Abstract
Chinese cabbage is an important vegetable from both a nutritional and an economic standpoint, with the leafy head serving as the primary harvested organ. However, the nutrient accumulation as well as influencing factors within the leafy head have not yet been elucidated. Thus, the distribution of metabolites (soluble sugars, minerals, carotenoids, vitamin C, flavonoid compounds, glucosinolates, and total phenolic compounds) were investigated in different leaf layers of Chinese cabbage with varying head types. The results showed that the inner leaves consistently displayed markedly higher levels of fructose and glucose when contrasted with the outer leaves. Similarly, there was an accumulation of glucosinolates in the inner leaves. By contrast, however, the antioxidants content exhibited a consistent decline from the outer leaves towards the central core, in line with the diminishing antioxidant capacity. This descending trend was also apparent in the mineral content, encompassing calcium, sodium, magnesium and sulfur. These results will provide dietary instruction, especially for consumers who have particular dietary needs.
1. Introduction
Chinese cabbage (Brassica rapa L. ssp. pekinensis) is globally prominent, is among the essential vegetables, and is celebrated for its ease of cultivation, abundant yields, delicate flavor, and health-promoting attributes. The primary edible portion of Chinese cabbage is the leafy head, which is composed of numerous gracefully curved leaves. Diverse beneficial components are present within this head, and these include soluble sugars, vitamins, minerals, and bioactive compounds known for their well-established health benefits, such as flavonoids, anthocyanins, carotenoids, and glucosinolates. The consumption of Chinese cabbage has been associated with a reduced risk of various diseases, including degenerative conditions, age-related chronic ailments [1], and various forms of cancer [2,3]. Notably, plant flavonoids are believed to have a potent inhibitory effect on alpha-amylase, leading to delayed carbohydrate digestion, which is advantageous for individuals with diabetes [4]. Glucosinolates, comprising a distinct component of cruciferous vegetables, have garnered significant attention owing to their potential anticancer properties [5,6,7]. Furthermore, phenolic compounds, carotenoids, and vitamin C are frequently associated with antioxidant activity, making Chinese cabbage a nutritious and health-enhancing vegetable [8,9].
Plants respond to a myriad of stimuli, encompassing both external and internal factors, through intricate metabolic processes. These processes, in turn, alter the nutritional composition and overall quality of the plants. Scientific research has been instrumental in illuminating the multifaceted effects of certain factors, such as varietal distinctions, agricultural practices, and cooking methods, on the nutritional and sensory attributes of Brassica vegetables. One such case comprised a comparison of the white and yellow inner leaves of Chinese cabbage, which revealed striking disparities. Specifically, the yellow inner leaf is distinguished by higher levels of glucosinolates and carotene, coupled with lower soluble sugar content [10]. Moreover, the chemical composition and sensory characteristics of individual plants can fluctuate depending on their developmental stage and the specific plant parts. In pak choi, mature leaves typically have higher concentrations of polyphenols and carotenoids, whereas emerging leaves display elevated levels of glucosinolates and glucosinolate hydrolysates [11]. Further, in pak choi, the abundance of hydroxycinnamic acid and flavonoids is greater in the leaves than in the petioles [12]. Moreover, the antioxidant content of the skin of Chinese cabbage surpasses that of the pulp [13]. In heading cabbage leaves, there is also variation in both primary and secondary metabolites among distinct leaf positions. For example, inner leaves tend to have higher fructose and glucose contents than their outer counterparts, whereas the glucosinolate levels in the outer leaves surpass those in the inner leaves [14].
As living standards improve, people are prioritizing high nutritional value in their food choices at an increasing rate [15]. As a result, the health benefits of Chinese cabbage have become a significant factor that influences consumers’ preferences. Consumers often select the inner leaf layers of Chinese cabbage based on experience, believing these leaves to be more tender and flavorful than the outer layers. However, this preference is largely subjective and lacks strong theoretical or scientific backing [16]. The perception that the inner leaves are superior is based more on tradition and personal preference than on objective data regarding the nutritional content in the inner and outer leaves. Unfortunately, the literature on the distribution and influencing factors of nutrients within the leafy head of Chinese cabbage is scarce, highlighting a gap between consumers’ need for dietary guidance and the lack of comprehensive information about the nutrition and bioactive chemical constituents within the leafy head. In this study, we aim to address this knowledge gap by conducting a comprehensive analysis of metabolites in the leafy head of Chinese cabbage. Considering that the distribution of metabolites within the leafy head may be influenced by development stages, light exposure, and photosynthetic efficiency, we measured three cabbage varieties with different heading types: the headed variety ‘H52’, the semi-headed variety ‘430’, and the loose-headed variety ‘TJLX’. Each variety differs in terms of the amount of light exposure achieved by the different leaf layers, and these differences may affect metabolite distribution. Our analysis will fill a significant gap in the literature by providing a detailed analysis of the nutrient distribution of the leafy heads of Chinese cabbage, offering new insights into internal and external factors that shape the metabolites distribution in leafy heads.
2. Materials and Methods
2.1. Plant Material Collection and Preparation
In this study, we conducted an analysis of three cultivars of Chinese cabbage: ‘H52’, ‘430’, and ‘TJLX’. The three cultivars represent important germplasm resources of Chinese cabbage collected by the Zhejiang Academy of Agricultural Science (ZAAS, Hangzhou, China). Among them, ‘H52’ and ‘430’ are Chinese cabbages with leafy heads, with the key distinction being that ‘H52’ is a heading variety, whereas ‘430’ is a semi-heading variety. ‘H52’ lacks exposure to light from the eighth leaf onward, while ‘430’ lacks exposure to light from the 13th leaf onward. In contrast, ‘TJLX’ is a Chinese cabbage with a loose- heading growth pattern. Seeds of these three cultivars were sown in a 72-hole seedling tray and the seedings were later transplanted to the experimental field of ZAAS in Hangzhou, located in the southern part of China.
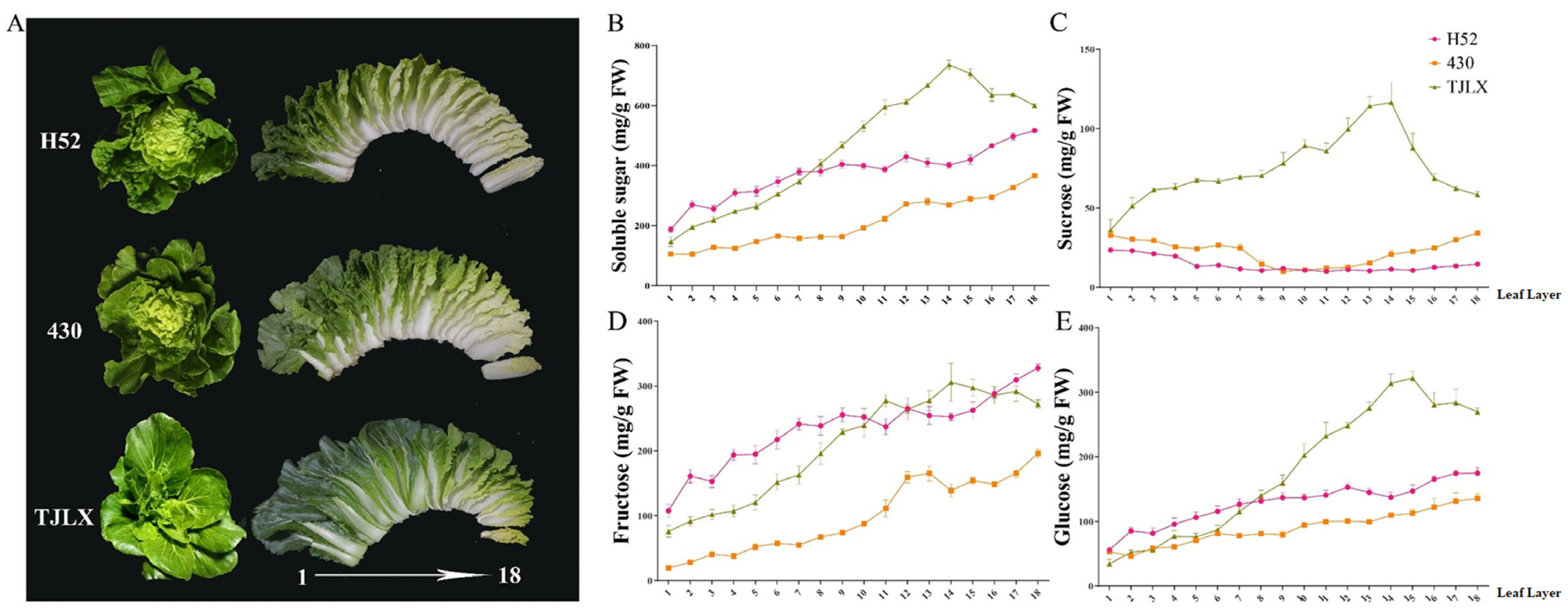
For plant material collection, we harvested the heads, along with their four outer rosette leaves, from the three cultivars of Chinese cabbage. These leaves were then sequentially separated into 18 leaf layers, starting from the outermost rosette leaves and progressing towards the inner part of the leaf head (Figure 1A). Each sample was a composite of materials from four Chinese cabbages, and we repeated this process three times to ensure that we achieved biological replicates. To preserve the collected leaf samples, we quickly stabilized them using liquid nitrogen and subsequently lyophilized them in an ultra-low-temperature freezer set at −80 °C, then ground them into powder. These samples were stored at −80 °C to await further analysis.

Figure 1.
(A) Sampling method: the leaves of Chinese cabbage varieties H52, 430, and TJLX were methodically separated into 18-leaf layers, beginning with the outer rosette leaves and advancing toward the inner portion of the leaf head. (B–E) Soluble sugar content in the leaf layers of the Chinese cabbage varieties H52, 430, and TJLX. Total soluble sugar (B), sucrose (C), fructose (D), and glucose (E) were included. Values are expressed as the mean ± SE (n = 3).
2.2. Soluble Sugar Assay
The soluble sugar content was determined according to previous reports [14]. First, we weighed 0.2 g of freeze-dried powder and then added 6 mL of 80% ethanol to the powder. The mixture was incubated in a water bath at 65 °C for 20 min and centrifuged at 8000× g for 10 min. After that, we collected the supernatant. The same procedure was repeated twice for extraction and precipitation. The supernatants were combined and concentrated to 1 mL, then the soluble sugars were analyzed using a high-performance liquid chromatography system consisting of a Waters 600 separation module and a Waters 2414 RI detector (Waters, Milford, MA, USA). A 20 μL sample was injected into a Sugarpak I column (300 mm × 6.5 mm, Waters, Milford, MA, USA) at a flow rate of 0.5 mL/min, and deionized water containing 0.0001 M calcium EDTA was used as the mobile phase. The sugar content was determined using the external standard method. Each sample was analyzed in triplicate, and the sugar concentration is expressed as mg/g dry weight (DW).
2.3. Carotenoids and Chlorophyll
Carotenoids and chlorophyll were extracted using ultrasonic extraction with 3 mL acetone according to the previous reports, with minor adjustments [17]. The ultrasonic power employed was 300 w, the temperature was maintained at 60 °C, and the extraction process lasted for 30 min. The separation of carotenoids and chlorophyll was performed using a Waters 600 high-performance liquid chromatography (HPLC) system with a Nova-Pak C18 column (3.9 × 150 mm, 4 μm; Waters, Milford, MA, USA) and a 2487 UV detector set at 448 and 428 nm. The flow rate was 0.5 mL/min with gradient elution using isopropyl alcohol and 80% acetonitrile.
2.4. Antioxidant and Antioxidant Capacity Assay
2.4.1. Vitamin C
The extraction of vitamin C was carried out as described in [18], with minor modifications. A solution containing 1% oxalic acid (w/v) was combined with a 0.1 g sample of freeze-dried Chinese cabbage, and the resulting supernatant was isolated for high-performance liquid chromatography (HPLC) analysis. The mobile phase employed during the analysis consisted of a 0.1% oxalic acid solution (w/v), flowing at a rate of 1.0 mL/min. The authentic ascorbic acid (Sigma-Aldrich, Shanghai, China) was used for the standard control group. The spectrophotometer measured the absorbance at a wavelength of 243 nm, allowing for the subsequent calculation of the vitamin C (VC) content within the sample.
2.4.2. Total Phenolic Compounds
The determination of total phenolic compounds was conducted using the Plant Total Phenol Content Assay Kit (AKPL016M, Boxbio, Beijing, China). According to the instructions, 0.1 g of the powdered sample was extracted with 2.5 mL of extracting solution (30% ethanol) utilizing the ultrasonic extraction method. The ultrasonic power employed was 300 w, the temperature was maintained at 60 °C, and the extraction process lasted for 30 min. The supernatant was collected through centrifugation.
In accordance with the kit’s instructions, the 5 mg/mL gallic acid standard solution was diluted with distilled water to 0.3, 0.2, 0.1, 0.05, 0.025, and 0.0125 mg/mL to prepare the standard dilutions. The concentration of total phenolic compounds was then measured using a spectrophotometer set at a wavelength of 760 nm. Using 0.3, 0.2, 0.1, 0.05, 0.025, and 0.0125 mg/mL as the x-axis (concentration) and their corresponding ΔA values as the y-axis (absorbance), the standard curve was plotted. Subsequently, the supernatant underwent further processing as directed by the provided instructions, and the resulting concentration was measured using a spectrophotometer. The acquired data were integrated into the previously established standard curve to accurately determine the total phenolic content within the sample.
2.4.3. Flavonoids
The determination of flavonoids compounds was conducted using the Plant Flavonoid Content Assay Kit (AKPL015M, Boxbio, Beijing, China). Firstly, 0.1 g of the powdered sample was extracted with 1 mL of ethanol (50%, v/v) solution utilizing the ultrasonic extraction method. The ultrasonic power employed was 300 w, the temperature was maintained at 60 °C, and the extraction process lasted for 30 min. The supernatant was collected through centrifugation. The absorbance of the standard sample was measured at a wavelength of 470 nm, and a standard curve was constructed. The flavonoid content in the sample was then determined using the prepared standard curve and absorbance values.
2.4.4. Total Antioxidant Capacity Assay
The principle of using the FRAP method to determine the total antioxidant capacity is that under acidic conditions, antioxidants can reduce Fe3+-TPTZ to produce blue Fe2+-TPTZ, and then determine blue Fe2+-TPTZ at 593 nm to obtain the total antioxidant capacity of the sample. According to this principle, we chose to use the Total Antioxidant Capacity (T-AOC) Assay Kit (AKAO012M, Boxbio, Beijing, China). We took 0.1 g freeze-dried powder and added 1 mL of pre-cooled extract for homogenization grinding, then we collected the supernatant according to the measurement procedure. The amount of total antioxidant capacity in the samples was calculated according to the algorithmic formulas provided in the manual.
2.5. Elements Determination
Firstly, 0.1 g of sample powder was subjected to digestion with 5 mL of nitric acid at a temperature of 140 °C. Following digestion, the resulting 5 mL solution was diluted to a final volume of 25 mL with distilled water. This prepared solution was then analyzed using inductively coupled plasma atomic emission spectrometry (ICP-AES) [19].
2.6. Principal Component Analysis (PCA) and Statistical Analysis
PCA-based Biplots were created to analyze the nutrient distribution within 18 leaf layers in H52, 430, and TJLX cultivars, from the outer layers to the inner layers, focusing on soluble sugar, plant pigments, plant antioxidant capacity, glucosinolate, and mineral content. The Biplots were created using GraphPad Prism 10.0.
Data were expressed as the mean ± standard error (SE) of three replicates. One-way ANOVA followed by Tukey’s test was analyzed through IBM SPSS Statistics 20 to obtain the relevant data, and differences were defined as significant at p < 0.05. The diagram was created using GraphPad Prism 8.0.
3. Results
3.1. Soluble Sugar Content
Soluble sugars significantly influence the taste, flavor, and nutritional value of Chinese cabbage. In this study, we used HPLC to quantify the levels of soluble sugars in the three Chinese cabbage varieties. The results indicated a gradual increase in the total soluble sugar content from the outer leaf layers to the inner heart leaf in both the H52 and 430 varieties (Figure 1B). Moreover, glucose and fructose were the dominant components, and their concentrations progressively increased from the outer leaves to the heart leaf (Figure 1C,D). Further, the sucrose content in both the outer and inner leaves of H52 and 430 remained relatively stable, with no significant differences observed (Figure 1E).
Importantly, in the TJLX variety, the total sugar content increased progressively from the outer leaf layers to the heart leaf, reaching its peak at the 14th leaf before it began to decline (Figure 1B). Notably, the relationship between the soluble sugar content and leaf age in the TJLX variety differed from that in the other two varieties. Across all three varieties, glucose, fructose, and sucrose levels continued to increase from the outer leaves to the inner leaves but reached their peak in the 14th leaf, which was followed by a decrease.
3.2. Vegetable Pigment
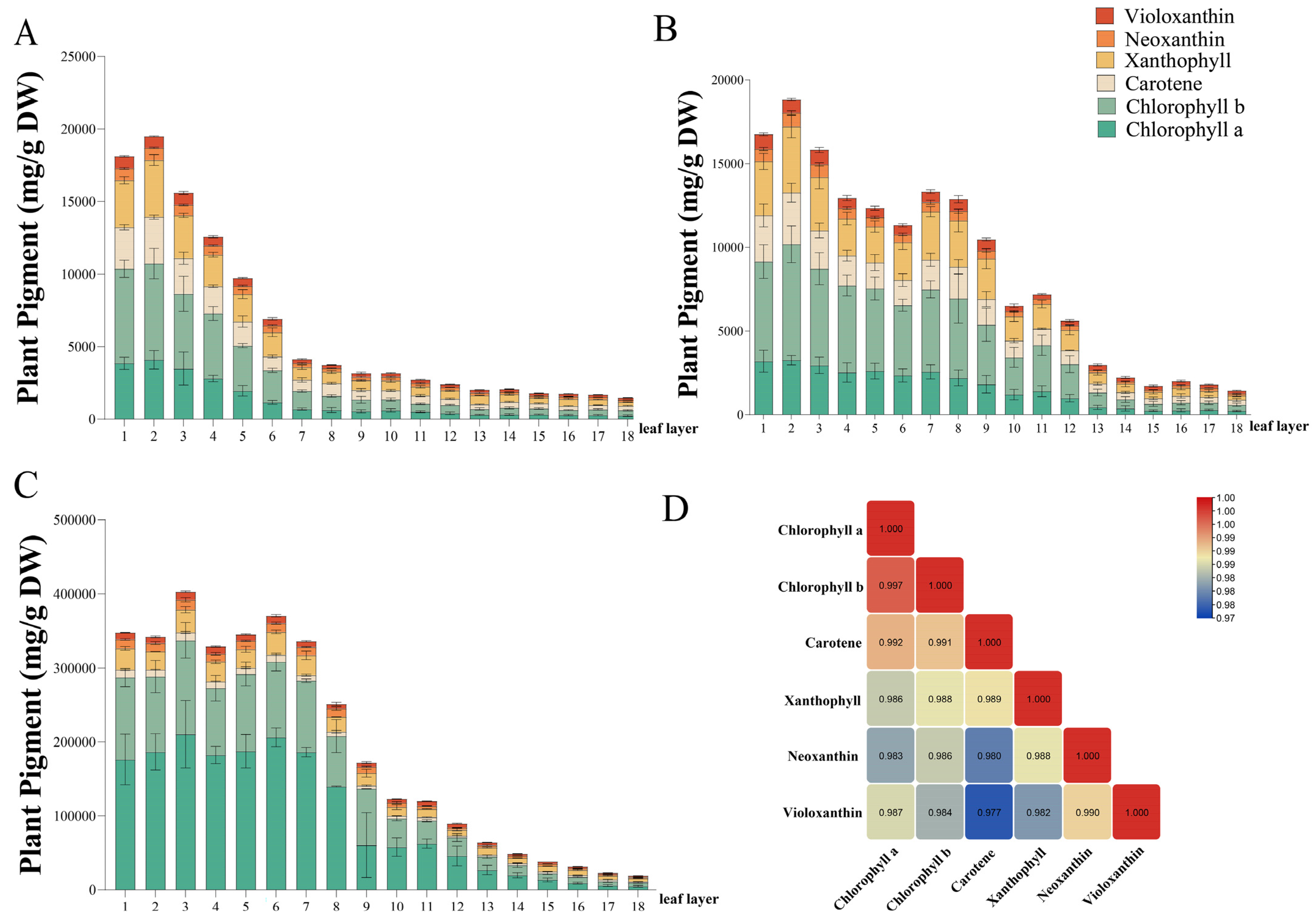
We conducted a study on the accumulation of chlorophyll (chlorophyll a and chlorophyll b) and carotenoids (β-carotene, neoxanthin, violaxanthin, and lutein) in the leaf layers of Chinese cabbage. The total pigment content in H52 cabbage plants decreased progressively from the second leaf to the innermost leaf (Figure 2A). After the second leaf, the 430 cabbage had the highest pigment content, but the pigment content fluctuated in subsequent leaves, showing an overall downward trend (Figure 2B). In the case of TJLX, the third leaf had the highest pigment content, and the pigment content in the first seven leaves remained relatively stable (Figure 2C). However, starting from the eighth leaf, there was a negative correlation between leaf age and plant pigment content.

Figure 2.
Accumulation and correlation of plant pigments in different leaves. The varieties are H52 (A), 430 (B), and TJLX (C), respectively. The correlation analysis of plant pigments was presented in the form of heat map (D). The values are expressed as the mean ± SE (n = 3).
Among these pigments, chlorophyll (a and b) dominates in different varieties, with lutein being the carotenoid with the highest content. Interestingly, the contents of four carotenoids and two chlorophylls exhibited a negative correlation with leaf age in this case, which was associated with the phenotype of Chinese cabbage. The outer leaves, which were exposed to more light, produced a significant amount of chlorophyll and carotenoids for photosynthesis. In contrast, the inner leaves, lacking light signals, experienced weakened photosynthesis, resulting in lower chlorophyll and carotenoid contents. Correlation analysis revealed that chlorophyll a, chlorophyll b, β-carotene, neoxanthin, violaxanthin, and lutein displayed significance among them (Figure 2D).
3.3. Vitamin C, Flavonoids, and Phenolic Contents
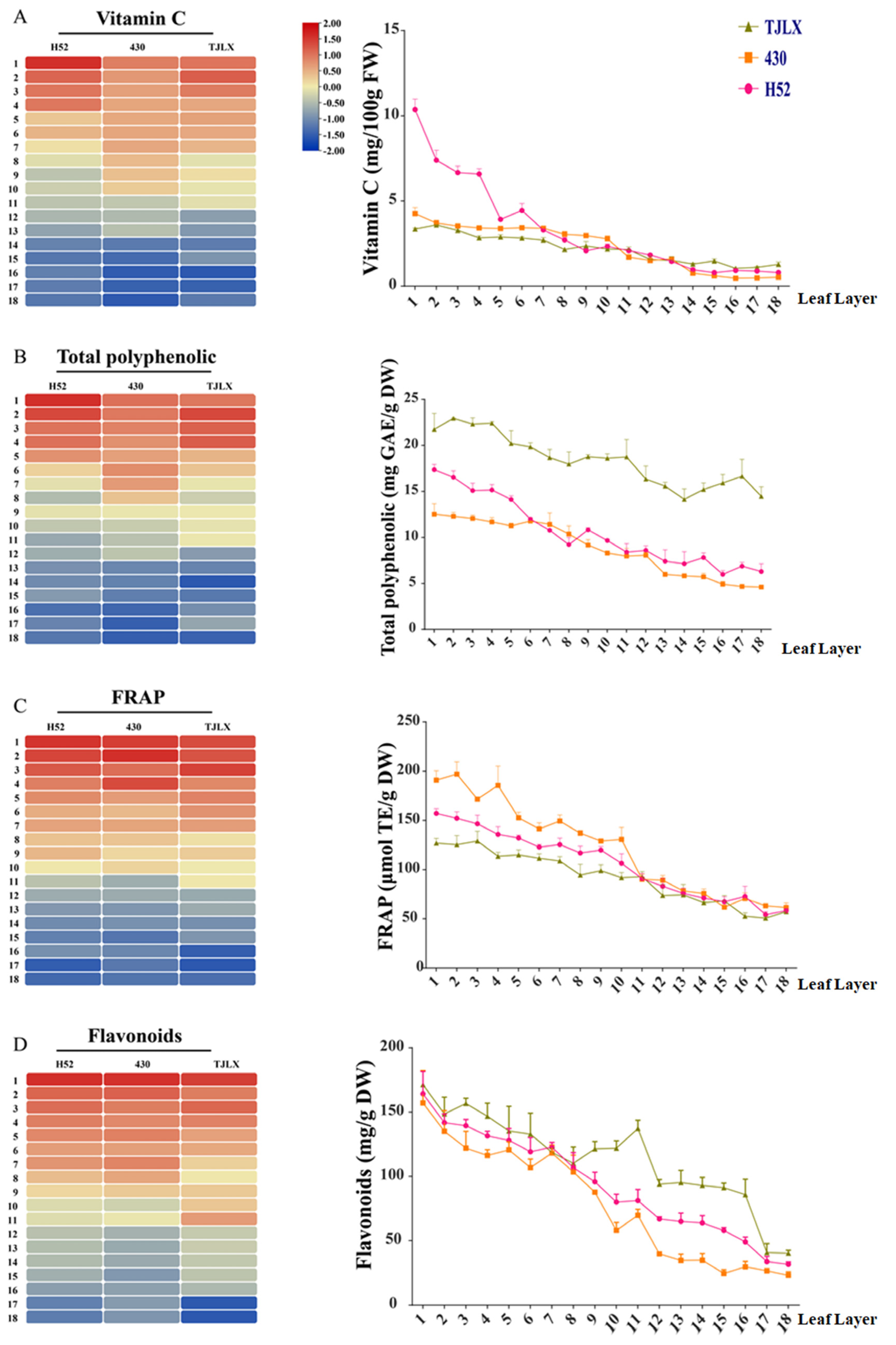
To evaluate the antioxidant capacity of the three distinct varieties, we measured the accumulation of various antioxidant compounds in the leaf layers of the samples, including vitamin C, flavonoids, and total phenols, as well as the overall antioxidant capacity. The heat maps (left) indicated a negative correlation between the accumulation of all antioxidant compounds and the leaf layer. The chart (right) shows the specific contents of vitamin C, flavonoids, and total phenols, as well as the total antioxidant capacity in the different types of Chinese cabbage (Figure 3).

Figure 3.
Distribution trends of vitamin C, flavonoids, phenolic substances, and total antioxidant capacity in the Chinese cabbage varieties H52, 430, and TJLX across each leaf layer. The content of vitamin C (A), phenolic substances (B), flavonoids (C) as well as the total antioxidant capacity (D) were displayed through heat maps (left) and chart (right), respectively.
3.4. Glucosinolate Content
The aliphatic glucosinolate levels in these three varieties, namely H52, 430, and TJLX, varied within the ranges of 0.293–0.474, 0.087–0.874, and 0–0.369 μmol/g DW, respectively (Table 1). Meanwhile, indole glucosinolate levels ranged from 0 to 0.054, 0.009 to 0.412, and 0.235 to 3.700 μmol/g DW, respectively, in these three varieties. In H52 Chinese cabbage, the total glucosinolate content showed minor fluctuations, and there was no significant difference between the inner and outer leaves. For the 430 Chinese cabbage variety, the total glucosinolate content varied, with a slight increase in the inner leaves compared to that in the outer leaves. In TJLX Chinese cabbage, the total glucosinolate content remained relatively stable in the first 14 leaves but showed a significant increase from the 15th to the 18th leaves.

Table 1.
Aliphatic (AGS), indole glucosinolate (IGS), and total glucosinolate (GS) contents (μmol/g DW) in different layers of the Chinese cabbage cultivars H52, 430, and TJLX.
3.5. Mineral Content
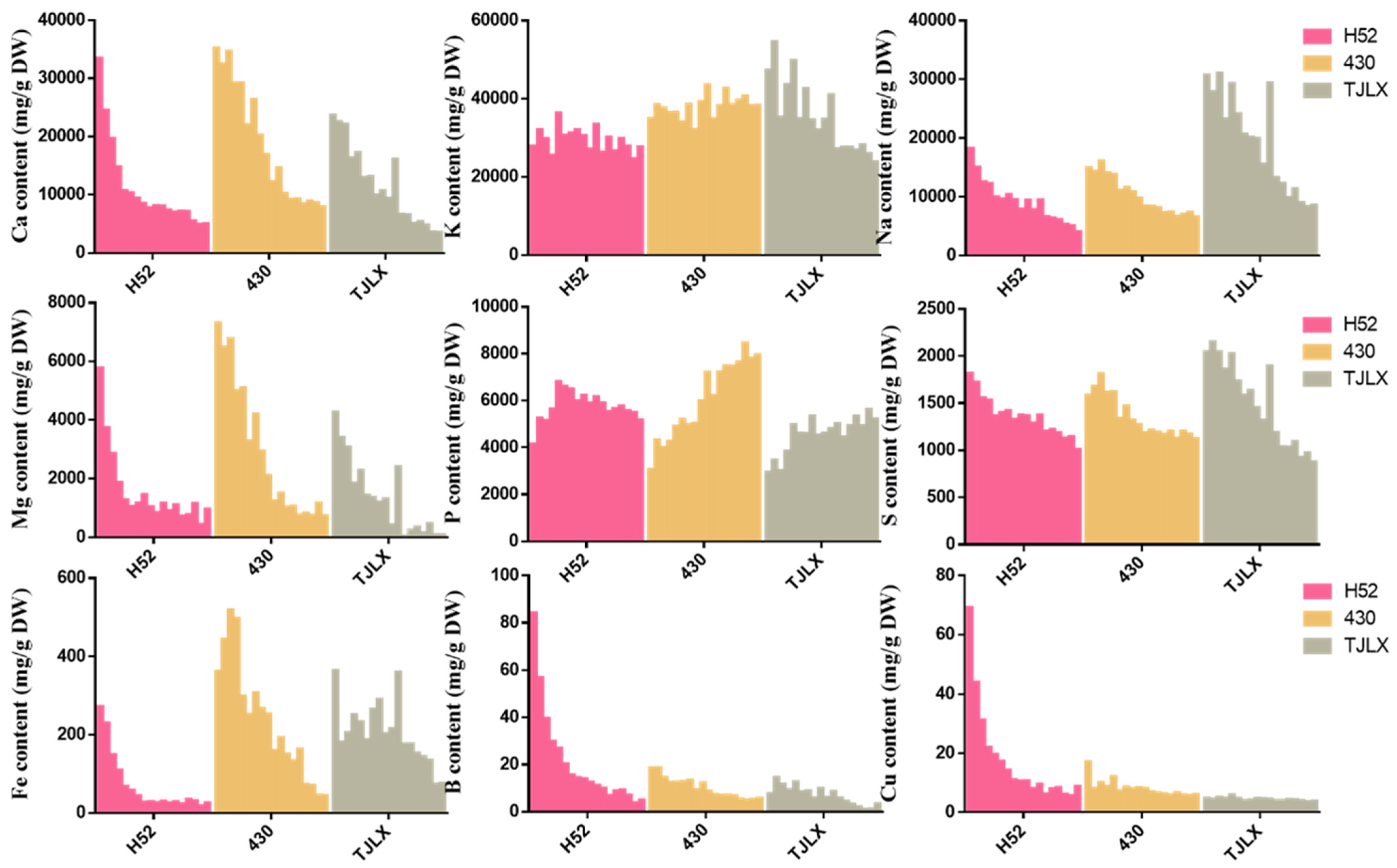
Minerals are essential for human growth, daily activities, and the maintenance of normal physiological functions in the body. The human body cannot produce minerals on its own; they must be obtained from food. We analyzed the contents of essential elements required by the human body in various leaf layers of the H52, 430, and TJLX cabbage varieties (Figure 4). These elements included calcium (Ca), potassium (K), sodium (Na), magnesium (Mg), phosphorus (P), and sulfur (S). Additionally, the concentrations of trace elements, including iron (Fe), boron (B), and copper (Cu), were measured. Among the macronutrients, K was the most abundant, followed by Ca and Na. Compared to the levels of K, the levels of Ca, Na, Mg, and S were relatively low. Moreover, the concentrations of Ca, Na, Mg, and P decreased continuously from the outer to the inner leaves, whereas the concentration of K remained relatively consistent across the different leaf layers.

Figure 4.
Mineral content of leaf layers of Chinese cabbage cultivars H52, 430 and TJLX.
Fe was most abundant among the detected trace micronutrients, and the accumulation of Fe, B, and Cu decreased toward the core in the H52 variety. With the exception of that in the first two leaves, the Fe content in 430 was slightly lower, and the distribution across the remaining leaf layers was similar to that in H52. Further, the B and Cu content remained consistent throughout the leaf layers. In TJLX, the concentrations of Ca, Na, Mg, and S decreased from the outer to the inner lobes, whereas the concentration of K remained relatively consistent among the different leaf layers. The distribution of P in the leaf layers differed from that of the other minerals. In H52 Chinese cabbage, the P content initially increased and then decreased, reaching its highest value in the sixth leaf layer. In 430 and TJLV, the P content fluctuated in each leaf layer but was generally positively correlated with the age of the leaf.
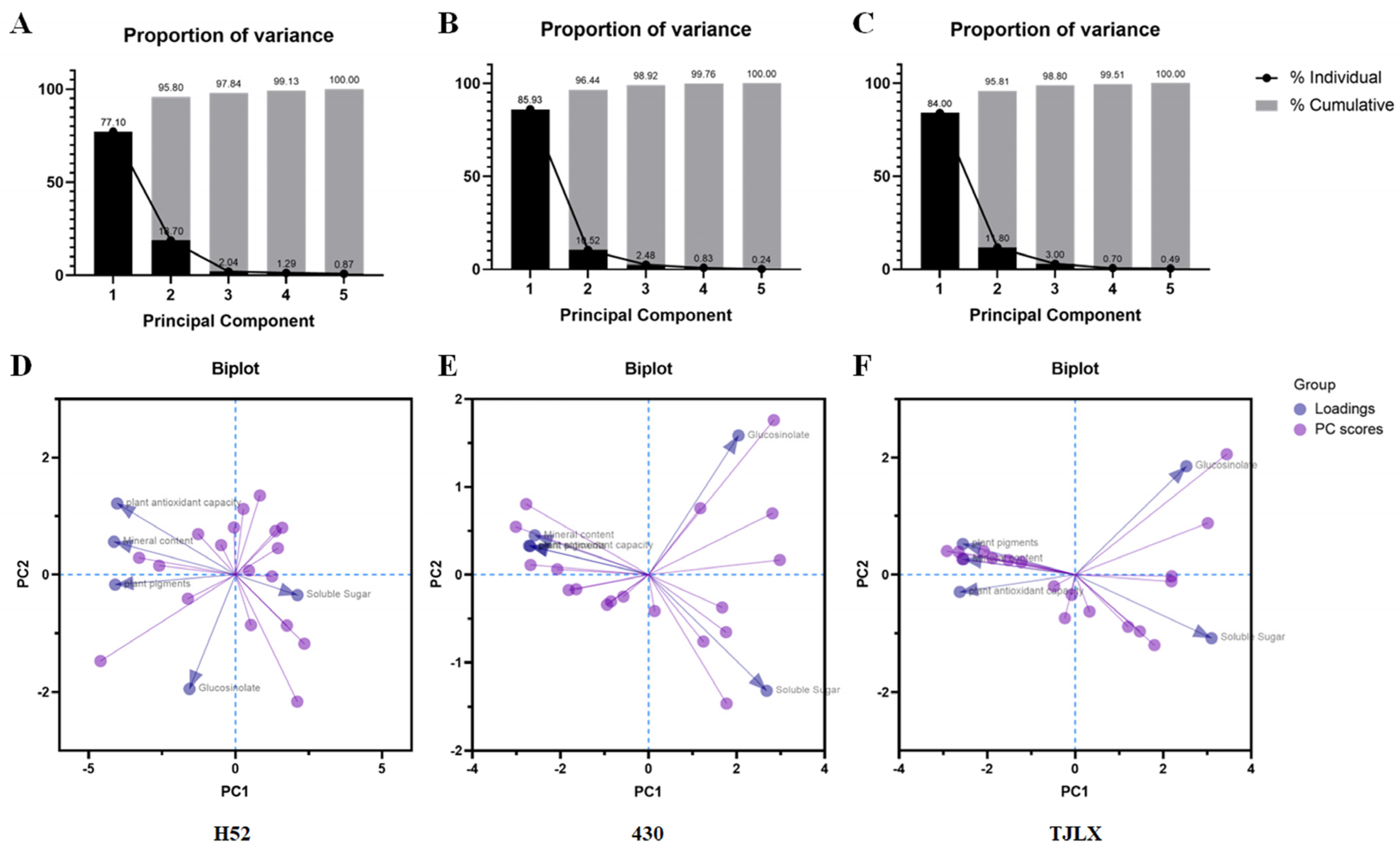
3.6. Principal Component Analysis (PCA)
Principal component analysis (PCA) was conducted to illustrate the relationships between metabolites and leaf layers in the H52, 430, and TJLX varieties (Figure 5). The first principal component (PC1) accounted for 77.1%, 85.93%, and 84.00% of the variance in the H52, 430, and TJLX varieties, respectively. When combined, PC1 and the second principal component (PC2) explained 95.8%, 96.44%, and 95.81% of the variance in the H52, 430, and TJLX varieties, respectively (Figure 5A–C). Biplot analysis revealed variations in metabolite levels across leaf layers in the H52, 430, and TJLX varieties (Figure 5D,E). We consolidated all of the measured traits into five variables: soluble sugar, plant pigments, glucosinolate content, mineral content, and plant antioxidant capacity. The PCA biplot showed that plant pigments, mineral content, and plant antioxidant capacity were positively correlated on PC1, while soluble sugar exhibited a negative correlation with these variables on PC1. Moreover, plant antioxidant capacity and glucosinolate content were the most distinguishing variables in the leaf layers of the H52 variety (Figure 5D), whereas soluble sugar and glucosinolate content were the key differentiating variables in the 430 and TJLX varieties. This indicates that these traits carried greater weight in the principal component analysis.

Figure 5.
PCA of nutrient distribution in the leaf layers of Chinese cabbage. The percentage of variance explained by the first five principal components (PC1 to PC5) for H52 (A), 430 (B), and TJLX (C). Biplot analysis of nutrient distribution across 18 leaf layers from the outer to the inner leaves in H52 (D), 430 (E), and TJLX (F), focusing on soluble sugar, plant pigments, plant antioxidant capacity, glucosinolate, and mineral content.
4. Discussion
Chinese cabbage, which originated in China, has been subjected to extensive domestication and purposeful selection, resulting in the development of a distinctive leafy head. As the leaf heads mature, the specific rosette leaves gradually curve upward and inward during the folding stage. This unique variation in curvature, which is distinct among the leaves and various parts of the petiole, results in the inner leaves being encased by the outer leaves, effectively protecting them from direct sunlight. Consequently, different types of head leaves in Chinese cabbage display diverse leaf characteristics, from the outermost to the innermost layers (Figure 1A). The primary objective of this study was to analyze the distribution of nutrients and phytochemicals within these head leaves of Chinese cabbage across different leaf layers and head types. The findings revealed distinct patterns of soluble sugar, vitamin, mineral, and bioactive compound accumulation in various cabbage types and leaf layers, and these patterns were influenced by factors such as the leaf age and the amount of light exposure.
Soluble sugar is a pivotal primary metabolic compound in Chinese cabbage that not only serves as a source of nutritional value but also exerts a significant effect on the taste and overall quality of this Chinese vegetable. Further, it plays a fundamental role in various metabolic pathways by providing both energy and a fundamental carbon structure [20,21]. Given the increasing emphasis on health, consumers are paying more attention to the sugar content of vegetables as a means of monitoring their carbohydrate intake. However, there is a dearth of research on the content and distribution of soluble sugars in Chinese cabbage leaves, particularly across different heading leaf types. In this study, the soluble sugar that predominantly accumulates in Chinese cabbage leaves was found to be fructose, followed by glucose. Interestingly, the total sugar content in the outer leaves was relatively low and gradually increased toward the center of the leaf head, which aligns with the sweetness and flavor of the inner leaves (Figure 1B–E). This distribution pattern is consistent with observations in other plants, such as Arabidopsis and Hevea brasiliensis, where a higher proportion of carbohydrates tends to be allocated to actively growing tissues, possibly because of the varying energy requirements during different stages of tissue development [22,23]. In addition, sucrose, which is the main photosynthetic product involved in the transport of sugar in plants, plays a pivotal role in the regulation of sugar signaling [24]. However, its content in Chinese cabbage leaves is relatively low, possibly because of its propensity to undergo hydrolysis to form fructose and glucose. Interestingly, the sucrose content exhibited a relatively stable pattern in the different leaf layers of the heading and semi-heading cabbages, whereas in the loose-heading cabbages, there was a gradual increase in sucrose content from the outer leaves toward the central leaves. This distinction could be attributed to the loose-heading cabbage receiving more sunlight than the heading and semi-heading Chinese cabbage. Accordingly, variations in light exposure could influence the distribution of sucrose within the leaf layers, thus contributing to this observed trend.
Vegetable coloration not only enhances the visual appeal of vegetables but also provides numerous health benefits to consumers. Chinese cabbage primarily contains two pigments: chlorophyll and carotenoids. The synthesis of chlorophyll, a crucial photosynthetic pigment, relies on light, resulting in higher chlorophyll levels in the outer leaves of Chinese cabbage heads. However, the curling of inner leaves restricts light exposure, leading to reduced chlorophyll production. This inhibition of chlorophyll production results in varying degrees of yellowing of the inner leaves, which reflects the lutein and carotene contents. Carotenoids—essential auxiliary light-absorbing pigments with photoprotective properties—significantly influence primary metabolite production [25]. Our study revealed that levels of carotenoids, including β-carotene, neoxanthin, violaxanthin, and lutein, were higher in the outer leaves than in the inner leaves (Figure 2). This distribution pattern might be attributed to the role of carotenoids in protecting plants from the damage caused by UV and visible-light exposure. Moreover, we observed a significant correlation between the chlorophyll content and carotenoid content. Interestingly, prior research on bioactive substances in Chinese cabbage has also identified significant correlations between carotenoids and chlorophyll, as well as a positive correlation with glucosinolates [26]. These correlations might explain why consumers prefer the yellow variety of Chinese cabbage because of its high nutritional value.
Vitamin C, phenolic compounds, and flavonoid compounds play crucial roles in vegetables as natural antioxidants, aiding in defense against oxidative stress and thereby protecting plant cells and tissues from harm caused by free radicals and oxidative molecules. Similarly, when humans consume vegetables, they consume antioxidants, which bolster the inherent protective mechanisms of the body and mitigate the adverse effects of oxidative stress on health [13,27]. In this study, a close correlation between vitamin C, phenolic compounds, and flavonoid compound accumulation and leaf age was identified. These antioxidants tended to accumulate more in the outer leaves and their levels gradually decreased moving from the outer leaves toward the inner leaves near the head (Figure 3). This phenomenon could be related to the photoprotective mechanism of vitamin C. Outer leaves receive more sunlight, promoting increased accumulation of vitamin C, whereas inner leaves are less influenced by light, leading to a relative decrease in the synthesis of vitamin C. Importantly, this study revealed a marked decline in the accumulation of total phenolic compounds as light intensity was diminished. This can be attributed to the distinct inward curling of Chinese cabbage leaves. Interestingly, previous research has indicated that light exposure results in elevated levels of acetyl-CoA and coumarin-CoA in Chinese cabbage leaves, both of which play roles in phenolic compound synthesis, potentially contributing to the higher phenolic compound contents in the outer leaves [28].
The beneficial impact of glucosinolates on cancer prevention highlights the significance of monitoring the natural occurrence of these compounds in cruciferous vegetables. Furthermore, the glucosinolate content plays a role in shaping the flavor of Chinese cabbage to some extent. In this study, it was observed that there were no significant differences in the total glucosinolate content between the inner and outer leaves of the heading leafy Chinese cabbage. On the other hand, in the semi-heading Chinese cabbage, the total glucosinolate content was slightly higher in the inner leaves compared to the outer leaves. Notably, for the loose-heading Chinese cabbage, the inner leaves near the core displayed significantly higher levels of glucosinolates than the outer leaves (Table 1). Moreover, the loose-heading Chinese cabbage exhibited higher total glucosinolate content compared to the heading and semi-heading Chinese cabbages, which may be linked to glucose levels. Significantly, previous research has suggested that glucose promotes the accumulation of both aliphatic and indole glucosinolate [29,30]. It is interesting to highlight that, as mentioned earlier, the glucose content in the loose-heading Chinese cabbage was notably higher than that in the heading leafy and semi-heading Chinese cabbages. Qualitative and quantitative analyses revealed that aliphatic glucosinolates were significantly more abundant than indole glucosinolates in various types of Chinese cabbage (Supplementary Table S1). This finding aligns with prior reports, underscoring that aliphatic glucosinolates are the predominant type of glucosinolates in Chinese cabbage [31].
Vegetables are widely recognized as one of the most cost-effective and healthy sources of essential micronutrients, including vitamins and minerals [32]. Scientific research has established that the human body requires a diverse array of essential elements to support the physiological and metabolic processes required for maintaining healthy bones, blood function, and overall tissue health [33]. In our study, we discovered that Chinese cabbage is notably rich in a range of essential minerals, including calcium, potassium, sodium, magnesium, P, S, iron, B, and copper. Among these minerals, Ca, K, and Na were found in the highest concentrations, followed by Mg, P, and S, whereas Fe, B, and Cu were detected at lower levels (Figure 4). The distribution patterns of these mineral elements varied among the different leaf layers of Chinese cabbage. Specifically, Ca, Na, Mg, S, Fe, B, and Cu gradually decreased as the leaf age increased, whereas P concentrations increased with the leaf age. Notably, high levels of K were consistently maintained throughout all the leaf layers of Chinese cabbage. Compared to cabbage and cauliflower, Chinese cabbage stands out due to its particularly rich mineral content, with K being especially abundant [34].
5. Conclusions
In summary, our research identifies distinctive patterns of metabolites in Chinese cabbage across various leaf layers. The inner leaves of leafy heads are particularly rich in soluble sugars and glucosinolates (which are known for their potential anticancer properties). In contrast, both the content of antioxidants and the overall antioxidant capacity consistently decrease from the outer leaves towards the central core. This downward trend is also observed in the mineral content, including calcium, sodium, magnesium, sulfur, iron, boron, and copper. Our data also suggested that the distribution pattern of metabolites in Chinese cabbage is influenced primarily by development stage and light exposure, which might need further investigation. These data offer dietary guidance for consumers with specific intake needs and may bridge the gap between traditional perceptions and scientific evidence, providing valuable insights for improving dietary recommendations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10090988/s1.
Author Contributions
Y.Z.: conceptualization, methodology, writing—original draft preparation; H.W.: conceptualization, validation, investigation, data curation, writing—review and editing; Y.M.: conceptualization, software, visualization, validation; Z.Y.: resources, methodology; J.L.: software, validation; P.T.: formal analyses, investigation; B.L.: conceptualization, supervision; J.Z.: project administration and funding acquisition, final approval of the version to be published; Q.H.: project administration and funding acquisition, final approval of the version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Zhejiang province Research and Development Program of “Lingyan” (NO. 2022C02030), Zhejiang province Major science and technology Program (NO. 2021C02065-5-2), State Key Laboratory of North China Crop Improvement and Regulation (“S&T Program of Hebei”, 23567601H).
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Björkman, M.; Klingen, I.; Birch, A.; Bones, A.; Bruce, T.; Johansen, T.; Meadow, R.; Mølmann, J.; Seljåsen, R.; Smart, L.; et al. Phytochemicals of Brassicaceae in plant protection and human health--influences of climate, environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Giovannucci, E.; Hunter, D.; Neuberg, D.; Su, L.; Christiani, D. Dietary intake of Cruciferous vegetables, Glutathione S-transferase (GST) polymorphisms and lung cancer risk in a Caucasian population. Cancer Causes 2004, 15, 977–985. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-Glucosidase and α-Amylase by Flavonoids. J. Nutr. Sci. Vitaminol. 2007, 52, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Wennberg, M.; Ekvall, J.; Olsson, K.; Nyman, M. Changes in carbohydrate and glucosinolate composition in white cabbage (Brassica oleracea var. capitata) during blanching and treatment with acetic acid. Food Chem. 2006, 95, 226–236. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Bartoszek, A.; Wolska, L.; Drzewiecki, J.; Gorinstein, S.; Namieśnik, J. Partial characterization of white cabbages (Brassica oleracea var. capitata f. alba) from different regions by glucosinolates, bioactive compounds, total antioxidant activities and proteins. Food Sci. Technol. 2008, 41, 1–9. [Google Scholar] [CrossRef]
- Seong, G.U.; Hwang, I.W.; Chung, S.-K. Antioxidant capacities and polyphenolics of Chinese cabbage (Brassica rapa L. ssp. Pekinensis) leaves. Food Chem. 2016, 199, 612–618. [Google Scholar] [CrossRef]
- Podsędek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Li, J.; Zhang, Y.; Zhou, D.; Li, C.; He, L.; Li, H.; Wang, F.; Gao, J. Identification of key genes controlling soluble sugar and glucosinolate biosynthesis in Chinese cabbage by integrating metabolome and genome-wide transcriptome analysis. Front. Plant Sci. 2022, 13, 1043489. [Google Scholar] [CrossRef]
- Heinze, M.; Hanschen, F.S.; Wiesner-Reinhold, M.; Baldermann, S.; Gräfe, J.; Schreiner, M.; Neugart, S. Effects of Developmental Stages and Reduced UVB and Low UV Conditions on Plant Secondary Metabolite Profiles in Pak Choi (Brassica rapa subsp. chinensis). J. Agric. Food Chem. 2018, 66, 1678–1692. [Google Scholar] [CrossRef] [PubMed]
- Harbaum, B.; Hubbermann, E.M.; Zhu, Z.; Schwarz, K. Free and bound phenolic compounds in leaves of pak choi (Brassica campestris L. ssp. chinensis var. communis) and Chinese leaf mustard (Brassica juncea Coss). Food Chem. 2008, 110, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Wu, J.; Gao, W.; Wei, J.; Yang, J.; Guo, C. Antioxidant Capacity of Different Fractions of Vegetables and Correlation with the Contents of Ascorbic Acid, Phenolics, and Flavonoids. J. Food Sci. 2011, 76, C1257–C1261. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yue, Z.; Zhong, X.; Lei, J.; Tao, P.; Li, B. Distribution of primary and secondary metabolites among the leaf layers of headed cabbage (Brassica oleracea var. capitata)—ScienceDirect. Food Chem. 2020, 312, 126028. [Google Scholar] [CrossRef] [PubMed]
- Brantsæter, A.L.; Ydersbond, T.A.; Hoppin, J.A.; Haugen, M.; Meltzer, H.M. Organic food in the diet: Exposure and health implications. Annu. Rev. Public Health 2016, 38, 295–313. [Google Scholar] [CrossRef]
- Swegarden, H.; Stelick, A.; Dando, R.; Griffiths, P.D. Bridging sensory evaluation and consumer research for strategic leafy brassica (brassica oleracea) improvement. J. Food Sci. 2019, 84, 3746–3762. [Google Scholar] [CrossRef]
- Benmeziane, A.; Boulekbache-Makhlouf, L.; Mapelli-Brahm, P.; Khodja, N.K.; Remini, H.; Madani, K. Extraction of carotenoids from cantaloupe waste and determination of its mineral composition. Food Res. Int. 2018, 111, 391–398. [Google Scholar] [CrossRef]
- Liu, H.; Meng, F.; Miao, H.; Chen, S.; Yin, T.; Hu, S.; Shao, Z.; Liu, Y.; Gao, L.; Zhu, C. Effects of postharvest methyl jasmonate treatment on main health-promoting components and volatile organic compounds in cherry tomato fruits. Food Chem. 2018, 263, 194–200. [Google Scholar] [CrossRef]
- Fan, K.; Wang, M.; Gao, Y.; Ning, Q.; Shi, Y. Transcriptomic and ionomic analysis provides new insight into the beneficial effect of Al on tea roots’ growth and nutrient uptake. Plant Cell Rep. 2019, 38, 715–729. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Liu, W. Sugar accumulation and characterization of metabolizing enzyme genes in leafy head of Chinese cabbage (Brassica campestris L. ssp. pekinensis). Hortic. Environ. Biotechnol. 2020, 62, 17–29. [Google Scholar] [CrossRef]
- Shen, J.; Zou, Z.; Zhang, X.; Zhou, L.; Wang, Y.; Fang, W.; Zhu, X. Metabolic analyses reveal different mechanisms of leaf color change in two purple-leaf tea plant (Camellia sinensis L.) cultivars. Hortic. Res. 2018, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Mainson, D.; Porcheron, B.; Maurousset, L.; Lemoine, R.; Pourtau, N. Carbon source-sink relationship in Arabidopsis thaliana: The role of sucrose transporters. Planta Int. J. Plant Biol. 2018, 247, 587–611. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Qi, J.; Fang, Y.; Xiao, X.; Li, J.; Lan, J.; Tang, C. Characterization of Sugar Contents and Sucrose Metabolizing Enzymes in Developing Leaves of Hevea brasiliensis. Front. Plant Sci. 2018, 9, 58. [Google Scholar] [CrossRef]
- Rolland, F.; Baenagonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and Novel Mechanisms. Annu. Rev. 2006, 57, 675–709. [Google Scholar] [CrossRef]
- Young, A.J. The photoprotective role of carotenoids in higher plants. Physiol. Plant. 1991, 83, 702–708. [Google Scholar] [CrossRef]
- Baek, S.A.; Jung, Y.H.; Lim, S.H.; Park, S.U.; Kim, J.K. Metabolic Profiling in Chinese Cabbage (Brassica rapa L. subsp. pekinensis) Cultivars Reveals that Glucosinolate Content Is Correlated with Carotenoid Content. Agric. Food Chem. 2016, 64, 4426–4434. [Google Scholar] [CrossRef]
- Huxley, R.R.; Neil, H.A.W. The relation between dietary flavonol intake and coronary heart disease mortality: A meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2003, 57, 904–908. [Google Scholar] [CrossRef]
- Kim, E.H.; Ro, H.M.; Kim, S.L.; Kim, H.S.; Chung, I.M. Analysis of isoflavone, phenolic, soyasapogenol, and tocopherol compounds in soybean [Glycine max (L.) Merrill] germplasms of different seed weights and origins. J. Agric. Food Chem. 2012, 60, 6045–6055. [Google Scholar]
- Wei, J.; Miao, H.; Wang, Q. Effect of glucose on glucosinolates, antioxidants and metabolic enzymes in Brassica sprouts. Sci. Hortic. 2011, 129, 535–540. [Google Scholar] [CrossRef]
- Miao, H.; Wei, J.; Zhao, Y.; Yan, H.; Sun, B.; Huang, J.; Wang, Q. Glucose signalling positively regulates aliphatic glucosinolate biosynthesis. J. Exp. Bot. 2013, 64, 1097–1109. [Google Scholar] [CrossRef]
- Lee, M.K.; Chun, J.H.; Byeon, D.H.; Chung, S.O.; Park, S.U.; Park, S.; Arasu, M.V.; Al-Dhabi, N.A.; Lim, Y.P.; Kim, S.J. Variation of glucosinolates in 62 varieties of Chinese cabbage (Brassica rapa L. ssp. pekinensis) and their antioxidant activity. Food Sci. Technol. 2014, 58, 93–101. [Google Scholar] [CrossRef]
- Flyman, M.V.; Afolayan, A.J. The implication of the mineral ratios of Cucumis myriocarpus Naud. and Pergularia daemia (Forsk.) Chiov. in human diets. J. Med. Food 2007, 10, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Mobeen; Wang, X.; Saleem, M.H.; Parveen, A.; Mumtaz, S.; Hassan, A.; Adnan, M.; Fiaz, S.; Ali, S.; Iqbal Khan, Z.; et al. Proximate Composition and Nutritive Value of Some Leafy Vegetables from Faisalabad, Pakistan. Sustainability 2021, 13, 8444. [Google Scholar] [CrossRef]
- Jie, W.; Zeci, L.; Jianhua, D.; Jian, L.; Ning, J.; Li, J.; Zhaozhuang, L.; Bo, Z.; Zhongqi, T.; Jihua, Y. A Comparative Study on the Nutrients, Mineral Elements, and Antioxidant Compounds in Different Types of Cruciferous Vegetables. Agronomy 2022, 12, 3121. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).