Abstract
Theanine, a unique non-protein amino acid, is specifically accumulated in tea plants during winter. This study explored the theanine accumulation patterns in ‘Longjing 43’ and ‘Huangjinya’ under different N supply conditions and analyzed the expression of genes involved in theanine biosynthesis during winter dormancy. We found that the two tea plant cultivars shared similar theanine accumulation patterns in winter. After 30 d of cultivation with various N forms and N deficiency, the theanine content in the tissues of both cultivars was highest in the control group, followed by NH4+ treatment. Furthermore, we noted that root growth of tea plants was inhibited to varying degrees under different N sources and N-deficient conditions. Gene expression analysis revealed that both N forms can induce the transcription of key genes, including CsADC, CsALT, CsCuAO, CsGDH2, CsPAO, CsNiR, CsNR, and CsTS1 in ‘Longjing 43’ and ‘Huangjinya’. The expression of these genes was strongly correlated with theanine levels under the N treatments. The winter theanine accumulation was finely tuned by the interplay of multiple related genes, with expression levels varying across different cultivars and tissues.
1. Introduction
L-Theanine, a water-soluble compound and non-protein amino acid, is primarily synthesized in the roots of tea plants (Camellia sinensis (L.) O. Kuntze). This compound, which is naturally found in tea plants, constitutes roughly 1%~2% of the dry weight of new shoots and represents about 70% of the total free amino acids in fresh tea leaves [1]. It is also known for its unique, refreshing flavor, a key factor in judging the quality of green tea. Meanwhile, theanine also plays a significant role in tea plant growth and stress resistance enhancement [2]. Theanine is a key constituent that gives tea its distinctive taste and is associated with numerous health benefits. Research has shown that it may help in combating cancer [3], acting as an antioxidant [4], supporting cardiovascular and cerebrovascular health [5], and protecting the liver and kidneys [6]. It also has a role in immune system regulation [7]. These health benefits and important physiological roles have made theanine a subject of considerable interest for the development of functional tea components.
Tea plants contain theanine across various tissues, and its concentration varies significantly [8]. This variation is influenced by factors such as genetic differences, tissue type, seasonal changes, and cultivation conditions [9]. Leaf color variations, especially yellowing and albinism, are valued for their potential in agriculture and commerce. These specific leaf color changes often indicate a higher theanine content, which is highly desirable in the tea industry [10,11]. Research showed that tea plant cultivars with yellow leaves typically had a higher theanine content than those with green leaves [12]. Theanine content in tea plants peaks in the spring during the annual cycle and then decreases as the year goes on [13]. Soil microbes in tea gardens can affect the nutrient uptake and metabolic processes of tea plants. Certain microbes from the rhizosphere of tea plants can significantly increase theanine levels and improve N resilience in Arabidopsis [14].
Theanine is produced in the roots of tea plants by theanine synthetase (TS), which combines ethylamine with L-glutamate. This compound is then transported to the tender buds and leaves that are eventually harvested [15]. The synthesis process is believed to be why theanine levels are high in the new spring growth. Key enzymes involved in theanine synthesis and breakdown include glutamine synthetase (GS), glutamate synthase (GOGAT), theanine synthetase (TS), theanine hydrolase (ThYD), and amine oxidase (AO) [16,17]. GS has a strong similarity to TS [18]. The proteins and enzymes involved in N metabolism, such as nitrate reductase (NR), nitrite reductase (NiR), nitrate transport proteins (NRT), and ammonium transport proteins (AMT), significantly affect theanine biosynthesis in tea plants [19].
In well-oxygenated soils, plants generally obtain N as nitrate. However, tea plants, valued for their leaves, are notable for their higher tolerance to ammonia and more efficient uptake of ammonium N [20,21]. When ammonium N is introduced, it can enhance photorespiration in tea plants, promoting a rapid conversion into theanine, proline, and glutamine. Among these, theanine accumulation in tea plants is significantly influenced by N levels and N forms [22]. This highlights the close relationship between N metabolism and theanine biosynthesis in tea plants. Research has shown that the application of ammonium (NH4+) and nitrate (NO3−) to tea plants quickly converts these into key amino acids (glutamine, theanine, arginine, aspartate, and glutamate) within just 2 h, and then transports them to the xylem for distribution [23]. It is important to note that the transport efficiency of these ions varies among different tea plant strains. The ability to transport NH4+ is a key factor affecting the plant’s assimilatory capacity and is directly related to its assimilation rate. To date, research on theanine synthesis has predominantly centered on single tea plant cultivars, with a primary emphasis on theanine accumulation in the young shoots and leaves during spring, due to their status as the principal economic harvest components. Therefore, we selected two markedly different tea plant cultivars (‘Longjing 43’ and ‘Huangjinya’) as research subjects. ‘Longjing 43’, a green-leaf tea cultivar, is widely cultivated due to its high yield and strong stress resistance. In contrast, ‘Huangjinya’, a yellow-leaf cultivar, is prized for its high theanine content and superior tea quality but is less widely planted because of its lower yield and weaker stress resistance [16]. Here, our research aims to explore the relationship between gene expression and theanine accumulation in different tea plant cultivars during winter, focusing on the response of theanine accumulation patterns in ‘Longjing 43’ and ‘Huangjinya’ to various N sources. Additionally, we will examine the correlation between the transcription levels of genes related to theanine synthesis and the actual theanine production.
2. Materials and Methods
2.1. Plant Material Culture and Sampling
In this study, two-year-old cutting seedlings of two C. sinensis cultivars (‘Longjing 43’ and ‘Huangjinya’) were chosen as the experimental subjects. Tea plant seedlings with healthy and uniform growth were selected for experimental treatment. The tea plants were hydroponically cultivated in the plant growth chamber of the State Key Laboratory of Crop Genetics & Germplasm Enhancement and Utilization at Nanjing Agricultural University, starting on 10 December during the winter season. The components of the complete nutrient solution were based on the method by Konishi et al. [24]. The pH of the hydroponic solution was maintained at 5.0. The tea plants were subjected to N supply treatments, which included sole ammonium N supply (NH4+) and sole nitrate N supply (NO3−). Ammonium and nitrate N were supplied to the nutrient solution as (NH4)2SO4 at 0.75 mM and Ca (NO3)2 at 0.75 mM, respectively. For the N-deficient treatment (-N), tea plants were grown in the solution without a N source. Tea plants grown in complete nutrient solution served as the control (CK). Each treatment consisted of 10 tea plant seedlings, and each was biologically replicated three times. After a 30-d treatment period, tea leaves and roots were harvested. The tea leaves were collected as a composite sample of the first four leaves. Immediately after collection, the tea plant specimens were flash frozen in liquid N and then stored at −80 °C refrigerator (Thermo Company, Waltham, MA, USA).
2.2. Determination of Theanine Content
The leaves and roots of tea plants from two tea plant cultivars were dried to constant weight in an oven at 80 °C for 24 h. According to Liu’s method, L-theanine was extracted from the leaves and roots separately [16]. Then, theanine content was determined by Ultra Performance Liquid Chromatography (UPLC) (Thermo Fisher Scientific, Waltham, MA, USA). The Waters ACQUITY UPLC H-class system from Waters Technologies, Milford, USA, coupled with a BEH C18 column and a fluorescence detector (Waters Technologies, Milford, MA, USA), was used for chromatographic separation and detection.
2.3. Identification and Primer Design of Relevant Genes
Drawing from the tea plant genome database and transcriptomic data [25,26,27], we collectively identified and screened seventeen pertinent genes, including CsADC, CsALT, CsCuAO, CsFd-GOGAT, CsNADH-GOGAT, CsGDH1, CsGDH2, CsGGT1, CsGGT3, CsPAO, CsGS1, CsGS2, CsNiR, CsNR, CsSAMDC, CsTS1, and CsTS2. The primer pairs of the identified genes for RT-qPCR were designed using Primer Premier 5.0 software. The principles of primer design were as follows: temperature range of 55 °C to 65 °C, lengths in the range of 18~24 bp, and GC content of 45 to 60%.
2.4. Total RNA Extraction and RT-qPCR Analysis
Total RNA was extracted from tea plant leaves by using an RNA extraction kit (Pudi Biotech Co., Ltd., Shanghai, China) following the manufacturer’s instructions. As shown in Table S1, we determined the RNA concentration and purity using a Nanodrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). One microgram of each RNA sample was reverse transcribed into cDNA by using the PrimeScript RT reagent kit (TaKaRa, Dalian, China), in accordance with the manufacturer’s instructions.
The specific quantitative primers were designed using Primer Premier 6.0 software, as shown in Table S2. The RT-qPCR primers for the related genes were verified for specificity using NCBI Primer-Blast (https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?) (accessed on 10 March 2024). The experiment was performed in 96-well plates using the Real-Time PCR detection system (Bio-Rad, CFX96, Boulder, CO, USA), according to the instructions provided with the SYBR Premix Ex Taq kit (Vazyme, Nanjing, China). The cDNA from the same samples used for physiological analysis was used as a template. The total RT-qPCR reaction mixture consisted of 10 µL of SYBR Premix Ex Taq, 2 µL of cDNA template, 7.2 µL of ddH2O, and 0.4 µL each of forward and reverse primers. The reaction protocol was as follows: initial denaturation at 95 °C for 30 s, denaturation at 95 °C for 10 s, and annealing at 60 °C for 30 s, for a total of 40 cycles. The fluorescence was measured continuously during the gradual warming from 65 to 95 °C. The expression levels of the target genes were normalized based on the expression levels of CsGAPDH in different samples [28].
2.5. Statistical Analysis
The relative expression levels of the genes were calculated and analyzed using the 2−ΔΔCT method [29]. SPSS 18.0 statistical analysis software was utilized to conduct a one-way randomized block analysis of variance (ANOVA) on the experimental results. Duncan’s multiple range test was performed at the 5% significance level to detect significant differences in theanine content and the expression of related genes under different N forms and N-deficient treatment in winter. The bar graphs were created using GraphPad Prism 8.3. Mantel’s test was employed to assess the correlation between the theanine content and the transcription levels of related genes in winter tea plants.
3. Results
3.1. Winter-Induced Differential Theanine Accumulation in Two C. sinensis Cultivars
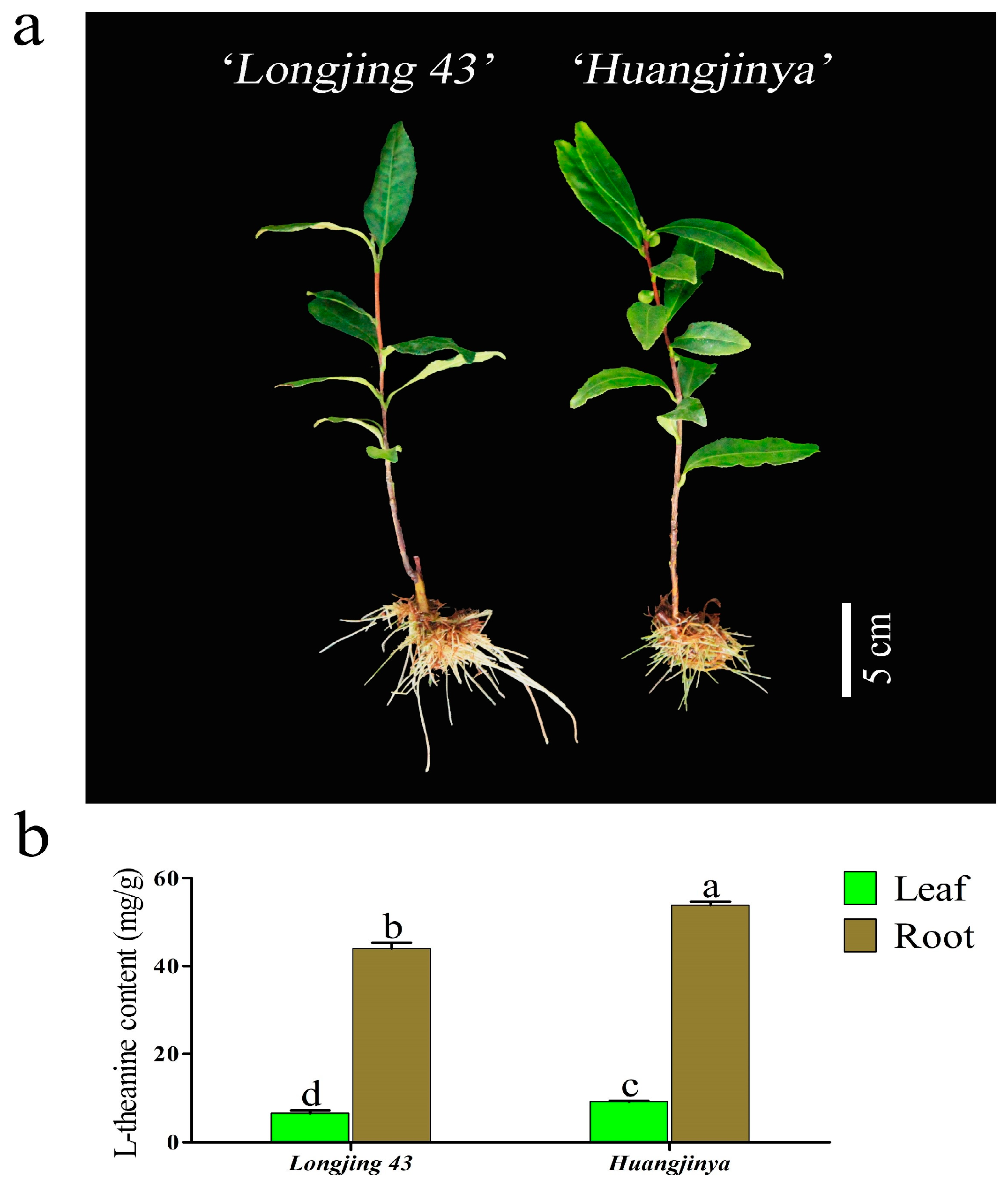
Theanine exhibited a unique accumulation pattern in the tea leaves and roots of the ‘Longjing 43’ and ‘Huangjinya’ varieties during their winter cultivation, as depicted in Figure 1. Contrary to the common assumption that leaves, as the edible parts of tea plants, would have higher theanine concentrations, the theanine content in the roots of both cultivars was significantly higher in the winter months. Specifically, the roots of ‘Longjing 43’ and ‘Huangjinya’ contained 6.71 times and 5.86 times more theanine than the leaves, respectively. These results suggest that theanine synthesis and accumulation primarily take place in the roots of tea plants during the winter season. The photoperiod-sensitive ‘Huangjinya’ cultivar showed a notable increase in theanine levels in both its leaves and roots, which were 1.40 times and 1.22 times higher than those in ‘Longjing 43’, respectively.
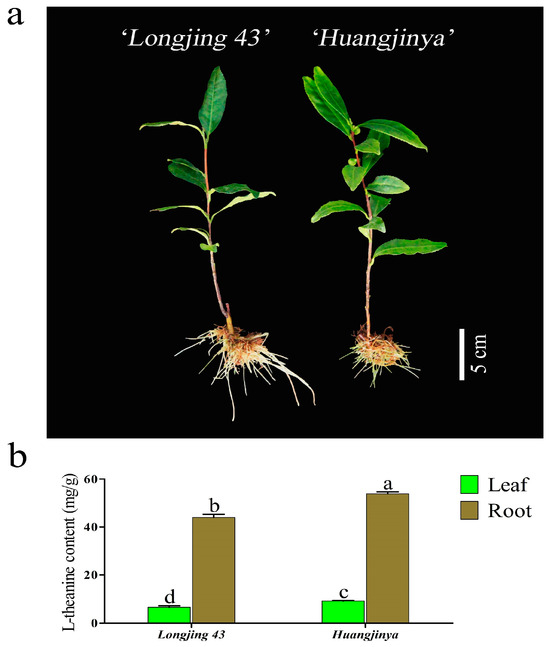
Figure 1.
The morphology and theanine content of two C. sinensis cultivars. (a) The phenotypes of ‘Longjing 43’ and ‘Huangjinya’ after 30 d of hydroponic cultivation. (b) Theanine content in leaves and roots of two C. sinensis cultivars. The different lowercase letters on the bar graph indicate significant differences at p < 0.05.
3.2. Morphology of Tea Plants and Theanine Accumulation Patterns Under Different N Forms and N Deficiency
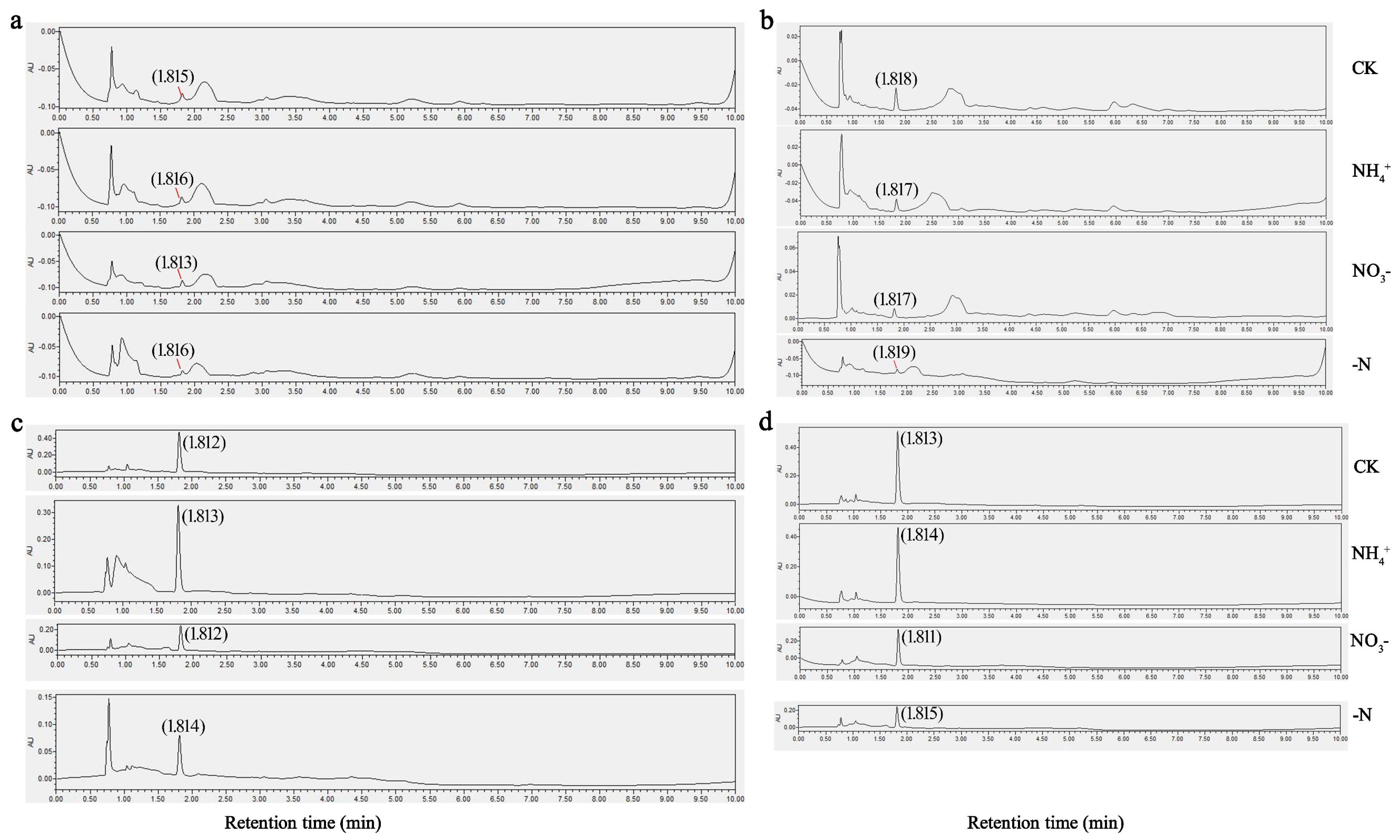
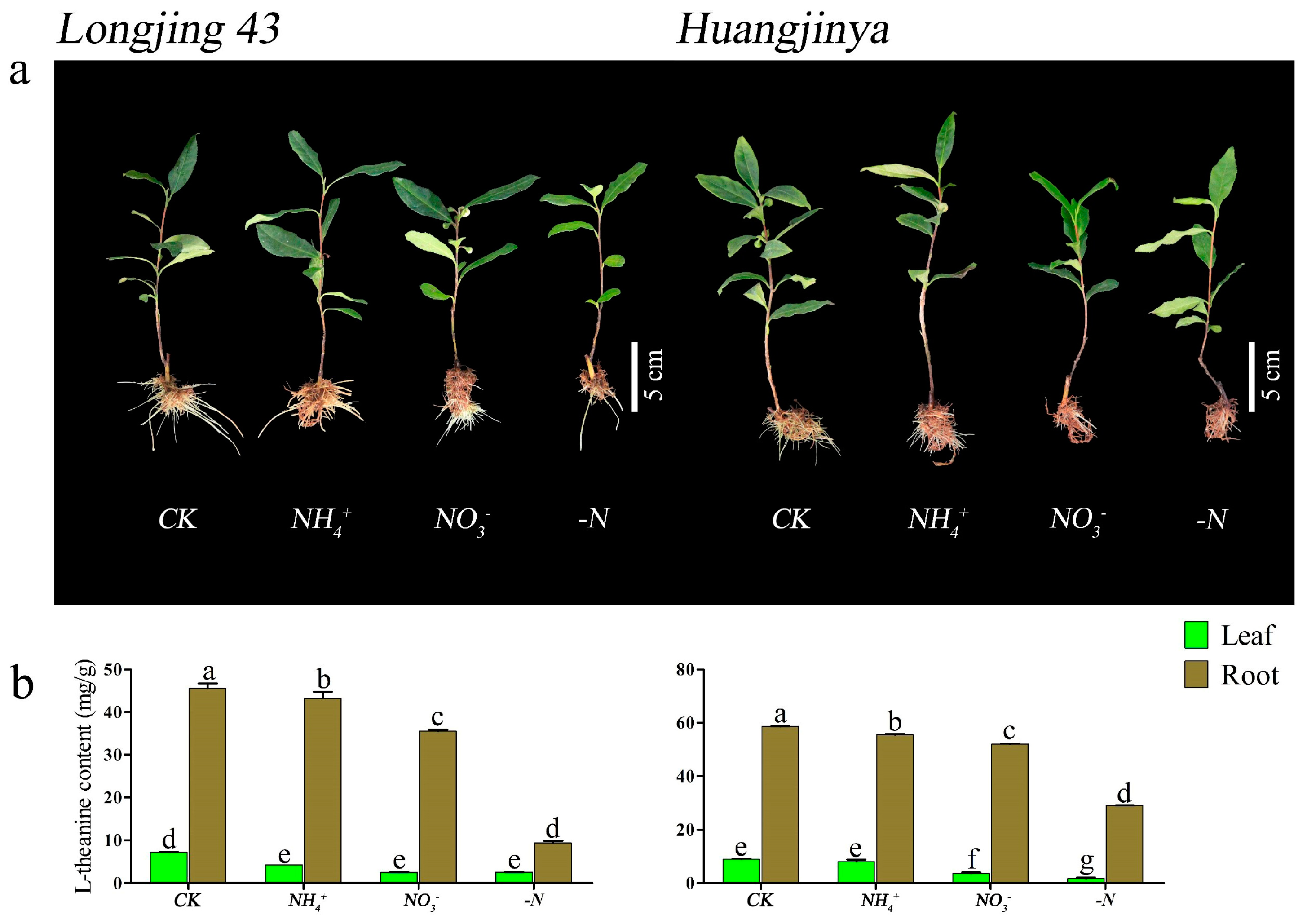
UPLC profiling of theanine concentrations in the foliage and root systems of two C. sinensis cultivars under different N forms and N deficiency consistently showed a peak elution at 1.81 min, with a precision of ±0.54 s (Figure 2). After 30 d of N treatment, both ‘Longjing 43’ and ‘Huangjinya’ displayed noticeable phenotypic responses, as shown in Figure 3a. In complete nutrient solution culture, both cultivars developed a denser and more abundant network of adventitious and lateral roots compared to other conditions. ‘Huangjinya’ showed comparatively subdued growth in its new roots. The adventitious roots of ‘Longjing 43’ were robust under NH4+ nourishment; however, there was a decrease in the lateral root system. ‘Huangjinya’ exhibited a more intricate and compact growth pattern in its new adventitious roots. When supplied with NO3−, ‘Longjing 43’ developed an increased number of delicate adventitious roots, which were notably devoid of lateral branches. The adventitious roots of ‘Huangjinya’ showed a significant reduction in growth under conditions of NO3− supply and N deficiency. ‘Longjing 43’ also experienced stunted root development in the absence of N. N deficiency was characterized by a yellowing of the leaves in both cultivars, indicating N starvation. These observations highlight the differential impact of N sources on the root system morphology of tea plants, with a notable suppression of adventitious root emergence and overall root growth under N-deficient conditions.
Figure 2.
Chromatographic analysis of theanine levels under different N forms and N deficiency. (a) The leaves of ‘Longjing 43’. (b) The leaves of ‘Huangjinya’. (c) The roots of ‘Longjing 43’. (d) The roots of ‘Huangjinya’. CK: complete nutrient supply, NH4+: ammonium N supply, NO3−: nitrate N supply, -N: N-free supply.
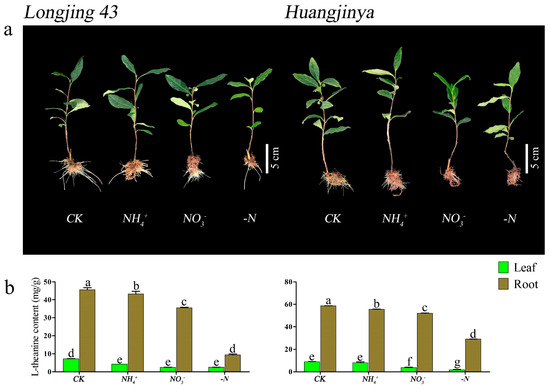
Figure 3.
The morphology and theanine accumulation dynamics of two C. sinensis cultivars under different N forms and N deficiency. (a) The phenotypes of two C. sinensis cultivars. (b) Theanine content in leaves and roots of two C. sinensis cultivars. CK: complete nutrient supply, NH4+: ammonium N supply, NO3−: nitrate N supply, -N: N-free supply. The different lowercase letters on the bar graph indicate significant differences at p < 0.05.
After 30 d of cultivation with different N forms and N deficiency, both ‘Longjing 43’ and ‘Huangjinya’ showed similar theanine accumulation profiles in their leaves and roots, as shown in Figure 3b. Compared to other treatments, the theanine content in the tissues of both cultivars was highest in the control group. ‘Longjing 43’ showed no significant difference in leaf theanine content among the other treatments; these levels were considerably lower than those in the CK condition. In contrast, ‘Huangjinya’ did not show significant variation in leaf theanine levels between the NH4+ treatment and CK; its levels were significantly higher compared to the NO3− and N deficiency. Both cultivars of C. sinensis had significantly higher theanine levels in their roots under NH4+ treatment compared to NO3− and N deficiency, with the latter showing the lowest theanine content. These findings indicate the substantial impact of N source variation on the theanine accumulation profile in tea plants.
3.3. Expression Profiling of Key Genes in ‘Longjing 43’ Tea Leaves and Roots Under Different N Forms and N Deficiency
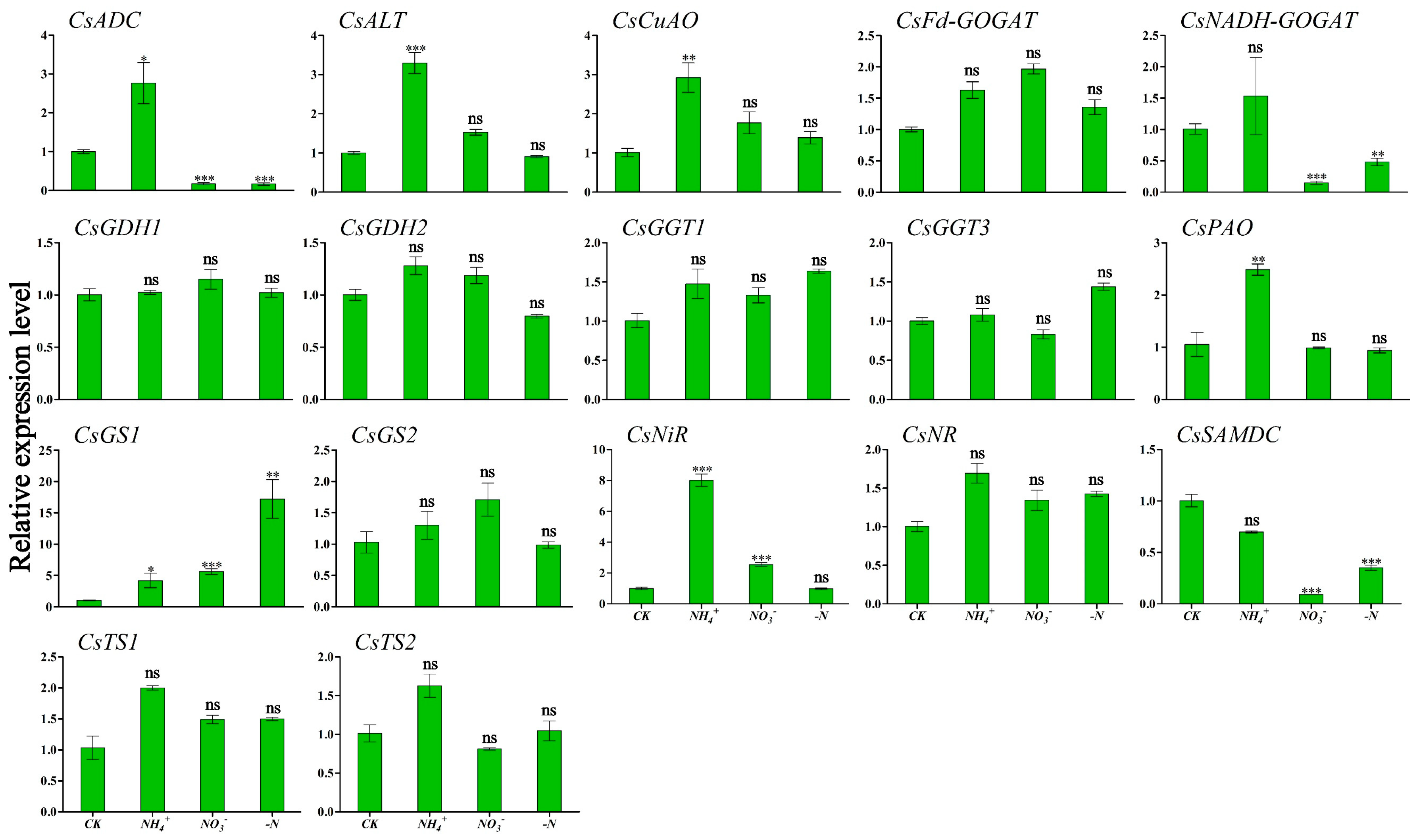
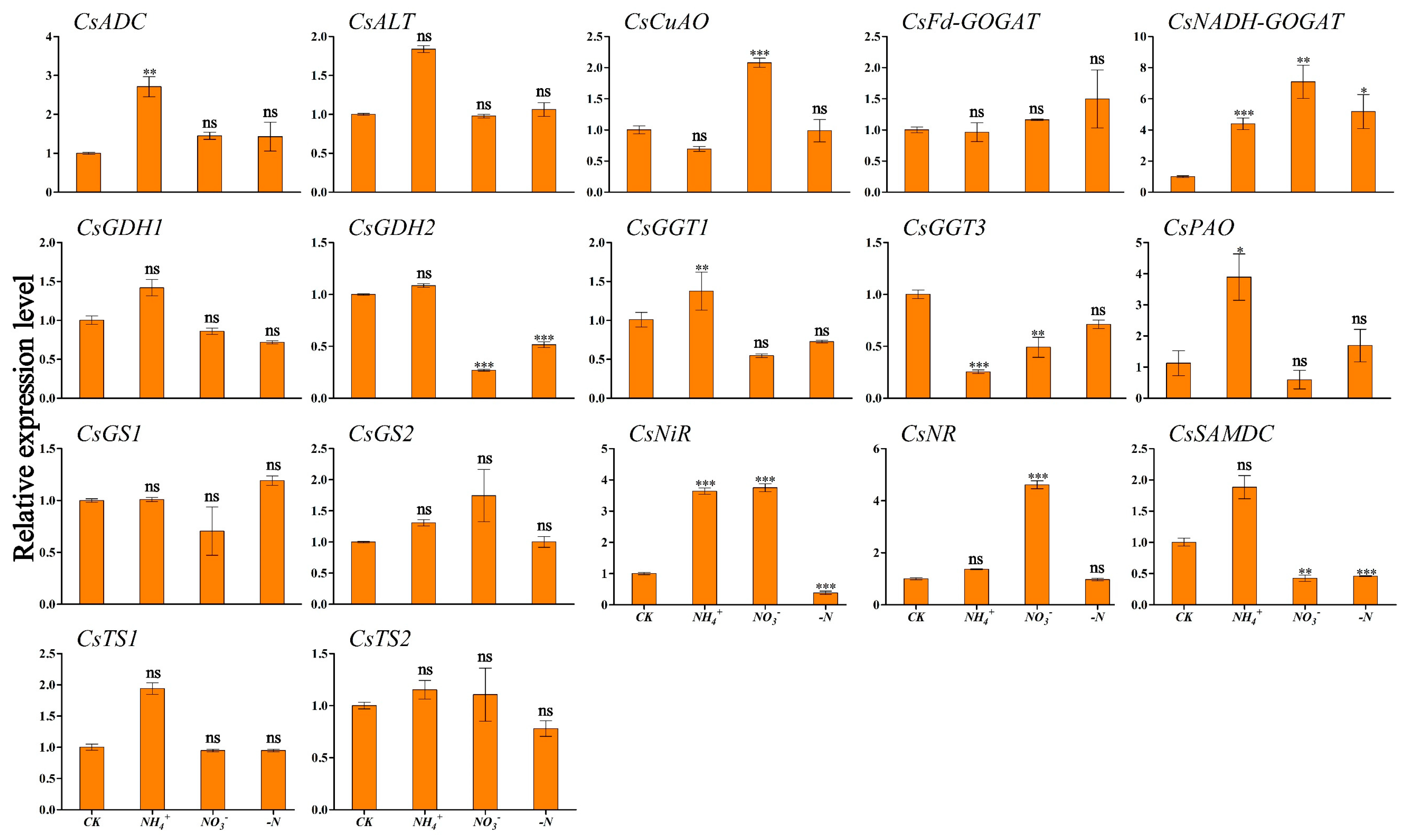
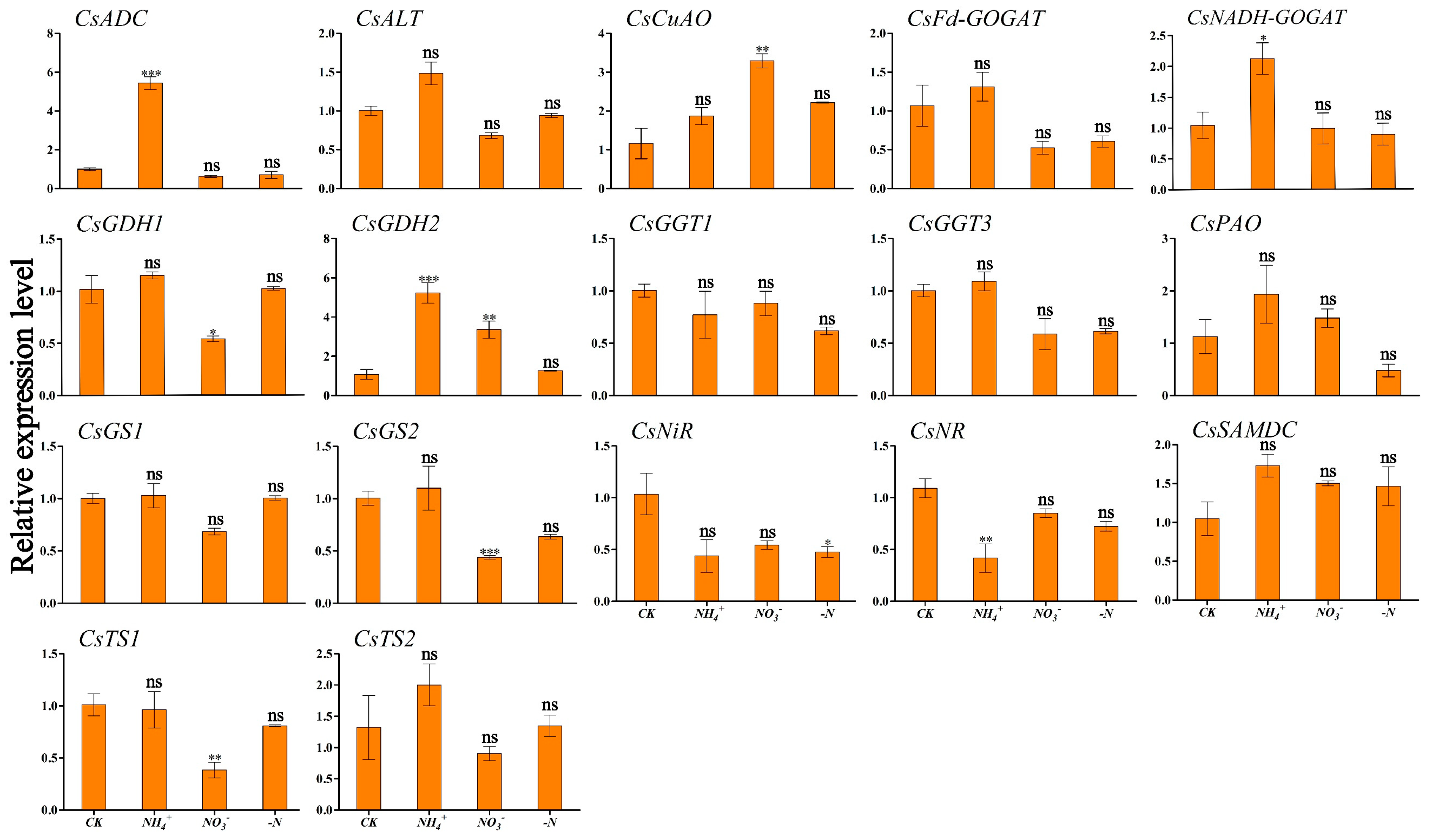
To clarify the relationship between winter theanine accumulation and gene expression, we performed RT-qPCR to detect the expression profiles of genes involved in theanine and N metabolism in ‘Longjing 43’ under different N forms and N deficiency. The results are presented in Figure 4 and Figure 5. CsADC, CsALT, CsGDH2, CsPAO, and CsTS1 exhibited similar expression patterns in response to N source signaling, with the highest transcription levels observed in both leaf and root tissues under NH4+ conditions. Within leaf tissues, CsCuAO and CsNiR reached peak relative expression under NH4+ enrichment (Figure 4). In contrast, CsGGT1 and CsSAMDC showed maximal expression in root tissues. CsNR, acting as a nitrate reductase gene, responded positively to NO3− provision, particularly showing its highest expression levels in the roots. Similarly, CsNADH-GOGAT achieved peak transcriptional activity in the roots under NO3− supplementation. The level was significantly different from those in the CK condition (Figure 5). In the absence of N deficiency, CsGS1 reached peak expression level in the leaves, which was statistically significant compared to the levels observed in the CK condition (Figure 4).
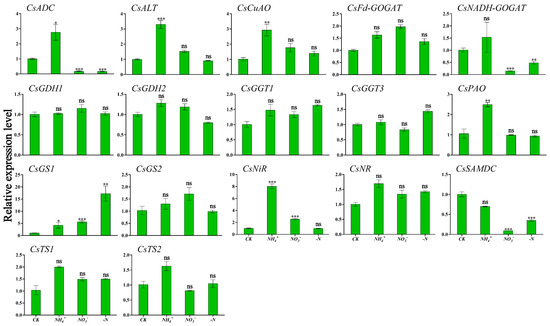
Figure 4.
Transcriptional profiling of genes in ‘Longjing 43’ tea leaves under different N forms and N deficiency. Gene expression of CK was set as the baseline with an arbitrary unit of 1 for comparative analysis. CK: complete nutrient supply, NH4+: ammonium N supply, NO3−: nitrate N supply, -N: N-free supply. * indicates that the value is significantly different from that of the CK (* p < 0.05; ** p < 0.01; *** p < 0.001), “ns” indicates that the value is not significantly different from that of the CK.
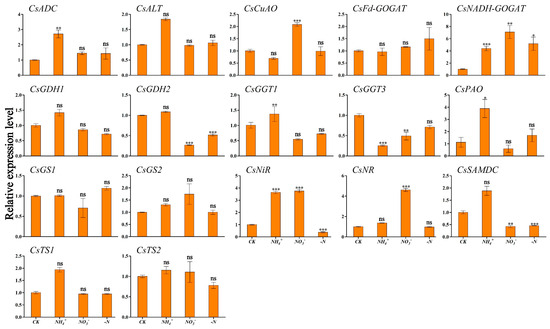
Figure 5.
Transcriptional profiling of genes in ‘Longjing 43’ tea roots under different N forms and N deficiency. Gene expression of CK was set as the baseline with an arbitrary unit of 1 for comparative analysis. CK: complete nutrient supply, NH4+: ammonium N supply, NO3−: nitrate N supply, -N: N-free supply. * indicates that the value is significantly different from that of the CK (* p < 0.05; ** p < 0.01; *** p < 0.001), “ns” indicates that the value is not significantly different from that of the CK.
3.4. Expression Profiling of Key Genes in ‘Huangjinya’ Tea Leaves and Roots Under Different N Forms and N Deficiency
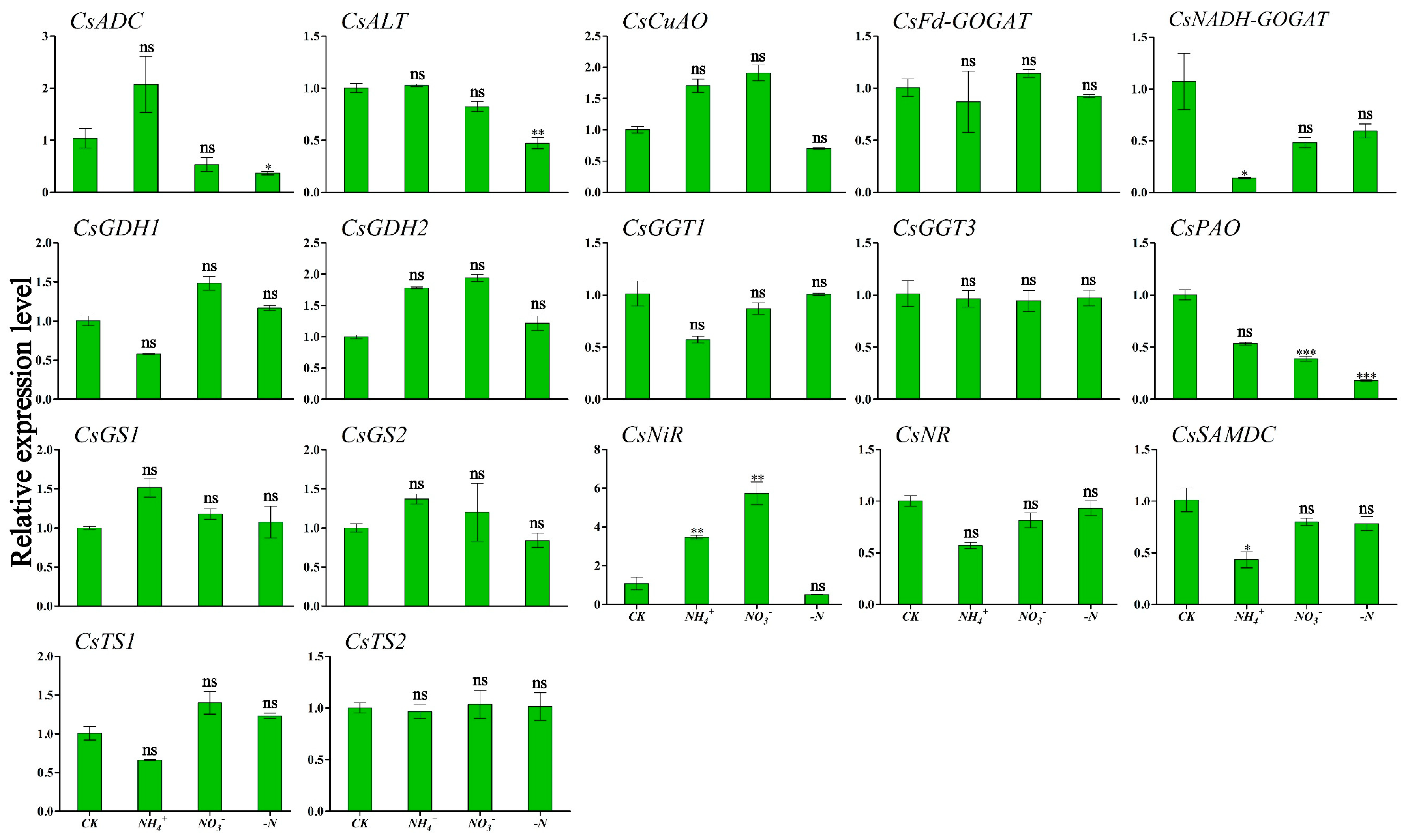
Figure 6 and Figure 7 show the expression patterns of theanine and N metabolism-related genes in ‘Huangjinya’ under different N forms and N deficiency. The analysis indicated that NH4+ supply was associated with the lowest expression levels of CsNADH-GOGAT and CsSAMDC in leaves, which were significantly different compared to CK (Figure 6). In root tissues, this treatment triggered peak transcriptional activity for CsADC, CsNADH-GOGAT, and CsGDH2, which exhibited significant variation from CK. CsCuAO, CsGDH1, CsGS2, and CsTS1 responded strongly to NO3− treatment in roots (Figure 7). Under this treatment, CsNiR showed the highest expression levels in leaves. Under N deficiency, CsADC and CsPAO in the leaves displayed significant down-regulation, with transcription levels markedly lower than those in the CK condition (Figure 6).
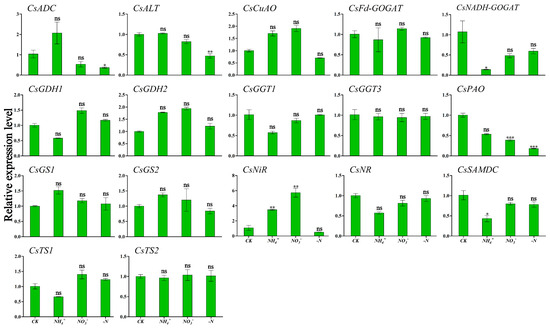
Figure 6.
Transcriptional profiling of genes in ‘Huangjinya’ tea leaves under different N forms and N deficiency. Gene expression of CK was set as the baseline with an arbitrary unit of 1 for comparative analysis. CK: complete nutrient supply, NH4+: ammonium N supply, NO3−: nitrate N supply, -N: N-free supply. * indicates that the value is significantly different from that of the CK (* p < 0.05; ** p < 0.01; *** p < 0.001), “ns” indicates that the value is not significantly different from that of the CK.
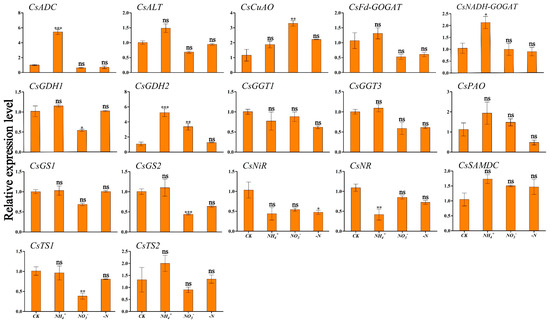
Figure 7.
Transcriptional profiling of genes in ‘Huangjinya’ tea roots under different N forms and N deficiency. Gene expression of CK was set as the baseline with an arbitrary unit of 1 for comparative analysis. CK: complete nutrient supply, NH4+: ammonium N supply, NO3−: nitrate N supply, -N: N-free supply. * indicates that the value is significantly different from that of the CK (* p < 0.05; ** p < 0.01; *** p < 0.001), “ns” indicates that the value is not significantly different from that of the CK.
3.5. Analysis of Differential Expression Patterns of Genes in Two C. sinensis Cultivars
The analysis of gene expression patterns related to theanine and N metabolism in two C. sinensis cultivars under different N forms and N deficiency revealed differential expression patterns. Under NH4+ treatment, CsNADH-GOGAT was highly expressed in the roots of ‘Huangjinya’. However, in the roots of ‘Longjing 43’, the expression of this gene was strongly induced by NO3− (Figure 5 and Figure 7). Compared to ‘Huangjinya’, the transcription level of CsGS1 in the leaves of ‘Longjing 43’ showed strong variation under different N treatments and peaked under N-deficient conditions (Figure 4 and Figure 6). Within the roots of two C. sinensis cultivars, CsGS1 did not display significant expression differences among the treatments. In ‘Huangjinya’, CsNiR demonstrated a positive response in leaf tissues to the supply of NH4+ and NO3−, particularly reaching peak transcription levels with NO3− treatment. Nevertheless, its relative expression in root tissues showed no significant changes under both treatments (Figure 6 and Figure 7). These findings highlight that CsNADH-GOGAT, CsGS1, and CsNiR display cultivar-specific and tissue-specific expression patterns in tea plants when subjected to various N source treatments. When supplied with NH4+, CsCuAO showed the highest relative expression levels in the leaves of ‘Longjing 43’, while this gene exhibited peak relative expression in the roots of ‘Longjing 43’ and ‘Huangjinya’ under NO3− treatment conditions (Figure 4, Figure 5 and Figure 7).
3.6. Theanine Biosynthesis Correlates with Gene Transcript Abundance in the Two C. sinensis Cultivars Under Different N Forms and N Deficiency
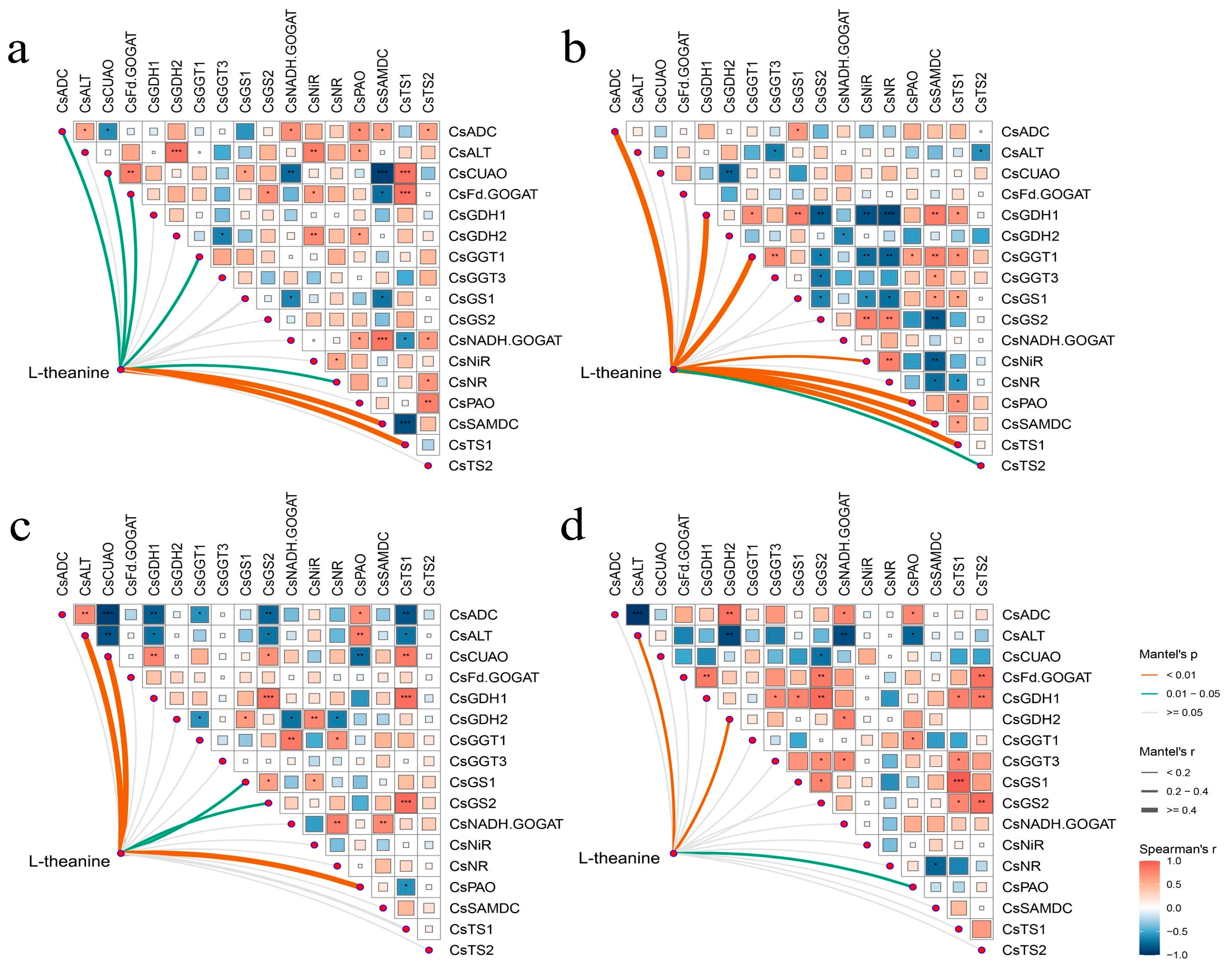
Mantel’s test delineated the correlation between specific genes and theanine accumulation in the leaves and roots of two tea cultivars under different N forms and N deficiency. In ‘Longjing 43’, seven genes correlated significantly with theanine levels in the leaves, with CsSAMDC and CsTS1 showing a highly significant association, as depicted in Figure 8a. Figure 8b illustrated that in ‘Longjing 43’, the accumulation of theanine in the roots was significantly influenced by a suite of genes, including CsADC, CsGDH1, CsGGT1, CsNiR, CsPAO, CsSAMDC, CsTS1, and CsTS2. In ‘Huangjinya’, CsALT, CsCuAO, CsGDH2, CsGS1, CsGS2, and CsPAO were identified as significant regulators of theanine accumulation under different N supply conditions, as shown in Figure 8c,d. Figure 8d highlights a significant correlation between CsALT, CsGDH2, and CsPAO with the theanine accumulation in the roots of ‘Huangjinya’.
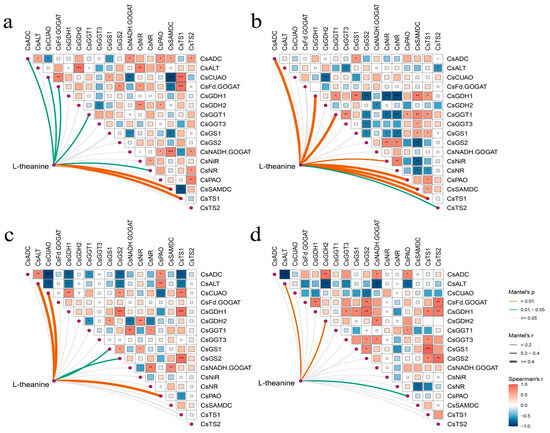
Figure 8.
Mantel’s test for gene transcript abundance and theanine accumulation correlation. (a) The leaves of ‘Longjing 43’, (b) The roots of ‘Longjing 43’, (c) The leaves of ‘Huangjinya’, (d) The roots of ‘Huangjinya’. Line colors indicate the significance levels of gene transcript abundance and theanine content (p < 0.01 denotes extremely significant differences, 0.01 < p < 0.05 indicates significant differences), and line width represents the Mantel’s r statistical measure. (* p < 0.05; ** p < 0.01; *** p < 0.001).
4. Discussion
Theanine is a key non-protein amino acid in tea plants, playing a vital role in N metabolism and is closely linked to the tea plant’s N assimilation. N is essential for tea plant growth and development and is a major factor in the synthesis of theanine [30]. Previous studies on theanine synthesis have mainly focused on individual tea plant cultivars, particularly on the accumulation in young spring shoots and leaves, which are the primary economic parts of the tea plants. While it was known that theanine is produced and stored in the roots during winter, the exact impact of different N sources on this synthesis process in winter was unclear. Understanding this mechanism is crucial for precision fertilization and sustainable tea cultivation.
In our study, we found that theanine was more abundant in the roots than in the leaves of both ‘Longjing 43’ and ‘Huangjinya’ during their winter growth cycle. This matches the findings of Li’s group. Their research examined theanine accumulation in 13 tea plant cultivars, looking at the roots and buds at four key times: 12 December, 1 March, 23 March, and 13 April. This period spans from winter to spring. They found a significant accumulation of theanine in the roots on 12 December and 1 March, while the content in the buds was lower at this time, increasing from 1 March to 23 March. This pattern shows that theanine enriches in the roots in winter and moves to the buds as spring begins [31,32]. Additionally, the study found that ‘Huangjinya’ accumulated more theanine in both the leaves and roots than ‘Longjing 43’. This difference in theanine accumulation patterns is largely due to genetic differences between the tea cultivars.
N is a key nutrient for the growth of tea plants and directly affects the synthesis of theanine [15,30]. Research has shown that ammonium and nitrate are the main forms of N that plants take up and use [33]. N deficiency significantly inhibits root growth, leading to underdeveloped root systems. Compared to normal N supply, sole NH4+ treatment limits primary root elongation but does not affect lateral root growth. The NO3− treatment, however, inhibits lateral root growth [34]. Consistent with prior studies, sole NH4+ and NO3− supply restricted the growth of tea plant adventitious roots to different extents in this study. This root architectural adjustment may be an adaptive response of tea plants to N supply changes and suggests variation in theanine accumulation in tea plants. Huang’s study with one-year-old ‘Yunnan large-leaf tea’ seedlings found that adding NH4+ and NO3− significantly increased the levels of proline, theanine, and glutamine in the leaves [22]. This finding was somewhat different from our results. In our study, both tea cultivars had the highest theanine accumulation in the leaves and roots under the CK. Applying NH4+ and NO3− notably increased the theanine content in the roots of tea plants, especially with NH4+. This winter enrichment occurs because tea plants tend to accumulate theanine in their roots, a trait of their preference for ammonium. As a plant that prefers ammonium, it efficiently absorbs NH4+ and converts it into glutamate. Glutamate then enters the theanine biosynthetic pathway. This regulatory mechanism helps the plant adjust to different N conditions.
The basic process of N assimilation in plants is managed by the GS/GOGAT cycle. This cycle changes NH4+ into glutamine, which is important for making many amino acids [35]. In this process, glutamate dehydrogenase (GDH) is very important. It helps incorporate ammonium into glutamate [36]. This is crucial for making theanine in tea plants. Wang et al. [37] showed that when NH4+ is available, the genes for GS and GOGAT, which are key in N metabolism, are used more in the young buds. The GOGAT gene is also greatly affected by NO3−. Although the GDH gene is used more when NO3− is added, this change is not large enough to be very noticeable. In our study, we observed that the CsNADH-GOGAT gene was mostly active in the roots of two C. sinensis cultivars. The genes for two types of glutamate dehydrogenase, CsGDH1 and CsGDH2, reacted to both NH4+ and NO3− in the tea plants we studied. All this shows that tea plants prefer to use ammonium for their N needs, which aligns with how plants usually handle N. This is also what Huang found, showing that NH4+ aids in how plants use N [22]. We also found that the genes CsADC, CsALT, CsCuAO, CsPAO, CsNiR, CsNR, and CsTS1 were more active when NH4+ and NO3− were added to the tea cultivars ‘Longjing 43’ and ‘Huangjinya’. This increased activity of these genes likely helped produce more theanine by enhancing the activity of key enzymes in the theanine synthesis process.
In our research, the genes related to theanine synthesis showed different expression levels under various treatments. They also exhibited different expression patterns in different parts of the tea plant and in different tea varieties. Glutamine synthetase (GS) and glutamate synthase (GOGAT) are important enzymes that work together to synthesize theanine. GS starts the process by synthesizing glutamine, which provides the N needed for theanine. GOGAT then maintains a steady supply of glutamate [38]. How these two enzymes work together is very important for synthesizing theanine efficiently, showing how they help with using N and amino acid synthesis. Fan et al. [39] found that different forms of glutamine synthetase gene (GS1.1, GS1.2, GS1.3) were used more in the leaves of tea plants when they were flowering. Within the scope of our research, CsGS1 was more responsive to different N sources in ‘Longjing 43’ and exhibited higher activity compared to ‘Huangjinya’. Liu et al. [16] compared theanine metabolism genes in three different tea plant cultivars, examining how these genes act in the same types of tissues. The CsNADH-GOGAT gene was less active in the stems of ‘Anjibaicha’ but very active in the roots of ‘Huangjinya’. Similar to the results of our study, CsNADH-GOGAT was more active in the roots than in the leaves in both tea cultivars. Additionally, CsNADH-GOGAT was most active in the roots of ‘Longjing 43’ with NO3−, but in ‘Huangjinya’, it was most active with NH4+. The differential activity suggests that the two tea cultivars may have distinct N assimilation pathways, which could be crucial for optimizing nitrogen use efficiency (NUE) in these varieties. NiR is a key gene in plants. It helps convert nitrite into ammonium. This change is important for how plants use N to grow [40]. CsNiR was most active in the leaves of ‘Huangjinya’ with NO3− and in ‘Longjing 43’ with NH4+. The differential activity of CsNiR underscores the importance of matching N forms to cultivar-specific requirements for optimal growth. Other related genes also exhibited differential activity in two C. sinensis cultivars, showing that each cultivar has its own way of using these genes. This all shows that how tea plants use N and how well they can grow might depend on how these genes are used. It also provides new insights for improving tea plants and managing N fertilizers in tea farming. The type of tissue, the special traits of each tea variety, and the changing seasons all play a role in how these genes are used. This complicates the process of theanine synthesis. By studying this, we gained insights into theanine accumulation patterns in winter and their relation to gene activity. This can help us better understand how tea plants function in winter and how to grow tea plants in a smarter and more sustainable way.
5. Conclusions
In summary, this study demonstrated that the synthesis and accumulation of theanine predominantly occur in the roots of tea plants during winter. Moreover, relative to CK, root growth was restricted to varying degrees under different N forms and N-deficient conditions. Compared to other treatments, theanine accumulation in tea plant roots peaked under complete nutrient solution culture (CK), followed by NH4+ treatment. We analyzed gene expressions and found that key genes such as CsADC, CsALT, CsCuAO, CsGDH2, CsPAO, CsNiR, CsNR, CsSAMDC, and CsTS1 were involved in theanine biosynthesis. Their expression levels were closely related to theanine levels under N treatments. The two tea plant cultivars, ‘Longjing 43’ and ‘Huangjinya’, responded differently to N sources, which might be due to their different N assimilation pathways. ‘Huangjinya’ accumulated more theanine in both the leaves and roots than ‘Longjing 43’. Notably, the two C. sinensis cultivars shared similar theanine accumulation patterns under different N forms and N deficiency conditions. The N assimilation process in tea plants was mainly managed by the GS/GOGAT cycle, where GDH played a crucial role in converting ammonia into glutamate, which is essential for theanine synthesis. This research revealed the pattern of theanine accumulation in winter and showed how different N forms and N deficiency influenced gene expression and theanine accumulation in tea plants. It provided important scientific evidence for precise fertilization and sustainable tea garden management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11040444/s1, Table S1. RNA quality analysis of tea plant leaves and roots. Table S2. Gene-targeted primers for RT-qPCR detection.
Author Contributions
Conceptualization, Y.C. and J.Z.; investigation, Y.C., J.L. and N.Y.; data curation, Z.H., W.L. and C.C.; writing—original draft, Y.C.; writing—review and editing, Y.W., X.C., X.L. and J.Z.; supervision, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Jiangsu Province Policy Guidance Program (No. SZ-LYG202126), the Jiangsu Horticultural Germplasm Conservation and Innovation Program (No. JSFEM-202212) and the Priority Academic Program Development of Jiangsu Higher Education Institutions Project (PAPD).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ashihara, H. Occurrence, biosynthesis and metabolism of theanine (γ-glutamyl-L-ethylamide) in plants: A comprehensive review. Nat. Prod. Commun. 2015, 10, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Ninomiya, K. Umami and food palatability. J. Nutr. 2000, 130, 921s–926s. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, P.; An, L.; Zhang, T.; Li, G. Chemoprotective effect of theanine in 1,2-dimethylhydrazine-induced colorectal cancer in rats via suppression of inflammatory parameters. J. Food. Biochem. 2022, 46, e14073. [Google Scholar] [CrossRef]
- Li, C.; Yan, Q.; Tang, S.; Xiao, W.; Tan, Z. L-theanine protects H9C2 cells from hydrogen peroxide-induced apoptosis by enhancing antioxidant capability. Med. Sci. Monit. 2018, 24, 2109–2118. [Google Scholar] [CrossRef]
- Ben, P.; Hu, M.; Wu, H.; Zhang, Z.; Gao, Y.; Luo, L.; Yin, Z. L-theanine down-regulates the JAK/STAT3 pathway to attenuate the proliferation and migration of vascular smooth muscle cells induced by angiotensin II. Biol. Pharm. Bull. 2018, 41, 1678–1684. [Google Scholar] [CrossRef]
- Altinkaynak, Y.; Kural, B.; Akcan, B.A.; Bodur, A.; Özer, S.; Yuluğ, E.; Munğan, S.; Kaya, C.; Örem, A. Protective effects of L-theanine against doxorubicin-induced nephrotoxicity in rats. Biomed. Pharmacother. 2018, 108, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tong, H.; Yan, Q.; Tang, S.; Han, X.; Xiao, W.; Tan, Z. L-theanine improves immunity by altering TH2/TH1 cytokine balance, brain neurotransmitters, and expression of phospholipase C in rat hearts. Med. Sci. Monit. 2016, 22, 8. [Google Scholar] [CrossRef]
- Yu, S.; Zhu, M.; Li, P.; Zuo, H.; Li, J.; Li, Y.; Peng, A.; Huang, J.; Fernie, A.R.; Liu, Z.; et al. Dissection of the spatial dynamics of biosynthesis, transport, and turnover of major amino acids in tea plants (Camellia sinensis). Hortic. Res. 2024, 11, uhae060. [Google Scholar] [CrossRef]
- Jiang, H.; Tian, Y.; Chen, L.; Liang, M. Diversity of tea landraces based on agronomic and quality traits in yunnan province. J. Plant Genet. Resour. 2013, 14, 634–640. [Google Scholar]
- Zeng, C.; Lin, H.; Liu, Z.; Liu, Z. Analysis of young shoots of ‘Anji Baicha’ (Camellia sinensis) at three developmental stages using nontargeted LC-MS-based metabolomics. J. Food. Sci. 2019, 84, 1746–1757. [Google Scholar] [CrossRef]
- Zeng, C.; Lin, H.; Liu, Z.; Liu, Z. Metabolomics analysis of Camellia sinensis with respect to harvesting time. Food. Res. Int. 2020, 128, 108814. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, L.; Lei, Y.; Ju, R.; Miao, S.; Jin, S. Integrated transcriptome and amino acid profile analyses reveal novel insights into differential accumulation of theanine in green and yellow tea cultivars. Tree Physiol. 2022, 42, 1501–1516. [Google Scholar] [CrossRef]
- Deng, W.; Ogita, S.; Ashihara, H. Biosynthesis of theanine (gammaethylamino-L-glutamic acid) in seedlings of Camellia sinensis. Phytochem. Lett. 2008, 1, 115–119. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, J.; Yu, Y.; Tian, Y.; Li, H.; Chen, X.; Li, W.; Liu, Y.; Lu, T.; He, B.; et al. Root microbiota of tea plants regulate nitrogen homeostasis and theanine synthesis to influence tea quality. Curr. Biol. 2024, 34, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ni, K.; Long, L.; Ruan, J. Nitrogen transport and assimilation in tea plant (Camellia sinensis): A review. Front. Plant Sci. 2023, 14, 1249202. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, Z.; Li, H.; Wang, Y.; Zhuang, J. L-theanine content and related gene expression: Novel insights into theanine biosynthesis and hydrolysis among different tea plant (Camellia sinensis L.) tissues and cultivars. Front. Plant Sci. 2017, 8, 498. [Google Scholar] [CrossRef]
- Dong, C.; Li, F.; Yang, T.; Feng, L.; Zhang, S.; Li, F.; Li, W.; Xu, G.; Bao, S.; Wan, X.; et al. Theanine transporters identified in tea plants (Camellia sinensis L.). Plant J. 2020, 101, 57–70. [Google Scholar] [CrossRef]
- Sasaoka, K.; Kito, M.; Onishi, Y. Some properties of the theanine synthesizing enzyme in tea seedlings. Agric. Biol. Chem. 1965, 29, 984–988. [Google Scholar] [CrossRef]
- Li, W.; Xiang, F.; Zhong, M.; Zhou, L.; Liu, H.; Li, S.; Wang, X. Transcriptome and metabolite analysis identifies nitrogen utilization genes in tea plant (Camellia sinensis). Sci. Rep. 2017, 7, 1693. [Google Scholar] [CrossRef]
- Liu, M.; Burgos, A.; Zhang, Q.; Tang, D.; Shi, Y.; Ma, L.; Yi, X.; Ruan, J. Analyses of transcriptome profiles and selected metabolites unravel the metabolic response to NH4+ and NO3− as signaling molecules in tea plant (Camellia sinensis L.). Sci. Hortic. 2017, 218, 293–303. [Google Scholar] [CrossRef]
- Ruan, J.; Gerendás, J.; Härdter, R.; Sattelmacher, B. Effect of nitrogen form and root-zone pH on growth and nitrogen uptake of tea (Camellia sinensis) plants. Ann. Bot. 2007, 99, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yao, Q.; Xia, E.; Gao, L. Metabolomics and transcriptomics analyses reveal nitrogen influences on the accumulation of flavonoids and amino acids in young shoots of tea plant (Camellia sinensis L.) associated with tea flavor. J. Agric. Food Chem. 2018, 66, 9828–9838. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Kato, T.; Xu, H. Transport of nitrogen assimilation in xylem vessels of green tea plants fed with NH4-N and NO3-N. Pedosphere 2008, 18, 222–226. [Google Scholar] [CrossRef]
- Konishi, S.; Miyamoto, S.; Taki, T. Stimulatory effects of aluminum on tea plants grown under low and high phosphorus supply. Soil Sci. Plant Nutr. 1985, 31, 361–368. [Google Scholar] [CrossRef]
- Wu, Z.; Li, X.; Liu, Z.; Xu, Z.; Zhuang, J. De novo assembly and transcriptome characterization: Novel insights into catechins biosynthesis in Camellia sinensis. BMC Plant Biol. 2014, 14, 277. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Yang, H.; Wang, S.; Zhao, J.; Liu, C.; Gao, L.; Xia, E.; Lu, Y.; Tai, Y.; She, G.; et al. Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality. Proc. Natl. Acad. Sci. USA 2018, 115, e4151–e4158. [Google Scholar] [CrossRef]
- Xia, E.; Tong, W.; Hou, Y.; An, Y.; Chen, L.; Wu, Q.; Liu, Y.; Yu, J.; Li, F.; Li, R.; et al. The reference genome of tea plant and resequencing of 81 diverse accessions provide insights into its genome evolution and adaptation. Mol. Plant 2020, 13, 1013–1026. [Google Scholar] [CrossRef]
- Wu, Z.; Tian, C.; Jiang, Q.; Li, X.; Zhuang, J. Selection of suitable reference genes for qRT-PCR normalization during leaf development and hormonal stimuli in tea plant (Camellia sinensis). Sci. Rep. 2016, 6, 19748. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Yang, T.; Su, Y.; Lin, S.; Zhang, S.; Zhang, Z. Nitrogen-regulated theanine and flavonoid biosynthesis in tea plant roots: Protein-level regulation revealed by multiomics analyses. J. Agric. Food Chem. 2021, 69, 10002–10016. [Google Scholar] [CrossRef]
- Li, F.; Dong, C.; Yang, T.; Ma, J.; Zhang, S.; Wei, C.; Wan, X.; Zhang, Z. Seasonal theanine accumulation and related gene expression in the roots and leaf buds of tea plants (Camellia sinensis L.). Front. Plant Sci. 2019, 10, 1397. [Google Scholar] [CrossRef]
- Lin, S.; Chen, Z.; Chen, T.; Deng, W.; Wan, X.; Zhang, Z. Theanine metabolism and transport in tea plants (Camellia sinensis L.): Advances and perspectives. Crit. Rev. Biotechnol. 2023, 43, 327–341. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, Y.; Huang, H. Differential contributions of NO3−/NH4+ to nitrogen use in response to a variable inorganic nitrogen supply in plantlets of two Brassicaceae species in vitro. Plant Methods 2019, 15, 86. [Google Scholar] [CrossRef]
- Gruber, B.D.; Giehl, R.F.H.; Friedel, S.; Nicolaus, V.W. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 2013, 163, 161–179. [Google Scholar] [CrossRef]
- Gutiérrez, R.A. Systems biology for enhanced plant nitrogen nutrition. Science 2012, 336, 1673–1675. [Google Scholar] [CrossRef]
- Fontaine, J.X.; Tercé-Laforgue, T.; Armengaud, P.; Clément, G.; Renou, J.P.; Pelletier, S.; Catterou, M.; Azzopardi, M.; Gibon, Y.; Lea, P.J.; et al. Characterization of a NADH-dependent glutamate dehydrogenase mutant of Arabidopsis demonstrates the key role of this enzyme in root carbon and nitrogen metabolism. Plant Cell 2012, 24, 4044–4065. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Lu, Y.; Qiu, Q.; Fan, D.; Wang, X.; Zheng, X. Influence of different nitrogen sources on carbon and nitrogen metabolism and gene expression in tea plants (Camellia sinensis L.). Plant Physiol. Biochem. 2022, 167, 561–566. [Google Scholar] [CrossRef]
- Gutiérrez, Á.G.; Cánovaset, F.M.; Ávila, C. Glutamate synthases from conifers: Gene structure and phylogenetic studies. BMC Genomics 2018, 19, 65. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, Q.; Liu, M.; Ma, L.; Shi, Y.; Ruan, J. Metabolomic and transcriptional analyses reveal the mechanism of C, N allocation from source leaf to flower in tea plant (Camellia sinensis L). J. Plant Physiol. 2019, 232, 200–208. [Google Scholar] [CrossRef]
- Ding, S.; Shao, X.; Li, J.; Ahammed, G.J.; Yao, Y.; Ding, J.; Hu, Z.; Yu, J.; Shi, K. Nitrogen forms and metabolism affect plant defence to foliar and root pathogens in tomato. Plant Cell Environ. 2021, 44, 1596–1610. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).