Lentil Biorooting Agents: An Ecological Alternative to Improve the Growth and Development of Italian Zucchini in Sustainable Production Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Crop Establishment Protocol
2.2. Preparation of Natural Rooting and Application of Treatments
2.3. Agronomic Analysis
2.4. Statistical Analysis
3. Results
3.1. Impact of Treatments on Height, Number of Leaves and Aboveground Biomass
3.2. Effects of Different Treatments on Root Growth and Biomass
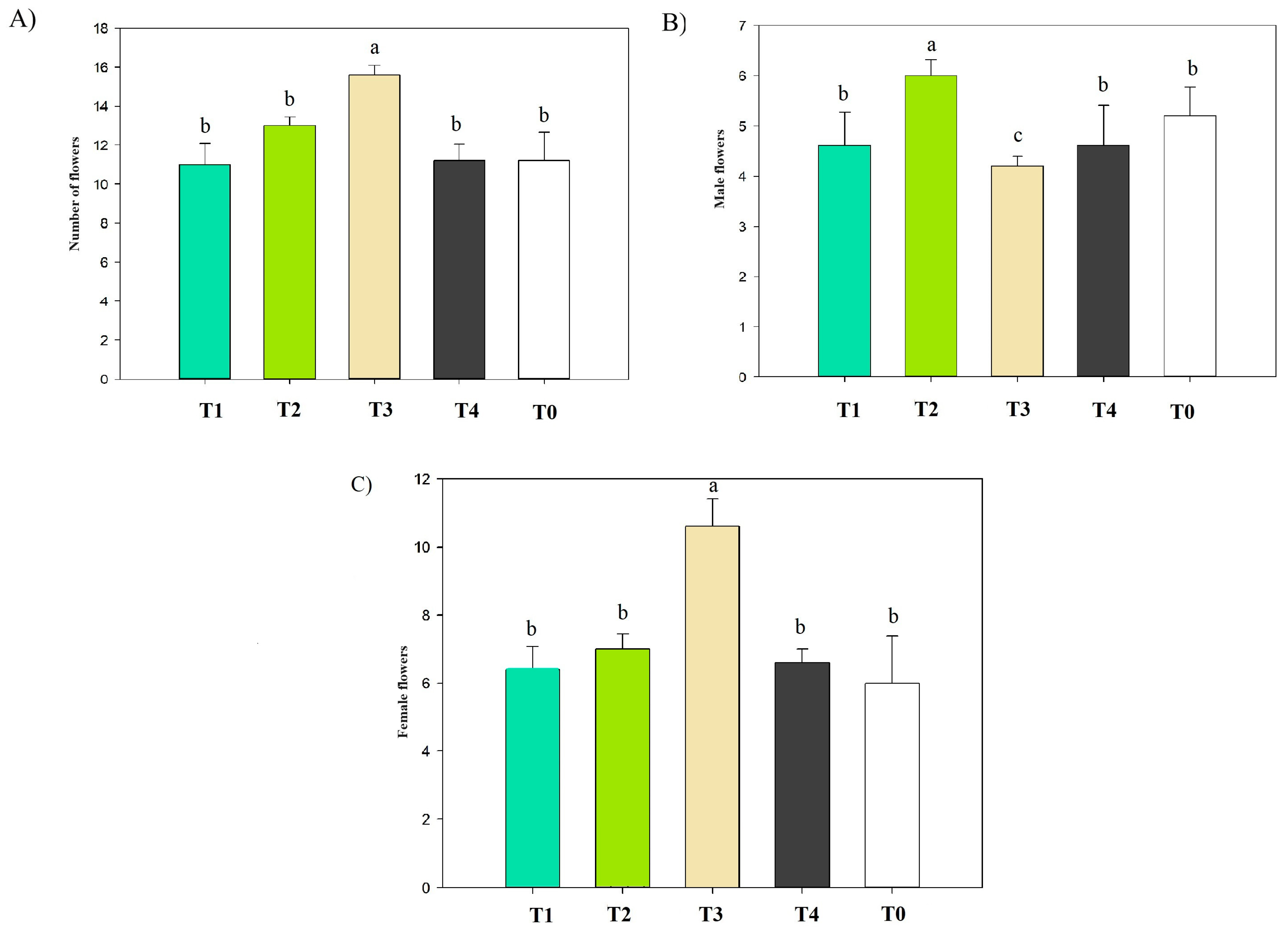
3.3. Effect of Treatments on Flower Production and Male and Female Flower Distributions
3.4. Correlations Between Growth and Development of Italian Zucchini Under Different Treatments
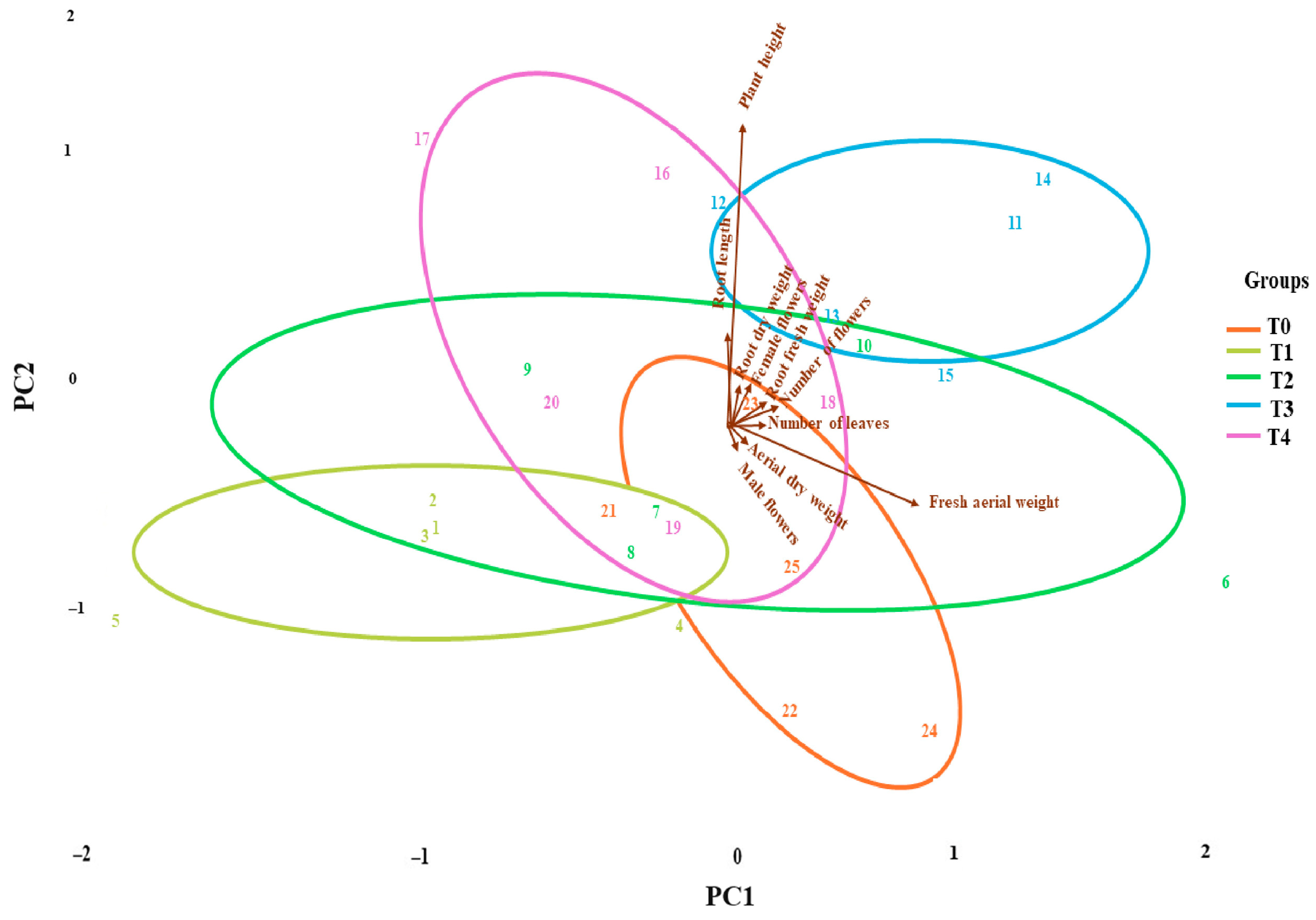
3.5. Treatment Differentiation Through Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Di Lorenzo, R.; Castaldo, L.; Sessa, R.; Ricci, L.; Vardaro, E.; Izzo, L.; Laneri, S. Chemical Profile and Promising Applications of Cucurbita pepo L. Flowers. Antioxidants 2024, 13, 1476. [Google Scholar] [CrossRef] [PubMed]
- Domblides, E.; Ermolaev, A.; Belov, S.; Kan, L.; Skaptsov, M.; Domblides, A. Efficient Methods for Evaluation on Ploidy Level of Cucurbita pepo L. Regenerant Plants Obtained in Unpollinated Ovule Culture In Vitro. Horticulturae 2022, 8, 1083. [Google Scholar] [CrossRef]
- Shehata, W.F.; Iqbal, Z.; Abdelbaset, T.E.; Saker, K.I.; El Shorbagy, A.E.; Soliman, A.M.; El-Ganainy, S.M. Identification of a Cucumber Mosaic Virus from Cucurbita pepo on New Reclamation Land in Egypt and the Changes Induced in Pumpkin Plants. Sustainability 2023, 15, 9751. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations, Statistics Division. Statistical Data on Crops, Tomatoes, World. 2025. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 5 March 2025).
- Rivera-Solís, L.L.; Benavides-Mendoza, A.; Robledo-Olivo, A.; González-Morales, S. La salud del suelo y el uso de bioestimulantes. Rev. Agrar. 2023, 20, 5–10. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Schmoll, M.; Esquivel-Ayala, B.A.; González-Esquivel, C.E.; Rocha-Ramírez, V.; Larsen, J. Mechanisms for plant growth promotion activated by Trichoderma in natural and managed terrestrial ecosystem. Microbiol. Res. 2024, 281, 127621. [Google Scholar] [CrossRef]
- Liu, M.; Chen, X.; Jiao, Y. Sustainable agriculture: Theories, methods, practices and policies. Agriculture 2024, 14, 473. [Google Scholar] [CrossRef]
- El Chami, D.; El Moujabber, M. Sustainable agriculture and climate resilience. Sustainability 2024, 16, 113. [Google Scholar] [CrossRef]
- Khan, M.T.; Aleinikovienė, J.; Butkevičienė, L.M. Innovative organic fertilizers and cover crops: Perspectives for sustainable agriculture in the Era of climate change and organic agriculture. Agronomy 2024, 14, 2871. [Google Scholar] [CrossRef]
- Gaeta, L.; Tarricone, L.; Persiani, A.; Fiore, A.; Montemurro, F.; De Benedetto, D.; Diacono, M. Sustainable Fertilization of Organic Sweet Cherry to Improve Physiology, Quality, Yield, and Soil Properties. Agronomy 2025, 15, 135. [Google Scholar] [CrossRef]
- Ahmad, N.; Jiang, Z.; Zhang, L.; Hussain, I.; Yang, X. Insights on phytohormonal crosstalk in plant response to nitrogen stress: A focus on plant root growth and development. Int. J. Mol. Sci. 2023, 24, 3631. [Google Scholar] [CrossRef]
- Xu, X.; Li, X.; Hu, X.; Wu, T.; Wang, Y.; Xu, X.; Han, Z. High miR156 expression is required for auxin-induced adventitious root formation via MxSPL26 independent of PINs and ARFs in Malus xiaojinensis. Front. Plant Sci. 2017, 8, 1059. [Google Scholar] [CrossRef]
- Zhao, Y. Essential roles of local auxin biosynthesis in plant development and in adaptation to environmental changes. Annu. Rev. Plant Biol. 2018, 69, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.; Zhou, H.; Jiang, C.; Zhao, S.; Wang, L.; Li, Q.; Lu, M.Z. The auxin receptor TIR 1 homolog (Pag FBL 1) regulates adventitious rooting through interactions with Aux/IAA 28 in Populus. Plant Biotechnol. J. 2019, 17, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Tinco Mamani, E. Propagación de estacas de higo (Ficus carica L.) bajo enraizadores naturales en distintos tiempos de sumersión. Rev. De Investig. E Innovación Agropecu. Y De Recur. Nat. 2024, 11, 47–56. [Google Scholar] [CrossRef]
- Guamán, R.; Leython, S.; Martínez, T. Enraizantes Naturales en Coffea canephora var. robusta (L. Linden). Investigatio 2019, 12, 93–102. [Google Scholar] [CrossRef]
- Nawroz Abdul-razzak, T.; Kamaran, S.R.; Djshwar, D.L.; Florian, M.W. Effects of oak leaf extract, biofertilizer, and soil containing oak leaf powder on tomato growth and biochemical characteristics under water stress conditions. Agriculture 2022, 12, 2082. [Google Scholar] [CrossRef]
- INEGI (Instituto Nacional de Estadística, Geografía e Informática). Anuario Estadístico y Geográfico del Estado de Hidalgo, México; Gobierno del Estado de Hidalgo: Higalgo, Mexico, 2017; Volume 1, p. 1674. Available online: https://www.inegi.org.mx/contenido/productos/prod_serv/contenidos/espanol/bvinegi/productos/nueva_estruc/anuarios_2017/702825095093.pdf (accessed on 5 March 2025).
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef]
- Hernández-Soto, I.; González-García, Y.; Juárez-Maldonado, A.; Hernández-Fuentes, A.D. Impact of Argemone mexicana L. on tomato plants infected with Phytophthora infestans. PeerJ 2024, 12, e16666. [Google Scholar] [CrossRef]
- Wijayabandara, K.; Campbell, S.; Vitelli, J.; Shabbir, A.; Adkins, S. Review of the biology, distribution, and management of the invasive fireweed (Senecio madagascariensis Poir). Plants 2021, 11, 107. [Google Scholar] [CrossRef]
- Barrios, E.A.M.; Vilca, L.C.; Huamani, O.M. Efecto de dos enraizantes naturales y uno sintético en la propagación de zarzamora (Rubus robustus C. Presl). Aporte Santiaguino 2022, 15, 72–86. [Google Scholar] [CrossRef]
- Galgano, F.; Tolve, R.; Scarpa, T.; Caruso, M.C.; Lucini, L.; Senizza, B.; Condelli, N. Extraction kinetics of total polyphenols, flavonoids, and condensed tannins of lentil seed coat: Comparison of solvent and extraction methods. Foods 2021, 10, 1810. [Google Scholar] [CrossRef]
- Żuchowski, J.; Pecio, Ł.; Reszczyńska, E.; Stochmal, A. New phenolic compounds from the roots of lentil (Lens culinaris). Helv. Chim. Acta 2016, 99, 674–680. [Google Scholar] [CrossRef]
- Del Rosario-Arellano, J.L.; Salazar-Ortiz, J.; Andrés-Meza, P.; Serna-Lagunes, R.; Borbonio-Fernández, V.; Real-Garrido, C.J.; Coria-Gil, N.A.B. Características agronómicas y forrajeras de variedades nativas de maíz en las montañas, Veracruz, México: Forage of native maize varieties. Acta Agrícola Y Pecu. 2024, 10, 1–12. [Google Scholar]
- Sharkey, T.D. The end game (s) of photosynthetic carbon metabolism. Plant Physiol. 2024, 195, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.R.; Koenig, D.; Sinha, N.R. LYRATE is a key regulator of leaflet initiation and lamina outgrowth in tomato. Plant Cell 2009, 21, 3093–3104. [Google Scholar] [CrossRef]
- Jian, S.; Wan, S.; Lin, Y.; Zhong, C. Nitrogen Sources Reprogram Carbon and Nitrogen Metabolism to Promote Andrographolide Biosynthesis in Andrographis paniculata (Burm. F.) Nees Seedlings. Int. J. Mol. Sci. 2024, 25, 3990. [Google Scholar] [CrossRef]
- Zhang, Q.; Gong, M.; Xu, X.; Li, H.; Deng, W. Roles of auxin in the growth, development, and stress tolerance of horticultural plants. Cells 2022, 11, 2761. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Moosa, A.; Ali, H.M.; Bermejo, N.F.; Munné-Bosch, S. Biostimulants: A sufficiently effective tool for sustainable agriculture in the era of climate change? PPB 2024, 211, 108699. [Google Scholar] [CrossRef]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent advances in the molecular effects of biostimulants in plants: An overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef]
- Martinez-Alonso, A.; Yepes-Molina, L.; Guarnizo, A.L.; Carvajal, M. Modification of gene expression of tomato plants through foliar flavonoid application in relation to enhanced growth. Genes 2023, 14, 2208. [Google Scholar] [CrossRef]
- Nicolas-Espinosa, J.; Garcia-Ibañez, P.; Lopez-Zaplana, A.; Yepes-Molina, L.; Albaladejo-Marico, L.; Carvajal, M. Confronting secondary metabolites with water uptake and transport in plants under abiotic stress. Int. J. Mol. Sci. 2023, 24, 2826. [Google Scholar] [CrossRef]
- Tyagi, K.; Maoz, I.; Kochanek, B.; Sela, N.; Lerno, L.; Ebeler, S.E.; Lichter, A. Cytokinin but not gibberellin application had major impact on the phenylpropanoid pathway in grape. Hortic. Res. 2021, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Colmenero-Flores, J.M.; Martínez, G.; Gamba, G.; Vázquez, N.; Iglesias, D.J.; Brumós, J.; Talón, M. Identification and functional characterization of cation–chloride cotransporters in plants. TPJ 2007, 50, 278–292. [Google Scholar] [CrossRef]
- Ingrisano, R.; Tosato, E.; Trost, P.; Gurrieri, L.; Sparla, F. Proline, Cysteine and Branched-Chain Amino Acids in Abiotic Stress Response of Land Plants and Microalgae. Plants 2023, 12, 3410. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Hernández, J.M.; Benítez-García, I.; Mazorra-Manzano, M.A.; Ramírez-Suárez, J.C.; Sánchez, E. Strategies for production, characterization and application of protein-based biostimulants in agriculture: A review. Chil. J. Agric. Res. 2020, 80, 274–289. [Google Scholar] [CrossRef]
- Williams, K.; Subramani, M.; Lofton, L.W.; Penney, M.; Todd, A.; Ozbay, G. Tools and Techniques to Accelerate Crop Breeding. Plants 2024, 13, 1520. [Google Scholar] [CrossRef] [PubMed]
- Aryan, S.; Gulab, G.; Safi, Z.; Durani, A.; Raghib, M.G.; Kakar, K.; Elansary, H.O. Enhancement of propagation using organic materials and growth hormone: A study on the effectiveness of growth and rooting of pomegranate cuttings. Horticulturae 2023, 9, 999. [Google Scholar] [CrossRef]
- Alvarado-Aguayo, A.; Munzón-Quintana, M. Evaluación de la efectividad de gel de sábila y agua de coco como enraizantes naturales en diferentes sustratos para propagación asexual de árboles de ficus benjamina. Agr. Costarr. 2020, 44, 65–78. [Google Scholar] [CrossRef]
- García-Cid, F.E.; Tapia-Zayago, F.A.; Pérez-Ríos, S.R.; Madariaga-Navarrete, A.; Cenobio-Galindo, A.d.J.; Hernández-Soto, I. Enraizantes a base de lenteja y sábila una alternativa ecológica en la producción de tomate. Boletín De Cienc. Agropecu. Del ICAP 2024, 10, 10–15. [Google Scholar] [CrossRef]



| Variable | (60.64%) | Component 2 (29.19%) |
|---|---|---|
| Plant height | 0.2882 | 0.0702 |
| Number of leaves | 0.3205 | −0.4690 |
| Fresh aerial weight | 0.4222 | −0.2522 |
| Aerial dry weight | 0.3658 | −0.3103 |
| Root length | 0.3321 | 0.2996 |
| Root fresh weight | 0.3001 | −0.1461 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Lemus, U.; Tapia-Zayago, F.A.; Pérez-Ríos, S.R.; Zaldívar-Ortega, A.K.; Rueda-Puente, E.O.; Hernández-Pérez, A.; González-Montiel, L.; Hernández-Soto, I. Lentil Biorooting Agents: An Ecological Alternative to Improve the Growth and Development of Italian Zucchini in Sustainable Production Systems. Horticulturae 2025, 11, 332. https://doi.org/10.3390/horticulturae11030332
González-Lemus U, Tapia-Zayago FA, Pérez-Ríos SR, Zaldívar-Ortega AK, Rueda-Puente EO, Hernández-Pérez A, González-Montiel L, Hernández-Soto I. Lentil Biorooting Agents: An Ecological Alternative to Improve the Growth and Development of Italian Zucchini in Sustainable Production Systems. Horticulturae. 2025; 11(3):332. https://doi.org/10.3390/horticulturae11030332
Chicago/Turabian StyleGonzález-Lemus, Uriel, Félix Antonio Tapia-Zayago, Sergio Rubén Pérez-Ríos, Ana Karen Zaldívar-Ortega, Edgar Omar Rueda-Puente, Aracely Hernández-Pérez, Lucio González-Montiel, and Iridiam Hernández-Soto. 2025. "Lentil Biorooting Agents: An Ecological Alternative to Improve the Growth and Development of Italian Zucchini in Sustainable Production Systems" Horticulturae 11, no. 3: 332. https://doi.org/10.3390/horticulturae11030332
APA StyleGonzález-Lemus, U., Tapia-Zayago, F. A., Pérez-Ríos, S. R., Zaldívar-Ortega, A. K., Rueda-Puente, E. O., Hernández-Pérez, A., González-Montiel, L., & Hernández-Soto, I. (2025). Lentil Biorooting Agents: An Ecological Alternative to Improve the Growth and Development of Italian Zucchini in Sustainable Production Systems. Horticulturae, 11(3), 332. https://doi.org/10.3390/horticulturae11030332









