MIR396d-p3 Negatively Regulates Apple Resistance to Colletotrichum gloeosporioides via MdUGT89A2 and MdRGA3
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material and Pathogen Cultures
2.2. sRNA-Seqencing and Identification of Differentially Expressed microRNAs (DE-miRNAs)
2.3. Bioinformatics Analysis
2.4. Rapid Amplification of cDNA Ends Assay
2.5. Plasmid Construction
2.6. Agrobacterium-Mediated Transient Transformation
2.7. Histochemical Staining and GUS Activity Quantification
2.8. RT-qPCR Assay
2.9. Statistical Analysis
3. Results
3.1. Analysis of Apple Small RNA Sequencing Response to C. gloeosporioides
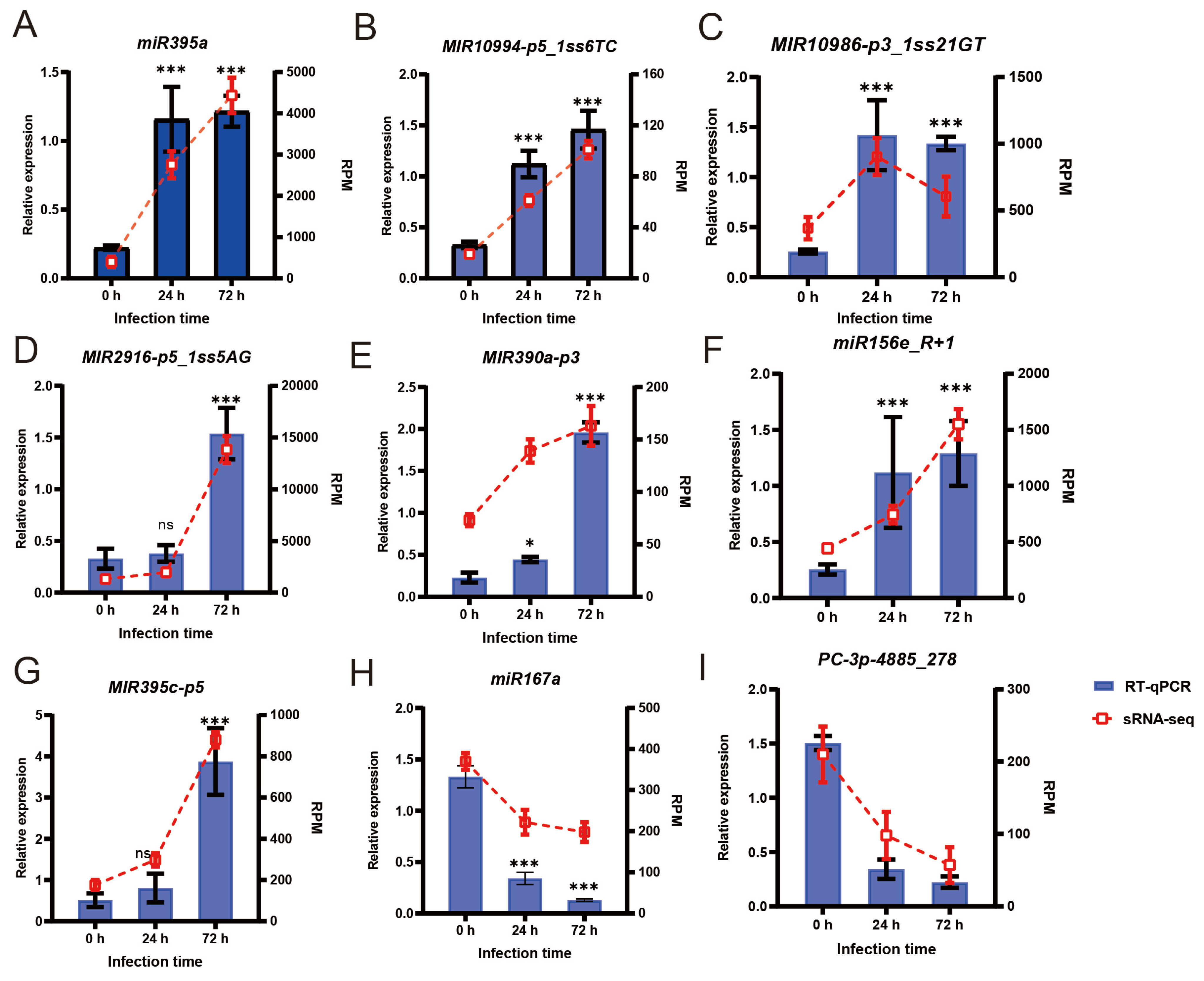
3.2. Validation of sRNA-Seq Data Using RT-qPCR
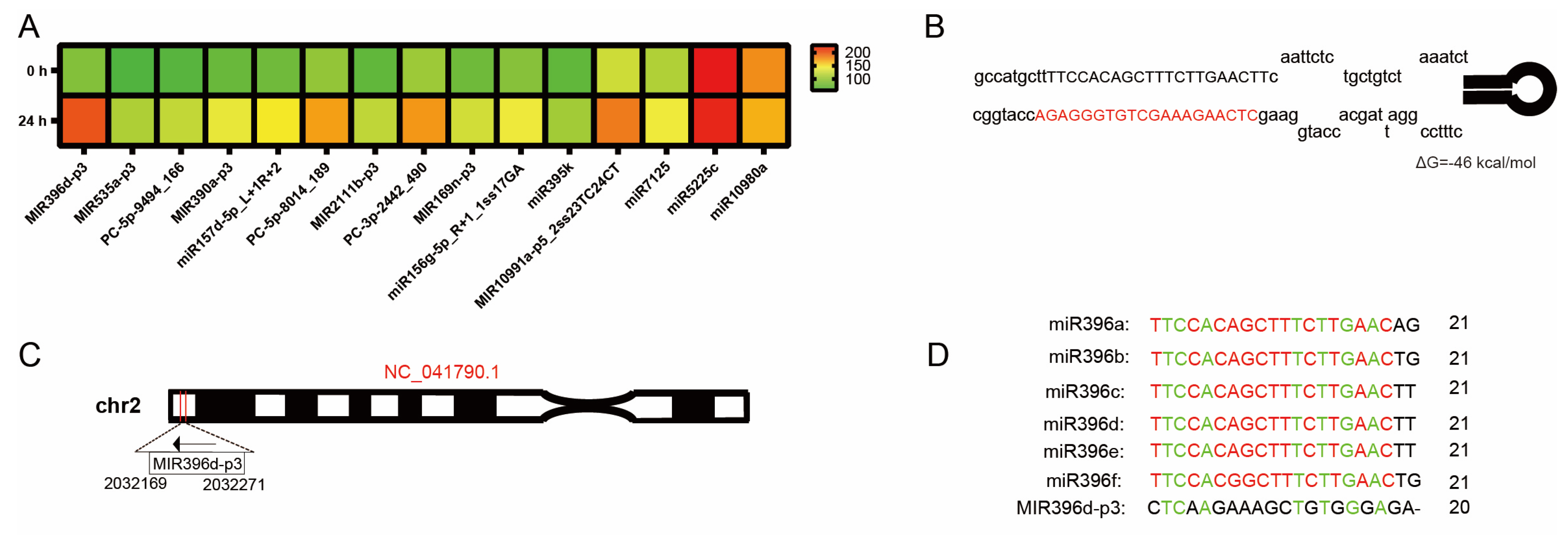
3.3. Identification and In Silico Analysis of miRNA Response to C. gloeosporioides
3.4. MIR396d-p3 Cleaves MdUGT89A2 and MdRGA3 Transcripts
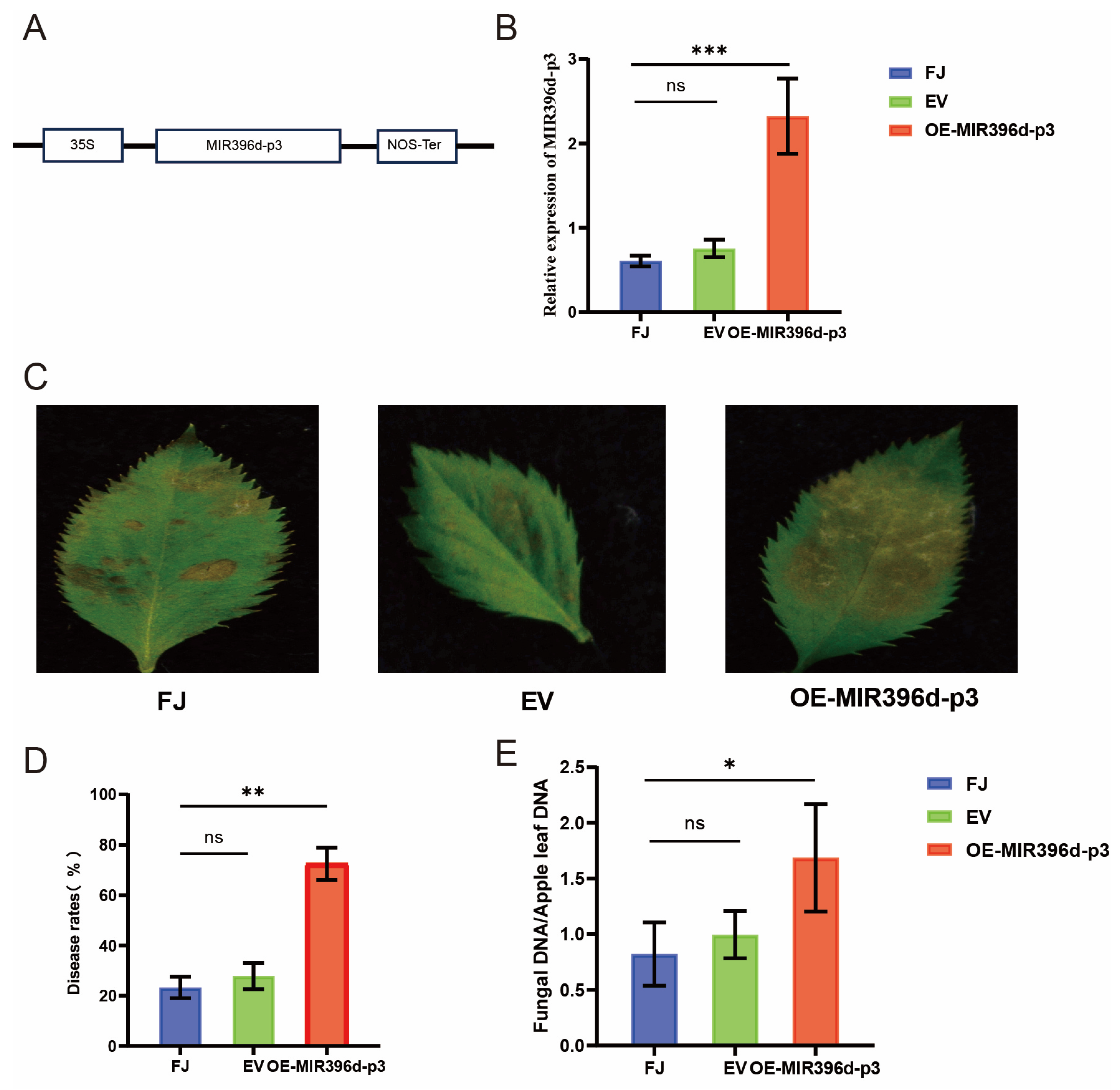
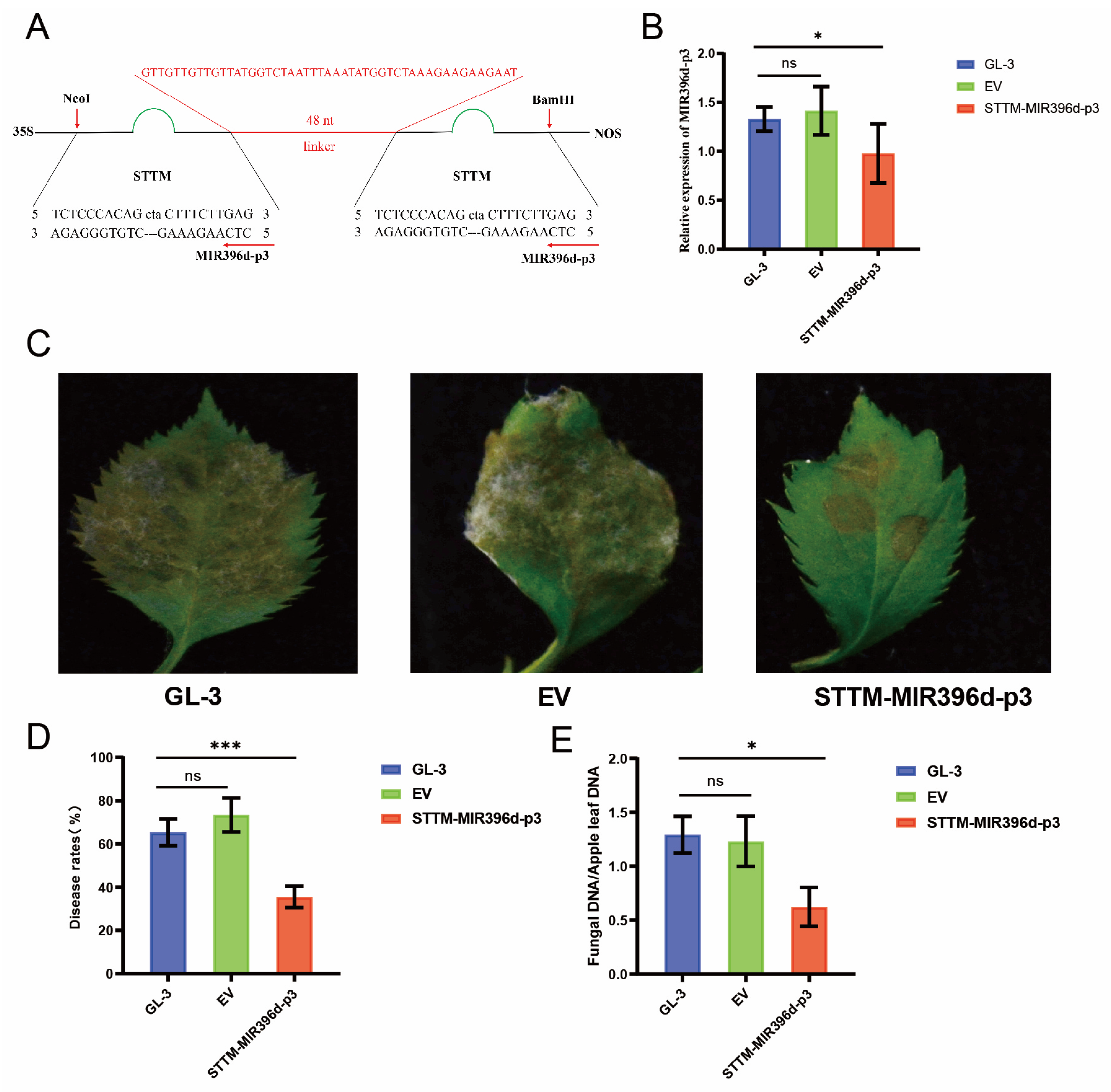
3.5. Responsiveness of MIR396d-p3 to C. gloeosporioides Infection
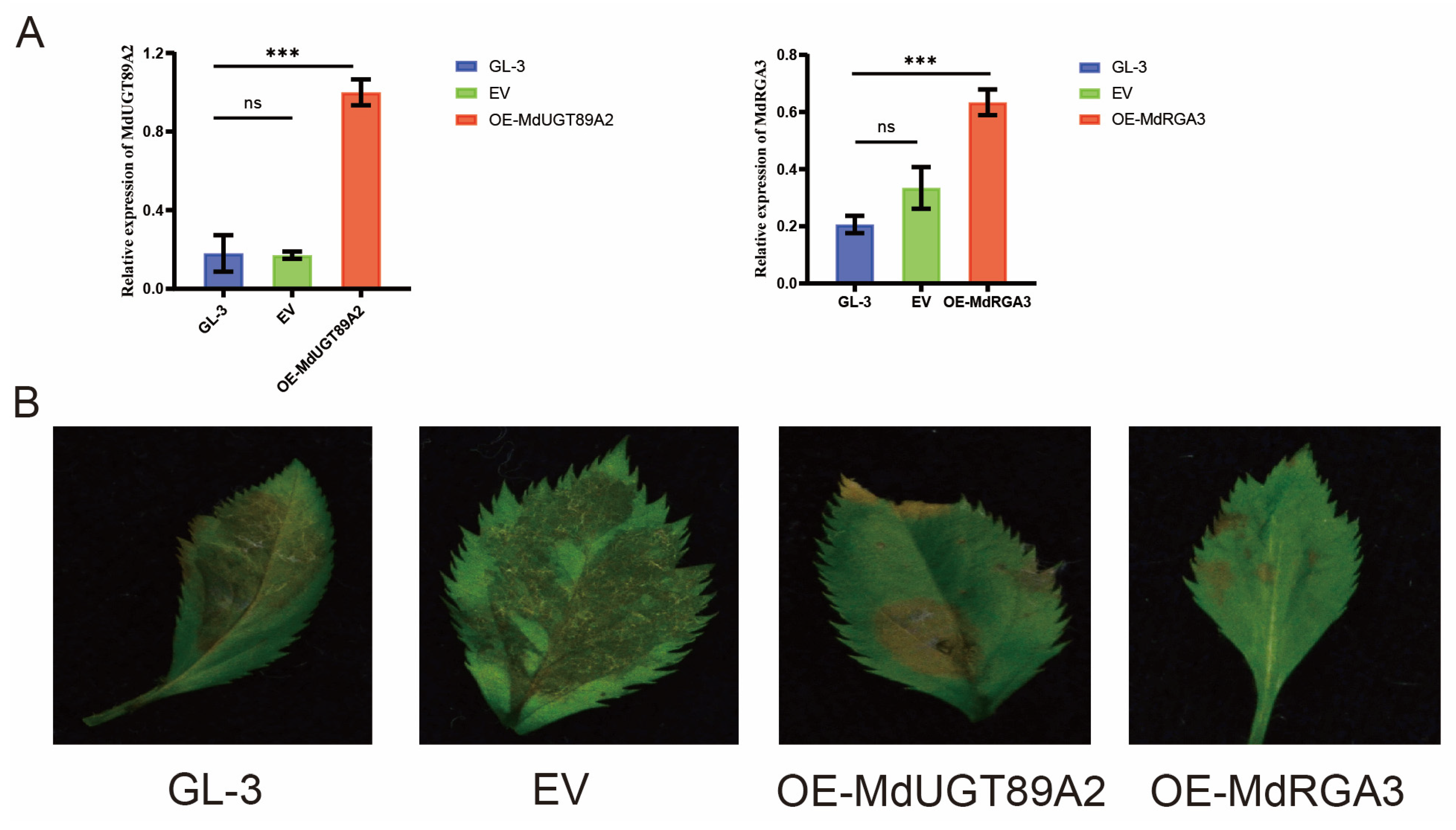
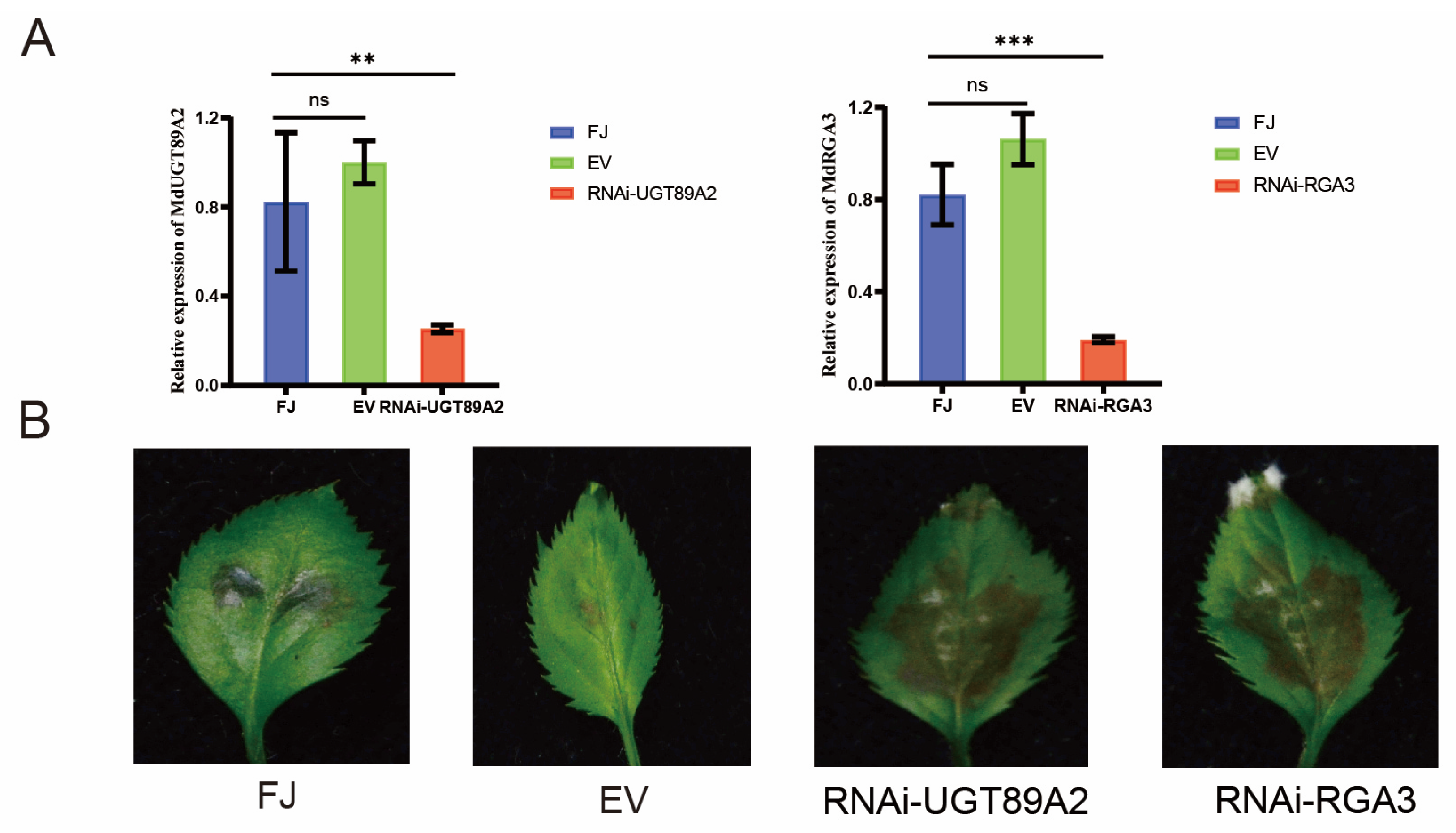
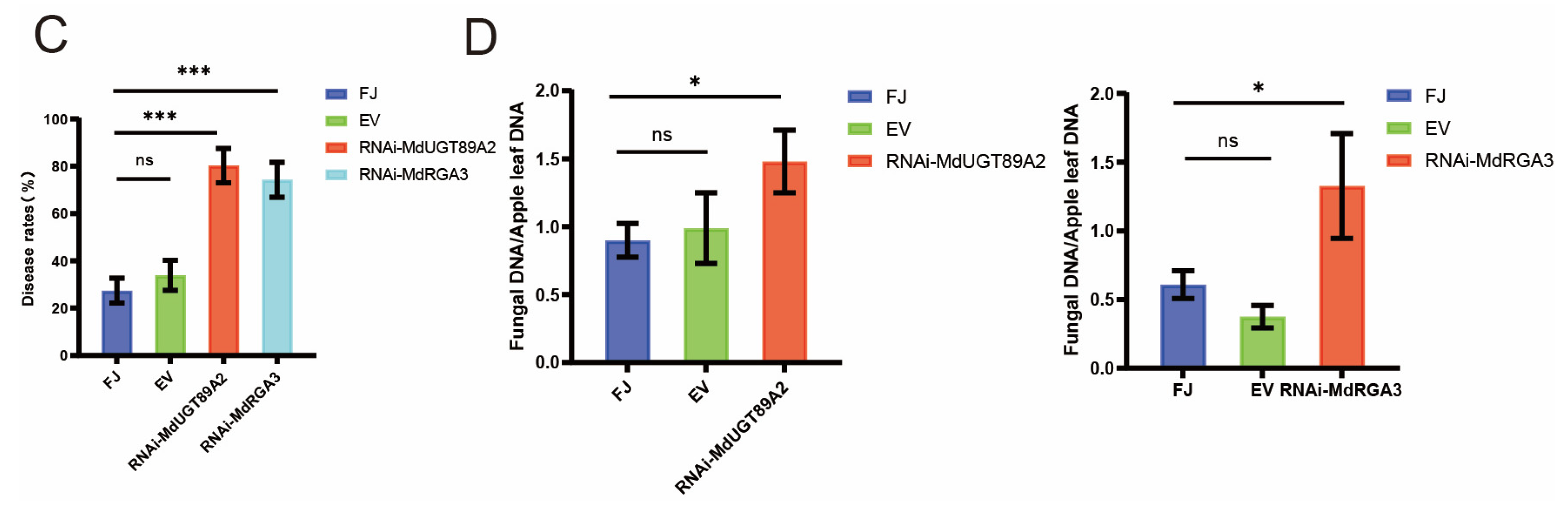
3.6. MdUGT89A2 and MdRGA3 Are Involved in the Resistance to C. gloeosporioides in Apple
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hamada, N.A.; Moreira, R.R.; Nesi, C.N.; May De Mio, L.L. Pathogen Dispersal and Glomerella Leaf Spot Progress Within Apple Canopy in Brazil. Plant Dis. 2019, 103, 3209–3217. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, F.; Su, Y.; Jiang, Q.; Yuan, Y.; Nie, X.; Zhou, Y.; Zhang, X.; Wang, Z.; Wang, F.; et al. MdIPT8, an Isopentenyl Transferase Enzyme, Enhances the Resistance of Apple to Colletotrichum Gloeosporioides Infection. Sci. Hortic. 2022, 303, 111245. [Google Scholar] [CrossRef]
- Shang, S.; Liu, G.; Zhang, S.; Liang, X.; Zhang, R.; Sun, G. A Fungal CFEM-containing Effector Targets NPR1 Regulator NIMIN2 to Suppress Plant Immunity. Plant Biotechnol. J. 2023, 22, 82–97. [Google Scholar] [CrossRef]
- Wang, B.; Li, B.-H.; Dong, X.-L.; Wang, C.-X.; Zhang, Z.-F. Effects of Temperature, Wetness Duration, and Moisture on the Conidial Germination, Infection, and Disease Incubation Period of Glomerella Cingulata. Plant Dis. 2015, 99, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, Y.; Cao, X.; Qi, Y. MicroRNAs and Their Regulatory Roles in Plant–Environment Interactions. Annu. Rev. Plant Biol. 2019, 70, 489–525. [Google Scholar] [CrossRef]
- Qiao, Y.; Xia, R.; Zhai, J.; Hou, Y.; Feng, L.; Zhai, Y.; Ma, W. Small RNAs in Plant Immunity and Virulence of Filamentous Pathogens. Annu. Rev. Phytopathol. 2021, 59, 265–288. [Google Scholar] [CrossRef]
- Yu, X.; Hou, Y.; Chen, W.; Wang, S.; Wang, P.; Qu, S. Malus Hupehensis miR168 Targets to ARGONAUTE1 and Contributes to the Resistance against Botryosphaeria Dothidea Infection by Altering Defense Responses. Plant Cell Physiol. 2017, 58, 1541–1557. [Google Scholar] [CrossRef]
- Yu, X.; Gong, H.; Cao, L.; Hou, Y.; Qu, S. MicroRNA397b Negatively Regulates Resistance of Malus Hupehensis to Botryosphaeria Dothidea by Modulating MhLAC7 Involved in Lignin Biosynthesis. Plant Sci. 2020, 292, 110390. [Google Scholar] [CrossRef]
- Zhou, T.; Cao, L.; Hu, K.; Yu, X.; Qu, S. miR164–NAC21/22 Module Regulates the Resistance of Malus Hupehensis against Alternaria Alternata by Controlling Jasmonic Acid Signaling. Plant Sci. 2023, 330, 111635. [Google Scholar] [CrossRef]
- Yu, X.; Hou, Y.; Cao, L.; Zhou, T.; Wang, S.; Hu, K.; Chen, J.; Qu, S. MicroRNA Candidate miRcand137 in Apple Is Induced by Botryosphaeria dothidea for Impairing Host Defense. Plant Physiol. 2022, 189, 1814–1832. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, C.; Zhang, Y.; Gu, Z.; Li, W.; Duan, X.; Wang, S.; Hao, L.; Wang, Y.; Wang, S.; et al. A Single-Nucleotide Polymorphism in the Promoter of a Hairpin RNA Contributes to Alternaria alternata Leaf Spot Resistance in Apple (Malus × Domestica). Plant Cell 2018, 30, 1924–1942. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Tang, J.; Li, T.; Zhang, Q. The microRNA miR482 Regulates NBS-LRR Genes in Response to ALT1 Infection in Apple. Plant Sci. 2024, 343, 112078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Y.; Zhang, Y.; Wu, C.; Wang, S.; Hao, L.; Wang, S.; Li, T. Md-miR156ab and Md-miR395 Target WRKY Transcription Factors to Influence Apple Resistance to Leaf Spot Disease. Front. Plant Sci. 2017, 8, 526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Q.; Hao, L.; Wang, S.; Wang, S.; Zhang, W.; Xu, C.; Yu, Y.; Li, T. A Novel miRNA Negatively Regulates Resistance to Glomerella Leaf Spot by Suppressing Expression of an NBS Gene in Apple. Hortic. Res. 2019, 6, 93. [Google Scholar] [CrossRef]
- Soto-Suárez, M.; Baldrich, P.; Weigel, D.; Rubio-Somoza, I.; San Segundo, B. The Arabidopsis miR396 Mediates Pathogen-Associated Molecular Pattern-Triggered Immune Responses against Fungal Pathogens. Sci. Rep. 2017, 7, 44898. [Google Scholar] [CrossRef]
- Dai, Z.; Tan, J.; Zhou, C.; Yang, X.; Yang, F.; Zhang, S.; Sun, S.; Miao, X.; Shi, Z. The OsmiR396–OsGRF8–OsF3H-flavonoid Pathway Mediates Resistance to the Brown Planthopper in Rice (Oryza sativa). Plant Biotechnol. J. 2019, 17, 1657–1669. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, T.; Yi, F.; Yu, J. SimiR396d Targets SiGRF1 to Regulate Drought Tolerance and Root Growth in Foxtail Millet. Plant Sci. 2023, 326, 111492. [Google Scholar] [CrossRef]
- Noon, J.B.; Hewezi, T.; Baum, T.J. Homeostasis in the Soybean miRNA396–GRFNetwork Is Essential for Productive Soybean Cyst Nematode Infections. J. Exp. Bot. 2019, 70, 1653–1668. [Google Scholar] [CrossRef]
- Dimunová, D.; Matoušková, P.; Podlipná, R.; Boušová, I.; Skálová, L. The Role of UDP-Glycosyltransferases in Xenobioticresistance. Drug Metab. Rev. 2022, 54, 282–298. [Google Scholar] [CrossRef]
- Bowles, D.; Isayenkova, J.; Lim, E.-K.; Poppenberger, B. Glycosyltransferases: Managers of Small Molecules. Curr. Opin. Plant Biol. 2005, 8, 254–263. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Lin, J.; Chen, L.; Li, Y.; Liu, Q.; Wang, G.; Xu, F.; Liu, L.; Hou, B. The Novel Pathogen-responsive Glycosyltransferase UGT73C7 Mediates the Redirection of Phenylpropanoid Metabolism and Promotes SNC1-dependent Arabidopsis Immunity. Plant J. 2021, 107, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Bethke, G.; Huang, Y.; Hensel, G.; Heinen, S.; Liu, C.; Wyant, S.R.; Li, X.; Quin, M.B.; McCormick, S.; Morrell, P.L.; et al. UDP-Glucosyltransferase HvUGT13248 Confers Type II Resistance to Fusarium Graminearum in Barley. Plant Physiol. 2023, 193, 2691–2710. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Qu, S.; Liu, F.; Sun, H.; Li, H.; Teng, W.; Zhan, Y.; Li, Y.; Han, Y.; Zhao, X. Multi-omics Analysis Identified the GmUGT88A1 Gene, Which Coordinately Regulates Soybean Resistance to Cyst Nematode and Isoflavone Content. Plant Biotechnol. J. 2025. early view. [Google Scholar] [CrossRef]
- McHale, L.; Tan, X.; Koehl, P.; Michelmore, R.W. Plant NBS-LRR Proteins: Adaptable Guards. Genome Biol. 2006, 7, 212. [Google Scholar] [CrossRef]
- Huang, S.; Jia, A.; Ma, S.; Sun, Y.; Chang, X.; Han, Z.; Chai, J. NLR Signaling in Plants: From Resistosomes to Second Messengers. Trends Biochem. Sci. 2023, 48, 776–787. [Google Scholar] [CrossRef]
- Wu, J.; Ji, Z.; Wang, N.; Chi, F.; Xu, C.; Zhou, Z.; Zhang, J. Identification of Conidiogenesis-Associated Genes in Colletotrichum Gloeosporioides by Agrobacterium Tumefaciens-Mediated Transformation. Curr. Microbiol. 2016, 73, 802–810. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, M.; Jia, X.; Hu, G.; Ren, F.; Fan, X.; Dong, Y. Integrated Transcriptome and Metabolome Dissecting Interaction between Vitis Vinifera L. and Grapevine Fabavirus. Int. J. Mol. Sci. 2023, 24, 3247. [Google Scholar] [CrossRef]
- Daccord, N.; Celton, J.-M.; Linsmith, G.; Becker, C.; Choisne, N.; Schijlen, E.; van de Geest, H.; Bianco, L.; Micheletti, D.; Velasco, R.; et al. High-Quality de Novo Assembly of the Apple Genome and Methylome Dynamics of Early Fruit Development. Nat. Genet. 2017, 49, 1099–1106. [Google Scholar] [CrossRef]
- Li, W.; Jia, Y.; Liu, F.; Wang, F.; Fan, F.; Wang, J.; Zhu, J.; Xu, Y.; Zhong, W.; Yang, J. Integration Analysis of Small RNA and Degradome Sequencing Reveals MicroRNAs Responsive to Dickeya Zeae in Resistant Rice. Int. J. Mol. Sci. 2019, 20, 222. [Google Scholar] [CrossRef]
- Dai, X.; Zhao, P.X. psRNATarget: A Plant Small RNA Target Analysis Server. Nucleic Acids Res. 2011, 39, W155–W159. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “One for All, All for One” Bioinformatics Platform for Biological Big-Data Mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS Fusions: Beta-Glucuronidase as a Sensitive and Versatile Gene Fusion Marker in Higher Plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.E.; Olsson, N.; Schlosser, J.; Peng, F.; Lund, S.T. An Optimized Grapevine RNA Isolation Procedure and Statistical Determination of Reference Genes for Real-Time RT-PCR during Berry Development. BMC Plant Biol. 2006, 6, 27. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, J.; Wang, K.; Li, D.; Yang, R.; Luo, H.; Zhang, W. MiR396-GRF Module Associates with Switchgrass Biomass Yield and Feedstock Quality. Plant Biotechnol. J. 2021, 19, 1523–1536. [Google Scholar] [CrossRef]
- Wang, L.; Hou, J.; Xu, H.; Zhang, Y.; Huang, R.; Wang, D.; He, X.-Q. The PtoTCP20-miR396d-PtoGRF15 Module Regulates Secondary Vascular Development in Populus. Plant Commun. 2023, 4, 100494. [Google Scholar] [CrossRef]
- Sun, L.; Zhu, M.; Zhou, X.; Gu, R.; Hou, Y.; Li, T.; Huang, H.; Yang, R.; Wang, S.; Zhao, W. The miR396a–SlGRF8 Module Regulates Sugar Accumulation in the Roots via SlSTP10 during the Interaction between Root-knot Nematodes and Tomato Plants. J. Integr. Plant Biol. 2024, 66, 2701–2715. [Google Scholar] [CrossRef]
- Wang, J.; Song, W.; Chai, J. Structure, Biochemical Function, and Signaling Mechanism of Plant NLRs. Mol. Plant 2023, 16, 75–95. [Google Scholar] [CrossRef]
- Zhang, J.; Coaker, G.; Zhou, J.-M.; Dong, X. Plant Immune Mechanisms: From Reductionistic to Holistic Points of View. Mol. Plant 2020, 13, 1358–1378. [Google Scholar] [CrossRef]
- Padmanabhan, C.; Zhang, X.; Jin, H. Host Small RNAs Are Big Contributors to Plant Innate Immunity. Curr. Opin. Plant Biol. 2009, 12, 465–472. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Tang, J.; Ji, Z.; Du, Y.; Cong, J.; Zhou, Z. MIR396d-p3 Negatively Regulates Apple Resistance to Colletotrichum gloeosporioides via MdUGT89A2 and MdRGA3. Horticulturae 2025, 11, 351. https://doi.org/10.3390/horticulturae11040351
Zhang B, Tang J, Ji Z, Du Y, Cong J, Zhou Z. MIR396d-p3 Negatively Regulates Apple Resistance to Colletotrichum gloeosporioides via MdUGT89A2 and MdRGA3. Horticulturae. 2025; 11(4):351. https://doi.org/10.3390/horticulturae11040351
Chicago/Turabian StyleZhang, Baodong, Jinqi Tang, Zhirui Ji, Yinan Du, Jialin Cong, and Zongshan Zhou. 2025. "MIR396d-p3 Negatively Regulates Apple Resistance to Colletotrichum gloeosporioides via MdUGT89A2 and MdRGA3" Horticulturae 11, no. 4: 351. https://doi.org/10.3390/horticulturae11040351
APA StyleZhang, B., Tang, J., Ji, Z., Du, Y., Cong, J., & Zhou, Z. (2025). MIR396d-p3 Negatively Regulates Apple Resistance to Colletotrichum gloeosporioides via MdUGT89A2 and MdRGA3. Horticulturae, 11(4), 351. https://doi.org/10.3390/horticulturae11040351




