The Effects of Increasing Boron on Growth, Yield, and Nutritional Value of Scallion (Allium cepa L.) Grown as a Bunch Harvest
Abstract
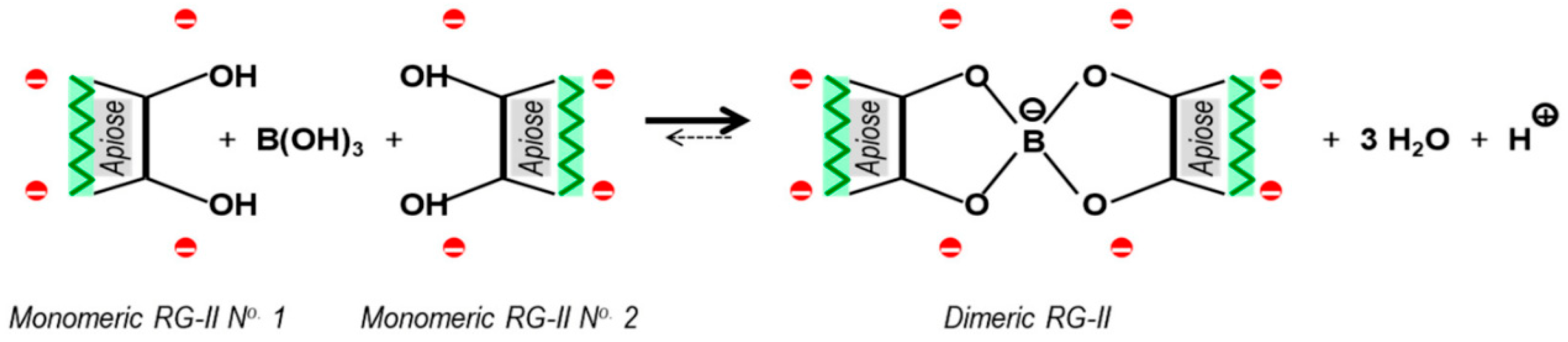
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sampling and Harvest of Plants
2.3. Determination of Photosynthetic Pigments
2.4. Determination of Membrane Damage
2.5. Determination of Nutrient Ion Concentrations
2.6. Determination of Bio-Concentration, Translocation, and Accumulation
2.7. Statistical Analysis
3. Results
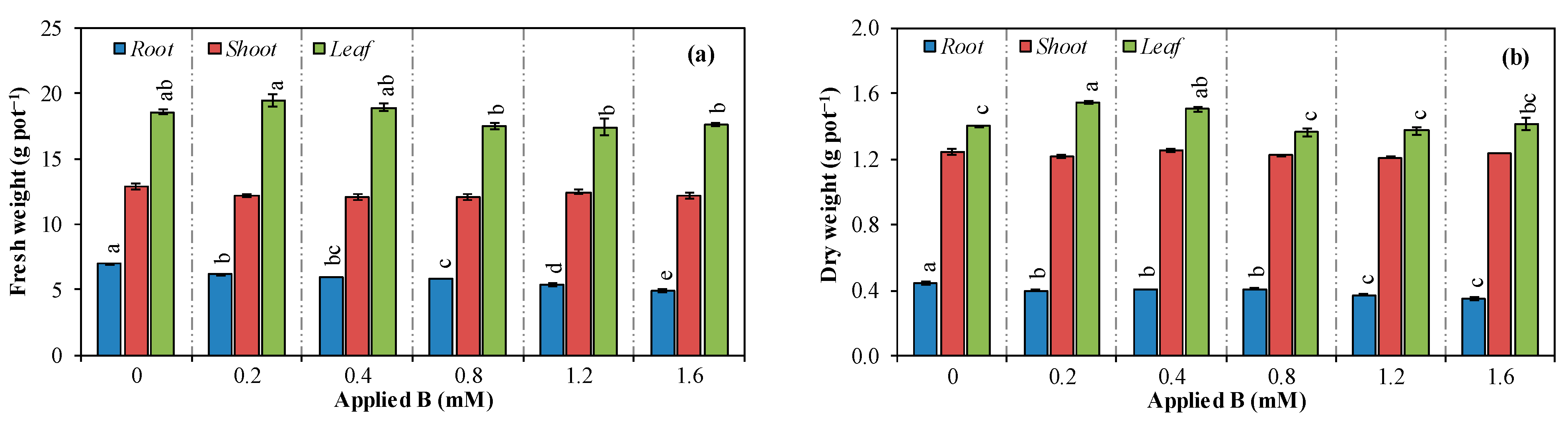
3.1. Vegetative Growth
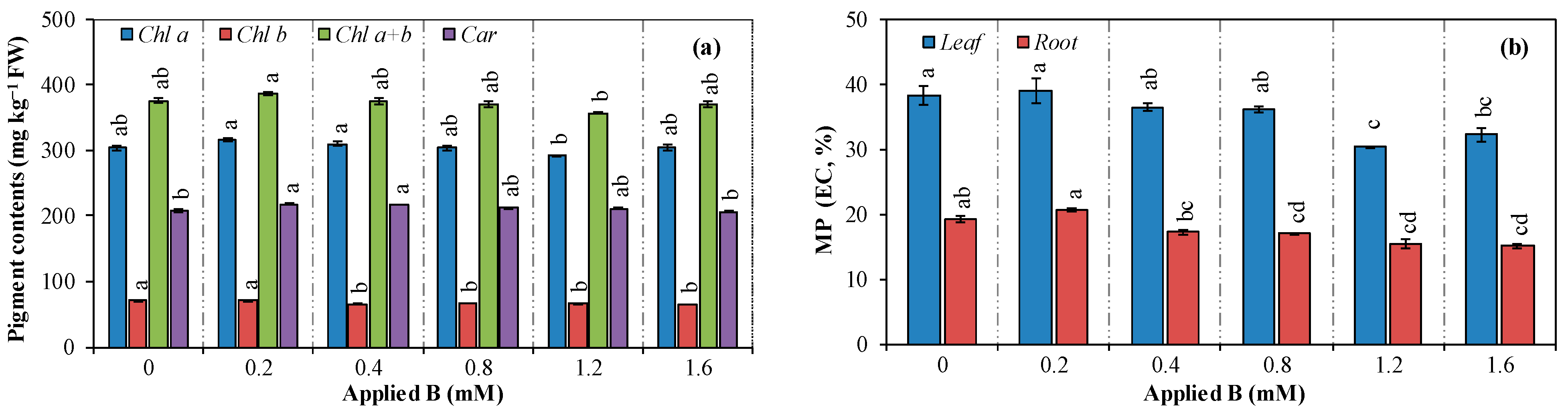
3.2. Photosynthetic Pigment Contents and Membrane Permeability
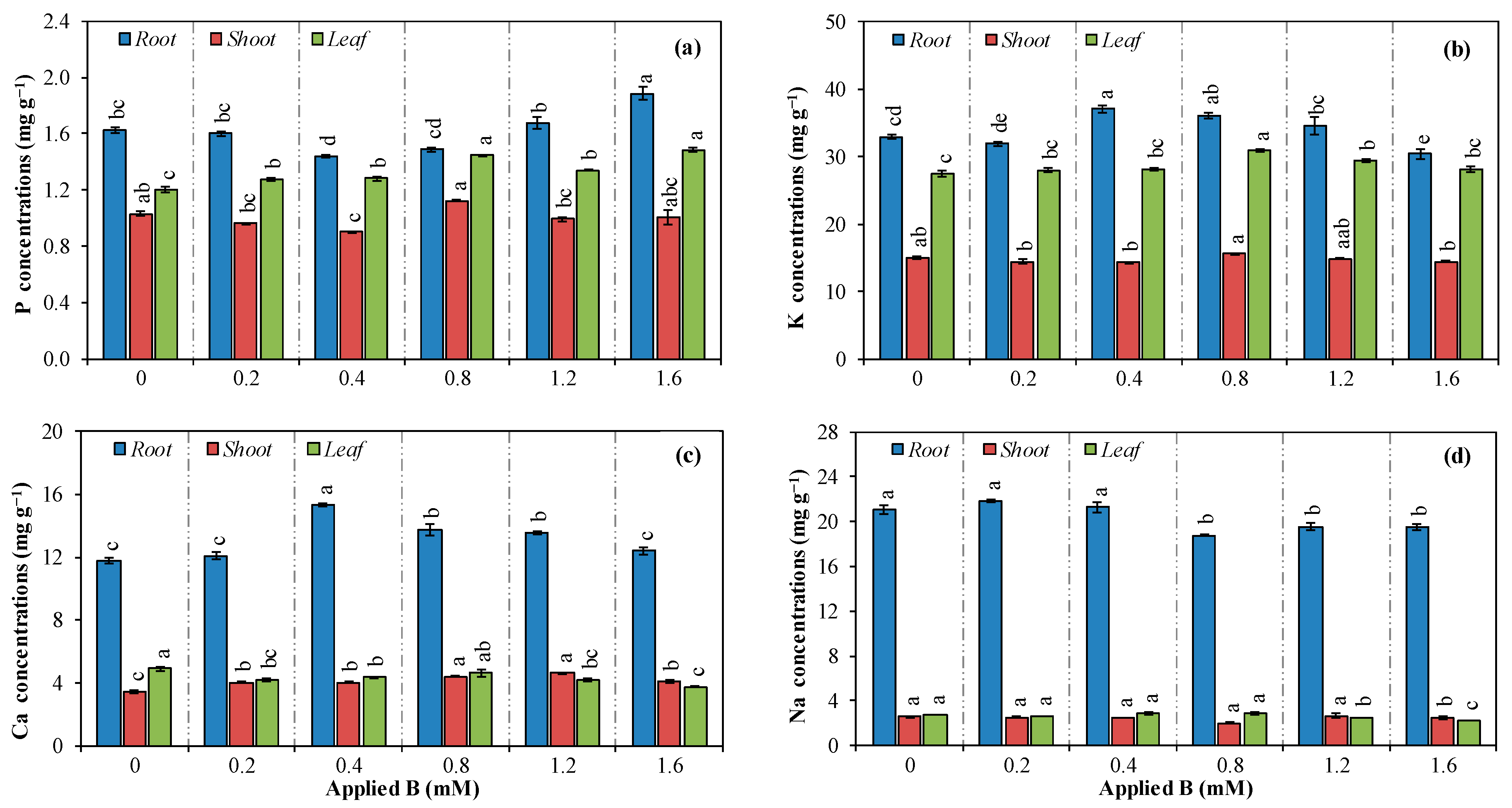
3.3. Concentration, Translocation, and Accumulation of Nutrients
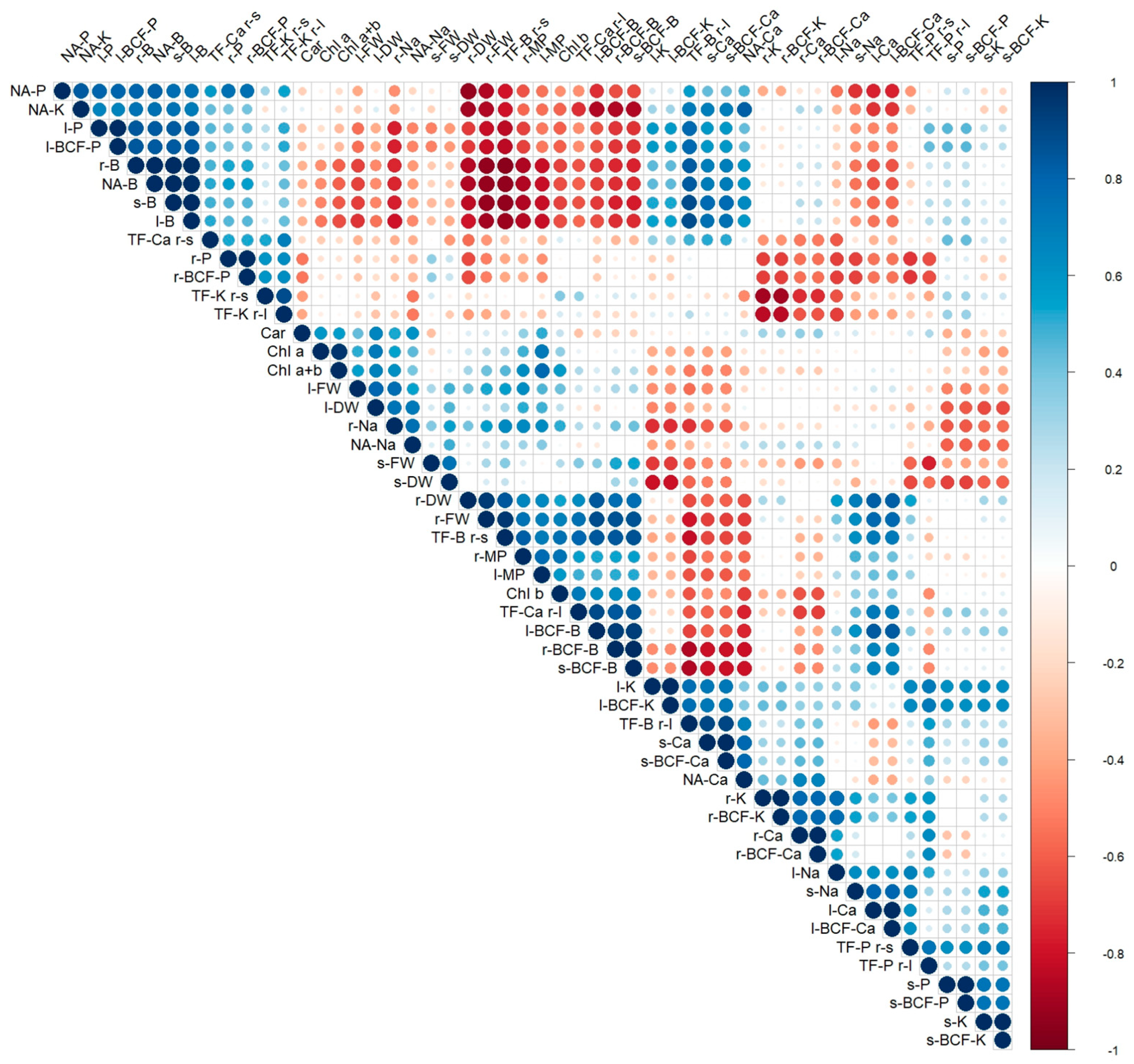
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goldbach, H.E.; Wimmer, M.A.; Findeklee, P. Discussion paper: Boron—How can the critical level be defined? J. Plant Nutr. Soil Sci. 2000, 163, 115–121. [Google Scholar] [CrossRef]
- Yau, S.K.; Hamblin, J.; Ryan, J. Phenotypic variation in boron toxicity tolerance in barley, durum, and bread wheat. Rachis 1994, 13, 20–25. [Google Scholar]
- Wimmer, M.A.; Goldberg, S.; Gupta, U.C. Boron. In Handbook of Plant Nutrition; Barker, A., Pilbeam, D., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 305–346. [Google Scholar]
- Loomis, W.D.; Durst, R.W. Chemistry and biology of boron. BioFactors 1992, 3, 229–239. [Google Scholar]
- Xiao, C.; Anderson, C.T. Roles of pectin in biomass yield and processing for biofuels. Front. Plant Sci. 2013, 4, 67. [Google Scholar] [CrossRef]
- Begum, R.A.; Fry, S.C. Arabinogalactan-proteins as boron-acting enzymes, cross-linking the rhamnogalacturonan-II domains of pectin. Plants 2023, 12, 3921. [Google Scholar] [CrossRef]
- O’Neill, M.A.; Eberhard, S.; Albersheim, P.; Darvill, A.G. Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science 2001, 294, 846–849. [Google Scholar] [CrossRef]
- Matsunaga, T.; Ishii, T.; Matsumoto, S.; Masanobu, H.; Darvill, A.; Albersheim, P.; O’Neill, M.A. Occurrence of the Primary Cell Wall Polysaccharide Rhamnogalacturonan II in Pteridophytes, Lycophytes, and Bryophytes. Implications for the Evolution of Vascular Plants. Plant Physiol. 2004, 134, 339–351. [Google Scholar] [CrossRef]
- Avci, U.; Peña, M.J.; O’Neill, M.A. Changes in the abundance of cell wall apiogalacturonan and xylogalacturonan and conservation of rhamnogalacturonan II structure during the diversification of the Lemnoideae. Planta 2018, 247, 953–971. [Google Scholar] [CrossRef]
- Begum, R.A.; Messenger, D.J.; Fry, S.C. Making and breaking of boron bridges in the pectic domain rhamnogalacturonan-II at apoplastic pH in vivo and in vitro. Plant J. 2023, 113, 1310–1329. [Google Scholar] [CrossRef]
- Hu, H.; Brown, P.H.; Labavitch, J.M. Species variability in boron requirement is correlated with cell wall pectin. J. Exp. Bot. 1996, 47, 227–232. [Google Scholar] [CrossRef]
- Brown, P.H.; Bellaloui, N.; Wimmer, M.A.; Bassil, E.S.; Ruiz, J.; Hu, H.; Pfeffer, H.; Dannel, F.; Römheld, V. Boron in plant biology. Plant Biol. 2002, 4, 205–223. [Google Scholar] [CrossRef]
- Brown, P.H.; Hu, H. Boron mobility and consequent management in different crops. Better Crops 1998, 82, 28–31. [Google Scholar]
- Brdar-Jokanović, M. Boron toxicity and deficiency in agricultural plants. Int. J. Mol. Sci. 2020, 21, 1424–1444. [Google Scholar] [CrossRef] [PubMed]
- Redgwell, R.J.; Selvendran, R.R. Structural features of cell wall polysaccharides of onion (Allium cepa). Carbohydr. Res. 1986, 157, 183–199. [Google Scholar] [CrossRef]
- Waldron, K.W.; Selvendran, R.R. Composition of the cell walls of different asparagus (Asparagus officinalis) tissues. Physiol. Plant. 1990, 80, 568–575. [Google Scholar] [CrossRef]
- Jones, J.B., Jr.; Wolf, B.; Mills, H.A. Plant Analysis Handbook. A Practical Sampling, Preparation, Analysis, and Interpretation Guide; Micro-Macro Publishing Inc.: Athens, GA, USA, 1991; pp. 127–189. [Google Scholar]
- González-Fontes, A.; Rexach, J.; Navarro-Gochicoa, M.T.; Herrera-Rodríguez, M.B.; Beato, V.M.; Maldonado, J.M.; Camacho-Cristóbal, J.J. Is boron involved solely in structural roles in vascular plants? Plant Signal. Behav. 2008, 3, 24–26. [Google Scholar] [CrossRef]
- Manna, D.; Maity, T.K. Growth, yield, and bulb quality of onion (Allium cepa L.) in response to foliar application of boron and zinc. J. Plant Nutr. 2016, 39, 438–441. [Google Scholar] [CrossRef]
- Francke, A.; Majkowska-Gadomska, J.; Kaliniewicz, Z.; Jadwisieńczak, K. No Effect of Biostimulants on the Growth, Yield, and Nutritional Value of Shallots Grown for Bunch Harvest. Agronomy 2022, 12, 1156. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 1–32. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Yan, B.; Dai, Q.; Liu, X.; Huang, S.; Wang, Z. Flooding-induced membrane damage, lipid oxidation, and activated oxygen generation in corn leaves. Plant Soil 1996, 179, 261–268. [Google Scholar] [CrossRef]
- Miller, R.O. High-temperature oxidation: Dry ashing. In Handbook of Reference Methods for Plant Analysis; Kalra, P.Y., Ed.; Taylor and Francis/CRC Press: Boca Raton, FL, USA, 1998; pp. 66–69. [Google Scholar]
- Soda, S.; Hamada, T.; Yamaoka, Y.; Ike, M.; Nakazato, H.; Saeki, Y.; Kasamatsu, T.; Sakurai, Y. Constructed wetlands for advanced treatment of wastewater with a complex matrix from a metal-processing plant: Bioconcentration and translocation factors of various metals in Acorus gramineus and Cyperus alternifolius. Ecol. Eng. 2012, 39, 63–70. [Google Scholar] [CrossRef]
- Herrera-Rodríguez, M.B.; González-Fontes, A.; Rexach, J.; Camacho-Cristobal, J.J.; Maldonado, J.M.; Navarro-Gochicoa, M.T. Role of boron in vascular plants and response mechanisms to boron stresses. Plant Stress 2010, 4, 115–122. [Google Scholar]
- Reid, R.; Fitzpatrick, K. Influence of leaf tolerance mechanisms and rain on boron toxicity in barley and wheat. Plant Physiol. 2009, 151, 413–420. [Google Scholar] [CrossRef]
- Francois, L.E. Yield and quality responses of garlic and onion to excess boron. HortScience 1991, 26, 547–549. [Google Scholar] [CrossRef]
- Trifunović-Momčilov, M.; Milošević, S.; Marković, M.; Đurić, M.; Jevremović, S.; Dragićević, I.Č.; Subotić, A.R. Changes in photosynthetic pigments content in non-transformed and AtCKX transgenic centaury (Centaurium erythraea Rafn) shoots grown under salt stress in vitro. Agronomy 2021, 11, 2056. [Google Scholar] [CrossRef]
- Ito, H.; Ohtsuka, T.; Tanaka, A. Conversion of chlorophyll b to chlorophyll a via 7-hydroxymethyl chlorophyll. J. Biol. Chem. 1996, 271, 1475–1479. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nakagawa, H.; Asaka, T.; Matoh, T. Borate ± rhamnogalacturonan II bonding reinforced by Ca2+ retains pectic polysaccharides in higher-plant cell walls. Plant Physiol. 1999, 119, 199–204. [Google Scholar] [CrossRef]
- Park, S.; Fischer, A.L.; Steen, C.J.; Iwai, M.; Morris, J.M.; Walla, P.J.; Niyogi, K.K.; Fleming, G.R. Chlorophyll-carotenoid excitation energy transfer in high-light-exposed thylakoid membranes investigated by snapshot transient absorption spectroscopy. J. Am. Chem. Soc. 2018, 140, 11965–11973. [Google Scholar] [CrossRef]
- Bolaños, L.; Lukaszewski, K.; Bonilla, I.; Blevins, D. Why boron? Plant Physiol. Biochem. 2004, 42, 907–912. [Google Scholar] [CrossRef]
- Brown, P.H.; Shelp, B.J. Boron mobility in plants. Plant Soil 1997, 193, 85–101. [Google Scholar] [CrossRef]
- Stangoulis, J.; Tate, M.; Graham, R.; Bucknall, M.; Palmer, L.; Boughton, B.; Reid, R. The mechanism of boron mobility in wheat and canola phloem. Plant Physiol. 2010, 153, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Cervilla, L.M.; Blasco, B.; Ríos, J.J.; Romero, L.; Ruiz, J.M. Oxidative stress and antioxidants in tomato (Solanum lycopersicum) plants subjected to boron toxicity. Ann. Bot. 2007, 100, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Hamurcu, M.; Demiral, T.; Hakki, E.E.; Turkmen, Ö.; Gezgin, S.; Bell, R.W. Oxidative stress responses in watermelon (Citrullus lanatus) as influenced by boron toxicity and drought. Zemdirbyste/Agriculture 2015, 102, 209–216. [Google Scholar] [CrossRef]
- Samet, H.; Çıkılı, Y. Response of Purslane (Portulaca oleracea L.) to Excess Boron and Salinity: Physiological Approach. Russ. J. Plant Physiol. 2019, 66, 316–325. [Google Scholar] [CrossRef]
- Paull, J.G.; Cartwright, B.; Rathjen, A.J. Responses of wheat and barley genotypes to toxic concentrations of soil boron. Euphytica 1988, 39, 137–144. [Google Scholar] [CrossRef]
- Nable, R.O. Resistance to boron toxicity amongst several barley and wheat cultivars: A preliminary examination of the resistance mechanism. Plant Soil 1988, 112, 45–52. [Google Scholar] [CrossRef]
- Subedi, K.D.; Gregory, P.J.; Gooding, M.J. Boron accumulation and partitioning in wheat cultivars with contrasting tolerance to boron deficiency. Plant Soil 1999, 214, 141–152. [Google Scholar] [CrossRef]
- Francois, L.E. Effect of excess boron on broccoli, cauliflower, and radish. J. Am. Soc. Hortic. Sci. 1986, 111, 494–498. [Google Scholar] [CrossRef]
- Huang, S.S.; Liao, Q.L.; Hua, M.; Wu, X.M.; Bi, K.S.; Yan, C.Y.; Chen, B.; Zhang, X.Y. Survey of heavy metal pollution and assessment of agricultural soil in Yangzhong district, Jiangsu Province, China. Chemosphere 2007, 67, 2148–2155. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zeng, G.; Chai, L.; Song, X.; Min, Z.; Xiao, X. Subcellular distribution and chemical forms of cadmium in Bechmeria nivea (L.) Gaud. Environ. Exp. Bot. 2008, 62, 389–395. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2000; p. 432. [Google Scholar]
- Kumar, A.; Singh, U.M.; Manohar, M.; Gaur, V.S. Calcium transport from source to sink: Understanding the mechanism(s) of acquisition, translocation, and accumulation for crop biofortification. Acta Physiol. Plant. 2015, 37, 1722. [Google Scholar] [CrossRef]
- González-Fontes, A.; Navarro-Gochicoa, M.T.; Camacho-Cristóbal, J.J.; Herrera-Rodríguez, M.B.; Quiles-Pando, C.; Rexach, J. Is Ca2+ involved in the signal transduction pathway of boron deficiency? New hypotheses for sensing boron deprivation. Plant Sci. 2014, 217, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Moradi, L.; Ehsanzadeh, P. Effects of Cd on photosynthesis and growth of safflower (Carthamus tinctorius L.) genotypes. Photosynthetica 2015, 53, 506–518. [Google Scholar] [CrossRef]
- García-Sánchez, F.; Simón-Grao, S.; Martínez-Nicolás, J.J.; Alfosea-Simón, M.; Liu, C.; Chatzissavvidis, C.; Pérez-Pérez, J.G.; Cámara-Zapata, J.M. Multiple stresses occurring with boron toxicity and deficiency in plants. J. Hazard. Mater. 2020, 397, 122713. [Google Scholar] [CrossRef]
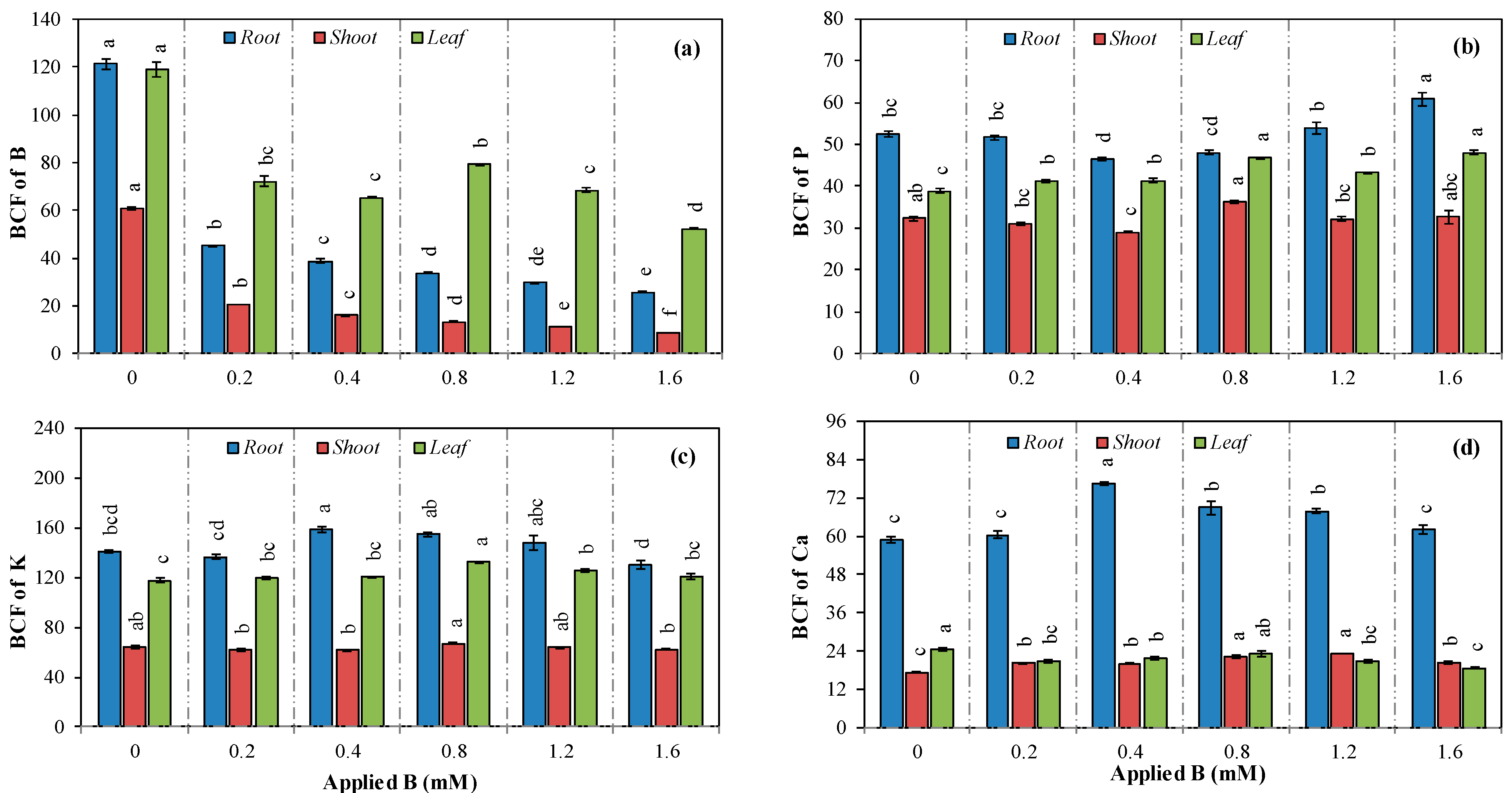

| Applied B (mM) | Boron Concentrations (mg kg−1) | Translocation Factor of B | |||
|---|---|---|---|---|---|
| Root | Shoot | Leaf | Root to Shoot | Root to Leaf | |
| 0 | 59.8 ± 1.01 f | 30.0 ± 0.26 e | 58.7 ± 5.07 e | 0.502 a | 0.984 d |
| 0.2 | 119.6 ± 1.52 e | 54.5 ± 0.34 d | 191.3 ± 13.2 d | 0.456 b | 1.601 c |
| 0.4 | 186.8 ± 4.14 d | 76.9 ± 0.40 c | 314.5 ± 4.00 c | 0.412 c | 1.685 c |
| 0.8 | 309.4 ± 4.38 c | 122.1 ± 2.31 b | 725.0 ± 1.80 b | 0.395 cd | 2.344 a |
| 1.2 | 400.8 ± 3.47 b | 151.8 ± 1.13 a | 922.1 ± 5.81 a | 0.379 d | 2.301 a |
| 1.6 | 456.6 ± 4.39 a | 155.0 ± 2.60 a | 931.3 ± 1.57 a | 0.340 e | 2.040 b |
| F-test | *** | *** | *** | *** | *** |
| Applied B (mM) | Translocation Factor of P | Translocation Factor of K | Translocation Factor of Ca | |||
|---|---|---|---|---|---|---|
| Root to Shoot | Root to Leaf | Root to Shoot | Root to Leaf | Root to Shoot | Root to Leaf | |
| 0 | 0.634 b | 0.740 c | 0.455 a | 0.836 bc | 0.295 b | 0.417 a |
| 0.2 | 0.600 bc | 0.800 c | 0.453 a | 0.878 ab | 0.334 a | 0.348 b |
| 0.4 | 0.626 b | 0.891 b | 0.388 b | 0.760 c | 0.263 c | 0.284 c |
| 0.8 | 0.756 a | 0.975 a | 0.433 ab | 0.856 ab | 0.321 ab | 0.337 b |
| 1.2 | 0.597 bc | 0.801 c | 0.433 ab | 0.851 abc | 0.341 a | 0.309 bc |
| 1.6 | 0.535 c | 0.788 c | 0.477 a | 0.928 a | 0.329 a | 0.302 bc |
| F-test | *** | *** | ** | *** | *** | *** |
| Applied B (mM) | Net Ion Accumulations via Roots (mg g−1 DW) | ||||
|---|---|---|---|---|---|
| B | P | K | Ca | Na | |
| 0 | 0.329 ± 0.004 f | 8.30 ± 0.21 c | 161.8 ± 2.59 b | 36.92 ± 0.72 b | 36.92 ± 0.63 ab |
| 0.2 | 1.028 ± 0.031 e | 9.48 ± 0.06 bc | 184.7 ± 1.63 a | 40.69 ± 0.53 a | 38.44 ± 0.62 ab |
| 0.4 | 1.584 ± 0.025 d | 8.94 ± 0.15 bc | 185.4 ± 2.51 a | 43.78 ± 0.55 a | 39.44 ± 0.74 a |
| 0.8 | 3.069 ± 0.024 c | 9.67 ± 0.09 b | 185.8 ± 1.64 a | 42.39 ± 1.00 a | 36.20 ± 0.55 b |
| 1.2 | 4.315 ± 0.062 b | 9.89 ± 0.18 b | 192.4 ± 4.22 a | 44.26 ± 0.83 a | 36.98 ± 0.23 ab |
| 1.6 | 4.774 ± 0.179 a | 11.48 ± 0.55 a | 195.8 ± 6.55 a | 42.01 ± 0.87 a | 36.22 ± 0.67 b |
| F-test | *** | *** | *** | *** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samet, H.; Çikili, Y. The Effects of Increasing Boron on Growth, Yield, and Nutritional Value of Scallion (Allium cepa L.) Grown as a Bunch Harvest. Horticulturae 2025, 11, 450. https://doi.org/10.3390/horticulturae11050450
Samet H, Çikili Y. The Effects of Increasing Boron on Growth, Yield, and Nutritional Value of Scallion (Allium cepa L.) Grown as a Bunch Harvest. Horticulturae. 2025; 11(5):450. https://doi.org/10.3390/horticulturae11050450
Chicago/Turabian StyleSamet, Halil, and Yakup Çikili. 2025. "The Effects of Increasing Boron on Growth, Yield, and Nutritional Value of Scallion (Allium cepa L.) Grown as a Bunch Harvest" Horticulturae 11, no. 5: 450. https://doi.org/10.3390/horticulturae11050450
APA StyleSamet, H., & Çikili, Y. (2025). The Effects of Increasing Boron on Growth, Yield, and Nutritional Value of Scallion (Allium cepa L.) Grown as a Bunch Harvest. Horticulturae, 11(5), 450. https://doi.org/10.3390/horticulturae11050450






