Physiological Investigation of Drought Stress Tolerance of ‘W. Murcott’ Mandarin Grafted onto ‘Carrizo’, ‘Sour Orange’, and ‘Volkameriana’ Rootstocks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Drought Treatment, and Determining Field Capacity of Soil
2.2. Plant Growth Measurements
2.3. Gas Exchange Measurements and Chlorophyll Concentration
2.4. Experimental Design and Data Analysis
3. Results
3.1. Plant Growth, Chlorophyll Concentration, and Chlorophyll Fluorescence
3.2. Leaf Gas Exchange Measurements
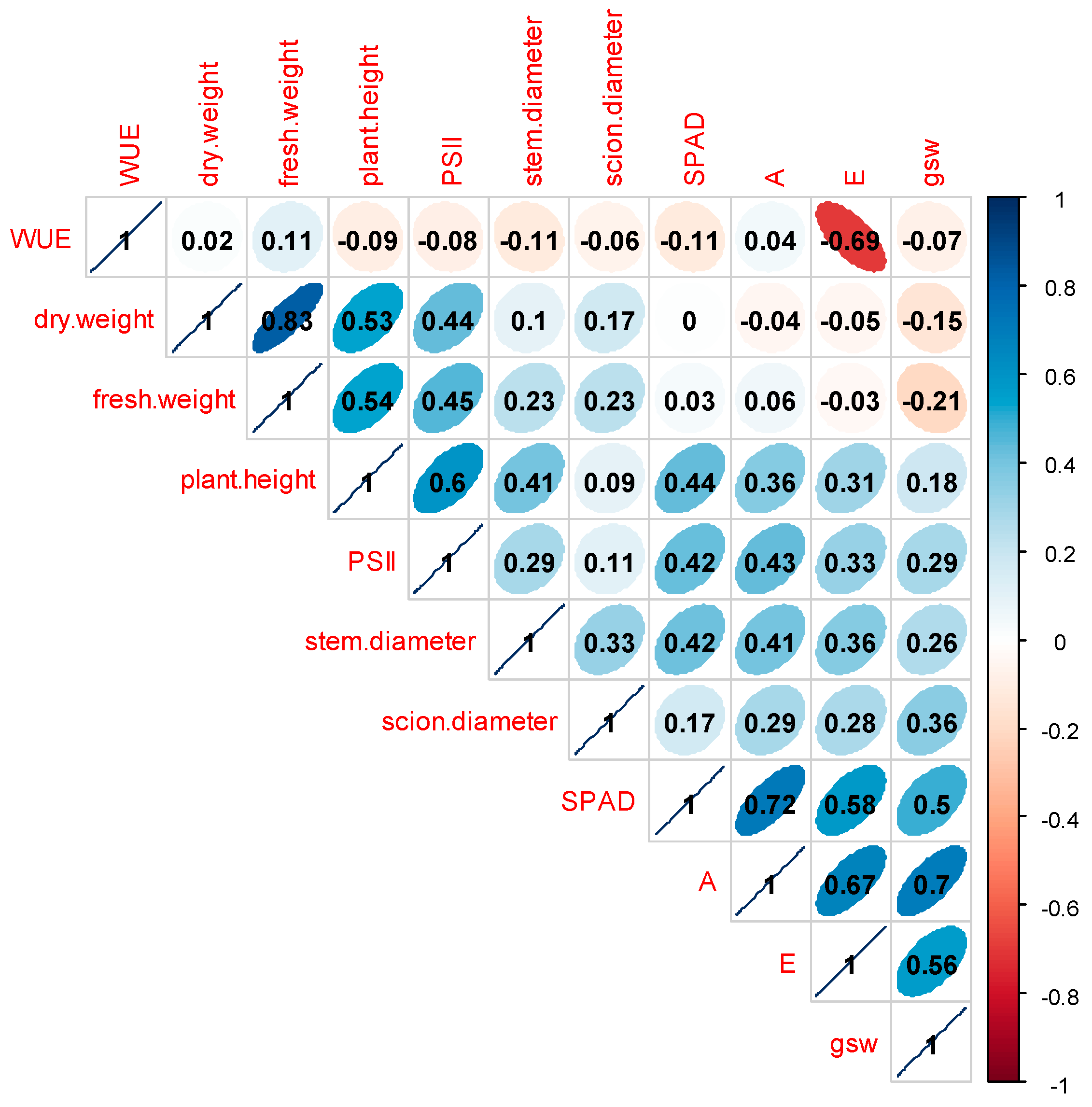
3.3. Correlation Coefficients Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. FAOSTAT-Agriculture Database (2022). Available online: http://faostat.fao.org/en/#data/QCL (accessed on 5 January 2025).
- Gimeno, J.; Gadea, J.; Forment, J.; Pérez-Valle, J.; Santiago, J.; Martínez-Godoy, M.A.; Yenush, L.; Bellés, J.M.; Brumós, J.; Colmenero-Flores, J.M.; et al. Shared and novel molecular responses of mandarin to drought. Plant Mol. Biol. 2009, 70, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef]
- Balfagón, D.; Zandalinas, S.I.; dos Reis de Oliveira, T.; Santa-Catarina, C.; Gómez-Cadenas, A.G. Reduction of heat stress pressure and activation of photosystem II repairing system are crucial for citrus tolerance to multiple abiotic stress combination. Physiol. Plant. 2022, 174, e13809. [Google Scholar] [PubMed]
- Romero, P.; Navarro, J.M.; Perez-Perez, J.; Garcia-Sanchez, F.; Gomez-Gomez, A.; Porras, I.; Martinez, V.; Botia, P. Deficit irrigation and rootstock: Their effects on water relations, vegetative development, yield, fruit quality and mineral nutrition of Clemenules mandarin. Tree Physiol. 2006, 26, 1537–1548. [Google Scholar]
- Garcıa-Sanchez, F.; Syvertsen, P.J.; Gimeno, V.; Botia, P.; Perez, G. Responses to Flooding and Drought Stres by Two Citrus Rootstock Seedlings with Different Water-Use Efficency. Physiol. Plant. 2007, 130, 532–542. [Google Scholar]
- Rodriguez-Gamir, J.; Primo-Millo, E.; Forner, J.B.; Forner-Giner, M.A. Citrus rootstock responses to water stress. Sci. Hortic. 2010, 126, 95–102. [Google Scholar]
- García-Tejero Iván, F.; Víctor, H.; Durán-Zuazo, J.; Muriel-Fernández, L.; Jiménez-Bocanegra, J.A. Linking canopy temperature and trunk diameter fluctuations with other physiological water status tools for water stress management in citrus orchards. Funct. Plant Biol. 2011, 38, 106–117. [Google Scholar] [CrossRef]
- Cerçi, S. Effects of Drought Stress on Some Photosynthetic Parameters and Plant Nutrient Concentrations in Different Citrus Rootstocks. Master’s Thesis, Institute of Science, Çukurova University, Adana, Turkey, 2012; 104p. [Google Scholar]
- Zandalinas, S.I.; Rivero, R.M.; Martínez, V.; Gómez-Cadenas, A.; Arbona, V. Tolerance of citrus plants to the combination of high temperatures and drought is associated to the increase in transpiration modulated by a reduction in abscisic acid levels. BMC Plant Biol. 2016, 16, 105. [Google Scholar] [CrossRef]
- Souza, J.D.; Silva, D.A.E.M.; Filho, M.A.C.; Morillon, R.; Bonatto, D.; Micheli, F.; Gesteria, A.D.S. Different adaptation strategies of two citrus scion/rootstock combinations in response to drought stress. PLoS ONE 2017, 12, e0177993. [Google Scholar]
- Korkmaz, N.; Aşkın, A.; Altunlu, H.; Polat, M.; Okatan, V.; Kahramanoglu, I. The effects of melatonin application on the drought stress of different citrus rootstocks. Turk. J. Agric. For. 2022, 46, 585–600. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.Y.; Wang, L.C.; Saleem, M.F.; Chen, M.; Wang, L. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Hu, F.; Zhang, Y.; Guo, J. Effects of drought stress on photosynthetic physiological characteristics, leaf microstructure, and related gene expression of yellow horn. Plant Signal. Behav. 2023, 18, e2215025. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, E. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Wu, X.; Yuan, J.; Luo, A.; Chen, Y.; Fan, Y. Drought stress and rewatering increase secondary metabolites and enzyme activity in dendrobium moniliforme. Ind. Crops Prod. 2016, 94, 385–393. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef]
- Cornic, G. Drought stress inhibits photosynthesis by decreasing stomatal aperture—Not by affecting ATP synthesis. Trends Plant Sci. 2000, 5, 187–188. [Google Scholar]
- Grassi, G.; Magnani, F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 2005, 28, 834–849. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Galmes, J.; Medrano, H.; Ribas-Carbó, M. Keeping a Positive Carbon Balance under Adverse Conditions: Responses of Photosynthesis and Respiration to Water Stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Ferguson, L.; Sakovich, N.; Roose, M. California Citrus Rootstocks; University of California Cooperative Extension Publication: Berkeley, CA, USA, 1990. [Google Scholar]
- von Broembsen. L.A. Citrus Rootstocks—The Choice You Have; S.A. Cooperative Citrus Exchange Ltd.: Pretoria, South Africa, 1985; p. 19. [Google Scholar]
- Castle, W.S.; Tucker, D.P.H.; Krezdom, A.H.; Youtsey, C.O. Rootstocks for Florida Citrus; University of Florida Cooperative Extension Service Publication: Gainesville, FL, USA, 1989; p. 41. [Google Scholar]
- Diaz-Curti, S.A.; Hernandez, C.; Lopez-Maza, I.; Salazar-Loredo, R.X.; Gonzalez-Sanchez, A.; Drought Tolerance of 27 Citrus Rootstocks Growing in a Nursery. International Society of Citriculture Abstracts. 2004; p. 145. Available online: https://www.ars.usda.gov/research/publications/publication/?seqNo115=163292 (accessed on 5 January 2025).
- Gonçalves, L.P.; Alves, T.O.; Martins, C.P.S.; de Sousa, A.O.; dos Santos, I.C.; Pirovani, C.P.; Almeida, A.F.; Filho, M.A.C.; Gesteira, A.S.; Soares Filho, S.; et al. Rootstock-induced physiological and biochemical mechanisms of drought tolerance in sweet orange. Acta Physiol. Plant. 2016, 38, 174. [Google Scholar] [CrossRef]
- Santana-Vieira, D.D.S.; Freschi, L.; Almeida, L.A.D.H.; Moraes, D.H.S.D.; Neves, D.M.; Santos, L.M.D.; Neves, D.M.; Santos, L.M.; Bertolde, F.Z.; Soares Filho, W.S.; et al. Survival strategies of citrus rootstocks subjected to drought. Sci. Rep. 2016, 6, 38775. [Google Scholar] [CrossRef]
- Sousa, A.D.O.; Silva, D.A.E.M.; Filho, M.A.C.; Costa, M.G.C.; Filho, W.D.S.S.; Micheli, F.; Maserti, B.; Gesteria, A.D.S. Metabolic responses to drought stress and rehydration in leaves and roots of three citrus scion/rootstock combinations. Sci. Hortic. 2022, 292, 110490. [Google Scholar] [CrossRef]
- Cimen, B.; Yesiloglu, T. Rootstock Breeding for Abiotic Stress Tolerance in Citrus; InTech: Houston, TX, USA, 2016. [Google Scholar]
- Klute, A. Methods of Soil Analysis. Part 1: Physical and Mineralogical Methods, 2nd ed.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1986. [Google Scholar]
- Magalhães Filho, J.R.; Amaral, L.R.; Machado, D.F.S.P.; Medina, C.L.; Machado, E.C. Water deficit, gas exchange and root growth in’ Valencia’ orange tree budded on two rootstocks. Bragantia 2008, 67, 75–82. [Google Scholar] [CrossRef]
- Kumar, S.; Awasthi, O.P.; Dubey, A.K.; Pandey, R.; Sharma, V.K.; Mishra, A.K.; Sharma, R.M. Root morphology and the effect of rootstocks on leaf nutrient acquisition of Kinnow mandarin (Citrus nobilis Loureiro × Citrus reticulata Blanco). J. Hortic. Sci. Biotechnol. 2017, 93, 100–106. [Google Scholar] [CrossRef]
- Monteoliva, M.I.; Guzzo, M.C.; Posada, G.A. Breeding for drought tolerance by monitoring chlorophyll content. Gene Technol. 2021, 10, 10–35248. [Google Scholar]
- Perez-Perez, J.G.; Syvertsen, J.P.; Botia, P.; Garcıa-Sanchez, F. Leaf Water Relations and Net Gas Exchange Responses of Salinized Carrizo citrange Seedlings During Drought Stress and Recovery. Ann. Bot. 2007, 100, 335–345. [Google Scholar] [PubMed]
- Kaçar, B.; Katkat, A.V.; Öztürk, Ş. Plant Physiology; Uludag University: Bursa, Turkey, 2002. [Google Scholar]
- Guo, C.; Liu, L.; Sun, H.; Wang, N.; Zhang, K.; Zhang, Y.; Zhu, J.; Li, A.; Bai, Z.; Liu, X.; et al. Predicting Fv/Fm and evaluating cotton drought tolerance using hyperspectral and 1D-CNN. Front. Plant Sci. 2022, 13, 1007150. [Google Scholar]
- Soto, A.C.; María Sara, M.; Ríos Rojas, M. Gas exchange and fluorescence in ‘sutil’ lime (Citrus aurantifolia Swingle) under different soil moisture levels. Bioagro 2022, 34, 195–206. [Google Scholar] [CrossRef]
- Vu, J.C.V.; Yelenosky, G. Biomass and photosynthesis responses of rough lemon (Citrus jambhiri Lush.) to growth regulators. Can. J. Plant Sci. 1988, 68, 261–266. [Google Scholar]
- Chen, Z.H.; Zhang, L.C.; Wu, G.L.; Zhang, S.L. Photosynthesis of Citrus Under Water Stres. Acta Agric.-Univ. Zheylangensis 1992, 18, 60–66. [Google Scholar]
- Moran, E.F.; Brondizio, E.S.; Mausel, P.; Wu, Y. Integrating Amazonian Vegetation, Land-use and Satellite Data. BioScience 1994, 44, 329–338. [Google Scholar]
- Erismann, N.; Machado, E.C.; Sant, M.L.; Tucci, A. Photosynthetic Limitation by CO2 Diffusion in Drought Stresses Orange Leaves on Three Rootstocks. Photosynth. Res. 2008, 96, 163–172. [Google Scholar]
- Kutlu, I. Drought Stress in Cereals. Turk. J. Sci. Rev. 2010, 3, 35–41. [Google Scholar]
- Kuşvuran, Ş.; Daşgan, H.Y.; Abak, K. Responses of Different Okra Genotypes to Drought Stress. In Proceedings of the VII. Vegetable Farming Symposium, Yalova, Türkiye, 26–29 August 2008; pp. 329–333. [Google Scholar]

| Dependent Variable | Independent Variable | ||
|---|---|---|---|
| R | DI | R × DI | |
| Plant height (cm) | 0.57 ns | 17.80 * | 0.99 ns |
| Rootstock diameter (mm) | 4.87 * | 9.84 * | 3.11 * |
| Scion diameter (mm) | 7.84 * | 2.70 ns | 1.32 ns |
| SPAD | 8.58 * | 16.73 * | 2.36 ns |
| Fv’/Fm’ | 0.26 ns | 31.46 * | 1.29 ns |
| Fresh weight (g) | 11.79 * | 16.28 * | 1.22 ns |
| Total dry weight (g) | 19.19 * | 12.89 * | 0.21 ns |
| PN | 10.91 * | 12.37 * | 0.92 ns |
| E | 2.80 ns | 3.56 * | 1.32 ns |
| gS | 3.30 ns | 1.94 ns | 1.07 ns |
| WUE | 0.06 ns | 0.14 ns | 0.67 ns |
| Rootstock | DI | Fresh Weight (g) | Dry Weight (g) | Plant Height (cm) |
|---|---|---|---|---|
| Carrizo | 174.80 a | 96.80 a | 7.00 | |
| Sour orange | 119.60 b | 53.07 b | 6.40 | |
| Volkameriana | 163.60 a | 83.07 a | 7.10 | |
| 40% | 117.87 c | 59.33 c | 5.17 b | |
| 50% | 153.73 b | 77.60 b | 6.13 b | |
| Control | 186.40 a | 96.00 a | 9.20 a | |
| Carrizo | 40% | 140.80 | 75.60 | 5.40 |
| 50% | 171.20 | 97.60 | 6.40 | |
| Control | 212.40 | 117.20 | 9.20 | |
| Sour orange | 40% | 98.80 | 37.20 | 4.60 |
| 50% | 125.60 | 48.80 | 5.80 | |
| Control | 134.40 | 73.20 | 8.80 | |
| Volkameriana | 40% | 114.00 | 65.20 | 5.50 |
| 50% | 164.40 | 86.40 | 6.20 | |
| Control | 212.40 | 97.60 | 9.60 | |
| LSD0.05 | Rootstock | 24.366 | 14.640 | ns |
| Treatment | 24.366 | 14.640 | 1.431 | |
| Interaction | ns | ns | ns |
| Rootstock | DI | Rootstock Diameter (mm) | Scion Diameter (mm) | SPAD | Fv’/Fm’ |
|---|---|---|---|---|---|
| Carrizo | 0.48 b | 0.26 b | 45.93 b | 0.52 | |
| Sour orange | 0.79 a | 0.32 b | 54.18 a | 0.53 | |
| Volkameriana | 0.81 a | 0.50 a | 48.62 b | 0.51 | |
| 40% | 0.47 b | 0.29 | 44.63 b | 0.40 c | |
| 50% | 0.64 b | 0.35 | 48.02 b | 0.55 b | |
| Control | 0.98 a | 0.44 | 56.07 a | 0.62 a | |
| Carrizo | 40% | 0.29 d | 0.28 | 42.26 | 0.38 |
| 50% | 0.55 bcd | 0.17 | 44.54 | 0.55 | |
| Control | 0.61 bcd | 0.32 | 50.98 | 0.65 | |
| Sour orange | 40% | 0.66 bcd | 0.26 | 46.88 | 0.39 |
| 50% | 0.82 bc | 0.32 | 50.08 | 0.56 | |
| Control | 0.90 b | 0.38 | 65.58 | 0.64 | |
| Volkameriana | 40% | 0.45 cd | 0.33 | 44.74 | 0.43 |
| 50% | 0.55 bcd | 0.56 | 49.45 | 0.54 | |
| Control | 1.43 a | 0.62 | 51.66 | 0.56 | |
| LSD0.05 | Rootstock | 0.239 | 0.129 | 4.121 | ns |
| Treatment | 0.239 | ns | 4.121 | 0.057 | |
| Interaction | 0.414 | ns | ns | ns |
| Rootstock | DI | PN | E | gSw | WUE |
|---|---|---|---|---|---|
| Carrizo | 5.25 c | 1.61 | 34.00 | 3.36 | |
| Sour orange | 6.57 a | 2.06 | 41.78 | 3.33 | |
| Volkameriana | 5.91 b | 1.91 | 39.67 | 3.23 | |
| 40% | 5.35 b | 1.68 b | 38.00 | 3.19 | |
| 50% | 5.67 b | 1.71 b | 35.67 | 3.42 | |
| Control | 6.70 a | 2.16 a | 41.78 | 3.22 | |
| Carrizo | 40% | 4.86 | 1.50 | 35.00 | 3.47 |
| 50% | 4.98 | 1.63 | 30.00 | 3.10 | |
| Control | 5.90 | 1.69 | 37.00 | 3.50 | |
| Sour orange | 40% | 6.02 | 1.87 | 41.67 | 3.44 |
| 50% | 6.00 | 1.62 | 35.33 | 3.71 | |
| Control | 7.68 | 2.70 | 48.33 | 2.85 | |
| Volkameriana | 40% | 5.18 | 1.77 | 37.33 | 2.94 |
| 50% | 6.02 | 1.87 | 41.67 | 3.44 | |
| Control | 6.52 | 2.10 | 40.00 | 3.31 | |
| LSD0.05 | Rootstock | 0.592 | ns | ns | ns |
| Treatment | 0.493 | 0.411 | ns | ns | |
| Interaction | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Incesu, M.; Cimen, B.; Yilmaz, B.; Yesiloglu, T.; Ilhan, M. Physiological Investigation of Drought Stress Tolerance of ‘W. Murcott’ Mandarin Grafted onto ‘Carrizo’, ‘Sour Orange’, and ‘Volkameriana’ Rootstocks. Horticulturae 2025, 11, 365. https://doi.org/10.3390/horticulturae11040365
Incesu M, Cimen B, Yilmaz B, Yesiloglu T, Ilhan M. Physiological Investigation of Drought Stress Tolerance of ‘W. Murcott’ Mandarin Grafted onto ‘Carrizo’, ‘Sour Orange’, and ‘Volkameriana’ Rootstocks. Horticulturae. 2025; 11(4):365. https://doi.org/10.3390/horticulturae11040365
Chicago/Turabian StyleIncesu, Meral, Berken Cimen, Bilge Yilmaz, Turgut Yesiloglu, and Merve Ilhan. 2025. "Physiological Investigation of Drought Stress Tolerance of ‘W. Murcott’ Mandarin Grafted onto ‘Carrizo’, ‘Sour Orange’, and ‘Volkameriana’ Rootstocks" Horticulturae 11, no. 4: 365. https://doi.org/10.3390/horticulturae11040365
APA StyleIncesu, M., Cimen, B., Yilmaz, B., Yesiloglu, T., & Ilhan, M. (2025). Physiological Investigation of Drought Stress Tolerance of ‘W. Murcott’ Mandarin Grafted onto ‘Carrizo’, ‘Sour Orange’, and ‘Volkameriana’ Rootstocks. Horticulturae, 11(4), 365. https://doi.org/10.3390/horticulturae11040365





