The Role and Regulatory Mechanism of Methionine Sulfoxide Reductase (Msr) in the Process of Chilling Injury of Fruits and Vegetables: A Review
Abstract
:1. Introduction
2. Msr in Plants
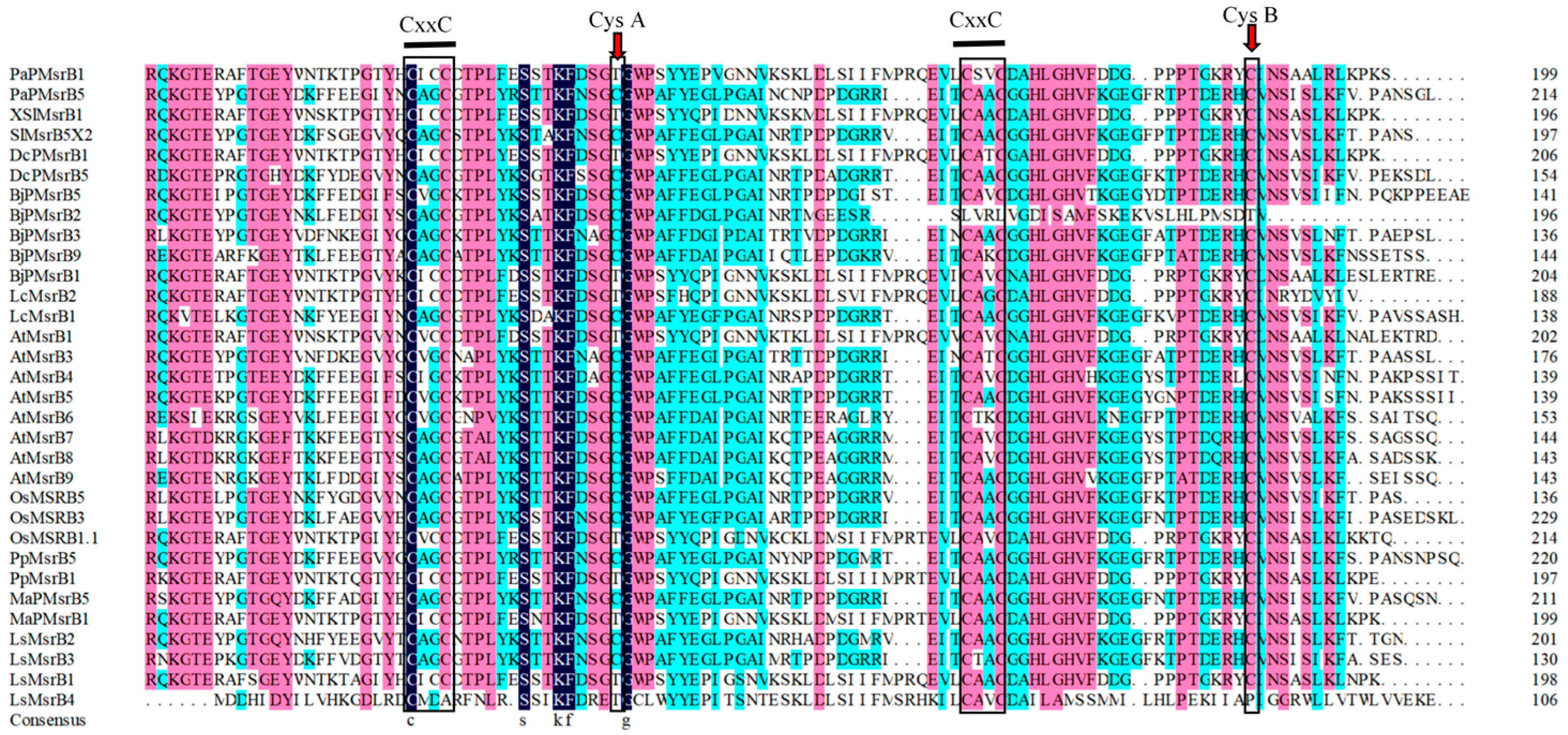
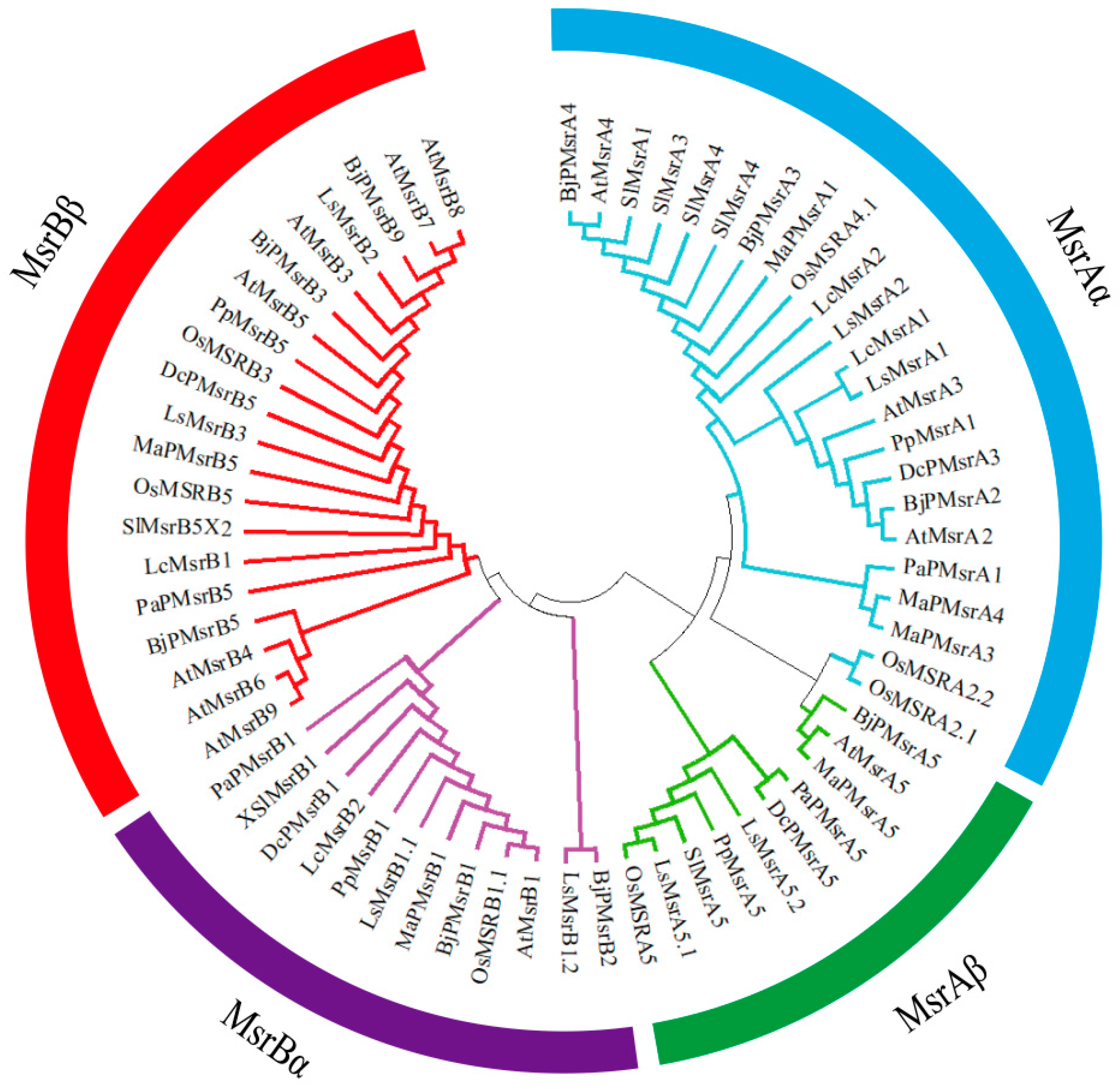
2.1. Classification and Distribution of Msr
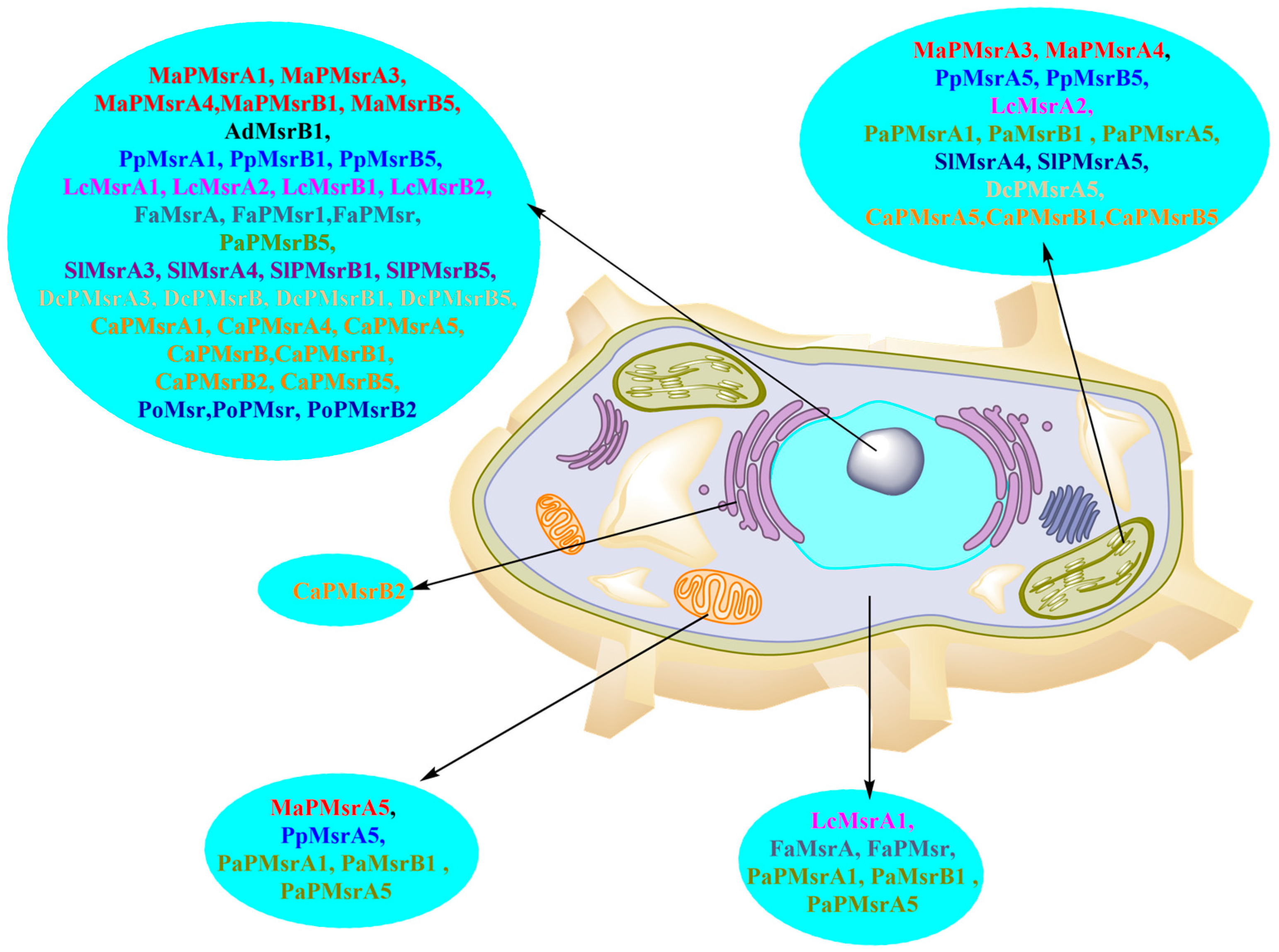
2.2. Subcellular Localization of Msr
3. Role of Msr in Response to Chilling Stress
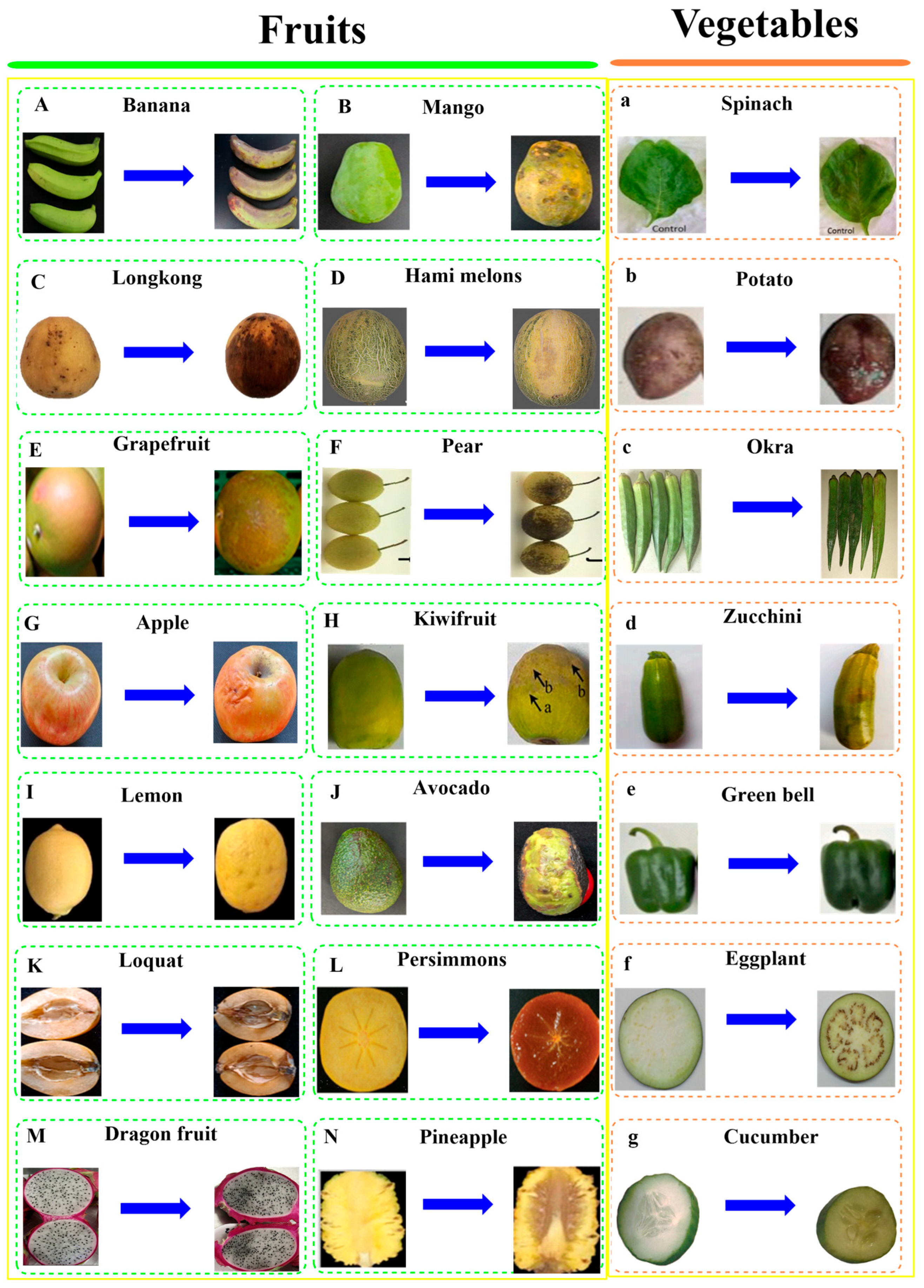
3.1. Postharvest CI of Fruits and Vegetables
- Severe tissue breakdown: in advanced cases, CI leads to extensive tissue damage. For example, cold-damaged olive fruit develops a sunken, wrinkled surface with brown discoloration in both the skin and flesh [64].
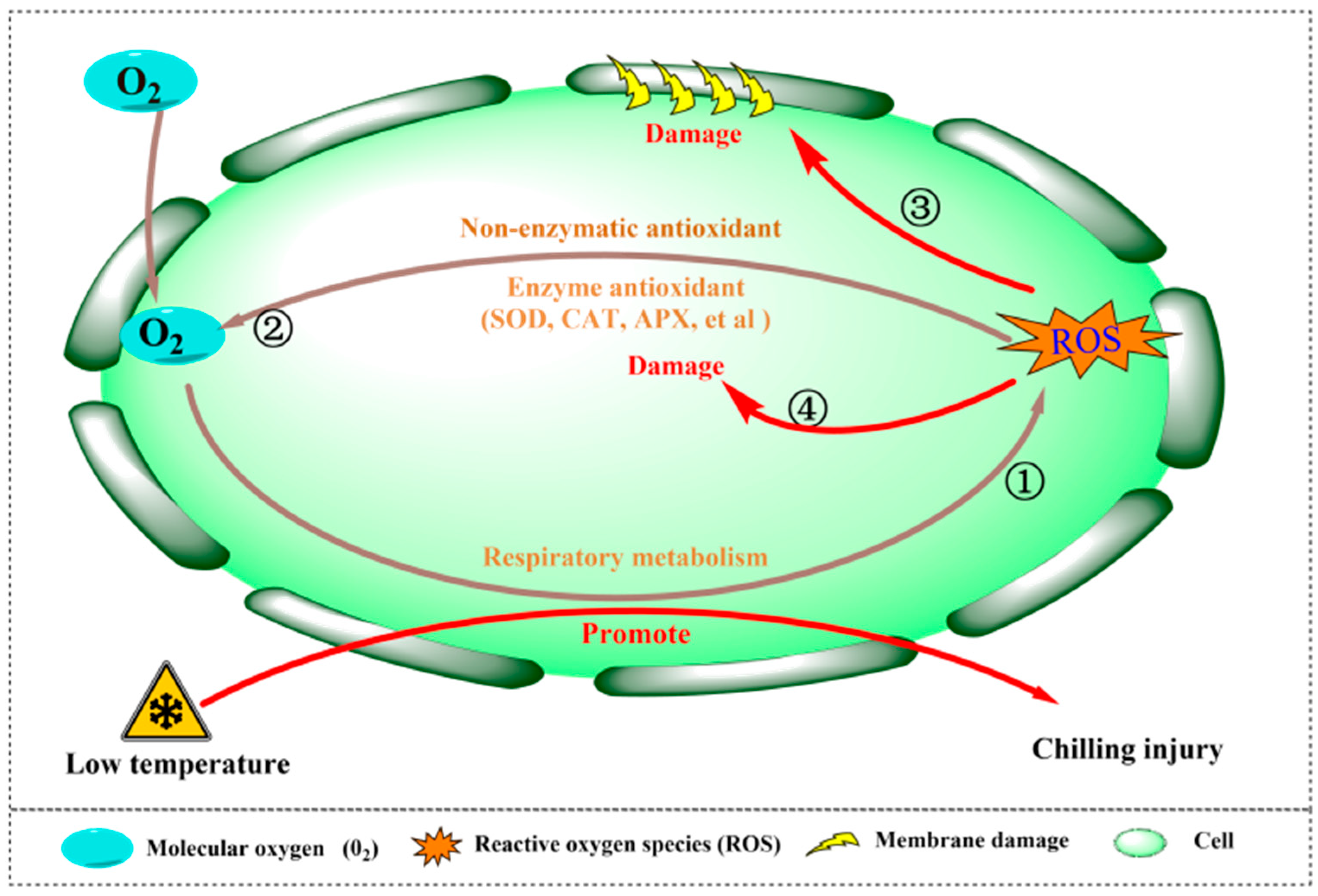
3.2. ROS and CI of Fruits and Vegetables
3.2.1. Definition, Types, and Production of ROS
3.2.2. Mechanism of ROS Causing Postharvest CI of Fruits and Vegetables
3.2.3. Repair Mechanism of Msr After Protein Oxidation in Fruits and Vegetables
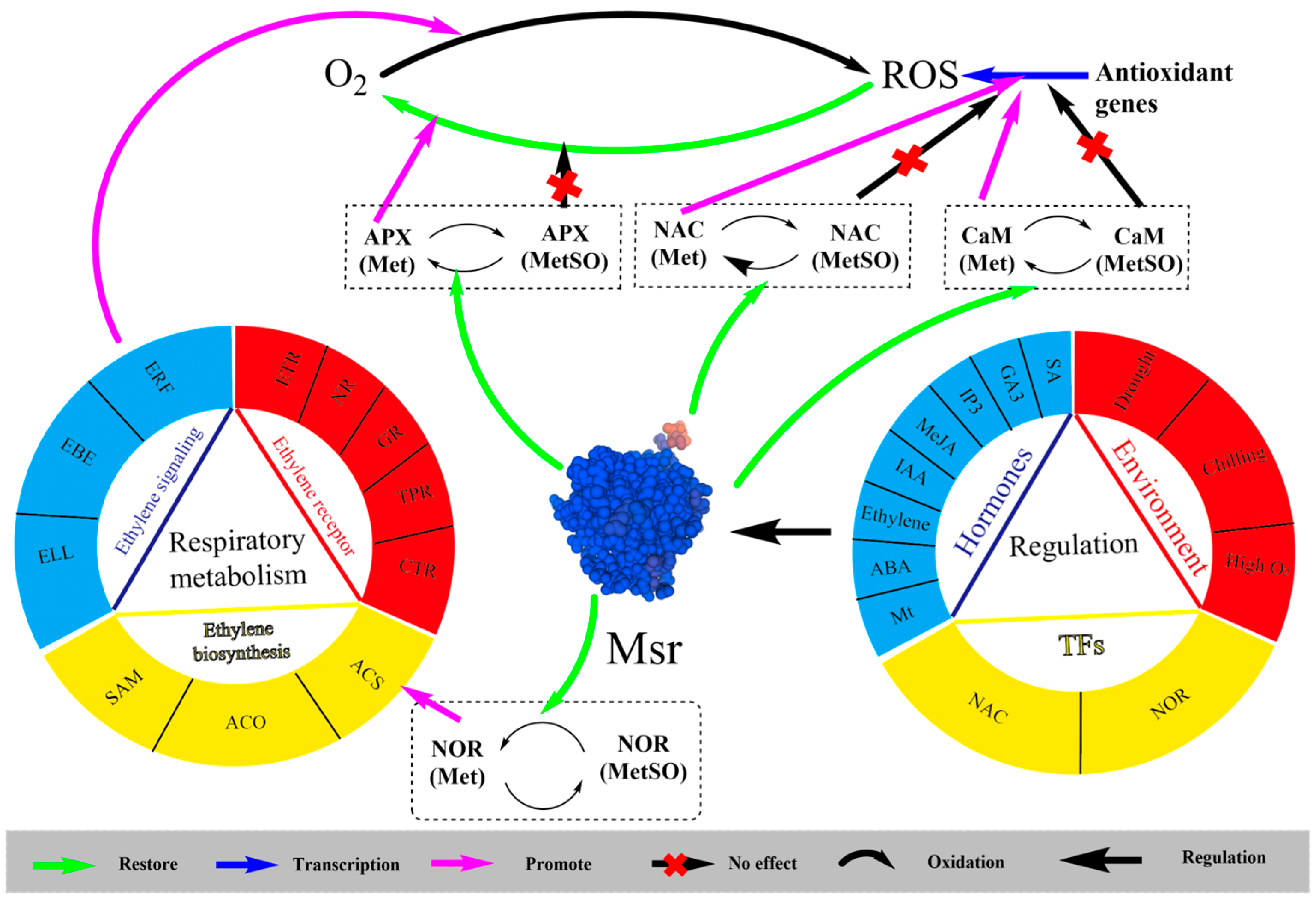
4. Regulation of Msr Genes
4.1. Upstream Regulatory Mechanisms of Msr
4.2. Substrate Protein of Msr
4.3. Msr-Mediated Regulation of CI in Postharvest Fruits and Vegetables
4.3.1. Msr Mediates the Regulation of NO Inhibition of CI in Postharvest Fruits and Vegetables
4.3.2. Msr Mediates the Regulation of MeJA Inhibition of CI in Postharvest Fruits and Vegetables
4.3.3. Msr Mediates the Regulation of GABA Inhibition of CI in Postharvest Fruits and Vegetables
4.3.4. Msr Mediates the Regulation of Melatonin Inhibition of CI in Postharvest Fruits and Vegetables
4.3.5. Msr Mediates the Regulation of Gibberellin Inhibition of CI in Postharvest Fruits and Vegetables
4.3.6. Potential Role of Msr in Brassinolide-Mediated CI Inhibition in Postharvest Fruits and Vegetables
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Species | Gene | NCBI Reference Sequence (mRNA/Protein) |
|---|---|---|
| Carica papaya L. | PaPMsrA5 | XM_022052179.1/ XP_021907871.1 |
| PaPMsrA1 | XM_022049933.1/ XP_021905625.1 | |
| Solanum lycopersicum L. | SlMsrA5 | NM_001320242.1/ NP_001307171.1 |
| SlMsrA4 | NM_001321043.1/ NP_001307972.1 | |
| SlMsrA3 | NM_001320370.1/ NP_001307299.1 | |
| SlMsrA2 | JN102298.1/ AEN03272.1 | |
| SlMsrA1 | NM_001320202.1/ NP_001307131.1 | |
| Daucus carota var. sativa Hoffm. | DcPMsrA3 | XM_017359291.2/ XP_017214780.1 |
| DcPMsrA5 | XM_017399723.2/ XP_017255212.1 | |
| Brassica juncea (L.) Czern. | BjPMsrA2 | XM_009124089.3/ XP_009122337.2 |
| BjPMsrA3 | XM_009132743.3/ XP_009130991.2 | |
| BjPMsrA5 | XM_009114216.3/ XP_009112464.1 | |
| BjPMsrA4 | XM_009139249.3/ XP_009137497.1 | |
| Litchi chinensis Sonn. | LcMsrA1 | KY475577.1/ AQP31371.1 |
| LcMsrA2 | KY475578.1/ AQP31372.1 | |
| LcMsrB1 | KY475579.1/ AQP31373.1 | |
| LcMsrB2 | MH396620.1/ QBB68762.1 | |
| Arabidopsis thaliana (L.) Heynh. | AtMsrA5 | NM_127359.5/ NP_179394.1 |
| AtMsrA4 | NM_118645.4/ NP_194243.1 | |
| AtMsrA2 | NM_120828.4/ NP_196363.1 | |
| AtMsrA3 | NM_120829.3/ NP_196364.1 | |
| AtMsrB1 | NM_104245.4/ NP_564640.2 | |
| AtMsrB2 | NM_001341518.1/ NP_001320026.1 | |
| AtMsrB3 | NM_116718.3/ NP_567271.1 | |
| AtMsrB4 | NM_116719.3/ NP_192390.1 | |
| AtMsrB5 | NM_116721.4/ NP_192392.1 | |
| AtMsrB6 | NM_116722.4/ NP_192393.2 | |
| AtMsrB7 | NM_118303.4/ NP_567637.1 | |
| AtMsrB8 | NM_118304.5/ NP_193915.1 | |
| Oryza sativa L. | OsMsrA5 | NM_001419584.1/ NP_001406513.1” |
| OsMsrA4.1 | XM_052278347.1/ XP_052134307.1 | |
| OsMsrA2.2 | XM_052297699.1/ XP_052153659.1 | |
| OsMsrA2.1 | NM_001419583.1/ NP_001406512.1 | |
| Prunus persica (L.) Batsch | PpMsrA5 | XM_007227483.2/ XP_007227545.1 |
| PpMsrA1 | XM_007218770.2/ XP_007218832.1 | |
| Musa acuminata ‘(AAA)’ | MaPMsrA1 | XM_009386413.3/ XP_009384688.2 |
| MaPMsrA4 | XM_009383708.3/ XP_009381983.2 | |
| MaPMsrA3 | XM_009403708.3/ XP_009401983.2 | |
| MaPMsrA5 | XM_009394013.3/ XP_009392288.2 | |
| Lactuca sativa L. | LsMsrA5.1 | Unpublished data |
| LsMsrA5.2 | Unpublished data | |
| LsMsrA1 | Unpublished data | |
| LsMsrA2 | Unpublished data | |
| Carica papaya L. | PaPMsrB1 | XM_022039393.1/ XP_021895085.1 |
| PaPMsrB5 | XM_022034123.1/ XP_021889815.1 | |
| Solanum lycopersicum L. | SlMsrB1 | XM_004244256.5/ XP_004244304.3 |
| SlPMsrB5 | XM_004229424.5/ XP_004229472.1 | |
| Daucus carota var. sativa Hoffm. | DcPMsrB1 | XM_017394132.2/ XP_017249621.1 |
| DcPMsrB5 | XM_017365040.2/ XP_017220529.1 | |
| Brassica juncea (L.) Czern. | BjPMsrB5 | XM_033286223.1/ XP_033142114.1 |
| BjPMsrB2 | XM_009122844.3/ XP_009121092.1 | |
| BjPMsrB3 | XM_009116296.3/ XP_009114544.1 | |
| BjPMsrB9 | XM_009110283.2/ XP_009108531.1 | |
| BjPMsrB1 | XM_009149339.3/ XP_009147587.1 | |
| Litchi chinensis Sonn. | LcMsrB2 | MH396620.1/ QBB68762.1 |
| LcMsrB1 | KY475579.1/ AQP31373.1 | |
| Arabidopsis thaliana (L.) Heynh. | AtMsrB1 | NM_104245.4/ NP_564640.2 |
| AtMsrB3 | NM_116718.3/ NP_567271.1 | |
| AtMsrB4 | NM_001340514.1/ NP_001329727.1 | |
| AtMsrB5 | NM_116721.4/ NP_192392.1 | |
| AtMsrB6 | NM_116722.4/ NP_192393.2 | |
| AtMsrB7 | NM_118303.4/ NP_567637.1 | |
| AtMsrB8 | NM_118304.5/ NP_193915.1 | |
| AtMsrB9 | NM_118305.3/ NP_567638.1 | |
| Oryza sativa L. | OsMSRB1.1 | NM_001423988.1/ NP_001410917.1 |
| OsMsrB3 | NM_001402525.1/ NP_001389454.1 | |
| OsMsrB5 | NM_001402157.1/ NP_001389086.1 | |
| Prunus persica (L.) Batsch | PpMsrB5 | XM_020570828.1/ XP_020426417.1 |
| PpMsrB1 | XM_007212038.2/ XP_007212100.1 | |
| Musa acuminata ‘(AAA)’ | MaPMsrB5 | XM_009413902.3/ XP_009412177.2 |
| MaPMsrB1 | XM_065153234.1/ XP_065009306.1 | |
| Lactuca sativa L. | LsMsrB1 | Unpublished data |
| LsMsrB2 | Unpublished data | |
| LsMsrB3 | Unpublished data | |
| LsMsrB4 | Unpublished data |
References
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Dat, J.; Vandenabeele, S.; Vranova, E.; Van Montagu, M.; Inze, D.; Van Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef]
- Henry, J.F.; Matilde, M.; Fulvio, U. Signaling Functions of Reactive Oxygen Species. Biochemistry 2010, 49, 835–842. [Google Scholar] [CrossRef]
- Mehdy, M.C. Active Oxygen Species in Plant Defense against Pathogens. Plant Physiol. 1994, 105, 467–472. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Cao, J.; Jiang, W. Advances in biochemical mechanisms and control technologies to treat chilling injury in postharvest fruits and vegetables. Trends Food Sci. Technol. 2021, 113, 355–365. [Google Scholar] [CrossRef]
- Xin, Q.; Zhou, X.; Jiang, W.; Zhang, M.; Sun, J.; Cui, K.; Liu, Y.; Jiao, W.; Zhao, H.; Liu, B. Effects of Reactive Oxygen Levels on Chilling Injury and Storability in 21 Apricot Varieties from Different Production Areas in China. Foods 2023, 12, 2378. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Bodbodak, S. Postharvest Heat Treatment for Mitigation of Chilling Injury in Fruits and Vegetables. Food Bioprocess Technol. 2014, 7, 37–53. [Google Scholar] [CrossRef]
- Kwon, S.J.; Kwon, S.I.; Bae, M.S.; Cho, E.J.; Park, O.K. Role of the Methionine Sulfoxide Reductase MsrB3 in Cold Acclimation in Arabidopsis. Plant Cell Physiol. 2007, 48, 1713–1723. [Google Scholar] [CrossRef]
- Rouhier, N.; Santos, C.V.D.; Tarrago, L.; Rey, P. Plant methionine sulfoxide reductase A and B multigenic families. Photosynth. Res. 2006, 89, 247–262. [Google Scholar] [CrossRef]
- Lee, S.; Li, C.; Koh, K.W.; Chuang, H.; Chen, Y.; Lin, C.; Chan, M. MSRB7 reverses oxidation of GSTF2/3 to confer tolerance of Arabidopsis thaliana to oxidative stress. J. Exp. Bot. 2014, 65, 5049–5062. [Google Scholar] [CrossRef]
- Rouhier, N.; Kauffmann, B.; Tete-Favier, F.; Palladino, P.; Gans, P.; Branlant, G.; Jacquot, J.; Boschi-Muller, S. Functional and Structural Aspects of Poplar Cytosolic and Plastidial Type A Methionine Sulfoxide Reductases. J. Biol. Chem. 2007, 282, 3367–3378. [Google Scholar] [CrossRef]
- Ding, P.; Gao, Y.; Zhu, J.; Chen, F.; Xia, G. Wheat methionine sulfoxide reductase genes and their response to abiotic stress. Mol. Breed. 2016, 36, 169. [Google Scholar] [CrossRef]
- Ding, D.; Sagher, D.; Laugier, E.; Rey, P.; Weissbach, H.; Zhang, X.H. Studies on the reducing systems for plant and animal thioredoxin-independent methionine sulfoxide reductases B. Biochem. Biophys. Res. Commun. 2007, 361, 629–633. [Google Scholar] [CrossRef]
- Sandrine, B.M.; Guy, B. Methionine sulfoxide reductase: Chemistry, substrate binding, recycling process and oxidase activity. Bioorg. Chem. 2014, 65, 5049–5062. [Google Scholar] [CrossRef]
- Min, D.; Li, F.; Ali, M.; Liu, J.; Fu, X.; Song, Y.; Ding, J.; Li, X.; Ji, N.; Zhang, X. Interaction of methionine sulfoxide reductase B5 with SlMYC2 stimulates the transcription of MeJA-mediated autophagy-related genes in tomato fruit. Hortic. Res. 2023, 10, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Duan, Y. The Role of Glycogen Synthase Kinase-3 in Gibberellic Acid-Induced Chilling Tolerance and Defense Response in Postharvest Peach Fruit. Food Bioprocess Technol. 2019, 12, 1733–1740. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.; Fu, X.; Min, D.; Ali, M.; Zhao, X.; Song, Y.; Ding, J.; Li, X.; Zhang, X. SlMsrB5 contributes to the chilling tolerance induced by methyl jasmonate in postharvest tomato fruit. Sci. Hortic. 2023, 322, 112386. [Google Scholar] [CrossRef]
- Xie, J.; Qin, Z.; Pan, J.; Li, J.; Li, X.; Khoo, H.E.; Dong, X. Melatonin treatment improves postharvest quality and regulates reactive oxygen species metabolism in “Feizixiao” litchi based on principal component analysis. Front Plant Sci. 2022, 13, 965345. [Google Scholar] [CrossRef]
- Zhang, X.H.; Weissbach, H. Origin and evolution of the protein-repairing enzymes methionine sulphoxide reductases. Biol. Rev. Camb. Philos. Soc. 2008, 83, 249–257. [Google Scholar] [CrossRef]
- Tarrago, L.; Laugier, E.; Rey, P. Protein-Repairing Methionine Sulfoxide Reductases in Photosynthetic Organisms: Gene Organization, Reduction Mechanisms, and Physiological Roles. Mol. Plant 2009, 2, 202–217. [Google Scholar] [CrossRef]
- Kalemba, E.; Stolarska, E. Regulation of Gene Expression of Methionine Sulfoxide Reductases and Their New Putative Roles in Plants. Int. J. Mol. Sci. 2019, 20, 1309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jia, P.; Jia, Y.; Li, Y.; Webster, K.A.; Huang, X.; Achary, M.; Lemanski, S.L.; Lemanski, L.F. Anoxia, acidosis, and intergenic interactions selectively regulate methionine sulfoxide reductase transcriptions in mouse embryonic stem cells. J. Cell Biochem. 2011, 112, 98–106. [Google Scholar] [CrossRef]
- Willems, P.; Messens, J. Protein Methionine Sulfoxide Dynamics in Arabidopsis thaliana under Oxidative Stress. Mol. Cell. Proteom. 2015, 14, 1217–1229. [Google Scholar] [CrossRef]
- Xiao, L.; Jiang, G.; Yan, H.; Lai, H.; Su, X.; Jiang, Y.; Duan, X. Methionine Sulfoxide Reductase B Regulates the Activity of Ascorbate Peroxidase of Banana Fruit. Antioxidants 2021, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, M. Cloning, Expression, and Characterization of a Methionine Sulfoxide Reductase B Gene from Nicotiana tabacum. Protein J. 2013, 32, 543–550. [Google Scholar] [CrossRef]
- Siddiqui, Z.S.; Cho, J.; Kwon, T.; Ahn, B.; Lee, K.; Jeong, M.; Ryu, T.; Lee, S.; Park, S.; Park, S. Physiological mechanism of drought tolerance in transgenic rice plants expressing Capsicum annuum methionine sulfoxide reductase B2 (CaMsrB2) gene. Acta Physiol. Plant 2014, 36, 1143–1153. [Google Scholar] [CrossRef]
- Dai, C. Expression Pattern of a Peptide Methionine Sulfoxide Reductase Gene from Tomato (Solanum lycopersicum) in Response to Abiotic and Oxidative Stresses. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 127–132. [Google Scholar] [CrossRef]
- Chu, H.D.; Nguyen, K.; Watanabe, Y.; Le, D.T.; Tran, L.P. Expression analyses of soybean genes encoding methionine-R-sulfoxide reductase under various conditions suggest a possible role in the adaptation to stress. Appl. Biol. Chem. 2016, 59, 681–687. [Google Scholar] [CrossRef]
- Venkatachalam, K. Exogenous nitric oxide treatment impacts antioxidant response and alleviates chilling injuries in longkong pericarp. Sci. Hortic. 2018, 237, 311–317. [Google Scholar] [CrossRef]
- Ding, P.; Fang, L.; Huang, S.; Zhu, J.; Wang, G.; Xia, G.; Chen, F. Wheat plastidial methionine sulfoxide reductase MSRB3.1 interacts with haem oxygenase 1 to improve osmotic stress tolerance in wheat seedlings. Environ. Exp. Bot. 2021, 188, 104528. [Google Scholar] [CrossRef]
- Wang, G.; Fu, X.; Zhao, W.; Zhang, M.; Chen, F. Ectopic Expression of Maize Plastidic Methionine Sulfoxide Reductase ZmMSRB1 Enhances Salinity Stress Tolerance in Arabidopsis thaliana. Plant Mol. Biol. Report. 2022, 40, 284–295. [Google Scholar] [CrossRef]
- Lopez, A.P.; Portales, R.B.; López-Ráez, J.A.; Medina-Escobar, N.; Blanco, J.M.O.; Franco, A.R. Characterization of a strawberry late-expressed and fruit-specific peptide methionine sulphoxide reductase. Physiol. Plant 2010, 126, 129–139. [Google Scholar] [CrossRef]
- Vieira Dos Santos, C.; Cuiné, S.; Rouhier, N.; Rey, P. The Arabidopsis Plastidic Methionine Sulfoxide Reductase B Proteins. Sequence and Activity Characteristics, Comparison of the Expression with Plastidic Methionine Sulfoxide Reductase A, and Induction by Photooxidative Stress. Plant Physiol. 2005, 138, 909–922. [Google Scholar] [CrossRef]
- Oh, S.; Baek, K.; Seong, E.S.; Joung, Y.H.; Choi, G.; Park, J.M.; Cho, H.S.; Kim, E.A.; Lee, S.; Choi, D. CaMsrB2, Pepper Methionine Sulfoxide Reductase B2, Is a Novel Defense Regulator against Oxidative Stress and Pathogen Attack. Plant Physiol. 2010, 154, 245–261. [Google Scholar] [CrossRef]
- Châtelain, E.; Satour, P.; Laugier, E.; Ly Vu, B.; Payet, N.; Rey, P.; Montrichard, F. Evidence for participation of the methionine sulfoxide reductase repair system in plant seed longevity. Proc. Natl. Acad. Sci. USA 2013, 110, 3633–3638. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhu, J.; Ding, P.; Chen, F.; Xia, G. The Brachypodium distachyon methionine sulfoxide reductase gene family. Genes Genom. 2017, 39, 975–985. [Google Scholar] [CrossRef]
- Romero, H.M.; Pell, E.J.; Tien, M. Expression profile analysis and biochemical properties of the peptide methionine sulfoxide reductase A (PMSRA) gene family in Arabidopsis. Plant Sci. 2006, 170, 705–714. [Google Scholar] [CrossRef]
- Taylor, A.B.; Benglis, D.M.; Dhandayuthapani, S.; Hart, P.J. Structure of Mycobacterium tuberculosis Methionine Sulfoxide Reductase A in Complex with Protein-Bound Methionine. J. Bacteriol. 2003, 185, 4119–4126. [Google Scholar] [CrossRef]
- Tarrago, L.; Laugier, E.; Zaffagnini, M.; Marchand, C.H.; Le Maréchal, P.; Lemaire, S.D.; Rey, P. Plant Thioredoxin CDSP32 Regenerates 1-Cys Methionine Sulfoxide Reductase B Activity through the Direct Reduction of Sulfenic Acid. J. Biol. Chem. 2010, 285, 14964–14972. [Google Scholar] [CrossRef]
- Xiao, T.; Mi, M.; Wang, C.; Qian, M.; Chen, Y.; Zheng, L.; Zhang, H.; Hu, Z.; Shen, Z.; Xia, Y. A methionine-R-sulfoxide reductase, OsMSRB5, is required for rice defense against copper toxicity. Environ. Exp. Bot. 2018, 153, 45–53. [Google Scholar] [CrossRef]
- Siddiqui, Z.S.; Oh, S.; Kim, E.; Jang, Y.; Lee, S.; Yun, D.; Kwon, T.; Wajid, D.; Ansari, H.H.; Park, S.; et al. Physiological and photochemical evaluation of pepper methionine sulfoxide reductase B2 (CaMsrB2) expressing transgenic rice in saline habitat. Plant Physiol. Biochem. 2021, 167, 198–209. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Y.; Wang, Y.; Chen, Y.; Chu, C. OsMSRA4.1 and OsMSRB1.1, two rice plastidial methionine sulfoxide reductases, are involved in abiotic stress responses. Planta 2009, 230, 227. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wu, Q.; Zhou, Y.; Yun, Z.; Duan, X.; Jiang, Y. l -Cysteine hydrochloride delays senescence of harvested longan fruit in relation to modification of redox status. Postharvest Biol. Technol. 2018, 143, 35–42. [Google Scholar] [CrossRef]
- Min, D.; Li, F.; Ali, M.; Zhang, X.; Liu, Y. Application of methyl jasmonate to control chilling tolerance of postharvest fruit and vegetables: A meta-analysis and eliciting metabolism review. Crit. Rev. Food Sci. Nutr. 2023, 33, 12878–12891. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, L.; Wu, C.; Shan, W.; Cai, D.; Lin, Z.; Wei, W.; Chen, J.; Lu, W.; Kuang, J. Methionine oxidation and reduction of the ethylene signaling component MaEIL9 are involved in banana fruit ripening. J. Integr. Plant Biol. 2023, 65, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Giné-Bordonaba, J.; Cantín, C.M.; Echeverría, G.; Ubach, D.; Larrigaudière, C. The effect of chilling injury-inducing storage conditions on quality and consumer acceptance of different Prunus persica cultivars. Postharvest Biol. Technol. 2016, 115, 38–47. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Mao, L.; Ying, T. Contribution of polyamines metabolism and GABA shunt to chilling tolerance induced by nitric oxide in cold-stored banana fruit. Food Chem. 2016, 197, 333–339. [Google Scholar] [CrossRef]
- Chen, H.; Lin, H.; Jiang, X.; Lin, M.; Fan, Z. Amelioration of chilling injury and enhancement of quality maintenance in cold-stored guava fruit by melatonin treatment. Food Chem. X 2022, 14, 100297. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, S.; Yuan, M.; Song, H.; Wang, T.; Zhang, W.; Zhang, Z. Effect of glycine betaine on chilling injury in relation to energy metabolism in papaya fruit during cold storage. Food Sci. Nutr. 2019, 7, 1123–1130. [Google Scholar] [CrossRef]
- Madhav, J.V.; Sethi, S.; Sharma, R.R.; Nagaraja, A.; Varghese, E. Influence of bilayer coating of salicylic acid and edible wax on chilling injury and functional attributes of guava. J. Food Process. Preserv. 2021, 7, e15610. [Google Scholar] [CrossRef]
- Nukuntornprakit, O.A.; Chanjirakul, K.; Van Doorn, W.G.; Siriphanich, J. Chilling injury in pineapple fruit: Fatty acid composition and antioxidant metabolism. Postharvest Biol. Technol. 2015, 99, 20–26. [Google Scholar] [CrossRef]
- Jiao, X.; Deng, B.; Zhang, L.; Gao, Z.; Feng, Z.; Wang, R. Melatonin and 1-Methylcyclopropene Improve the Postharvest Quality and Antioxidant Capacity of ‘Youhou’ Sweet Persimmons during Cold Storage. Int. J. Fruit Sci. 2022, 22, 809–825. [Google Scholar] [CrossRef]
- Jin, P.; Wang, K.; Shang, H.; Tong, J.; Zheng, Y. Low-temperature conditioning combined with methyl jasmonate treatment reduces chilling injury of peach fruit. J. Sci. Food Agric. 2009, 89, 1690–1696. [Google Scholar] [CrossRef]
- Xia, J.A.; Yang, Y.W.; Cao, H.X.; Han, C.; Ge, D.K.; Zhang, W.Y. Visible-near infrared spectrum-based classification of apple chilling injury on cloud computing platform. Comput. Electron. Agric. 2018, 145, 27–34. [Google Scholar] [CrossRef]
- Nie, X.; Zhao, Y.; Li, Y.; Lv, H. 1-Methylcyclopropylene and heat treatment alleviate chilling injury in purple sweet potato by regulating ROS homeostasis. Sci. Hortic. 2024, 324, 112606. [Google Scholar] [CrossRef]
- Zhu, Z.; Ding, Y.; Zhao, J.; Nie, Y.; Zhang, Y.; Sheng, J.; Tang, X. Effects of Postharvest Gibberellic Acid Treatment on Chilling Tolerance in Cold-Stored Tomato (Solanum lycopersicum L.) Fruit. Food Bioprocess Technol. 2016, 9, 1202–1209. [Google Scholar] [CrossRef]
- Malekzadeh, P.; Khosravi-Nejad, F.; Hatamnia, A.A.; Sheikhakbari Mehr, R. Impact of postharvest exogenous γ-aminobutyric acid treatment on cucumber fruit in response to chilling tolerance. Physiol. Mol. Biol. Plants 2017, 23, 827–836. [Google Scholar] [CrossRef]
- Ge, W.; Zhao, Y.; Kong, X.; Sun, H.; Luo, M.; Yao, M.; Wei, B.; Ji, S. Combining salicylic acid and trisodium phosphate alleviates chilling injury in bell pepper (Capsicum annuum L.) through enhancing fatty-acid desaturation efficiency and water retention—ScienceDirect. Food Chem. 2020, 327, 127057. [Google Scholar] [CrossRef]
- Xiao, L.; Jiang, X.; Deng, Y.; Xu, K.; Duan, X.; Wan, K.; Tang, X. Study on Characteristics and Lignification Mechanism of Postharvest Banana Fruit during Chilling Injury. Foods 2023, 12, 1097. [Google Scholar] [CrossRef]
- Huang, T.; Liu, G.; Zhu, L.; Liu, J.; Xiang, Y.; Xu, X.; Zhang, Z. Mitigation of chilling injury in mango fruit by methyl jasmonate is associated with regulation of antioxidant capacity and energy homeostasis. Postharvest Biol. Technol. 2024, 211, 112801. [Google Scholar] [CrossRef]
- Sun, H.J.; Luo, M.L.; Zhou, X.; Zhou, Q.; Ji, S.J. Influence of Melatonin Treatment on Peel Browning of Cold-Stored “Nanguo” Pears. Food Bioprocess Technol. 2020, 13, 1478–1490. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, C.; Brummell, D.A.; Shuning, Q.I.; Duan, Y. Salicylic acid treatment mitigates chilling injury in peach fruit by regulation of sucrose metabolism and soluble sugar content. Food Chem. 2021, 358, 129867. [Google Scholar] [CrossRef]
- Sangprayoon, P.; Supapvanich, S.; Youryon, P.; Wongs Aree, C.; Boonyaritthongchai, P. Efficiency of salicylic acid or methyl jasmonate immersions on internal browning alleviation and physicochemical quality of Queen pineapple cv. “Sawi” fruit during cold storage. J. Food Biochem. 2019, 43, e13059. [Google Scholar] [CrossRef]
- Fan, Z.; Lin, B.; Lin, H.; Lin, M.; Chen, J.; Lin, Y. γ-Aminobutyric acid treatment reduces chilling injury and improves quality maintenance of cold-stored Chinese olive fruit. Food Chem. X 2022, 13, 100208. [Google Scholar] [CrossRef]
- Ming, N.; Fengxian, T.; Qin, Z.; Xinxin, Z.; Liping, Y.; Wenchao, C.; Chunhui, S. The quality of Gold Queen Hami melons stored under different temperatures. Sci. Hortic. 2019, 243, 140–147. [Google Scholar] [CrossRef]
- Lado, J.; Gurrea, A.; Zacarías, L.; Rodrigo, M.J. Rodrigo Influence of the storage temperature on volatile emission, carotenoid content and chilling injury development in Star Ruby red grapefruit. Food Chem. 2019, 295, 72–81. [Google Scholar] [CrossRef]
- Jiao, J.; Jin, M.; Liu, H.; Suo, J.; Yin, X.; Zhu, Q.; Rao, J. Application of melatonin in kiwifruit (Actinidia chinensis) alleviated chilling injury during cold storage. Sci. Hortic. 2022, 296, 110876. [Google Scholar] [CrossRef]
- Liao, L.; Li, S.; Li, Y.; Huang, Z.; Li, J.; Xiong, B.; Zhang, M.; Sun, G.; Wang, Z. Pre- or Post-Harvest Treatment with MeJA Improves Post-Harvest Storage of Lemon Fruit by Stimulating the Antioxidant System and Alleviating Chilling Injury. Plants 2022, 21, 2840. [Google Scholar] [CrossRef]
- Guo, X.; Tseung, C.; Zare, A.; Liu, T. Hyperspectral image analysis for the evaluation of chilling injury in avocado fruit during cold storage. Postharvest Biol. Technol. 2023, 206, 112548. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, Y.; Yi, B.; Zhao, Y.; Bao, Y.; Wu, Z.; Zheng, Y.; Jin, P. Exogenous phytosulfokine α alleviates chilling injury of loquat fruit via regulating sugar, proline, polyamine and γ-aminobutyric acid metabolisms. Food Chem. 2024, 436, 137729. [Google Scholar] [CrossRef]
- Balois-Morales, R.; Peña, C.; Baruch Arroyo-Peña, V. Symptoms and sensitivity to chilling injury of pitahaya (Hylocereus undatus (haw.) britton & rose) fruits during postharvest. Agrociencia 2013, 8, 795–813. Available online: https://api.semanticscholar.org/CorpusID:83613141 (accessed on 2 January 2025).
- Yusof, N.L. Effect of Vacuum Impregnation with Sucrose and Plant Growth Hormones to Mitigate the Chilling Injury in Spinach Leaves. Appl. Sci. 2021, 11, 10410. [Google Scholar] [CrossRef]
- Concellon, A.; Zaro, M.J.; Chaves, A.R.; Vicente, A.R. Changes in quality and phenolic antioxidants in dark purple American eggplant (Solanum melongena L. cv. Lucia) as affected by storage at 0 °C and 10 °C. Postharvest Biol. Technol. 2012, 66, 35–41. [Google Scholar] [CrossRef]
- Kaushik, D.; Aryadeep, R. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Cheng, Y.; Song, C. Hydrogen peroxide homeostasis and signaling in plant cells. Sci. China C Life Sci. 2006, 49, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wrzaczek, M.; Overmyer, K.; Rvi, J.K.I.S. Plant ROS and RNS: Making plant science more radical than ever. Physiol. Plant 2010, 4, 357–359. [Google Scholar] [CrossRef]
- Ralay, R.H.; Rakotoarison, O.; Tesse, A.; Schott, C.; Randriantsoa, A.; Lobstein, A.; Andriantsitohaina, R. Cedrelopsis grevei induced hypotension and improved endothelial vasodilatation through an increase of Cu/Zn SOD protein expression. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H775–H781. [Google Scholar] [CrossRef]
- Shahida, M.N.; Siti, N.; Ding, P. Effects of temperature and storage duration on keeping quality of Malaysian grown passion fruit (Passiflora edulis Sims f. flavicarpa Deg.). Acta Hortic. 2021, 1327, 557–562. [Google Scholar] [CrossRef]
- Bai, C.; Wu, C.; Ma, L.; Fu, A.; Zheng, Y.; Han, J.; Li, C.; Yuan, S.; Zheng, S.; Gao, L. Transcriptomics and metabolomics analyses provide insights into postharvest ripening and senescence of tomato fruit under low temperature. Hortic. Plant J. 2023, 9, 109–121. [Google Scholar] [CrossRef]
- Hu, D.; Guo, Q.; Zhang, Y.; Chen, F. Maize Methionine Sulfoxide Reductase Genes ZmMSRA2 and ZmMSRA5.1 Involved in the Tolerance to Osmotic or Salinity Stress in Arabidopsis and Maize. Plant Mol. Biol. Report. 2022, 41, 118–133. [Google Scholar] [CrossRef]
- Kaya, A.; Lee, B.C.; Gladyshev, V.N. Regulation of Protein Function by Reversible Methionine Oxidation and the Role of Selenoprotein MsrB1. Antioxid. Redox Signal. 2015, 23, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Johnson, L.C.; Weissbach, H.; Brot, N.; Lively, M.O.; Lowther, W.T. Free methionine-(R)-sulfoxide reductase from Escherichia coli reveals a new GAF domain function. Proc. Natl. Acad. Sci. USA 2007, 104, 9597–9602. [Google Scholar] [CrossRef]
- Ding, P.; Fang, L.; Wang, G.; Li, X.; Huang, S.; Gao, Y.; Zhu, J.; Xiao, L.; Tong, J.; Chen, F.; et al. Wheat methionine sulfoxide reductase A4.1 interacts with heme oxygenase 1 to enhance seedling tolerance to salinity or drought stress. Plant Mol. Biol. 2019, 101, 203–220. [Google Scholar] [CrossRef]
- Tarrago, L.; Kaya, A.; Weerapana, E.; Marino, S.M.; Gladyshev, V.N. Methionine sulfoxide reductases preferentially reduce unfolded oxidized proteins and protect cells from oxidative protein unfolding. J. Biol. Chem. 2012, 287, 24448–24459. [Google Scholar] [CrossRef]
- Lee, E.S.; Park, J.H.; Wi, S.D.; Chae, H.B.; Paeng, S.K.; Bae, S.B.; Phan, K.A.T.; Lee, S.Y. Arabidopsis Disulfide Reductase, Trx-h2, Functions as an RNA Chaperone under Cold Stress. Appl. Sci. 2021, 11, 6865. [Google Scholar] [CrossRef]
- Oh, J.; Hong, S.; Lee, Y.; Koh, E.; Kim, K.; Seo, Y.W.; Chung, N.; Jeong, M.; Jang, C.S.; Lee, B.; et al. Modulation of gene expressions and enzyme activities of methionine sulfoxide reductases by cold, ABA or high salt treatments in Arabidopsis. Plant Sci. 2005, 169, 1030–1036. [Google Scholar] [CrossRef]
- Elbagoury, M.M.; Turoop, L.; Runo, S.; Sila, D.N. Regulatory influences of methyl jasmonate and calcium chloride on chilling injury of banana fruit during cold storage and ripening. Food Sci. Nutr. 2021, 9, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Wang, M.H. Characterization and functional analysis of methionine sulfoxide reductase A gene family in tomato. Mol. Biol. Rep. 2012, 39, 6297–6308. [Google Scholar] [CrossRef]
- Qin, G.; Meng, X.; Wang, Q.; Tian, S. Oxidative damage of mitochondrial proteins contributes to fruit senescence: A redox proteomics analysis. J. Proteome Res. 2009, 8, 2449. [Google Scholar] [CrossRef]
- Jiao, C.; Duan, Y. The Role of IP3 in NO-Enhanced Chilling Tolerance in Peach Fruit. J. Agric. Food Chem. 2019, 67, 8312–8318. [Google Scholar] [CrossRef]
- Sadanandom, A.; Poghosyan, Z.; Fairbairn, D.J.; Murphy, D.J. Differential Regulation of Plastidial and Cytosolic Isoforms of Peptide Methionine Sulfoxide Reductase in Arabidopsis. Plant Physiol. 2000, 123, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zeng, J.; Li, Z.; Song, Y.; Yan, H.; He, J.; Jiang, Y.; Duan, X. Redox Regulation of the NOR Transcription Factor Is Involved in the Regulation of Fruit Ripening in Tomato 1. Plant Physiol. 2020, 2, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.L.; Wang, W.Q.; Liu, X.F.; Duan, X.W.; Allan, A.C.; Grierson, D.; Yin, X.R. An ethylene-hypersensitive methionine sulfoxide reductase regulated by NAC transcription factors increases methionine pool size and ethylene production during kiwifruit ripening. New Phytol. 2021, 232, 237–251. [Google Scholar] [CrossRef]
- Jiang, G.; Wu, F.; Li, Z.; Li, T.; Gupta, V.K.; Duan, X.; Jiang, Y. Sulfoxidation Regulation of Musa acuminata Calmodulin (MaCaM) Influences the Functions of MaCaM-Binding Proteins. Plant Cell Physiol. 2018, 59, 1214–1224. [Google Scholar] [CrossRef]
- Rey, P.; Tarrago, L. Physiological Roles of Plant Methionine Sulfoxide Reductases in Redox Homeostasis and Signaling. Antioxidants 2018, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, M.T.; Martínez-Téllez, M.A.; Zacarías, L. Abscisic Acid in the Response of ‘Fortune’ Mandarins to Chilling. Effect of Maturity and High-Temperature Conditioning. J. Sci. Food Agric. 1997, 73, 494–502. [Google Scholar] [CrossRef]
- Zhang, T.; Che, F.; Zhang, H.; Pan, Y.; Xu, M.; Ban, Q.; Han, Y.; Rao, J. Effect of nitric oxide treatment on chilling injury, antioxidant enzymes and expression of the CmCBF1 and CmCBF3 genes in cold-stored Hami melon (Cucumis melo L.) fruit. Postharvest Biol. Technol. 2017, 127, 88–98. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Hu, M.; Pan, Y.; Jiang, Y.; Zhang, Z.; Jiang, G. Nitric oxide is involved in melatonin-induced cold tolerance in postharvest litchi fruit. Postharvest Biol. Technol. 2023, 196, 112157. [Google Scholar] [CrossRef]
- Ghorbani, B.; Pakkish, Z.; Khezri, M. Nitric oxide increases antioxidant enzyme activity and reduces chilling injury in orange fruit during storage. N. Z. J. Crop Hortic. Sci. 2018, 46, 101–116. [Google Scholar] [CrossRef]
- Jiao, C. IP3 mediates NO-enhanced chilling tolerance in postharvest kiwifruit. Postharvest Biol. Technol. 2021, 176, 111463. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Khan, Z.U.; Mao, L.; Ying, T. Effect of nitric oxide on energy metabolism in postharvest banana fruit in response to chilling stress. Postharvest Biol. Technol. 2015, 108, 21–27. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, Z.; Swinny, E.E. Postharvest nitric oxide fumigation delays fruit ripening and alleviates chilling injury during cold storage of Japanese plums (Prunus salicina Lindell). Postharvest Biol. Technol. 2009, 53, 101–108. [Google Scholar] [CrossRef]
- Xu, M.; Dong, J.; Zhang, M.; Xu, X.; Sun, L. Cold-induced endogenous nitric oxide generation plays a role in chilling tolerance of loquat fruit during postharvest storage. Postharvest Biol. Technol. 2012, 65, 5–12. [Google Scholar] [CrossRef]
- Wang, D.; Li, L.; Xu, Y.; Limwachiranon, J.; Li, D.; Ban, Z.; Luo, Z. Effect of Exogenous Nitro Oxide on Chilling Tolerance, Polyamine, Proline, and gamma-Aminobutyric Acid in Bamboo Shoots (Phyllostachys praecox f. prevernalis). J. Agric. Food Chem. 2017, 65, 5607–5613. [Google Scholar] [CrossRef]
- Jiao, C.; Chai, Y.; Duan, Y. Inositol 1, 4, 5-Trisphosphate Mediates Nitric-Oxide-Induced Chilling Tolerance and Defense Response in Postharvest Peach Fruit. J. Agric. Food Chem. 2019, 17, 4764–4773. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Fortiz, J.; Cruz, R.; Baez, R.; Wang, C.Y. Methyl Jasmonate Reduces Chilling Injury and Maintains Postharvest Quality of Mango Fruit. J. Agric. Food Chem. 2000, 48, 515–519. [Google Scholar] [CrossRef]
- Zhou, J.; Min, D.; Li, Z.; Fu, X.; Zhao, X.; Wang, J.; Zhang, X.; Li, F.; Li, X. Effects of chilling acclimation and methyl jasmonate on sugar metabolism in tomato fruits during cold storage. Sci. Hortic. 2021, 289, 110495. [Google Scholar] [CrossRef]
- Fan, L.; Wang, Q.; Lv, J.; Gao, L.; Zuo, J.; Shi, J. Amelioration of postharvest chilling injury in cowpea (Vigna sinensis) by methyl jasmonate (MeJA) treatments. Sci. Hortic. 2016, 203, 95–101. [Google Scholar] [CrossRef]
- Cao, S.; Cai, Y.; Yang, Z.; Zheng, Y. MeJA induces chilling tolerance in loquat fruit by regulating proline and γ-aminobutyric acid contents. Food Chem. 2012, 133, 1466–1470. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Wang, K.; Rui, H.; Tang, S. Effect of methyl jasmonate on cell wall modification of loquat fruit in relation to chilling injury after harvest. Food Chem. 2010, 118, 641–647. [Google Scholar] [CrossRef]
- Zhang, X.; Sheng, J.; Li, F.; Meng, D.; Shen, L. Methyl jasmonate alters arginine catabolism and improves postharvest chilling tolerance in cherry tomato fruit. Postharvest Biol. Technol. 2012, 64, 160–167. [Google Scholar] [CrossRef]
- Yang, A.; Cao, S.; Yang, Z.; Cai, Y.; Zheng, Y. γ-Aminobutyric acid treatment reduces chilling injury and activates the defence response of peach fruit. Food Chem. 2011, 129, 1619–1622. [Google Scholar] [CrossRef]
- Soleimani Aghdam, M.; Naderi, R.; Sarcheshmeh, M.A.A.; Babalar, M. Amelioration of postharvest chilling injury in anthurium cut flowers by γ-aminobutyric acid (GABA) treatments. Postharvest Biol. Technol. 2015, 110, 70–76. [Google Scholar] [CrossRef]
- Khaliq, G.; Ali, S.; Ejaz, S.; Abdi, G.; Faqir, Y.; Ma, J.; Siddiqui, M.W.; Ali, A. γ-Aminobutyric acid is involved in overlapping pathways against chilling injury by modulating glutamate decarboxylase and defense responses in papaya fruit. Front. Plant Sci. 2023, 14, 1233477. [Google Scholar] [CrossRef]
- Ngaffo Mekontso, F.; Duan, W.; Cisse, E.H.M.; Chen, T.; Xu, X. Alleviation of Postharvest Chilling Injury of Carambola Fruit by γ-aminobutyric Acid: Physiological, Biochemical, and Structural Characterization. Front. Nutr. 2021, 8, 752583. [Google Scholar] [CrossRef]
- Rastegar, S.; Khankahdani, H.H.; Rahimzadeh, M. Effect of γ-aminobutyric acid on the antioxidant system and biochemical changes of mango fruit during storage. J. Food Meas. Charact. 2020, 14, 778–789. [Google Scholar] [CrossRef]
- Ge, Y.; Duan, B.; Li, C.; Tang, Q.; Li, X.; Wei, M.; Chen, Y.; Li, J. γ-Aminobutyric acid delays senescence of blueberry fruit by regulation of reactive oxygen species metabolism and phenylpropanoid pathway. Sci. Hortic. 2018, 240, 303–309. [Google Scholar] [CrossRef]
- Al Shoffe, Y.; Nock, J.F.; Zhang, Y.; Watkins, C.B. Pre- and post-harvest γ-aminobutyric acid application in relation to fruit quality and physiological disorder development in ‘Honeycrisp’ apples. Sci. Hortic. 2021, 289, 110431. [Google Scholar] [CrossRef]
- Meng, D.M.; Wang, H.D.; Zhang, Y.X.; Xi, Z.A.; Yang, R.; Sheng, J.P.; Zhang, X.H.; Ding, Y.; Wang, J.P.; Fan, Z.C. Ornithine decarboxylase is involved in methyl jasmonate-regulated postharvest quality retention in button mushrooms (Agaricus bisporus). J. Sci. Food Agric. 2019, 99, 790–796. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Huang, X.; Yang, K.; Gao, S.; Du, R. Effect of exogenous γ-aminobutyric acid (GABA) treatment on chilling injury and antioxidant capacity in banana peel. Sci. Hortic. 2014, 168, 132–137. [Google Scholar] [CrossRef]
- Jiao, C. γ-Aminobutyric acid boosts chilling tolerance by promoting the methionine sulfoxide reductase-thioredoxin reductase system in peach fruit. Hortic. Environ. Biotechnol. 2022, 63, 353–361. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, C.; Yang, R.; Zhao, S.; Han, X.; Wang, Z.; Li, S.; Gao, H. Endogenous salicylic acid mediates melatonin-induced chilling-and oxidative-stress tolerance in harvested kiwifruit. Postharvest Biol. Technol. 2023, 201, 112341. [Google Scholar] [CrossRef]
- Guillén, F.; Medina-Santamarina, J.; García-Pastor, M.E.; Chen, N.J.; Uruu, G.; Paull, R.E. Postharvest melatonin treatment delays senescence and increases chilling tolerance in pineapple. Lwt 2022, 169, 113989. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Q.; Chen, W.; Guo, Q.; Xia, Y.; Wu, D.; Jing, D.; Liang, G. Melatonin treatment maintains quality and delays lignification in loquat fruit during cold storage. Sci. Hortic. 2021, 284, 110126. [Google Scholar] [CrossRef]
- Madebo, M.P.; Luo, S.; Wang, L.; Zheng, Y.; Jin, P. Melatonin treatment induces chilling tolerance by regulating the contents of polyamine, γ-aminobutyric acid, and proline in cucumber fruit. J. Integr. Agric. 2021, 20, 3060–3074. [Google Scholar] [CrossRef]
- Yan, R.; Li, S.; Cheng, Y.; Kebbeh, M.; Huan, C.; Zheng, X. Melatonin treatment maintains the quality of cherry tomato by regulating endogenous melatonin and ascorbate-glutathione cycle during room temperature. J. Food Biochem. 2022, 46, e14285. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, A.; Wang, B.; Zhang, H.; Zheng, Y.; Wang, L. Melatonin mobilizes the metabolism of sugars, ascorbic acid and amino acids to cope with chilling injury in postharvest pear fruit. Sci. Hortic. 2024, 323, 112548. [Google Scholar] [CrossRef]
- Kebbeh, M.; Dong, J.; Huan, C.; Liu, Y.; Zheng, X. Melatonin treatment alleviates chilling injury in mango fruit ‘Keitt’ by modulating proline metabolism under chilling stress. J. Integr. Agric. 2023, 22, 935–944. [Google Scholar] [CrossRef]
- Jannatizadeh, A.; Aghdam, M.S.; Luo, Z.; Razavi, F. Impact of Exogenous Melatonin Application on Chilling Injury in Tomato Fruits During Cold Storage. Food Bioprocess Technol. 2019, 12, 741–750. [Google Scholar] [CrossRef]
- Kong, X.; Ge, W.; Wei, B.; Zhou, Q.; Zhou, X.; Zhao, Y.; Ji, S. Melatonin ameliorates chilling injury in green bell peppers during storage by regulating membrane lipid metabolism and antioxidant capacity. Postharvest Biol. Technol. 2020, 170, 111315. [Google Scholar] [CrossRef]
- Ali, S.; Nawaz, A.; Naz, S.; Ali, M.; Ejaz, S.; Azam, M.; Razzaq, K. Exogenous melatonin mitigates chilling injury in zucchini fruit by enhancing antioxidant system activity, promoting endogenous proline and GABA accumulation, and preserving cell wall stability. Postharvest Biol. Technol. 2023, 204, 112445. [Google Scholar] [CrossRef]
- Gao, H.; Lu, Z.M.; Yang, Y.; Wang, D.N.; Cao, W. Melatonin treatment reduces chilling injury in peach fruit through its regulation of membrane fatty acid contents and phenolic metabolism. Food Chem. 2017, 245, 659–666. [Google Scholar] [CrossRef]
- Jiao, C.; Duan, Y. Guanosine 3′,5′-cyclic monophosphate mediates gibberellic acid-induced chilling tolerance and defense response in postharvest peach fruit. Postharvest Biol. Technol. 2019, 155, 80–85. [Google Scholar] [CrossRef]
- Ding, Y.; Sheng, J.; Li, S.; Nie, Y.; Zhao, J.; Zhu, Z.; Wang, Z.; Tang, X. The role of gibberellins in the mitigation of chilling injury in cherry tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2015, 101, 88–95. [Google Scholar] [CrossRef]
- Oien, D.B.; Moskovitz, J. Substrates of the methionine sulfoxide reductase system and their physiological relevance. Curr. Top. Dev. Biol. 2008, 80, 93–133. [Google Scholar] [CrossRef]
- Gao, H.; Kang, L.; Liu, Q.; Cheng, N.; Wang, B.; Cao, W. Effect of 24-epibrassinolide treatment on the metabolism of eggplant fruits in relation to development of pulp browning under chilling stress. J. Food Sci. Technol. 2014, 52, 3394–3401. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, S.; Niu, C.; Chen, H.; Zhu, F.; Farouk, A.; Lu, J.; Chen, C.; Ban, Z.; Huang, J. Brassinolide Alleviates Chilling Injury of Sweet Cherry (Prunus avium L. cv. Tieton) during Cold Storage. Horticulturae 2024, 10, 675. [Google Scholar] [CrossRef]
- Xu, X.; Guo, C.; Ma, C.; Li, M.; Chen, Y.; Liu, C.; Chu, J.; Yao, X. Brassinolide Soaking Reduced Nitrite Content and Extended Color Change and Storage Time of Toona sinensis Bud during Low Temperature and Near Freezing-Point Temperature Storage. Int. J. Mol. Sci. 2022, 23, 13110. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.; Lv, X.; Cheng, N.; Peng, B.; Cao, W. Effect of 24-epibrassinolide on chilling injury of peach fruit in relation to phenolic and proline metabolisms. Postharvest Biol. Technol. 2016, 111, 390–397. [Google Scholar] [CrossRef]
- Hu, S.; Wang, T.; Shao, Z.; Meng, F.; Chen, H.; Wang, Q.; Zheng, J.; Liu, L. Brassinosteroid Biosynthetic Gene SlCYP90B3 Alleviates Chilling Injury of Tomato (Solanum lycopersicum) Fruits during Cold Storage. Antioxidants 2022, 11, 115. [Google Scholar] [CrossRef]
- Chakraborty, N.; Ganguly, R.; Sarkar, A.; Dasgupta, D.; Sarkar, J.; Acharya, K.; Burachevskaya, M.; Minkina, T.; Keswani, C. Multifunctional Role of Brassinosteroids in Plant Growth, Development, and Defense. J. Plant Growth Regul. 2025, 24, 11593–11594. [Google Scholar] [CrossRef]
- Liu, Z.; Rouhier, N.; Couturier, J. Dual Roles of Reducing Systems in Protein Persulfidation and Depersulfidation. Antioxidants 2025, 14, 101. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, F.; Shuai, L.; Yusof, M.T.; Ramli, N.S.; Misran, A.; Liu, Y.; He, M.; Liang, Y.; Dek, M.S.P. The Role and Regulatory Mechanism of Methionine Sulfoxide Reductase (Msr) in the Process of Chilling Injury of Fruits and Vegetables: A Review. Horticulturae 2025, 11, 422. https://doi.org/10.3390/horticulturae11040422
Yin F, Shuai L, Yusof MT, Ramli NS, Misran A, Liu Y, He M, Liang Y, Dek MSP. The Role and Regulatory Mechanism of Methionine Sulfoxide Reductase (Msr) in the Process of Chilling Injury of Fruits and Vegetables: A Review. Horticulturae. 2025; 11(4):422. https://doi.org/10.3390/horticulturae11040422
Chicago/Turabian StyleYin, Feilong, Liang Shuai, Mohd Termizi Yusof, Nurul Shazini Ramli, Azizah Misran, Yunfen Liu, Meiying He, Yuanli Liang, and Mohd Sabri Pak Dek. 2025. "The Role and Regulatory Mechanism of Methionine Sulfoxide Reductase (Msr) in the Process of Chilling Injury of Fruits and Vegetables: A Review" Horticulturae 11, no. 4: 422. https://doi.org/10.3390/horticulturae11040422
APA StyleYin, F., Shuai, L., Yusof, M. T., Ramli, N. S., Misran, A., Liu, Y., He, M., Liang, Y., & Dek, M. S. P. (2025). The Role and Regulatory Mechanism of Methionine Sulfoxide Reductase (Msr) in the Process of Chilling Injury of Fruits and Vegetables: A Review. Horticulturae, 11(4), 422. https://doi.org/10.3390/horticulturae11040422






