Abstract
The objective of this study was to investigate the impact of nutrient starvation on the growth, biochemical, metabolomic, and biological traits of Lavandula viridis L’Hér and Thymus lotocephalus G. López and R. Morales in vitro cultures. In both species, a reduction in shoot growth and in the production of chlorophyll and carotenoids was observed in cultures grown under nutrient-deficient media (especially Fe and N) compared to those grown under control conditions. The highest levels of hydrogen peroxide and lipid peroxidation, two indicators of oxidative stress, were observed in L. viridis cultures grown under N deficiency and in T. lotocephalus under P and Fe limitation. The results demonstrated that nutrient deficiency led to a 72% and 62% increase in the quantified phenolic compounds in L. viridis and T. lotocephalus, respectively. The highest concentrations of the major compound in both species—rosmarinic acid—were observed in cultures grown under Mg-deficient (60.7 ± 1.0 mg/gDW) and Fe-deficient (50.0 ± 0.4 mg/gDW) conditions in L. viridis and T. lotocephalus, respectively. Furthermore, nutrient starvation enhanced the capacity of green extracts to inhibit three enzymes (tyrosinase, elastase, and hyaluronidase) associated with anti-aging and their antioxidant properties.
1. Introduction
Lamiaceae is a vast family of dicotyledonous plants that includes 236 genera and 7280 species, mostly herbs and shrubs [1]. The genera Thymus and Lavandula are two of particular significance within this family, encompassing numerous species that are widely distributed across the globe, notably in the Mediterranean region [2,3]. Two Mediterranean species, Lavandula viridis L’Hér and Thymus lotocephalus G. López and R. Morales, are exclusively found in the Iberian Peninsula and the Algarve region (southern region of Portugal), respectively. Despite remaining relatively unexplored, both species are of significant interest due to their diverse biological properties. These include the capacity to inhibit enzymes associated with diabetes and neurological diseases, as well as antioxidant, antibacterial, antifungal, anti-protozoal, and nematocidal activities [4,5,6,7,8,9,10,11,12,13,14,15]. Additionally, these species offer protection against ultraviolet radiation [12], making them a promising resource for various industrial applications. Nevertheless, the potential application of these two species in the cosmetic sector has yet to be fully established, despite the growing demand for innovative anti-aging products, particularly those of natural origin [12,16]. Furthermore, it is becoming increasingly important to select an environmentally friendly solvent for the extraction of plant-derived bioactive components for use in cosmetic products. Petroleum is the source of the solvents most commonly utilized in the extraction of biocompounds across numerous industries, including cosmetics. Despite their potent extraction and dissolution capabilities, these solvents are highly detrimental to the environment and human health. Recently, Natural Deep Eutectic Solvents (NADES) have emerged as novel green alternatives to conventional solvents, characterized by their natural origin and biodegradable and non-toxic properties [17].
The aging process of the skin is characterized by alterations in the dermal connective tissue, as well as the degradation of elastin and hyaluronic acid, due to the oxidative stress caused by reactive oxygen species (ROS) and other natural processes. This results in the appearance of skin laxity and deep wrinkles [18]. Another issue pertaining to the aging process of the skin is the overproduction of melanin in the melanocytes, which results in the formation of blemishes due to alterations in pigmentation. Tyrosinase is the primary regulatory enzyme responsible for this process [12]. In addition to their hydrating and antioxidant properties, plant extracts are believed to confer further benefits in the context of cosmeceutical treatments. As functional components, they may, for instance, protect skin proteins or polysaccharides from enzymatic degradation caused by exposure to ultraviolet rays or other environmental-related stresses. Alternatively, they could delay or stop the activities of anti-aging-related enzymes, including elastase (Ela), hyaluronidase (Hyal), and tyrosinase (Tyr) [19]. However, the biological characteristics of plants associated with the presence of secondary metabolites are susceptible to changes in the environment, which can impact their industrial applications. This was previously demonstrated by our group in studies examining the impact of varying environmental factors associated with climate change on the production of volatiles and phenolics (and their biological activities) in Lamiaceae plants [12,13,20].
A number of mineral nutrients are essential for plants to grow and develop properly. These are classified as macronutrients [nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and sulfur (S)] and micronutrients [chlorine (Cl), iron (Fe), boron (B), manganese (Mn), zinc (Z), copper (Cu), nickel (Ni), and molybdenum (Mo)] [21]. Macronutrients (required in higher amounts) are used to generate structural and energetic components essential to plant growth and development. In contrast, micronutrients (required in trace amounts) have a higher connection to enzymatic reactions [21,22]. In addition, nutrients play a pivotal role in primary metabolism and are also involved in secondary metabolism, signal transduction, hormone reception, energy metabolism, and molecular regulation. Consequently, deficiencies in any of the nutrients can have a significant impact on plant physiology [23]. The agricultural sector is confronting a multitude of concerning challenges, largely due to the prevalence of environmental issues that are adversely impacting the global landscape. The limitation of nutrients in the soil is the result of the interaction of various stress factors, including salinity and drought, which impact the availability and transportation of nutrients [24]. In response to low nutrient availability in the soil, roots engage in chemical communication and chain reactions, utilizing phytohormones, ROS, carbohydrates, and transcription factors to regulate nutrient levels [21]. In response to the accumulation of ROS, plants accumulate both enzymatic and non-enzymatic (secondary metabolites) antioxidant elements in order to cope with oxidative stress [25]. A number of studies have validated the efficacy of in vitro culture as a means of producing secondary metabolites (and stimulating their production by modifying the culture conditions) while avoiding any adverse impact on the natural populations of the species. Furthermore, the large-scale production of uniform plants is feasible through controlled in vitro cultivation, which is not affected by seasonal or geographic variations [13]. The objective of this study was to evaluate the growth, biochemical (photosynthetic pigments and oxidative stress), phenolic profile, and bioactive (antioxidant capacity, and Tyr, Ela, and Hyal inhibition) responses of in vitro cultures of L. viridis and T. lotocephalus to different nutrient starvation conditions, after growing in culture media without N, P, Ca, Mg, or Fe sources. To the best of our knowledge, this is the inaugural investigation into the impact of the nutrient starvation effect on these species, along with the potential of their extracts to inhibit elastase and hyaluronidase. Furthermore, to the best of our knowledge, this is also the inaugural investigation into the impact of Fe, Ca, or P deficiencies on the biosynthesis of rosmarinic acid (the most prevalent biocompound in both species under examination in the present study).
2. Materials and Methods
2.1. Plant Material and In Vitro Culture Conditions
Shoots of T. lotocephalus and L. viridis were multiplied in MS medium [26] and MS with 0.2 mg/L 6-benzyladenine (BA), respectively, according to Dias et al. [27] and Coelho et al. [28]. Both media contained 2% (w/v) sucrose and 0.7% (w/v) agar [9]. To assess the impact of nutrient deprivation, the cultures of both species were cultivated in multiplication media but lacking the sources of nitrogen (-N) [KNO3 and NH4NO3], phosphorus (-P) [KH2PO4], calcium (-Ca) [CaCl2.2H2O], magnesium (-Mg) [MgSO4.7H2O], or iron (-Fe) [FeSO4.7H2O]. The control was established using multiplication media of each species with the full MS composition (all nutrients). The culture media were distributed in 12 Erlenmeyer flasks (80 mL) and sterilized by autoclaving at 121 °C for 20 min. In vitro cultures (7 shoots in each Erlenmeyer) were incubated at 25 ± 2 °C with 16 h light (cool white fluorescent lamps, 40 µmol m−2 s−1) for 7 weeks. Following a seven-week period, a number of parameters were assessed in shoots cultivated in all the aforementioned culture media.
2.2. Growth Parameters
The number of shoots and the length of the longest shoot produced per culture, as well as the total fresh and dry weight, were analyzed. To determine dry weight, about 80% of the shoots were oven-dried at 40 °C until a constant weight was achieved.
2.3. Photosynthetic Pigments and Oxidative Stress Indicators
The contents of chlorophyll and carotenoids, malondialdehyde (MDA), and hydrogen peroxide (H2O2) were evaluated according to Mansinhos et al. [13] using fresh material.
Twenty-five milligrams of plant material were macerated in 4 mL of pure acetone to extract the photosynthetic pigments. The absorbance was then measured using three different wavelengths (470, 644.8, and 661.6 nm) using a T70+ UV/Vis Spectrophotometer (PG Instruments Ltd., Leicestershire, UK). For the analysis of oxidative stress markers (H2O2 and MDA), 0.1 g of plant material was mixed with 1 mL TCA (0.1%, w/v), subjected to centrifugation, and the resulting supernatant was collected. For the H2O2 assay, 200 µL of the supernatant was homogenized with an equal volume of potassium phosphate buffer (10 mM). After 30 min, the absorbance was read at 390 nm. In the case of the lipid peroxidation assay (MDA), 500 µL of the supernatant was mixed with 500 µL of TCA (5%, w/v) + TBA (20%, w/v) (positive control) or with 500 µL of TCA (20%, w/v) (negative control), and the mixture was heated at 95 °C for 30 min. Subsequently, the reaction was stopped by placing the mixture on ice and the absorbance was measured at 440, 532, and 660 nm. The results of the H2O2 assay were expressed as micromoles of H2O2 equivalents per gram of fresh weight (µmol/gFW), while those of the MDA assay were expressed as nanomoles of MDA equivalents per gram of fresh weight (nmol/gFW).
2.4. Phenolic Extraction and Quantification
2.4.1. Green Extraction of Phenolic Compounds
The plant material was dried (40 °C) in an oven dryer until a constant weight was achieved, after which it was pulverized to a particle size of less than 2 mm. An Ultrasound-Assisted Extraction (UAE) (Elmasonic S 100 (H, Elma Hans Schmidbauer GmbH & Co. KG, Singen, Germany) was performed according to Mansinhos et al. [12], using the NADES proline–lactic acid [1:1, with 30% (w/w) water] as solvent, given its good performance in extracting phenolics from Lamiaceae species in previous studies and its environmentally friendly nature. To this end, 250 mg of the plant material was mixed with 10 mL of the NADES solution. Following filtration using Whatman No. 1 filter paper (Whatman Int. Ltd., Maidstone, UK), the extracts were stored at −20 °C until required for use.
2.4.2. Analysis of Individual Phenolics by HPLC-HRMS
The chromatographic technique HPLC-HRMS was used following the procedure of Mansinhos et al. [13] to analyze the phenolic profile of the plant extracts. The analysis was performed on a Dionex Ultimate 3000 HPLC system equipped with an HPLC pump and an autosampler (operating at 10 °C) (Thermo Scientific, San Jose, CA, USA). The separation of samples was conducted on a 150 × 4.6 mm i.d. 5 µm 100 Kinetex column (A C18) (Phenomenex, UK) at 40 °C, with a flow rate of 1 mL/min. Two mobile phases, Milli-Q double distilled water (solvent A) and acetonitrile (solvent B), both containing 0.1% formic acid, were present in the solvent system. The gradient mode was as follows: 0 min—90% A; 10 min—74% A; 22 min—35% A; 30 min—5% A; 40 min—5% A; 40.1 min—90% A; and 45 min—90% A. The column flow rate at 0.2 mL/min was targeted to an Exactive Orbitrap mass spectrometer (Thermo Fisher Scientific, San José, CA, USA) supplied with a heated electrospray ionization probe (HESI). The auto MS/MS (100–1000 m/z) scan mode was used to study negative ions. Complete scans were recorded with a resolution of 50,000, and a full automatic gain control (AGC) target of one million charges and using two microscans. Additionally, the analyses relied on in-source collision-induced dissociation scans (25 eV) with a 4000 V spray voltage, a 150 °C heater, a 320 °C capillary temperature, 25 units of sheath gas flow, and 5 units of auxiliary gas. For data processing and acquisition, Xcalibur software, version 3.0 (Thermo Fisher Scientific, San José, CA, USA) was the software utilized. Every week, ready-to-use calibration mixtures of Pierce LQT ESI Positive Ion Calibration Solution and Pierce ESI Negative Ion Calibration Solution (Thermo Fisher Scientific, San José, CA, USA) were used to externally calibrate the Exactive Orbitrap. To evaluate and confirm that the analytical procedure was correctly carried out, a quality control (QC) sample was used. This sample was made up of identical aliquots from a representative pool of the samples (plant extracts). Throughout the run, the QC sample was injected on a frequent basis to track drifts and measure the variance of a metabolite characteristic (below 20%). The compounds were identified using standards in conjunction with retention time and precise mass. When the standards were not available, the compound was first roughly identified by comparing the defined accurate mass of the molecular ion with the theoretical precise mass of the molecular ion. It was then looked up in a number of metabolite databases, including PubChem, Phenol Explorer, Metlin, and ChemSpider. The compound identification was carried out following the MSI MS levels previously established by Sumner et al. [29]. The compounds’ chemical formula, theoretical exact mass, delta ppm, retention time (RT), and MSI MI levels are summarized in Table S1. The assumptions used to quantify the phenolic compounds, namely linear range, intercept, slope, R2, limits of detection (LOD), and quantification (LOQ) are summarized in Table S2. LOD and LOQ ranged from 2.06 to 153.40 µg/L and from 6.24 to 464.85 µg/L, respectively. Tables S3 (L. viridis) and S4 (T. lotocephalus) provide the detailed results expressed in micrograms per gram of dry weight (µg/gDW) or milligram per gram of dry weight (mg/gDW).
2.5. Antioxidant Capacity of Green Extracts
The antioxidant activity of plant extracts was accessed according to the methodology proposed by Mansinhos et al. [12], which employed four distinct assays with varying modes of action: Oxygen Radical Absorbance Capacity (ORAC) (a hydrogen atom transfer (HAT)-based methodology), Ferric Reducing Antioxidant Power (FRAP) (a single electron transfer (SET)-based method), and 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) (a combination of the two indicated (HAT and SET) mechanisms). The results were determined as milligrams of Trolox (for ORAC, DPPH, ABTS assays) or ascorbic acid (for FRAP) equivalents per gram of dry weight (mgTE/gDW or mgAAE/gDW).
2.6. Anti-Aging Enzyme Inhibitory Capacity of Green Extracts
The evaluation of the inhibitory activities of tyrosinase (Tyr), elastase (Ela), and hyaluronidase (Hyal) was conducted using a microplate reader (SynergyTM HTX Multi-Mode Microplate Reader, BioTek Instruments, Inc., Winooski, VT, USA) in accordance with the methodologies established by Gonçalves et al. [9], Chiocchio et al. [30], and Liyanaarachchi et al. [31], with minor modifications. For the assessment of Tyr inhibitory activity, 50 µL of sodium phosphate buffer (blank), plant extract, or kojic acid (positive control) was mixed with 50 µL of mushroom tyrosinase (46 U/mL) and 80 µL of sodium phosphate buffer (20 mM, pH 6.8). After 10 min, 80 µL of L-DOPA (2.5 mM) was added, and the mixture was incubated for a further 10 min. Subsequently, the absorbance was recorded at 475 nm. The inhibition assay for Ela was conducted as follows. A total of 15 µL of porcine pancreatic elastase (0.01 U) and 30 µL of Tris buffer (blank), plant extract, or quercetin (positive control) were incubated at 25 °C for 10 min with 105 µL of Tris buffer (0.1 M, pH 8.1). Fifty microliters of the substrate N-succinyl-Ala-Ala-Ala-p-nitroanilide (2 mM) were added to the reaction mixture. After an incubation period of 5 min at 25 °C, the variation in absorbance was monitored at 420 nm in a microplate reader under a constant temperature of 25 °C (T0 and T5). For the Hyal assay, 10 µL of freshly prepared type-1-S bovine testes hyaluronidase (4200 U/mL) was dissolved in acetate buffer (0.1 M, pH 3.5) and homogenized with 50 μL of DMSO (5%) (blank), plant extract, or tannic acid (positive control) dissolved in DMSO (5%). The mixture was then incubated at 37 °C for 20 min. Subsequently, 25 µL of calcium chloride (12.5 mM) was added to the reaction, and a further incubation was performed for 10 min at the same temperature. The Ca2+ activated enzyme was treated with 50 µL of the substrate hyaluronic acid [3 mg/mL, dissolved in acetate buffer] and incubated at 37 °C for 40 min. Following the incubation period, the reaction was terminated by the addition of 10 µL of sodium hydroxide (0.9 M) and 20 μL of sodium borate (0.2 M) followed by heating for 3 min at 100 °C. After cooling on fresh water for 2 min, 50 µL of ρ-dimethylaminobenzaldehyde solution [(250 mg of p-dimethylaminobenzaldehyde in 21.88 mL of pure acetic acid and 3.12 mL of hydrochloric acid (10 N)] was added and the mixture was incubated at 37 °C for 10 min. The final reading was taken at 585 nm and the results were expressed as a percentage inhibition (%) for the three enzymes.
2.7. Statistical Analysis
The data are presented as mean ± standard error (SE) and evaluated using one-way analysis of variance (ANOVA) and Duncan’s New Multiple Range Test (ρ < 0.05) (n = 3). The multivariate data analysis was performed using the Polar Heatmap Dendrograms and Heatmaps functions of the OriginPro software, version 2022 (OriginLab Corporation, Northampton, MA, USA).
3. Results and Discussion
3.1. Plant Growth and Levels of Photosynthetic Pigments
The growth of plants can be influenced by many abiotic stress factors. The limitation of several nutrients in the substrate of plants has been demonstrated to both stimulate and hinder plant growth, depending on the specific nutrients present, their concentration, the duration of exposure, and the species of plant in question [22,32,33,34]. Figure 1 illustrates the visual appearance of L. viridis and T. lotocephalus in vitro-regenerated shoots cultured in media devoid of diverse nutrients for a 7-week period.

Figure 1.
Shoots of Lavandula viridis and Thymus lotocephalus grown in control medium (CT) and media without sources of nitrogen (-N), phosphorus (-P), calcium (-Ca), magnesium (-Mg), or iron (-Fe). The scale bar corresponds to 1 cm.
The number of regenerated shoots, length of the longest shoot, and biomass produced [fresh weight (FW) and dry weight (DW)] were compared and subjected to thorough analysis, as indicated in Table 1.

Table 1.
Shoot growth parameters of Lavandula viridis and Thymus lotocephalus grown in control medium (CT) and media without sources of nitrogen (-N), phosphorus (-P), calcium (-Ca), magnesium (-Mg), or iron (-Fe).
The number of shoots produced in both species was found to be negatively affected by the limitation of all nutrients, with the exception of Ca, which had no significant impact on the number of shoots produced in L. viridis (6.29 ± 0.70 vs. 5.77 ± 0.55) and resulted in a higher number than the control in T. lotocephalus (more than 60%) (Table 1).
With regard to the length of the shoots, in both species, the longest was observed in the control group and the shortest in the N deficiency group. In both L. viridis and T. lotocephalus cultures, the absence of Ca in the culture media stimulated the production of biomass. However, as can be observed in Figure 1, this higher production is partially related to hyperhydricity, a plant disorder characterized by stiff transparent shoots with small internodes due to the increased water content (which overestimates the biomass results). This is commonly caused by the accumulation of ethylene [12,35]. A deficiency in calcium has been previously associated with this phenomenon in other studies, which has been justified by the fact that low Ca levels decrease cell motility and pectin formation, thereby compromising cell wall and membrane integrity and reducing transpiration [35]. A similar outcome was observed in a study conducted with Stevia rebaudiana, wherein the exclusion of Ca from the MS medium led to an enhancement in the length of shoots, the number of nodes and leaves, as well as FW and DW [32]. Conversely, the absence of N, P, K, Ca, or Mg in the culture medium resulted in an inhibition of plant growth on Brassica rapa L. ssp. pekinensis shoots, with the greatest impact observed in response to Ca deficiency [22].
The biometric features of both species tested in this study were particularly affected by the absence of N (Table 1). Furthermore, negative effects on plant growth parameters have been observed in other species belonging to distinct families that have been subjected to N limitation. These include Eucomis autumnalis (Mill.) Chitt (Asparagaceae), Tulbaghia ludwigiana Harv. and Tulbaghia violacea Harv. (Amaryllidaceae) [33], Arabidopsis thaliana (Brassicaceae) [34], B. rapa (Brassicaceae) [22], Lactuca sativa L. (Asteraceae), and Castilleja tenuiflora Benth (Orobanchaceae) [36]. In contrast, the absence of this macronutrient in the media does not affect the growth of Japanese mint [37] or chamomile [38]. Similarly, in the case of T. lotocephalus, as occurred for N, the absence of P and Fe also resulted in a significant reduction in the number of shoots (88% less for P and 84.5% for Fe) and biomass produced (97.3% less FW for P and 96.5% for Fe) (Table 1). Similar outcomes were observed in other plant species subjected to P or Fe stress [22,33,34,39,40,41].
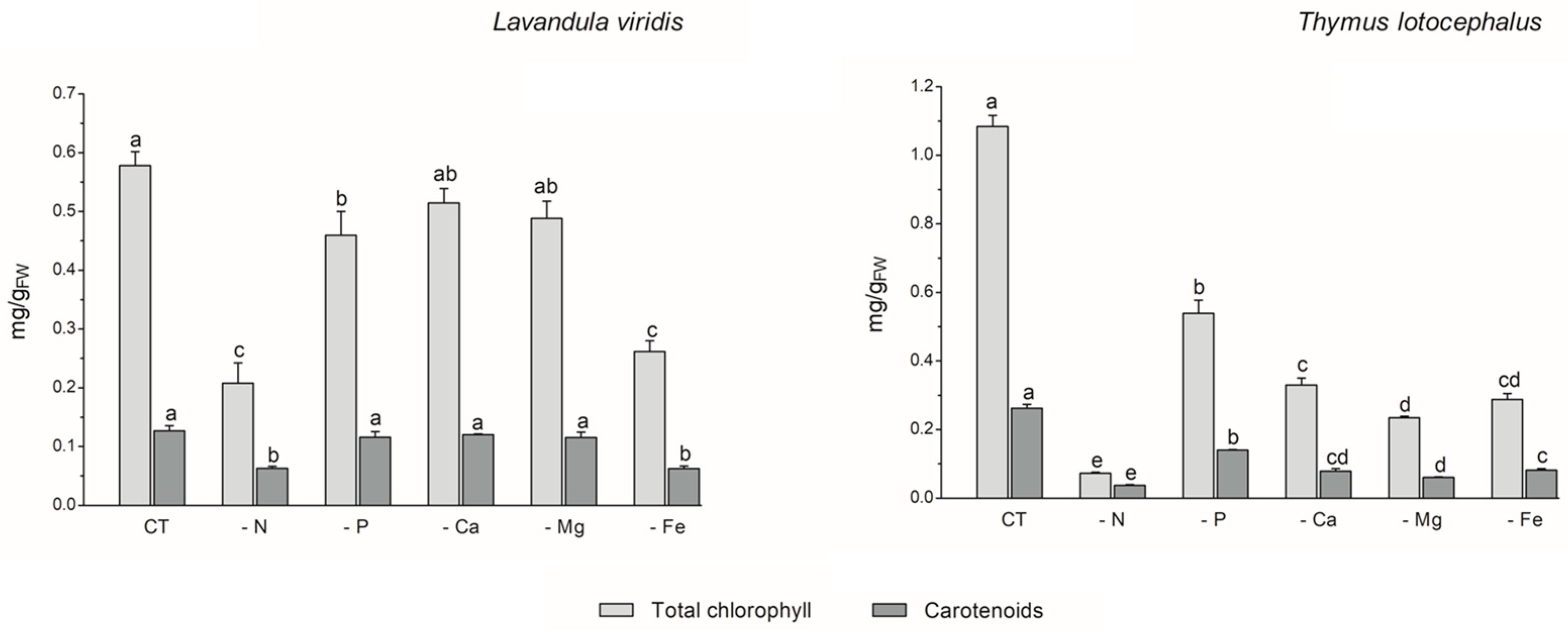
The process of photosynthesis, in which chlorophylls play a pivotal role in light capture, is of paramount importance to the growth and productivity of plants. Consequently, it can be employed as a reliable indicator of stress tolerance [33]. In L. viridis, the highest content of total chlorophyll content was observed in the control (0.58 ± 0.02 mg/gFW), with no significant differences evident between cultures grown under Ca (0.51 ± 0.02 mg/gFW) or Mg (0.49 ± 0.03 mg/gFW) deficiency (Figure 2).
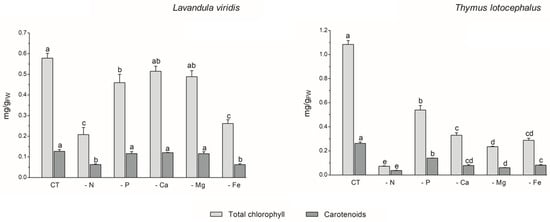
Figure 2.
Total chlorophyll and carotenoid contents of Lavandula viridis and Thymus lotocephalus grown in control medium (CT) and media without sources of nitrogen (-N), phosphorus (-P), calcium (-Ca), magnesium (-Mg), or iron (-Fe). Values are expressed as mean ± SE. The results for each variable were examined using one-way analysis of variance (ANOVA), and graph bars with different letters (a–d) are substantially different at p < 0.05 (Duncan’s New Multiple Range Test).
Conversely, deficiencies in N or Fe resulted in the lowest levels of carotenoid production (65.4% and 55.3% reductions, respectively) and total chlorophyll (64.0% and 54.8% reductions, respectively) in the shoots of this species. In the case of T. lotocephalus, the current findings indicated that the deficiency of the five nutrients, especially N or Mg, resulted in a notable reduction in the concentration of the photosynthetic pigments, chlorophylls (by 50.2–93.3%), and carotenoids (by 46.7–85.7%). Indeed, as was the case with growth, the absence of N had the greatest detrimental impact on the production of photosynthetic pigments in both species. These results were anticipated, given that N is a major component of nucleic acids, amino acids, proteins, and chlorophyll. It can therefore be regarded as the major limiting macronutrient for plant growth and development [36], and its absence can even be fatal [33]. The findings of Aremu et al. [33] indicate that N stress significantly reduced the photosynthetic pigment content of Tulbaghia spp. compared to P stress, which is in accordance with the present results. Additionally, the N deficiency had a detrimental impact on the chlorophyll content of C. tenuiflora cultured in a RITA® temporary immersion system [36].
The negative impact of Fe deficiency on pigment production observed in this study can be explained by the fact that this nutrient is a crucial cofactor in photosystem complexes and is found in nearly every component of the electron transport chain in the chloroplast, where photosynthesis occurs [42]. As stated by Briat et al. [42], Fe is present in the three primary complexes of the photosynthetic apparatus. Specifically, two Fe atoms are located in the photosystem II (PSII) complex, six in the cytochrome b6f (Cyt b6f) complex, and fourteen are present in the photosystem I (PSI) complex. The current findings are consistent with those of previous studies on other plant species. For example, Cucumis sativus L. [43], Sulla carnosa [44], potato calli [39], fennel [45], and peanut [40] plants grown under Fe limitation have been observed to register a decrease in total chlorophyll content compared to the control.
With regard to Mg, this macronutrient constitutes a central element of chlorophyll and an activator for upwards of three hundred enzymes, including ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) [46]. The results obtained for T. lotocephalus are in accordance with those observed for Citrus sinensis, whereby a deficiency in Mg resulted in a reduction in the chlorophyll content [46].
3.2. Oxidative Stress Caused by Nutrient Starvation
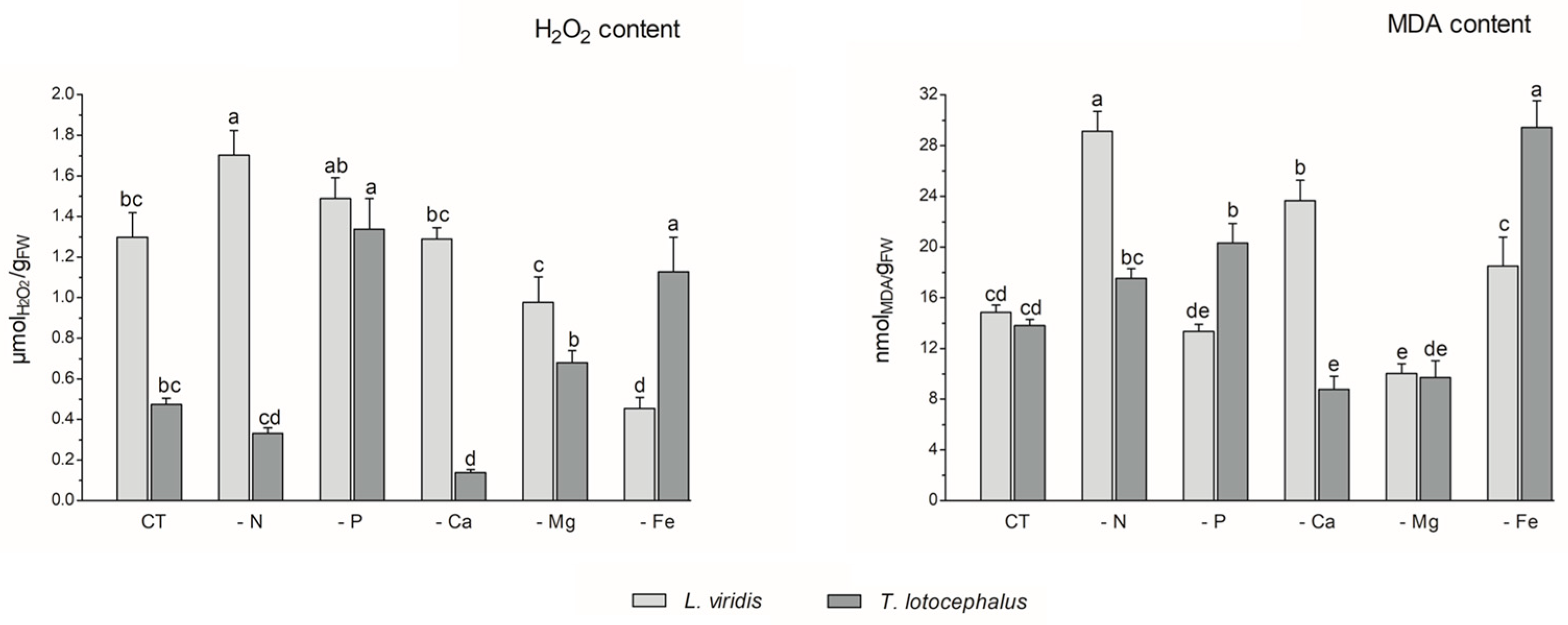
Abiotic stress can result in the production of high levels of reactive oxygen species (ROS), including hydrogen peroxide (H2O2), hydroxyl radical (OH−), superoxide anions (O−), and singlet oxygen (1O2), which can cause damage to plant cells and impair pigment production [12]. In this study, the absence of P (1.34 ± 0.12 µmol/gFW) or Fe (1.13 ± 0.14 µmol/gFW) in the culture media resulted in an increase in H2O2 content in T. lotocephalus in comparison to the control (0.47 ± 0.03 µmol/gFW) (Figure 3).
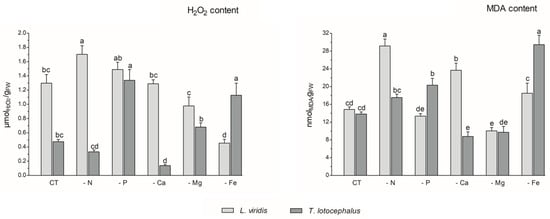
Figure 3.
Hydrogen peroxide (H2O2) and malondialdehyde (MDA) contents of Lavandula viridis and Thymus lotocephalus grown in control medium (CT) and media without sources of nitrogen (-N), phosphorus (-P), calcium (-Ca), magnesium (-Mg), or iron (-Fe). Values are expressed as mean ± SE. The results for each species were examined using one-way analysis of variance (ANOVA), and graph bars with different letters (a–e) are substantially different at p < 0.05 (Duncan’s New Multiple Range Test).
Conversely, the Ca deficiency (0.14 ± 0.02 µmol/gFW) resulted in a reduction in this parameter. In accordance with the findings of this study, the exposure of tomato plants to P limitation [41], as well as peanut plants [40] and potato calli [39] to Fe deficiency, has been shown to result in a significant improvement in ROS accumulation. In L. viridis, the highest production of H2O2 was observed in plants subjected to N limitation (1.70 ± 0.12 µmolH2O2/gFW), which was significantly different from the control (1.51 ± 0.09 µmol/gFW). In contrast, the content of H2O2 was significantly decreased in this species when Mg (0.98 ± 0.13 µmol/gFW) or Fe (0.46 ± 0.05 µmol/gFW) starvation was imposed, in comparison to the control. While the present study did not analyze the antioxidant enzyme system or the thiol-based antioxidant system, it is plausible that these systems may play a role in protecting L. viridis under conditions of Mg and/or Fe starvation. This hypothesis is supported by observations in other species that have experienced similar nutrient deficits [47,48]. Furthermore, the literature suggests that the overexpression of antioxidant metabolites and antioxidant enzymes resulting from nutrient deficiencies may provide sufficient protection against oxidative stress [47]. In light of the aforementioned statement and in accordance with the data presented in Figure 4, it can be posited that certain phenolic compounds may also play a role in the protection of L. viridis against hydrogen peroxide. This is evidenced by the negative correlation observed between these compounds and the H2O2 content. With regard to N starvation, these findings are in alignment with the existing bibliographic data on shoots from Matricaria chamomilla L. [38] and C. tenuiflora [36], which demonstrated elevated H2O2 production across all N-deficient variants.
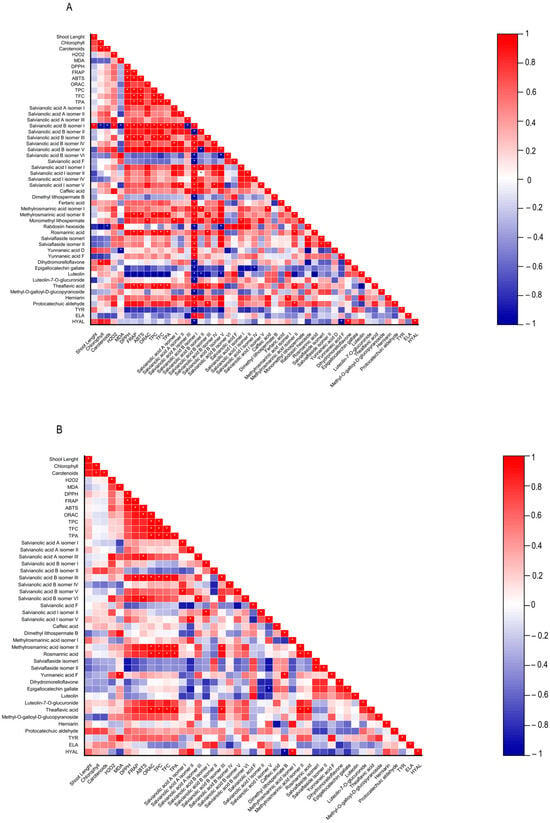
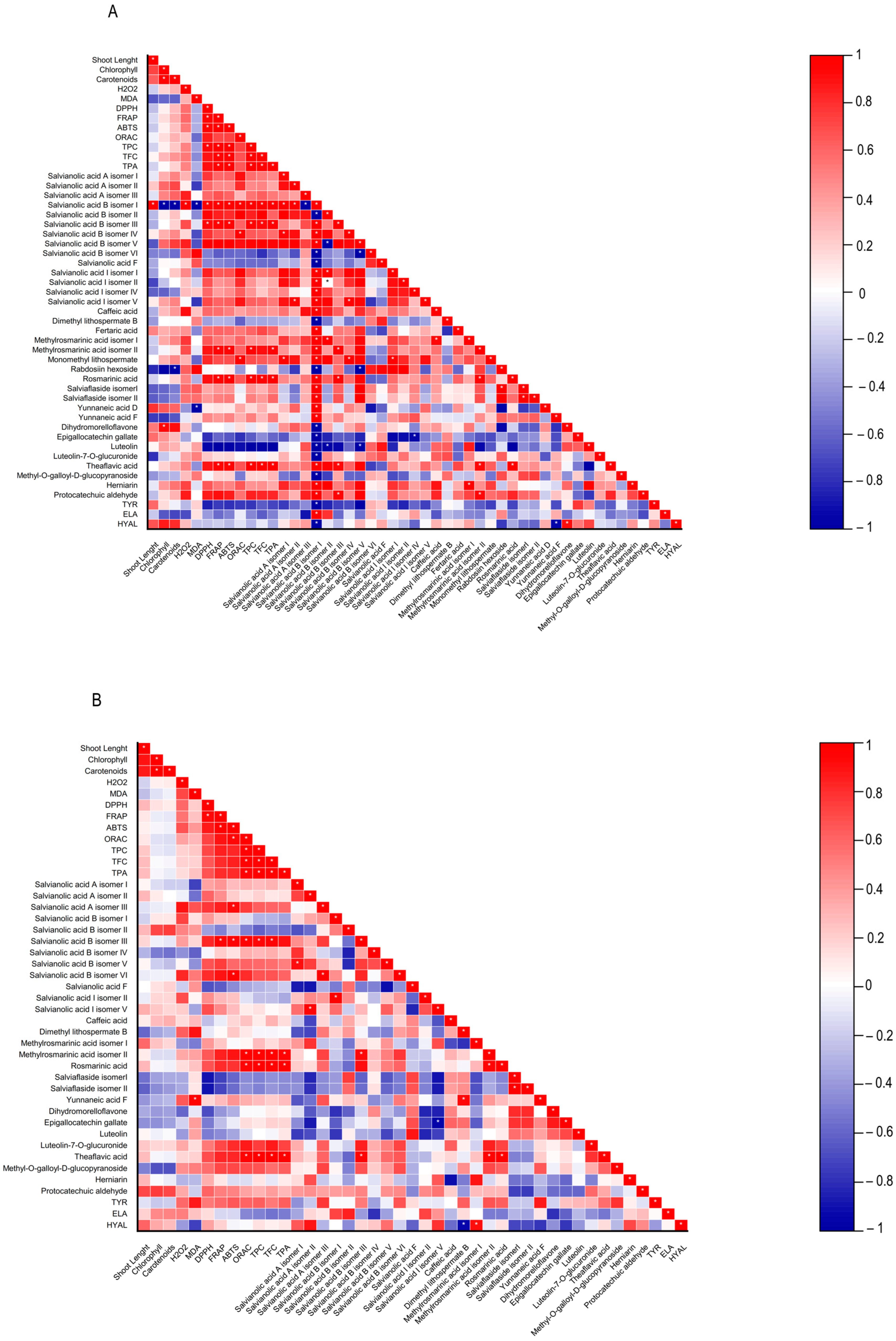
Figure 4.
Heatmap corresponding to Pearson’s correlation between the shoot length, contents in chlorophylls, carotenoids, hydrogen peroxide (H2O2) and lipid peroxidation (MDA), biological properties of plant extracts (antioxidant activity (DPPH, FRAP, ABTS, and ORAC) and anti-aging enzyme (tyrosinase, elastase, and hyaluronidase) inhibitory capacity, contents in total phenolics, flavonoids, phenolic acids, and the individual phenolic compounds identified by HPLC-HRMS from (A) Lavandula viridis and (B) Thymus lotocephalus grown in control medium (CT) and media without sources of nitrogen (-N), phosphorus (-P), calcium (-Ca), magnesium (-Mg), or iron (-Fe). * Correlation is significant (p ≤ 0.01).
The production of ROS during abiotic stress results in the overproduction of malondialdehyde (MDA). This compound is regarded as a biomarker of oxidative damage and the extent of lipid peroxidation, given that the concentration of MDA increases in conjunction with the advancement of membrane–lipid peroxidation [9,49]. As observed for the H2O2 content, the media lacking Fe (29.45 ± 2.09 nmol/gFW) or P (20.32 ± 1.55 nmoL/gFW) exhibited the highest MDA levels, in comparison with the control (13.80 ± 0.49 nmol/gFW), in T. lotocephalus (Figure 3). These findings indicate the presence of oxidative stress and support the hypothesis that elevated levels of H2O2 can damage cellular membranes by generating hydroxyl radicals (OH−) and promoting lipid peroxidation [44]. The results indicate that the disruption of thylakoid membranes in chloroplasts by lipid peroxidation, which resulted in a reduction in chlorophyll production, was substantiated by the inverse relationship between MDA and photosynthetic pigments (Figure 4), particularly in L. viridis. Furthermore, a loss of membrane integrity was also previously observed in these two Lamiaceae species under heat stress [18]. Similarly, the findings of other authors indicated that Fe deficiency elevated MDA levels in S. carnosa [44] and peanut plants [40], and a similar outcome was observed in Solanum lycopersicum L. grown under P limitation [41]. In contrast to the effects of Fe or P limitation, Ca limitation resulted in a reduction in the oxidation of lipids in T. lotocephalus (8.77 ± 1.04 nmol/gFW).
In L. viridis, the highest levels of MDA were observed in cultures grown in N-deficient (29.14 ± 1.56 nmol/gFW) or Ca-deficient media (23.68 ± 1.60 nmol/gFW) (Figure 3). Similar results were observed in the blueberry Legacy cultivar, where lipid peroxidation was more pronounced in plants grown in N-deficient conditions [50]. In contrast, the results obtained in Ocimum basilicum L. (Lamiaceae) demonstrated that limiting N considerably reduced the degree of lipid peroxidation in shoots and roots for all studied genotypes (Small-leaved basil, Genovese, Dark Opal, and Lemon basil) [49]. Similarly, as observed in the case of L. viridis, the MDA content was found to be reduced following Mg starvation.
3.3. Phenolic Profile and Antioxidant Activity of the Green Extracts
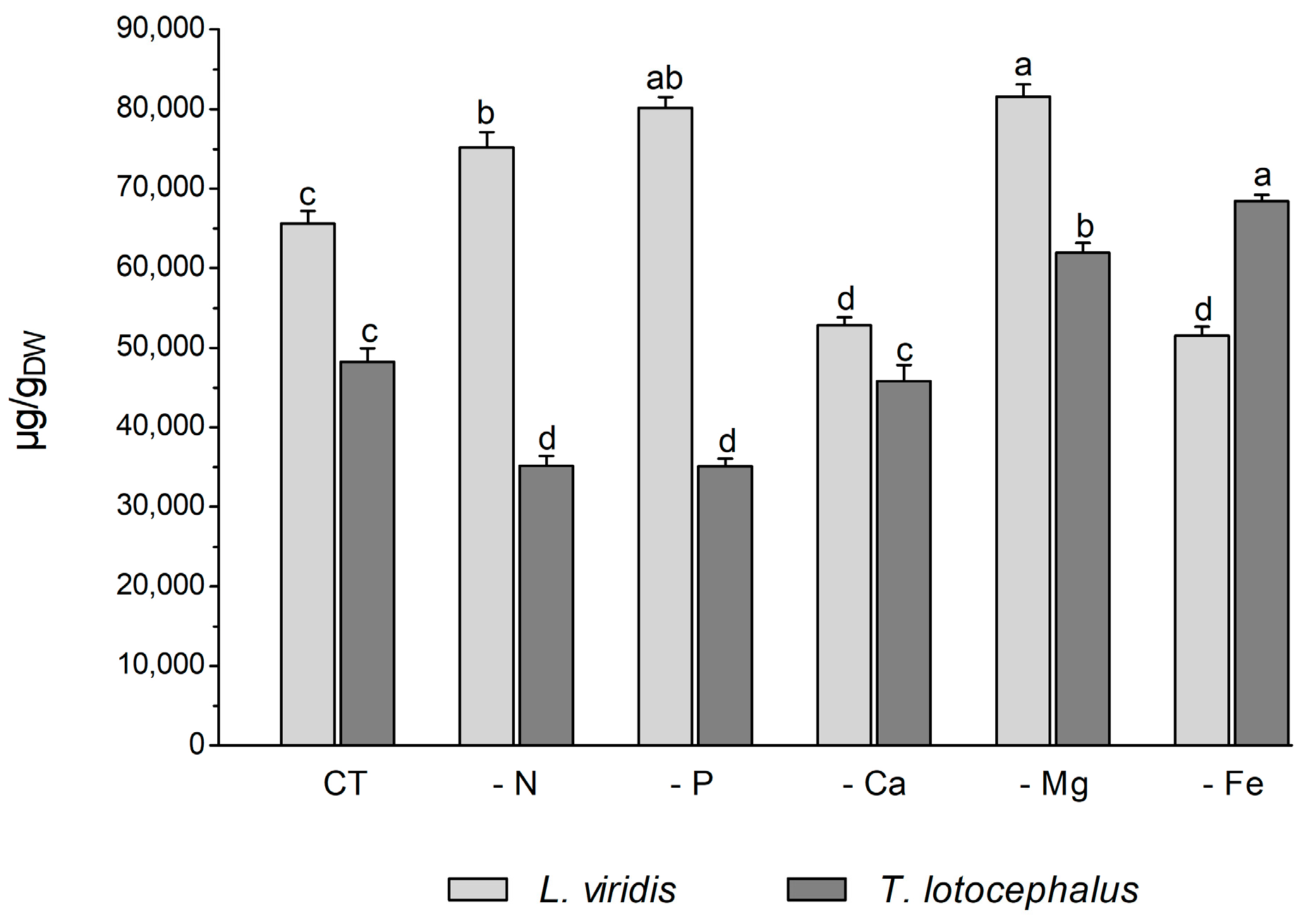
Plants usually react to oxidative stress by inducing secondary metabolite production. L viridis and T. lotocephalus are a rich source of phenolic compounds, particularly rosmarinic acid. A total of 35 phenolic compounds were identified in L. viridis extracts (Table S1, Supporting Information), of which 34 were quantified. In T. lotocephalus, 22 phenolics were quantified out of the 29 initially identified. The total phenolic content, as determined by HPLC-HRMS, ranged from 52.8 to 81.6 mg/gDW in L. viridis and from 35.1 to 68.4 mg/gDW in T. lotocephalus. These findings are illustrated in Figure 5 and described in detail in Tables S3 and S4, respectively. The findings of this study illustrate the pivotal role that phenolics play in enabling both species to withstand nutrient deficiencies. The proportion of quantified compounds in L. viridis and T. lotocephalus that exhibited an increase following exposure to stressful nutrient conditions was 72% and 62%, respectively.
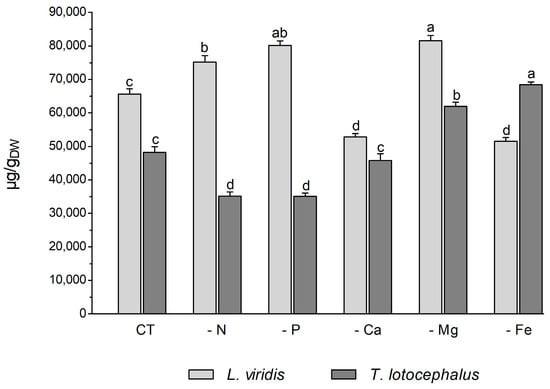
Figure 5.
Total phenolic content determined by HPLC-HRMS of green extracts from Lavandula viridis and Thymus lotocephalus grown in control medium (CT) and media without sources of nitrogen (-N), phosphorus (-P), calcium (-Ca), magnesium (-Mg), or iron (-Fe). Values are expressed as mean ± SE. The results for each species were examined using one-way analysis of variance (ANOVA), and graph bars with different letters (a–d) are substantially different at p < 0.05 (Duncan’s New Multiple Range Test).
In L. viridis, the highest concentration of phenolic compounds was obtained in cultures grown in MS media under Mg (81.6 ± 1.5 mg/gDW) or P (80.2 ± 1.3 mg/gDW) starvation, which was superior in approximately 20% to the control (Figure 5). The largest production of phenolics was observed under Mg or P deprivation, and the lowest MDA content was also noted under these two nutrient deficits. This may demonstrate the role of phenolics in counteracting lipid peroxidation. This assertion is corroborated by the robust negative correlation evident in the heatmap of L. viridis between lipid peroxidation and phenolic production (Figure 4). Similarly, deficiencies in Mg or P have been shown to result in increased phenolic content in citrus [46] and strawberry plants [51], respectively. In addition to its role as a component of primary metabolites, N is also a crucial factor in the synthesis of secondary metabolites. In comparison to the control, N deficiency also resulted in an increase in total phenolic compounds in L. viridis. This finding is consistent with the results of other authors who have demonstrated an inverse relationship between low N availability and phenolic biosynthesis [36,38,49,52,53]. One hypothesis to explain this phenomenon is that low N availability promotes the synthesis of metabolites with C, H, and O structures, which in turn favors the synthesis of terpenes and phenolic compounds. With regard to metabolites containing N, such as alkaloids, cyanogenic components, and non-protein amino acids, their biosynthesis will be inhibited [21]. In contrast to the effects of Mg, P, or N starvation, the total phenolic content was found to decrease following exposure of L. viridis to Ca or Fe deficiencies (Figure 5). The reduction in phenolic production under conditions of Ca or Fe starvation may indicate that these compounds are utilized to mitigate the elevated levels of MDA observed in L. viridis cells. This hypothesis is supported by the strong negative correlation between lipid peroxidation and phenolics observed in this species (Figure 4). These findings differ from those observed in S. rebaudiana, where the Ca-deficient medium was found to stimulate the production of these compounds [32]. Nevertheless, the inhibition of phenolic production was also observed in other species subjected to Fe limitation, namely S. carnosa [44] and Foeniculum vulgare Mill [45].
The accumulation of phenolics in T. lotocephalus cultures was significantly increased by Mg and Fe starvation, particularly micronutrient deficiency, which stimulated the production of more than 29.5% of these compounds compared to the control (Figure 5). Similarly, the Fe-deficient medium has been demonstrated to enhance the production of phenolics in strawberry plants [51] and potato calli [39]. Conversely, N or P limitation was observed to decrease phenolic accumulation in T. lotocephalus, as has been documented in other species subjected to N or P stresses, namely E. autumnalis, T. ludwigiana, and T. violacea [33]. Kováčik et al. [54] observed that nutrient deprivation resulted in elevated total phenolics and phenolic acids, which are associated with elevated/preserved phenylalanine ammonium lyase (PAL) activity (the first key enzyme involved in the synthesis of phenolics). An increase in PAL activity has been previously observed in T. lotocephalus and L. viridis subjected to other abiotic stress, namely temperature [13].
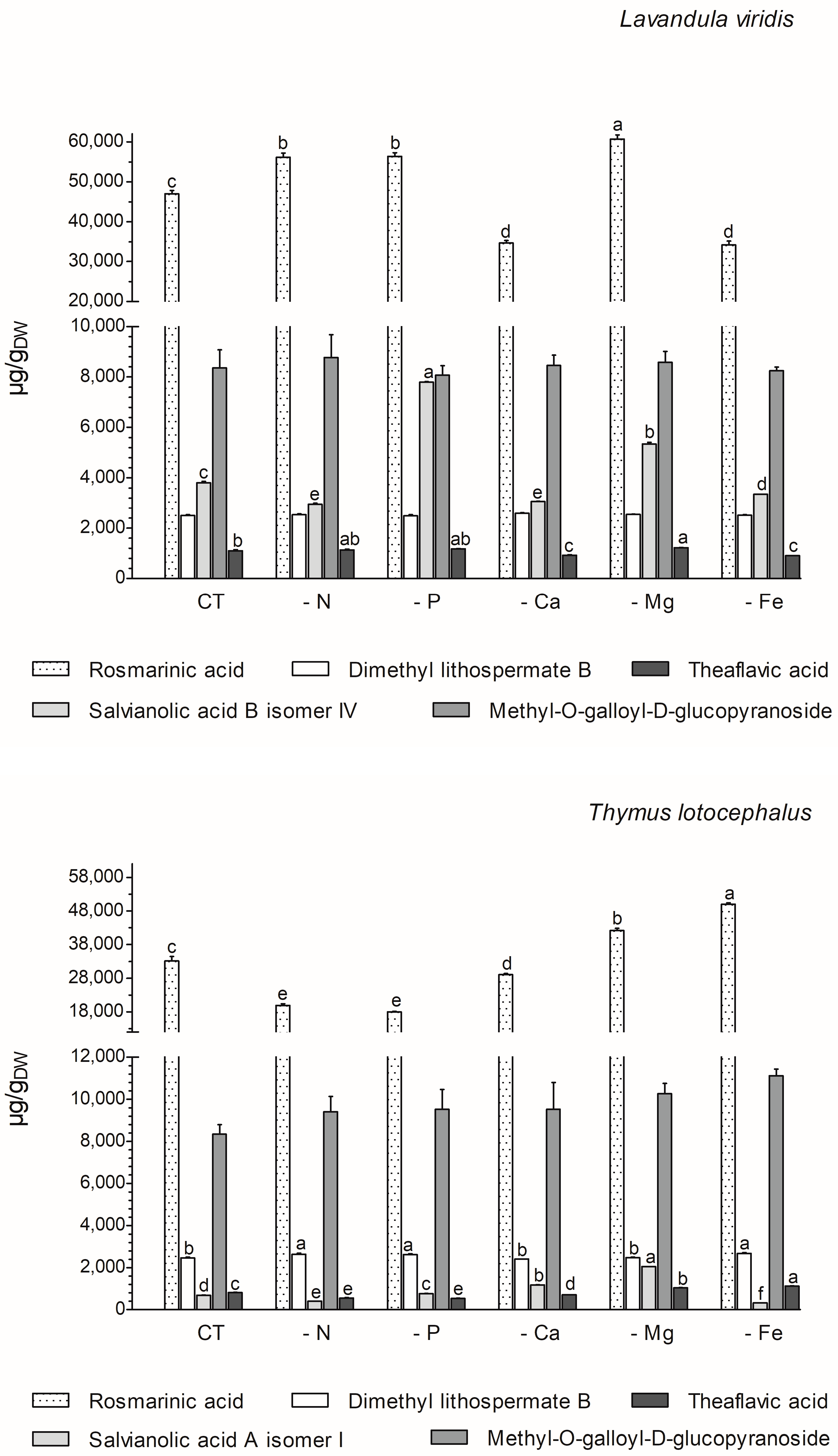
The majority of the phenolics produced by these two species (77% in L. viridis and 84% in T. lotocephalus) belong to the class of phenolic acids. The majority of these (68.6% for L. viridis and 57.1% for T. lotocephalus of total phenolics) are rosmarinic acid (RA) derivatives (RA coupled with additional aromatic moieties and its derivatives) [55]. The phenolic acid RA (or α-o-caffeoyl-3,4-dihydroxyphenyllactic acid), which is synthesized through the esterification of caffeic acid, represents the major compound in L. viridis (comprising 67–74% of the total compounds) and T. lotocephalus (comprising 51–73% of the total compounds). Its production was stimulated by the limitation of the nutrients in the culture media, namely Mg, N, or P in L. viridis and Mg or Fe in T. lotocephalus (Figure 5 and Figure 6, and Tables S3 and S4). The highest concentration of RA in L. viridis was observed in the Mg-deficient medium (60.7 ± 1.0 mg/gDW), representing a significant increase of over 22.7% compared to the control (46.9 ± 0.9 mg/gDW). In T. lotocephalus, the culture medium lacking Fe was the most effective in stimulating the production of RA (50.0 ± 0.4 mg/gDW), with an accumulation of over 33.6% compared to the control (33.2 ± 1.3 mg/gDW). Similarly, the biosynthesis of RA has been observed to be stimulated by nutrient limitation in other species belonging to the Lamiaceae family, including Origanum vulgare L. [56], Perilla frutescens [57], and O. basilicum [49]. Nevertheless, to the best of our knowledge, this is the first study to report that Fe or P starvation is an effective method for stimulating RA. While less abundant than rosmarinic acid, methyl-O-galloyl-D-glucopyranoside was also a prominent compound in both species (Figure 6 and Tables S3 and S4), as previously identified by our research group [12,13]. However, in both species, the production of this galloyl ester was not affected by nutrient starvation, with no statistically significant difference observed compared to the controls. It is noteworthy that the resilience or lack of efficacy of this compound to abiotic stress has been previously documented in L. viridis subjected to temperature stress [13] and in T. lotocephalus exposed to drought [12].
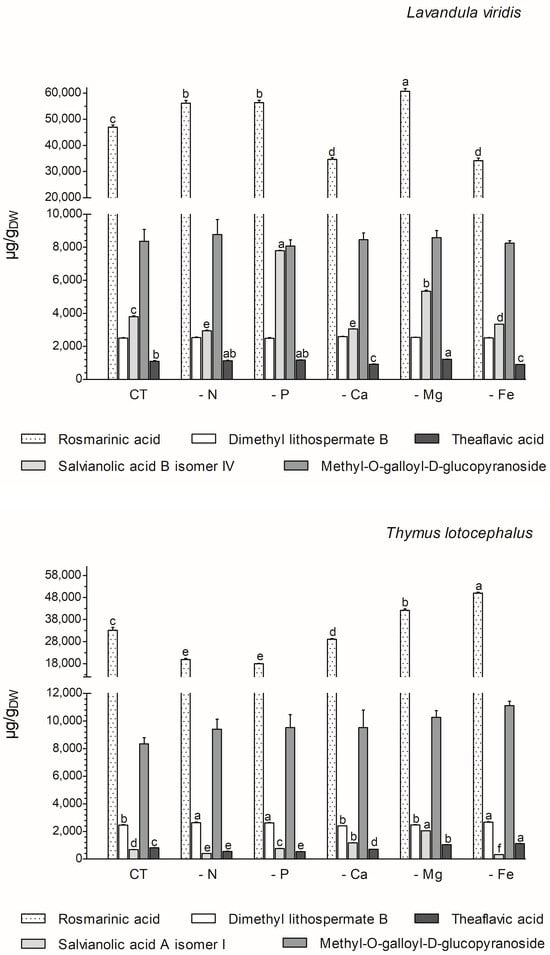
Figure 6.
Variation in main phenolic compounds determined by HPLC-HRMS of green extracts from Lavandula viridis and Thymus lotocephalus grown in control medium (CT) and media without sources of nitrogen (-N), phosphorus (-P), calcium (-Ca), magnesium (-Mg), or iron (-Fe). Values are expressed as mean ± SE. The results for each species were examined using one-way analysis of variance (ANOVA), and graph bars with different letters (a–f) are substantially different at p < 0.05 (Duncan’s New Multiple Range Test).
The two species under investigation in the present study are rich in different salvianolic acids, which are known as RA derivatives. The most abundant salvianolic acids in L. viridis and T. lotocephalus, respectively, are salvianolic acid B (isomer IV) (3.0–7.8 mg/gDW) and salvianolic acid A (isomer I) (0.4–2.1 mg/gDW) (Figure 6 and Tables S3 and S4). The highest production of salvianolic acid B (isomer IV) was obtained in L. viridis grown under P deficiency (7.8 ± 0.02 mg/gDW), which corresponds to more than 50% of that produced by the control (Figure 6 and Figure 7, and Tables S3 and S4). The production of salvianolic acid A (isomer I) was markedly enhanced under Mg deficiency conditions in T. lotocephalus exhibiting a 66.3% increase relative to the control (Figure 6 and Figure 7, and Tables S3 and S4). Another RA derivative and abundant compound (the fourth in L. viridis and the third in T. lotocephalus) was identified in both species: dimethyl lithospermate B. The nutrient-limiting conditions in the culture media did not result in alterations to the production of this RA derivative in L. viridis. However, the accumulation of this compound was increased in T. lotocephalus when the cultures were subjected to N, P, or Fe starvation (Figure 5 and Figure 6, and Tables S3 and S4).
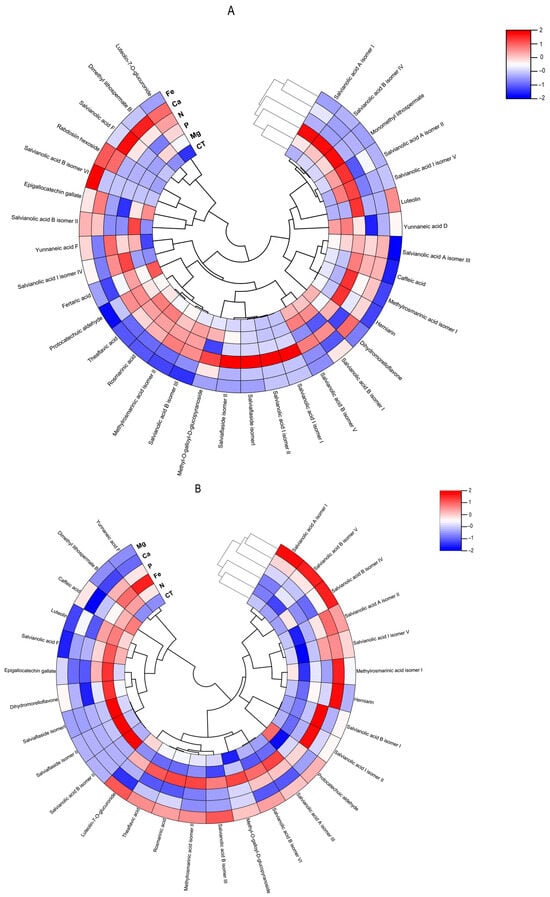
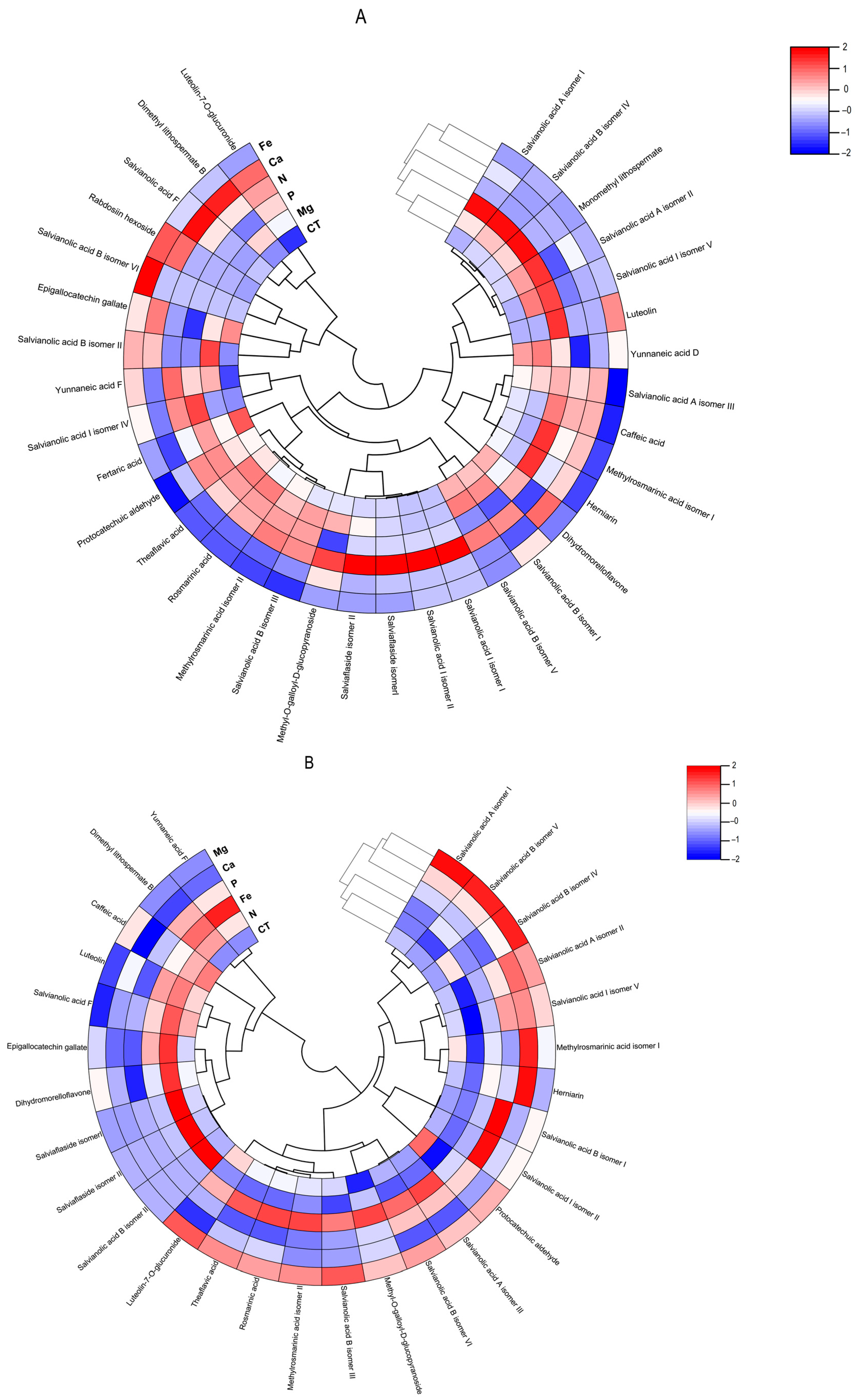
Figure 7.
Polar heatmap with dendrogram indicting the clustering of distance (or similarity) among extracts and individual phenolic compounds. The polar heatmap corresponds to Pearson’s correlation of the compounds identified by HPLC-HRMS in the extracts from (A) Lavandula viridis and (B) Thymus lotocephalus grown in control medium (CT) and media without sources of nitrogen (-N), phosphorus (-P), calcium (-Ca), magnesium (-Mg), or iron (-Fe).
RA and its derivatives have been shown to possess a number of biological properties, including anti-inflammatory, antibacterial, antiviral, antioxidant, and antiangiogenic activities. Furthermore, preclinical pharmacological studies have indicated that RA and its derivatives represent a promising strategy for cancer treatment and prevention. However, their low bioavailability limits their pharmacological effects, necessitating the development of innovative approaches for nanoformulation, for instance [55]. In addition to salvianolic acids and dimethyl lithospermate B, other RA derivatives were identified and quantified (although in smaller quantities) in both species, namely methylrosmarinic acid, monomethyl lithospermate, salviaflaside, and yunnaneic acid F. Furthermore, the RA derivatives rabdosiin hexoside and yunnaneic acid D were identified and quantified in L. viridis (Tables S3 and S4).
The flavonoid theaflavic acid represents the fifth major compound of both studied species, whose production was stimulated by the limitation of Mg in L. viridis and Mg or Fe in T. lotocephalus (Figure 5 and Figure 6, and Tables S3 and S4). Some of the identified compounds in both species under investigation are being reported for the first time. To the best of our knowledge, this is the first occasion on which dimethyl lithospermate B has been identified in the Lavandula and Thymus genera. However, it has previously been identified in other species of the Lamiaceae family, namely Salvia miltiorrhiza [58]. Furthermore, this is also the first identification of monomethyl lithospermate in the Lavandula genus and T. lotocephalus, although it has been identified in other Thymus species [59,60]. The identification of rabdosiin hexoside in the Lavandula genus represents a novel finding. Yunnaneic acid D and yunnaneic acid F were identified for the first time in L. viridis species, although both have been previously identified in Lavandula austroapennina [61]. Furthermore, this is also the inaugural report identifying yunnaneic acid F in Thymus species. The results of the hierarchical cluster analysis presented in the form of a polar heatmap with a dendrogram (Figure 7) indicate that, based on the HPLC-HRMS results, the extracts from L. viridis with a composition more similar are CT and Mg, followed by P and N. This corresponds to the four nutrients whose absence most stimulated the production of phenolics. Conversely, cultures derived from Ca- or Fe-deficient media exhibited a high degree of similarity, likely due to the limited accumulation of phenolic compounds, as indicated by the negative correlations (Figure 7). With regard to T. lotocephalus, the greatest distance is observed between the CT-N-Fe and Ca-Mg-P clusters.
A low MDA level may be indicative of the functionality of the enzymatic antioxidant system, thereby enabling the plant to defend itself against stress. Furthermore, a reduction in MDA accumulation may be associated with an increase in phenolic content and the associated antioxidant activity. The results obtained with L. viridis (Figure 4) are in accordance with those previously reported by Jakovljević et al. [49], who observed a negative correlation between MDA and phenolic contents in basil cultivars subjected to stressful nutrient conditions. A negative correlation between MDA and phenolic contents indicates that phenolics can act as antioxidants, scavenging ROS and thus decreasing lipid peroxidation. The negative correlation found between the results of the four antioxidant capacity assays and MDA contents in L. viridis, as observed in our study, lends further support to this hypothesis. Similarly, in a previous study, phenolic compounds and the associated antioxidant capacity of extracts from this species indicated that they play an important role in protecting the in vitro cultures against lipid peroxidation caused by temperature stress [13]. The protection of this species from lipid peroxidation induced by nutrient starvation appears to be primarily attributed to the action of specific isomers of salvianolic acids (A, B and I), yunnaneic acid D, fertaric acid, monomethyl lithospermate, dihydromorelloflavone, theaflavic acid, and rosmarinic acid (Figure 4). Some of these compounds have previously been demonstrated to elicit a defensive response in L. viridis against the deleterious effects on lipid membranes induced by elevated temperatures [13]. In the case of T. lotocephalus, a negative correlation between MDA and total phenolic accumulations or antioxidant activity was not observed, suggesting that other main protective mechanisms may be at play in this species. These could include the enzymatic antioxidant system, such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), etc. Nevertheless, some individual phenolics from T. lotocephalus appear to possess the ability to safeguard this species from lipid peroxidation, including some isomers of the salvianolic acids A, B, and I, methylrosmarinic acid, herniarin, and protocatechuic aldehyde (Figure 4).
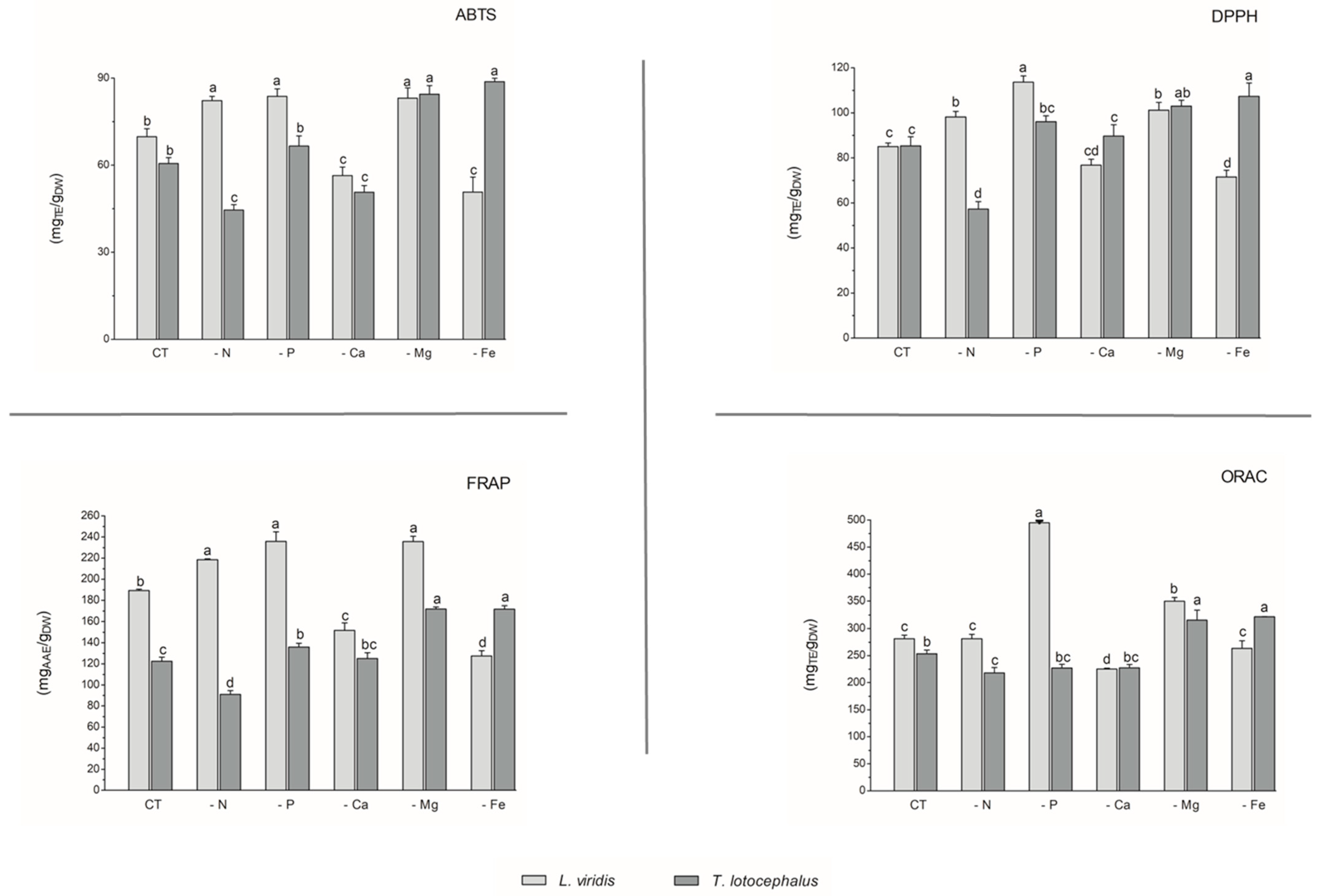
With regard to antioxidant activity, a robust positive correlation was identified between the four antioxidant assays tested (DPPH, FRAP, ABTS, and ORAC) and the total phenolic, flavonoid, and phenolic acid contents in both species (Figure 4). This finding substantiates the antioxidant potential of phenolic compounds derived from the L. viridis and T. lotocephalus extracts. In particular, salvianolic acids A, B, and I, methylrosmarinic acid, monomethyl lithospermate, rosmarinic acid, theaflavic acid, and protocatechuic aldehyde were identified as the compounds that most contributed to the antioxidant capacity of L. viridis. The most important compounds in T. lotocephalus were salvianolic acids A and B, methylrosmarinic acid, rosmarinic acid, theaflavic acid, luteolin-7-O-glucuronide, and methyl-O-galloyl-D-glucopyranoside. The antioxidant capacity of plant extracts in L. viridis was found to be stimulated by N, P, or Mg limitations, but conversely, Ca or Fe deficiencies were observed to have a detrimental effect (Figure 8). The antioxidant activity of T. lotocephalus extracts was found to be elevated in the presence of Mg or Fe starvation, as evidenced by the four assays employed (ABTS, DPPH, FRAP, and ORAC). Conversely, the lowest antioxidant capacity of T. lotocephalus was observed in extracts from cultures grown in N-limited media. Nutrient-limiting conditions, specifically N [49,52,53], Ca [32], Fe [44,51], or K [33], have also been shown to significantly enhance the antioxidant properties of diverse plants.
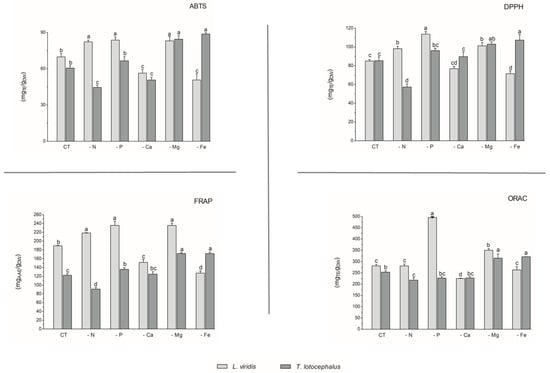
Figure 8.
Antioxidant capacity assessed by 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP), and oxygen radical absorbance capacity (ORAC) methodologies of the extracts from Lavandula viridis and Thymus lotocephalus grown in control medium (CT) and media without sources of nitrogen (-N), phosphorus (-P), calcium (-Ca), magnesium (-Mg), or iron (-Fe). Values are expressed as mean ± SE. The results for each species were examined using one-way analysis of variance (ANOVA), and graph bars with different letters (a–d) are substantially different at p < 0.05 (Duncan’s New Multiple Range Test).
3.4. Effect of Nutrient Deficiency on the Inhibitory Anti-Aging Enzymatic Capacity
The present study was conducted to investigate anti-tyrosinase (Tyr), anti-hyaluronidase (Hyal), and anti-elastase (Ela) activities. To the best of our knowledge, this is the first report on the impact of nutrient deficiency on the inhibition of these four enzymes. Furthermore, it is the inaugural report assessing the bioactive potential of L. viridis and T. lotocephalus toward the inhibition of the enzymes Hyal and Ela. Furthermore, the utilization of innovative and environmentally friendly solvents (Natural Deep Eutectic Solvents, NADES) in Lavandula and Thymus extracts with the potential to inhibit Hyal and Ela enzymes is being reported for the first time. Our group has previously employed NADES to extract phenolic compounds with the capacity to inhibit Tyr in T. lotocephalus [12] and Lavandula pedunculata [17]. The natural origin, biodegradability, and non-toxicity of NADES present untapped potential for the development of novel extracts with distinctive phytochemical profiles and biological characteristics, which could be employed safely in a variety of industries, including cosmetics [12].
As illustrated in Table 2, the inhibitory effect towards Tyr ranges from 17.08 to 39.35% in L. viridis and from 20.17 to 61.14% in T. lotocephalus, exhibiting the most pronounced inhibitory activity among the three tested enzymes.

Table 2.
Tyrosinase, hyaluronidase, and elastase inhibitory activities (%) of the extracts from Lavandula viridis and Thymus lotocephalus grown in control medium (CT) and media without sources of nitrogen (-N), phosphorus (-P), calcium (-Ca), magnesium (-Mg), or iron (-Fe).
The extracts from cultures grown under control media and Fe-deficient conditions in L. viridis were demonstrated to be the most potent in inhibiting Tyr, a result that was replicated in T. lotocephalus subjected to Fe limitation. The highest correlation with Tyr inhibition in L. viridis was observed for epigallocatechin gallate and luteolin. In T. lotocephalus, several compounds demonstrated a good correlation, including yunnaneic acid F, methyl-O-galloyl-D-glucopyranoside, salvianolic acid A (isomer III) and B (isomer VI), theflavic acid, dimethyl lithospermate B, methylrosmarinic acid, and rosmarinic acid (Figure 4). Some of these compounds have previously been shown to be correlated with Tyr inhibition in other investigations of T. lotocephalus species [12]. Previous studies have reported the anti-Tyr effect of other Lavandula [3,62,63,64,65] and Thymus species [2,60,66,67,68,69,70,71,72,73,74,75], but these studies used water or organic solvents to extract the biocompounds.
A review of the literature revealed no reports on Hyal inhibition by other Lavandula spp. extracts. Only one study was identified concerning the capacity of the essential oils from Lavandula hybrida, Lavandula latifolia, and Lavandula hybrida to inhibit Hyal [76]. With regard to the Thymus genera, only one study was identified that examined the capacity of T. vulgaris to inhibit Hyal. The authors of this study reported that the water and acetone–water (25:75%) extracts (100 µg/mL) (which have high dielectric constants) demonstrated notable inhibition of Hyal (>85%), in contrast to hexane, ethyl acetate, acetone, and acetone–water (75:25 and 50:50%) [18].
In the present study, the most effective inhibition of Hyal by L. viridis extracts was observed in cultures grown under control conditions (74.98 ± 4.14%) or Ca-deficient conditions (71.70 ± 7.61%). These results were comparable to those obtained with the positive control (tannic acid, 73.15 ± 0.61%), which was applied at the same concentration as the plant extracts (500 µg/mL). Similarly, the most significant anti-Hyal activity of T. lotocephalus extracts was observed in cultures subjected to Ca limitation (73.44 ± 5.49%) (Table 2). In the case of L. viridis, the flavonoids dihydromorelloflavone and luteolin demonstrated a significant strong correlation with Hyal inhibition, particularly the former. In T. lotocephalus, the methylrosmarinic acid isomer I exhibited the strongest correlation. Furthermore, the salvianolic acids A (isomers I and II) and I (isomer V) and herniarin were identified as promising candidates for the development of Hyal inhibitors (Figure 4). It has previously been demonstrated that luteolin [19,77,78] and salvianolic acid A [79] are effective targets for the inhibition of Hyal.
The Ela inhibitory capacity of the extracts of both species was found to be the lowest among the three tested enzymes. Nevertheless, the most effective anti-Ela results were observed in cultures of L. viridis (20.48 ± 0.05%) and T. lotocephalus (23.00 ± 0.29%) cultivated under Fe or P starvation, respectively (Table 2). Pearson’s correlation analysis revealed that salvianolic acids B (isomers I and II) exhibited the highest correlation with Ela inhibition in both species (Figure 4). This RA derivative has previously demonstrated efficacy in the reduction in porcine pancreatic elastase-induced emphysema in the lungs of rats [80]. Furthermore, salvianolic acid I (isomer II) may also play a significant role in the anti-Ela activity of T. lotocephalus extracts. Similar to our own findings, the inhibition of Ela was found to be poor in Thymus carnosus [71]. In contrast, greater capacities to inhibit Ela were identified in other Lavandula and Thymus species, including Lavandula officinalis [64], Thymus vulgaris [18], T. brachychilus [66], T. pulegioides [74], T. pubescens [2], and T. fragrantissimus [73].
4. Conclusions
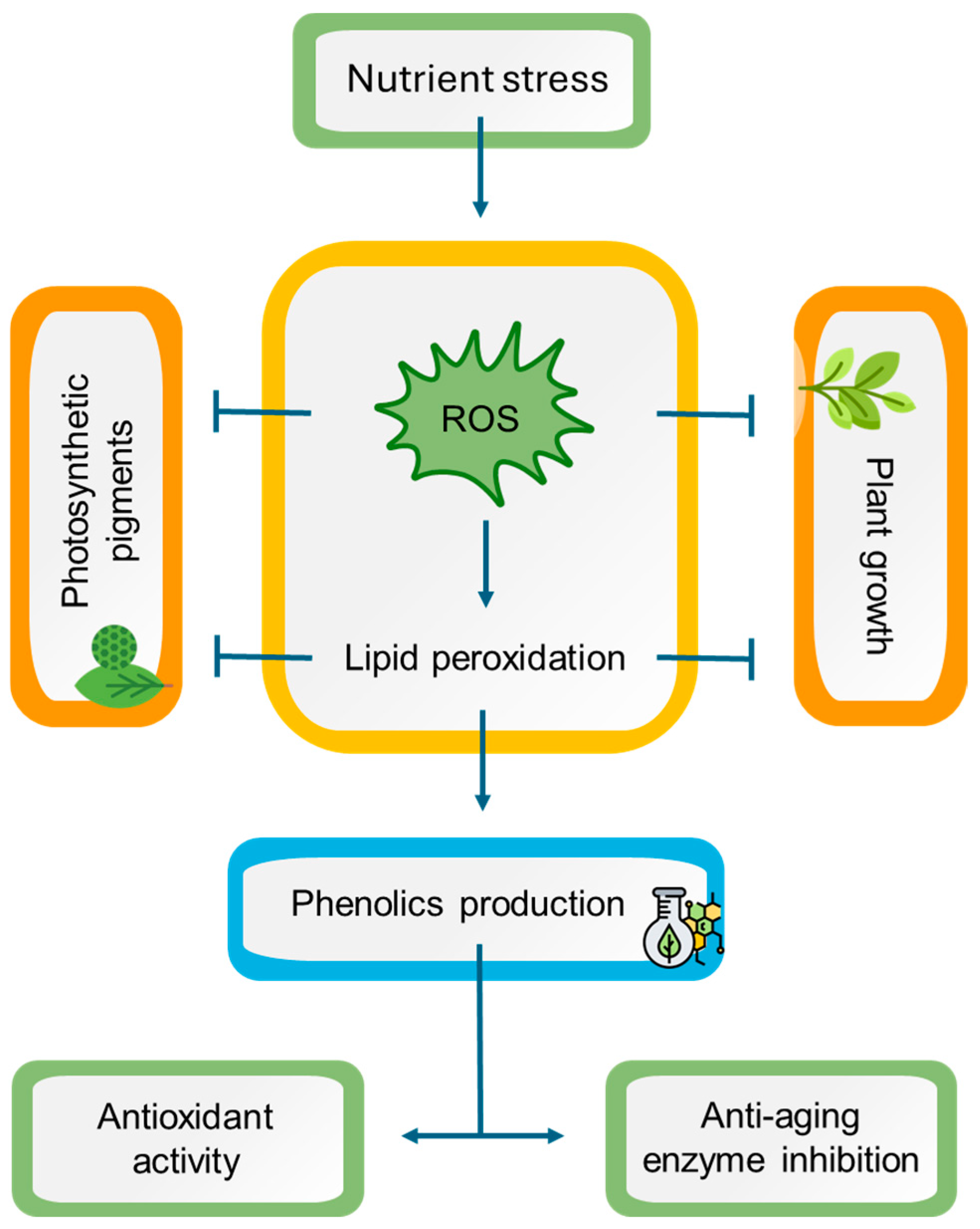
This is the first report to investigate the impact of the nutrient starvation effect on two endemic aromatic and medicinal plants from the Mediterranean, namely L. viridis and T. lotocephalus. The findings indicate that a deficiency in nutrients resulted in oxidative stress in both species, with ROS accumulation, which is directly linked to lipid peroxidation. As a consequence, a detrimental impact on plant growth and pigment production was observed. Conversely, oxidative stress resulted in enhanced production of phenolic compounds (e.g., rosmarinic acid and its derivatives salvianolic acids A, B, F, and I, methylrosmarinic acid, salviaflaside, and yunnaneic acid F, as well as theaflavic acid and herniarin), which in general also augmented the antioxidant and anti-aging enzyme inhibition capabilities of plant extracts (Figure 9). In light of these findings, it can be posited that the extracts of these species prepared with green solvents have the potential to be used for cosmetic applications. Furthermore, it can be suggested that in vitro culture associated with temporary nutrient starvation represents an effective platform for the production of phenolic compounds with bioactivity in Lamiaceae species.
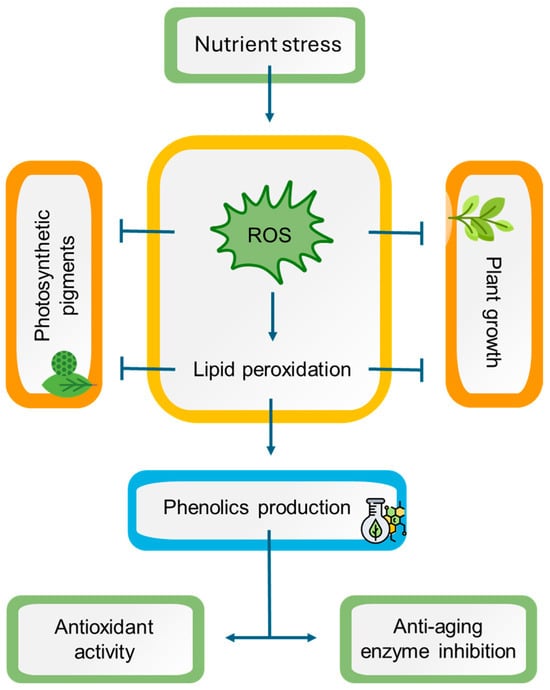
Figure 9.
A working model representing the potential mechanisms of nutrient stress in Lavandula viridis and Thymus lotocephalus. Nutrient starvation induces oxidative stress by triggering reactive oxygen species (ROS) accumulation, leading to lipid peroxidation with subsequent impairment in photosynthetic pigment production and plant growth. On the other hand, oxidative stress causes an improvement in phenolic compound production, enhancing the antioxidant and anti-aging enzyme inhibition potentials of plant extracts. Notes: (⟶) induction; ( ) inhibition.
) inhibition.
 ) inhibition.
) inhibition.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10090947/s1, Table S1: HPLC-HRMS data of identified phenolic compounds in Lavandula viridis and Thymus lotocephalus extracts; Table S2: Summary of HPLC-HRMS criterion for quantification of phenolic compounds in Lavandula viridis and Thymus lotocephalus extracts; Table S3: Quantity (µg/gDW or mg/gDW*) of the phenolic compounds in Lavandula viridis extracts grown in control medium (CT) and media without sources of nitrogen (-N), phosphorus (-P), calcium (-Ca), magnesium (-Mg) or iron (-Fe); Table S4: Quantities of (µg/gDW or mg/gDW*) phenolic compounds in Thymus lotocephalus extracts grown in control medium (CT) and media without sources of nitrogen (-N), phosphorus (-P), calcium (-Ca), magnesium (-Mg) or iron (-Fe).
Author Contributions
I.M.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Roles/Writing—original draft. S.G.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Funding acquisition, Project administration, Resources, Supervision, Writing—review and editing. R.R.-S.: Formal analysis, Investigation, Methodology, Software, Validation; Writing—review and editing. G.P.-C.: Validation, Writing—review and editing. J.M.M.-R.: Validation, Funding acquisition, Project administration, Resources, Writing—review and editing. A.R.: Conceptualization, Investigation, Validation, Funding acquisition, Project administration, Resources, Supervision, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed by National Funds through FCT-Foundation for Science and Technology under the Projects LA/P/0121/2020 (https://doi.org/10.54499/LA/P/0121/2020) and UIDB/05183/2020 (https://doi.org/10.54499/UIDB/05183/2020). Inês Mansinhos (https://doi.org/10.54499/SFRH/BD/145243/2019) and Sandra Gonçalves (CEECINST/00052/2021) acknowledge the financial support from FCT. Raquel Rodríguez Solana was supported by the grant RYC2022-036888-I, funded by MCIU/AEI/10.13039/501100011033 and by the FSE+.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Berry, P.E. “Lamiales”. Encyclopedia Britannica. Available online: https://www.britannica.com/plant/Lamiales/Main-families (accessed on 20 August 2024).
- Yigitkan, S.; Akdeniz, M.; Yener, I.; Seker, Z.; Yilmaz, M.A.; Firat, M.; Kavak, D.E.; Yilmaz Koseoglu, P.; Ertas, A.; Kolak, U.; et al. Comprehensive Study of Chemical Composition and Biological Activity of Thymus pubescens Boiss. et Kotschy Ex Čelak. S. Afr. J. Bot. 2022, 149, 425–434. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Goméz-García, R.; Machado, M.; Nunes, C.; Ribeiro, S.; Nunes, J.; Oliveira, A.L.S.; Pintado, M. Lavandula Pedunculata Polyphenol-Rich Extracts Obtained by Conventional, MAE and UAE Methods: Exploring the Bioactive Potential and Safety for Use a Medicine Plant as Food and Nutraceutical Ingredient. Foods 2023, 12, 4462. [Google Scholar] [CrossRef] [PubMed]
- Aazza, S.; El-Guendouz, S.; Miguel, M.G.; Antunes, M.D.; Faleiro, M.L.; Correia, A.I.; Figueiredo, A.C. Antioxidant, Anti-Inflammatory and Anti-Hyperglycaemic Activities of Essential Oils from Thymbra capitata, Thymus albicans, Thymus caespititius, Thymus carnosus, Thymus lotocephalus and Thymus mastichina from Portugal. Nat. Prod. Commun. 2016, 11, 1029–1038. [Google Scholar] [CrossRef]
- Barbosa, P.; Lima, A.S.; Vieira, P.; Dias, L.S.; Tinoco, M.T.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C.; Mota, M. Nematicidal Activity of Essential Oils and Volatiles Derived from Portuguese Aromatic Flora against the Pinewood Nematode, Bursaphelenchus Xylophilus. J. Nematol. 2010, 42, 8–16. [Google Scholar]
- Costa, P.; Goncalves, S.; Grosso, C.; Andrade, P.B.; Valentao, P.; Gabriela Bernardo-Gil, M.; Romano, A. Chemical Profiling and Biological Screening of Thymus Lotocephalus Extracts Obtained by Supercritical Fluid Extraction and Hydrodistillation. Ind. Crop. Prod. 2012, 36, 246–256. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Andrade, P.B.; Valentão, P.; Romano, A. Inhibitory Effect of Lavandula viridis on Fe(2+)-Induced Lipid Peroxidation, Antioxidant and Anti-Cholinesterase Properties. Food Chem. 2011, 126, 1779–1786. [Google Scholar] [CrossRef]
- Costa, S.; Cavadas, C.; Cavaleiro, C.; Salgueiro, L.; do Céu Sousa, M. In Vitro Susceptibility of Trypanosoma brucei brucei to Selected Essential Oils and Their Major Components. Exp. Parasitol. 2018, 190, 34–40. [Google Scholar] [CrossRef]
- Gonçalves, S.; Mansinhos, I.; Rodríguez-Solana, R.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Romano, A. Impact of Metallic Nanoparticles on In Vitro Culture, Phenolic Profile and Biological Activity of Two Mediterranean Lamiaceae Species: Lavandula viridis L’Hér and Thymus lotocephalus G. López and R. Morales. Molecules 2021, 26, 6427. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.; Mansinhos, I.; Rodríguez-Solana, R.; Pérez-Santín, E.; Coelho, N.; Romano, A. Elicitation Improves Rosmarinic Acid Content and Antioxidant Activity in Thymus lotocephalus Shoot Cultures. Ind. Crop. Prod. 2019, 137, 214–220. [Google Scholar] [CrossRef]
- Machado, M.; Martins, N.; Salgueiro, L.; Cavaleiro, C.; Sousa, M.C. Lavandula Luisieri and Lavandula viridis Essential Oils as Upcoming Anti-Protozoal Agents: A Key Focus on Leishmaniasis. Appl. Sci. 2019, 9, 3056. [Google Scholar] [CrossRef]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Duarte, H.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Response of Thymus lotocephalus In Vitro Cultures to Drought Stress and Role of Green Extracts in Cosmetics. Antioxidants 2022, 11, 1475. [Google Scholar] [CrossRef] [PubMed]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Impact of Temperature on Phenolic and Osmolyte Contents in In Vitro Cultures and Micropropagated Plants of Two Mediterranean Plant Species, Lavandula viridis and Thymus lotocephalus. Plants 2022, 11, 3516. [Google Scholar] [CrossRef]
- Mateus, D.M.R.; Ferraz, E.; Perna, V.; Sales, P.; Hipólito-Correia, V. Essential Oils and Extracts of Plants as Biocides against Microorganisms Isolated from the Ruins of the Roman City of Conímbriga (Portugal). Environ. Sci. Pollut. Res. 2024, 31, 40669–40677. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Canhoto, J.; Vale-Silva, L.; Silva, M.J.; Pinto, E.; Salgueiro, L. Chemical Composition and Antifungal Activity of the Essential Oils of Lavandula viridis L’Hér. J. Med. Microbiol. 2011, 60, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Karatoprak, G.Ş.; Yücel, Ç.; Göger, F.; Sobarzo-Sánchez, E.; Küpeli Akkol, E. Potential Antioxidant and Enzyme Inhibitory Effects of Nanoliposomal Formulation Prepared from Salvia aramiensis Rech. f. Extract. Antioxidants 2020, 9, 293. [Google Scholar] [CrossRef]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Ultrasonic-Assisted Extraction and Natural Deep Eutectic Solvents Combination: A Green Strategy to Improve the Recovery of Phenolic Compounds from Lavandula pedunculata Subsp. Lusitanica (Chaytor) Franco. Antioxidants 2021, 10, 582. [Google Scholar] [CrossRef] [PubMed]
- Duque, L.; Bravo, K.; Osorio, E. A Holistic Anti-Aging Approach Applied in Selected Cultivated Medicinal Plants: A View of Photoprotection of the Skin by Different Mechanisms. Ind. Crop. Prod. 2017, 97, 431–439. [Google Scholar] [CrossRef]
- Juszczak, A.M.; Marijan, M.; Jakupović, L.; Tomczykowa, M.; Tomczyk, M.; Zovko Končić, M. Glycerol and Natural Deep Eutectic Solvents Extraction for Preparation of Luteolin-Rich Jasione Montana Extracts with Cosmeceutical Activity. Metabolites 2023, 13, 32. [Google Scholar] [CrossRef]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Moreno-Rojas, J.M.; Romano, A. Environmental Factors Related to Climate Change Alter the Chemical Composition and Biological Activity of Lavandula viridis L’Hér Essential Oil. Agriculture 2024, 14, 1067. [Google Scholar] [CrossRef]
- Aguirre-Becerra, H.; Vazquez-Hernandez, M.C.; Saenz de la O, D.; Alvarado-Mariana, A.; Guevara-Gonzalez, R.G.; Garcia-Trejo, J.F.; Feregrino-Perez, A.A. Role of Stress and Defense in Plant Secondary Metabolites Production. In Bioactive Natural Products for Pharmaceutical Applications; Pal, D., Nayak, A.K., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 151–195. ISBN 978-3-030-54027-2. [Google Scholar]
- Sung, J.; Yun, H.; Back, S.; Fernie, A.R.; Kim, Y.X.; Lee, Y.; Lee, S.; Lee, D.; Kim, J. Changes in Mineral Nutrient Concentrations and C-N Metabolism in Cabbage Shoots and Roots Following Macronutrient Deficiency. J. Plant Nutr. Soil Sci. 2018, 181, 777–786. [Google Scholar] [CrossRef]
- Barzana, G.; Rios, J.J.; Lopez-Zaplana, A.; Nicolas-Espinosa, J.; Yepes-Molina, L.; Garcia-Ibañez, P.; Carvajal, M. Interrelations of Nutrient and Water Transporters in Plants under Abiotic Stress. Physiol. Plant. 2021, 171, 595–619. [Google Scholar] [CrossRef] [PubMed]
- Mansinhos, I.; Gonçalves, S.; Romano, A. Impact of Abiotic Stresses on In Vitro Production of Secondary Metabolites. In Vitro Propagation and Secondary Metabolite Production from Medicinal Plants: Current Trends (Part 1); Anis, M., Khanam, M., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2024; pp. 62–90. ISBN 978-981-5165-23-4. [Google Scholar]
- Mansinhos, I.; Gonçalves, S.; Romano, A. How Climate Change-Related Abiotic Factors Affect the Production of Industrial Valuable Compounds in Lamiaceae Plant Species: A Review. Front. Plant Sci. 2024, 15, 1370810. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Dias, M.C.; Almeida, R.; Romano, A. Rapid Clonal Multiplication of Lavandula viridis L’Hér through In Vitro Axillary Shoot Proliferation. Plant Cell Tissue Organ Cult. 2002, 68, 99–102. [Google Scholar] [CrossRef]
- Coelho, N.; Goncalves, S.; Elena Gonzalez-Benito, M.; Romano, A. Establishment of an In Vitro Propagation Protocol for Thymus lotocephalus, a Rare Aromatic Species of the Algarve (Portugal). Plant Growth Regul. 2012, 66, 69–74. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics Off. J. Metabolomic Soc. 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Screening of a Hundred Plant Extracts as Tyrosinase and Elastase Inhibitors, Two Enzymatic Targets of Cosmetic Interest. Ind. Crops Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Liyanaarachchi, G.D.; Samarasekera, J.K.R.R.; Mahanama, K.R.R.; Hemalal, K.D.P. Tyrosinase, Elastase, Hyaluronidase, Inhibitory and Antioxidant Activity of Sri Lankan Medicinal Plants for Novel Cosmeceuticals. Ind. Crops Prod. 2018, 111, 597–605. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Deng, X.; Adeel, M.; Rizwan, M.; Shakoor, N.; Yang, Y.; Javed, R. Influence of Calcium and Magnesium Elimination on Plant Biomass and Secondary Metabolites of Stevia Rebaudiana Bertoni. Biotechnol. Appl. Biochem. 2022, 69, 2008–2016. [Google Scholar] [CrossRef]
- Aremu, A.O.; Masondo, N.A.; Van Staden, J. Physiological and Phytochemical Responses of Three Nutrient-Stressed Bulbous Plants Subjected to Vermicompost Leachate Treatment. Acta Physiol. Plant. 2014, 36, 721–731. [Google Scholar] [CrossRef]
- Carrera, D.Á.; Oddsson, S.; Grossmann, J.; Trachsel, C.; Streb, S. Comparative Proteomic Analysis of Plant Acclimation to Six Different Long-Term Environmental Changes. Plant Cell Physiol. 2018, 59, 510–526. [Google Scholar] [CrossRef] [PubMed]
- Teixeira da Silva, J.A.; Nezami-Alanagh, E.; Barreal, M.E.; Kher, M.M.; Wicaksono, A.; Gulyás, A.; Hidvégi, N.; Magyar-Tábori, K.; Mendler-Drienyovszki, N.; Márton, L.; et al. Shoot Tip Necrosis of In Vitro Plant Cultures: A Reappraisal of Possible Causes and Solutions. Planta 2020, 252, 47. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Rodriguez, E.; Rosa Lopez-Laredo, A.; Medina-Perez, V.; Trejo-Tapia, G.; Luis Trejo-Espino, J. Influence of Spermine and Nitrogen Deficiency on Growth and Secondary Metabolites Accumulation in Castilleja Tenuiflora Benth. Cultured in a RITA (R) Temporary Immersion System. Eng. Life Sci. 2019, 19, 944–954. [Google Scholar] [CrossRef]
- Sommano, S.R.; Kanthawang, N.; Janpen, C.; Norkum ai, P.; Wongkaew, M.; Inkham, C.; Van Doan, H.; Cheewangkoon, R. Physiological and Oxidative Responses of Japanese Mint Grown under Limited Water and Nitrogen Supplies in an Evaporated Greenhouse System. Front. Sustain. Food Syst. 2022, 5, 808327. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Štork, F.; Hedbavny, J. Nitrate Deficiency Reduces Cadmium and Nickel Accumulation in Chamomile Plants. J. Agric. Food Chem. 2011, 59, 5139–5149. [Google Scholar] [CrossRef]
- Boamponsem, G.A.; Leung, D.W.M.; Lister, C. Relationships among Iron Deficit-Induced Potato Callus Growth Inhibition, Fe Distribution, Chlorosis, and Oxidative Stress Amplified by Reduced Antioxidative Enzyme Activities. Plant Cell Tissue Organ Cult. PCTOC 2018, 132, 393–412. [Google Scholar] [CrossRef]
- Song, Y.; Dong, Y.; Kong, J.; Tian, X.; Bai, X.; Xu, L. Effects of Root Addition and Foliar Application of Nitric Oxide and Salicylic Acid in Alleviating Iron Deficiency Induced Chlorosis of Peanut Seedlings. J. Plant Nutr. 2017, 40, 63–81. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Y.; Zhao, X.; Jin, X.; Hou, L.; Shi, Y.; Ahammed, G.J. Silicon Compensates Phosphorus Deficit-Induced Growth Inhibition by Improving Photosynthetic Capacity, Antioxidant Potential, and Nutrient Homeostasis in Tomato. Agronomy 2019, 9, 733. [Google Scholar] [CrossRef]
- Briat, J.-F.; Dubos, C.; Gaymard, F. Iron Nutrition, Biomass Production, and Plant Product Quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef]
- Bityutskii, N.; Pavlovic, J.; Yakkonen, K.; Maksimović, V.; Nikolic, M. Contrasting Effect of Silicon on Iron, Zinc and Manganese Status and Accumulation of Metal-Mobilizing Compounds in Micronutrient-Deficient Cucumber. Plant Physiol. Biochem. 2014, 74, 205–211. [Google Scholar] [CrossRef]
- Jelali, N.; Wasli, H.; Youssef, R.B.; Hessini, K.; Cardoso, S.M. Iron Deficiency Modulates Secondary Metabolites Biosynthesis and Antioxidant Potential in Sulla carnosa L. Primed with Salicylic Acid. Appl. Sci. 2022, 12, 10351. [Google Scholar] [CrossRef]
- Wasli, H.; Jelali, N.; Saada, M.; Ksouri, R.; Cardoso, S.M. Insights on the Adaptation of Foeniculum vulgare Mill to Iron Deficiency. Appl. Sci. 2021, 11, 7072. [Google Scholar] [CrossRef]
- Li, C.-P.; Qi, Y.-P.; Zhang, J.; Yang, L.-T.; Wang, D.-H.; Ye, X.; Lai, N.-W.; Tan, L.-L.; Lin, D.; Chen, L.-S. Magnesium-Deficiency-Induced Alterations of Gas Exchange, Major Metabolites and Key Enzymes Differ among Roots, and Lower and Upper Leaves of Citrus Sinensis Seedlings. Tree Physiol. 2017, 37, 1564–1581. [Google Scholar] [CrossRef]
- Cai, Y.-T.; Zhang, H.; Qi, Y.-P.; Ye, X.; Huang, Z.-R.; Guo, J.-X.; Chen, L.-S.; Yang, L.-T. Responses of Reactive Oxygen Species and Methylglyoxal Metabolisms to Magnesium-Deficiency Differ Greatly among the Roots, Upper and Lower Leaves of Citrus Sinensis. BMC Plant Biol. 2019, 19, 76. [Google Scholar] [CrossRef]
- Muneer, S.; Lee, B.-R.; Kim, K.-Y.; Park, S.-H.; Zhang, Q.; Kim, T.-H. Involvement of Sulphur Nutrition in Modulating Iron Deficiency Responses in Photosynthetic Organelles of Oilseed Rape (Brassica napus L.). Photosynth. Res. 2014, 119, 319–329. [Google Scholar] [CrossRef]
- Jakovljević, D.; Topuzović, M.; Stanković, M. Nutrient Limitation as a Tool for the Induction of Secondary Metabolites with Antioxidant Activity in Basil Cultivars. Ind. Crops Prod. 2019, 138, 111462. [Google Scholar] [CrossRef]
- Yañez-Mansilla, E.; Cartes, P.; Reyes-Díaz, M.; Ribera-Fonseca, A.; Alberdi, M. Photosynthetic and Antioxidant Performance Are Differentially Affected by Short-Term Nitrogen Supply in Highbush Blueberry Cultivars. Cienc. E Investig. Agrar. 2014, 41, 61–70. [Google Scholar] [CrossRef]
- Valentinuzzi, F.; Mason, M.; Scampicchio, M.; Andreotti, C.; Cesco, S.; Mimmo, T. Enhancement of the Bioactive Compound Content in Strawberry Fruits Grown under Iron and Phosphorus Deficiency. J. Sci. Food Agric. 2015, 95, 2088–2094. [Google Scholar] [CrossRef]
- Galieni, A.; Di Mattia, C.; De Gregorio, M.; Speca, S.; Mastrocola, D.; Pisante, M.; Stagnari, F. Effects of Nutrient Deficiency and Abiotic Environmental Stresses on Yield, Phenolic Compounds and Antiradical Activity in Lettuce (Lactuca sativa L.). Sci. Hortic. 2015, 187, 93–101. [Google Scholar] [CrossRef]
- Ordóñez-Díaz, J.L.; Cardeñosa, V.; Muñoz-Redondo, J.M.; Ferreres, F.; Pereira-Caro, G.; Medrano, E.; Moreno-Rojas, J.M.; Moreno, D.A. Impact of Abiotic Stresses (Nitrogen Reduction and Salinity Conditions) on Phenolic Compounds and Antioxidant Activity of Strawberries. Processes 2021, 9, 1044. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Bačkor, M. Nitric Oxide Signals ROS Scavenger-Mediated Enhancement of PAL Activity in Nitrogen-Deficient Matricaria chamomilla Roots: Side Effects of Scavengers. Free Radic. Biol. Med. 2009, 46, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, S.; Iqbal, J.; Abbasi, B.A.; Ullah, Z.; Yaseen, T.; Kanwal, S.; Mahmood, T.; Sydykbayeva, S.; Ydyrys, A.; Almarhoon, Z.M.; et al. Rosmarinic Acid and Its Derivatives: Current Insights on Anticancer Potential and Other Biomedical Applications. Biomed. Pharmacother. 2023, 162, 114687. [Google Scholar] [CrossRef]
- Lattanzio, V.; Cardinali, A.; Ruta, C.; Fortunato, I.M.; Lattanzio, V.M.T.; Linsalata, V.; Cicco, N. Relationship of Secondary Metabolism to Growth in Oregano (Origanum vulgare L.) Shoot Cultures under Nutritional Stress. Environ. Exp. Bot. 2009, 65, 54–62. [Google Scholar] [CrossRef]
- Suh, D.H.; Kim, Y.X.; Jung, E.S.; Lee, S.; Park, J.; Lee, C.H.; Sung, J. Characterization of Metabolic Changes under Low Mineral Supply (N, K, or Mg) and Supplemental LED Lighting (Red, Blue, or Red–Blue Combination) in Perilla Frutescens Using a Metabolomics Approach. Molecules 2020, 25, 4714. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.G.; Tran, P.T.; Lee, J.-H.; Min, B.S.; Kim, J.A. Anti-Inflammatory Activity of Caffeic Acid Derivatives Isolated from the Roots of Salvia Miltiorrhiza Bunge. Arch. Pharm. Res. 2018, 41, 64–70. [Google Scholar] [CrossRef]
- Jaouadi, R.; Silva, A.M.S.; Boussaid, M.; Yahia, I.B.H.; Cardoso, S.M.; Zaouali, Y. Differentiation of Phenolic Composition Among Tunisian Thymus algeriensis Boiss. et Reut. (Lamiaceae) Populations: Correlation to Bioactive Activities. Antioxidants 2019, 8, 515. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Ruiz-Medina, A.; Zengin, G.; Ak, G.; Jugreet, S.; Mahomoodally, M.F.; Emre, G.; Orlando, G.; Libero, M.L.; Nilofar; et al. New Biological and Chemical Evidences of Two Lamiaceae Species (Thymbra Capitata and Thymus Sipyleus Subsp. Rosulans): In Vitro, In Silico and Ex Vivo Approaches. Molecules 2022, 27, 9029. [Google Scholar] [CrossRef]
- Gravina, C.; Formato, M.; Piccolella, S.; Fiorentino, M.; Stinca, A.; Pacifico, S.; Esposito, A. Lavandula austroapennina (Lamiaceae): Getting Insights into Bioactive Polyphenols of a Rare Italian Endemic Vascular Plant. Int. J. Mol. Sci. 2023, 24, 8038. [Google Scholar] [CrossRef] [PubMed]
- El Aanachi, S.; Gali, L.; Rammali, S.; Bensouici, C.; Aassila, H.; Dari, K. In Vitro Study of the Antioxidant, Photoprotective, Anti-Tyrosinase, and Anti-Urease Effects of Methanolic Extracts from Leaves of Six Moroccan Lamiaceae. J. Food Meas. Charact. 2021, 15, 1785–1795. [Google Scholar] [CrossRef]
- Lee, C.J.; Chen, L.G.; Chang, T.L.; Ke, W.M.; Lo, Y.F.; Wang, C.C. The Correlation between Skin-Care Effects and Phytochemical Contents in Lamiaceae Plants. Food Chem. 2011, 124, 833–841. [Google Scholar] [CrossRef]
- Salem, M.A.; Radwan, R.A.; Mostafa, E.S.; Alseekh, S.; Fernie, A.R.; Ezzat, S.M. Using an UPLC/MS-Based Untargeted Metabolomics Approach for Assessing the Antioxidant Capacity and Anti-Aging Potential of Selected Herbs. RSC Adv. 2020, 10, 31511–31524. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Yagi, S.; Selvi, S.; Cziáky, Z.; Jeko, J.; Sinan, K.I.; Topcu, A.A.; Erci, F.; Boczkaj, G. Elucidation of Chemical Compounds in Different Extracts of Two Lavandula Taxa and Their Biological Potentials: Walking with Versatile Agents on the Road from Nature to Functional Applications. Ind. Crop. Prod. 2023, 204, 117366. [Google Scholar] [CrossRef]
- Akdeniz, M.; Yigitkan, S.; Yilmaz, M.A.; Yener, I.; Varhan Oral, E.; Firat, M.; Erdogan Orhan, I.; Kolak, U.; Ertas, A. A Comprehensive Study on Chemical and Biological Investigation of Thymus Brachychilus Jalas: A Rich Source of Ursolic and Oleanolic Acids. Anal. Lett. 2024, 57, 2677–2693. [Google Scholar] [CrossRef]
- Akin, M.; Saki, N. Antimicrobial, DPPH Scavenging and Tyrosinase Inhibitory Activities of Thymus vulgaris, Helichrysum arenarium and Rosa damascena Mill. Ethanol Extracts by Using TLC Bioautography and Chemical Screening Methods. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 204–216. [Google Scholar] [CrossRef]
- Akman, T.Ç.; Şimşek, S.; Akşit, Z.; Akşit, H.; Aydin, A.; Tüfekçi, A.R.; Adem, S.; Yilmaz, M.A. Liquid Chromatography–Tandem Mass Spectrometry Profile and Antioxidant, Antimicrobial, Antiproliferative, and Enzyme Activities of Thymus pectinatus and Thymus convolutus: In Vitro and In Silico Approach. J. Sci. Food Agric. 2024, 104, 4039–4049. [Google Scholar] [CrossRef]
- Fayez, S.; Fahmy, N.M.; Zengin, G.; Yagi, S.; Uba, A.I.; Eldahshan, O.A.; Koyuncu, I.; Temiz, E.; Dall’Acqua, S.; Sut, S.; et al. LC-MS/MS and GC-MS Profiling, Antioxidant, Enzyme Inhibition, and Antiproliferative Activities of Thymus leucostomus H Ausskn. & V Elen. Extracts. Arch. Pharm. 2023, 356, 2300444. [Google Scholar] [CrossRef]
- Küçükaydın, S.; Çayan, F.; Tel-Çayan, G.; Duru, M.E. HPLC-DAD Phytochemical Profiles of Thymus cariensis and T. cilicicus with Antioxidant, Cytotoxic, Anticholinesterase, Anti-Urease, Anti-Tyrosinase, and Antidiabetic Activities. S. Afr. J. Bot. 2021, 143, 155–163. [Google Scholar] [CrossRef]
- Martins-Gomes, C.; Steck, J.; Keller, J.; Bunzel, M.; Santos, J.A.; Nunes, F.M.; Silva, A.M. Phytochemical Composition and Antioxidant, Anti-Acetylcholinesterase, and Anti-α-Glucosidase Activity of Thymus carnosus Extracts: A Three-Year Study on the Impact of Annual Variation and Geographic Location. Antioxidants 2023, 12, 668. [Google Scholar] [CrossRef]
- Senol, S.F.; Orhan, I.E.; Ozgen, U.; Renda, G.; Bulut, G.; Guven, L.; Karaoglan, E.S.; Sevindik, H.G.; Skalicka-Wozniak, K.; Koca Caliskan, U.; et al. Memory-Vitalizing Effect of Twenty-Five Medicinal and Edible Plants and Their Isolated Compounds. S. Afr. J. Bot. 2016, 102, 102–109. [Google Scholar] [CrossRef]
- Silva, A.M.; Félix, L.M.; Teixeira, I.; Martins-Gomes, C.; Schäfer, J.; Souto, E.B.; Santos, D.J.; Bunzel, M.; Nunes, F.M. Orange Thyme: Phytochemical Profiling, In Vitro Bioactivities of Extracts and Potential Health Benefits. Food Chem. X 2021, 12, 100171. [Google Scholar] [CrossRef]
- Taghouti, M.; Martins-Gomes, C.; Schäfer, J.; Félix, L.M.; Santos, J.A.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Thymus pulegioides L. as a Rich Source of Antioxidant, Anti-Proliferative and Neuroprotective Phenolic Compounds. Food Funct. 2018, 9, 3617–3629. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Atasagun, B.; Zakariyyah Aumeeruddy, M.; Saleem, H.; Mollica, A.; Babak Bahadori, M.; Mahomoodally, M.F. Phenolic Profiling and In Vitro Biological Properties of Two Lamiaceae Species (Salvia modesta and Thymus argaeus): A Comprehensive Evaluation. Ind. Crop. Prod. 2019, 128, 308–314. [Google Scholar] [CrossRef]
- Carrasco, A.; Tomas, V.; Tudela, J.; Miguel, M.G. Comparative Study of GC-MS Characterization, Antioxidant Activity and Hyaluronidase Inhibition of Different Species of Lavandula and Thymus Essential Oils. Flavour Fragr. J. 2016, 31, 57–69. [Google Scholar] [CrossRef]
- Jusri, R.; Widodo, W.S.; Widowati, W.; Armansyah, A.; Sormin, D.E.; Fachrial, E.; Lister, I.N.E. Comparison of Antioxidant and Anti-Hyaluronidase Potentials of Pineapple Core Extract (Ananas comosus (L.) Merr.) and Luteolin. Maj. Kedokt. Bdg. 2019, 51, 63–69. [Google Scholar] [CrossRef]
- Wölfle, U.; Heinemann, A.; Esser, P.R.; Haarhaus, B.; Martin, S.F.; Schempp, C.M. Luteolin Prevents Solar Radiation-Induced Matrix Metalloproteinase-1 Activation in Human Fibroblasts: A Role for P38 Mitogen-Activated Protein Kinase and Interleukin-20 Released from Keratinocytes. Rejuvenation Res. 2012, 15, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Aoshima, H.; Miyase, T.; Warashina, T. Caffeic Acid Oligomers with Hyaluronidase Inhibitory Activity from Clinopodium gracile. Chem. Pharm. Bull. 2012, 60, 499–507. [Google Scholar] [CrossRef]
- Dhapare, S.; Li, H.; Sakagami, M. Salvianolic Acid B as an Anti-Emphysema Agent II: In Vivo Reversal Activities in Two Rat Models of Emphysema. Pulm. Pharmacol. Ther. 2018, 53, 52–60. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).