Aromatic and Nutritional Composition of Edible Flowers of Garden Garlic and Wild Leek
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.3. Volatile Profile Analysis
2.4. Data Analysis
3. Results
3.1. Nutritional Compositions, Minerals and Bioactive Components
3.2. Volatile Profile
4. Discussion
4.1. Nutritional Compositions
4.2. Bioactive Components and Minerals
4.3. Volatile Fraction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinakin, D.J.; Kumar, V.; Suri, S.; Sharma, R.; Kaushal, M. Nutraceutical potential of tree flowers: A comprehensive review on biochemical profile, health benefits, and utilization. Food Res. Int. 2020, 127, 108724. [Google Scholar] [CrossRef] [PubMed]
- Pinedo-Espinoza, J.M.; Gutiérrez-Tlahque, J.; Santiago-Saenz, Y.O.; Aguirre-Mancilla, C.L.; Reyes-Fuentes, M.; López-Palestina, C.U. Nutritional composition, bioactive compounds and antioxidant activity of wild edible flowers consumed in semiarid regions of Mexico. Plant Foods Hum. Nutr. 2020, 75, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Lara-Cortés, E.; Martín-Belloso, O.; Osorio-Díaz, P.; Barrera-Necha, L.L.; Sánchez-López, J.A.; Bautista-Baños, S. Antioxidant capacity nutritional and functional composition of edible dahlia flowers. Rev. Chapingo Ser. Hortic. 2014, 20, 101–116. [Google Scholar] [CrossRef]
- Kumari, P.; Bhargava, B. Phytochemicals from edible flowers: Opening a new arena for healthy lifestyle. J. Funct. Foods 2021, 78, 104375. [Google Scholar] [CrossRef]
- Kandylis, P. Phytochemicals and antioxidant properties of edible flowers. Appl. Sci. 2022, 12, 9937. [Google Scholar] [CrossRef]
- Guiné, R.P.; Florença, S.G.; Ferrão, A.C.; Bizjak, M.Č.; Vombergar, B.; Simoni, N.; Vieira, V. Factors affecting eating habits and knowledge of edible flowers in different countries. Open Agric. 2021, 6, 67–81. [Google Scholar] [CrossRef]
- Nicknezhad, S.; Hashemabadi, D.; Allahyari, M.S.; Marzban, S.; Hassen, T.B.; Surujlal, J. Sensorial analysis of factors influencing consumers’ perceptions toward the consumption of edible flowers in Iran. JAFR 2023, 12, 100580. [Google Scholar] [CrossRef]
- Mulík, S.; Hernández-Carrión, M.; Pacheco-Pantoja, S.E.; Aguilar-Ruiz, N.; Ozuna, C. Culinary uses of Mexican edible flowers: Recipe analysis. Int. J. Gastron. Food Sci. 2022, 28, 100539. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.; et al. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef]
- Lopez-Hortas, L.; Rodriguez, P.; Diaz-Reinoso, B.; Gaspar, M.C.; de Sousa, H.C.; Braga, M.E.; Dominguez, H. Supercritical fluid extraction as a suitable technology to recover bioactive compounds from flowers. J. Supercrit. Fluids 2022, 188, 105652. [Google Scholar] [CrossRef]
- Multisona, R.R.; Shirodkar, S.; Arnold, M.; Gramza-Michalowska, A. Clitoria ternatea flower and its bioactive compounds: Potential use as microencapsulated ingredient for functional foods. Appl. Sci. 2023, 13, 2134. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Barral, M.; Carpena, M.; Gullón, P.; Fraga-Corral, M.; Otero, P.; Prieto, M.A.; Simal-Gandara, J. Traditional plants from Asteraceae family as potential candidates for functional food industry. Food Funct. 2021, 12, 2850–2873. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.L.; Chen, S.G.; Xie, Y.Q.; Chen, F.; Zhao, Y.Y.; Luo, C.X.; Gao, Y.Q. Total phenolic, flavonoid and antioxidant activity of 23 edible flowers subjected to in vitro digestion. J. Funct. Foods 2015, 17, 243–259. [Google Scholar] [CrossRef]
- Pires, T.C.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.; Santos-Buelga, C.; Ferreira, I.C. Edible flowers as sources of phenolic compounds with bioactive potential. Food Res. Int. 2018, 105, 580–588. [Google Scholar] [CrossRef]
- Zhang, N.; He, Z.; He, S.; Jing, P. Insights into the importance of dietary chrysanthemum flower (Chrysanthemum morifolium cv. Hangju)-wolfberry (Lycium barbarum fruit) combination in antioxidant and anti-inflammatory properties. Food Res. Int. 2019, 116, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Chetia, I.; Vijayakumar, A.; Badwaik, L.S. Edible flowers’ flavor, safety and their utilization as functional ingredients: A review. J. Food Sci. Technol. 2025, 62, 11–23. [Google Scholar] [CrossRef]
- Garba, U.; Yunusa, A.K. Allium flowers. In Edible Flowers; Gupta, A.K., Kumar, V., Naik, B., Mishra, P., Eds.; Elsevier: New York, NY, USA, 2024; pp. 9–28. [Google Scholar] [CrossRef]
- Kubec, R.; Krejčová, P.; Mansur, L.; García, N. Flavor precursors and sensory-active sulfur compounds in alliaceae species native to South Africa and South America. J. Agric. Food Chem. 2013, 61, 1335–1342. [Google Scholar] [CrossRef]
- Dey, P.; Khaled, K.L. An extensive review on Allium ampeloprasum a magical herb. Int. J. Sci. Res. 2015, 4, 371–377. [Google Scholar]
- Pires, T.C.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C. Edible flowers: Emerging components in the diet. Trends Food Sci. Technol. 2019, 93, 244–258. [Google Scholar] [CrossRef]
- Lockowandt, L.; Pinela, J.; Roriz, C.L.; Pereira, C.; Abreu, R.M.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Bredol, M.; Ferreira, I.C. Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: The blue flowers and the unexplored non-edible part. Ind. Crops Prod. 2019, 128, 496–503. [Google Scholar] [CrossRef]
- Yang, P.F.; Yang, Y.N.; Feng, Z.M.; Jiang, J.S.; Zhang, P.C. Six new compounds from the flowers of Chrysanthemum morifolium and their biological activities. Bioorg. Chem. 2019, 82, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, C.; Zhou, J.; Zhou, F.; Gui, A.; Chu, H.; Shao, Q. Chrysanthemum morifolium as a traditional herb: A review of historical development, classification, phytochemistry, pharmacology and application. J. Ethnopharmacol. 2024, 330, 118198. [Google Scholar] [CrossRef]
- Antarkar, S.; Sharma, A.; Bhargava, A.; Gupta, H.; Tomar, R.; Srivastava, S. Physico-chemical and Nutritional Evaluation of Cookies with Different Levels of Rosehip and Hibiscus Powder Substitution. Arch. Curr. Res. Int. 2019, 17, 1–10. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A. Anti-hyperglycemic and anticholinergic effects of natural antioxidant contents in edible flowers. Antioxidants 2019, 8, 308. [Google Scholar] [CrossRef]
- Fernandes, L.; Ramalhosa, E.; Baptista, P.; Pereira, J.A.; Saraiva, J.A.; Casal, S.I. Nutritional and nutraceutical composition of pansies (Viola × wittrockiana) during flowering. J. Food Sci. 2019, 84, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zhang, M.; Ju, R.; Wang, Y.; Chitrakar, B.; Wang, B. Effect of different drying methods on the quality of restructured rose flower (Rosa rugosa) chips. Drying Technol. 2020, 38, 1632–1643. [Google Scholar] [CrossRef]
- Marchioni, I.; Gabriele, M.; Carmassi, G.; Ruffoni, B.; Pistelli, L.; Pistelli, L.; Najar, B. Phytochemical, Nutritional and Mineral Content of Four Edible Flowers. Foods 2024, 13, 939. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Official Analytical Chemists, 18th ed.; Association of Official Analytical Chemist (AOAC International): Washington, DC, USA, 2005. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Arnous, A.; Makris, D.P.; Kefalas, P. Correlation of pigment and flavanol content with antioxidant properties in selected aged regional wines from Greece. J. Food Compos. Anal. 2002, 15, 655–665. [Google Scholar] [CrossRef]
- Moreno, E.; Fita, A.; González-Mas, M.C.; Rodríguez-Burruezo, A. HS-SPME study of the volatile fraction of Capsicum accessions and hybrids in different parts of the fruit. Sci. Hortic. 2012, 135, 87–97. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; Alamar, M.C.; Gutiérrez, A.; Granell, A. Comparative analysis of the volatile fraction of fruit juice from different Citrus species. PLoS ONE 2011, 6, e22016. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.; Andrade, C.; Aguiar, T.; Cavestré, R.; Guimaraes, M.; Cardoso, W. Postharvest of Edible Flowers. Pesqui. Agropecu. Bras. 2022, 57, e02953. [Google Scholar] [CrossRef]
- TRAXCO. Cultivo de Flores Comestibles. Available online: https://www.traxco.es/blog/produccion-agricola/cultivar-flores-comestibles (accessed on 29 January 2025).
- Rop, O.; Mlcek, J.; Jurikova, T.; Neugebauerova, J. Edible Flowers-A new Promising source of Mineral Elements in Human Nutrition. Molecules 2012, 17, 6672–6683. [Google Scholar] [CrossRef]
- Grzeszczuk, M.; Wesołowska, A.; Jadczak, D.; Jakubowska, B. Nutritional value of chive edible flowers. Acta Sci. Pol. Hortorum Cultus 2011, 10, 85–94. [Google Scholar]
- Fomina, T.I.; Kukushkina, T.A. Edible Flowers of Onions (Allium L.) as a Source of Biologically Active Substances. Russ. J. Org. Chem. 2022, 48, 1405–1410. [Google Scholar] [CrossRef]
- Haytowitz, D.B.; Ahuja, J.K.; Wu, X.; Somanchi, M.; Nickle, M.; Nguyen, Q.A.; Roseland, J.M.; Williams, J.R.; Patterson, K.Y.; Li, Y.; et al. USDA National Nutrient Database for Standard Reference, Legacy Release; Nutrient Data Laboratory, Beltsville Human Nutrition Research Center, ARS, USDA: Beltsville, MD, USA, 2019. [CrossRef]
- Lara-Cortés, E.; Osorio-Díaz, P.; Jiménez-Aparicio, A.; Bautista-Baños, S. Contenido nutricional, propiedades funcionales y conservación de flores comestibles. Revisión. Arch. Latinoam. Nutr. 2013, 63, 197–208. [Google Scholar]
- Sotelo, A.; López-García, S.; Basurto-Peña, F. Content of nutrient and antinutrient in edible flowers of wild plants in Mexico. Plant Foods Hum. Nutr. 2007, 62, 133–138. [Google Scholar] [CrossRef]
- González-Barrio, R.; Periago, M.J.; Luna-Recio, C.; Garcia-Alonso, F.J.; Navarro-González, I. Chemical composition of the edible flowers, pansy (Viola wittrockiana) and snapdragon (Antirrhinum majus) as new sources of bioactive compounds. Food Chem. 2018, 252, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak, A.; Grezeszczuk, M.E. Nutritional and biological value of five edible flower species. Not. Bot. Horti. Agrobo. 2019, 47, 128–134. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Rezende, F.A.G.G.; Moura, M.A.F.; Dominguete, L.C.B.; Sande, D. Edible flowers: Bioactive profile and its potential to be used in food development. Food Res. Int. 2020, 129, 108868. [Google Scholar] [CrossRef]
- Chensom, S.; Okumura, H.; Mishima, T. Primary screening of antioxidant activity, total polyphenol content, carotenoid content, and nutritional composition of 13 edible flowers from Japan. Prev. Nutr. Food Sci. 2019, 24, 171. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Spence, C.; Levitan, C.A. Exploring the links between colours and tastes/flavours. J. Percept. Imaging. 2022, 5, 1–16. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Koprowska, K.; Gottschling, A.; Janda-Milczarek, K. Edible Flowers as a Source of Dietary Fibre (Total, Insoluble and Soluble) as a Potential Athlete’s Dietary Supplement. Nutrients 2022, 14, 2470. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Carvalho, A.M.; Ferreira, I.C. Studies on chemical constituents and bioactivity of Rosa micrantha: An alternative antioxidants source for food, pharmaceutical, or cosmetic applications. J. Agric. Food Chem. 2010, 58, 6277–6284. [Google Scholar] [CrossRef] [PubMed]
- Mlcek, J.; Rop, O. Fresh edible flowers of ornamental plants—A new source of nutraceutical foods. Trends Food Sci. Technol. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Grzeszczuk, M.; Stefaniak, A.; Pachlowska, A. Biological value of various edible flower species. Acta Sci. Pol. Hortoru. 2016, 15, 109–119. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, C.; Chen, K.; Li, X. Flavonoids, phenolics, and antioxidant capacity in the flower of Eriobotrya japonica Lindl. Int. J. Mol. Sci. 2011, 12, 2935–2945. [Google Scholar] [CrossRef]
- Rivas-García, L.; Navarro-Hortal, M.D.; Romero-Márquez, J.M.; Forbes-Hernández, T.Y.; Varela-López, A.; Llopis, J.; Sánchez-González, C.; Quiles, J.L. Edible flowers as a health promoter: An evidence-based review. Trends Food Sci. Technol. 2021, 117, 46–59. [Google Scholar] [CrossRef]
- Janarny, G.; Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S. Nutraceutical potential of dietary phytochemicals in edible flowers—A review. J. Food Biochem. 2021, 45, e13642. [Google Scholar] [CrossRef]
- Wen, H.; Li, S.; Wei, Y.; Dong, J.; Liang, Z.; Guo, L.; He, H.; Zhang, Y. Yunnan edible flowers and their potential in future foods: Focus on ethnological applications, chemical and pharmacological research. J. Future Foods 2025, 5, 119–133. [Google Scholar] [CrossRef]
- Paiva, L.; Rego, C.; Lima, E.; Marcone, M.; Baptista, J. Comparative analysis of the polyphenols, caffeine, and antioxidant activities of green tea, white tea, and flowers from Azorean Camellia sinensis varieties affected by different harvested and processing conditions. Antioxidants 2021, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Kołton, A.; Długosz-Grochowska, O.; Wojciechowska, R.; Czaja, M. Biosynthesis Regulation of Folates and Phenols in Plants. Sci. Hortic. 2022, 291, 110561. [Google Scholar] [CrossRef]
- Frías-Moreno, M.N.; Parra-Quezada, R.A.; González-Aguilar, G.; Ruíz-Canizales, J.; Molina-Corral, F.J.; Sepulveda, D.R.; Salas-Salazar, N.; Olivas, G.I. Quality, bioactive compounds, antioxidant capacity, and enzymes of raspberries at different maturity stages, effects of organic vs. Conventional fertilization. Foods 2021, 10, 953. [Google Scholar] [CrossRef]
- Chen, Z.; Świsłocka, R.; Choińska, R.; Marszałek, K.; Dąbrowska, A.; Lewandowski, W.; Lewandowska, H. Exploring the correlation between the molecular structure and biological activities of metal–phenolic compound complexes: Research and description of the role of metal ions in improving the antioxidant activities of phenolic compounds. Int. J. Mol. Sci. 2024, 25, 11775. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Deng, M.; Lv, Z.; Peng, Y. Evaluation of antioxidant activities of extracts from 19 Chinese edible flowers. SpringerPlus 2014, 3, 315. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent developments in effective antioxidants: The structure and antioxidant properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef]
- García-Pérez, M.E.; Niokhor, D.P.; Stevanovic, T. Comparative study of antioxidant capacity of yellow birch twigs extracts at ambient and high temperatures. Food chem. 2008, 107, 344–351. [Google Scholar] [CrossRef]
- Tariq, A.; Zeng, F.; Graciano, C.; Ullah, A.; Sadia, S.; Ahmed, Z.; Murtaza, G.; Zhang, Z. Regulation of metabolites by nutrients in plants. In Plant Ionomics: Sensing, Signaling, and Regulation; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2023; pp. 1–18. [Google Scholar] [CrossRef]
- Voon, H.C.; Rajeev, B.; Karim, A.A.; Rosma, A. Composition of tree peony (Paeonia suffruticosa) and Chinese apple flower (Malus spp.) buds. Int. Food Res. J. 2013, 20, 1173–1179. [Google Scholar]
- Navarro-González, I.; González-Barrio, R.; García-Valverde, V.; Bautista-Ortín, A.B.; Periago, M.J. Nutritional composition and antioxidant capacity in edible flowers: Characterisation of phenolic compounds by HPLC-DAD-ESI/MSn. Int. J. Mol. Sci. 2015, 16, 805–822. [Google Scholar] [CrossRef]
- Kara, D. Evaluation of trace metal concentrations in some herbs and herbal teas by principal component analysis. Food Chem. 2009, 114, 347–354. [Google Scholar] [CrossRef]
- Rafiee, H.; Mehrafarin, A.; Labbafi, M.; Qaderi, A.; Badi, H.N. Mineral elements and biochemical analysis of Calendula officinalis L. affected by bio-stimulators. Trakia J. Sci. 2015, 13, 27–35. [Google Scholar] [CrossRef]
- Ražić, S.; Dogo, S.; Slavković, L.; Popović, A. Inorganic analysis of herbal drugs. Part I. Metal determination in herbal drugs originating from medicinal plants of the family Lamiacae. J. Serb. Chem. Soc. 2005, 70, 1347–1355. [Google Scholar] [CrossRef]
- Özcan, M.M.; Akbulut, M. Estimation of minerals, nitrate and nitrite contents of medicinal and aromatic plants used as spices, condiments and herbal tea. Food Chem. 2007, 106, 852–858. [Google Scholar] [CrossRef]
- Bulduk, I. Determination of trace element levels in flowers and leaves of vicia faba by ICP-MS. Prog. Chem. Biochem. Res. 2020, 3, 221–228. [Google Scholar]
- Grzeszczuk, M.; Stefaniak, A.; Meller, E.; Wysocka, G. Mineral composition of some edible flowers. J. Elementol. 2018, 23, 151–162. [Google Scholar] [CrossRef]
- Araújo, S.; Matos, C.; Correia, E.; Antunes, M.C. Evaluation of phytochemicals content, antioxidant activity and mineral composition of selected edible flowers. Qual. Assur. Saf. Crops Foods 2019, 11, 471–478. [Google Scholar] [CrossRef]
- Spanish Food Composition Database. Available online: https://www.bedca.net/bdpub (accessed on 29 January 2025).
- Schade, F.; Legge, R.L.; Thompson, J.E. Fragrance volatiles of developing and senescing carnation flowers. Phytochemistry 2001, 56, 703–710. [Google Scholar] [CrossRef]
- Usami, A.; Kashima, Y.; Marumoto, S.; Miyazawa, M. Characterization of aroma-active compounds in dry flower of Malva sylvestris L. by GC-MS-O analysis and OAV calculations. J. Oleo Sci. 2013, 62, 563–570. [Google Scholar] [CrossRef]
- Ge, L.; Lin, B.; Mo, J.; Chen, Q.; Su, L.; Li, Y.; Yang, K. Composition and antioxidant and antibacterial activities of essential oils from three yellow Camellia species. Trees 2019, 33, 205–212. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, B.; Huang, W.; Amrouche, A.T.; Maurizio, B.; Simal-Gandara, J.; Tundis, R.; Jianbo, X.; Zou, L.; Lu, B. Edible flowers as functional raw materials: A review on anti-aging properties. Trends Food Sci. Technol. 2020, 106, 30–47. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Malheiro, R.; Rodrigues, N.; Saraiva, J.A.; Ramalhosa, E. Borage, calendula, cosmos, Johnny Jump up, and pansy flowers: Volatiles, bioactive compounds, and sensory perception. Eur. Food. Res. Technol. 2019, 245, 593–606. [Google Scholar] [CrossRef]
- Zhang, S.S.; Guo, S.; Zheng, Z.J.; Liu, S.J.; Hou, Y.F.; Ho, C.T.; Bai, N.S. Characterization of volatiles in Allium tenuissimum L. flower by headspace-gas chromatography-olfactometry-mass spectrometry, odor activity values, and the omission and recombination experiments. LWT 2021, 151, 112144. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Ding, D.; Su, J.; Zhao, Z. Volatile profiles of Allium tenuissimum L. flower fried by four different oils, using SPME–GC–MS, and sensory evaluation coupled with partial least squares regression. J. Food Compos. Anal. 2022, 109, 104461. [Google Scholar] [CrossRef]
- Marchioni, I.; Najar, B.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Bioactive compounds and aroma profile of some Lamiaceae edible flowers. Plants 2020, 9, 691. [Google Scholar] [CrossRef]
- Abbas, F.; Zhou, Y.; O’Neill Rothenberg, D.; Alam, I.; Ke, Y.; Wang, H.C. Aroma components in horticultural crops: Chemical diversity and usage of metabolic engineering for industrial applications. Plants 2023, 12, 1748. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Ishak, I.; Harith, N.M.; Kuan, G.L.P. Comparison of bioactive compounds and sensory evaluation on edible flowers tea infusion. Ital. J. Food Sci. 2019, 31, 264–273. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, C.; Cheng, B.; Wan, H.; Luo, L.; Pan, H.; Zhang, Q. Volatile compound analysis and aroma evaluation of tea-scented roses in China. Ind. Crops Prod. 2020, 155, 112735. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; López-Cañavate, M.E.; Guirao-Martínez, J.; Roca, M.J.; Aguayo, E. Aloe vera Flowers, a Byproduct with Great Potential and Wide Application, Depending on Maturity Stage. Foods 2020, 9, 1542. [Google Scholar] [CrossRef]
- Fraternale, D.; Dufat, H.; Albertini, M.C.; Bouzidi, C.; D’Adderio, R.; Coppari, S.; Barbara, D.G.; Melandri, D.; Ramakrishna, S.; Colomba, M. Chemical composition, antioxidant and anti-inflammatory properties of Monarda didyma L. essential oil. PeerJ 2022, 10, e14433. [Google Scholar] [CrossRef]
- Ivanova, A.; Mikhova, B.; Najdenski, H.; Tsvetkova, I.; Kostova, I. Chemical composition and antimicrobial activity of wild garlic Allium ursinum of Bulgarian origin. Nat. Prod. Commun. 2009, 4, 1934578X0900400808. [Google Scholar] [CrossRef]
- Snoussi, M.; Trabelsi, N.; Dehmeni, A.; Benzekri, R.; Bouslama, L.; Hajlaoui, B.; Al-sieni, A.; Papetti, A. Phytochemical analysis, antimicrobial and antioxidant activities of Allium roseum var. odoratissimum (Desf.) Coss extracts. Ind. Crops Prod. 2016, 89, 533–542. [Google Scholar] [CrossRef]
- Emir, A.; Emir, C.; Yıldırım, H. Chemical and biological comparison of different parts of two Allium species: Allium paniculatum L. subsp. villosulum (Hal.) Stearn and Allium paniculatum L. subsp. paniculatum L. Chem. Pap. 2021, 75, 411–419. [Google Scholar] [CrossRef]

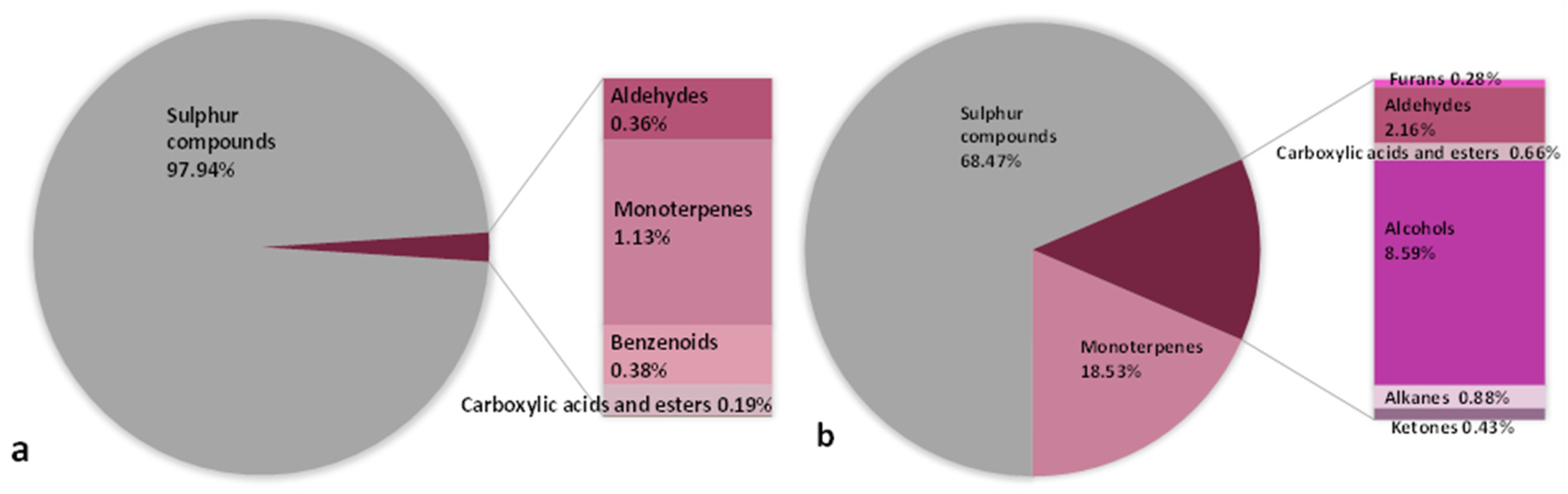

| Parameter | Garden Garlic Flowers | Wild Leek Flowers | |||
|---|---|---|---|---|---|
| Mean ± SD | CV (%) | Mean ± SD | CV (%) | p-Value | |
| Nutritional value (g 100 g−1 dw) | |||||
| Moisture | 82.192 a ± 0.697 | 0.848 | 77.317 b ± 0.067 | 0.087 | 0.0003 |
| Dry matter | 17.808 b ± 0.697 | 3.913 | 22.683 a ± 0.067 | 0.297 | 0.0003 |
| Ash | 6.607 a ± 0.161 | 2.441 | 3.056 b ± 0.433 | 14.181 | 0.0002 |
| Crude protein | 10.915 b ± 0.476 | 4.366 | 19.280 a ± 0.703 | 3.646 | 0.0001 |
| Fat | 1.398 a ± 0.170 | 12.156 | 1.773 a ± 0.201 | 11.322 | 0.0688 |
| Crude fiber | 16.237 a ± 0.609 | 3.753 | 10.058 b± 0.804 | 7.990 | 0.0004 |
| Carbohydrates | 64.843 a ± 1.263 | 1.948 | 65.833 a ± 1.626 | 2.470 | 0.4518 |
| Energy value (kcal 100 g−1) | 348.084 b ± 1.030 | 0.296 | 376.522 a ± 3.298 | 0.876 | 0.0001 |
| Bioactive components | |||||
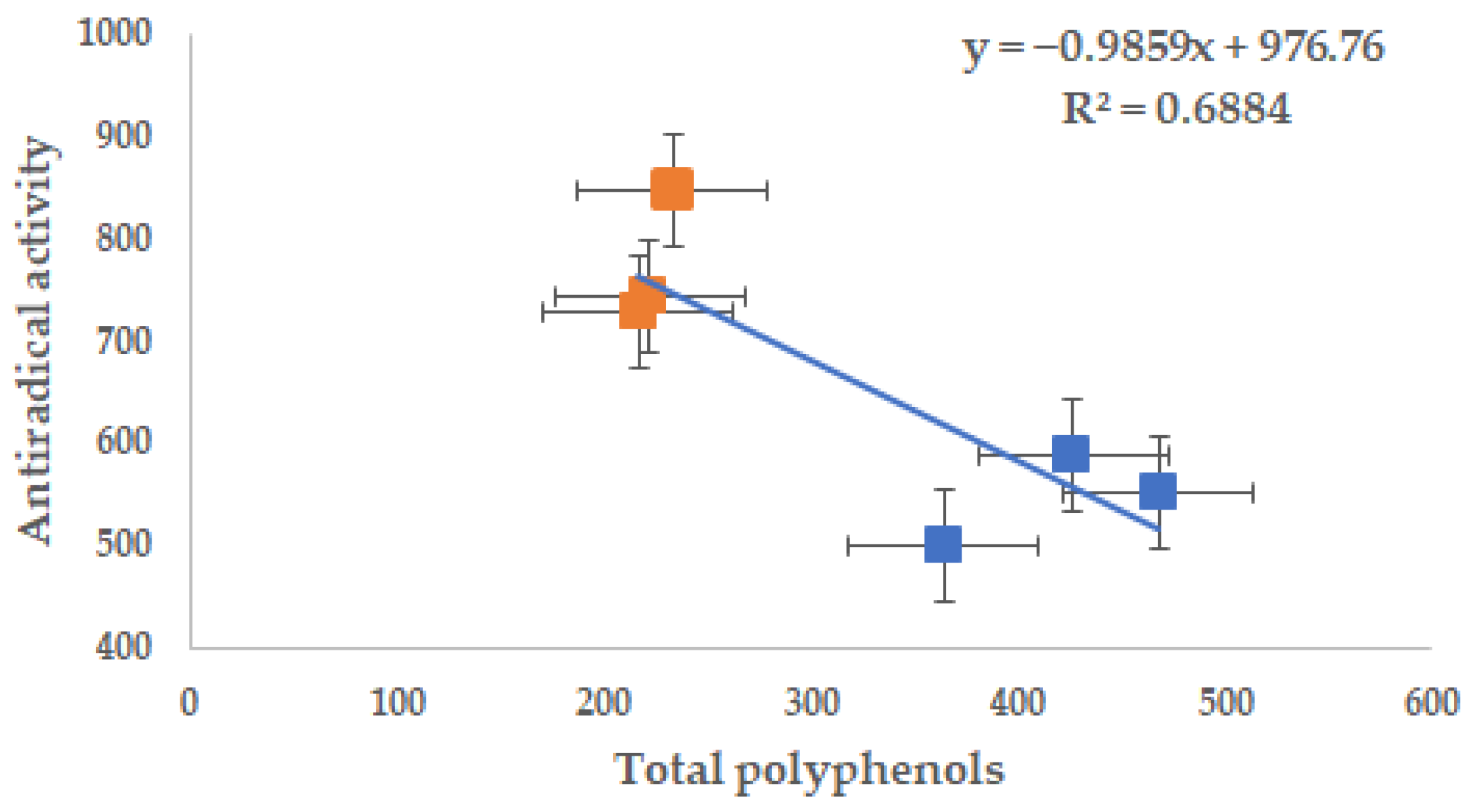
| Total polyphenols (mg EAG·100 g−1 dw) | 1258.400 b ± 8.547 | 0.679 | 1850.320 a ± 225.664 | 12.196 | 0.0105 |
| Antiradical activity by DPPH assay (μmol TE·100 g−1 dw) | 4333.86 a ± 184.327 | 4.253 | 2407.22 b ± 194.04 | 8.061 | 0.0002 |
| Macrominerals (mg 100 g−1 dw) | |||||
| K | 330.004 a ± 49.876 | 15.114 | 370.815 a ± 22.743 | 6.133 | 0.2667 |
| P | 327.733 b ± 9.135 | 2.787 | 305.904 a ± 5.725 | 1.871 | 0.0247 |
| Ca | 590.033 a ± 84.538 | 14.328 | 305.663 b ± 7.051 | 2.307 | 0.0044 |
| Mg | 157.246 a ± 23.442 | 14.908 | 156.545 a ± 3.424 | 2.187 | 0.9616 |
| Na | 53.043 a ± 7.042 | 13.276 | 25.143 b ± 4.894 | 19.466 | 0.0049 |
| Microminerals (mg 100 g−1 dw) | |||||
| Fe | 32.786 a ± 5.731 | 17.479 | 3.396 b ± 0.432 | 12.738 | 0.0009 |
| Cu | 0.773 a ± 0.143 | 18.531 | 0.413 b ± 0.024 | 5.815 | 0.0127 |
| Zn | 1.952 b ± 0.346 | 17.741 | 2.895 a ± 0.035 | 1.226 | 0.0094 |
| Mn | 1.245 a ± 0.208 | 16.685 | 0.753 b ± 0.039 | 5.144 | 0.0157 |
| B | 1.853 a ± 0.319 | 17.224 | 1.542 a ± 0.041 | 2.673 | 0.1699 |
| Cr | 0.061 a ± 0.012 | 20.651 | 0.023 b ± 0.006 | 25.082 | 0.0096 |
| Mo | 0.069 a ± 0.019 | 27.964 | 0.011 b ± 0.002 | 19.735 | 0.0066 |
| Se | 0.0099 | - | 0.000 | - | - |
| Heavy metals (mg 100 g−1 dw) | |||||
| Cd | 0.009 b ± 0.001 | 15.629 | 0.025 a ± 0.001 | 6.073 | 0.0002 |
| Pb | 0.215 a ± 0.034 | 15.704 | 0.133 b ± 0.018 | 13.701 | 0.0208 |
| Hg | 0.0030 | - | 0.000 | - | - |
| Chemical Family | Volatile Compound | RI | IM * | Aroma | Flowers ** | p-Value | |
|---|---|---|---|---|---|---|---|
| Garden Garlic | Wild Leek | ||||||
| Alcohols | (Z)-3-hexen-1-ol | 857 | RS | powerful, fresh, green grass | not detected | 116.199 ± 45.975 | |
| 1-hexanol | 868 | RS | freshly cut grass | not detected | 20.430 ± 9.981 | ||
| Aldehydes | hexanal | 800 | RS | green, fresh, fatty, fruity | not detected | 4.381 ± 1.726 | |
| nonanal | 1104 | RS | waxy, rose | 7.476 b ± 1.265 | 25.984 a ± 6.048 | 0.0133 | |
| decanal | 1206 | RS | sweet, waxy | 10.801 a ± 1.534 | 3.930 b ± 1.266 | 0.0081 | |
| Benzenoids | benzaldehyde | 893 | MS | almond | 17.779 ± 5.078 | not detected | |
| benzyl alcohol | 1036 | MS | green musty | 3.607 | traces | ||
| Carboxylic acids and esters | nonanoic acid | 1273 | MS | dairy products, fatty | not detected | 7.016 ± 5.942 | |
| (Z)-3-hexenyl acetate | 1005 | MS | green fruity, sweet | 9.721 a ± 3.527 | 5.331 a ± 0.940 | 0.2750 | |
| Furans | 2-pentylfuran | 993 | RS | fruity, green, earthy | not detected | 6.890 ± 3.490 | |
| Ketones | 3-pentanone | 688 | MS | acetone-like | not detected | 10.302 ± 0.227 | |
| Monoterpenes | p-cymene | 1025 | MS | sweet, soft, fresh, lemon, | not detected | 4.503 ± 0.692 | |
| limonene | 1030 | RS | citrus, herbal, floral | 6.126 b ± 1.218 | 83.278 a ± 27.126 | 0.0159 | |
| (E)-β-ocimene | 1049 | RS | herbal | 5.499 a ± 2.248 | 14.689 a ± 13.704 | 0.4023 | |
| (Z)-β-ocimene | 1038 | RS | floral, sweet, herbal, warm | 45.208 a ± 8.541 | 192.146 a ± 176.494 | 0.3048 | |
| Alkanes | tridecane | 1300 | MS | green | not detected | 1.696 | |
| tetradecane | 1400 | MS | mild, waxy | not detected | 13.418 ± 3.999 | ||
| Sulphur compounds | dimethyl disulfide | 746 | MS | garlic | 184.875 ± 68.515 | not detected | |
| allyl methyl disulfide | 920 | MS | pungent, garlic-like | 1318.892 ± 163.894 | not detected | ||
| methyl propyl disulfide | 932 | MS | onion, garlic | 231.565 a ± 163.894 | 37.246 b ± 20.600 | 0.0042 | |
| dimethyl trisulfide | 970 | MS | cabbage | 54.580 ± 7.514 | not detected | ||
| diallyl disulfide | 1081 | RS | garlic | 2563.305 ± 649.833 | not detected | ||
| dipropyl disulfide | 1107 | RS | burnt onion, green onion | 208.128 a ± 75.650 | 730.151 a ± 408.425 | 0.1502 | |
| allyl methyl trisulfide | 1142 | MS | garlic | 305.459 ± 28.413 | not detected | ||
| methyl propyl trisulfide | 1150 | MS | warm, herbaceous, onion | 56.361 a ± 3.983 | 54.502 a ± 43.337 | 0.9547 | |
| dipropyl trisulfide | 1328 | MS | garlic-like | not detected | 260.442 ± 21.481 | ||
| diallyl tetrasulfide | 1532 | MS | garlic, onion, green, metallic | 19.945 ± 2.882 | not detected | ||
| dipropyl tetrasulfide | 1570 | MS | pungent sulfur-like, onion | not detected | 6.489 ± 2.829 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambrano Núñez, T.M.; Morales Noriega, A.M.; García-Martínez, M.D.; Raigón Jiménez, M.D. Aromatic and Nutritional Composition of Edible Flowers of Garden Garlic and Wild Leek. Horticulturae 2025, 11, 323. https://doi.org/10.3390/horticulturae11030323
Zambrano Núñez TM, Morales Noriega AM, García-Martínez MD, Raigón Jiménez MD. Aromatic and Nutritional Composition of Edible Flowers of Garden Garlic and Wild Leek. Horticulturae. 2025; 11(3):323. https://doi.org/10.3390/horticulturae11030323
Chicago/Turabian StyleZambrano Núñez, Telmo Marcelo, Adriana Margarita Morales Noriega, María Dolores García-Martínez, and María Dolores Raigón Jiménez. 2025. "Aromatic and Nutritional Composition of Edible Flowers of Garden Garlic and Wild Leek" Horticulturae 11, no. 3: 323. https://doi.org/10.3390/horticulturae11030323
APA StyleZambrano Núñez, T. M., Morales Noriega, A. M., García-Martínez, M. D., & Raigón Jiménez, M. D. (2025). Aromatic and Nutritional Composition of Edible Flowers of Garden Garlic and Wild Leek. Horticulturae, 11(3), 323. https://doi.org/10.3390/horticulturae11030323








