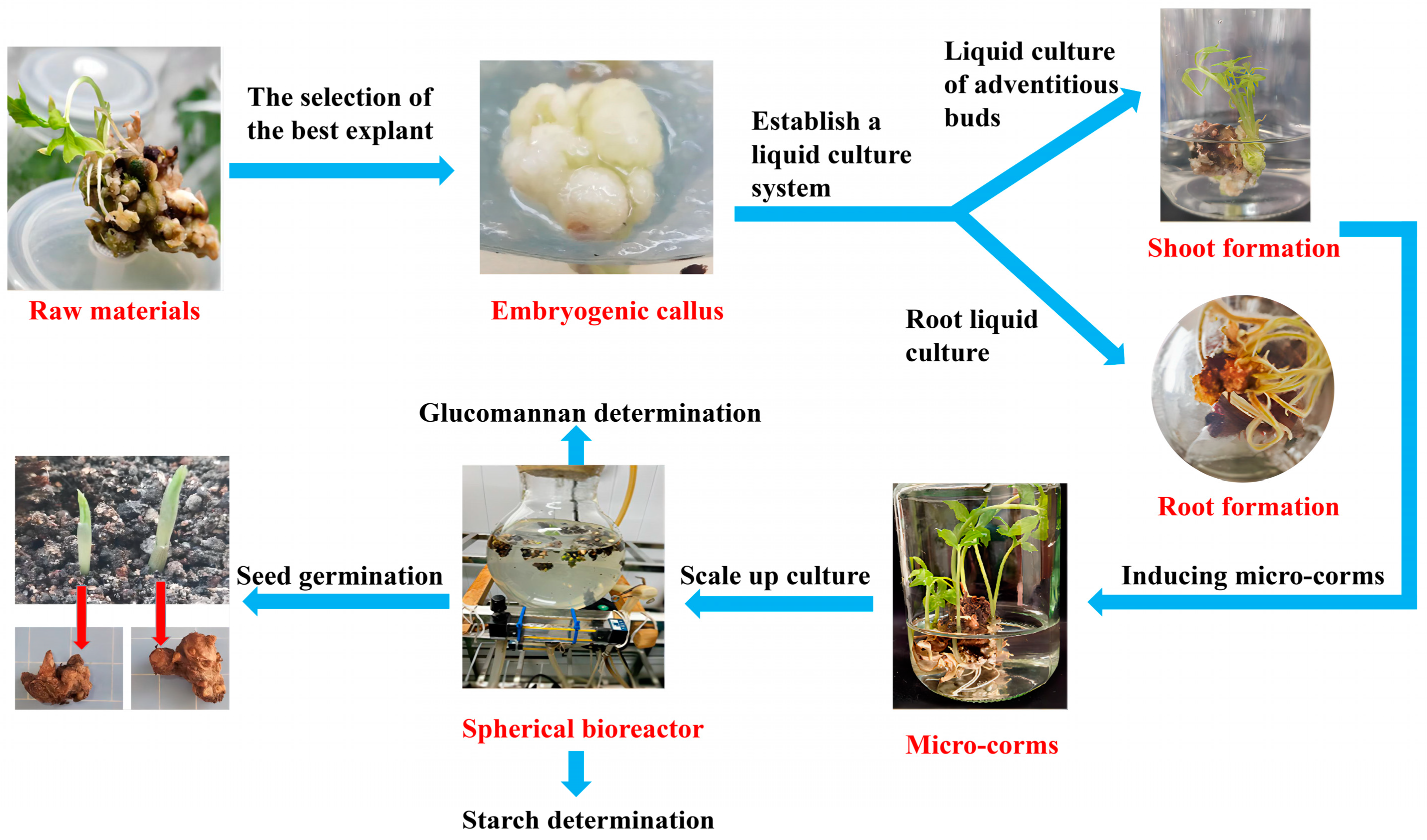
Bioreactor-Based Liquid Culture and Production of Konjac Micro-Corm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Liquid Culture for Adventitious Shoot Inductions
2.3. Liquid Culture for Adventitious Root Formation
2.4. Liquid Culture for Micro-Corm Generations
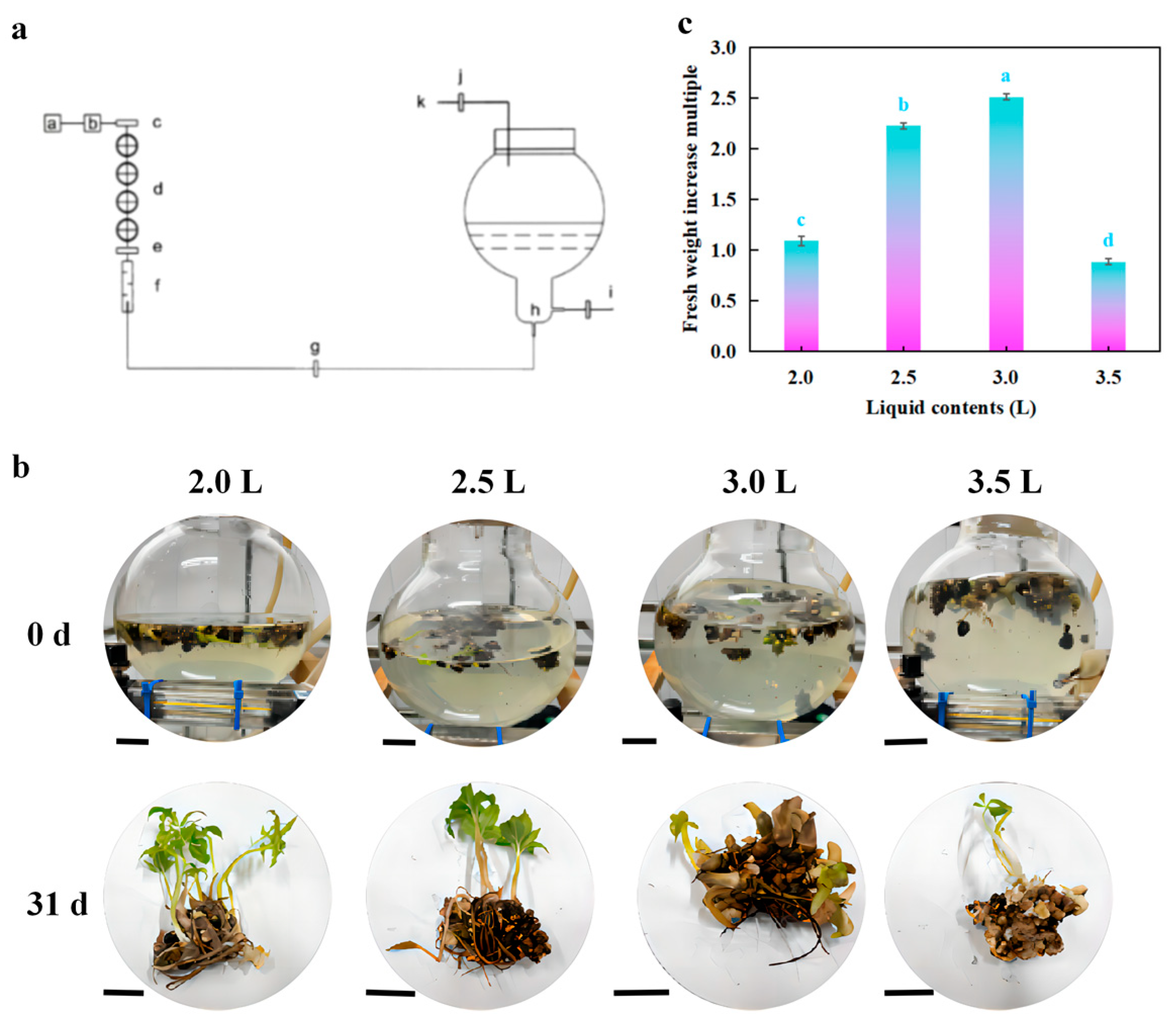
2.5. Bioreactor-Based Scale-Up Culture of Micro-Corms
2.6. Determination of Glucomannan Content
2.7. Statistical Analyses
3. Results
3.1. The Induction of Adventitious Shoots Under Liquid Culture
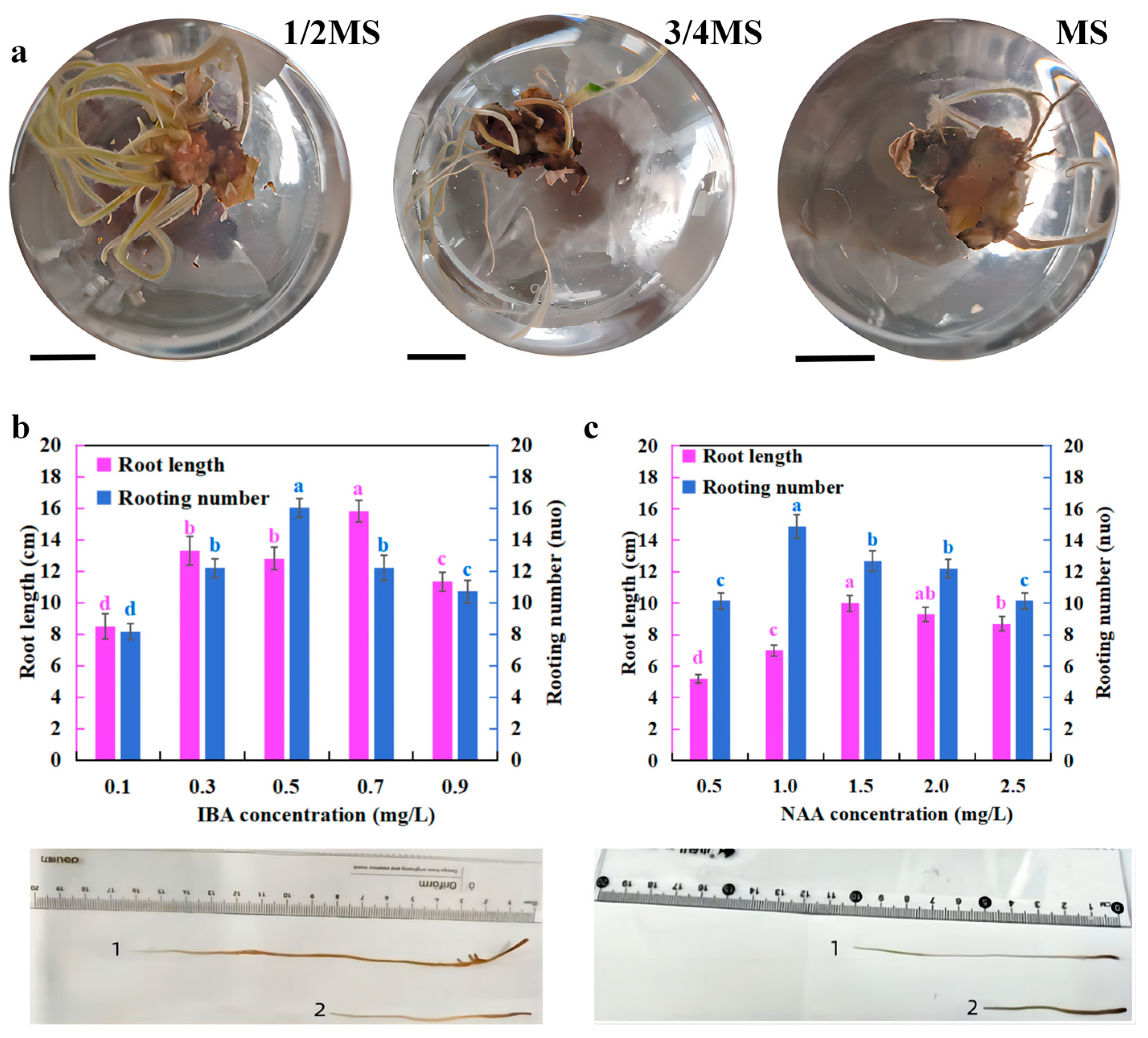
3.2. The Formation of Adventitious Roots in Liquid Culture
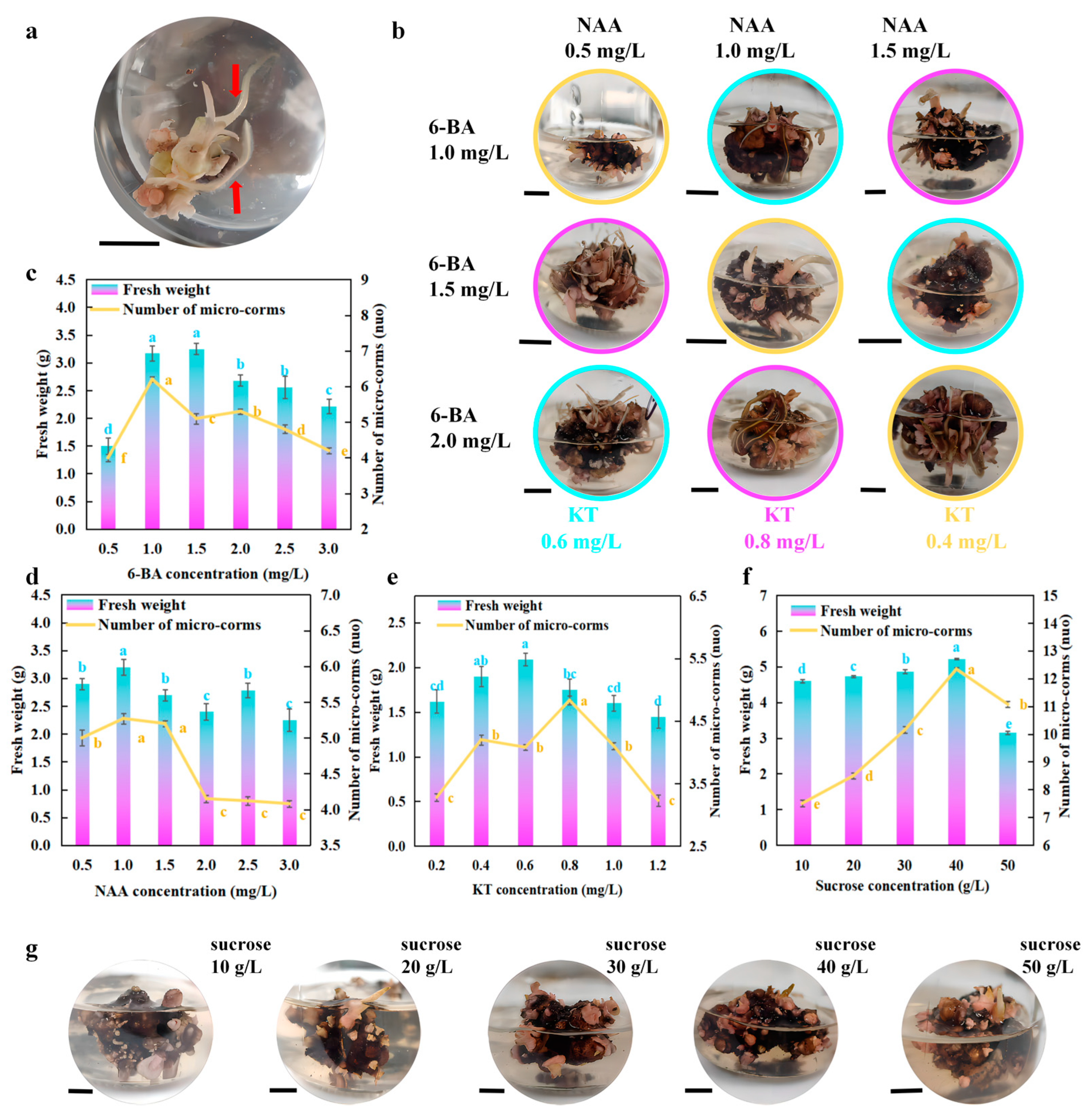
3.3. Konjac Micro-Corm Induction and Growth Under Liquid Culture
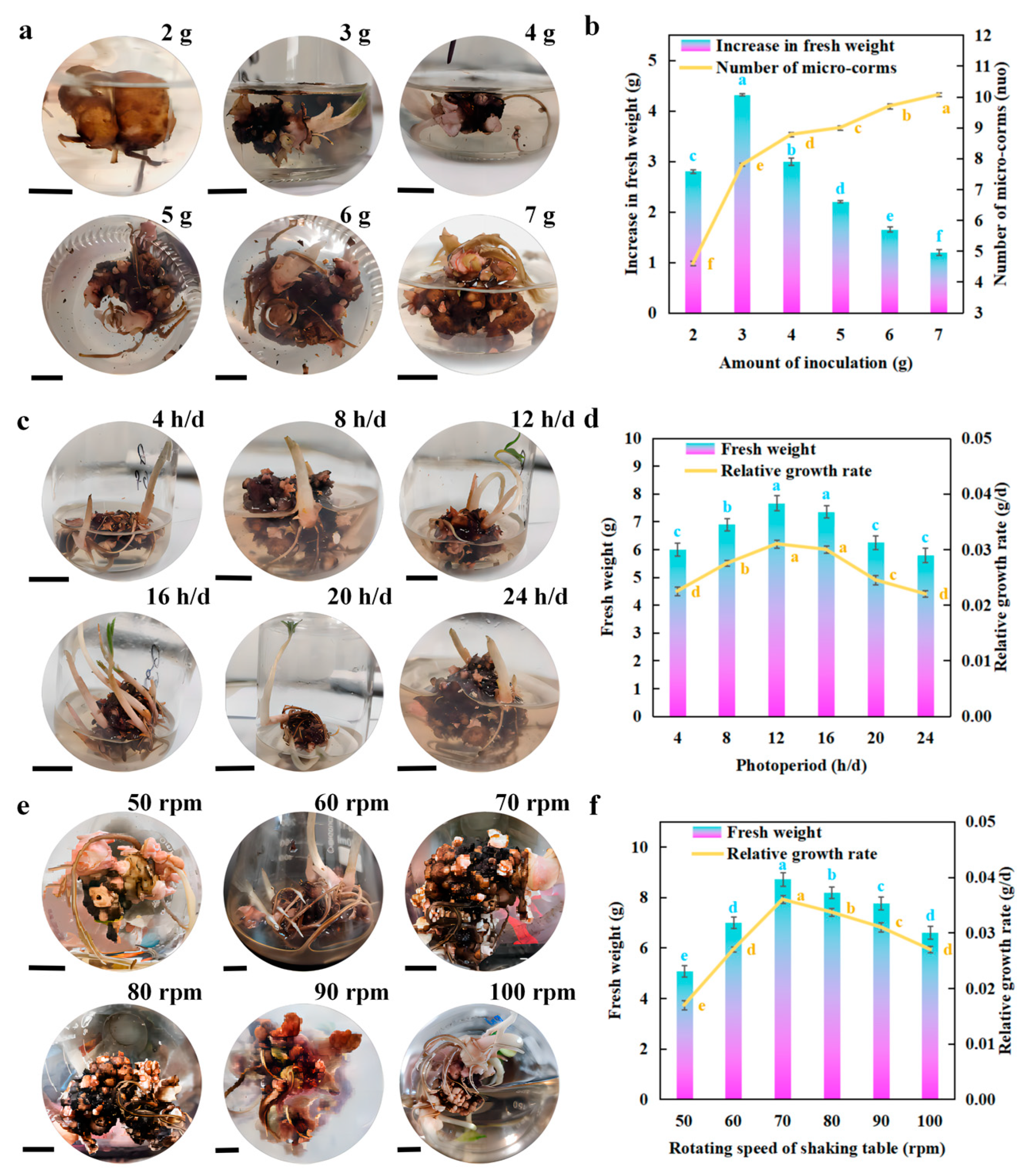
3.4. Scale-Up Culture of Micro-Corms in Spherical Bioreactor and Identification of Main Storage Substances
4. Discussion
4.1. Liquid Culture of Adventitious Bud Induction
4.2. Liquid Culture for Induced Rooting
4.3. Liquid Culture Induced by Micro-Corms
4.4. Scale-Up Culture of Micro-Corms in Spherical Bioreactor and Identification of Main Storage Substances
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6-BA | 6-Benzylaminopurine |
| 2,4-D | 2,4-Dichlorophenoxyacetic acid |
| IBA | Indole-3-butyric acid |
| MS | Murashige and Skoog |
| NAA | 1-Naphthaleneacetic acid |
| KT | Kinetin |
| PGRs | Plant growth regulators |
References
- Chotigamas, T.; Sirisansaneeyakul, S.; Sripaoraya, S.; Gateprasert, M.; Vanichsriratana, W. Process development for konjac (Amorphophallus oncophyllus) glucomannan production using cell suspension culture technique. Mitochondrial DNA Part B Resour. 2024, 9, 522–526. [Google Scholar]
- Gao, Y.; Yin, S. Assembly and analysis of the chloroplast genome of Amorphophallus kachinensis engler & gehrmann (Araceae) from Southwestern China: Implications for conservation and utilization. Mitochondrial DNA Part B Resour. 2024, 9, 452–456. [Google Scholar] [CrossRef]
- Sun, L.; Xiao, Q.L.; Zhang, T.; Fan, J.Q.; Deng, L.G.; Shen, Y.F.; Liu, X. First report of Fusarium tricinctum causing leaf blight on Amorphophallus konjac in hunan province of China. Plant Dis. 2023, 108, 228. [Google Scholar] [CrossRef]
- Oksel, C.; Avin, F.A.; Liyanapathiranage, P.; Shreckhise, J.; Baysal-Gurel, F. First report of Achromobacter xylosoxidans causing bacterial stem and leaf blight on Cyrilla arida in Tennessee and the United States. Plant Dis. 2024, 108, 1094. [Google Scholar] [CrossRef] [PubMed]
- Al-Qasab, G.H.; Rasheed, J.H. Regeneration of cowpea Vigna radiata L. plant from cell suspension. J. Res. Appl. Sci. Biotechnol. 2023, 2, 179–185. [Google Scholar] [CrossRef]
- Panggabean, N.H.; Panggabean, N.H.; Nurwahyuni, I.; Elimasni; Basyuni, M. Somatic embryogenesis of oil palm (Elaeis guineensis jacq.) from bud explants using suspension culture. OnLine J. Biol. Sci. 2023, 23, 236–242. [Google Scholar] [CrossRef]
- Makowski, W.; Królicka, A.; Tokarz, B.; Szopa, A.; Ekiert, H.; Tokarz, K.M. Temporary immersion bioreactors as a useful tool for obtaining high productivity of phenolic compounds with strong antioxidant properties from Pontechium maculatum. Plant Cell Tissue Organ Cult. 2023, 153, 525–537. [Google Scholar] [CrossRef]
- Kim, J.-H.; Han, J.-E.; Murthy, H.N.; Kim, J.-Y.; Kim, M.-J.; Jeong, T.-K.; Park, S.-Y. Production of secondary metabolites from cell cultures of Sageretia thea (osbeck) M.C. Johnst. using balloon-type bubble bioreactors. Plants 2023, 12, 1390. [Google Scholar] [CrossRef] [PubMed]
- Kochan, E.; Szymańska, G.; Kwiatkowski, P.; Sienkiewicz, M. Linalool as a novel natural factor enhancing ginsenoside production in hairy root cultures of American ginseng. Plant Cell Tiss Organ Cult. 2023, 153, 285–293. [Google Scholar] [CrossRef]
- Li, D.; Mohammadi, M.A.; Qin, Y.; Zhang, Z. Somatic embryogenesis and indirect in vitro plant regeneration in Amorphophallus konjac K. Koch by one-step seedling formation. Horticulturae 2021, 7, 497. [Google Scholar] [CrossRef]
- Oktavia, G.A.E.; Junairiah, J.; Manuhara, Y.S.W. Amorphophallus muelleri blume shoot induction on different media types and plant growth regulator combinations. AGRIVITA J. Agric. Sci. 2024, 46, 458–469. [Google Scholar] [CrossRef]
- Krasniqi, D.L.; Kocaoğlu, B.; İşleyen, K.; Uras, D.; Yılmaz, E.; Ünek, C.; Nikerel, E.; Özdemir, B.S. Scaling up plant molecular farming via bioprocessing of plant cell suspension culture. In Applications of Plant Molecular Farming; Springer: Singapore, 2024; pp. 163–189. [Google Scholar]
- Huiban, F.A.; Roberta, T.M.; Camen, D.D.; Camelia, T. Bioreactors–a new biotechnological tool for standardized production of plant bioactive compounds. J. Hortic. For. Biotechnol. 2023, 27, 93–98. [Google Scholar]
- Chua, M.; Chan, K.; Hocking, T.J.; Williams, P.A.; Perry, C.J.; Baldwin, T.C. Methodologies for the extraction and analysis of konjac glucomannan from corms of Amorphophallus konjac K. Koch. Carbohydr. Polym. 2012, 87, 2202–2210. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Bioreactor systems for micropropagation of plants: Present scenario and future prospects. Front. Plant Sci. 2023, 14, 1159588. [Google Scholar] [CrossRef]
- Hwang, H.-D.; Kwon, S.-H.; Murthy, H.N.; Yun, S.-W.; Pyo, S.-S.; Park, S.-Y. Temporary immersion bioreactor system as an efficient method for mass production of in vitro plants in horticulture and medicinal plants. Agronomy 2022, 12, 346. [Google Scholar] [CrossRef]
- De Carlo, A.; Tarraf, W.; Lambardi, M.; Benelli, C. Temporary immersion system for production of biomass and bioactive compounds from medicinal plants. Agronomy 2021, 11, 2414. [Google Scholar] [CrossRef]
- Ozyigit, I.I.; Dogan, I.; Hocaoglu-Ozyigit, A.; Yalcin, B.; Erdogan, A.; Yalcin, I.E.; Cabi, E.; Kaya, Y. Production of secondary metabolites using tissue culture-based biotechnological applications. Front. Plant Sci. 2023, 14, 1132555. [Google Scholar] [CrossRef]
- Yaganza, E.S.; Tweddell, R.J.; Arul, J. Postharvest application of organic and inorganic salts to control potato (Solanum tuberosum L.) storage soft rot: Plant tissue-salt physicochemical interactions. J. Agric. Food Chem. 2014, 62, 9223–9231. [Google Scholar] [CrossRef]
- Nie, J.; Pan, Y.; Shi, J.; Guo, Y.; Yan, Z.; Duan, X.; Xu, M. A comparative study on the uptake and toxicity of nickel added in the form of different salts to maize seedlings. Int. J. Environ. Res. Public Health 2015, 12, 15075–15087. [Google Scholar] [CrossRef]
- Ansari, S.A.; Singh, S.; Rani, A. Inorganic salts influence IAA ionization and adventitious rhizogenesis in Pongamia pinnata. J. Plant Physiol. 2004, 161, 117–120. [Google Scholar] [CrossRef]
- Lukatkin, A.; Mokshin, E.; Bolshakova, E.; Da Silva, J. Effects of inorganic salts concentration and alternative plant growth regulators on the in vitro organogenesis of a new hybrid Cymbidium. BTA 2019, 100, 279–288. [Google Scholar] [CrossRef]
- Gaspar, T.; Kevers, C.; Penel, C.; Greppin, H.; Reid, D.M.; Thorpe, T.A. Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cell. Dev. Biol.-Plant 1996, 32, 272–289. [Google Scholar] [CrossRef]
- Wei, K.; Ruan, L.; Wang, L.; Cheng, H. Auxin-induced adventitious root formation in nodal cuttings of Camellia sinensis. IJMS 2019, 20, 4817. [Google Scholar] [CrossRef]
- Ivanchenko, M.G.; Den Os, D.; Monshausen, G.B.; Dubrovsky, J.G.; Bednářová, A.; Krishnan, N. Auxin increases the hydrogen peroxide (H2O2) concentration in tomato (Solanum Lycopersicum) root tips while inhibiting root growth. Ann. Bot. 2013, 112, 1107–1116. [Google Scholar] [CrossRef]
- Parrotta, L.; Faleri, C.; Del Duca, S.; Cai, G. Depletion of sucrose induces changes in the tip growth mechanism of tobacco pollen tubes. Ann. Bot. 2018, 122, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Liu, Y.; Su, L.; Liu, X.; Chu, Q.; He, Z.; Zhou, X.; Lu, W.; Jiang, C.; Zheng, W. Physiological, anatomical and quality indexes of root tuber formation and development in chayote (Sechium edule). BMC Plant Biol. 2023, 23, 413. [Google Scholar] [CrossRef] [PubMed]
- Castillo, S.E.; Tovar, J.C.; Shamin, A.; Gutirerrez, J.; Pearson, P.; Gehan, M.A. A protocol for Chenopodium quinoa pollen germination. Plant Methods 2022, 18, 65. [Google Scholar] [CrossRef]
- La, D.; Psa, F.; Bmm, D.; Alm, A.; Fg, S.; Rg, A.; Ps, P.; Ar, N. The impact of carbon source on cell growth and the production of bioactive compounds in cell suspensions of Hancornia speciosa Gomes. Sci. Rep. 2021, 11, 24315. [Google Scholar] [CrossRef]
- ‘Aizat Norhisham, D.; Md Saad, N.; Ahmad Usuldin, S.R.; Vayabari, D.A.G.; Ilham, Z.; Ibrahim, M.F.; Show, P.-L.; Wan-Mohtar, W.A.A.Q.I. Performance of Malaysian kenaf Hibiscus cannabinus callus biomass and exopolysaccharide production in a novel liquid culture. Bioengineered 2023, 14, 2262203. [Google Scholar] [CrossRef]
- Waresindo, W.X.; Priyanto, A.; Sihombing, Y.A.; Hapidin, D.A.; Edikresnha, D.; Aimon, A.H.; Suciati, T.; Khairurrijal, K. Konjac glucomannan-based hydrogels with health-promoting effects for potential edible electronics applications: A mini-review. Int. J. Biol. Macromol. 2023, 248, 125888. [Google Scholar] [CrossRef]
- Ueno, H.; Haraguchi, N.; Azuma, M.; Shiiya, T.; Noda, T.; Ebihara, E.; Uehira, Y.; Uchida, T.; Sasaba, K.; Nakamura, M.; et al. Active consumption of konjac and konjac products improves blood glucose control in patients with type 2 diabetes mellitus. J. Am. Nutr. Assoc. 2023, 42, 123–129. [Google Scholar] [CrossRef]
- Sakamoto, A.; Uzuhashi, S.; Hoshi, H.; Kubota, M.; Horie, H.; Kakishima, M. First report of rust caused by Puccinia paullula on Monstera deliciosa and M. adansonii in Japan. Plant Dis. 2023, 107, 570. [Google Scholar] [CrossRef]
- Pereira, J.; Mouazen, A.M.; Foo, M.; Ahmed, H. A framework of artificial light management for optimal plant development for smart greenhouse application. PLoS ONE. 2021, 16, e0261281. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Van Labeke, M.-C.; Höfte, M. Timing of light quality affects susceptibility to Botrytis cinerea in strawberry leaves. J. Photochem. Photobiol. B Biol. 2020, 211, 111988. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. The control of seed dormancy and germination by temperature, light and nitrate. Bot. Rev. 2020, 86, 39–75. [Google Scholar] [CrossRef]
- Maurya, J.P.; Bhalerao, R.P. Photoperiod- and temperature-mediated control of growth cessation and dormancy in trees: A molecular perspective. Ann. Bot. 2017, 120, 351–360. [Google Scholar] [CrossRef]
- Adams, S.R.; Langton, F.A. Photoperiod and plant growth: A review. J. Hortic. Sci. Biotechnol. 2005, 80, 2–10. [Google Scholar] [CrossRef]
- Ochieno, D.M.W. Soil microbes determine outcomes of pathogenic interactions between Radopholus similis and Fusarium oxysporum V5w2 in tissue culture banana rhizospheres starved of nitrogen, phosphorus, and potassium. Front. Sustain. Food Syst. 2022, 6, 706072. [Google Scholar] [CrossRef]
- Hanrahan-Tan, D.G.; Henderson, L.; Kertesz, M.A.; Lilje, O. The effects of nitrogen and phosphorus on colony growth and zoospore characteristics of soil chytridiomycota. J. Fungi 2022, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V.; Berkov, S.; Georgiev, M.; Burrus, M.; Codina, C.; Bastida, J.; Ilieva, M.; Pavlov, A. Optimized nutrient medium for galanthamine production in Leucojum aestivum L. in vitro shoot system. Z. Naturforschung C 2009, 64, 219–224. [Google Scholar] [CrossRef]
- Yang, S.; Tao, J.; Liu, X.; Guo, D.; Zheng, J. Effects of carbon source and nitrogen source on callus growth and flavonoid content in Glycyrrhiza uralensis. Chin. J. Chin. Mater. Med. 2006, 31, 1857–1859. [Google Scholar]
- García-Ramírez, Y. Temporary immersion system for biomass production of Salvia spp.: A mini-review. Methods Mol. Biol. 2024, 2759, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Caselles, C.; Burgos, L.; Sánchez-Balibrea, I.; Egea, J.A.; Faize, L.; Martín-Valmaseda, M.; Bogdanchikova, N.; Pestryakov, A.; Alburquerque, N. The effect of silver nanoparticle addition on micropropagation of apricot cultivars (Prunus armeniaca L.) in semisolid and liquid media. Plants 2023, 12, 1547. [Google Scholar] [CrossRef]
- Motolinía-Alcántara, E.A.; Castillo-Araiza, C.O.; Rodríguez-Monroy, M.; Román-Guerrero, A.; Cruz-Sosa, F. Engineering considerations to produce bioactive compounds from plant cell suspension culture in bioreactors. Plants 2021, 10, 2762. [Google Scholar] [CrossRef] [PubMed]
- Pożoga, M.; Olewnicki, D.; Wójcik-Gront, E.; Latocha, P. An Efficient method of Pennisetum × advena “Rubrum” plantlets production using the temporary immersion bioreactor systems and agar cultures. Plants 2023, 12, 1534. [Google Scholar] [CrossRef]




| Level | IBA (mg/L) | NAA (mg/L) | Inorganic Salt (mg/L) |
|---|---|---|---|
| 1 | 0.3 | 1.0 | 1/2MS |
| 2 | 0.5 | 1.5 | 3/4MS |
| 3 | 0.7 | 2.0 | MS |
| Level | 6-BA (mg/L) | NAA (mg/L) | KT (mg/L) |
|---|---|---|---|
| 1 | 1.0 | 0.5 | 0.4 |
| 2 | 1.5 | 1.0 | 0.6 |
| 3 | 2.0 | 1.5 | 0.8 |
| Group | Level | Factor Concentration (mmol/L) | Increase in Fresh Weight (g) | Relative Growth Rate (g/d) | ||
|---|---|---|---|---|---|---|
| NH4NO3 | KNO3 | Nitrogen | ||||
| Nitrogen | 1 | 5.2 | 4.7 | 15 | 3.13 ± 0.02 f | 0.001 |
| 2 | 10.3 | 9.4 | 30 | 4.54 ± 0.06 d | 0.0138 | |
| 3 | 15.5 | 14.1 | 45 | 5.62 ± 0.01 c | 0.021 | |
| 4 | 20.6 | 18.8 | 60 | 5.98 ± 0.05 b | 0.023 | |
| 5 | 30.9 | 28.2 | 90 | 6.31 ± 0.04 a | 0.025 | |
| 6 | 41.2 | 37.6 | 120 | 4.03 ± 0.03 e | 0.010 | |
| NO3−/NH4+ | 1 | 1:1 | 4.78 ± 0.02 e | 0.016 | ||
| 2 | 2:1 | 7.21 ± 0.03 a | 0.029 | |||
| 3 | 3:1 | 6.31 ± 0.12 b | 0.025 | |||
| 4 | 4:1 | 6.02 ± 0.16 c | 0.023 | |||
| 5 | 5:1 | 5.70 ± 0.04 d | 0.021 | |||
| 6 | 90:0 | 1.81 ± 0.09 f | −0.020 | |||
| KH2PO4 | 1 | 0.25 | 2.19 ± 0.09 d | −0.010 | ||
| 2 | 0.5 | 3.20 ± 0.03 c | 0.002 | |||
| 3 | 0.75 | 5.23 ± 0.05 b | 0.019 | |||
| 4 | 1.0 | 7.01 ± 0.27 a | 0.029 | |||
| 5 | 1.25 | 6.89 ± 0.15 a | 0.028 | |||
| 6 | 1.5 | 4.91 ± 0.31 b | 0.016 | |||
| Level | NH4NO3 (mmol/L) | KNO3 (mmol/L) | KH2PO4 (mmol/L) |
|---|---|---|---|
| 1 | 10.3 | 9.4 | 0.75 |
| 2 | 15.5 | 14.1 | 1.0 |
| 3 | 20.6 | 18.8 | 1.25 |
| 6-BA (mg/L) | NAA (mg/L) | Induction Rate (%) | Number of Bud (nuo) | Fresh Weight (g) | Bud Morphology |
|---|---|---|---|---|---|
| 0.5 | 91.0 ± 0.07 e | 10 ± 0.12 a | 1.67 ± 0.10 c | A higher number of bud spots, tender, with no obvious micro-corm formation | |
| 1.0 | 98.7 ± 0.02 a | 8 ± 0.02 b | 2.09 ± 0.03 a | The number of bud spots is moderate, but the bud spots are generally more mature, conducive to the next step of differentiation. | |
| 1.5 | 93.8 ± 0.02 d | 3 ± 0.04 e | 1.88 ± 0.03 b | The number of bud spots is relatively low, and the growth is slow. | |
| 2.0 | 95.2 ± 0.04 c | 2 ± 0.14 f | 1.45 ± 0.02 de | Few bud spots, and the buds are immature. | |
| 2.5 | 96.4 ± 0.01 b | 5 ± 0.05 b | 1.52 ± 0.11 d | Numerous bud spots with obvious micro-corm growth, exhibiting signs of aging. | |
| 3.0 | 90.3 ± 0.03 f | 6 ± 0.07 c | 1.36 ± 0.06 e | Small and numerous bud spots, with no obvious micro-corm formation. | |
| 1 | 0.5 | 100 | 28.01 ± 0.05 a | 4.09 ± 0.09 a | Many bud spots, and there is obvious konjac formation below the bud spots |
| 1 | 1.0 | 100 | 6.00 ± 0.21 c | 2.78 ± 0.15 c | Few bud spots, but the Amorphophallus are larger and more pronounced. |
| 1 | 1.5 | 100 | 16.53 ± 0.16 b | 3.32 ± 0.14 b | Numerous bud spots, but no obvious formation of konjac. |
| Experiment Number | IBA A | NAA B | Inorganic Salt C | Root Length (cm) | Number of Roots (nuo) |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 21.21 | 20.17 |
| 2 | 1 | 2 | 2 | 15.32 | 15.54 |
| 3 | 1 | 3 | 3 | 6.35 | 10.75 |
| 4 | 2 | 1 | 3 | 8.52 | 18.01 |
| 5 | 2 | 2 | 1 | 24.19 | 16.45 |
| 6 | 2 | 3 | 2 | 17.28 | 12.38 |
| 7 | 3 | 1 | 2 | 13.74 | 14.83 |
| 8 | 3 | 2 | 3 | 4.63 | 8.96 |
| 9 | 3 | 3 | 1 | 20.78 | 9.15 |
| Root length | |||||
| K1 | 42.88 | 43.47 | 66.18 | ||
| K2 | 49.99 | 44.14 | 46.32 | ||
| K3 | 32.94 | 44.41 | 19.50 | ||
| R | 3.61 | 0.31 | 15.56 | ||
| Primary and secondary factors | C > A > B | ||||
| Optimal combination | A2B3C1 | ||||
| Number of roots | |||||
| K1 | 46.46 | 53.01 | 45.77 | ||
| K2 | 46.84 | 46.82 | 42.75 | ||
| K3 | 42.94 | 32.38 | 37.72 | ||
| R | 1.3 | 6.88 | 2.68 | ||
| Primary and secondary factors | B > C > A | ||||
| Optimal combination | A2B1C1 | ||||
| Group | Source | Sum of Squares | df | Mean Square | F | Sig. | Significance |
|---|---|---|---|---|---|---|---|
| Root length | A | 15.906 | 2 | 7.953 | 35.461 | 0.118 | |
| B | 0.086 | 2 | 0.043 | 0.191 | 0.850 | ||
| C | 236.273 | 2 | 118.136 | 526.767 | 0.031 | * | |
| Error | 0.224 | 1 | 0.224 | ||||
| Total | 1891.458 | 8 | |||||
| Total corrected | 344.666 | 7 | |||||
| Number of roots | A | 41.794 | 2 | 20.897 | 176.147 | 0.006 | ** |
| B | 72.261 | 2 | 36.130 | 304.554 | 0.003 | ** | |
| C | 11.025 | 2 | 5.512 | 46.466 | 0.021 | * | |
| Error | 0.237 | 2 | 0.119 | ||||
| Total | 1896.043 | 9 | |||||
| Total corrected | 125.317 | 8 |
| Experiment Number | 6-BA A | NAA B | KT C | Fresh Weight (g) | Number of Micro-Corm (nuo) |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 7.31 | 7.23 |
| 2 | 1 | 2 | 2 | 6.28 | 8.79 |
| 3 | 1 | 3 | 3 | 9.52 | 7.09 |
| 4 | 2 | 1 | 3 | 8.67 | 12.41 |
| 5 | 2 | 2 | 1 | 7.90 | 10.53 |
| 6 | 2 | 3 | 2 | 10.54 | 15.87 |
| 7 | 3 | 1 | 2 | 9.32 | 19.90 |
| 8 | 3 | 2 | 3 | 9.65 | 18.16 |
| 9 | 3 | 3 | 1 | 11.42 | 19.66 |
| Fresh weight | |||||
| K1 | 23.11 | 25.30 | 26.63 | ||
| K2 | 27.11 | 23.83 | 26.14 | ||
| K3 | 30.39 | 31.48 | 27.84 | ||
| R | 2.43 | 2.55 | 0.57 | ||
| Primary and secondary factors | B > A > C | ||||
| Optimal combination | A3B3C3 | ||||
| Number of micro-corms | |||||
| K1 | 23.11 | 39.54 | 37.42 | ||
| K2 | 38.81 | 37.48 | 44.56 | ||
| K3 | 57.72 | 42.62 | 37.66 | ||
| R | 11.53 | 1.71 | 2.38 | ||
| Primary and secondary factors | A > C > B | ||||
| Optimal combination | A3B3C2 | ||||
| Group | Source | Sum of Squares | df | Mean Square | F | Sig. | Significance |
|---|---|---|---|---|---|---|---|
| Fresh weight | A | 10.986 | 2 | 5.493 | 49.651 | 0.020 | * |
| B | 8.862 | 2 | 4.431 | 40.051 | 0.024 | * | |
| C | 0.510 | 2 | 0.255 | 2.307 | 0.302 | ||
| Error | 0.221 | 2 | 0.111 | ||||
| Total | 742.577 | 9 | |||||
| Total corrected | 20.580 | 8 | |||||
| Number of micro-corms | A | 4.461 | 2 | 2.231 | 1.587 | 0.387 | |
| B | 200.214 | 2 | 100.107 | 71.214 | 0.014 | * | |
| C | 10.961 | 2 | 5.480 | 3.899 | 0.204 | ||
| Error | 2.811 | 2 | 1.406 | ||||
| Total | 1808.862 | 9 | |||||
| Total corrected | 218.448 | 8 |
| Experiment Number | NH4NO3 A | KNO3 B | KH2PO4 C | Increase in Fresh Weight (g) |
|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 8.34 |
| 2 | 1 | 2 | 2 | 7.39 |
| 3 | 1 | 3 | 3 | 6.54 |
| 4 | 2 | 1 | 3 | 10.32 |
| 5 | 2 | 2 | 1 | 11.10 |
| 6 | 2 | 3 | 2 | 7.09 |
| 7 | 3 | 1 | 2 | 6.13 |
| 8 | 3 | 2 | 3 | 9.21 |
| 9 | 3 | 3 | 1 | 6.64 |
| Increase in fresh weight | ||||
| K1 | 22.27 | 24.79 | 26.08 | |
| K2 | 28.51 | 27.70 | 20.61 | |
| K3 | 21.98 | 20.27 | 26.07 | |
| R | 2.18 | 2.48 | 1.82 | |
| Primary and secondary factors | B > A > C | |||
| Optimal combination | A2B2C1 | |||
| Source | Sum of Squares | df | Mean Square | F | Sig. | Significance |
|---|---|---|---|---|---|---|
| A | 9.074 | 2 | 4.537 | 69.358 | 0.014 | * |
| B | 9.345 | 2 | 4.672 | 71.431 | 0.014 | * |
| C | 6.637 | 2 | 3.318 | 50.733 | 0.019 | * |
| Error | 0.131 | 2 | 0.065 | |||
| Total | 613.410 | 9 | ||||
| Total corrected | 25.186 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Sun, X.; Pan, Y.; Liu, C.; Su, L.; Zhang, Z. Bioreactor-Based Liquid Culture and Production of Konjac Micro-Corm. Horticulturae 2025, 11, 235. https://doi.org/10.3390/horticulturae11030235
Sun Y, Sun X, Pan Y, Liu C, Su L, Zhang Z. Bioreactor-Based Liquid Culture and Production of Konjac Micro-Corm. Horticulturae. 2025; 11(3):235. https://doi.org/10.3390/horticulturae11030235
Chicago/Turabian StyleSun, Yuqi, Xian Sun, Yufan Pan, Changbin Liu, Lingye Su, and Zongshen Zhang. 2025. "Bioreactor-Based Liquid Culture and Production of Konjac Micro-Corm" Horticulturae 11, no. 3: 235. https://doi.org/10.3390/horticulturae11030235
APA StyleSun, Y., Sun, X., Pan, Y., Liu, C., Su, L., & Zhang, Z. (2025). Bioreactor-Based Liquid Culture and Production of Konjac Micro-Corm. Horticulturae, 11(3), 235. https://doi.org/10.3390/horticulturae11030235








