Double Grafting: A Synthesis of Applications and Future Research Horizons
Abstract
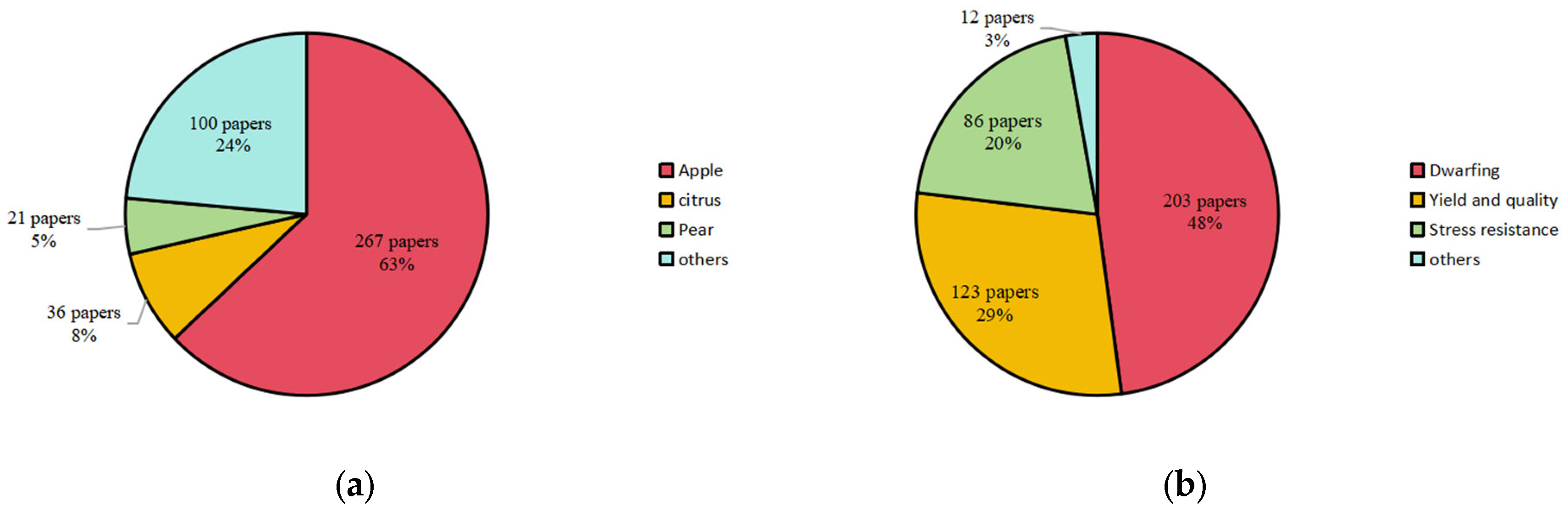
1. Introduction
2. Effects of Double Grafting on Plant Physiology
2.1. Healing Process
2.2. Photosynthetic Function
2.3. Nutrient Uptake and Transportation
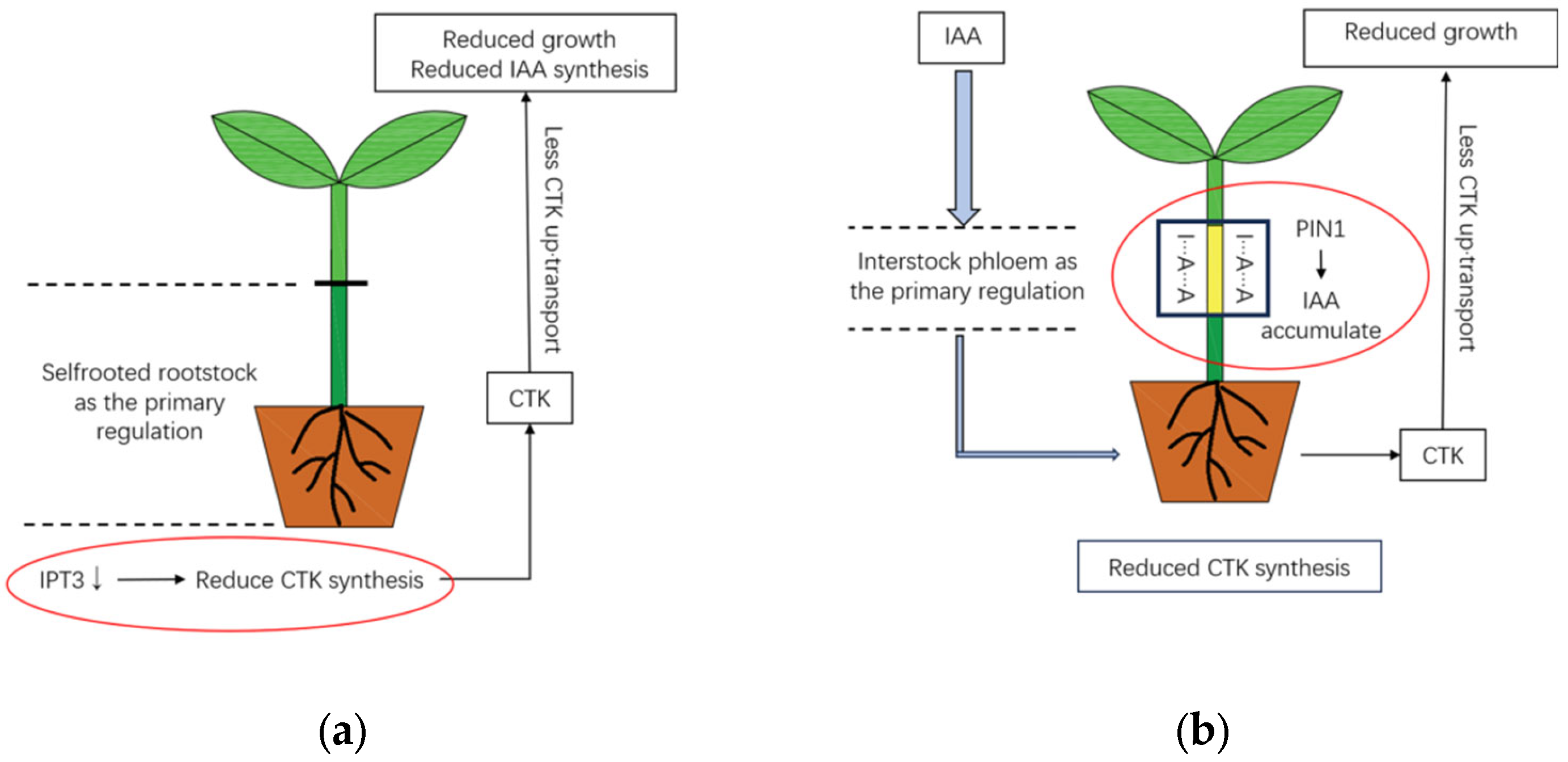
2.4. Phytohormone Regulation
2.5. Gene Expression and Biomass Metabolism
3. Application of Double Grafting
3.1. Dwarfing
3.2. Regulation of Flower Bud Differentiation
3.3. Enhancing Stress Tolerance
3.4. Enhancement of Yield and Quality
3.5. As a Mediator of Non-Compatible Grafting
3.6. Microinterstocks
4. Research Directions for Subsequent Studies on Double Grafting
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldschmidt, E.E. Plant Grafting: New Mechanisms, Evolutionary Implications. Front. Plant Sci. 2014, 5, 727. [Google Scholar] [CrossRef] [PubMed]
- Miodragović, M.; Magazin, N.; Keserović, Z.; Milić, B.; Popović, B.; Blagojević, B.; Kalajdžić, J. The Early Performance and Fruit Properties of Apricot Cultivars Grafted on Prunus spinosa L. Interstock. Sci. Hortic. 2019, 250, 199–206. [Google Scholar] [CrossRef]
- De Francesco, A.; Simeone, M.; Gómez, C.; Costa, N.; García, M.L. Transgenic Sweet Orange Expressing Hairpin CP-mRNA in the Interstock Confers Tolerance to Citrus Psorosis Virus in the Non-Transgenic Scion. Transgenic. Res. 2020, 29, 215–228. [Google Scholar] [CrossRef]
- Guilherme, D.D.O.; Marinho, C.S.; Biazatt, M.A.; Campos, G.S.; Bremenkamp, C.A. Produção de Mudas de Laranjeira Pêra Por Meio Do Método de Interenxertia. Cienc. Rural. 2014, 44, 414–417. [Google Scholar] [CrossRef]
- Popara, G.; Magazin, N.; Keserović, Z.; Milić, B.; Milović, M.; Kalajdžić, J.; Manojlović, M. Rootstock and Interstock Effects on Plum Cv. ‘Čačanska Lepotica’ Young Tree Performance and Fruit Quality Traits. Erwerbs-Obstbau 2020, 62, 421–428. [Google Scholar] [CrossRef]
- Sun, W.; Cao, Y.; Du, P.; Li, Z.; Xu, Z.; Zhou, S.; Li, Z.; Zhang, X.; Liang, B. Evaluation of Phlorizin Stress Resistance and Mineral Nutrient Utilization Efficiency in Apple Seedlings with Different Dwarfing Interstocks. Sci. Hortic. 2025, 340, 113947. [Google Scholar] [CrossRef]
- Hayat, F.; Li, J.; Iqbal, S.; Peng, Y.; Hong, L.; Balal, R.M.; Khan, M.N.; Nawaz, M.A.; Khan, U.; Farhan, M.A.; et al. A Mini Review of Citrus Rootstocks and Their Role in High-Density Orchards. Plants 2022, 11, 2876. [Google Scholar] [CrossRef]
- Kirca, L.; Karadeniz, T. Histological Investigation of the Compatibility of Prunus spinosa as Interstock with Almond Cultivars. Appl. Fruit Sci. 2024, 66, 1305–1316. [Google Scholar] [CrossRef]
- Serivichyaswat, P.T.; Kareem, A.; Feng, M.; Melnyk, C.W. Auxin Signaling in the Cambium Promotes Tissue Adhesion and Vascular Formation during Arabidopsis Graft Healing. Plant Physiol. 2024, 196, 754–762. [Google Scholar] [CrossRef]
- Wang, L.; Liao, Y.; Liu, J.; Zhao, T.; Jia, L.; Chen, Z. Advances in Understanding the Graft Healing Mechanism: A Review of Factors and Regulatory Pathways. Hortic. Res. 2024, 11, uhae175. [Google Scholar] [CrossRef]
- Feng, M.; Augstein, F.; Kareem, A.; Melnyk, C.W. Plant Grafting: Molecular Mechanisms and Applications. Mol. Plant 2024, 17, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.K.; Melnyk, C.W. The Role of Plant Hormones during Grafting. J. Plant. Res. 2018, 131, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, S.; Fevereiro, P.; Kragler, F.; Pina, A. Plant Grafting and Graft Incompatibility: A Review from the Grapevine Perspective. Sci. Hortic. 2022, 299, 111019. [Google Scholar] [CrossRef]
- Li, H.; Li, D.; Wang, R.; Ma, C. Effects of different dwarfing interstocks on Whangkumbae pear growth and correlation analysis of graft-compatibility. Acta Agric. Zhejiangensis 2022, 34, 1175–1182. [Google Scholar] [CrossRef]
- Wang, T.; Deng, L.; Huang, S.; Xiong, B.; Ihtisham, M.; Zheng, Z.; Zheng, W.; Qin, Z.; Zhang, M.; Sun, G.; et al. Genetic Relationship, SPAD Reading, and Soluble Sugar Content as Indices for Evaluating the Graft Compatibility of Citrus Interstocks. Biology 2022, 11, 1639. [Google Scholar] [CrossRef]
- Wang, T.; Jin, Z.; Yuan, Y.; Deng, L.; Sun, G.; He, S.; Liao, L.; Wang, J.; Xiong, B.; Wang, Z. Interstock-Mediated Graft Incompatibility: Insights into Photosynthetic Pigments, Carbohydrates, Antioxidant Defense Systems, and Hormones Response Mechanisms in Citrus. Plants 2025, 14, 522. [Google Scholar] [CrossRef]
- Rong, Y.; Liao, L.; Li, S.; Wei, W.; Bi, X.; Sun, G.; He, S.; Wang, Z. Comparative Transcriptomic and Physiological Analyses Reveal Key Factors for Interstocks to Improve Grafted Seedling Growth in Tangor. Int. J. Mol. Sci. 2023, 24, 6533. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Qin, S.J.; Ma, X.X.; Zhang, J.E.; Zhou, P.; Sun, M.; Wang, B.S.; Zhou, H.F.; Lyu, D.G. Effect of Interstocks on the Photosynthetic Characteristics and Carbon Distribution of Young Apple Trees during the Vigorous Growth Period of Shoots. Europ. J. Hort. Sci. 2015, 80, 296–305. [Google Scholar] [CrossRef]
- Fayek, M.A.; Rashedy, A.A.; Ali, A.E.M. Alleviating the Adverse Effects of Deficit Irrigation in Flame Seedless Grapevine via Paulsen Interstock. Rev. Bras. Frutic. 2022, 44, e-839. [Google Scholar] [CrossRef]
- Calderón, F.J.; Weibel, A.M.; Trentacoste, E.R. Effects of Different Interstock Length on Vegetative Growth and Flowering in Peach Cv. Pavie Catherine. Sci. Hortic. 2021, 285, 110174. [Google Scholar] [CrossRef]
- Casierra–Posada, F.; Guzmán, J.A. Effect of Rootstock and Interstock on Mthe Fruit Quality of Mango (Mangifera Indica L.). Agron. Colomb. 2009, 27, 367–374. [Google Scholar]
- Zhou, S.; Shen, Z.; Yin, B.; Liang, B.; Li, Z.; Zhang, X.; Xu, J. Effects of Dwarfing Interstock Length on the Growth and Fruit of Apple Tree. Horticulturae 2023, 9, 40. [Google Scholar] [CrossRef]
- Li, C.; Yang, T.; Gao, J.; Wang, Q.; Cai, H.; Du, X.; Wang, S.; Gong, G.; Wang, X.; Zhang, Y.; et al. Dwarfing Mechanism of Apple Dwarf Rootstock: Research Progress. Chin. Agric. Sci. Bull. 2017, 33, 86. [Google Scholar] [CrossRef]
- Doley, D. Effects of Rootstocks and Interstock on Cell Dimensions in Scion Stems of Apple (Malus pumila Mill). New Phytol. 1974, 73, 173–194. [Google Scholar] [CrossRef]
- Shen, Y.; Zhuang, W.; Tu, X.; Gao, Z.; Xiong, A.; Yu, X.; Li, X.; Li, F.; Qu, S. Transcriptomic Analysis of Interstock-Induced Dwarfism in Sweet Persimmon (Diospyros kaki Thunb.). Hortic. Res. 2019, 6, 51. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, W.; Tahir, M.M.; Chen, X.; Saudreau, M.; Zhang, D.; Costes, E. Contributions of Leaf Distribution and Leaf Functions to Photosynthesis and Water-Use Efficiency from Leaf to Canopy in Apple: A Comparison of Interstocks and Cultivars. Front. Plant Sci. 2023, 14, 1117051. [Google Scholar] [CrossRef]
- Gimeno, V.; Syvertsen, J.P.; Simon, I.; Martinez, V.; Camara-Zapata, J.M.; Nieves, M.; Garcia-Sanchez, F. Interstock of ‘Valencia’ Orange Affects the Flooding Tolerance in ‘Verna’ Lemon Trees. HortScience 2012, 47, 403–409. [Google Scholar] [CrossRef]
- Ma, L.; Hou, C.W.; Zhang, X.Z.; Li, H.L.; Han, D.G.; Wang, Y.; Han, Z.H. Seasonal Growth and Spatial Distribution of Apple Tree Roots on Different Rootstocks or Interstems. J. Am. Soc. Hortic. Sci. 2013, 138, 79–87. [Google Scholar] [CrossRef]
- Zapata, J.M.C.; Cerdá, A.; Nieves, M. Interstock-Induced Mechanism of Increased Growth and Salt Resistance of Orange (Citrus Sinensis) Trees. Tree Physiol. 2004, 24, 1109–1117. [Google Scholar] [CrossRef]
- Guo, Z.; Qin, Y.; Lv, J.; Wang, X.; Dong, H.; Dong, X.; Zhang, T.; Du, N.; Piao, F. Luffa Rootstock Enhances Salt Tolerance and Improves Yield and Quality of Grafted Cucumber Plants by Reducing Sodium Transport to the Shoot. Environ. Pollut. 2023, 316, 120521. [Google Scholar] [CrossRef]
- Ji, J.; He, X.; Liu, H.; Li, Z.; Zhou, S.; Zhang, X.; Xu, J.; Liang, B. Influence of Dwarfing Interstock on the Tolerance and Nutrient Utilization Efficiency of Apple Trees under Drought Stress. Sci. Hortic. 2023, 315, 111984. [Google Scholar] [CrossRef]
- Li, H.L.; Zhang, H.; Yu, C.; Ma, L.; Wang, Y.; Zhang, X.Z.; Han, Z.H. Possible Roles of Auxin and Zeatin for Initiating the Dwarfing Effect of M9 Used as Apple Rootstock or Interstock. Acta. Physiol. Plant. 2012, 34, 235–244. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Z.; Li, Y.; Li, W.; Sun, L.; Xu, J.; Mao, J.; Chen, B. Modification of Fruit Quality and Leaf Mineral Composition of ‘Nagafu No.2’ Apple Grafted onto Four Dwarfing Interstocks. J. Plant. Growth Regul. 2023, 42, 7240–7256. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, X.; Wu, T.; Xu, X.; Han, Z.; Wang, Y. Methylation Effect on IPT5b Gene Expression Determines Cytokinin Biosynthesis in Apple Rootstock. Biochem. Biophys. Res. Commun. 2017, 482, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Sharma, N.C.; Sharma, D.P.; Kumar, P.; Chand, K.; Thakur, H. Dwarfism Mechanism in Malus Clonal Rootstocks. Planta 2024, 260, 133. [Google Scholar] [CrossRef]
- Ren, X.F.; Li, B.Z.; Zhang, L.S.; Han, M.Y.; Li, X.W. Effects of Planting Depth of Apple Dwarfing Rootstock on Root Growth, Hormone Content, Fruit Yields and Quality. Acta Hortic. Sin. 2013, 40, 2127. [Google Scholar]
- Li, Q.; Gao, Y.; Wang, K.; Feng, J.; Sun, S.; Lu, X.; Liu, Z.; Zhao, D.; Li, L.; Wang, D. Transcriptome Analysis of the Effects of Grafting Interstocks on Apple Rootstocks and Scions. Int. J. Mol. Sci. 2023, 24, 807. [Google Scholar] [CrossRef]
- Liao, L.; Li, Y.; Bi, X.; Xiong, B.; Wang, X.; Deng, H.; Zhang, M.; Sun, G.; Jin, Z.; Huang, Z.; et al. Transcriptome Analysis of Harumi Tangor Fruits: Insights into Interstock-Mediated Fruit Quality. Front. Plant Sci. 2022, 13, 995913. [Google Scholar] [CrossRef]
- Jing, J.; Liu, M.; Yin, B.; Liang, B.; Li, Z.; Zhang, X.; Xu, J.; Zhou, S. Effects of 10 Dwarfing Interstocks on Cold Resistance of ‘Tianhong 2’ Apple. Horticulturae 2023, 9, 827. [Google Scholar] [CrossRef]
- Wang, L.; Guan, M.; Zhong, D.; Liu, S. Effects of Interstock Grafting on Cucumber Resistance to Powdery Mildew. J. Shandong Agric. Univ. (Nat. Sci. Ed.) 2020, 51, 206–211. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, H.; Galarneau, E.R.-A.; Yang, Y.; Li, D.; Song, J.; Ma, C.; Zhang, S.; Wang, R. Metabolome Analysis Reveals Important Compounds Related to Dwarfing Effect of Interstock on Scion in Pear. Ann. Appl. Biol. 2021, 179, 108–122. [Google Scholar] [CrossRef]
- Li, W.; Chu, C.; Li, H.; Zhang, H.; Sun, H.; Wang, S.; Wang, Z.; Li, Y.; Foster, T.M.; López-Girona, E.; et al. Near-Gapless and Haplotype-Resolved Apple Genomes Provide Insights into the Genetic Basis of Rootstock-Induced Dwarfing. Nat. Genet. 2024, 56, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Shi, Y.; Yin, B.; Zhou, S.; Li, Z.; Zhang, X.; Xu, J. Effect of Different Dwarfing Interstocks on the Vegetative Growth and Nitrogen Utilization Efficiency of Apple Trees under Low-Nitrate and Drought Stress. Sci. Hortic. 2022, 305, 111369. [Google Scholar] [CrossRef]
- Ou, C.; Wang, F.; Wang, J.; Li, S.; Zhang, Y.; Fang, M.; Ma, L.; Zhao, Y.; Jiang, S. A de Novo Genome Assembly of the Dwarfing Pear Rootstock Zhongai 1. Sci. Data 2019, 6, 281. [Google Scholar] [CrossRef]
- Liao, L.; Li, J.; Lan, X.; Li, Y.; Li, Y.; Huang, Z.; Jin, Z.; Yang, Y.; Wang, X.; Zhang, M.; et al. Exogenous Melatonin and Interstock Treatments Confer Chilling Tolerance in Citrus Fruit during Cold Storage. Sci. Hortic. 2024, 327, 112802. [Google Scholar] [CrossRef]
- Nee, C. 039 Interstock Preservation of Plant Germplasm. HortScience 1994, 29, 433d–433. [Google Scholar] [CrossRef]
- Zhao, D.; Yuan, J.; Xu, K.; Cheng, C.; Li, H. Selection of Morphological, Physiological and Biochemical Indices: Evaluating Dwarfing Apple Interstocks in Cold Climate Zones. N. Z. J. Crop Hortic. Sci. 2016, 44, 291–311. [Google Scholar] [CrossRef]
- Di Vaio, C.; Cirillo, C.; Buccheri, M.; Limongelli, F. Effect of Interstock (M.9 and M.27) on Vegetative Growth and Yield of Apple Trees (Cv “Annurca”). Sci. Hortic. 2009, 119, 270–274. [Google Scholar] [CrossRef]
- Yuan, J.-C.; Cheng, C.-G.; Zhao, D.-Y.; Liu, S.-T.; Li, E.-M. Effects of different interstocks on the growth, yield, and fruit quality of Hanfu apple. Ying Yong Sheng Tai Xue Bao 2021, 32, 3145–3151. [Google Scholar] [CrossRef]
- Oliveira, J.A.A.; Bruckner, C.H.; Silva, D.F.P.D.; Santos, C.E.M.D.; Soares, W.D.S.; Nunes, L.V. Performance of Interstocks in the Plant Development and Fruit Quality of Plum Trees. Acta Sci. Agron. 2019, 41, 39928. [Google Scholar] [CrossRef]
- Edstrom, J.P.; Connell, J.H.; Micke, W.C.; Yeager, J.T. ‘Marianna 2624’ Plum as a Rootstock for Almond. Horttech 1999, 9, 127. [Google Scholar] [CrossRef]
- Yilmaz, B.; Kamiloğlu, M.; Çimen, B.; Incesu, M.; Yesiloglu, T.; Tuzcu, O. Effects of different interstock lengths on the yield, fruit quality and tree size of Kütdiken lemon trees in Turkey. J. Glob. Agric. Ecol. 2015, 3, 91–96. [Google Scholar]
- Kamiloğlu, M.; Yeşiloğlu, T. Effect of Interstocks In Photosynthesis and Growth Rates for ‘Navelina’ Orange And ‘Kütdiken’ Lemon. Türk Tarım Doğa Bilimleri Dergisi 2014, 1, 939–946. [Google Scholar]
- Shokrollah, H.; Lee Abdullah, T.; Sijam, K.; Akmar Abdullah, S.N. Potential Use of Selected Citrus Rootstocks and Interstocks against HLB Disease in Malaysia. Crop Prot. 2011, 30, 521–525. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Riquelme, M.T.; Porras, I.; Ferreres, F. Effect of the Rootstock and Interstock Grafted in Lemon Tree (Citrus limon (L.) Burm.) on the Flavonoid Content of Lemon Juice. J. Agric. Food Chem. 2004, 52, 324–331. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, Q.; Sun, M.; Du, R.; Jin, W.; Liu, S. Transcriptome and Metabolome Profiling Reveal the Effects of Hormones on Current-Year Shoot Growth in Chinese ‘Cuiguan’ Pear Grafted onto Vigorous Rootstock ‘Duli’ and Dwarf Rootstock ‘Quince A’. BMC Plant. Biol. 2024, 24, 169. [Google Scholar] [CrossRef]
- Guo, L.; Chen, P.; Sun, Y.; Liu, S.; Li, F.; Chen, Y.; Li, X. Effects of Different Interstocks on Tree Growth and Fruit Quality of Jinqiushatangju. Acta Agric. Univ. Jiangxiensis 2021, 43, 1278–1286. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Li, Y.; Chen, Z.; Yue, Y.; Zhai, J.; Zhao, Z.; Ji, X. Effects of Different Rootstocks on Fruit Quality of Jianxiang Kiwifruit. South China Fruits 2024, 53, 161–168+175. [Google Scholar] [CrossRef]
- Singh, N.P.; Srivastava, R.P. A New Approach towards Double Grafting in Mango. Curr. Sci. 1980, 1, 678–679. [Google Scholar]
- Fayek, M.A.; Ali, A.E.M.; Rashedy, A.A. Physiological and Chemical Performance of the Flame Seedless Grapevine Cultivar in the Presence of Paulsen 1103 as the Interstock. Ciênc. Agrotec. 2022, 46, e021621. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Sun, X.; Liu, S. Effects of different grafting methods and different interstock graftings on fruit quality and volatile compounds in cucumber. Plant Physiol. J. 2019, 55, 867–874. [Google Scholar] [CrossRef]
- Miguel, A.; Marsal, J.I.; Maroto Borrego, J.V.; López-Galarza, S.; San Bautista, A.; Bono, M. Evaluación del injerto intermedio como solución a los problemas de afinidad del melón piel de sapo injertadoJornadas del grupo de horticultura. Fund. Rural. Valencia 2008, 50, 237–240. [Google Scholar]
- Miguel, A.; Marsal, J.I.; San Bautista, A.; López-Galarza, S.; Pascual, B.; Maroto, J.V. Double Grafting As A Method To Solve Affinity Problems In “Piel De Sapo” Melon Cultivars. Acta Hortic. 2011, 898, 287–290. [Google Scholar] [CrossRef]
- German, M.A.; Dai, N.; Matsevitz, T.; Hanael, R.; Petreikov, M.; Bernstein, N.; Ioffe, M.; Shahak, Y.; Schaffer, A.A.; Granot, D. Suppression of Fructokinase Encoded by LeFRK2 in Tomato Stem Inhibits Growth and Causes Wilting of Young Leaves. Plant J. 2003, 34, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Hayat, F.; Iqbal, S.; Coulibaly, D.; Razzaq, M.K.; Nawaz, M.A.; Jiang, W.; Shi, T.; Gao, Z.; Hayat, F.; Iqbal, S.; et al. An Insight into Dwarfing Mechanism: Contribution of Scion-Rootstock Interactions toward Fruit Crop Improvement. Fruit Res. 2021, 1, 1–11. [Google Scholar] [CrossRef]
- Karlıdağ, H.; Aslantaş, R.; Eşitken, A. Effects of Interstock (M9) Length Grafted onto MM106 Rootstock on Sylleptic Shoot Formation, Growth and Yield in Some Apple Cultivars. J. Agric. Sci. 2014, 4, 70–73. [Google Scholar]
- Dong, Y.; Ye, X.; Xiong, A.; Zhu, N.; Jiang, L.; Qu, S. The Regulatory Role of Gibberellin Related Genes DKGA2ox1 and MIR171f_3 in Persimmon Dwarfism. Plant Sci. 2021, 310, 110958. [Google Scholar] [CrossRef]
- Wu, T.; He, H.; Dang, J.; Li, X.; He, Q.; Guo, Q.; Liang, G.; Huang, X.; Peng, W. Seedling growth and photosynthetic efficiency of loquat plants with different ploidy of “Hua Bai 1” as interstock. South China Fruits 2023, 52, 92–96. [Google Scholar] [CrossRef]
- Mahmoudi Meimand, M.J.; Shamshiri, M.H. Effects of Rootstock and Interstock on Pollen Traits and Flowering Synchronization in Pistachio (Pistacia vera L.). Erwerbs-Obstbau 2019, 61, 267–271. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Z.; Du, P.; Ji, J.; Sun, W.; Xu, J.; Liang, B. Effects of Different Dwarfing Interstocks on the Rhizosphere, Endophytic Bacteria, and Drought Resistance of Apple Trees. Microbiol. Res. 2024, 283, 127690. [Google Scholar] [CrossRef]
- Shaltiel-Harpaz, L.; Gerchman, Y.; Ibdah, M.; Kedoshim, R.; Rachmany, D.; Hatib, K.; Bar-Ya’akov, I.; Soroker, V.; Holland, D. Grafting on Resistant Interstocks Reduces Scion Susceptibility to Pear Psylla, Cacopsylla Bidens. Pest Manag. Sci. 2018, 74, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.; Martínez-García, P.J.; Martínez-Gómez, P.; Dicenta, F. Plum Pox Virus (Sharka) Resistance in Peach by Grafting ‘Garrigues’ Almond as Interstock. Sci. Hortic. 2024, 338, 113749. [Google Scholar] [CrossRef]
- Marcon, J.L.; Kretzschmar, A.A.; Hipólito, J.d.S.; Rufato, A.D.R.; Rufato, L.; Wurz, D.A. Increasing the Length of EM-9 Interstock Enhances Production Efficiency in Imperial Gala Apples. Rev. Ceres 2019, 66, 178–183. [Google Scholar] [CrossRef]
- Lu, X.; Gao, Y.; Wang, K.; Sun, S.; Liu, Z.; Yan, P.; Feng, J.; Li, Q.; Li, L.; Wang, D. Dwarf Interstocks Improve Aroma Quality of ‘Huahong’ Apple (Malus × Domestica). Agriculture 2022, 12, 1710. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.; Ren, X.; Bao, L.; Zhang, L.; Zhang, L.; Han, M.; Zhang, D. Root Growth, Yield and Fruit Quality of “Red Fuji” Apple Trees in Relation to Planting Depth of Dwarfing Interstock on the Loess Plateau. Eur. J. Hortic. Sci. 2015, 80, 109–116. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Kumar, R.; Wamiq, M.; Gautam, P.; Gangwar, V. Graft Incompatibility Problem in Fruit Propagation and Scion-Stock Relationship; AkiNik Publications: Delhi, India, 2023; ISBN 978-93-5570-527-3. [Google Scholar]
- Girardi, E.A.; Mourão Filho, F.D.A.A. Production of Interstocked “Pera” Sweet Orange Nursey Trees on “Volkamer” Lemon and “Swingle” Citrumelo Rootstocks. Sci. Agric. 2006, 63, 5–10. [Google Scholar] [CrossRef]
- Exadaktylou, E.; Thomidis, T.; Grout, B.; Tsipouridis, C. Methods to Propagate the Cherry Rootstock Gisela 5 by Using Root Cuttings and Application of Micrografting. Adv. Hortic. Sci. 2007, 21, 51–54. [Google Scholar]
- Zlesak, D.C. Rose. In Flower Breeding and Genetics: Issues, Challenges and Opportunities for the 21st Century; Anderson, N.O., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 695–740. ISBN 978-1-4020-4428-1. [Google Scholar]

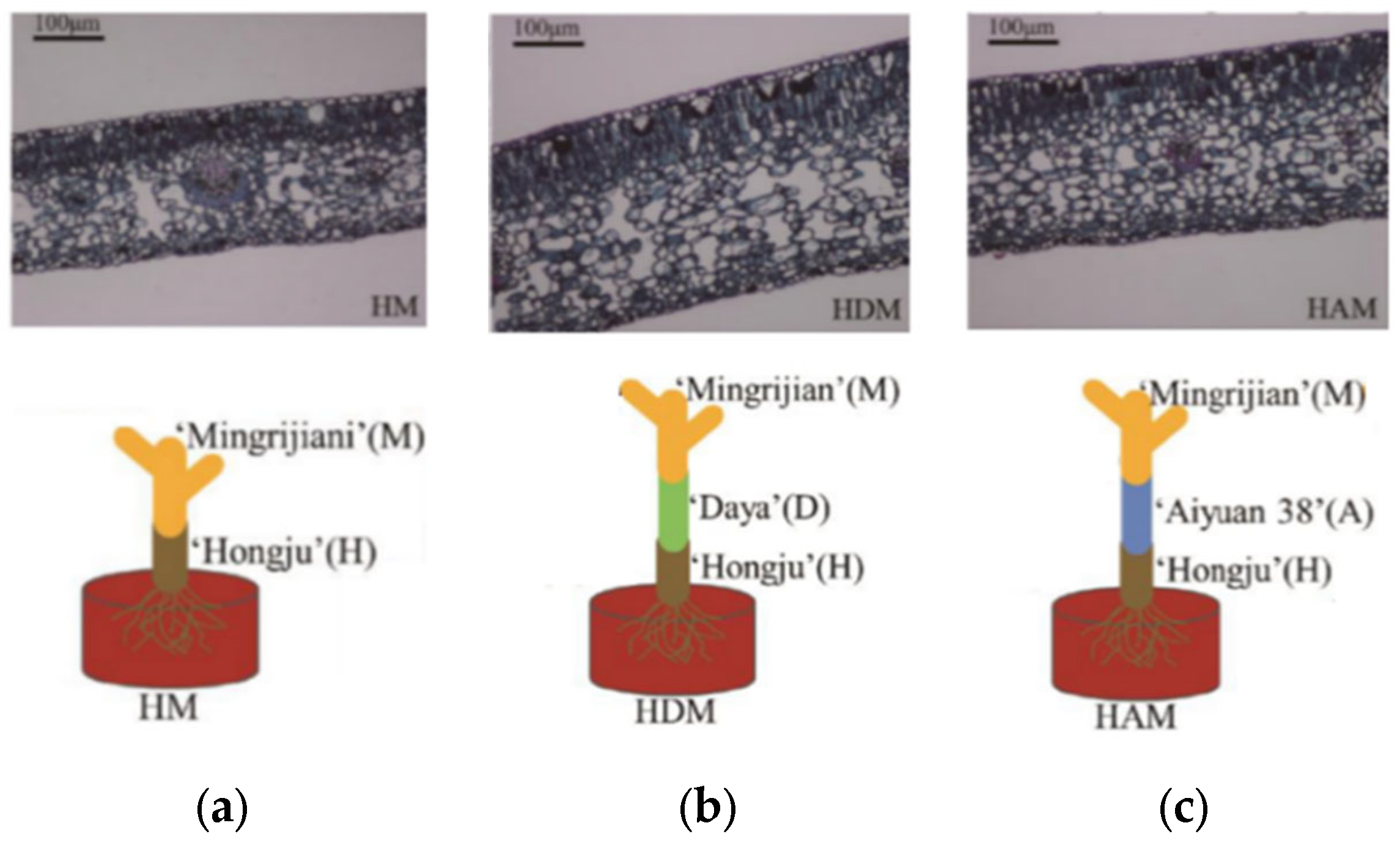
| Type | Species | Interstock | Role |
|---|---|---|---|
| Fruit Trees | Apple | Jizhen 1 and Jizhen 2 | Improved fruit quality and cold resistance [43] |
| SH6 | Increased keratin and wax content in fruit [47] | ||
| M27 | Enhanced fruit yield and quality [48] | ||
| GM256 | Dwarfing, cold resistance, enhanced fruit yield and quality [49] | ||
| Plum | UFV 186 and UFV 286 | Dwarfing [50] | |
| Havens 2B | Enhanced grafting compatibility [51] | ||
| Citrus | Paradisi Macf. | Enhanced fruit yield [52] | |
| Rubidoux trifoliate | Dwarfing [53] | ||
| C. grandis | Againsted disease [54] | ||
| Lemon | Washington Navel | Improved fruit flavor quality [55] | |
| Pear | Cuiguan | Dwarfing [56] | |
| OHF51 | Dwarfing, enhanced grafting compatibility [41] | ||
| Zhongai 1 | Dwarfing [44] | ||
| Sugar orange | Nanfeng | Enhanced fruit yield and quality [57] | |
| Kiwifruit | Miliang 1 | Enhanced fruit yield and quality [58] | |
| Mango | Bapakkai | Rapid propagation [59] | |
| Grape | Paulsen 1103 | Enhanced grafting compatibility [60] | |
| Vegetables | Melon | Oriental Crisp and Sweet | Enhanced fruit appearance and flavor quality [61] |
| Sienne | Enhanced grafting compatibility and yield [62] | ||
| Troubadour | Enhanced grafting compatibility [63] | ||
| Tomato | FK-3a | Inhibit growth [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Zhao, Y.; Xu, B.; Tan, S.; Tong, J.; Zhang, C. Double Grafting: A Synthesis of Applications and Future Research Horizons. Horticulturae 2025, 11, 366. https://doi.org/10.3390/horticulturae11040366
Yu J, Zhao Y, Xu B, Tan S, Tong J, Zhang C. Double Grafting: A Synthesis of Applications and Future Research Horizons. Horticulturae. 2025; 11(4):366. https://doi.org/10.3390/horticulturae11040366
Chicago/Turabian StyleYu, Jialing, Yinglei Zhao, Baoyu Xu, Shiyi Tan, Junhua Tong, and Chenghao Zhang. 2025. "Double Grafting: A Synthesis of Applications and Future Research Horizons" Horticulturae 11, no. 4: 366. https://doi.org/10.3390/horticulturae11040366
APA StyleYu, J., Zhao, Y., Xu, B., Tan, S., Tong, J., & Zhang, C. (2025). Double Grafting: A Synthesis of Applications and Future Research Horizons. Horticulturae, 11(4), 366. https://doi.org/10.3390/horticulturae11040366





