclopedia Centenario de Nayarit, 1st ed.; Ladrón, L.C.P., Ed.; Consejo Estatal para la Cultura y Las Artes de Nayarit: Tepic, Mexico, 2017; Volume 91, pp. 25–30. [Google Scholar]
- Vargas-Torres, A.; Becerra-Loza, A.S.; Sayago-Ayerdi, S.G.; Palma-Rodríguez, H.M.; García-Magaña, M.d.L.; Montalvo-González, E. Combined effect of the application of 1-MCP and different edible coatings on the fruit quality of jackfruit bulbs (Artocarpus heterophyllus Lam) during cold storage. Sci. Hortic. 2017, 214, 221–227. [Google Scholar] [CrossRef]
- Barros-Castillo, J.C.; Calderón-Santoyo, M.; Cuevas-Glory, L.F.; Pino, J.A.; Ragazzo-Sánchez, J.A. Volatile profiles of five jackfruit (Artocarpus heterophyllus Lam.) cultivars grown in the Mexican Pacific area. Food Res. Int. 2021, 139, 109961. [Google Scholar] [CrossRef] [PubMed]
- SIAP. Boletín De Export. Jackfruit . 2017. Available online: https://www.gob.mx/cms/uploads/attachment/file/229919/Boletin_de_exportaciones_jackfruit_2017_06.pdf (accessed on 25 August 2024).
- Morelos-Flores, D.A.; Nolasco-González, Y.; Gutiérrez-Martínez, P.; Hernández-Fuentes, L.M.; Montalvo-González, E.; García-Magaña, M.L. Study of marketing simulation in jackfruit (Artocarpus heterophyllus Lam) treated with 1–methylcyclopropene. Rev. Int. Investig. Innovación Tecnológica 2021, 9, 90–111. [Google Scholar]
- Morelos-Flores, D.A.; Anzaldo-Mendiola, R.L.; Montalvo-González, E.; Zamora-Gasga, V.M.; Chacón-López, M.A.; Santacruz-Varela, A.; García-Magaña, M.D.L. Characterization and antioxidant capacity of phenolic compounds of jackfruit genotypes from Nayarit, Mexico. Food Chem. Adv. 2023, 3, 100470. [Google Scholar] [CrossRef]
- Morelos-Flores, D.A.; Montalvo-González, E.; Chacón-López, M.A.; Santacruz-Varela, A.; Zamora-Gasga, V.M.; Torres-Garcia, G.; García-Magaña, d.L.M. Jackfruit in Mexico: Characterization of four genotypes from the south of Nayarit. Fruits 2024, 79, 1–9. [Google Scholar] [CrossRef]
- Salgotra, R.K.; Chauhan, B.S. Genetic Diversity, Conservation, and Utilization of Plant Genetic Resources. Genes 2023, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Pandey, A.; Choudhary, S.B.; Kumar, S.; Tribhuvan, K.U.; Mishra, D.C.; Bhati, J.; Kumar, M.; Tomar, J.; Bishnoi, S.; et al. Development of genic-SSR markers and their application in revealing genetic diversity and population structure in an Eastern and North-Eastern Indian collection of Jack (Artocarpus heterophyllus Lam.). Ecol. Indic. 2021, 131, 108143. [Google Scholar] [CrossRef]
- Palupi, D.; Rahayu, S.S.B.; Daryono, B.S. Genetic diversity in jackfruit (Artocarpus heterophyllus Lam.) based on molecular characters in Indonesia. SABRAO J. Breed Genet. 2019, 51, 57–67. [Google Scholar]
- Kavya, K.; Shyamalamma, S.; Gayatri, S. Morphological and molecular genetic diversity analysis using SSR markers in Jackfruit (Artocarpus heterophyllus Lam.) genotypes for pulp colour. Indian J. Agric. Res. 2019, 53, 8–16. [Google Scholar] [CrossRef]
- CONAGUA. Servicio Meteorologico Nacional, Normales Climatológica por Estado. 2023. Available online: https://smn.conagua.gob.mx/es/informacion-climatologica-por–estado?estado=nay (accessed on 10 July 2023).
- Love, K.; Paull, R.E. Jackfruit; College of Tropical Agriculture and Human Resources: Honolulu, HI, USA, 2011; pp. 1–7. [Google Scholar]
- IPGRI Artocarpus heterophyllus. In Powdered Crude Drug Microscopy of Leaves and Barks; International Plant Genetic Resources Institute: Rome, Italy, 2000; p. 64. [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Rockville, MA, USA, 2005. [Google Scholar]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz-Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Montreau, F.R. Sur le dosage des composés phénoliques totaux dans les vins par la méthode Folin-Ciocalteu. OENO One 1972, 24, 397–404. [Google Scholar] [CrossRef]
- García, J.R.; De la Rosa, L.A.; González-Barrios, A.G.; Herrera-Duenez, B.; López-Díaz, J.A.; González-Aguilar, G.A.; Ruíz-Cruz, S.; Álvarez-Parrilla, E. Cuantificación de polifenoles y capacidad antioxidante en duraznos comercializados en ciudad Juárez, México. Tecnociencia Chihuah. 2011, 5, 67–75. [Google Scholar]
- Philip, T.; Chen, T. Development of a Method for the Quantitative Estimation of Provitamin A Carotenoids in Some Fruits. J. Food Sci. 1988, 53, 1703–1706. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G. Antioxidant Phytochemicals in Fruits and Vegetables: Diet and Health Implications. HortScience 2000, 35, 588–592. [Google Scholar] [CrossRef]
- Alvarez-Parrilla, E.; De La Rosa, L.A.; Legarreta, P.; Saenz, L.; Rodrigo-García, J.; González-Aguilar, G.A. Daily consumption of apple, pear and orange juice differently affects plasma lipids and antioxidant capacity of smoking and non-smoking adults. Int. J. Food Sci. Nutr. 2010, 61, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Mercado Mercado, G.; López Teros, V.; Montalvo González, E.; González Aguilar, G.A.; Álvarez Parrilla, E.; Sáyago Ayerdi, S.G. Efecto de la extracción asistida por ultrasonido en la liberación y bioaccesibilidad in vitro de carotenoides, en bebidas elaboradas con mango (Mangifera indica L.) ‘Ataulfo’. Nova Sci. 2018, 10, 100–132. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Anuragi, H.; Dhaduk, H.L.; Kumar, S.; Dhruve, J.J.; Parekh, M.J.; Sakure, A.A. Molecular diversity of Annona species and proximate fruit composition of selected genotypes. 3 Biotech 2016, 6, 204. [Google Scholar] [CrossRef]
- Talamantes-Sandoval, C.A.; Cortés-Cruz, M.; Balois-Morales, R.; López-Guzmán, G.G.; Palomino-Hermosillo, Y.A. Molecular analysis of genetic diversity in soursop (Annona muricata L.) using SRAP markers. Rev. Fitotec. Mex. 2019, 42, 209–214. [Google Scholar] [CrossRef]
- Lira-Ortiz, R.; Cortés-Cruz, M.A.; López-Guzmán, G.G.; Palomino-Hermosillo, Y.A.; Sandoval-Padilla, I.; Ochoa-Jiménez, V.A.; Sánchez-Herrera, L.M.; Balois-Morales, R.; Berumen-Varela, G. Genetic diversity of soursop populations (Annona muricata L.) in Nayarit, Mexico using SSR and SRAP markers. Acta Biol. Colomb. 2022, 27, 104–112. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Rohlf, F.J. NTSYS-pc, Version 1.80; Distribution by Exeter Software: New York, NY, USA, 1993. [Google Scholar]
- Dey, B.; Baruah, K. Morphological Characterization of Jackfruit (Artocarpus heterophyllus Lam.) of Assam. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1005–1016. [Google Scholar] [CrossRef]
- Mahla, J.S.; Soni, N.V.; Patel, P.C.; Patel, A.V.; Dasalania, J.P.; Roul, S. Variability, character association and path analysis for Annona yield and quality attributes. Emergent Life Sci. Res. 2022, 8, 229–239. [Google Scholar] [CrossRef]
- Marais, D.L.D.; Lasky, J.R.; Verslues, P.E.; Chang, T.Z.; Juenger, T.E. Interactive effects of water limitation and elevated temperature on the physiology, development and fitness of diverse accessions of Brachypodium distachyon. New Phytol. 2017, 214, 132–144. [Google Scholar] [CrossRef] [PubMed]
- De Tepic, I.T.; de Oca, M.M.-M.; Osuna-García, J.; Hernández-Estrada, A.; Ochoa-Villarreal, M.; Tovar-Gómez, B. Efecto del 1-Metilciclopropeno (1-MCP) Sobre la Fisiología y Calidad de Frutos de Jaca (Artocarpus heterophyllus Lam.). Rev. Chapingo. Ser. Hortic. 2007, 13, 165–170. [Google Scholar] [CrossRef]
- Salehi, F. Recent Advances in the Modeling and Predicting Quality Parameters of Fruits and Vegetables during Postharvest Storage: A Review. Int. J. Fruit Sci. 2020, 20, 506–520. [Google Scholar] [CrossRef]
- Ray, B.J.; Chandra, K.; Viswavidyalaya, A.; Choudhury, S.G.; Kalindi, D. Physiology and Biochemistry of Fruit ripening: A Review. Scientist 2023, 2, 197–204. [Google Scholar] [CrossRef]
- Xanthopoulos, G.T.; Templalexis, C.G.; Aleiferis, N.P.; Lentzou, D.I. The contribution of transpiration and respiration in water loss of perishable agricultural products: The case of pears. Biosyst. Eng. 2017, 158, 76–85. [Google Scholar] [CrossRef]
- Lufu, R.; Ambaw, A.; Opara, U.L. Mechanisms and modelling approaches to weight loss in fresh fruit: A review. Technol. Hortic. 2024, 4, e006. [Google Scholar] [CrossRef]
- Elevitch, C.R.; Manner, H.I. Artocarpus heterophyllus (jackfruit). Species Profiles Pac. Isl. Agrofor. 2006, 1, 16. [Google Scholar]
- Ebrahimi, P.; Shokramraji, Z.; Tavakkoli, S.; Mihaylova, D.; Lante, A. Chlorophylls as Natural Bioactive Compounds Existing in Food By-Products: A Critical Review. Plants 2023, 12, 1533. [Google Scholar] [CrossRef]
- Asmady, N.H.; Abidin, M.Z. Effect of Vacuum Packaging on Sensory and Texture Properties of Fresh Cut Jackfruit. Sci. Technol. 2023, 3, 452–459. [Google Scholar] [CrossRef]
- Tatarowska, B.; Milczarek, D.; Wszelaczyńska, E.; Pobereżny, J.; Keutgen, N.; Keutgen, A.J.; Flis, B. Carotenoids Variability of Potato Tubers in Relation to Genotype, Growing Location and Year. Am. J. Potato Res. 2019, 96, 493–504. [Google Scholar] [CrossRef]
- Zafar, J.; Aqeel, A.; Shah, F.I.; Ehsan, N.; Gohar, U.F.; Moga, M.A.; Festila, D.; Ciurea, C.; Irimie, M.; Chicea, R. Biochemical and immunological implications of lutein and zeaxanthin. Int. J. Mol. Sci. 2021, 22, 10910. [Google Scholar] [CrossRef] [PubMed]
- González-Peña, M.A.; Ortega-Regules, A.E.; de Parrodi, C.A.; Lozada-Ramírez, J.D. Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12, 313. [Google Scholar] [CrossRef]
- Li, X.; Xu, C.; Korban, S.S.; Chen, K. Regulatory mechanisms of textural changes in ripening fruits. CRC Crit. Rev. Plant. Sci. 2010, 29, 222–243. [Google Scholar] [CrossRef]
- Su, Q.; Li, X.; Wang, L.; Wang, B.; Feng, Y.; Yang, H.; Zhao, Z. Variation in Cell Wall Metabolism and Flesh Firmness of Four Apple Cultivars during Fruit Development. Foods 2022, 11, 3518. [Google Scholar] [CrossRef]
- Bemmo, U.L.K.; Bindzi, J.M.; Kamseu, P.R.T.; Ndomou, S.C.H.; Tambo, S.T.; Zambou, F.N. Physicochemical properties, nutritional value, and antioxidant potential of jackfruit (Artocarpus heterophyllus) pulp and seeds from Cameroon eastern forests. Food Sci. Nutr. 2023, 11, 4722–4734. [Google Scholar] [CrossRef]
- Famiani, F.; Farinelli, D.; Frioni, T.; Palliotti, A.; Battistelli, A.; Moscatello, S.; Walker, R.P. Malate as substrate for catabolism and gluconeogenesis during ripening in the pericarp of different grape cultivars. Biol. Plant 2016, 60, 155–162. [Google Scholar] [CrossRef]
- Mirza, A.; Tripathi, K.; Kumar, P.; Kumar, R.; Saxena, R.; Kumar, A.; Badoni, H.; Goyal, B. Efficacy of jackfruit components in prevention and control of human disease: A scoping review. J. Educ. Health Promot. 2023, 12, 361. [Google Scholar] [CrossRef]
- Seleim, M.A.A.; Hassan, M.A. Physicochemical Properties and Nutritional Evaluation of Jackfruits (Artocarpus heterophyllus L.). Int. Adv. Res. J. Sci. Eng. Technol. 2019, 6, 75–84. [Google Scholar] [CrossRef]
- Saxena, A.; Bawa, A.S.; Raju, P.S. Jackfruit (Artocarpus heterophyllus Lam.). In Postharvest Biology and Technology od Tropical and Subtropical Fruits; Woodhead Publishing Limited: Sawston, UK, 2011. [Google Scholar] [CrossRef]
- Saxena, A.; Bawa, A.; Raju, P. Phytochemical changes in fresh-cut jackfruit (Artocarpus heterophyllus L.) bulbs during modified atmosphere storage. Food Chem. 2009, 115, 1443–1449. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Nieto Ramírez, M.I.; García Trejo, J.F.; Caltzontzin Rabell, V.; Chávez Jaime, R.; Estrada Sánchez, M.D.L.L. Efecto de las condiciones de cultivo en la producción de fenoles, flavonoides totales y su capacidad antioxidante en el árnica (Heterotheca inuloides). Rev. Mex. Cienc. Agric. 2018, 21, 4296–4305. [Google Scholar] [CrossRef][Green Version]
- Zhao, X.; Li, Y.; Zhang, M.M.; He, X.; Ahmad, S.; Lan, S.; Liu, Z.J. Research advances on the gene regulation of floral development and color in orchids. Gene 2023, 888, 147751. [Google Scholar] [CrossRef] [PubMed]
- Stanley, L.; Yuan, Y.W. Transcriptional Regulation of Carotenoid Biosynthesis in Plants: So Many Regulators, So Little Consensus. Front. Plant Sci. 2019, 10, 1017. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhang, J.; Nageswaran, D.; Li, L. Carotenoid metabolism and regulation in horticultural crops. Hortic. Res. 2015, 2, 15036. [Google Scholar] [CrossRef] [PubMed]
- Francenia Santos Sánchez, N.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; Shalaby, E., Ed.; Intechopen: London, UK, 2019. [Google Scholar]
- Kesawat, M.S.; Satheesh, N.; Kherawat, B.S.; Kumar, A.; Kim, H.U.; Chung, S.M.; Kumar, M. Regulation of Reactive Oxygen Species during Salt Stress in Plants and Their Crosstalk with Other Signaling Molecules—Current Perspectives and Future Directions. Plants 2023, 12, 864. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Phenolic Compounds from New Natural Sources—Plant Genotype and Ontogenetic Variation. Molecules 2023, 28, 1731. [Google Scholar] [CrossRef]
- Amevoin, K.; Agboyi, L.K.; Gomina, M.; Kounoutchi, K.; Bassimbako, K.H.; Djatoite, M.; Dawonou, A.V.; Tagba, A. Fruit fly surveillance in Togo (West Africa): State of diversity and prevalence of species. Int. J. Trop. Insect Sci. 2021, 41, 3105–3119. [Google Scholar] [CrossRef]


| Name of the Primers | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| LMCH 144 | GTTTGGAAGAGTCGCAGGAT | ACTGTAAAACGCAGACCAAGAT |
| LMCH 128 | CTTGTTAAAATGGCTGTTACT | GCATTGAGCTGACATAACTC |
| LMCH 122 | AGCAAAGATAAAGAGAAGATAA | ATCCAAGCCTATTAACAACT |
| LMCH 114 | AAAATGTAGTGTGAAAGATGAC | GTCCATCAGTTTTAAGTGC |
| LMCH 112 | TAACCGTGATCTACAATAAT | TTGCATACATTTTCCTATTT |
| LMCH 96 | AGAAGCTGGGAAACAAAACA | ATTCTGGCTTTTAATTGAGGA |
| LMCH 79 | GAAGCAAGTAGACACGTAGTA | AGGGTTGGTATTTCTTTATAGT |
| LMCH 78 | ATTTGATTGATTGATTTCCTA | CTTTTGCTTTCTTTCACATC |
| LMCH 71 | AGATAACACCCGCCCACTAT | ACAACTTTTCTCCCAACCTATC |
| LMCH 70 | GAAGTTTAGAGGCGATTCC | TTTTGCCACTTTACTGTCAC |
| LMCH 69 | AGCTTTAGCCATGAATTAGA | GAAAGGCTGACGAGATATAA |
| LMCH 43 | CTAGTTCCAAGACGTGAGAGAT | ATAGGAATAAGGGACTGTTGAG |
| LMCH 36 | ATAGAAGATTTACCCAGGAG | GTAAGTAGCTGATTGTTGATCT |
| LMCH 29 | GTACCATCTTTTAGGAAATC | TGCAATCTATGTTAGTCAC |
| Name of the Primers | Markers (Forward–Reverse) | Sequence |
|---|---|---|
| SRAPS 1 | Forward (5′-3′) me1 | TGAGTCCAAACCGGAT |
| Reverse (5′-3) em1 | GACTGCGTACGAATTAA | |
| SRAPS 2 | Forward (5′-3′) me1 | TGAGTCCAAACCGGATA |
| Reverse (5′-3′) em15 | GACTGCGTACGAATTCTG | |
| SRAPS 3 | Forward (5′-3′) me3 | TGAGTCCAAACCGGAAT |
| Reverse (5′-3) em1 | GACTGCGTACGAATTAA | |
| SRAPS 4 | Forward (5′-3′) me3 | TGAGTCCAAACCGGAAT |
| Reverse (5′-3′) em15 | GACTGCGTACGAATTCTG | |
| SRAPS 5 | Forward (5′-3′) me4 | TGAGTCCAAACCGGACC |
| Reverse (5′-3′) em1 | GACTGCGTACGAATTAA | |
| SRAPS 6 | Forward (5′-3′) me4 | TGAGTCCAAACCGGACC |
| Reverse (5′-3′) em15 | GACTGCGTACGAATTCTG |
| Genotype | Number of Heterozygous Loci | Total Number of Loci | Heterozygosity |
|---|---|---|---|
| Agüitada | 3 | 8 | 0.375 |
| Rumina | 4 | 8 | 0.500 |
| Licenciada | 5 | 8 | 0.625 |
| Karlita | 4 | 8 | 0.500 |
| Agüitada | Rumina | Licenciada | Karlita | |
|---|---|---|---|---|
| Agüitada | 1.0000 | |||
| Rumina | 0.4815 | 1.000 | ||
| Licenciada | 0.6667 | 0.3571 | 1.0000 | |
| Karlita | 0.4483 | 0.3793 | 0.4815 | 1.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morelos-Flores, D.A.; Montalvo-González, E.; Chacon-López, M.A.; Santacruz-Varela, A.; Zamora-Gasga, V.M.; Berumen-Varela, G.; García-Magaña, M.d.L. Jackfruit Genotypes in Southern Nayarit: A Comparative Study of Morphological, Physiological, Physicochemical, Phytochemical, and Molecular Assessments. Horticulturae 2024, 10, 918. https://doi.org/10.3390/horticulturae10090918
Morelos-Flores DA, Montalvo-González E, Chacon-López MA, Santacruz-Varela A, Zamora-Gasga VM, Berumen-Varela G, García-Magaña MdL. Jackfruit Genotypes in Southern Nayarit: A Comparative Study of Morphological, Physiological, Physicochemical, Phytochemical, and Molecular Assessments. Horticulturae. 2024; 10(9):918. https://doi.org/10.3390/horticulturae10090918
Chicago/Turabian StyleMorelos-Flores, David Antonio, Efigenia Montalvo-González, Martina Alejandra Chacon-López, Amalio Santacruz-Varela, Víctor Manuel Zamora-Gasga, Guillermo Berumen-Varela, and María de Lourdes García-Magaña. 2024. "Jackfruit Genotypes in Southern Nayarit: A Comparative Study of Morphological, Physiological, Physicochemical, Phytochemical, and Molecular Assessments" Horticulturae 10, no. 9: 918. https://doi.org/10.3390/horticulturae10090918
APA StyleMorelos-Flores, D. A., Montalvo-González, E., Chacon-López, M. A., Santacruz-Varela, A., Zamora-Gasga, V. M., Berumen-Varela, G., & García-Magaña, M. d. L. (2024). Jackfruit Genotypes in Southern Nayarit: A Comparative Study of Morphological, Physiological, Physicochemical, Phytochemical, and Molecular Assessments. Horticulturae, 10(9), 918. https://doi.org/10.3390/horticulturae10090918









