Interactions of Fe and Zn Nanoparticles at Physiochemical, Biochemical, and Molecular Level in Horticultural Crops Under Salt Stress: A Review
Abstract
1. Introduction
2. Impact of Soil Salinity on Horticultural Crops
3. Different Methods to Mitigate Salt Stress
4. Genetic Engineering in Coping Salt Stress in Horticultural Crops
5. Application of Nanoparticles and Their Impacts Against Salt Stress
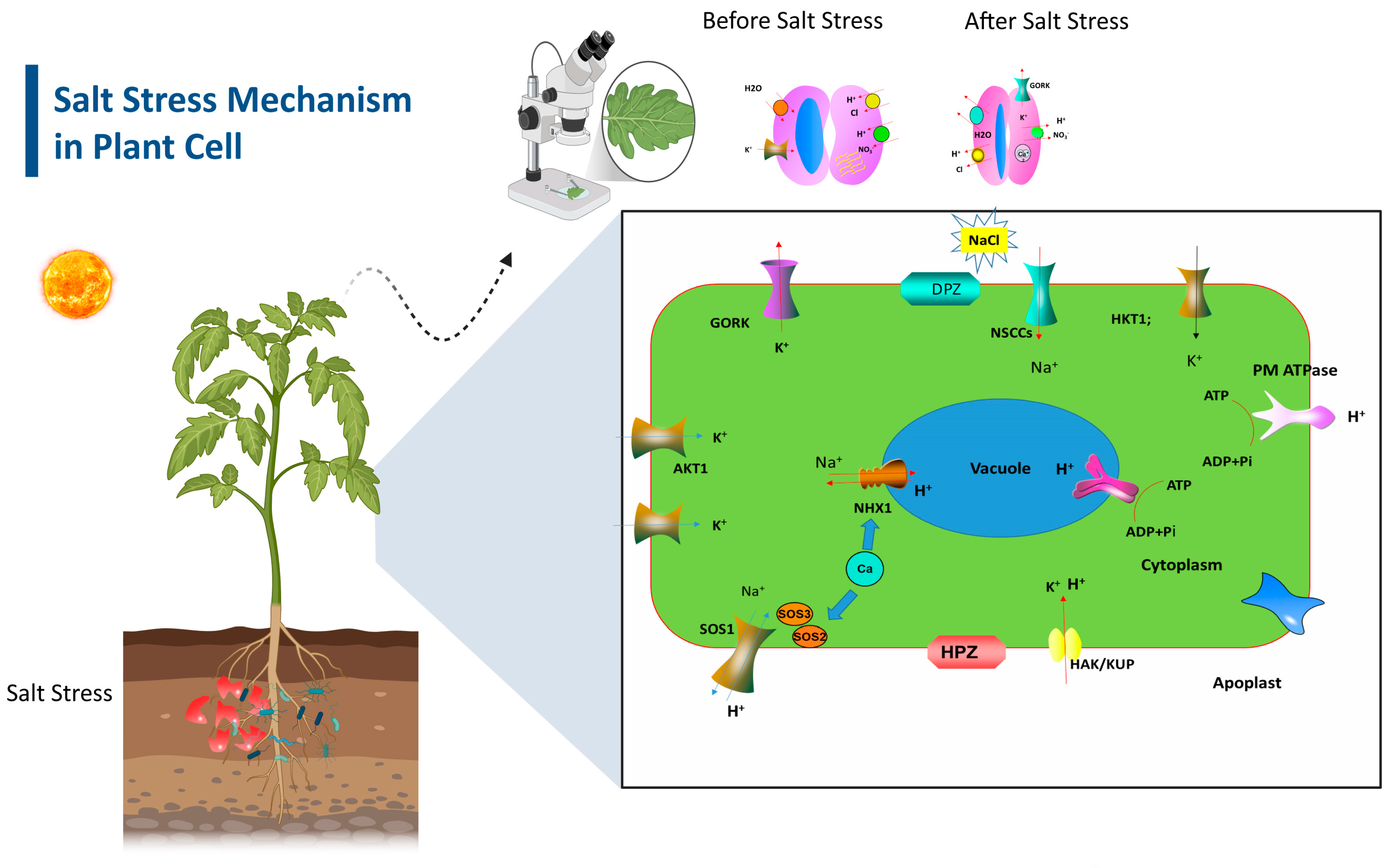
6. Inoculation, Uptake Mechanism, and Salt Mitigation Effect
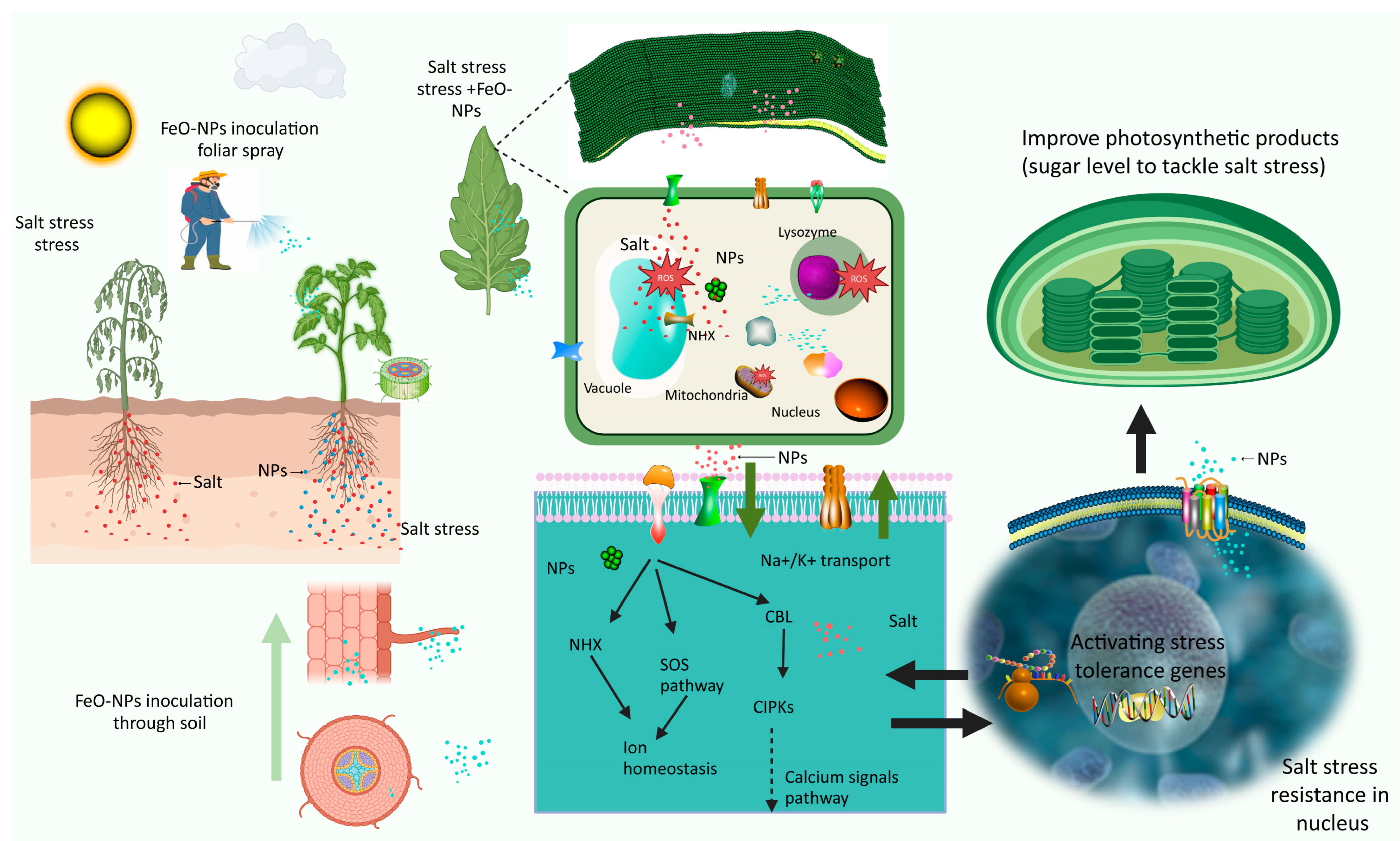
7. Iron Oxide Nanoparticles’ Role of Mitigating Salt Stress in Horticultural Crop
| Crop | Inoculation Method | FeO-NPs Concentration | NaCl Concentration | Physiological Attributes | Reference |
|---|---|---|---|---|---|
| Trachyspermum ammi L. | Foliar spray | 5, 10, and 15 μM/L | 0, 25, 50, and 75 mM | Improved uptake of beneficial nutrients like Fe, N, P, K, and Zn plant growth parameters (e.g., fresh root and shoot weight, root shoot length, and leaf number of leaves under severe salt stress) | [89] |
| Pistacia vera L. | Hydroponic | 2.9 mg/L | 0, 100, and 200 mM | Reduced oxidative stress and enhanced chlorophyll content | [90] |
| Trachyspermum ammi L. | Foliar application | 4, 8, and 12 dS/m | 3 mM | Increased endogenous SA levels, Fe content, and K+ intake and maintained the K+/Na+ ratio and antioxidant enzyme activity | [88] |
| Dracocephalum moldavica L. | Foliar application | 30, 60, and 90 mg/L | 50–100 mM | Enhanced the antioxidant enzymatic activities and phenolic compounds flavonoid and anthocyanin | [86] |
| Lycopersicon esculentum L. | Priming | 25, 50, and 100 mg/kg soil | 200 mM | Antioxidants and osmoregulatory substances significantly improved | [91] |
| Aloe vera L. | Foliar application | 2 mg/L | 50–100 mM | Alleviated the damaging effects of salinity | [92] |
| Lycopersicon esculentum L. | Soil drench | 100 mg/L | 200 mM | Impact on growth, biosynthesis of chlorophyll content, and metabolic activity | [93] |
| Fragaria x ananassa Duch. | MS medium | 0, 0.08, and 0.8 ppm | 0, 50 and 100 mM | Enhanced the antioxidant enzymatic activities and soluble sugar contents | [94] |
| Phoenix dactylifera L. | MS medium | 1 mg/L | 1% | Increased antioxidant activity | [95] |
| Moring oleifera L. | Foliar treatment | 0, 20, 40, and 60 ppm | Saline mix soil | Enhanced crude protein, fiber, and ash percentages, as well as the activity of antioxidant enzymes | [96] |
| Vitis vinifera L. | Irrigation | 0, 0.08, and 0.8 ppm | 100 mM | Improved ion homeostasis | [97] |
| Vitis trifolia L. | Irrigation | 0.8 ppm | 0, 50, and 100 mM | Improved micronutrients in stressful conditions | [98] |
| Zea mays L. | Foliar application | 0, 15, and 30 ppm | 0, 50, 100 and 150 mM | It reduced the adverse effects of salinity stress by effectively maintaining the regulation of Na+ concentration, reduction in MDA levels, and H2O2 concentration | [99] |
| Triticum aestivum L. | Seed priming | 0 and 500 mg/L | 75 mM | Enhanced antioxidant activities and gene expression | [100] |
| Gossypium hirsutum L. | Irrigation | 0.3 mg and 100 mg/L | 10 mM | Increasing leaf number and fresh leaf weight, even under stress | [101] |
| Helianthus annuus L. | Foliar spray | 2 g/L | 100 mM | Shoot dry weight and seed yield of sunflower | [102] |
| Phaseolus vulgaris L. | Foliar spray | 0, 10, 20, and 30 µM | 200 mM | Seed germination percentage, shoot length, carbohydrate in the shoot and root, and soluble proteins in the shoot and root | [103] |
| Luffa cylindrica L. | Foliar spray | 0.01, 0.025, 0.05, and 0.1 ppm | 60 mM | Photosynthetic activity, pigments, primary and secondary metabolites, and antioxidant enzymatic activity | [104] |
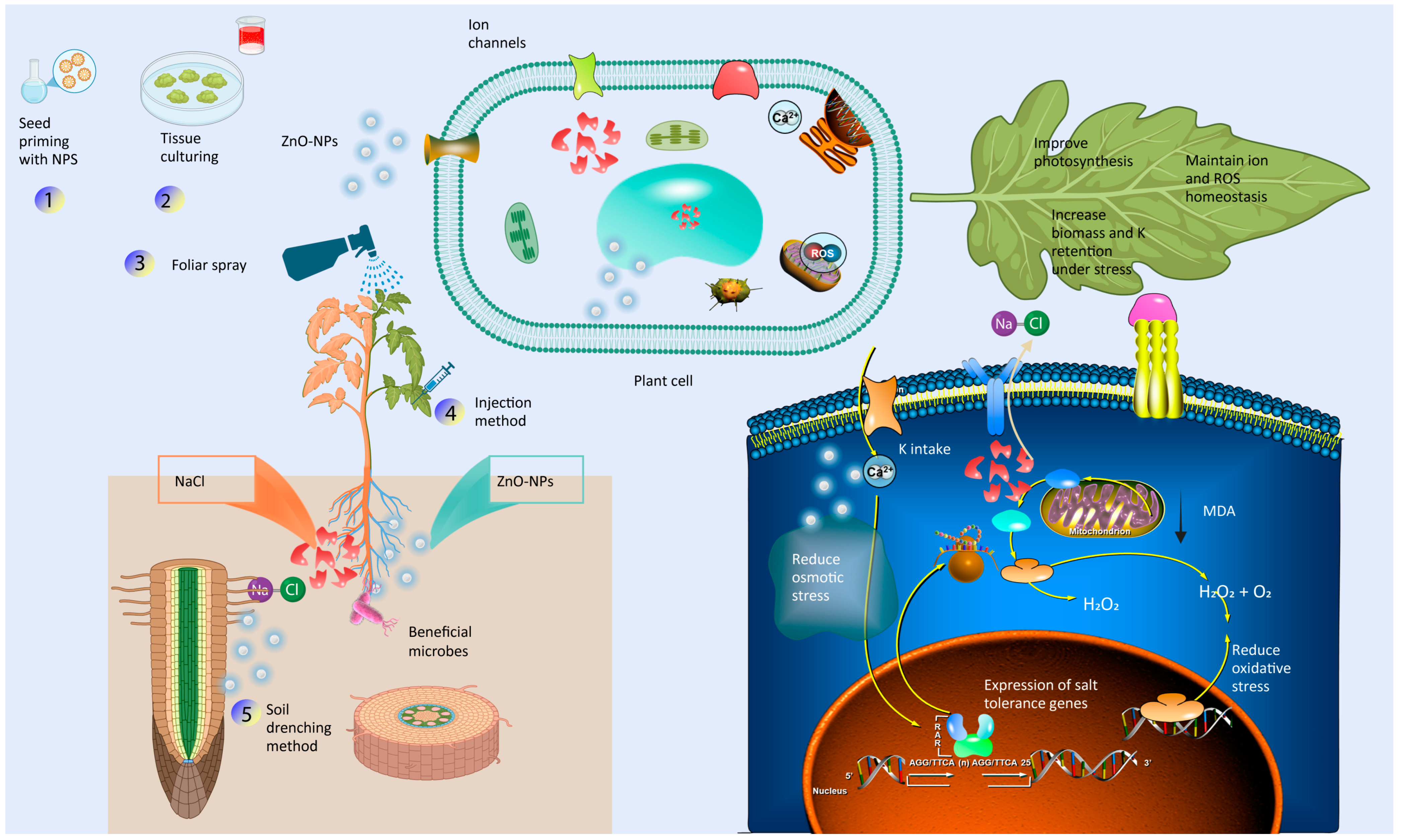
8. Mitigation Impact of Zinc Oxide Nanoparticles in Horticultural Crop
| Crop | Inoculation Method | ZnO-NPs Concentration | NaCl Concentration | Physiological Attributes | Reference |
|---|---|---|---|---|---|
| Solanum melongena L. | Seed priming | 0.42, 0.49, 0.50, and 0.57 mg/g | 50 and 100 mM | ZnO-NPs improved photosynthetic pigments, biosynthesis of chlorophyll contents, total soluble sugars, proteins, and proline contents | [108] |
| Capsicum annum L. | Foliar spray | 0, 1000, and 2000 ppm | 25, 50, and 75 mM | Improved antioxidant enzymatic system, water holding capacity, retention of K+ and proline content, reduction in electrolyte leakage (EL), Na+, and MDA level | [109] |
| Phaseolus vulgaris L. | Foliar spray | 0, 10, 20, and 30 µM | 200 mM | Enhanced the total chlorophyll contents, carbohydrates, and nitrogen in the shoot and root of the plant | [103] |
| Raphanus sativus L. | Supplemented with soil | 1% w/w | 100 mM | Promoted biomass and antioxidant activities | [110] |
| Vicia faba L. | Foliar application | 50 and 100 mg/L | 150 mM | Improved plant vegetative growth as well as the accumulation of antioxidants enzymes, osmolytes, and secondary metabolites and reduced reactive oxygen species level | [111] |
| Cucumis sativus L. | Foliar application | 25 and 100 mg/L | 200 mM | Improved proline contents, total sugar, glycine betaine, free amino acids, and antioxidant enzymes | [112] |
| Carthamus tinctorius L. | Foliar application | 17 mg/L | 250 mM | Reduction in the Na+ concentration in leaves and roots (74–60%) | [113] |
| Lagenaria siceraria L. | Soil drenching | 250, 500, and 750 mg/L | 500 mg/L | Increased total soluble sugars (23%) and chlorophyll (31%) while keeping the gas exchange parameters constant | [114] |
| Glycine max L. | Presoaking | 25, 50, 100, and 200 mg/L | 250 mM | Improved crop yield and enhanced antioxidant enzyme activities | [115] |
| Fragaria x ananassa Duch. | Ms media | 0, 15, and 30 mg/L | 0, 35, and 70 mM | Treatment resulted in increased proline content, peroxidase (POD), and catalase (CAT) levels | [116] |
| Coriandrum sativum L. | Ms media | 100 mg/L | 50 mM | Enhanced free radical scavenging activity reduced by up to 55%, antioxidant potential by up to 35%, and reduced power by 20% in leaves | [117] |
| Brassica napus L. | Priming | 100 mg/L | 150 mM/L | Enhanced plant growth development and gene expression | [118] |
| Solanum lycopersicum L. | Injection method | 0, 20, and 40 mg/L | 250 mM NaCl | Improved the endophytic bacteria and promoted plant growth under stress conditions | [119] |
| Camelina sativa L. | Foliar application | 0, 20, 40, and 80 mg/L | 0, 50, and 100 mM | Enhanced the macronutrients and plant growth | [120] |
| Olea europaea L. | Foliar application | 200 mg/L | Saline soil | Enhanced the oil and total soluble solids (TSS) percentages of olive fruit as well as the N and P mineral content of the leaves | [121] |
| Pisum sativum L. | Seed priming | 50 and 100 ppm | 50 and 100 mM | Decreased levels of MDA, glycine betaine, and hydrogen peroxide | [122] |
| Oryza sativa L. | modified Hogland’s solution) | 50 mg/L | 0, 60, 80, and 100 mM | Improved antioxidant system that decreased MDA, proline, and H2O2 levels while lowering oxidative stress | [123] |
| Triticum aestivum L. | Supplemented with soil | 0.12 g/pot | 10 dS/m | Increased the total chlorophyll content by 24.6 and 10%, the height of the plant at the vegetative development stage and maturation stages by 34.6 and 37.4%, the length of the shoots and spikes by 30.7 to 27.6%, and the fresh and dry weights of the roots by 74.5 to 63.1% | [124] |
| Zea mays L. | Foliar application | 0, 50, and 100 mg/L | 60 and 120 mM | Significantly impacted features of plant development, physiological function, nutritional profiles, antioxidant activity, plant yield, and traits that contribute to yield | [125] |
| Hordeum vulgare L. | Seed priming | 100 mg/kg | 100 mM | Mitigation of salt adverse effect; improved fresh biomass, photosynthesis, and antioxidant enzymatic activities by reducing ROS level | [126] |
| Solanum tubersum L. | Soil drenching method | 20, 12, and 15 ppm | Saline soil | Enhanced physiological characteristics, shoot dry weight, number of stems per plant, relative water content of leaves, photosynthetic rate, stomatal conductance, and chlorophyll content | [127] |
| Gossipyum barbadense L. | Foliar application | 100 and 200 ppm | EC 52 dS/m (20%) | ZnO-NPs promoted the dry mass of root, stem and leaves; improved shoot/root ratio | [128] |
| Helianthus annuus L. | Foliar application | 2 g/L | 0 and 100 mM | Improved plant physiological attributes and antioxidant activities | [129] |
| Medicago sativa L. | Foliar application | 25 and 50 mg/L | S100 | Increased the levels of osmolytes (proline, total soluble sugar, and total soluble protein) | [130] |
| Phaseolus vulgaris L. | Foliar application | 25, 50, 100, and 200 mg/L | 200 mM | Increased total soluble sugar contents, proline contents, and antioxidant enzyme activity by promoting the physiological growth parameters | [107] |
| Vicia faba L. | Foliar application | 50 and 100 mg/L | 150 mM | Promoted fresh biomass and antioxidant activities | [111] |
| Abelmoschus esculentus L. | Foliar application | 10 mg/L | 0, 10, 25, 50, 75, and 100% SW | Significantly enhanced the antioxidant activities and total soluble sugar contents | [131] |
| Moringa peregrina L. | Foliar application | 30, 60, and 90 mg/L | 3000, 6000, and 9000 mg/L | Reduction in Na+ while increasing the uptake of beneficial nutrients (Fe, N, P, K, Zn, Mn, Mg) | [132] |
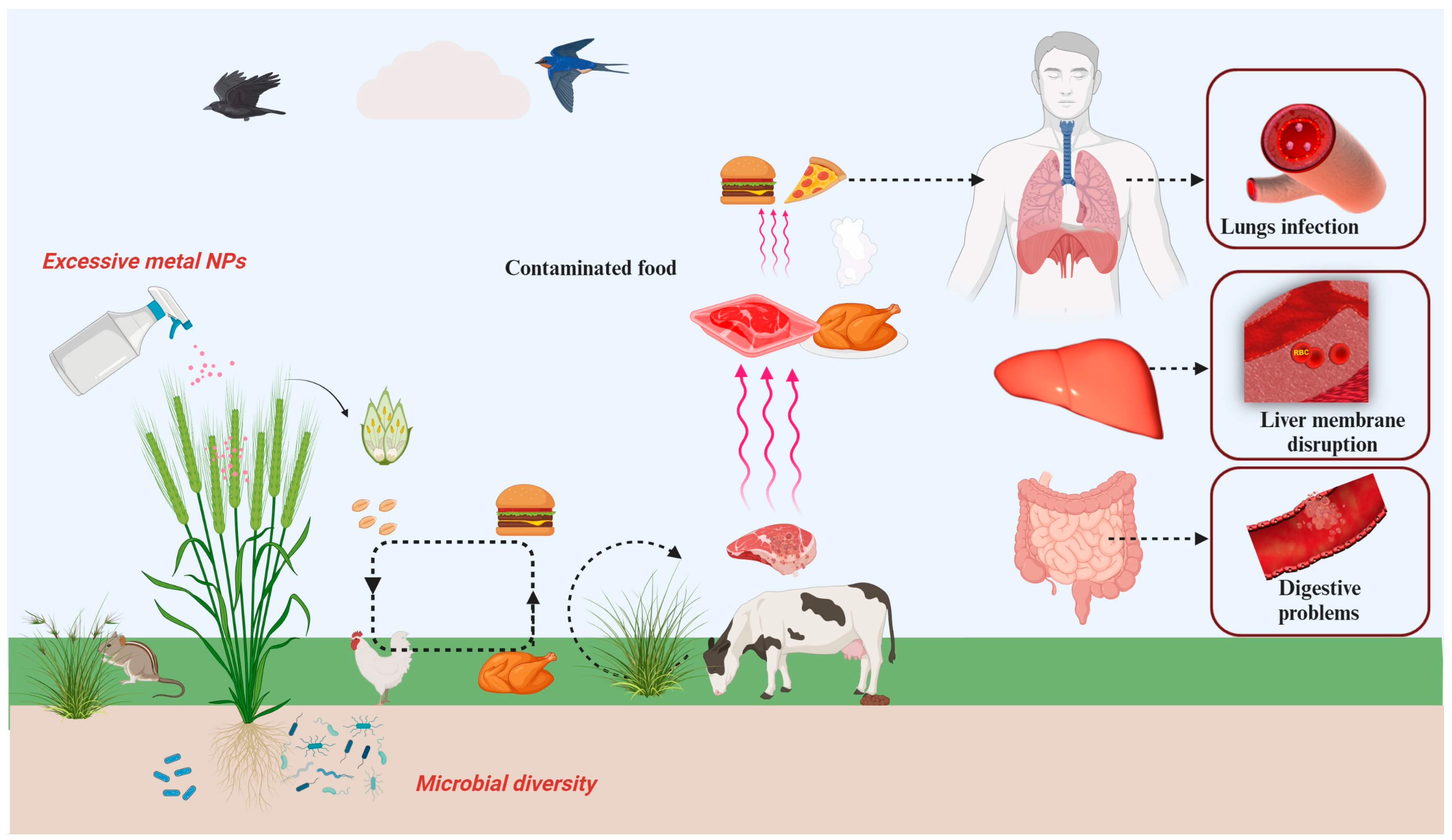
9. Global Risk, Limitations, and Challenges of NPs Application in Future Agriculture
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, Z.-Y.; Rao, M.P.N.; Wang, H.-F.; Fang, B.-Z.; Liu, Y.-H.; Li, L.; Xiao, M.; Li, W.-J. Transcriptomic analysis of two endophytes involved in enhancing salt stress ability of Arabidopsis thaliana. Sci. Total Environ. 2019, 686, 107–117. [Google Scholar] [CrossRef]
- Hayat, K.; Zhou, Y.; Menhas, S.; Bundschuh, J.; Hayat, S.; Ullah, A.; Wang, J.; Chen, X.; Zhang, D.; Zhou, P. Pennisetum giganteum: An emerging salt accumulating/tolerant non-conventional crop for sustainable saline agriculture and simultaneous phytoremediation. Environ. Pollut. 2020, 265, 114876. [Google Scholar] [CrossRef]
- Bhattarai, S.; Biswas, D.; Fu, Y.-B.; Biligetu, B. Morphological, Physiological, and Genetic Responses to Salt Stress in Alfalfa: A Review. Agronomy 2020, 10, 577. [Google Scholar] [CrossRef]
- Hoffman, G.J.; Shalhevet, J. Controlling Salinity. In Design and Operation of Farm Irrigation Systems, 2nd ed.; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2007; pp. 160–207. [Google Scholar]
- Tripler, E.; Shani, U.; Ben-Gal, A.; Mualem, Y. Apparent steady state conditions in high resolution weighing-drainage lysimeters containing date palms grown under different salinities. Agric. Water Manag. 2012, 107, 66–73. [Google Scholar] [CrossRef]
- Wani, S.H.; Kumar, V.; Khare, T.; Guddimalli, R.; Parveda, M.; Solymosi, K.; Suprasanna, P.; Kishor, P.K. Engineering salinity tolerance in plants: Progress and prospects. Planta 2020, 251, 76. [Google Scholar] [CrossRef] [PubMed]
- Isayenkov, S.V.; Maathuis, F.J. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef]
- Shabala, S.; Pottosin, I. Regulation of potassium transport in plants under hostile conditions: Implications for abiotic and biotic stress tolerance. Physiol. Plant. 2014, 151, 257–279. [Google Scholar] [CrossRef]
- Chérel, I.; Gaillard, I. The complex fine-tuning of K+ fluxes in plants in relation to osmotic and ionic abiotic stresses. Int. J. Mol. Sci. 2019, 20, 715. [Google Scholar] [CrossRef]
- Xicluna, J.; Lacombe, B.; Dreyer, I.; Alcon, C.; Jeanguenin, L.; Sentenac, H.; Thibaud, J.-B.; Chérel, I. Increased functional diversity of plant K+ channels by preferential heteromerization of the shaker-like subunits AKT2 and KAT2. J. Biol. Chem. 2007, 282, 486–494. [Google Scholar] [CrossRef]
- Lebaudy, A.; Pascaud, F.; Véry, A.-A.; Alcon, C.; Dreyer, I.; Thibaud, J.-B.; Lacombe, B. Preferential KAT1-KAT2 heteromerization determines inward K+ current properties in Arabidopsis guard cells. J. Biol. Chem. 2010, 285, 6265–6274. [Google Scholar] [CrossRef] [PubMed]
- Shah, I.H.; Ashraf, M.; Sabir, I.A.; Manzoor, M.A.; Malik, M.S.; Gulzar, S.; Ashraf, F.; Iqbal, J.; Niu, Q.; Zhang, Y. Green synthesis and Characterization of Copper oxide nanoparticles using Calotropis procera leaf extract and their different biological potentials. J. Mol. Struct. 2022, 1259, 132696. [Google Scholar] [CrossRef]
- Manzoor, N.; Ahmed, T.; Noman, M.; Shahid, M.; Nazir, M.M.; Ali, L.; Alnusaire, T.S.; Li, B.; Schulin, R.; Wang, G. Iron oxide nanoparticles ameliorated the cadmium and salinity stresses in wheat plants, facilitating photosynthetic pigments and restricting cadmium uptake. Sci. Total Environ. 2021, 769, 145221. [Google Scholar] [CrossRef] [PubMed]
- Shah, I.H.; Manzoor, M.A.; Sabir, I.A.; Ashraf, M.; Gulzar, S.; Chang, L.; Zhang, Y. A green and environmental sustainable approach to synthesis the Mn oxide nanomaterial from Punica granatum leaf extracts and its in vitro biological applications. Environ. Monit. Assess. 2022, 194, 921. [Google Scholar] [CrossRef] [PubMed]
- Shah, I.H.; Sabir, I.A.; Ashraf, M.; Rehman, A.; Ahmad, Z.; Azam, M.; Ashraf, G.A.; ur Rasheed, H.; Li, G.; Mouna, J. Phyto-fabrication of copper oxide nanoparticles (NPs) utilizing the green approach exhibits antioxidant, antimicrobial, and antifungal activity in Diospyros kaki fruit. Fruit Res. 2024, 4, e022. [Google Scholar] [CrossRef]
- Shah, I.H.; Manzoor, M.A.; Sabir, I.A.; Ashraf, M.; Liaquat, F.; Gulzar, S.; Chang, L.; Zhang, Y. Phytotoxic effects of chemically synthesized copper oxide nanoparticles induce physiological, biochemical, and ultrastructural changes in Cucumis melo. Environ. Sci. Pollut. Res. 2023, 30, 51595–51606. [Google Scholar] [CrossRef]
- Pandey, R.K.; Prajapati, V.K. Molecular and immunological toxic effects of nanoparticles. Int. J. Biol. Macromol. 2018, 107, 1278–1293. [Google Scholar] [CrossRef]
- Syu, Y.-Y.; Hung, J.-H.; Chen, J.-C.; Chuang, H.-W. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol. Biochem. 2014, 83, 57–64. [Google Scholar] [CrossRef]
- Munns, R.; Schachtman, D.; Condon, A. The significance of a two-phase growth response to salinity in wheat and barley. Funct. Plant Biol. 1995, 22, 561–569. [Google Scholar] [CrossRef]
- Shah, I.H.; Manzoor, M.A.; Jinhui, W.; Li, X.; Hameed, M.K.; Rehaman, A.; Li, P.; Zhang, Y.; Niu, Q.; Chang, L. Comprehensive review: Effects of climate change and greenhouse gases emission relevance to environmental stress on horticultural crops and management. J. Environ. Manag. 2024, 351, 119978. [Google Scholar] [CrossRef]
- Yang, S.; Xiong, X.; Arif, S.; Gao, L.; Zhao, L.; Shah, I.H.; Zhang, Y. A calmodulin-like CmCML13 from Cucumis melo improved transgenic Arabidopsis salt tolerance through reduced shoot’s Na+, and also improved drought resistance. Plant Physiol. Biochem. 2020, 155, 271–283. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Naveed, M.; Sajid, H.; Mustafa, A.; Niamat, B.; Ahmad, Z.; Yaseen, M.; Kamran, M.; Rafique, M.; Ahmar, S.; Chen, J.-T. Alleviation of Salinity-Induced Oxidative Stress, Improvement in Growth, Physiology and Mineral Nutrition of Canola (Brassica napus L.) Through Calcium-Fortified Composted Animal Manure. Sustainability 2020, 12, 846. [Google Scholar] [CrossRef]
- Hayat, K.; Bundschuh, J.; Jan, F.; Menhas, S.; Hayat, S.; Haq, F.; Shah, M.A.; Chaudhary, H.J.; Ullah, A.; Zhang, D. Combating soil salinity with combining saline agriculture and phytomanagement with salt-accumulating plants. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1085–1115. [Google Scholar] [CrossRef]
- Reddy, I.N.B.L.; Kim, B.-K.; Yoon, I.-S.; Kim, K.-H.; Kwon, T.-R. Salt tolerance in rice: Focus on mechanisms and approaches. Rice Sci. 2017, 24, 123–144. [Google Scholar] [CrossRef]
- Chantre Nongpiur, R.; Lata Singla-Pareek, S.; Pareek, A. Genomics approaches for improving salinity stress tolerance in crop plants. Curr. Genom. 2016, 17, 343–357. [Google Scholar] [CrossRef]
- Fita, A.; Rodríguez-Burruezo, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Breeding and domesticating crops adapted to drought and salinity: A new paradigm for increasing food production. Front. Plant Sci. 2015, 6, 978. [Google Scholar] [CrossRef]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Nikam, T.D.; Suprasanna, P. Looking at halophytic adaptation to high salinity through genomics landscape. Curr. Genom. 2017, 18, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Li, Y.; Liu, B.; Kong, F.; Tran, L.S.P. Adaptive mechanisms of soybean grown on salt-affected soils. Land Degrad. Dev. 2018, 29, 1054–1064. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Kumar, P.; Lata, C.; Kumar, S. Effect of individual and interactive alkalinity and salinity on physiological, biochemical and nutritional traits of marvel grass. Indian J. Exp. Biol. 2018, 56, 573–581. [Google Scholar]
- Mansour, M.M.F.; Ali, E.F. Evaluation of proline functions in saline conditions. Phytochemistry 2017, 140, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Derbali, W.; Goussi, R.; Koyro, H.-W.; Abdelly, C.; Manaa, A. Physiological and biochemical markers for screening salt tolerant quinoa genotypes at early seedling stage. J. Plant Interact. 2020, 15, 27–38. [Google Scholar] [CrossRef]
- Paiva, A.L.S.; Passaia, G.; Lobo, A.K.M.; Jardim-Messeder, D.; Silveira, J.A.; Margis-Pinheiro, M. Mitochondrial glutathione peroxidase (OsGPX3) has a crucial role in rice protection against salt stress. Environ. Exp. Bot. 2019, 158, 12–21. [Google Scholar] [CrossRef]
- Li, W.; Tran, L.-S.P. Effects of ethylene on seed germination of halophyte plants under salt stress. In Ethylene Signaling; Springer: Berlin/Heidelberg, Germany, 2017; pp. 253–259. [Google Scholar]
- Mann, A.; Kumar, N.; Lata, C.; Kumar, A.; Kumar, A.; Meena, B. Functional annotation of differentially expressed genes under salt stress in Dichanthium annulatum. Plant Physiol. Rep. 2019, 24, 104–111. [Google Scholar] [CrossRef]
- Li, S.; Khoso, M.A.; Xu, H.; Zhang, C.; Liu, Z.; Wagan, S.; Dinislam, K.; Liu, L. WRKY Transcription Factors (TFs) as Key Regulators of Plant Resilience to Environmental Stresses: Current Perspective. Agronomy 2024, 14, 2421. [Google Scholar] [CrossRef]
- Yihong, J.; Zhen, L.; Chang, L.; Ziying, S.; Ning, Z.; Meiqing, S.; Yuhui, L.; Lei, W. Genome-wide identification and drought stress-induced expression analysis of the NHX gene family in potato. Front. Genet. 2024, 15, 1396375. [Google Scholar] [CrossRef]
- Yun Li, H.; Xia Si, D. Effect of potassium on uptake and translocation of sodium and potassium in Chinese cabbage under NaCl stress. J. Plant Nutr. 2019, 42, 250–260. [Google Scholar] [CrossRef]
- Plett, D.; Safwat, G.; Gilliham, M.; Møller, I.S.; Roy, S.; Shirley, N.; Jacobs, A.; Johnson, A.; Tester, M. Improved salinity tolerance of rice through cell type-specific expression of AtHKT1; 1. PLoS ONE 2010, 5, e12571. [Google Scholar] [CrossRef]
- Mian, A.; Oomen, R.J.; Isayenkov, S.; Sentenac, H.; Maathuis, F.J.; Véry, A.A. Over-expression of an Na+-and K+-permeable HKT transporter in barley improves salt tolerance. Plant J. 2011, 68, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; James, R.A.; Xu, B.; Athman, A.; Conn, S.J.; Jordans, C.; Byrt, C.S.; Hare, R.A.; Tyerman, S.D.; Tester, M. Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nat. Biotechnol. 2012, 30, 360. [Google Scholar] [CrossRef] [PubMed]
- James, R.A.; Blake, C.; Byrt, C.S.; Munns, R. Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1; 4 and HKT1; 5), decrease Na+ accumulation in bread wheat leaves under saline and waterlogged conditions. J. Exp. Bot. 2011, 62, 2939–2947. [Google Scholar] [CrossRef]
- Campbell, M.T.; Bandillo, N.; Al Shiblawi, F.R.A.; Sharma, S.; Liu, K.; Du, Q.; Schmitz, A.J.; Zhang, C.; Véry, A.-A.; Lorenz, A.J. Allelic variants of OsHKT1; 1 underlie the divergence between indica and japonica subspecies of rice (Oryza sativa) for root sodium content. PLoS Genet. 2017, 13, e1006823. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, L.; Zong, J.; Chen, J.; Guo, H.; Guo, A.; Liu, J. Heterologous expression of the halophyte Zoysia matrella H+-pyrophosphatase gene improved salt tolerance in Arabidopsis thaliana. Plant Physiol. Biochem. 2015, 91, 49–55. [Google Scholar] [CrossRef]
- Kashtoh, H.; Baek, K.-H. Structural and functional insights into the role of guard cell ion channels in abiotic stress-induced stomatal closure. Plants 2021, 10, 2774. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Zhang, L.; Cao, Y.; Huang, D.; Tang, K. Molecular cloning and stress-dependent regulation of potassium channel gene in Chinese cabbage (Brassica rapa ssp. Pekinensis). J. Plant Physiol. 2006, 163, 968–978. [Google Scholar] [CrossRef]
- Pilot, G.; Gaymard, F.; Mouline, K.; Chérel, I.; Sentenac, H. Regulated expression of Arabidopsis Shaker K+ channel genes involved in K+ uptake and distribution in the plant. Plant Mol. Biol. 2003, 51, 773–787. [Google Scholar] [CrossRef]
- Obata, T.; Kitamoto, H.K.; Nakamura, A.; Fukuda, A.; Tanaka, Y. Rice shaker potassium channel OsKAT1 confers tolerance to salinity stress on yeast and rice cells. Plant Physiol. 2007, 144, 1978–1985. [Google Scholar] [CrossRef]
- Khan, I.; Hasan, M.; Kausar, R.; Shehzad, J.; Mustafa, G. Plant molecular responses to nanoparticle stress. In Plant and Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2022; pp. 239–264. [Google Scholar]
- Li, P.; Luo, T.; Pu, X.; Zhou, Y.; Yu, J.; Liu, L. Plant transporters: Roles in stress responses and effects on growth and development. Plant Growth Regul. 2021, 93, 253–266. [Google Scholar] [CrossRef]
- Singh, A.; Rajput, V.D.; Sharma, R.; Ghazaryan, K.; Minkina, T. Salinity stress and nanoparticles: Insights into antioxidative enzymatic resistance, signaling, and defense mechanisms. Environ. Res. 2023, 235, 116585. [Google Scholar] [CrossRef]
- Singh, A.; Agrawal, S.; Solanki, S.; Rajput, V.D.; Minkina, T.; Mandzhieva, S.; Elshikh, M.S.; El-Ramady, H.R.; Al Tawaha, A.R.M.; Ghazaryan, K. Nanobiotechnology Approaches for Enhancing Crop Resilience to Salinity Stress. In Plant Stress Tolerance; CRC Press: Boca Raton, FL, USA, 2025; pp. 465–502. [Google Scholar]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.D.; Véry, A.A.; Wang, L.M.; Deng, Y.W.; Sentenac, H.; Huang, D.F. A K+ channel from salt-tolerant melon inhibited by Na+. New Phytol. 2011, 189, 856–868. [Google Scholar] [CrossRef] [PubMed]
- Julkowska, M.M.; Testerink, C. Tuning plant signaling and growth to survive salt. Trends Plant Sci. 2015, 20, 586–594. [Google Scholar] [CrossRef]
- Gao, L.W.; Yang, S.L.; Wei, S.W.; Huang, D.F.; Zhang, Y.D. Supportive role of the Na+ transporter CmHKT1; 1 from Cucumis melo in transgenic Arabidopsis salt tolerance through improved K+/Na+ balance. Plant Mol. Biol. 2020, 103, 561–580. [Google Scholar] [CrossRef]
- Maathuis, F.J.; Amtmann, A. K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- Wang, L.; Wei, S.; Chen, J.; Zhang, Y.; Huang, D. Regulation of the inward rectifying K+ channel MIRK and ion distribution in two melon cultivars (Cucumis melo L.) under NaCl salinity stress. Acta Physiol. Plant. 2013, 35, 2789–2800. [Google Scholar] [CrossRef]
- Véry, A.-A.; Nieves-Cordones, M.; Daly, M.; Khan, I.; Fizames, C.; Sentenac, H. Molecular biology of K+ transport across the plant cell membrane: What do we learn from comparison between plant species? J. Plant Physiol. 2014, 171, 748–769. [Google Scholar] [CrossRef]
- Franco, J.A.; Fernandez, J.A.; Bañón, S.N. Relationship between the Effects of Salinity on Seedling LeafArea and Fruit. HortScience 1997, 32, 4. [Google Scholar] [CrossRef]
- Navarro, J.; Botella, M.; Martinez, V. Yield and fruit quality of melon plants grown under saline conditions in relation to phosphate and calcium nutrition. J. Hortic. Sci. Biotechnol. 1999, 74, 573–578. [Google Scholar] [CrossRef]
- Kaya, C.; Tuna, A.L.; Ashraf, M.; Altunlu, H. Improved salt tolerance of melon (Cucumis melo L.) by the addition of proline and potassium nitrate. Environ. Exp. Bot. 2007, 60, 397–403. [Google Scholar] [CrossRef]
- Li, H.; Ding, X.; Wang, C.; Ke, H.; Wu, Z.; WANG, Y.; Liu, H.; Guo, J. Control of tomato yellow leaf curl virus disease by Enterobacter asburiaeBQ9 as a result of priming plant resistance in tomatoes. Turk. J. Biol. 2016, 40, 150–159. [Google Scholar] [CrossRef]
- Ali, M.; Kamran, M.; Abbasi, G.H.; Saleem, M.H.; Ahmad, S.; Parveen, A.; Malik, Z.; Afzal, S.; Ahmar, S.; Dawar, K.M. Melatonin-induced salinity tolerance by ameliorating osmotic and oxidative stress in the seedlings of two tomato (Solanum lycopersicum L.) cultivars. J. Plant Growth Regul. 2021, 40, 2236–2248. [Google Scholar] [CrossRef]
- Shah, I.H.; Ashraf, M.; Khan, A.R.; Manzoor, M.A.; Hayat, K.; Arif, S.; Sabir, I.A.; Abdullah, M.; Niu, Q.; Zhang, Y. Controllable synthesis and stabilization of Tamarix aphylla-mediated copper oxide nanoparticles for the management of Fusarium wilt on musk melon. 3 Biotech. 2022, 12, 128. [Google Scholar] [CrossRef]
- Singh, A.; Tiwari, S.; Pandey, J.; Lata, C.; Singh, I.K. Role of nanoparticles in crop improvement and abiotic stress management. J. Biotechnol. 2021, 337, 57–70. [Google Scholar] [CrossRef]
- Srihasam, S.; Thyagarajan, K.; Korivi, M.; Lebaka, V.R.; Mallem, S.P.R. Phytogenic generation of NiO nanoparticles using Stevia leaf extract and evaluation of their in-vitro antioxidant and antimicrobial properties. Biomolecules 2020, 10, 89. [Google Scholar] [CrossRef]
- Stewart, J.; Hansen, T.; McLean, J.E.; McManus, P.; Das, S.; Britt, D.W.; Anderson, A.J.; Dimkpa, C.O. Salts affect the interaction of ZnO or CuO nanoparticles with wheat. Environ. Toxicol. Chem. 2015, 34, 2116–2125. [Google Scholar] [CrossRef]
- Khan, A.R.; Salam, A.; Li, G.; Iqbal, B.; Ulhassan, Z.; Liu, Q.; Azhar, W.; Liaquat, F.; Shah, I.H.; ul Hassan, S.S. Nanoparticles and their crosstalk with stress mitigators: A novel approach towards abiotic stress tolerance in agricultural systems. Crop J. 2024, 12, 1280–1298. [Google Scholar] [CrossRef]
- Zia-ur-Rehman, M.; Anayatullah, S.; Irfan, E.; Hussain, S.M.; Rizwan, M.; Sohail, M.I.; Jafir, M.; Ahmad, T.; Usman, M.; Alharby, H.F. Nanoparticles assisted regulation of oxidative stress and antioxidant enzyme system in plants under salt stress: A review. Chemosphere 2023, 314, 137649. [Google Scholar] [CrossRef]
- Etesami, H.; Fatemi, H.; Rizwan, M. Interactions of nanoparticles and salinity stress at physiological, biochemical and molecular levels in plants: A review. Ecotoxicol. Environ. Saf. 2021, 225, 112769. [Google Scholar] [CrossRef] [PubMed]
- Shah, I.H.; Sabir, I.A.; Rehman, A.; Hameed, M.K.; Albashar, G.; Manzoor, M.A.; Shakoor, A. Co-application of copper oxide nanoparticles and Trichoderma harzianum with physiological, enzymatic and ultrastructural responses for the mitigation of salt stress. Chemosphere 2023, 336, 139230. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Anbazhagan, V.; Dhankher, O.P.; Prasad, P.V. Uptake, Translocation, Toxicity, and Impact of Nanoparticles on Plant Physiological Processes. Plants 2024, 13, 3137. [Google Scholar] [CrossRef] [PubMed]
- Nazeer, A.; Ahmad, F.; Verma, N.; Ahmad, S. Nanomaterials and Plant Biomolecules: Basics of Interactions. In Targeted Delivery of Nanopesticides and Nanofertilizers in Sustainable Agricultural Farming; Springer: Berlin/Heidelberg, Germany, 2023; pp. 9–49. [Google Scholar]
- Banerjee, K.; Pramanik, P.; Maity, A.; Joshi, D.; Wani, S.H.; Krishnan, P. Methods of using nanomaterials to plant systems and their delivery to plants (mode of entry, uptake, translocation, accumulation, biotransformation and barriers). In Advances in Phytonanotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 123–152. [Google Scholar]
- Nazari, M.; Bickel, S.; Benard, P.; Mason-Jones, K.; Carminati, A.; Dippold, M.A. Biogels in soils: Plant mucilage as a biofilm matrix that shapes the rhizosphere microbial habitat. Front. Plant Sci. 2022, 12, 798992. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yue, L.; Dhankher, O.P.; Xing, B. Nano-enabled improvements of growth and nutritional quality in food plants driven by rhizosphere processes. Environ. Int. 2020, 142, 105831. [Google Scholar] [CrossRef]
- Benelli, G. Mode of action of nanoparticles against insects. Environ. Sci. Pollut. Res. 2018, 25, 12329–12341. [Google Scholar] [CrossRef]
- Wei, L.; Liu, J.; Jiang, G. Nanoparticle-specific transformations dictate nanoparticle effects associated with plants and implications for nanotechnology use in agriculture. Nat. Commun. 2024, 15, 7389. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, H.; Zhang, C. Zinc oxide nanoparticles enhance plasmid transfer among growth-promoting endophytes in Arabidopsis thaliana. Sci. Total Environ. 2024, 913, 169682. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Khader, A.; Mahmoud, E. Green iron oxide nanoparticles and magnetic nanobiochar: Enhancing tomato performance, phytochemicals, and root-knot nematode resistance. BMC Plant Biol. 2024, 24, 469. [Google Scholar] [CrossRef]
- Moradbeygi, H.; Jamei, R.; Heidari, R.; Darvishzadeh, R. Investigating the enzymatic and non-enzymatic antioxidant defense by applying iron oxide nanoparticles in Dracocephalum moldavica L. plant under salinity stress. Sci. Hortic. 2020, 272, 109537. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Gaur, S.; Singh, S.; Yadav, V.; Liu, S.; Singh, V.P.; Sharma, S.; Srivastava, P.; Prasad, S.M. Acquisition and homeostasis of iron in higher plants and their probable role in abiotic stress tolerance. Front. Environ. Sci. 2018, 5, 86. [Google Scholar] [CrossRef]
- Abdoli, S.; Ghassemi-Golezani, K.; Alizadeh-Salteh, S. Responses of ajowan (Trachyspermum ammi L.) to exogenous salicylic acid and iron oxide nanoparticles under salt stress. Environ. Sci. Pollut. Res. 2020, 27, 36939–36953. [Google Scholar] [CrossRef] [PubMed]
- Moloudzadeh, R.; Fathi, S.; Yari, F.; Najafian, S.; Seyedi, A. The potential of coated iron nanoparticles for modulating of negative effects of salinity stress in Ajowan. Hortic. Environ. Biotechnol. 2024, 65, 747–760. [Google Scholar] [CrossRef]
- Karimi, S.; Tavallali, V.; Ferguson, L.; Mirzaei, S. Developing a nano-Fe complex to supply iron and improve salinity tolerance of pistachio under calcium bicarbonate stress. Commun. Soil Sci. Plant Anal. 2020, 51, 1835–1851. [Google Scholar] [CrossRef]
- Shahzad, R.; Harlina, P.W.; Khan, S.U.; Koerniati, S.; Hastilestari, B.R.; Ningrum, R.A.; Wahab, R.; Djalovic, I.; Prasad, P.V. Iron oxide nanoparticles alleviate salt-alkaline stress and improve growth by modulating antioxidant defense system in cherry tomato. J. Plant Interact. 2024, 19, 2375508. [Google Scholar] [CrossRef]
- Ebrahimzadeh, A.; Ghorbanzadeh, S.; Vojodi Mehrabani, L.; Sabella, E.; De Bellis, L.; Hassanpouraghdam, M.B. KNO3, nano-Zn, and Fe foliar application influence the growth and physiological responses of Aloe vera under salinity. Agronomy 2022, 12, 2360. [Google Scholar] [CrossRef]
- Singh, N.; Singh, M.K.; Raghuvansi, J.; Yadav, R.K.; Azim, Z. Green synthesis of nano iron oxide using Emblica officinalis L. fruit extract and its impact on growth, chlorophyll content, and metabolic activity of Solanum lycopersicum L. J. Appl. Biol. Biotechnol. 2024, 12, 173–181. [Google Scholar] [CrossRef]
- Dedejani, S.; Mozafari, A.A.; Ghaderi, N. Salicylic acid and iron nanoparticles application to mitigate the adverse effects of salinity stress under in vitro culture of strawberry plants. Iran. J. Sci. Technol. Trans. A Sci. 2021, 45, 821–831. [Google Scholar] [CrossRef]
- Al–Mayahi, A.M.W. Combined efficiency of iron nanoparticles (IONPs) and salicylic acid (SA) on in vitro propagation of date palm (Phoenix dactylifera L.) under combined drought and salinity. S. Afr. J. Bot. 2023, 162, 324–333. [Google Scholar] [CrossRef]
- Tawfik, M.; Mohamed, M.H.; Sadak, M.S.; Thalooth, A.T. Iron oxide nanoparticles effect on growth, physiological traits and nutritional contents of Moringa oleifera grown in saline environment. Bull. Natl. Res. Cent. 2021, 45, 177. [Google Scholar] [CrossRef]
- Mozafari, A.-A.; Ghadakchi asl, A.; Ghaderi, N. Grape response to salinity stress and role of iron nanoparticle and potassium silicate to mitigate salt induced damage under in vitro conditions. Physiol. Mol. Biol. Plants 2018, 24, 25–35. [Google Scholar] [CrossRef]
- Ghadakchi asl, A.; Mozafari, A.A.; Ghaderi, N. Iron nanoparticles and potassium silicate interaction effect on salt-stressed grape cuttings under in vitro conditions: A morphophysiological and biochemical evaluation. In Vitro Cell. Dev. Biol.-Plant 2019, 55, 510–518. [Google Scholar] [CrossRef]
- Mukhtiar, A.; Alawadi, H.F.; Naqve, M.; Seleiman, M.F.; Mahmood, A.; Majeed, M.I.; Hafeez, M.B.; Naeem, K. Role of iron oxide nanoparticles in maize (Zea mays L.) to enhance salinity stress tolerance. Not. Bot. Horti Agrobot. Cluj-Napoca 2024, 52, 13695. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Shmarev, A.N.; Ivanov, A.A.; Zharmukhamedov, S.K.; Strokina, V.; Kosobryukhov, A.; Yu, M.; Allakhverdiev, S.I.; Shabala, S. Effects of iron oxide nanoparticles (Fe3O4) and salinity on growth, photosynthesis, antioxidant activity and distribution of mineral elements in wheat (Triticum aestivum). Funct. Plant Biol. 2023, 50, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Gören, H.K.; Canavar, Ö.; Tan, U. Mitigating Salinity Stress in Cotton (Gossypium hirsutum L.) with K-humate and Iron Oxide Nanoparticles. Turk. J. Agric. Nat. Sci. 2024, 11, 1275–1283. [Google Scholar] [CrossRef]
- Torabian, S.; Farhangi-Abriz, S.; Zahedi, M. Efficacy of FeSO 4 nano formulations on osmolytes and antioxidative enzymes of sunflower under salt stress. Indian J. Plant Physiol. 2018, 23, 305–315. [Google Scholar] [CrossRef]
- Al-huraby, A.I. Environmental Physiological Studies on the Phaseolus vulgaris L. Plant Under Salinity and the Treatment of Iron Oxide Nanoparticles. Ph.D. Dissertation, King Abdulaziz University Jeddah, Jeddah, Saudi Arabia, 2022. [Google Scholar]
- Waqas, M.; Ali, N.; Ashraf, M.Y.; Usman, S.; Shah, A.A.; Raja, V.; El-Sheikh, M.A. Impact of iron sulfate (FeSO4) foliar application on growth, metabolites and antioxidative defense of Luffa cylindrica (Sponge gourd) under salt stress. Sci. Rep. 2024, 14, 26001. [Google Scholar] [CrossRef]
- Sofy, M.R.; Elhindi, K.M.; Farouk, S.; Alotaibi, M.A. Zinc and paclobutrazol mediated regulation of growth, upregulating antioxidant aptitude and plant productivity of pea plants under salinity. Plants 2020, 9, 1197. [Google Scholar] [CrossRef]
- Sehrish, A.K.; Ahmad, S.; Nafees, M.; Mahmood, Z.; Ali, S.; Du, W.; Naeem, M.K.; Guo, H. Alleviated cadmium toxicity in wheat (Triticum aestivum L.) by the coactive role of zinc oxide nanoparticles and plant growth promoting rhizobacteria on TaEIL1 gene expression, biochemical and physiological changes. Chemosphere 2024, 364, 143113. [Google Scholar] [CrossRef]
- Gupta, A.; Bharati, R.; Kubes, J.; Popelkova, D.; Praus, L.; Yang, X.; Severova, L.; Skalicky, M.; Brestic, M. Zinc oxide nanoparticles application alleviates salinity stress by modulating plant growth, biochemical attributes and nutrient homeostasis in Phaseolus vulgaris L. Front. Plant Sci. 2024, 15, 1432258. [Google Scholar] [CrossRef]
- Anwar, T.; Qureshi, H.; Fatimah, H.; Siddiqi, E.H.; Anwaar, S.; Moussa, I.M.; Adil, M.F. Elucidating effect of ZnO-Nanoparticles and melatonin on physiological adjustments and growth of Solanum melongena under salinity stress. Sci. Hortic. 2023, 322, 112455. [Google Scholar] [CrossRef]
- Rasouli, F.; Asadi, M.; Hassanpouraghdam, M.B.; Aazami, M.A.; Ebrahimzadeh, A.; Kakaei, K.; Dokoupil, L.; Mlcek, J. Foliar application of ZnO-NPs influences chlorophyll fluorescence and antioxidants pool in Capsicum annum L. under salinity. Horticulturae 2022, 8, 908. [Google Scholar] [CrossRef]
- Taqdees, Z.; Khan, J.; Kausar, S.; Afzaal, M.; Akhtar, I. Silicon and zinc nanoparticles-enriched miscanthus biochar enhanced seed germination, antioxidant defense system, and nutrient status of radish under NaCl stress. Crop Pasture Sci. 2022, 73, 556–572. [Google Scholar] [CrossRef]
- Mogazy, A.M.; Hanafy, R.S. Foliar spray of biosynthesized zinc oxide nanoparticles alleviate salinity stress effect on Vicia faba plants. J. Soil Sci. Plant Nutr. 2022, 22, 2647–2662. [Google Scholar] [CrossRef]
- Mahawar, L.; Živčák, M.; Barboricova, M.; Kovár, M.; Filaček, A.; Ferencova, J.; Vysoká, D.M.; Brestič, M. Effect of copper oxide and zinc oxide nanoparticles on photosynthesis and physiology of Raphanus sativus L. under salinity stress. Plant Physiol. Biochem. 2024, 206, 108281. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, H.; Mazher, J.; Azmat, A.; Nosheen, A.; Naz, R.; Hassan, M.N.; Noureldeen, A.; Ahmad, P. Combined application of zinc oxide nanoparticles and biofertilizer to induce salt resistance in safflower by regulating ion homeostasis and antioxidant defence responses. Ecotoxicol. Environ. Saf. 2021, 218, 112262. [Google Scholar] [CrossRef]
- Javeed, A.; Ahmed, S.; Sardar, R. Alleviation of salinity stress in zinc oxide nanoparticle-treated Lagenaria siceraria L. by modulation of physiochemical attributes, enzymatic and non-enzymatic antioxidative system. Funct. Plant Biol. 2023, 50, 941–954. [Google Scholar] [CrossRef]
- Gaafar, R.; Diab, R.; Halawa, M.; Elshanshory, A.; El-Shaer, A.; Hamouda, M. Role of zinc oxide nanoparticles in ameliorating salt tolerance in soybean. Egypt. J. Bot. 2020, 60, 733–747. [Google Scholar] [CrossRef]
- Zeid, I.M.A.; Mohamed, F.H.; Metwali, E.M. Responses of two strawberry cultivars to NaCl-induced salt stress under the influence of ZnO nanoparticles. Saudi J. Biol. Sci. 2023, 30, 103623. [Google Scholar]
- Hanif, S.; Sajjad, A.; Javed, R.; Mannan, A.; Zia, M. Proline doped ZnO nanocomposite alleviates NaCl induced adverse effects on morpho-biochemical response in Coriandrum sativum. Plant Stress 2023, 9, 100173. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; Wang, C.; Hashem, A.M.; Tabl, K.M.; Nishawy, E.; Kuai, J.; Zhou, G.; Wang, B. Selenium and zinc oxide nanoparticles modulate the molecular and morpho-physiological processes during seed germination of Brassica napus under salt stress. Ecotoxicol. Environ. Saf. 2021, 225, 112695. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour, A.; Haliloglu, K.; Tolga Cinisli, K.; Ozkan, G.; Ozturk, H.I.; Pour-Aboughadareh, A.; Poczai, P. Application of zinc oxide nanoparticles and plant growth promoting bacteria reduces genetic impairment under salt stress in tomato (Solanum lycopersicum L.‘Linda’). Agriculture 2020, 10, 521. [Google Scholar] [CrossRef]
- Fatemeh, R.; Hadi, A.; Latifeh, P. Effects of foliar application of ZnO nanoparticles on secondary metabolite and micro-elements of camelina (Camelina sativa L.) under salinity stress. J. Stress Physiol. Biochem. 2020, 16, 54–69. [Google Scholar]
- Al-Saif, A.M.; Sas-Paszt, L.; Mosa, W.F. Olive Performance Under the Soil Application of Humic Acid and the Spraying of Titanium and Zinc Nanoparticles Under Soil Salinity Stress. Horticulturae 2024, 10, 295. [Google Scholar] [CrossRef]
- Mustafa, G.; Chaudhari, S.K.; Manzoor, M.; Batool, S.; Hatami, M.; Hasan, M. Zinc oxide nanoparticles mediated salinity stress mitigation in Pisum sativum: A physio-biochemical perspective. BMC Plant Biol. 2024, 24, 835. [Google Scholar] [CrossRef]
- Singh, A.; Sengar, R.S.; Rajput, V.D.; Minkina, T.; Singh, R.K. Zinc oxide nanoparticles improve salt tolerance in rice seedlings by improving physiological and biochemical indices. Agriculture 2022, 12, 1014. [Google Scholar] [CrossRef]
- Adil, M.; Bashir, S.; Bashir, S.; Aslam, Z.; Ahmad, N.; Younas, T.; Asghar, R.M.A.; Alkahtani, J.; Dwiningsih, Y.; Elshikh, M.S. Zinc oxide nanoparticles improved chlorophyll contents, physical parameters, and wheat yield under salt stress. Front. Plant Sci. 2022, 13, 932861. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Ahmad, A.; Alhammad, B.A.; Tola, E. Exogenous application of zinc oxide nanoparticles improved antioxidants, photosynthetic, and yield traits in salt-stressed maize. Agronomy 2023, 13, 2645. [Google Scholar] [CrossRef]
- Qian, J.; Shan, R.; Shi, Y.; Li, H.; Xue, L.; Song, Y.; Zhao, T.; Zhu, S.; Chen, J.; Jiang, M. Zinc Oxide Nanoparticles Alleviate Salt Stress in Cotton (Gossypium hirsutum L.) by Adjusting Na+/K+ Ratio and Antioxidative Ability. Life 2024, 14, 595. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Abdeldaym, E.A.; Abdelaziz, S.M.; El-Sawy, M.B.; Mottaleb, S.A. Synergetic effects of zinc, boron, silicon, and zeolite nanoparticles on confer tolerance in potato plants subjected to salinity. Agronomy 2019, 10, 19. [Google Scholar] [CrossRef]
- Hussein, M.; Abou-Baker, N. The contribution of nano-zinc to alleviate salinity stress on cotton plants. R. Soc. Open Sci. 2018, 5, 171809. [Google Scholar] [CrossRef] [PubMed]
- Torabian, S.; Zahedi, M.; Khoshgoftarmanesh, A. Effect of Foliar Spray of Zinc Oxide on some Antiox Idant Enzymes Activity of Sunflower Under Salt Stress; Jomo Kenyatta University of Agriculture and Technology: Juja, Kenya, 2018. [Google Scholar]
- Hassan, M.U.; Kareem, H.A.; Hussain, S.; Guo, Z.; Niu, J.; Roy, M.; Saleem, S.; Wang, Q. Enhanced salinity tolerance in Alfalfa through foliar nano-zinc oxide application: Mechanistic insights and potential agricultural applications. Rhizosphere 2023, 28, 100792. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; Alzahrani, H.S. The potential mitigation effect of ZnO nanoparticles on [Abelmoschus esculentus L. Moench] metabolism under salt stress conditions. Saudi J. Biol. Sci. 2020, 27, 3132–3137. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.S.; El-Feky, S.A.; Darwish, E. Alleviation of salt stress on Moringa peregrina using foliar application of nanofertilizers. J. Hortic. For. 2015, 7, 36–47. [Google Scholar]
- Singh, A.; Rajput, V.D.; Agrawal, S.; Ghazaryan, K.; Minkina, T.; Al Tawaha, A.R.M.; Chauhan, A.; Mandzhieva, S.S.; Singh, R.K.; Papadakis, M. Nanoparticles mediated salt stress resilience: A holistic exploration of physiological, biochemical, and Nano-omics approaches. Rev. Environ. Contam. Toxicol. 2024, 262, 19. [Google Scholar] [CrossRef]
- Khan, M.; Khan, M.S.A.; Borah, K.K.; Goswami, Y.; Hakeem, K.R.; Chakrabartty, I. The potential exposure and hazards of metal-based nanoparticles on plants and environment, with special emphasis on ZnO NPs, TiO2 NPs, and AgNPs: A review. Environ. Adv. 2021, 6, 100128. [Google Scholar] [CrossRef]
- Gowtham, H.; Shilpa, N.; Singh, S.B.; Aiyaz, M.; Abhilash, M.; Nataraj, K.; Amruthesh, K.; Ansari, M.A.; Alomary, M.N.; Murali, M. Toxicological effects of nanoparticles in plants: Mechanisms involved at morphological, physiological, biochemical and molecular levels. Plant Physiol. Biochem. 2024, 210, 108604. [Google Scholar] [CrossRef]
- Aslam, M.; Aslam, A.; Sheraz, M.; Ali, B.; Ulhassan, Z.; Najeeb, U.; Zhou, W.; Gill, R.A. Lead toxicity in cereals: Mechanistic insight into toxicity, mode of action, and management. Front. Plant Sci. 2021, 11, 587785. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, J.; Xu, L.; Li, P.; Xing, W.; ur Rahman, S.; Ahmad, N.; Naeem, M.; Lu, J.; Rehman, A. Interactions of Fe and Zn Nanoparticles at Physiochemical, Biochemical, and Molecular Level in Horticultural Crops Under Salt Stress: A Review. Horticulturae 2025, 11, 442. https://doi.org/10.3390/horticulturae11040442
Weng J, Xu L, Li P, Xing W, ur Rahman S, Ahmad N, Naeem M, Lu J, Rehman A. Interactions of Fe and Zn Nanoparticles at Physiochemical, Biochemical, and Molecular Level in Horticultural Crops Under Salt Stress: A Review. Horticulturae. 2025; 11(4):442. https://doi.org/10.3390/horticulturae11040442
Chicago/Turabian StyleWeng, Jinyang, Lu Xu, Pengli Li, Wei Xing, Saeed ur Rahman, Naveed Ahmad, Muhammad Naeem, Jun Lu, and Asad Rehman. 2025. "Interactions of Fe and Zn Nanoparticles at Physiochemical, Biochemical, and Molecular Level in Horticultural Crops Under Salt Stress: A Review" Horticulturae 11, no. 4: 442. https://doi.org/10.3390/horticulturae11040442
APA StyleWeng, J., Xu, L., Li, P., Xing, W., ur Rahman, S., Ahmad, N., Naeem, M., Lu, J., & Rehman, A. (2025). Interactions of Fe and Zn Nanoparticles at Physiochemical, Biochemical, and Molecular Level in Horticultural Crops Under Salt Stress: A Review. Horticulturae, 11(4), 442. https://doi.org/10.3390/horticulturae11040442










