Abstract
This study investigated the germination characteristics and genetic variability of Cerastium candidissimum, a Greek endemic species with potential for ornamental horticulture. The seeds were collected from three populations of Mount Hymettus, M. Parnitha, and M. Parnassos. The cardinal temperatures for germination, the effect of seed storage duration, and population-specific germination responses were examined. Germination trials were conducted in vitro on half-strength Murashige and Skoog medium, with seeds tested after dark and dry room storage periods of 6, 18, and 30 months. Seeds from Mount Parnitha exhibited high germination rates (81–94%) within a temperature range of 10–20 °C after 6 and 18 months of storage. Similarly, seeds from Mount Parnassos demonstrated optimal germination (81.3–94.0%) at 10–20 °C after 6 months of storage, though an 18-month storage period shifted the optimal range to 15–20 °C (67–71%). In contrast, the Mount Hymettus population exhibited the lowest germination percentages, with 6-month-old seeds reaching only 47.3% germination at 20 °C, declining to 34% at 15 °C after 18 months, and near-zero germination after 30 months. The time required for 50% germination (T50) ranged from 4 to 8 days at 20 °C across all populations but increased as incubation temperature decreased (4–18 days at 15 °C; 8–18 days at 10 °C). The molecular analysis revealed that the primers used presented high polymorphism (49.0%), and a total of 136 amplified markers were produced. Individuals from different populations were grouped in three different branches. These findings indicate population-level variability in germination traits, likely reflecting genetic and ecological differences. The high germination rates of Parnitha and Parnassos’ populations support their potential use in floriculture. Conversely, the low germination success of the Hymettus population suggests higher environmental stress or genetic constraints, warranting further investigation into its possible classification as a distinct ecotype.
1. Introduction
The introduction of new ornamental species, particularly under increasing anthropogenic pressure and global climate change, could benefit both the floriculture industry and ex situ biodiversity conservation strategies. Species originating from the wild hold significant potential for ornamental use. Additionally, integrating wild species into urban and suburban environments can contribute to sustainability by providing essential biodiversity services. Mediterranean ecosystems have been a significant biodiversity tank since ancient times, providing suitable species for exploitation [1,2].
The genus Cerastium L. (Caryophyllaceae) includes more than 65 species native to Europe, with the Balkan Peninsula serving as the primary center of diversity (34 species) and endemism (17 species) [3,4,5,6]. Greece alone hosts 25 Cerastium species, 16 of which are endemic [7,8,9,10]. Several perennial Cerastium species are valued for their ornamental qualities, including their compact, cushion-like growth habits, silver-green foliage, and abundant white flowers. In contrast, many annual Cerastium species are regarded as weeds [11]. Among the aforementioned ornamental perennial species, Cerastium tomentosum and C. biebersteinii, both belonging to Cerastium subg. Cerastium, are widely cultivated as groundcovers in commercial floriculture [12,13]. However, despite their high esthetic appeal, these species can be short-lived and susceptible to rapid decline. Moreover, their ability to escape cultivation and their propensity to establish self-sustaining populations has led to their classification as invasive species in certain regions [14,15].
Cerastium candidissimum Correns is a perennial herbaceous species endemic to central and southern Greece. It occurs in temperate and sub-Mediterranean grasslands and high-mountain vegetation, typically inhabiting dry, rocky limestone areas, often within Abies cephalonica woodland clearings and alpine pastures at elevations of 1000–2300 m [16]. The species forms dense, tufted clumps and grows to a height of 15–30 cm, with numerous ascending stems bearing sessile, lanceolate leaves. Its cymoid inflorescences, which develop at the stem tips between May and August, consist of 3–7 strikingly white flowers. The entire plant is covered with a dense indumentum of short white hairs, giving it a characteristic silvery-white appearance [16,17]. Due to its unique esthetic appeal, this showy Greek endemic species could be introduced into commercial floriculture as a novel pot plant or even as a cut flower (Figure 1).

Figure 1.
Cerastium candidissimum individuals in full bloom (June 2020, Mount Parnassos, 1700 m a.s.l.; 38°33′06.2″ N, 22°34′46.8″ E).
Closely resembling the Italian endemic Cerastium tomentosum, C. candidissimum is of considerable horticultural interest due to its refined appearance, lower invasiveness, and adaptability to warm, dry summers. These attributes make it particularly suitable for use as a groundcover, in mixed perennial plantings, in rock gardens, and on green roofs, especially in nutrient-poor and exposed environments [18]. Notably, it has been recently reported that C. candidissimum is among the top 10 Greek endemic plant taxa sold in the international plant trade based on pricing criteria [19]. Furthermore, as a native species, its cultivation requires relatively low production costs for growers [20].
C. candidissimum faces significant threats due to anthropogenic pressures, leading to the decline of many natural populations. Kougioumoutzis et al. [21] have recommended its inclusion in the list of endangered Greek endemic species. Many C. candidissimum populations are small and isolated, with their reproductive success further constrained by intensive grazing and insect predation [22]. Previous studies have investigated the in vitro propagation potential of different C. candidissimum populations, including the development of an encapsulation technique for node explants [23]. Additionally, seeds have proven to be an excellent resource for in vitro experimentation in various horticultural species [24,25,26,27,28,29,30,31,32]. Although germination data exist for 60 Cerastium taxa in seed databases such as the Society for Ecological Restoration and the Royal Botanic Gardens’ Seed Information Database, C. candidissimum is notably absent from these records [33]. To date, no published studies have specifically examined the germination ecology of C. candidissimum.
Seed germination, particularly in conjunction with seed storage, plays a crucial role in propagation studies [34,35,36]. The development of species-specific propagation protocols and long-term conservation strategies—such as ex situ storage in seed banks—require precise knowledge of the target species’ germination ecophysiology [37,38,39,40]. In Mediterranean montane and alpine species such as C. candidissimum, temperature is one of the most critical ecological factors influencing germination. The timing of seedling emergence must align with the narrow bioclimatic window that ensures successful establishment and survival [41].
Seed-based propagation is advantageous, as it requires minimal plant material, making it an environmentally sustainable approach. Additionally, seed propagation facilitates the selection of novel genotypes with desirable ornamental or commercial traits [42]. Various PCR-based methods have been employed to assess genetic variation among plant populations, including those propagated through in vitro culture. Among these, random amplified polymorphic DNA (RAPD) analysis is a cost-effective and rapid technique for evaluating genetic relationships at the subspecies or variety level [43,44]. RAPD markers have been widely used to assess population differentiation in woody species [45,46,47,48,49]. For example, Oliya et al. [50] used RAPD analysis to evaluate the genetic stability of in vitro-propagated Rhynchostylis retusa. Molecular studies involving various genetic markers have also been conducted in Cerastium, with RAPD analysis successfully applied to Cerastium arcticum [51].
The present study aimed to (1) investigate the germination capacity of C. candidissimum by determining its optimal and cardinal germination temperatures across seed accessions from three populations; (2) assess the effect of seed storage duration in dark, dry room conditions on germination performance; and (3) evaluate the potential of RAPD markers for identifying genetically distinct populations with valuable traits. The ultimate goal of this research is to promote the introduction of C. candidissimum into the floriculture industry while enhancing our understanding of its germination ecology and genetic diversity.
2. Materials and Methods
2.1. Seed Collection
Seeds of C. candidissimum were collected at different times, depending on the species’ flowering period, when seeds had reached full maturity. Seeds were harvested from three populations located on the slopes of Mount Hymettus, M. Parnitha, and M. Parnassos. Specifically, seeds from Hymettus were collected at 900 m a.s.l. (37°57′23.0″ N, 23°49′03.6″ E) on 30 June 2020; seeds from Parnitha were collected at 1200 m a.s.l. (38°09′55.3″ N, 23°42′59.4″ E) on 15 July 2020; and seeds from Parnassos were collected at 1700 m a.s.l. (38°33′06.2″ N, 22°34′46.8″ E) on 25 July 2020. Collection was performed from 30 to 40 randomly selected individuals per population according to the ENSCONET protocol and associated criteria [52]. After their collection, the seeds were transported to the laboratory, where they were manually separated from the pericarp. They were then placed in Petri dishes and stored under controlled conditions in a growth chamber at 25 °C and 30% relative humidity in complete darkness. Germination experiments were conducted after storage periods of 6, 18, and 30 months.
2.2. In Vitro Germination
Prior to germination experiments, seeds were surface-disinfected by an initial 10 s wash with 90.0% v/v ethanol solution, followed by immersion for 10 min in a 20.0% (v/v) solution of commercial bleach (4.6% w/v sodium hypochlorite) and finally rinsed three times (3.0 min per rinse) with sterile double-distilled water. Then, they were cultured in vitro in 9 cm Petri dishes containing solid Murashige and Skoog (MS) medium [53] at half strength (MS/2), hormone-free, and solidified with 8 g L−1 agar. The Petri dishes were incubated under controlled conditions at six different temperatures (10 °C, 15 °C, 20 °C, 25 °C, 30 °C, and 35 °C) under 16 h cool white fluorescent light (37.5 μmol m−2 s−1/8 h dark photoperiod), following the methodology of Bertsouklis and Panagaki [54]. Germination was assessed at 6, 18, and 30 months after seed storage. A seed was considered germinated when a radicle of at least 2 mm emerged, as per the criteria established by the International Seed Testing Association [55]. Germination was recorded every two days over a total period of 36 days. The time required for 50% of the final germination (T50) was calculated according to Soltani et al. [56], providing a comparative measure of germination speed. A total of 150 seeds per treatment were used (6 Petri dishes per treatment with 25 seeds per dish). The germination speed index (GSI) was determined using the Maguire formula [57]:
where G1, G2, … Gn represent the number of normal seedlings counted at each observation, and N1, N2, … Nn represent the number of days from sowing to each observation. The statistical significance of results was assessed using an analysis of variance (ANOVA), with mean comparisons performed using Tukey’s HSD test at p < 0.05. Germination speed indices (GSIs) were expressed as mean ± standard error.
GSI = G1/N1 + G2/N2 + … + Gn/Nn
Canonical discriminant analysis (CDA) was used to highlight the variation among different populations and minimize the three populations’ variation [58]. Four variables were used, namely germination (%), the GSI, T50, and days for full germination. In order to classify the three populations by CDA, Wilks’ Lambda method was applied using JMP 11.0 software. The Wilks’ Lambda estimates the performance of the discriminant analyses calculating the following ratio: within-group variation/total variation. Hence, the significance of the discriminant function can be evaluated providing the chance-corrected percentage of agreement between real and predicted groups [59].
2.3. DNA Extraction and RAPD Analysis
Plant material for DNA extraction was collected from in vitro-grown seedlings that were 50 days old. A total of 30 individuals (10 from each population) were sampled. The collected material was flash-frozen in liquid nitrogen and stored at −80 °C until further processing. Genomic DNA was extracted from 0.1 g of leaf tissue using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany), following the manufacturer’s protocol. Amplification reactions were performed according to the methodology described by Stavrakakis and Biniari [60]. Twenty 10-mer oligonucleotide arbitrary primers were used for the amplification of RAPD sequences. Twelve primers that could generate strongly amplified, polymorphic, and reproducible bands were selected for formal amplification (Table 1). The reactions were repeated two times with independently isolated genomic DNA as templates.

Table 1.
Results of the RAPD analysis using the 12 selected primers, including their nucleotide sequences, number of amplified fragments, and number and percentage of polymorphic bands.
2.4. Data Collection and Statistical Analysis
Germination percentage was recorded every two days for a total of 36 days. Data collection was conducted with 6 replicates per treatment (25 seeds per replicate), using a total of 2700 seeds per population. A factorial experiment was designed for each population, incorporating two main factors, namely temperature (10, 15, 20, 25, 30, and 35 °C) and storage period (6, 18, and 30 months). The experiments followed a completely randomized design (CRD). A two-way ANOVA was used to assess the significance of temperature and storage effects at each storage period. Mean differences were evaluated using Tukey’s HSD test at p ≤ 0.05. Germination percentage data were arcsine-transformed prior to statistical analysis to ensure the homogeneity of variance. Results were expressed as the mean ± standard error. For RAPD analysis, a binary matrix was generated for each plant based on the presence (1) or absence (0) of RAPD bands. The resulting data were analyzed using NTSYS-pc version 2.11f [61]. Cluster analysis was performed using the unweighted pair group method with arithmetic mean (UPGMA), generating a dendrogram based on the genetic distance matrix. Genetic similarity (GS) was calculated using the simple matching (SM) coefficient [62]. Additionally, principal coordinate analysis (PCA) was conducted to visualize genetic relationships among populations.
3. Results
3.1. In Vitro Germination
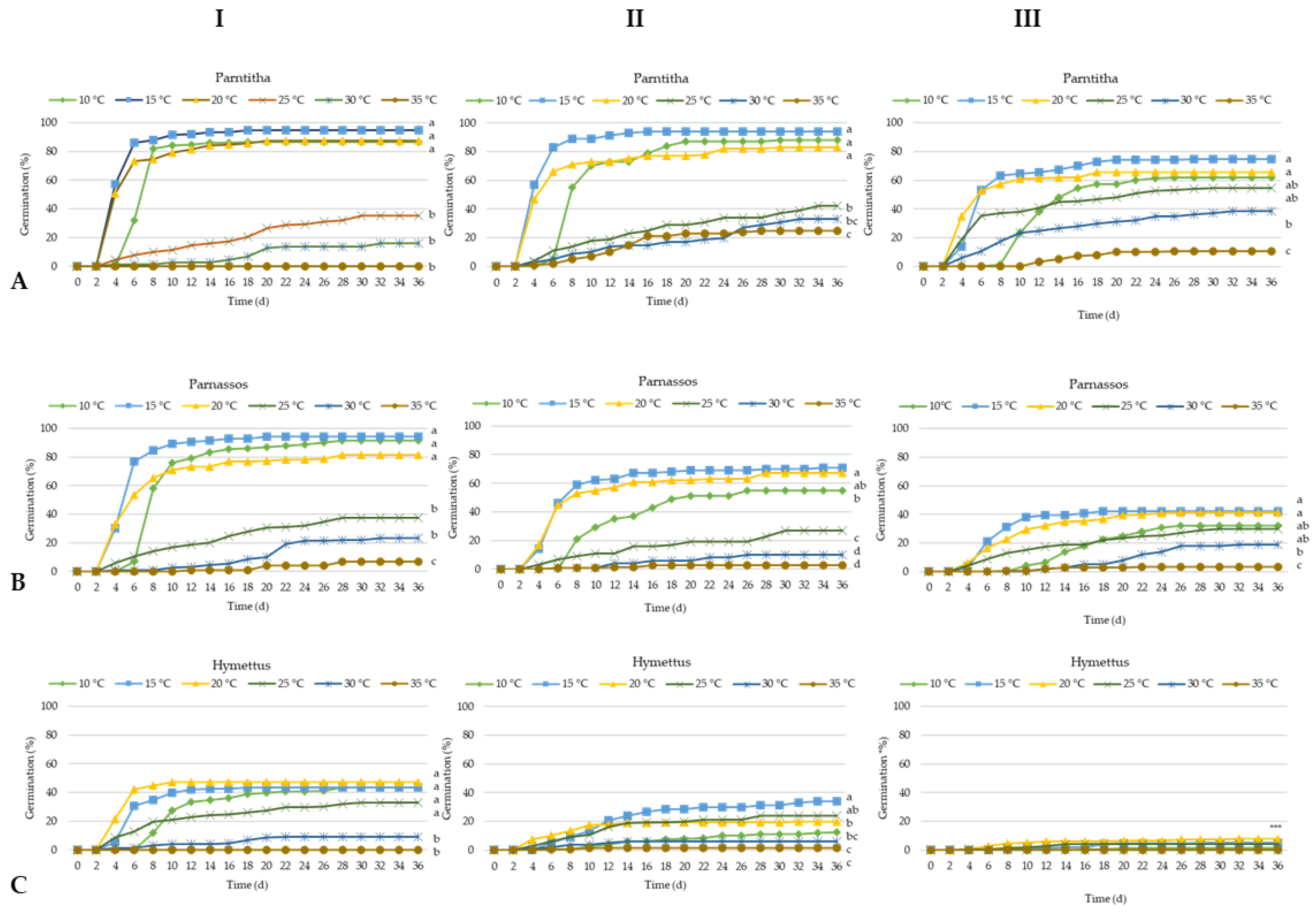
A two-way analysis of variance (ANOVA) revealed a significant interaction between temperature and storage period on seed germination percentage (G%), T50, the germination speed index (GSI), and overall germination success across the studied populations. Seeds from the Parnitha population exhibited high germination rates (81% to 94%) at temperatures ranging from 10 to 20 °C after 6 and 18 months of storage. After 30 months, germination declined to 74.6% at 15 °C and 65.3% at 20 °C, with some germination observed at 35 °C after 18 and 30 months (25% and 11%, respectively; Figure 2A and Figure 3).
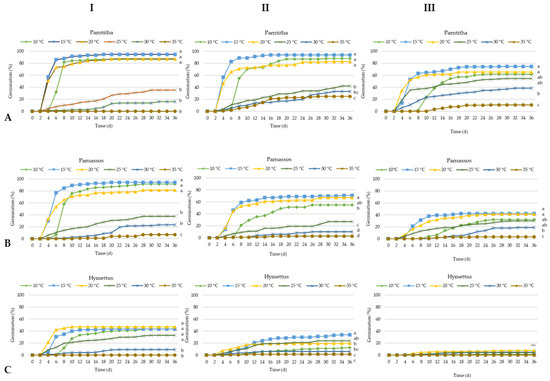
Figure 2.
Germination time course curves of Cerastium candidissimum seeds, as affected by storage period (I: 6 months storage; II: 18 months storage; III: 30 months storage), provenance ((A) Parnitha, (B) Parnassos, (C) Hymettus), and incubation temperature (10, 15, 20, 25, 30, or 35 °C) on half-strength Murashige and Skoog media. n = 6, 25 seeds/Petri dish (total 150 seeds per treatment. Two-way ANOVA results for each population: Ftemperature × storage period ***; Fone way: ***, ***: significant at p ≤ 0.001. Mean separation in seeds by Tukey’s HSD at p ≤ 0.05; means followed by the same letter are not significant.

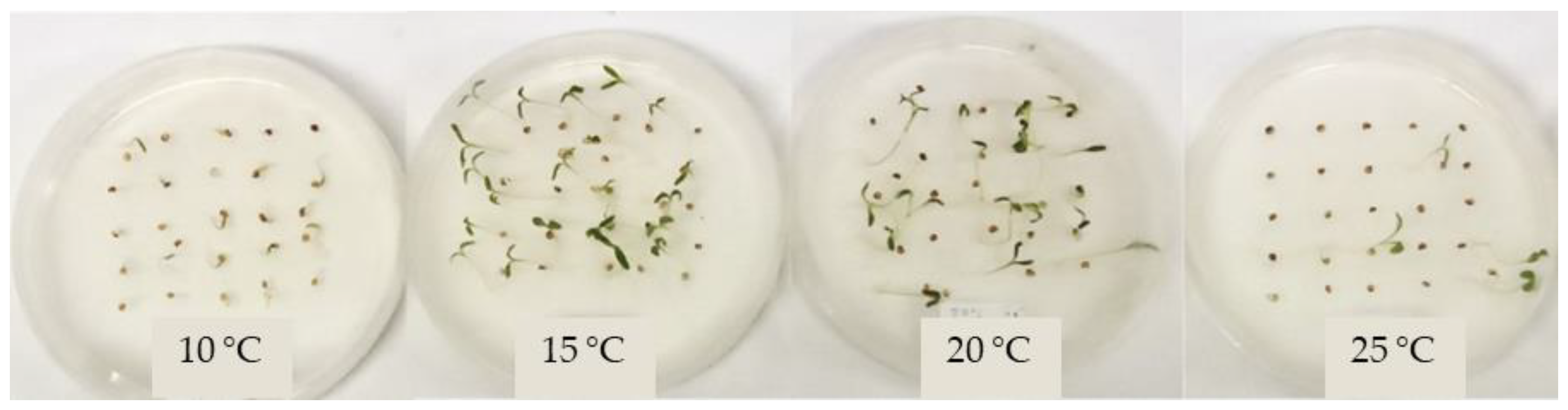
Figure 3.
Germination of C. candidissimum seeds from the Parnitha population after 6 months of storage at 10, 15, 20, and 25 °C (from left to right) during the 20th day of their in vitro incubation.
Seeds from the Parnassos population displayed a similar germination pattern. After 6 months of storage, germination remained high (81.3% to 94%) within the 10–20 °C range. Following 18 months, the highest germination occurred at 20 °C (71%), with no significant difference from 15 °C (67%). Germination decreased at lower temperatures and fell below 27% at temperatures exceeding 25 °C. After 30 months, germination did not surpass 42% at 15 and 20 °C (Figure 2B).
The Hymettus population exhibited the lowest germination rates. Six-month-old seeds achieved a maximum germination of 47.3% at 20 °C, which further declined to 34% at 15 °C after 18 months. Following 30 months of storage, Hymettus seeds showed germination rates below 10% and were practically non-viable (Figure 2C).
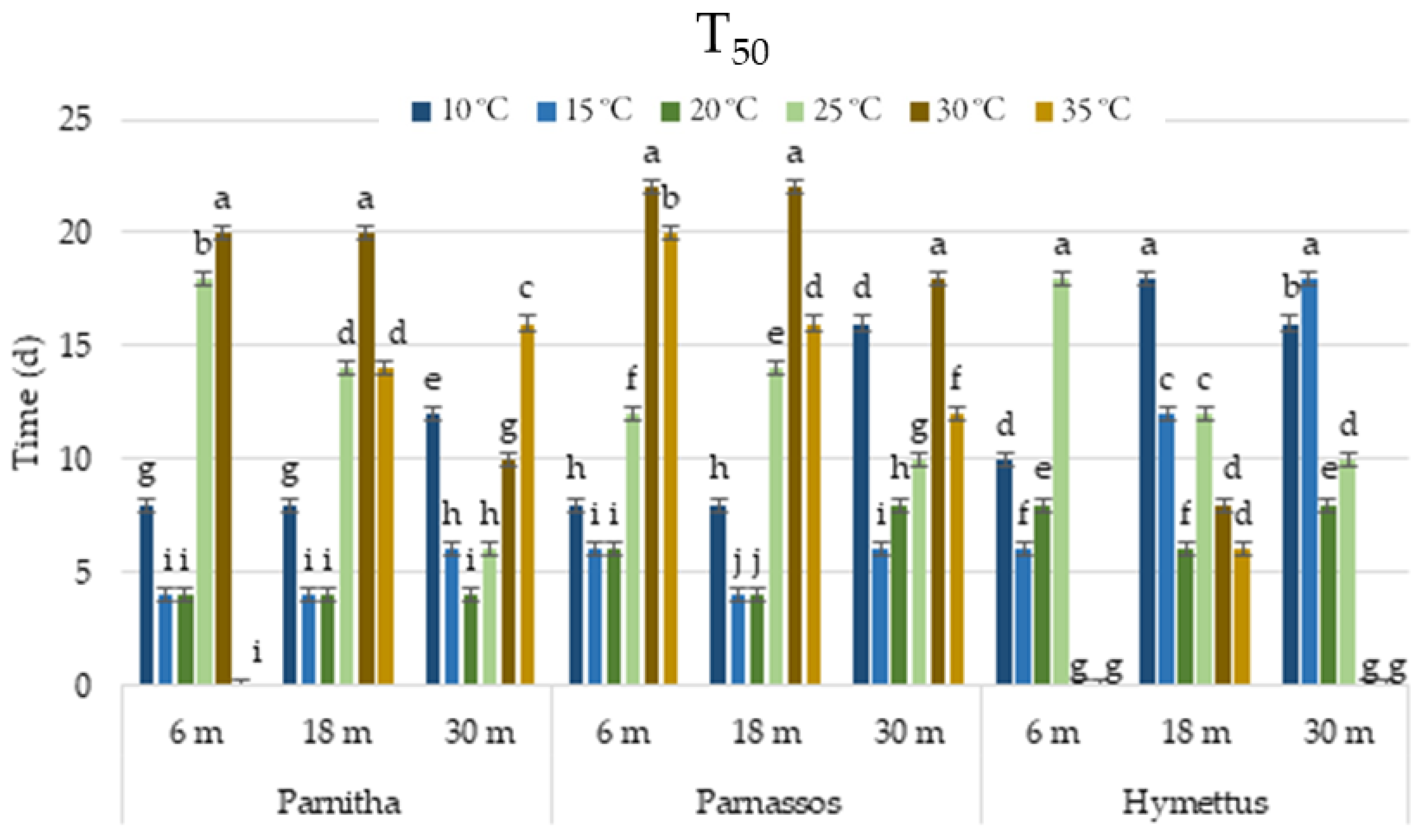
The germination speed, reflected by T50 values, ranged from 4 to 8 days at 20 °C across all populations and storage treatments. However, T50 increased as incubation temperatures decreased, extending to 4–18 days at 15 °C and 8–18 days at 10 °C. Germination speed was lowest at 20–35 °C. Seeds from Parnitha germinated fastest at 15 and 20 °C after 6 or 18 months, and at 20 °C after 30 months (T50 = 4 days). Similarly, Parnassos seeds germinated most rapidly at 15 and 20 °C after 18 months, whereas Hymettus seeds germinated faster at 15 °C and 20 °C after 6 and 18 months, respectively (Figure 4).
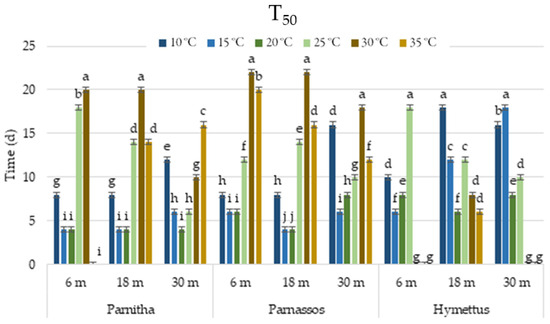
Figure 4.
Time (days taken for a cumulative germination of 50% (T50) of Cerastium candidissimum seeds, as affected by provenance (Parnitha, Hymettus, Parnassos), incubation temperature (10, 15, 20, 25, 30, or 35 °C), and storage time (6, 18, or 30 months). Two-way ANOVA results for Parnitha: Fstorage × temperature ***, Fone-way ANOVA ***; Parnassos: Fstorage × temperature ***, Fone-way ANOVA ***; Hymettus: Fstorage × temperature ***, Fone-way ANOVA ***; *** significant at p ≤ 0.001; n = 6, 25 seeds/Petri dish (total 150 seeds per treatment). Mean separation in seeds by Tukey’s HSD at p ≤ 0.05; means followed by the same letter are not significantly different. Standard errors of the means are shown for each temperature.
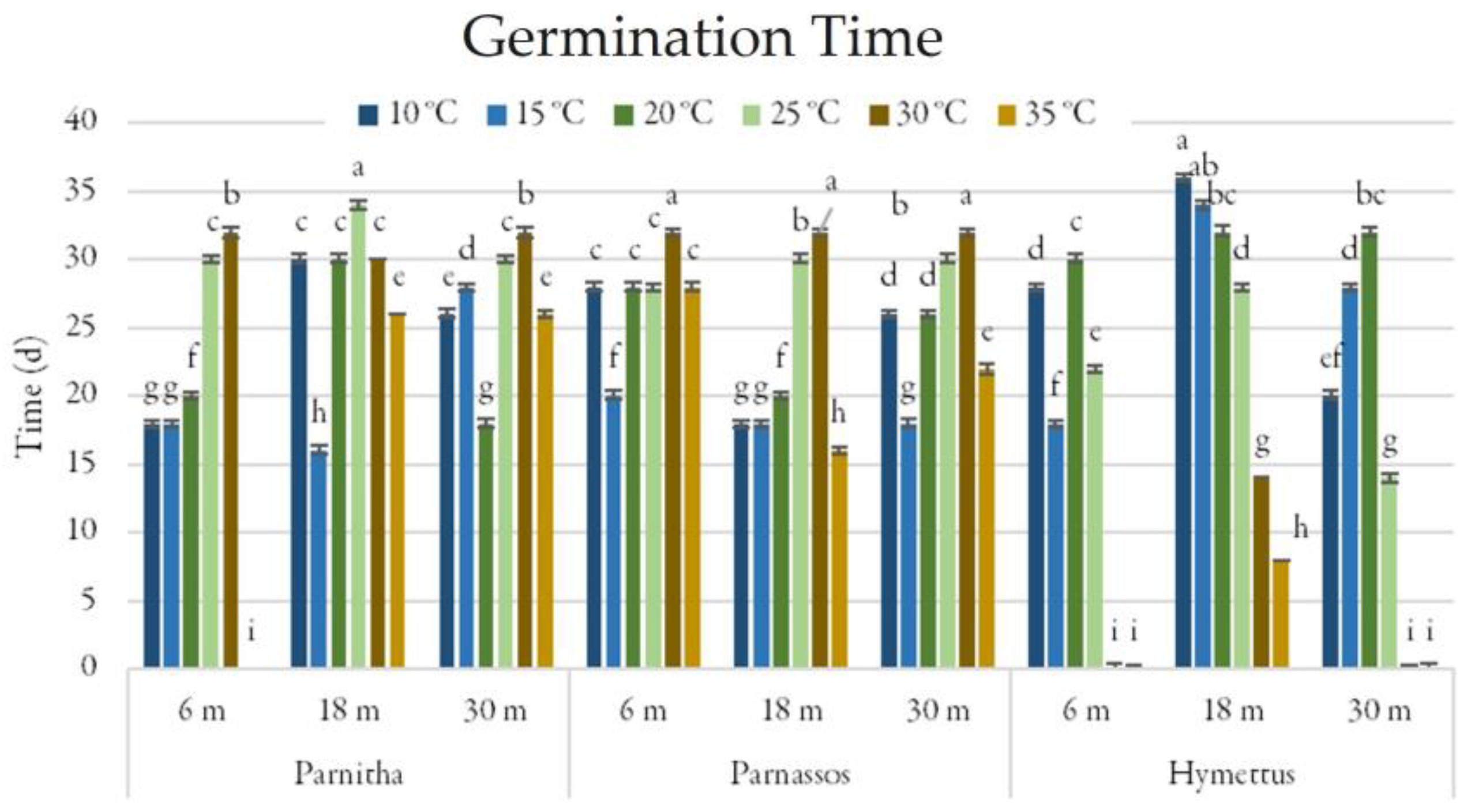
The germination period (GP) varied between 16 and 32 days. Parnitha seeds exhibited the shortest germination period when 18-month-old seeds were incubated at 15 °C (16 days). Parnassos and Hymettus populations also showed reduced germination periods after 18 months (GP = 16 and 8 days, respectively; Figure 5).
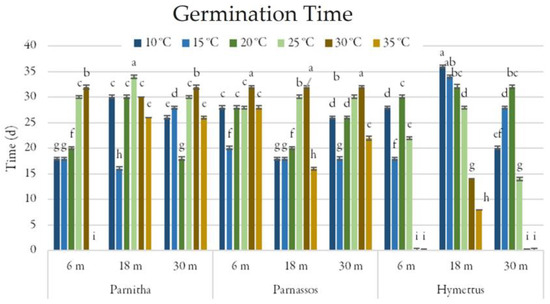
Figure 5.
Time (days) taken for full germination of Cerastium candidissimum seeds, as affected by provenance (Parnitha, Hymettus, Parnassos), incubation temperature (10, 15, 20, 25, 30, or 35 °C), and storage time (6, 18, or 30 months). Two-way ANOVA results for Parnitha: Fstorage × temperature ***, Fone-way ANOVA ***; Parnassos: Fstorage × temperature ***, Fone-way ANOVA ***; Hymettus: Fstorage × temperature ***, Fone-way ANOVA ***; *** significant at p ≤ 0.001; n = 6, 25 seeds/Petri dish (total 150 seeds per treatment). Mean separation in seeds by Tukey’s HSD at p ≤ 0.05; means followed by the same letter are not significantly different. Standard errors of the means are shown for each temperature.
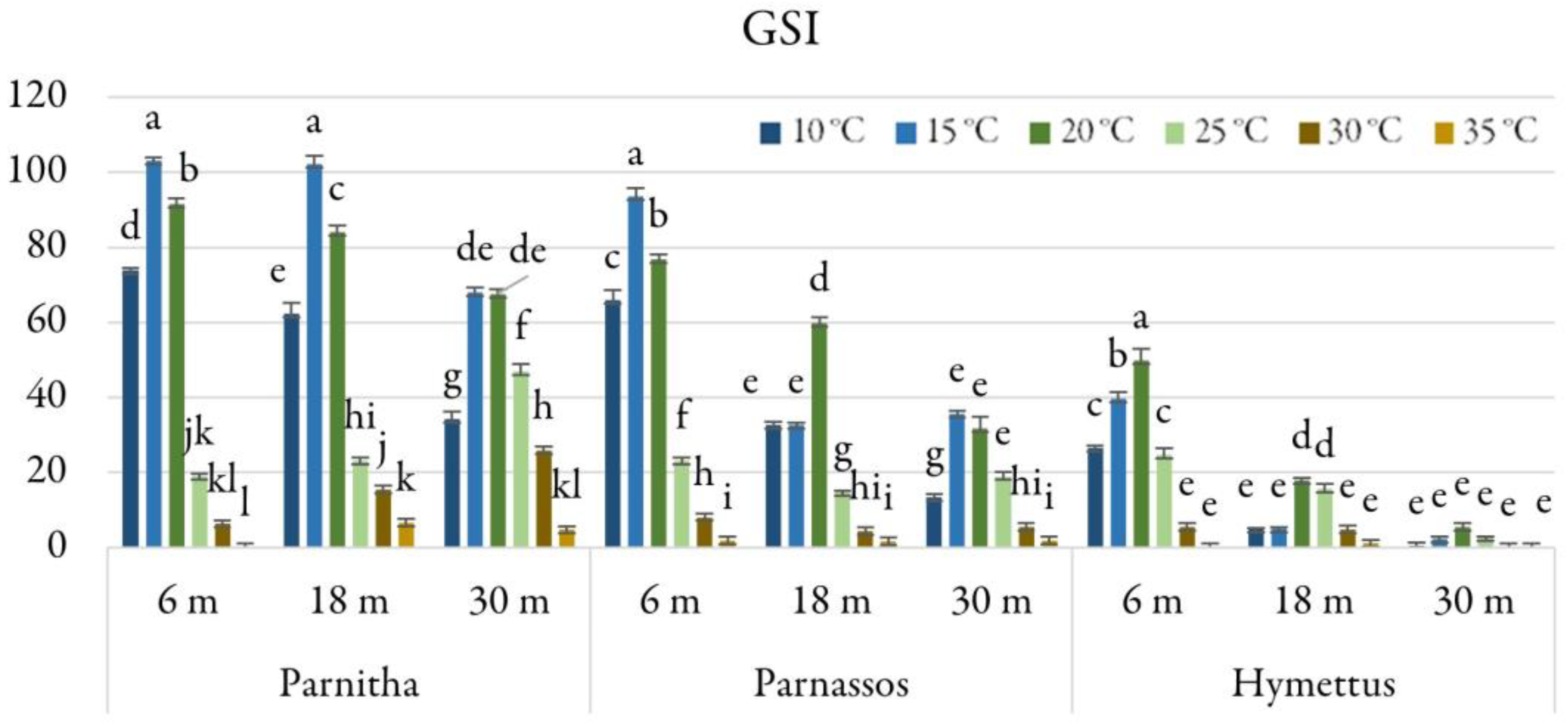
Analysis of the GSI revealed that Parnitha seeds stored for 6 and 18 months had the highest germination speed at 15 °C (GSI = 103.05 and 102.13, respectively). Parnassos seeds displayed the highest GSI (91.57) after 6 months at 15 °C. The highest GSI for Hymettus seeds was recorded at 20 °C (GSI = 50), approximately half of the values observed in Parnitha and Parnassos (Figure 6).
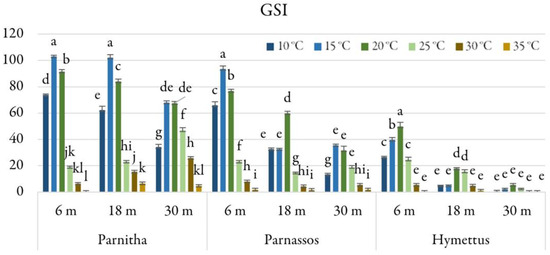
Figure 6.
Germination speed index (GSI) of Cerastium candidissimum seeds, as affected by provenance (Parnitha, Hymettus, Parnassos), incubation temperature (10, 15, 20, 25, 30, or 35 °C), and storage time (6, 18, or 30 months). Two-way ANOVA results for Parnitha: Fstorage × temperature ***, Fone-way ANOVA ***; Parnassos: Fstorage × temperature ***, Fone-way ANOVA ***; Hymettus: Fstorage × temperature ***, Fone-way ANOVA ***; *** significant at p ≤ 0.001; n = 6, 25 seeds/Petri dish (total 150 seeds per treatment). Mean separation in seeds by Tukey’s HSD at p ≤ 0.05; means followed by the same letter are not significantly different. Standard errors of the means are shown for each temperature.
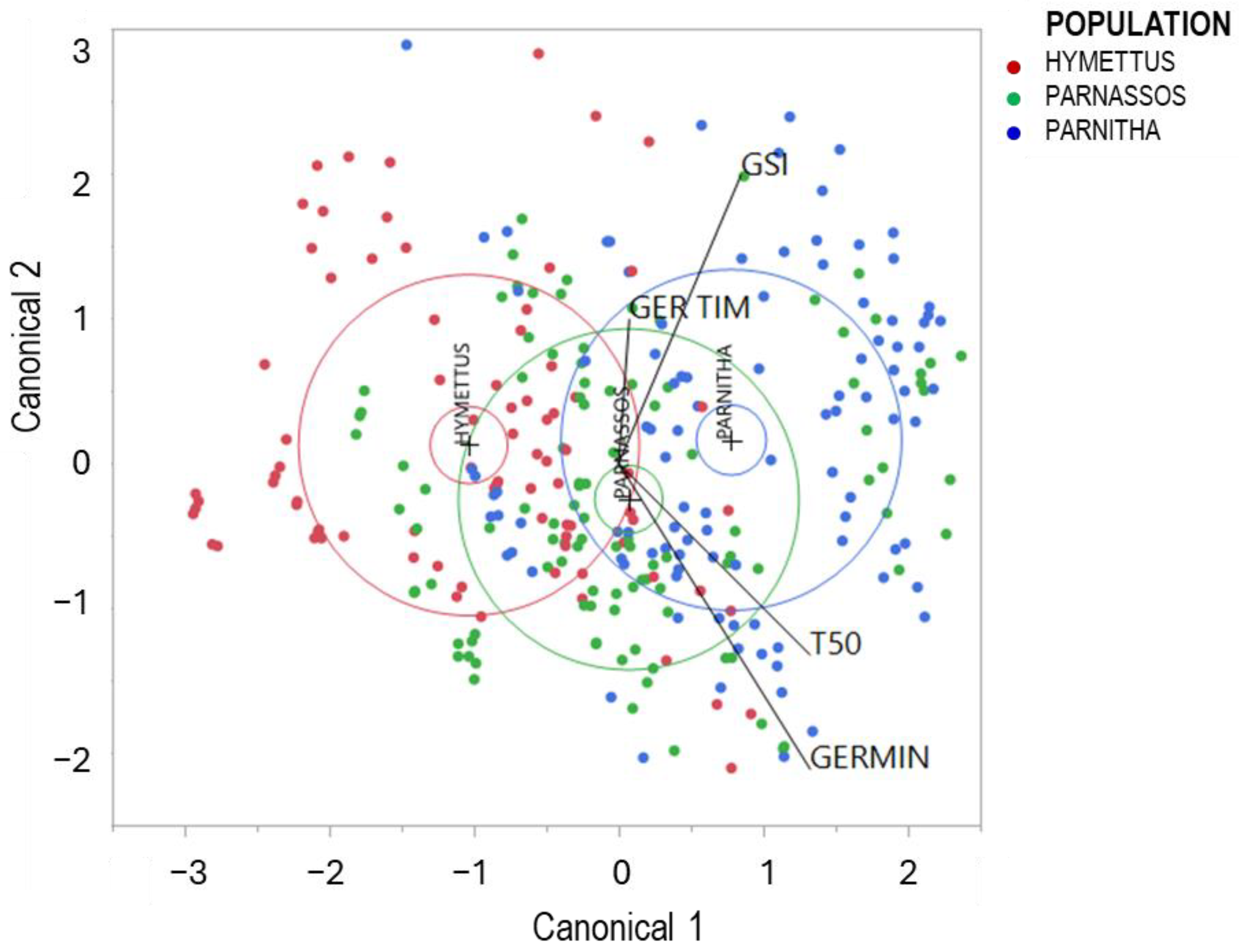
Canonical discriminant analysis (CDA) effectively classified the three populations into distinct groups. Three well-defined clusters were observed along the first canonical axis, explaining 93.46% of the total variation (Figure 7).
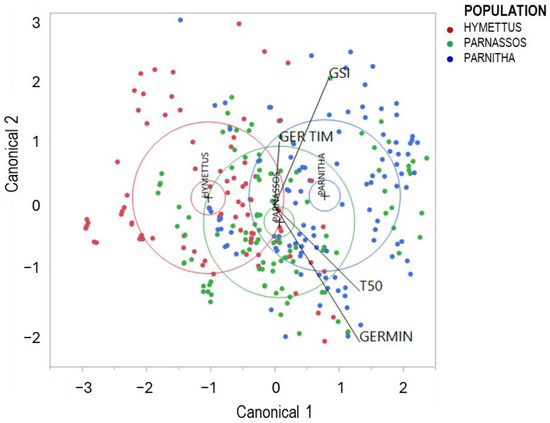
Figure 7.
Discriminant analysis of germination data separating the three populations of Cerastium candidissimum. GERMIN: germination (%); T50: time taken for a cumulative germination of 50.0%; GER TIM: full germination period; GSI: germination speed index (F18.4877 = 576, p < 0.0001).
3.2. DNA Extraction and RAPD Analysis
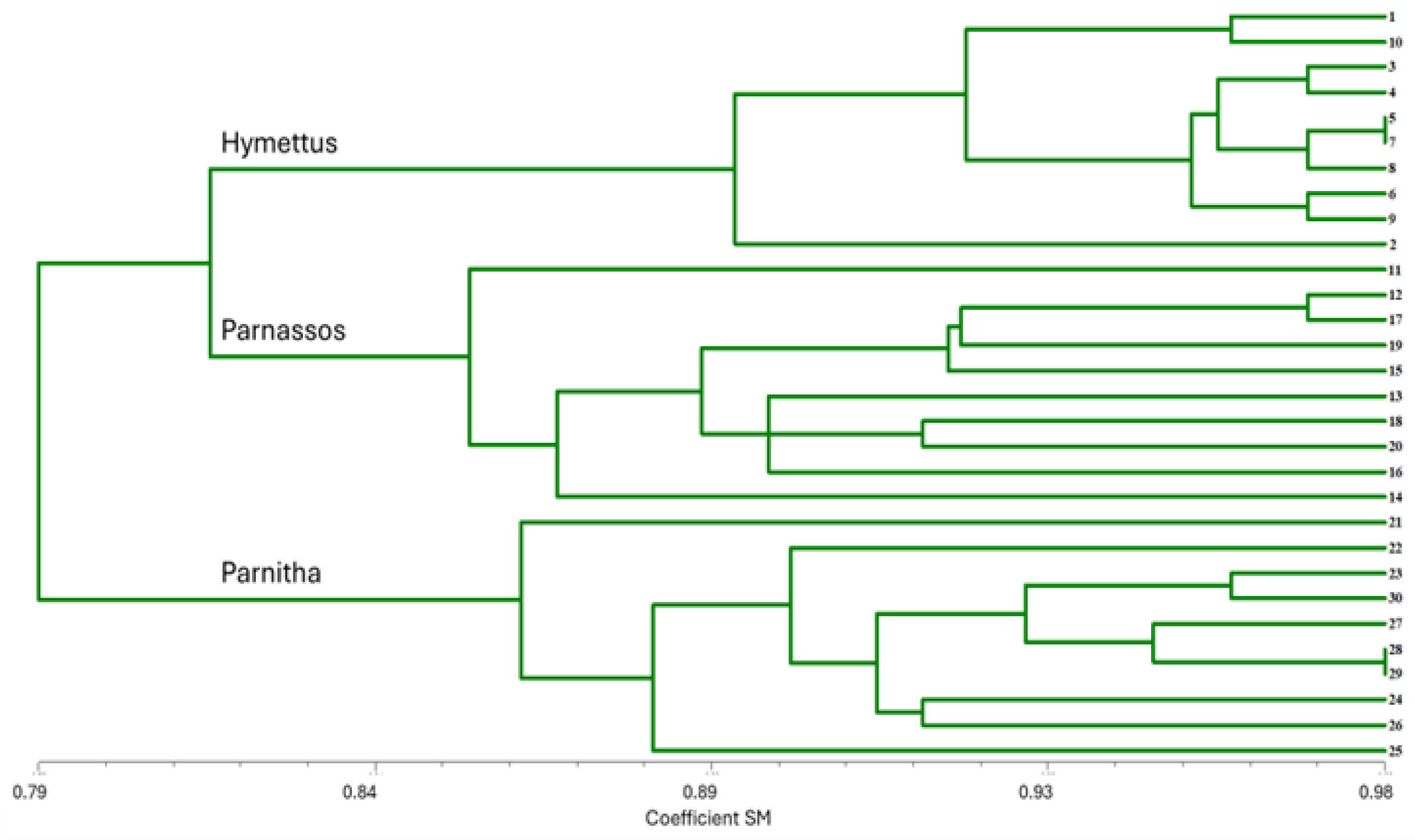
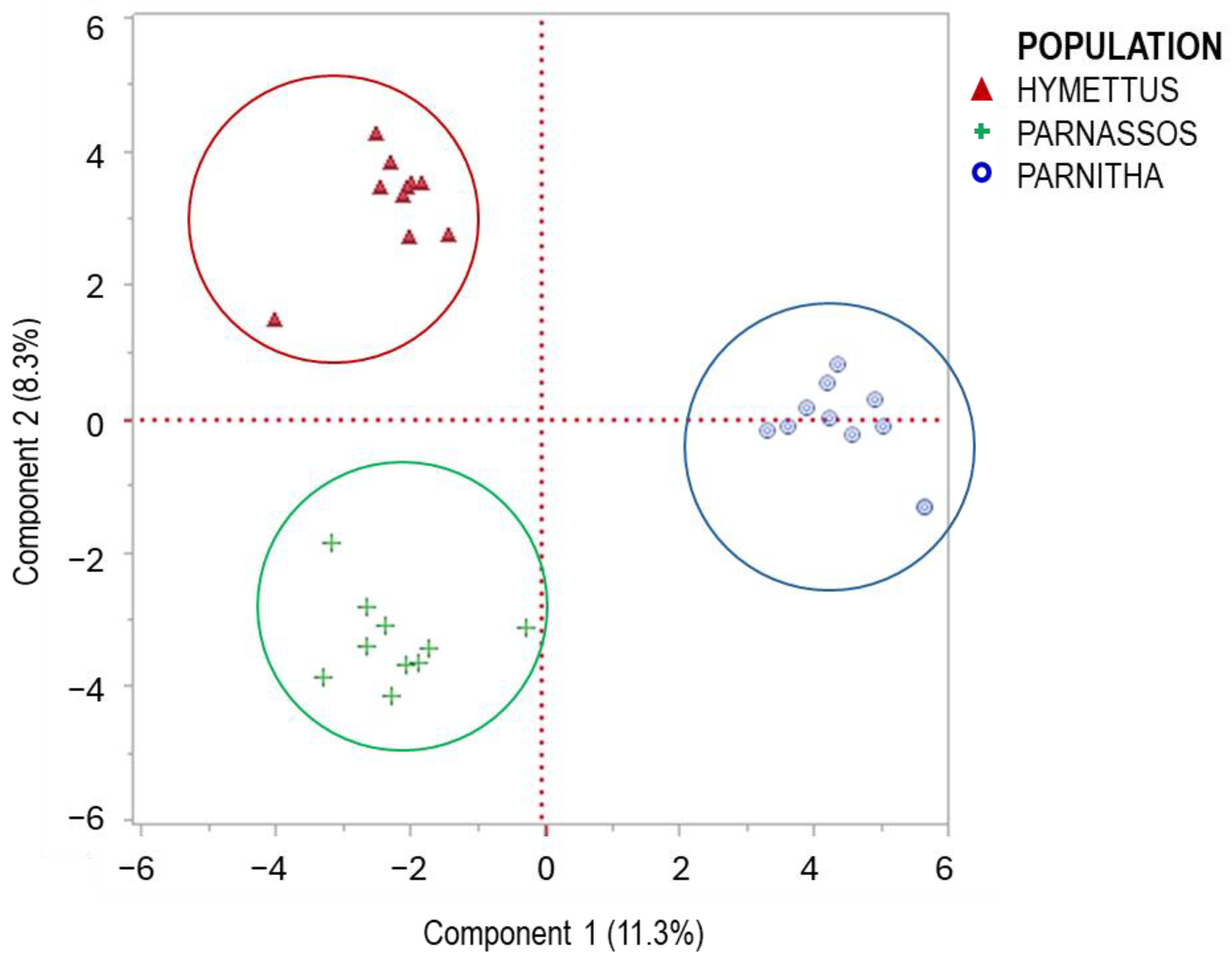
The RAPD analysis identified significant genetic variation among the three populations. The primers used exhibited polymorphism at 49.0%, generating 136 amplified markers (Table 1). The highest levels of polymorphism were observed in primers 1225 and OPM-11 (66.6% and 62.5%, respectively), while OPF-02 and OPF-05 also displayed substantial polymorphism (60%). The highest numbers of bands (>15) were produced by primers 1224, OPF-01, OPF-06, and OPM-11, indicating strong discriminatory power. Primers 1225, 1226, OPF-02, and OPF-05 produced 10–15 bands, whereas primers 1227, OPF-03, OPF-04, and OPM-18 exhibited lower polymorphism (6–10 bands). The UPGMA dendrogram grouped individuals from different populations into three main branches. The Parnitha population formed a distinct branch, whereas Parnassos and Hymettus populations clustered together in a separate branch (Figure 8). Despite the greater geographic distance between Parnassos and Hymettus, their genetic similarity suggests a possible historical connection. Principal coordinate analysis (PCA) further confirmed the separation of the three populations, with the first principal component explaining 11.3% of the total variation (Figure 9). The differentiation in germination behavior supports the hypothesis that Hymettus populations may represent a distinct ecotype.
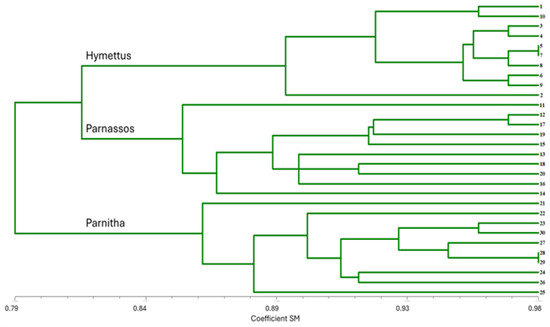
Figure 8.
UPGMA dendrogram of 30 Cerastium candidissimum individuals based on RAPD molecular analysis for SM, showing genetic similarity between individuals studied. 1, 2, 3…10: number of individuals from the Parnassos population; 11, 12, 13…20: number of individual from the Hymettus population; 21, 22, 23…30: number of individual from the Parnitha population.
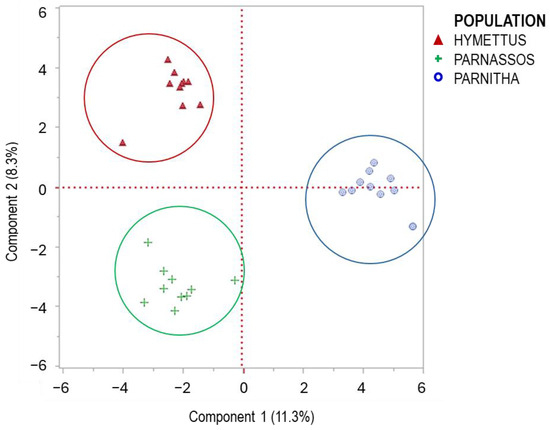
Figure 9.
PCA evaluation of the primers used and their contribution to the variability of the populations studied. The PCA plot, constructed from 10 individuals per population, shows relationships among three different populations of Cerastium candidissimum individuals.
4. Discussion
This study defined, for the first time, the temperature range required for the seed germination of C. candidissimum. The results demonstrated that the upper threshold for germination in the three populations approached 35 °C (Figure 2), while high germination percentages recorded at 10 °C suggest that the lower threshold may be below this temperature. The interaction between temperature and storage period significantly affected all estimated germination parameters, including final germination percentage, the germination speed index (GSI), time to 50% germination (T50), and total germination period. Therefore, the combined effects of these two factors were crucial in determining germination success.
Seed germination occurred over a relatively short period and within a broad temperature range, with no pre-treatments required. Six-month-old seeds exhibited high germination percentages (>80%) at temperatures between 10 °C and 20 °C, contrasting with the alpine species Cerastium dinaricum, which is reported to require cold stratification to germinate [37]. Given the high final germination percentages recorded in Parnitha and Parnassos populations, their seeds can be classified as non-dormant according to the standard definition of dormancy [63]. Furthermore, the Parnitha population maintained germination rates near 80% even after 18 months of storage, whereas Parnassos and Hymettus populations exhibited a more pronounced gradual decline in germinability over time.
The observed optimal germination temperatures (10–15 °C) are consistent with other Greek endemic drought-tolerant species, such as Lithodora zahnii and Dianthus fruticosus, which also demonstrate preference for lower germination temperatures [64,65]. This adaptation aligns with the ecological strategies of Mediterranean plants, as high summer temperatures often coincide with prolonged droughts, which can increase seedling mortality if germination occurs at inappropriate times. Similar germination patterns have been observed in Watsonia species native to South Africa, where germination preferences are linked to seasonal rainfall patterns, favoring either winter or summer emergence [66]. The germination behavior of C. candidissimum populations from Parnitha and Parnassos aligns with this Mediterranean adaptation strategy, as evidenced by their optimal germination temperatures. Moreover, the Seed Information Database (SID) reports that C. tomentosum, a species closely related to C. candidissimum, exhibits optimal germination at 15 °C in similar ecological conditions [33].
In contrast, the Hymettus population exhibited significantly lower germination percentages compared to the other two populations. Several factors may explain this trend, including adverse environmental conditions at the collection site during seed setting and potential inbreeding depression due to small population size [67,68]. Many Mediterranean species experience seasonal drought stress, making temperature a critical determinant of seed germination success. Environmental constraints during seed maturation, coupled with genetic differences, may contribute to the reduced viability of Hymettus seeds over time [36,69,70]. Notably, extending the storage period from 6 to 30 months further diminished Hymettus seed germination, ultimately reducing it to near zero. This decline could be linked to lower endosperm content, resource availability during seed development, or site-specific habitat characteristics [71,72]. Additionally, the differential germination ability observed across populations suggests the potential for local adaptation to specific environmental conditions [73].
Another plausible explanation for the low germinability of the Hymettus population relates to genetic variation within the species. Previous studies have proposed that differences in germination traits may indicate ecotypic differentiation [74,75]. If this hypothesis holds and could be further related to evidence for unique morphological and/or functional traits, it could support the classification of the Hymettus population as a distinct ecotype. The results of discriminant analysis further emphasize the distinct germination responses among populations, corroborating similar studies on Mediterranean xerophytic species such as Origanum vulgare [76].
RAPD molecular analysis identified 12 polymorphic markers, which were selected for a full analysis. The UPGMA clustering revealed two major groups, one comprising the Parnitha population and the other containing two subclusters—Parnassos and Hymettus populations (Figure 8). Interestingly, this genetic grouping contradicts the actual geographical distances among populations, as Parnassos and Hymettus are not the most proximate populations. However, high genetic similarity between geographically distant populations has been observed in other species of the genus, such as Cerastium arcticum and C. alpinum, likely due to historical dispersal events, a more widespread occurrence during glacial maxima, and shared evolutionary pressures [51,77].
The results of the RAPD analysis suggest that the lower germination success of the Hymettus population is potentially linked to physiological limitations, such as reduced endosperm content or environmental stressors affecting seed development. Additionally, low germinability may represent an adaptive response to local conditions, including water scarcity, which can influence seed longevity and viability [37,78].
Regarding the low value of the first principal component of the PCA explaining 11.3% of the total variation (Figure 9), it reveals that there is a significant portion of the total variance and genetic differentiation across multiple components rather than a single axis. The PCA indicated that 30 components were necessary to explain >60% of variation, revealing a high level of genetic diversity possibly attributed to the biogeography and evolution of C. candidissimum, as it has been mentioned for other native species [79]. Hence, the preservation of the populations is necessary to protect the genetic diversity of the species, as it has been proposed for other native species, i.e., Anisodus tanguticus and Foeniculum vulgare [80,81]. Still, the low PCA variance of the first component could be examined in further detail. Further molecular and morphological analyses are needed to explore intraspecific variation and elucidate the phylogeographic relationships among these populations.
The integration of novel ornamental species into floriculture requires a comprehensive understanding of their propagation potential. Wild species intended for commercial cultivation must undergo artificial selection, breeding, and controlled propagation to ensure consistency in horticultural traits [19]. In this regard, RAPD markers have proven useful for identifying genotypes with desirable ornamental characteristics, which could facilitate the selection of commercially valuable clones. Additionally, populations with low germination potential, such as those from Hymettus, should be prioritized for conservation efforts to prevent genetic erosion. Future research should focus on elucidating the causes of low germination in the Hymettus population and developing targeted conservation strategies. Finally, further molecular and morphological analyses are needed to explore intraspecific variation and elucidate the phylogeographic relationships among these populations, preferably incorporating additional populations of the species within a wide and representative geographical and elevational profile.
The introduction of C. candidissimum into the floriculture and landscape architecture industries could be facilitated through the collection and storage of wild seeds for two to three years, providing a reliable source of propagating material for growers. Moreover, elite genotypes with superior ornamental traits could be selectively cultivated for commercialization. RAPD markers offer an efficient, cost-effective approach for molecular studies, enabling the identification of genotypes with valuable traits. The increasing threat of wildfires in the Hymettus and Parnitha regions underscores the urgency of conservation studies on the germination and genetic structure of C. candidissimum populations.
5. Conclusions
This study revealed significant variability in the germination behavior of Cerastium candidissimum across three populations, highlighting the influence of environmental and genetic factors. The high germination percentages observed in Parnitha and Parnassos populations suggest that seeds from these sites could serve as a valuable resource for ornamental horticulture and conservation efforts. Conversely, the markedly lower germination rates in the Hymettus population indicate greater environmental pressures and potential genetic constraints. RAPD analysis confirmed high genetic polymorphism among populations and provided valuable insights into their genetic structure. The findings suggest that Hymettus populations may represent a distinct ecotype, warranting further investigation. Future research should focus on optimizing propagation techniques, assessing genetic stability, and developing conservation strategies for C. candidissimum. Given its horticultural potential, the species could be integrated into commercial floriculture, offering an attractive, drought-tolerant option for landscape applications.
Author Contributions
Conceptualization, K.B. and A.-E.B.; methodology, K.B., S.T., A.-E.B. and E.K.; formal analysis, K.B. and S.T.; investigation, K.B., S.T. and A.-E.B.; data curation, K.B., S.T., A.-E.B. and E.K.; writing—original draft preparation, K.B., S.T., A.-E.B. and E.K.; writing—review and editing, K.B., S.T., A.-E.B. and E.K.; visualization, K.B., S.T. and A.-E.B.; supervision, K.B.; project administration, K.B.; funding acquisition, K.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Heywood, V.; Skoula, M. The MEDUSA Network: Conservation and sustainable use of wild plants of the Mediterranean region. In Perspectives on New Crops and New Uses; Kanick, J., Ed.; ASHS Press: Alexandria, VA, USA, 1999; pp. 148–151. [Google Scholar]
- Krigas, N.; Tsoktouridis, G.; Anestis, I.; Khabbach, A.; Libiad, M.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Lamchouri, F.; Tsiripidis, I.; Tsiafouli, M.A.; et al. Exploring the potential of neglected local endemic plants of three Mediterranean regions in the ornamental sector: Value chain feasibility and readiness timescale for their sustainable exploitation. Sustainability 2021, 13, 2539. [Google Scholar] [CrossRef]
- Iatrou, G. The richness and the rarity of the Greek flora. In Directive 92/43/EEC: The Greek Habitat Project Natura 2000: An Overview; Dafis, S., Papastergiadou, E., Georgiou, K., Babalonas, D., Georgiadis, T., Papageorgiou, M., Lazaridou, T., Tsiaoussi, V., Eds.; Life Contract B4-3200/94/756, Commission of the European Communities DG XI, The Goulandris Natural History Museum-Greek Biotope/Wetland Centre: Thessaloniki, Greece, 1996; pp. 437–438. [Google Scholar]
- Bittrich, V. Caryophyllaceae. In Flowering Plants: Dicotyledons—Magnoliid, Hamamelid, and Caryophyllid Families; Kubitzki, K., Rohwer, J.G., Bittrich, V., Eds.; Springer: Berlin, Germany, 1993; pp. 206–236. [Google Scholar]
- Greenberg, A.K.; Donoghue, M.J. Molecular systematics and character evolution in Caryophyllaceae. Taxon 2011, 60, 1637–1652. [Google Scholar] [CrossRef]
- World Flora Online (WFO). Available online: http://www.worldfloraonline.org (accessed on 2 July 2024).
- Niketić, M.; Siljak-Yakovlev, S.; Frajman, B.; Lazarević, M.; Stevanović, B.; Tomović, G.; Stevanović, V. Towards resolving the systematics of Cerastium subsection Cerastium (Caryophyllaceae): A cytogenetic approach. Bot. J. Linn. Soc. 2013, 172, 205–224. [Google Scholar] [CrossRef]
- Surina, B.; Schönswetter, P.; Schneeweiss, G.M. Quaternary range dynamics of ecologically divergent species (Edraianthus serpyllifolius and E. tenuifolius, Campanulaceae) within the Balkan refugium. J. Biogeog. 2011, 38, 1381–1393. [Google Scholar] [CrossRef]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular Plants of Greece: An Annotated Checklist; Botanic Garden and Botanical Museum Berlin-Dahlem; Hellenic Botanical Society: Athens, Greece, 2013. [Google Scholar]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular plants of Greece: An annotated checklist—Supplement. Willdenowia 2016, 46, 301–347. [Google Scholar] [CrossRef]
- Khalaf, M.; Stace, C.A. The distinction between Cerastium tomentosum L. and C. biebersteinii DC. (Caryophyllaceae), and their occurrence in the wild in Britain. Watsonia 2001, 23, 481–491. [Google Scholar]
- Rice, G. RHS Encyclopedia of Perennials; Dorling Kindersley: London, UK, 2011; p. 496. [Google Scholar]
- Oschmann, C.; Kobayashi, N.; Perkuhn, C.; Grüneberg, H.; Wissemeier, A.H. Study to expand the range of wild plants for extensive roof greening systems using superabsorbent polymers (SAP). Acta Hortic. 2009, 813, 421–426. [Google Scholar] [CrossRef]
- Staats, D.; Klett, J.E. Water conservation potential and quality of non-turf groundcovers versus Kentucky bluegrass under increasing levels of drought stress. J. Environ. Hortic. 1995, 13, 181–185. [Google Scholar] [CrossRef]
- Verlinden, M.; Nijs, I. Alien plant species favored over congeneric natives under experimental climate warming in temperate Belgium. Biol. Invasions 2010, 12, 2777–2787. [Google Scholar] [CrossRef]
- Strid, A. Flora Hellenica; Koeltz Scientific Books: Koenigstein, Germany, 1997; p. 547. [Google Scholar]
- Khalaf, M.K. Biosystematic Studies in Cerastium tomentosum Group (Caryophyllaceae). Ph.D. Thesis, University of Leicester, Leicester, UK, 1993. Available online: https://hdl.handle.net/2381/35321 (accessed on 23 December 2022).
- Hayes, A. Cerastium candidissimum. 2017. Available online: https://www.anniesannuals.com/plants/view/?id=2132 (accessed on 10 September 2024).
- Krigas, N.; Menteli, V.; Vokou, D. The electronic trade in Greek endemic plants: Biodiversity, commercial, and legal aspects. Econ. Bot. 2014, 68, 85–95. [Google Scholar] [CrossRef]
- Darras, A.I. Implementation of sustainable practices in ornamental plant cultivation worldwide: A critical review. Agronomy 2020, 10, 1570. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Strid, A.; Dimopoulos, P. Extinction risk assessment of the Greek endemic flora. Biology 2021, 10, 195. [Google Scholar] [CrossRef]
- Samaropoulou, S. Cytological Study of Endemic and Rare Plants in Kefalonia. Master’s Thesis, Patras University, Patras, Greece, 2014. (In Greek). [Google Scholar]
- Bertsouklis, K.; Tsopela, S. In Vitro propagation of three populations of the endangered Greek endemic Cerastium candidissimum and short-term storability of alginate-encapsulated shoot explants for exploitation and conservation. Horticulturae 2023, 9, 273. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Geneve, R.L. Hartmann & Kester’s Plant Propagation: Principles and Practices, 8th ed.; Prentice Hall: Hoboken, NJ, USA, 2014. [Google Scholar]
- Cabahug, R.A.M.; Nam, S.Y.; Lim, K.B.; Jeon, J.K.; Hwang, Y.J. Propagation techniques for ornamental succulents. Kor. Fl. Assoc. 2018, 26, 90–101. [Google Scholar] [CrossRef]
- Bertsouklis, K.; Theodorou, P.; Aretaki, P.-E. In vitro propagation of the Mount Parnitha endangered species Sideritis raeseri subsp. attica. Horticulturae 2022, 8, 1114. [Google Scholar] [CrossRef]
- Bertsouklis, K.; Vlachou, G.; Trigka, M.; Papafotiou, M. In Vitro studies on seed germination of the Mediterranean species Anthyllis barba-jovis to facilitate its introduction into the floriculture industry. Horticulturae 2022, 8, 889. [Google Scholar] [CrossRef]
- Bertsouklis, K.; Naksi, K.; Aretaki, P.-E. In vitro germination and regeneration of Senna artemisioides, a valuable leguminous ornamental shrub. Not. Bot. Horti. Agrobot. Cluj. Napoca 2023, 51, 12992. [Google Scholar] [CrossRef]
- Bertsouklis, K.; Trigka, M.; Bazanis, A.E.; Akoumianaki-Ioannidou, A. In Vitro germination and micropropagation of the Balkan endemic Lilium chalcedonicum L., a potential ornamental lily. Not. Bot. Horti. Agrobot. Cluj. Napoca 2024, 52, 13596. [Google Scholar] [CrossRef]
- Pipinis, E.; Stampoulidis, A.; Kotoula, A.A.; Milios, E.; Kostas, S.; Hatzilazarou, S.; Papaioannou, E.; Papaeirinaios, A.; Kitikidou, K.; Radoglou, K. Seed germination behavior and molecular analysis of four populations of Arbutus andrachne species from Greece and cultivation practices for producing high-quality plants. Agriculture 2023, 13, 1428. [Google Scholar] [CrossRef]
- Alexopoulos, A.A.; Mavrommati, E.; Kartsonas, E.; Petropoulos, S.A. Effect of temperature and sucrose on in vitro seed germination and bulblet production of Pancratium maritimum L. Agronomy 2022, 12, 2786. [Google Scholar] [CrossRef]
- Bazanis, A.-E.; Papafotiou, M. In Vitro germination, micropropagation, and addressing hyperhydricity in the Balkan native Dianthus cruentus, a plant with high ornamental and xeriscaping potential. Horticulturae 2024, 10, 813. [Google Scholar] [CrossRef]
- Society for Ecological Restoration. International Network for Seed-Based Restoration and Royal Botanic Gardens Kew. Seed Information Database (SID). Available online: https://ser-sid.org/ (accessed on 27 February 2025).
- Pence, V.C. In vitro collecting (IVC). I. The effect of collecting method and antimicrobial agents on contamination in temperate and tropical collections. In Vitro Cell. Dev. Biol. Plant 2005, 41, 324–332. [Google Scholar] [CrossRef]
- Walters, C.; Wheeler, L.M.; Grotenhuis, J.M. Longevity of seeds stored in a genebank: Species characteristics. Seed Sci. Res. 2005, 15, 1–20. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Breaking seed dormancy during dry storage: A useful tool or major problem for successful restoration via direct seeding? Plants 2020, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Fišer-Pečnikar, Ž.; Balant, M.; Glasnović, P.; Surina, B. Seed dormancy and germination of the rare, high-elevation Balkan endemic Cerastium dinaricum (Caryophyllaceae). Biologia 2018, 73, 937–943. [Google Scholar] [CrossRef]
- Vlachou, G.; Martini, A.Ν.; Dariotis, Ε.; Papafotiou, Μ. Comparative evaluation of seed germination of five Mediterranean sage species (Salvia sp.) native to Greece. Acta Hortic. 2020, 1298, 593–598. [Google Scholar] [CrossRef]
- Stoian-Dod, R.L.; Dan, C.; Morar, I.M.; Sestras, A.F.; Truta, A.M.; Roman, G.; Sestras, R.E. Seed germination within genus Rosa: The complexity of the process and influencing factors. Horticulturae 2023, 9, 914. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Krigas, N.; Tsoktouridis, G.; Maloupa, E.; Grigoriadou, K. Seed germination trials and ex situ conservation of local prioritized endemic plants of Crete (Greece) with commercial interest. Seeds 2022, 1, 279–302. [Google Scholar] [CrossRef]
- Giménez-Benavides, L.; Escudero, A.; Pérez-García, F. Seed germination of high mountain Mediterranean species: Altitudinal, interpopulation, and interannual variability. Ecol. Res. 2005, 20, 433–444. [Google Scholar] [CrossRef]
- Sarasan, V.; Kite, G.C.; Sileshi, G.W.; Stevenson, P.C. Applications of phytochemical and in vitro techniques for reducing over-harvesting of medicinal and pesticidal plants and generating income for the rural poor. Plant Cell Rep. 2011, 30, 1163–1172. [Google Scholar] [CrossRef]
- Williams, J.G.K.; Kubelik, A.R.; Livak, K.J.; Rafalski, J.A.; Tingey, S.V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucl. Acids Res. 1990, 18, 6531–6535. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Nisar, M. Assessment of plant genetic variations using molecular markers: A review. J. Appl. Biol. Biotechnol. 2020, 8, 99–109. [Google Scholar] [CrossRef]
- Vázquez, J.L.H.; Gómez-Mercado, F.; Guerrero, J.-L.G.; Rodríguez-García, I.; García-Maroto, F. Genetic relationships and population structure within taxa of the endemic Sideritis pusilla (Lamiaceae) assessed using RAPDs. Botanical Bot. J. Linn. Soc. 1999, 129, 345–358. [Google Scholar] [CrossRef]
- Parab, G.; Krishnan, S. Assessment of genetic variation among populations of Rhynchostylis retusa, an epiphytic orchid from Goa, India, using ISSR and RAPD markers. Ind. J. Biotechnol. 2008, 7, 313–319. [Google Scholar]
- Boulila, A.; Béjaoui, A.; Messaoud, C.; Machraoui, M.; Boussaid, M. Genetic diversity and population structure of Teucrium polium (Lamiaceae) in Tunisia. Biochem. Genet. 2010, 48, 57–70. [Google Scholar] [CrossRef]
- Bertsouklis, K.F.; Papafotiou, M. Morphometric and molecular analysis of the three Arbutus species of Greece. Not. Bot. Horti. Agrobot. Cluj. Napoca 2016, 44, 423–430. [Google Scholar] [CrossRef]
- Ramesh, P.; Mallikarjuna, G.; Sameena, S.; Kumar, A.; Gurulakshmi, K.; Reddy, B.V.; Reddy, P.C.O.; Sekhar, A.C. Advancements in molecular marker technologies and their applications in diversity studies. J. Biosci. 2020, 45, 123. [Google Scholar] [CrossRef]
- Oliya, B.K.; Chand, K.; Thakuri, L.S.; Baniya, M.K.; Sah, A.K.; Pant, B. Assessment of genetic stability of micropropagated plants of Rhynchostylis retusa (L.) using RAPD markers. Sci. Horticult. 2021, 281, 110008. [Google Scholar] [CrossRef]
- Hagen, A.R.; Giese, H.; Brochmann, C. Trans-Atlantic dispersal and phylogeography of Cerastium arcticum (Caryophyllaceae) inferred from RAPD and SCAR markers. Am. J. Bot. 2001, 88, 103–112. [Google Scholar] [CrossRef]
- ENSCONET. Seed Collecting Manual for Wild Species; Royal Botanic Gardens, Kew (UK), Universidad Politécnica de Madrid (Spain), Eds.; ENSCONET: Richmond, UK, 2009; pp. 1–36. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Bertsouklis, K.; Panagaki, K.-P. In Vitro germination and propagation of Dyckia brevifolia, an ornamental and endangered bromeliad. Horticulturae 2022, 8, 390. [Google Scholar] [CrossRef]
- International Seed Testing Association. International rules for seed testing. Seed Sci. Technol. 1999, 27, 333. [Google Scholar]
- Soltani, A.; Galeshi, S.; Zeinali, E.; Latifi, N. Genetic variation for and interrelationships among seed vigor traits in wheat from the Caspian Sea coasts of Iran. Seed Sci. Technol. 2001, 29, 653662. [Google Scholar]
- Maguire, J.D. Speed of germination—Aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Li, L.; Boyd, C.E.; Odom, J.; Dong, S. Identification of Ictalurid catfish fillets to rearing location using elemental profiling. J. World Aquac. Soc. 2013, 44, 405–414. [Google Scholar] [CrossRef]
- Khemiri, S.; Gaamour, A.; Ben Abdallah, L.; Fezzani, S. The use of otolith shape to determine stock structure of Engrauli sencrasicolus along the Tunisian coast. Hydrobiologia 2018, 821, 73–82. [Google Scholar] [CrossRef]
- Stavrakakis, M.N.; Biniari, K. Genetic study of the grape cultivars belonging to the muscat family by random amplified polymorphic DNA markers. Vitis 1998, 37, 119–122. [Google Scholar]
- Rohlf, F.J. NTSYS-pc: Numerical Taxonomy and Multivariate Analysis System, Version 1.80; Exeter Software: Setauket, NY, USA, 1992. [Google Scholar]
- Sokal, R.R.; Sneath, P.H. Principles of Numerical Taxonomy; W.H. Freeman and Company: San Francisco, CA, USA, 1963. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 2014. [Google Scholar] [CrossRef]
- Papafotiou, M.; Kalantzis, A. Studies on in vitro propagation of Lithodorazahnii. Acta Horticult. 2009, 813, 465–470. [Google Scholar] [CrossRef]
- Papafotiou, M.; Stragas, J. Seed germination and in vitro propagation of Dianthus fruticosus L. Acta Horticult. 2009, 813, 481–484. [Google Scholar] [CrossRef]
- Ascough, G.D.; Erwin, J.E.; Van Staden, J. Temperature-dependent seed germination in Watsonia species related to geographic distribution. S. Afr. J. Bot. 2007, 73, 650–653. [Google Scholar] [CrossRef][Green Version]
- Oostermeijer, J.G.B.; Van Eijck, M.W.; Den Nijs, J.C.M. Offspring fitness in relation to population size and genetic variation in the rare perennial plant species Gentiana pneumonanthe (Gentianaceae). Oecologia 1994, 97, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Ouborg, N.J.; Van Treuren, R. Variation in fitness-related characters among small and large populations of Salvia pratensis. J. Ecol. 1995, 83, 369–380. [Google Scholar] [CrossRef]
- Cogoni, D.; Mattana, E.; Fenu, G.; Bacchetta, G. From seed to seedling: A critical transitional stage for the Mediterranean psammophilous species Dianthus morisianus (Caryophyllaceae). Plant Biosyst. 2012, 146, 910–917. [Google Scholar] [CrossRef]
- Nelson, S.K.; Kanno, Y.; Seo, M.; Steber, C.M. Seed dormancy loss from dry after-ripening is associated with increasing gibberellin hormone levels in Arabidopsis thaliana. Front. Plant Sci. 2023, 14, 1145414. [Google Scholar] [CrossRef] [PubMed]
- Helenurm, K.; Schaal, B.A. Genetic load, nutrient limitation, and seed production in Lupinus texensis (Fabaceae). Am. J. Bot. 1996, 83, 1585–1595. [Google Scholar] [CrossRef]
- Oostermeijer, J.G.B.; Luitjen, S.H.; Krenova, Z.V.; Den Nijs, H.C.M. Relationships between population and habitat characteristics and reproduction of the rare Gentiana pneumonanthe L. Cons. Biol. 1998, 12, 1042–1053. [Google Scholar] [CrossRef]
- Pérez-García, F.; Hornero, J.; González-Benito, M.E. Interpopulation variation in seed germination of five Mediterranean Labiatae shrubby species. Isr. J. Plant Sci. 2003, 51, 117–124. [Google Scholar] [CrossRef]
- Turesson, G. The genotypical response of the plant species to the habitat. Hereditas 1922, 3, 211–350. [Google Scholar] [CrossRef]
- Shin, C.J.; Kim, J.G. Ecotypic differentiation in seed and seedling morphology and physiology among Cicuta virosa populations. Aq. Bot. 2013, 111, 74–80. [Google Scholar] [CrossRef]
- Bischoff, A.; Müller-Schärer, H. Testing population differentiation in plant species—How important are environmental maternal effects? Oikos 2010, 119, 445–454. [Google Scholar] [CrossRef]
- Milarska, S.E.; Androsiuk, P.; Bednarek, P.T.; Larson, K.; Giełwanowska, I. Genetic variation of Cerastium alpinum L. from Babia Góra, a critically endangered species in Poland. J. Appl. Gen. 2023, 64, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Estrelles, E.; Güemes, J.; Riera, J.; Boscaiu, M.; Ibars, A.M.; Costa, M. Seed germination behavior in Sideritis from different Iberian habitats. Not. Bot. Horti. Agrobot. Cluj. Napoca 2010, 38, 9–13. [Google Scholar] [CrossRef]
- Venkataramana, K.B.; Babrekar, P.P.; Lakhanpaul, S. Study of genetic diversity in Indian and exotic sesame (Sesamum indicum L.) germplasm using random amplified polymorphic DNA (RAPD) markers. Euphytica 1999, 110, 21–34. [Google Scholar] [CrossRef]
- Bahmani, K.; Izadi-Darbandi, A.; Sadat-Noori, S.A.; Jafari, A.A. Assessment of the Genetic Diversity in Iranian Fennels by RAPD Markers. J. Herbs Spices Med. Plants 2013, 19, 275–285. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, L.; Meng, L.; Liu, J. Genetic variation in the endangered Anisodus tanguticus (Solanaceae), an alpine perennial endemic to the Qinghai-Tibetan Plateau. Genetica 2008, 132, 123–129. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).