Abstract
Indoor vertical farms (IVFs) provide the conditions for producing seedlings of good quality. However, their effectiveness depends on the daily light integral (DLI) and nutrient management. This study examined the effects of DLI and nutrient solution electrical conductivity (EC) on tomato and bell pepper seedlings produced in an IVF or a greenhouse. Seedlings in the greenhouse were harvested 45 (tomato) and 55 (bell pepper) days after sowing, while those in the IVF were harvested after 30 and 40 days, respectively. The optimal EC was 2.0 for tomato and 2.4 dS m−1 for bell pepper. Tomato seedlings showed a decreased shoot-to-root ratio in the IVF. Tomatoes in the IVF reached 241% higher total biomass than greenhouse seedlings at 31.7 mol m−2 d−1, while bell peppers had an increase of 333% at 39.6 mol m−2 d−1; however, a DLI of 23.7 mol m−2 d−1 was enough to cause an increase of 153% and 264%, respectively. Nutrient concentration decreased in IVF seedlings, which was attributed to a dilution effect; in contrast, the nutrient content of tomato and bell pepper were highest when grown in the IVF when irrigated with solutions at 2.0 dS m−1 and a DLI of 31.7 mol m−2 d−1.
1. Introduction
Tomato (Solanum lycopersicum L.) and bell pepper (Capsicum annuum L.) are widely cultivated due to their high demand and economic profitability. Successful greenhouse or open-field cultivation of these crops requires the transplantation of high-quality seedlings to ensure good stand establishment and yields [1,2,3,4]. Vigorous seedlings that quickly adapt after transplantation and develop into productive plants are obtained when seeds have a high germination rate and receive adequate nutritional management, along with optimal light and temperature conditions. Seedling producers aim to regulate both shoot and root growth to obtain high-quality plants capable of achieving successful harvests [5]. To minimize post-transplant shock, seedlings with compact growth, excellent shoot and root development, and a low shoot-to-root ratio [2] are preferred. The optimal size (height) of bell pepper seedlings ranges from 10.2 to 11.4 cm [1], while for tomato, its size ranges from 9.5 to 11.5 cm [2].
Modern agriculture is increasingly adopting advanced technologies to cultivate plants under controlled conditions in high-density cropping systems, ensuring higher yields even under adverse environmental conditions [6]. Indoor vertical farming (IVF) is an alternative production strategy to meet global food demands, integrating lighting, hydroponic techniques, climate control, and automation, to optimize resource efficiency and minimize environmental impact [7]. Compared to open-field cultivation, IVF systems result in increased crop yield by utilizing the vertical space and enabling year-round production [8]. In IVFs, solar radiation is replaced by artificial lighting and production may be highly automated, while agricultural inputs can be minimized and the controlled environment protects crops from extreme climatic conditions [9]. Climate change, increasing agribusiness costs, and intensified urbanization, alongside municipal and industrial competition for resources, could force the partial displacement of conventional open-field and greenhouse production systems towards IVFs, which offer the potential for sustained high productivity with enhanced efficiency in water and other agricultural resources [10].
A key factor in IVF success is the use of light-emitting diodes (LEDs) for artificial lighting [6]. Lighting with LEDs is preferred due to their long lifespan, low heat emission, high energy efficiency, and ability to deliver specific light spectra and intensities [11,12,13]. Light intensity, spectral distribution, and photoperiod duration, combined with agronomic practices, shape plant responses to light [6,14]. The daily light integral (DLI) is a function of light intensity and duration [15] and represents the accumulated number of photons in the photosynthetic photon flux density (PPFD) range delivered to a given area and integrated over one day [16,17]. The DLI serves as a reference for optimal light requirements for plant growth [18] and has become a widely adopted parameter for assessing the irradiance delivered to horticultural crops [19] because of its influence on plant growth, development, yield, and quality [20] due to its relationship with photosynthetic rates and crop productivity. The assimilation of light is crucial for the performance of plants in IVF and the economical sustainability of this production system [21]; for this reason, implementing lighting methods to minimize LED fixture usage is critical for reducing investment and energy expenses [17] in indoor systems.
In IVFs, the DLI can be adjusted by prolonging the photoperiod or augmenting the PPFD [22]. Research has shown that an increased DLI enhances the growth of tomato and bell pepper seedlings. For example, Yan et al. [23] found that bell pepper seedlings achieved adequate shoot fresh weight, seedling quality index, chlorophyll a content, and root volume at a DLI of 14.4 mol m−2 d−1. Similarly, Zhang et al. [24] reported that high-quality tomato seedlings were produced with a DLI of 13.12 mol m−2 d−1 using LED lamps set at 200 µmol m−2 s−1 and an 18 h photoperiod. In contrast, for commercial greenhouse production, higher DLIs are required for tomato and bell pepper cultivation, as these horticultural species necessitate a DLI of 20 to 30 mol m−2 d−1, because of their higher planting densities [25].
A significant drawback of vertical farming is the substantial electricity costs necessary to meet daily energy demands for illumination [26,27], coupled with considerable initial setup expenditures. Consequently, further studies are required on DLI strategies to determine the ideal circumstances for IVFs, improving their efficacy and expanding its usefulness in horticulture crop production. Indoor vertical farming frequently integrates hydroponic or soilless cultivation methods to enhance water and nutrient utilization efficiency. Investigating appropriate nutritional methods under varying light circumstances is necessary, as the response of seedlings to the DLI may depend on nutrient supply [23], making nutrient concentration an important factor to be considered in IVFs in order to avoid nutrient deficits and minimize waste [28]. This study aimed to assess the viability of developing tomato and bell pepper seedlings in an IVF system utilizing artificial illumination, examining the impact of LED-enhanced DLI and nutrient solution concentration, as measured by electrical conductivity (EC), on plant growth and nutrient status.
2. Materials and Methods
2.1. Study Site and Plant Material
This study was conducted in the Vertical Farm Laboratory and under greenhouse conditions at Universidad Autónoma Agraria Antonio Narro, located in Saltillo, Coahuila, México (25°21′13″ N, 101°02′01″ W), at an altitude of 1765 m above sea level.
Tomato (cv. El Cid) and bell pepper (cv. California Wonder) seeds were sown at a depth of 1 cm in 200-cell polystyrene trays filled with sphagnum peat (Premier Horticulture Inc., Quakertown, PA, USA), previously adjusted to a pH of 5.8 and an EC of 0.25 dS m−1.
2.2. Indoor Vertical Farm System and Growing Conditions
This study was conducted in a vertical rack system (1.72 m length × 0.80 m width × 2.45 m height) consisting of three stacked aluminum trays (0.80 m width × 1.72 m length × 0.095 m depth) separated by 0.45 m (Karma Verde Fresh, Monterrey, México). The trays were covered with a black acrylonitrile butadiene styrene plastic, precisely fitted within the aluminum structure.
Environmental conditions in the IVF and the greenhouse were maintained as shown in Table 1. Temperature was monitored with a data logger (WatchDog model 1000, Spectrum Technologies Inc., Plainfield, IL, USA) and controlled with two 1-ton minisplit air conditioners (Whirlpool, model WA5260Q, Grand Rapids, MI, USA). Relative humidity was controlled using an ultrasonic humidifier (MistCloud, model MXCUD-001-001, Atizapán de Zaragoza, México) and a 25 cm diameter extractor, which was connected to a programmable digital humidity controller (IHC-200, Inkbird Tech, Shenzhen, China). Air circulation was maintained using fans, ensuring an average CO2 concentration at 400 ppm (Telaire, model 7001, Amphenol Advanced Sensors, St. Marys, PA, USA).

Table 1.
Average daily light integral (DLI) treatments calculated from the photosynthetic photon flux density (PPFD) obtained with light-emitting diode lamps in the indoor vertical farm (30 cm from the light source) or from natural light in the greenhouse, along with average temperature (Temp) and relative humidity (RH) maintained during the study for tomato (Solanum lycopersicum L.) and bell pepper (Capsicum annuum L.) seedlings.
2.3. Daily Light Integral and Nutrient Solution Electrical Conductivity Treatments
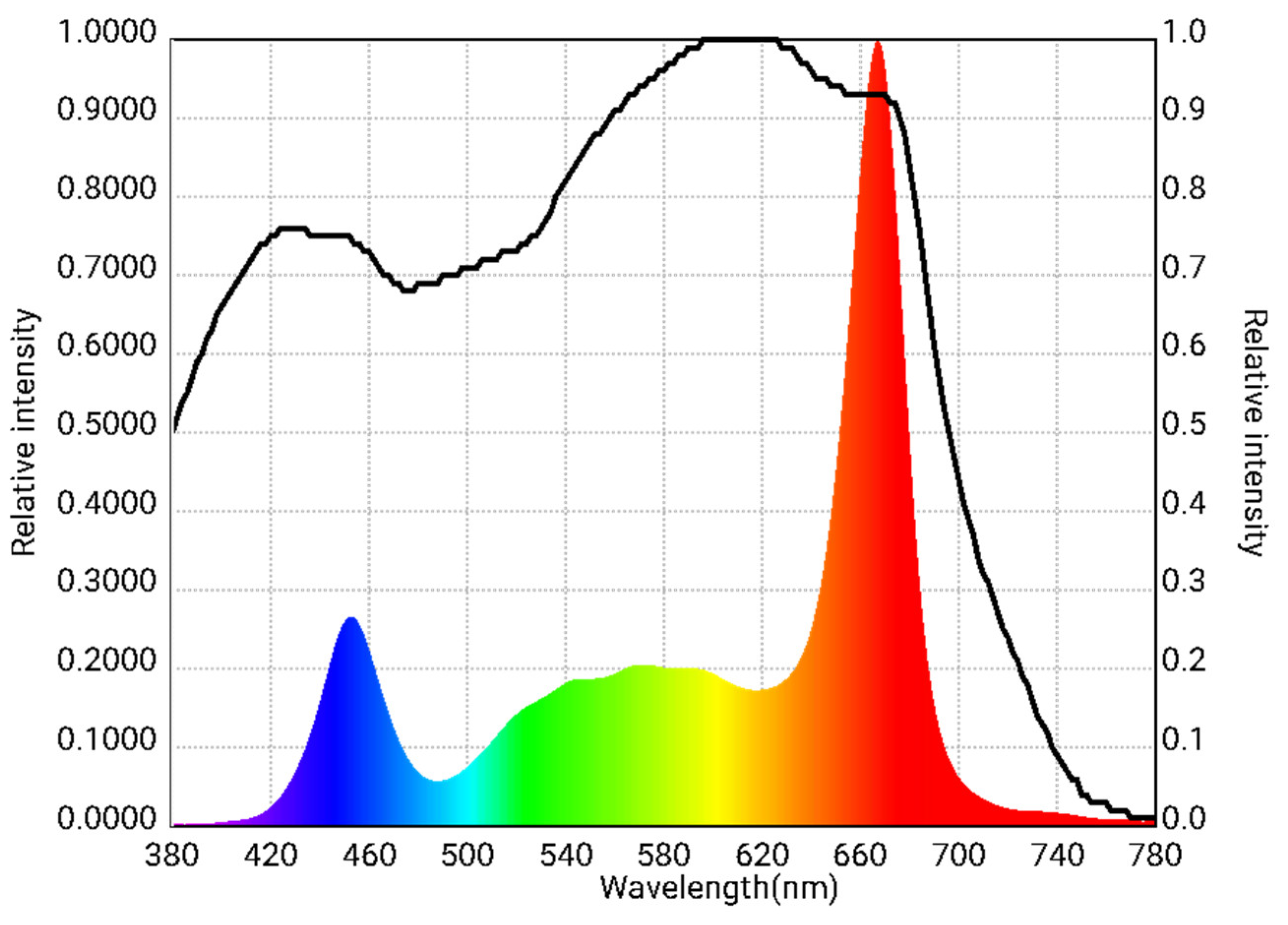
Three different PPFDs were achieved by installing 6, 8, or 10 LED lamps per level, positioned above each tray but below the one above it. A spectroradiometer (Asensetek Lighting Passport Pro Standard, Next Generation LED, Aalst, Belgium) was used to characterize the spectral distribution of the LED lamps. The device operated within a measurement range of 380–780 nm with an accuracy of ±5%. The PPFD was measured across the entire surface of each level, which was divided into a grid of 18 equally spaced sections (each section was 26.7 cm × 29.7 cm), at a height of 30 cm from the light source. The LED lamps emitted a spectral distribution of 12.6% blue light (400–500 nm), 26.1% green light (500–600 nm) and 61.3% red light (600–700 nm) (Figure 1). Illumination was maintained continuously (24 h per day) from seedlings’ emergence until harvest, resulting in three DLI treatments (shown in Table 1). As a control, tomato and bell pepper seedlings were also grown in a “low-tech” tunnel-type greenhouse with a polyethylene cover; the greenhouse had a fan-and-pad cooling system and a heating unit for temperature control. Natural sunlight in the greenhouse provided the average DLI and PPFD, along with temperatures and relative humidity shown in Table 1, which were recorded using a data logger (WatchDog model 1000, Spectrum Technologies Inc., Plainfield, IL, USA) (Table 1).
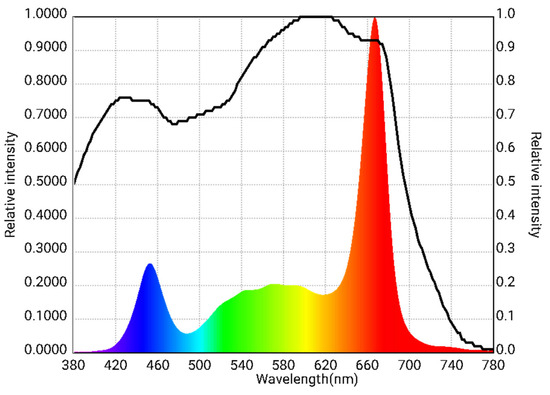
Figure 1.
Relative spectral distribution of light measured with a spectroradiometer at 30 cm from LED lamps used to produce three daily light integral treatments.
Three different ECs in the nutrient solution were evaluated under each DLI treatment. The base nutrient solution contained 12 meq L−1 NO3−, 1 meq L−1, H2PO4−, 7 meq L−1 SO42−, 7 meq L−1 K+, 9 meq L−1 Ca2+, 4 meq L−1 Mg2+, 5.3 mg L−1 Fe-EDTA, 0.4 mg L−1 Zn-EDTA, 2.6 mg L−1 Mn-EDTA, 0.5 mg L−1 Cu-EDTA, 0.2 mg L−1 B (Na2[B4O5 (OH)4] ·8H2O), and 0.2 mg L−1 Mo (Na2MoO4). This full-strength nutrient solution had an EC of 2.0 dS m−1. Two additional solutions were prepared via reducing or increasing macronutrient concentration by 20%, resulting in ECs of 1.6 and 2.4 dS m−1, respectively. The composition of tap water was considered to calculate the nutrient solution composition, and the pH was adjusted to 5.8 by controlling the total alkalinity of water to 1.0 meq L−1.
2.4. Growth Parameters and Nutrient Status
At harvest, leaves, stems, and roots were separated and dried at 60 °C for 72 h in an oven, and the dry weight was determined with a precision balance (A&D Weighing, model GF-200, Cole Parmer, Chicago, IL, USA). The stem diameter was measured with a digital vernier caliper, while the shoot length and root volume were measured using a graduated ruler and a graduated cylinder, respectively. The shoot-to-root ratio was calculated with the dry weight of the shoot divided by the dry weight of the root.
Dried tissue samples were ground to pass through a 40-mesh screen (Mini Willey Mill, Thomas Scientific, Swedesboro, NJ, USA) for mineral analysis. The concentrations of phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), zinc (Zn), copper (Cu), boron (B), and molybdenum (Mo) were determined from 0.25 g of ground seedling tissue digested in 2 mL of a 2:1 mixture of H2SO4 and HClO4, as well as 1 mL of 30% H2O2. The digest was diluted to 25 mL with distilled and filtered water before nutrient analysis was conducted in an inductively coupled plasma atomic emission spectrometer (Liberty Model, VARIAN, Santa Clara, CA, USA) [29]. Nitrogen (N) concentration was determined using the semi-micro Kjeldahl’s method [30]. The total nutrient content per plant was calculated by adjusting concentration data according to the total dry weight of the seedlings.
2.5. Experimental Design and Statistical Analysis
Each level in the IVF had LED lamps generating three DLI treatments, combined with three nutrient solution ECs. In both the greenhouse and in the IVF, the treatments were distributed in a completely randomized experimental design with a factorial arrangement. Each experimental unit consisted of one 200-cell tray with three replicates; measurements were taken from 10 independent seedling samples from each of the three replications. Data were analyzed using a two-way analysis of variance (ANOVA) (SAS v. 9.0, SAS Institute Inc., Cary, NC, USA). When significant differences were detected, Tukey’s test (p < 0.05) was used to separate the means.
3. Results and Discussion
3.1. Harvest Time
Tomato (Figure 2) and bell pepper (Figure 3) seedlings were considered harvest-ready when their roots fully explored the substrate in each cell of the polystyrene tray. Tomato seedlings grown in the IVF were ready for harvest after 30 days (Figure 2A), whereas those in the greenhouse reached this stage 45 days after sowing (Figure 2B). Similarly, bell pepper seedlings were harvested at 40 days in the IVF (Figure 3A) and at 55 days in the greenhouse (Figure 3B). These results highlight one of the key advantages of IVF over greenhouse production: faster growth rates due to the higher light utilization efficiency achieved under optimized environmental conditions [31]. These findings align with previous reports by Chen et al. [32], which indicate that IVFs with LED lighting significantly shorten the growth cycle, reducing the time required to produce transplant-ready seedlings.

Figure 2.
Representative tomato (Solanum lycopersicum L.) seedlings at harvest collected from the indoor vertical farm (A) grown with a daily light integral of 31.7 mol m−2 d−1 and from the greenhouse (B) with a daily light integral of 25.9 mol m−2 d−1.

Figure 3.
Representative bell pepper (Capsicum annuum L) seedlings at harvest time collected from the indoor vertical farm (A) grown with a daily light integral of 39.6 mol m−2 d−1 and from the greenhouse (B) with a daily light integral of 22.6 mol m−2 d−1.
3.2. Biomass Accumulation
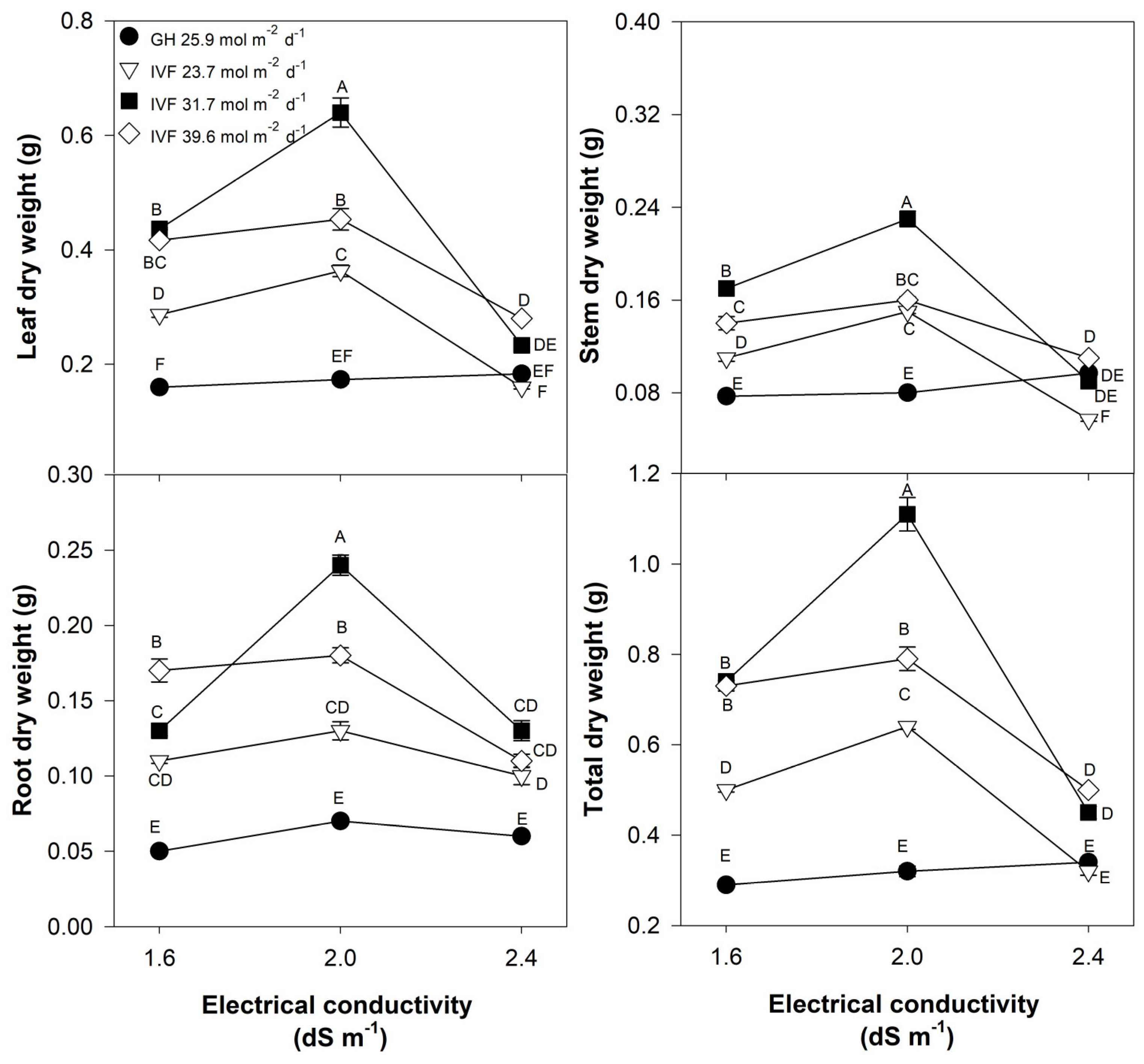
The leaf, stem, root, and total dry weight of tomato seedlings responded similarly to the DLI and the EC of the nutrient solution (Table 2; Figure 4). In general, all DLIs applied in the IVF showed significantly higher biomass accumulation across all plant parts compared to those developed in the greenhouse. However, seedlings receiving a DLI of 31.7 mol m−2 d−1 exhibited the highest total biomass, surpassing greenhouse-grown seedlings by 241% (Table 2). When averaged across all DLI levels, the optimal EC for tomato seedlings was of 2.0 dS m−1 (Table 2).

Table 2.
Effects of the daily light integral (DLI; mol m−2 d−1), provided through natural light under greenhouse conditions (GH) or through light-emitting diode lamps in an indoor vertical farm (IVF), and electrical conductivity (EC; dS m−1) of the nutrient solution on the dry weight (DW) and other growth parameters in tomato (Solanum lycopersicum L.) seedlings.
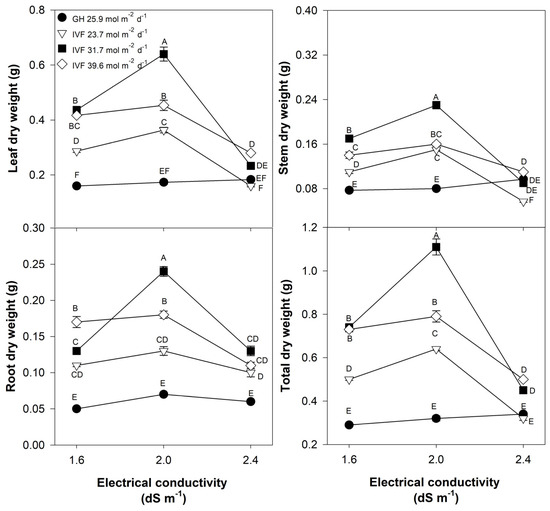
Figure 4.
Effects of the daily light integral, provided through natural light under greenhouse conditions (GH) or through light-emitting diode lamps in an indoor vertical farm (IVF), and electrical conductivity of the nutrient solution on the dry weight of plant parts (leaf, stem, root, and total weight) in tomato (Solanum lycopersicum L.) seedlings. Bars represent the standard error of the mean. Letters represent mean separation according to Tukey’s multiple comparison test with p < 0.05.
A significant DLI × EC interaction was observed (Table 2), indicating that the lowest dry weigh of the greenhouse seedlings was independent of the EC (Figure 4). In contrast, seedlings grown in the IVF had increased biomass production as the DLI increased to 31.7 mol m−2 d−1, except when the nutrient solution was at 2.4 dS m−1. The highest biomass accumulation across all plant parts occurred in seedlings grown with a DLI of 31.7 mol m−2 d−1 and EC of 2.0 dS m−1 (Figure 4).
These findings align with Ke et al. [33], who reported increased dry weight in dwarf tomatoes with a DLI of 40.32 mol m−2 d−1 under LED illumination; such results are similar to those observed in the current study, as the dry weight was highest when the DLI was 31.7 mol m−2 d−1. Regardless of the DLI, our results demonstrated that highly concentrated nutrient solutions were harmful to tomato seedlings, which we attributed to the high EC that results when the ion concentration was raised to increase the EC. Tomato is considered a moderately salt-sensitive plant, tolerating up to 2.5 dS m−1 [34], which was confirmed in the present study, as the best EC for the seedling development phase was 2.0 dS m−1.
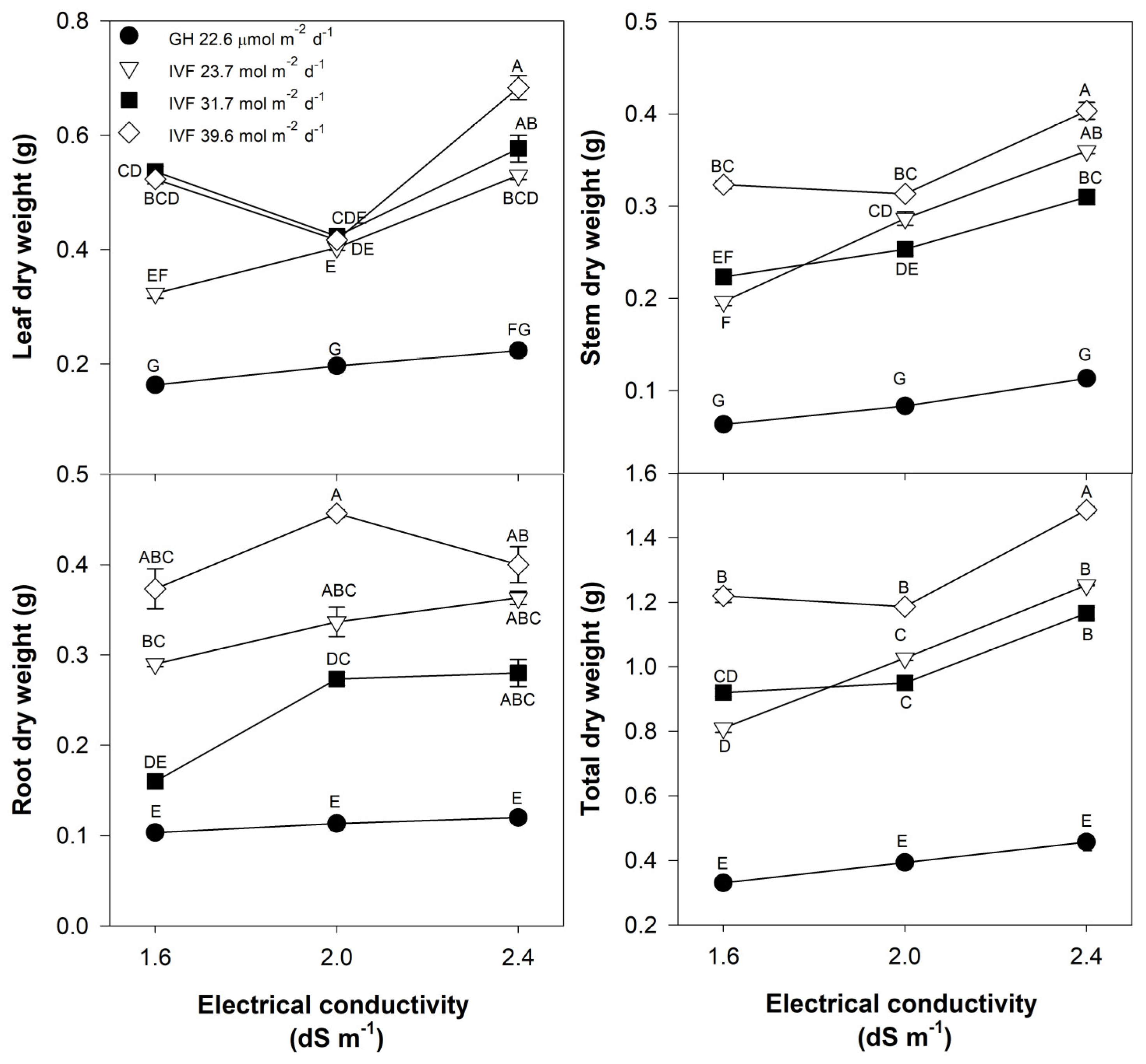
Bell pepper seedlings exhibited a growth pattern similar to tomato seedlings, with IVF-grown plants accumulating significantly higher biomass in all plant parts, compared to those developed in the greenhouse (Table 3). However, in contrast to tomato, bell pepper seedlings required the highest tested DLI (39.6 mol m−2 d−1) to achieve maximum biomass, surpassing that of greenhouse-grown seedlings by 333% (Table 3). Moreover, unlike tomato, bell pepper seedlings exhibited the highest total biomass with an EC of 2.4 dS m−1, indicating a greater tolerance to higher EC (Table 3).

Table 3.
Effects of the daily light integral (DLI; mol m−2 d−1), provided through natural light under greenhouse conditions (GH) or through light-emitting diode lamps in an indoor vertical farm (IVF), and electrical conductivity (EC; dS m−1) of the nutrient solution on the dry weight (DW) and other growth parameters in bell pepper (Capsicum annuum L.) seedlings.
Despite the consistently lower biomass accumulation in greenhouse-grown seedlings, regardless of the EC, the positive effect of increasing DLI on dry weight in the IVF, leaf, stem, and total dry weight of bell pepper seedlings increased as EC increased (Figure 5). The highest total dry weight in bell pepper also occurred under a DLI of 39.6 mol m−2 d−1 and an EC of 2.4 dS m−1. Nonetheless, root dry weight showed a distinct pattern, as it reached its maximum under 39.6 mol m−2 d−1, but EC had no significant effects (Figure 5). Our findings show that bell pepper is a species that, compared to tomato, requires a larger supply of nutrients, even during the phase of seedling development, which may be due to its 94% higher total biomass, compared to that of tomato seedlings. The higher growth of bell pepper in response to a higher EC may be because it is a species with high nutritional needs [35]. Savvas et al. [36] reported similar conclusions, as during the fruiting phase, tomato had a daily requirement of 90–130 mg N, 25–30 mg P, and 150–320 mg K, whereas pepper had higher nutrient demands: 180 mg N, 30 mg P, 220 mg K, 80 mg Ca, and 23 mg Mg. Tomato (Figure 4) and bell pepper (Figure 5) seedlings cultivated in the greenhouse demonstrated negligible responses to EC; this may be attributed to the diminished growth of the plants in the greenhouse, preventing the differentiation of the effects related to the EC of the nutrient solution.
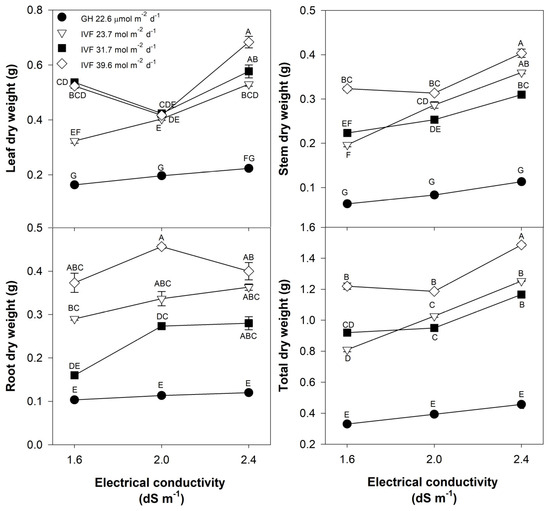
Figure 5.
Effects of the daily light integral, provided through natural light under greenhouse conditions (GH) or through light-emitting diode lamps in an indoor vertical farm (IVF), and the electrical conductivity of the nutrient solution on the dry weight of plant parts (leaf, stem, root, and total weight) in bell pepper (Capsicum annuum L.) seedlings. Bars represent the standard error of the mean. Letters represent mean separation according to Tukey’s multiple comparison test with p < 0.05.
Previous studies have reported that tomato and bell pepper seedlings perform better when grown under a DLI of 13.12 mol m−2 d−1 [24] and 14.4 mol m−2 d−1 [23], respectively. Our results showed that both species exhibited enhanced seedling growth at much higher DLIs, 31.7 and 39.6 mol m−2 d−1, respectively. This higher DLI required for maximum growth may be explained by the higher plant density obtained on the germination trays used in the present experiment, as the standard for tomato and bell pepper seedling production in México is in 200-cell trays, compared to those used by Yan et al. [23] and Zhang et al. [24] with only 72 cells. Nonetheless, if energy costs are of concern for commercial growers to use such high DLIs, our results show that a DLI as low as 23.7 mol m−2 d−1 was still enough to cause a total dry weight increase of 153% and 264% in tomato and bell pepper seedlings, respectively, compared to those from the greenhouse.
Tomato [37] and bell pepper are categorized as a day-neutral species; nevertheless, extended photoperiods or constant light exposure have been shown to negatively affect plants, resulting in mottled leaf chlorosis/necrosis, diminished leaf growth, reduced plant vigor, and disrupted plant’s circadian rhythms [38,39,40]. Plants typically respond to circadian rhythms to regulate the timing of biological processes like growth, development, flowering, and photosynthesis [41]. Nonetheless, it is reported that during the domestication of tomatoes, there was a notable deceleration of circadian rhythms, leading to modified sensitivity that allowed this species to adapt more efficiently to changes in day duration at latitudes beyond their native range [42]. It has been demonstrated that under prolonged photoperiods, an allele (EID1) is reported to enhance the performance of tomato plants; this allele was selected during the domestication of tomato in order to slow circadian rhythms and to acclimate the plants for cultivation in regions with extended summer days (longer photoperiods) when compared to that of the equator, its center of origin [43]. Veléz-Ramírez [44] stated that certain wild accessions of tomato, together with specific modern inbred lines and F1 hybrids, exhibit tolerance to continuous lighting.
Besides the reduced sensitivity of tomatoes to circadian rhythms under continuous lighting, the absence of adverse symptoms observed in the current experiment in seedlings exposed to photoperiods exceeding the limits set by Cruz and Gómez [40] may be ascribed to the shorter experimental duration in the IVF (30 and 40 days for tomato and bell pepper, respectively), as seedling production occurs within less lengthy timeframes compared to commercial fruit production. Indeed, Lanoue et al. [39] demonstrated that fruit yield under continuous lighting remained unaffected during the first month of harvest, while the impact of more prolonged photoperiods became evident in subsequent months. Bell pepper has also been observed to thrive under continuous lighting conditions with a nocturnal light intensity of 150 μmol m−2 s−1 of blue light, without exhibiting visible damage [45]. Our findings align with those by Aguirre-Bercerra et al. [22], as tomato seedlings demonstrated enhanced biomass when cultivated under uninterrupted 24 h photoperiods, a phenomenon attributed by the authors to continuous photosynthesis and carbon assimilation. Similarly, Hwang et al. [46] detected that photoperiods longer than 20 h resulted in enhanced growth of tomato and red pepper seedlings, which is a widely used technique in greenhouse and closed production systems to enhance growth and yield in many species; according to the authors, this is due to the fact that after reaching the light saturation point only the photoperiod will increase the net photosynthesis rate.
3.3. Shoot-to-Root Ratio
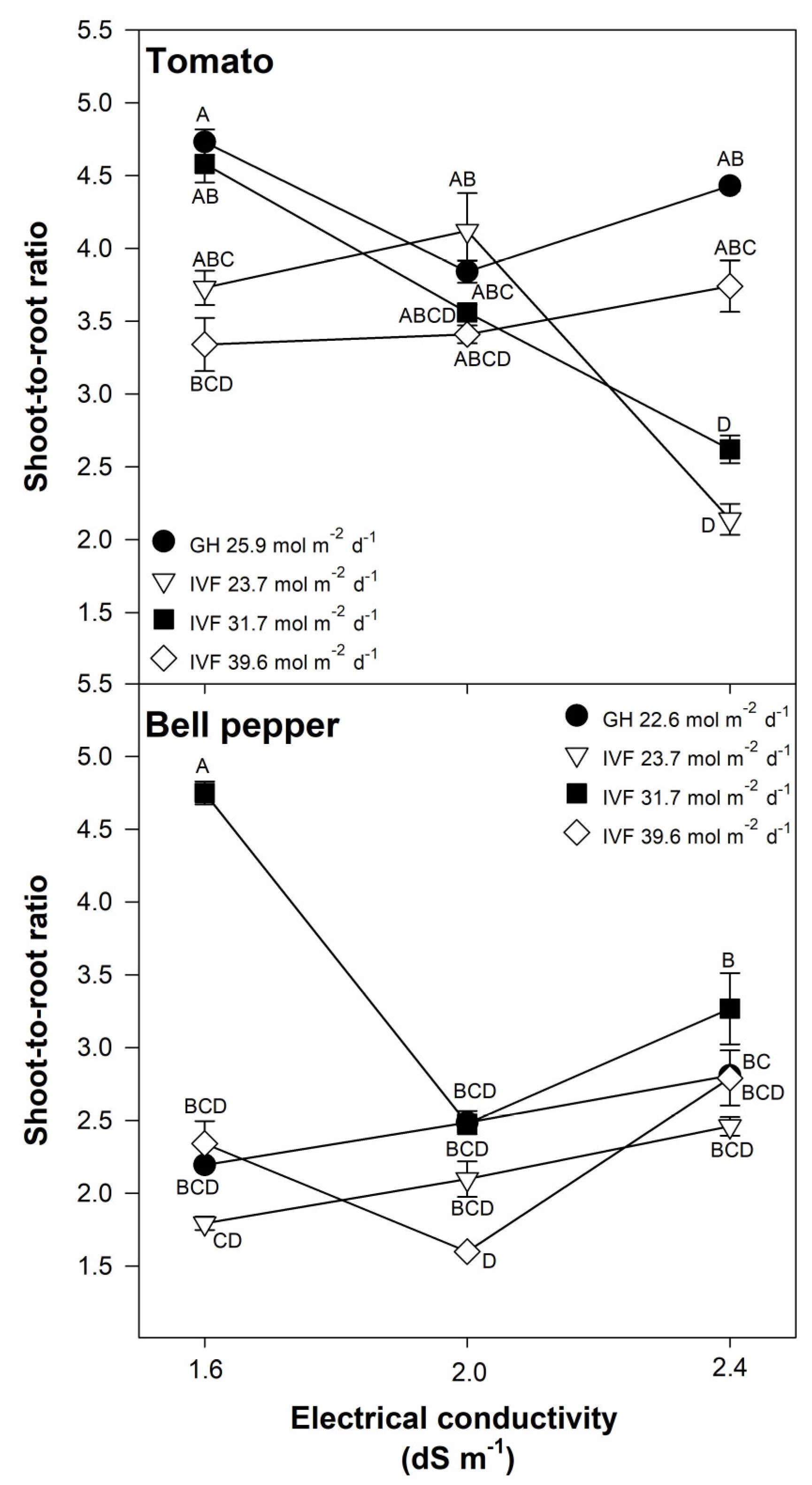
The shoot-to-root ratio was significantly influenced by the DLI, EC, and their interaction in both tomato (Table 2) and bell pepper (Table 3) seedlings. Tomato seedlings grown in the IVF under a DLI of 23.7 or 31.7 mol m−2 d−1 exhibited a lower shoot-to-root ratio when the nutrient solution had the highest EC (Figure 6). The nutrient solution EC had no significant effect on seedlings grown in the IVF under a DLI of 39.6 mol m−2 d−1 or in the greenhouse under 25.9 mol m−2 d−1 (Figure 6). These results indicate that under LED lighting with a DLI of 23.7 or 31.7 mol m−2 d−1, increased EC promotes root development more than shoot growth.
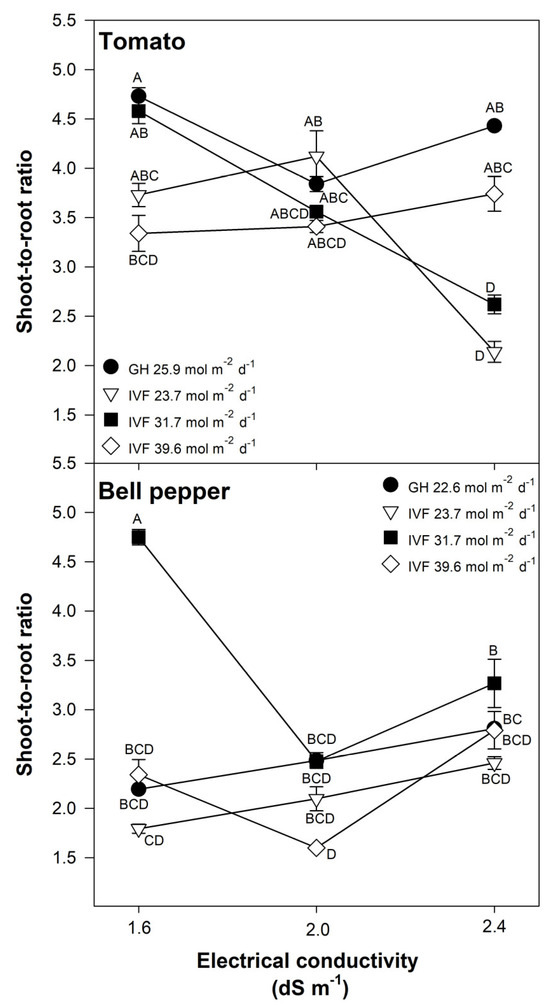
Figure 6.
Effects of the daily light integral, provided through natural light under greenhouse conditions (GH) or through light-emitting diode lamps in an indoor vertical farm (IVF), and electrical conductivity of the nutrient solution on the shoot-to-root ratio in tomato (Solanum lycopersicum L.) and bell pepper (Capsicum annuum L.) seedlings. Bars represent the standard error of the mean. Letters represent mean separation according to Tukey’s multiple comparison test with p < 0.05.
For bell pepper, the highest shoot-to-root ratio occurred when seedlings were grown under a DLI of 31.7 mol m−2 d−1 and an EC of 1.6 dS m−1 (Figure 6). For the remaining DLI and EC combinations, the ratio was lower, indicating stronger root development relative to shoot growth (Figure 6). The lowest shoot-to-root ratio was observed in seedlings grown under a DLI of 39.6 mol m−2 d−1 and an EC of 2.0 dS m−1 (Figure 6).
High-quality seedlings for transplants should have compact growth and shoot length, and well-developed roots, resulting in a low shoot-to-root ratio, which minimizes transplant shock [2]. Zhang et al. [24] reported shoot-to-root ratios of 4.75−5.98 in tomato seedlings grown under DLIs of 12.99−13.17 mol m−2 d−1, with the lowest ratio observed when PPFD was 300.7 µmol m−2 s−1 during a photoperiod of 12 h. Miyama [47] reported that a higher shoot-to-root ratio was associated with increased leaf intumescence in tomato seedlings grown under LED lighting, concluding that high shoot-to-root ratios are undesirable for seedling production, as weak roots impair transplant success. Brazaityte et al. [48] observed that raising the blue light to 100% of the light spectrum, resulted in increased shoot-to-root ratios (5.18−7.17) in kale (Brassica napus L.) and mustard (Brassica juncea L.) produced as microgreens, which were regarded as being of lower quality; however, proportions of red light as small as 25% were enough to modify the balance between the shoot and the root. This finding suggests that LED light with a high proportion of blue light should be avoided in order to produce compact shoots and well-developed roots; this may explain our results as the LED lamps used in the present study contained on average an 12.6% of blue light and 61.3% of red light.
Our results showed that seedlings with a better balance of the shoot in relation to root biomass are produced in IVF conditions than in the greenhouse. For tomato, this was when the solutions had an EC of 2.4 dS m−1 and a DLI of 23.7 or 31.7 mol m−2 d−1, whereas for bell pepper, it was with a DLI of 39.6 mol m−2 d−1 and an EC of 2.0 dS m−1 (Figure 6). A higher shoot-to-root ratio is detrimental to seedling quality and subsequent establishment when they are transplanted; this is because the reduced biomass accumulation in the root delays the time for removing them from the germination trays, while the shoot continues to develop, resulting in weakened, spindly, and/or etiolated seedlings. Additionally, a high shoot-to-root ratio can lead to decreased nutrient uptake and water absorption due to the poorly developed roots, further hindering seedling establishment as transplants are more susceptible to environmental stressors.
3.4. Shoot Length, Stem Diameter, and Root Volume
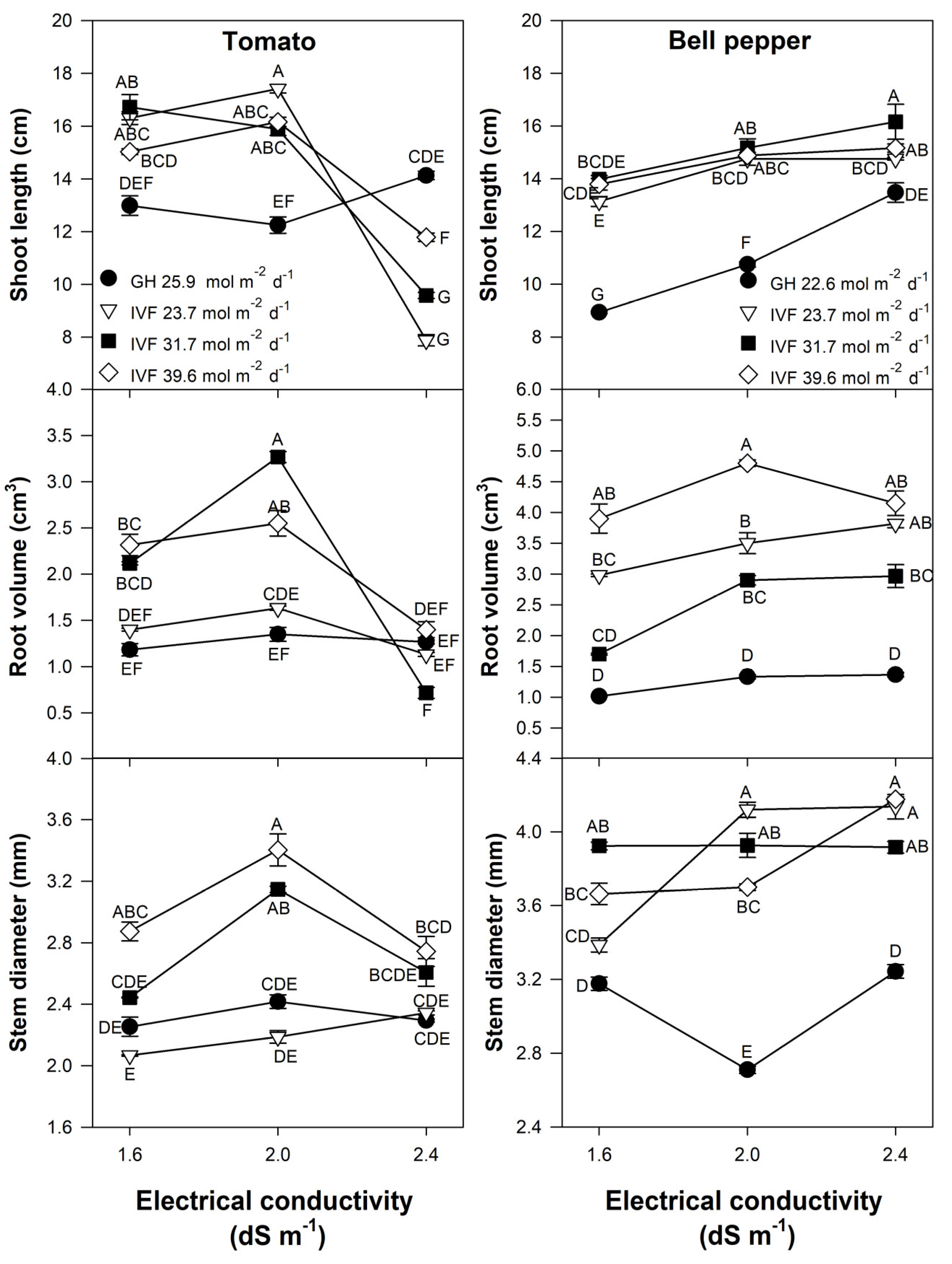
Shoot length, stem diameter, and root volume were significantly affected by the DLI, EC, and the interaction of both factors in tomato (Table 2) and bell pepper (Table 3) seedlings. In general, the length and the diameter of the stems, along with the root volume, were higher in seedlings developed in the IVF than in the greenhouse, whereas the EC that promoted a higher length and diameter of the stems and the root volume was 2.0 and 2.4 dS m−1 in tomato and bell pepper, respectively.
Root volume exhibited similar tendencies as shoot length as, compared to the greenhouse, IVF-grown seedlings exhibited greater root volume when the DLI was 31.7 and 39.6 mol m−2 d−1 and they were fed with nutrient solutions of 1.6 or 2.0 dS m−1 (Figure 7); increasing the nutrient solution EC to 2.4 dS m−1 resulted in decreased root volume (Figure 7). Root volume also increased in IVF-grown bell pepper seedlings, particularly under a DLI of 39.6 mol m−2 d−1, and was further enhanced at an EC of 2.0 or 2.4 dS m−1 (Figure 7). The effect of DLI on root growth may be attributed to light intensity and quality influencing carbohydrate transport through the phloem, as demonstrated by Lanoue et al. [49] in tomato seedlings. Similar reports were published by Miotto et al. [50], who indicated that sucrose and PPFD have a synergistic effect on root morphology and growth in Arabidopsis [Arabidopsis thaliana (L.) Heynh.]. Additionally, Huang et al. [51] demonstrated that an increase in PPFD led to increased root growth of up to 60% in bamboo [Pleioblastus pygmaeus (Miq.) Nakai].
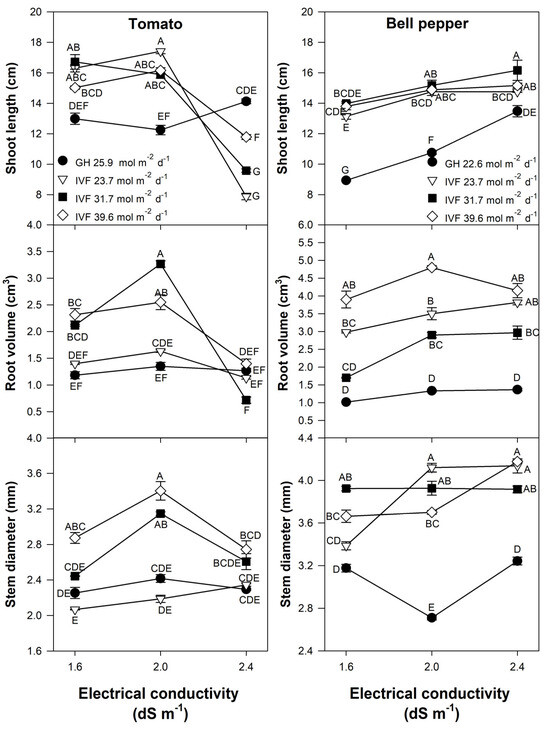
Figure 7.
Effects of the daily light integral, provided through natural light under greenhouse conditions (GH) or through light-emitting diode (LED) lamps in an indoor vertical farm (IVF), and electrical conductivity (EC) of the nutrient solution on growth parameters (shoot length, root volume, and stem diameter) in tomato (Solanum lycopersicum L.) and bell pepper (Capsicum annuum L.) seedlings. Bars represent the standard error of the mean. Letters represent mean separation according to Tukey’s multiple comparison test with p < 0.05.
In tomatoes grown in the IVF, seedlings irrigated with nutrient solutions at an EC of 2.0 dS m−1 and exposed to a DLI of 31.7 or 39.6 mol m−2 d−1 developed the largest stem diameter. However, increasing the EC to 2.4 dS m−1 resulted in decreased diameter (Figure 7). The EC of the nutrient solution had no significant effect on stem thickness in seedlings grown under 23.7 mol m−2 d−1 or in a greenhouse. Bell pepper seedlings in the IVF had a higher stem diameter than those grown under natural light in the greenhouse (Figure 7). When cultivated under a DLI 23.7 or 39.6 mol m−2 d−1, stem thickness increased as the EC increased. In contrast, greenhouse-grown seedlings did not exceed the stem diameter of those cultivated under artificial lighting (Figure 7). Hwang et al. [46] examined the effects of various DLIs, reporting that stem thicknesses greater than 2.5 mm in tomato and 2.8 mm in pepper seedlings were observed under DLI ranges of 18.0 to 21.6 mol m−2 d−1, and 10.8 to 21.6 mol m−2 d−1, respectively. The present study’s results exceeded those diameters reported by Hwang et al. [46], where tomato and bell pepper reached 3.40 mm and 4.14 mm, respectively. Ding et al. [52] reported that pepper seedlings fertigated with nutrient solutions at 2.9 dS m−1 reached a stem diameter of 9 mm, which was greater than those recorded in the present study. Additionally, in cherry tomato seedlings, Fan et al. [53] showed that the highest assessed PPFD (550 µmol m−2 s−1) was associated with increased stem diameter, indicating that stem thickness is positively correlated with high light intensity. Similar trends were observed in the present study, as larger stem diameters in tomato were obtained with the higher light intensities that we used to achieve 31.7 and 39.6 mol m−2 d−1, provided that the EC was 2.0 dS m−1. In bell pepper, however, no significant DLI effect was observed at high EC levels.
Compared to greenhouse-produced plant material, IVF-grown tomato seedlings had longer shoots when the EC was 1.6 or 2.0 dS m−1, regardless of DLI (Figure 7). However, increasing the solution EC to 2.4 dS m−1 led to a significant reduction in the length of indoor-developed seedlings, which were even shorter than those grown in the greenhouse (Figure 7). Greenhouse-grown bell pepper seedlings had shorter shoots than those cultivated in the IVF (Figure 7). Unlike tomato, increasing EC significantly increased shoot length in both greenhouse and IVF-grown seedlings, regardless of DLI. Seedlings developed in the IVF showed that the DLI had no significant effect on the shoot length in both tomato and bell pepper seedlings. Similar results were reported by Ke et al. [33] in dwarf tomatoes, where after nine days of treatment, a DLI ranging from 17.28 to 40.32 mol m−2 d−1 had no effect on stem length. However, other studies have shown contrasting results. Hwang et al. [46] reported that the longest stems in tomato seedlings (15 cm) were obtained with a DLI of 14.4 mol m−2 d−1, using LED lights with a PPFD of 200 µmol m−2 s−1 and a 20 h photoperiod; meanwhile, red pepper seedlings reached 12 cm in length with DLIs ranging from 7.2 to 14.4 mol m−2 d−1 under the same photoperiod but with a PPFD of 100 to 200 µmol m−2 s−1. Standard commercial practices prefer seedlings of compact growth, with optimal shoot and root development. However, authors differ in their recommendations for adequate seedling size. According to Agehara and Leskovar [1,2], bell pepper seedling length should range from 10.2 to 11.4 cm, while for tomatoes, it ranges from 9.5 to 11.5 cm. In contrast, Kim and Hwang [54] suggested that high-quality tomato seedlings should measure between 13 and 15 cm in height, while Carballo et al. [55] reported an ideal length of 13 to 16 cm for bell pepper. In addition to shoot length, high-quality tomato seedlings should have ‘sturdy’ stems with a minimum diameter of 3 mm, uniform leaf size, three to four leaves, excellent root development, and a dark green color [54]. For bell pepper seedlings, the minimum stem diameter should also be 3 mm, with at least two well-developed true leaves and a minimum root length of 5.6 cm [55]. Our results indicate that to obtain tomato seedlings with a stem length < 12.0 cm under LED lighting, the EC should be 2.4 dS m−1, whereas for bell pepper seedlings, the shortest length was observed at an EC of 1.6 dS m−1.
Blue light has been demonstrated to promote the development of shorter stems, while red light has been associated with the elongation of plants [56], and to achieve a more compact seedling morphology, the proportion of blue light in LED lamps should be increased from 27% to 61% of the total spectrum, while red light should be reduced accordingly to decrease stem elongation [57]. Jin et al. [58] observed that as the proportion of red LED light decreased, the height of cucumber (Cucumis sativus L.) seedlings decreased. The authors reported that the highest height was 6.22 cm 19 days after sowing when red light comprised 90% of the total spectrum and blue light comprised the remaining 10%. In a separate study, increasing the proportion of blue LED light by 61% resulted in a 55% reduction in plant height and a more compact morphology in tomatoes [57]. Studies have also shown that blue light in the range of 26% to 66% caused reduced lettuce (Lactuca sativa L.) shoot biomass and leaf area, while leaf thickness was increased [59]. In line with the previous report, spinach plants under a higher blue-to-red light ratio (3:1) also resulted in decreased leaf length and width, as well as lower biomass accumulation [60]. According to Izzo et al. [59], this effect of blue light is driven by a developmental limitation in radiation capture by the leaves as the epidermal cell area is decreased in response to blue light. These reports may explain why tomato and bell pepper seedlings in the present study had longer stems compared to those grown under natural lighting in the greenhouse, as the LED lamps we used contained only 12.6% of blue light, whereas the red light was at 61.3%. Our results are in line with reports by Son et al. [61], indicating that the stems of tomato seedlings were taller when the blue light proportion, in relation to red light, was low (13%, 26%, or 35%), and by Nasiri et al. [62], who reported that tomato seedlings showed increased height when blue light was 33% and were shorter when it was at 66%.
The decreased growth and biomass of tomato seedlings cultivated in the greenhouse may also be attributed to the elevated daytime temperatures observed during the study (Table 1). The findings of Gupta et al. [63] demonstrated that elevated root media temperatures expedite seed germination, while Hwang et al. [46] indicated that a daytime temperature of 27 °C results in a reduction in dry weight in tomato seedlings compared to those cultivated at 23 or 25 °C; this decrease in dry weight is exacerbated under higher photon flux densities. However, the authors also indicated that bell pepper seedlings exhibited little response to temperatures between 23 and 27 °C. [46].
3.5. Nutrient Status
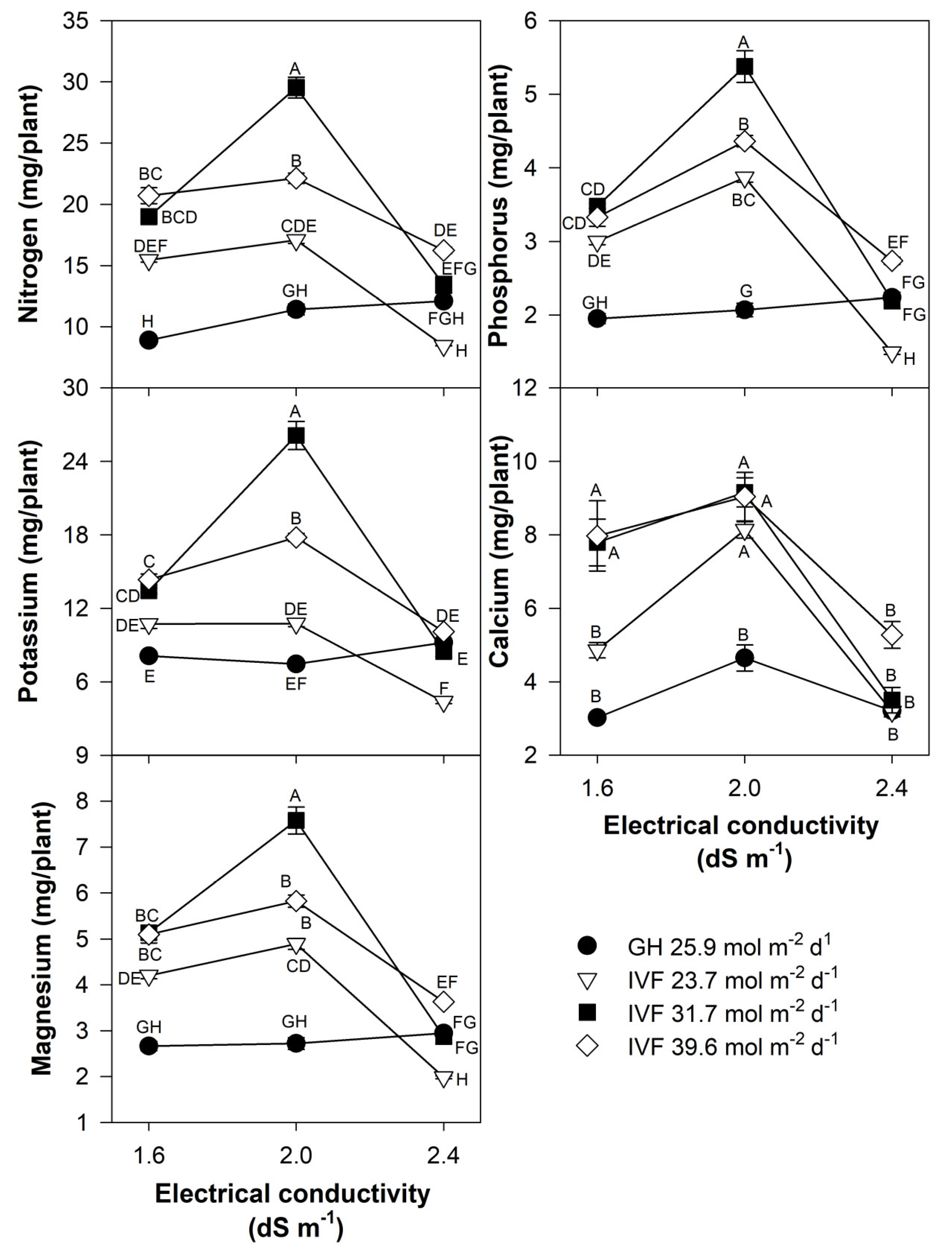
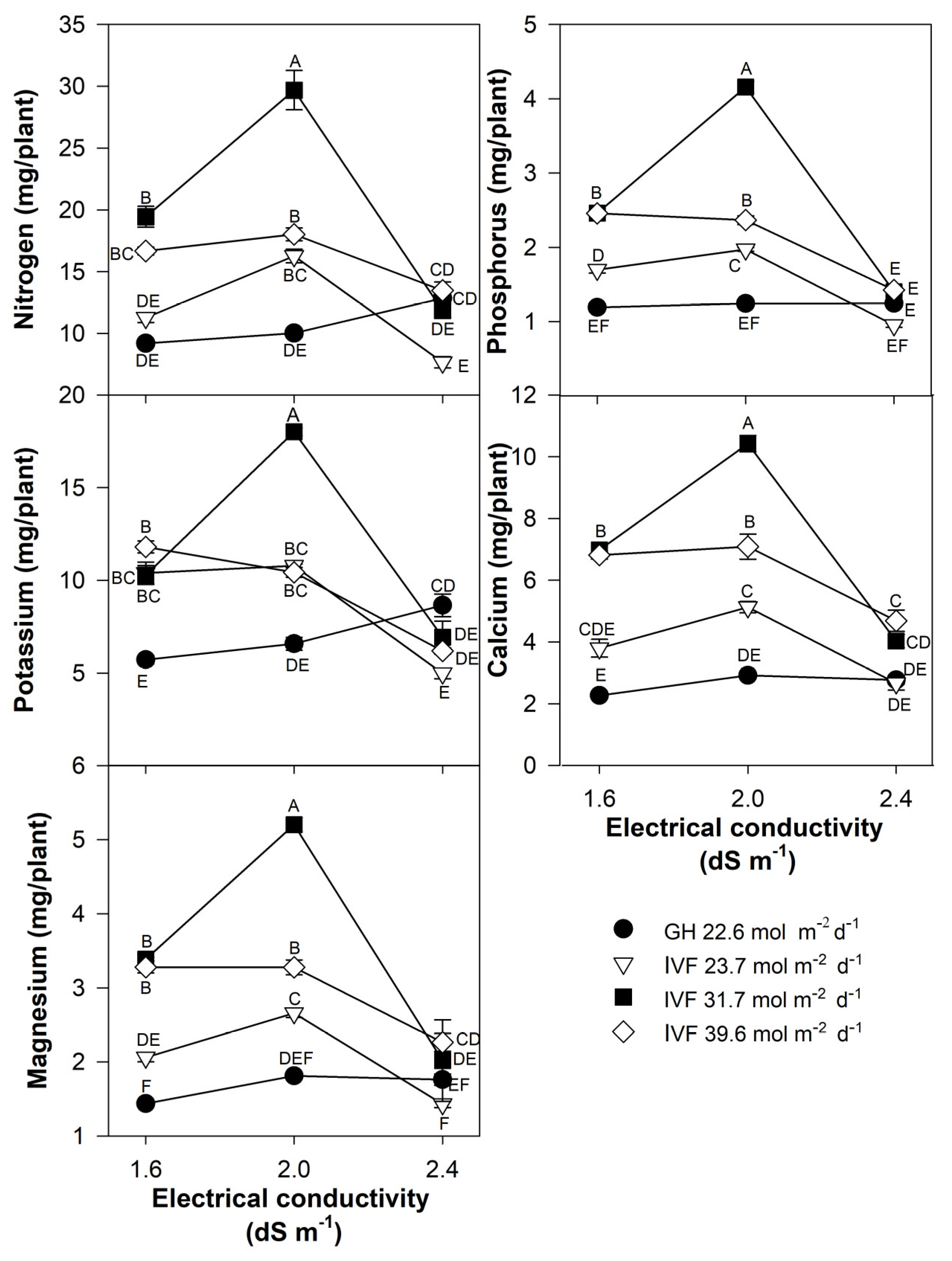
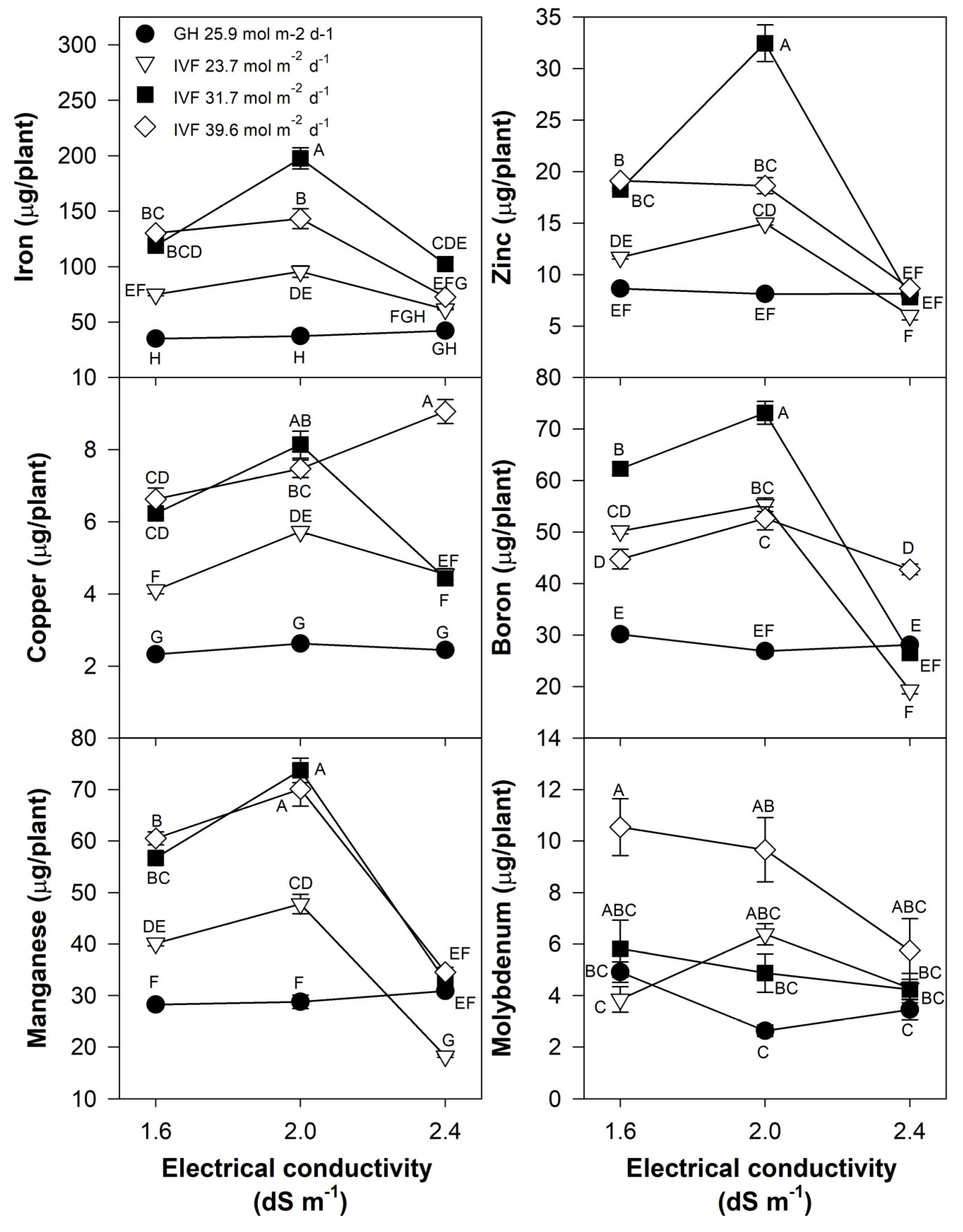
The concentrations of N, P, K, Mg, Mn, and Zn in tomato (Table 4) and bell pepper (Table 5) seedlings showed similar trends, with lower nutrient concentrations in plants grown in the IVF compared to those in the greenhouse. A similar trend was observed for B in tomato (Table 4) and Cu in bell pepper (Table 5). Calcium concentrations remained unchanged in bell pepper (Table 5), whereas in tomato, they decreased when seedlings were cultivated in the IVF under 31.7 mol m−2 d−1 (Table 4). Irrigation with a solution of 2.4 dS m−1 resulted in a reduction in K, Ca, Mg, B, Mn, and Zn in tomato (Table 4), whereas in bell pepper, P, B, and Zn concentrations decreased (Table 5). Micronutrients, including Cu and Fe in tomato (Table 4) and B and Fe in bell pepper (Table 5), demonstrated the opposite trend, showing increased concentrations in seedlings cultivated in the IVF. The treatments applied had no effect on Mo levels in either tomato or bell pepper.

Table 4.
Effect of the daily light integral (DLI; mol m−2 d−1) and electrical conductivity (EC; dS m−1) of the nutrient solution on nutrient concentration in tomato (Solanum lycopersicum L.) seedlings grown under natural light in the greenhouse (GH) or under artificial lighting in an indoor vertical farm (IVF).

Table 5.
Effect of the daily light integral (DLI; mol m−2 day−1) and electrical conductivity (EC; dS m−1) of the nutrient solution on nutrient concentration in bell pepper (Capsicum annuum L.) seedlings grown under natural light in the greenhouse (GH) or under artificial lighting in an indoor vertical farm (IVF).
The response of bell pepper seedlings to DLI is reported to be influenced by nutrient supply, particularly N. At a low DLI of 14.4 mol m−2 d−1, plant growth necessitated elevated N concentrations of 15 mmol L−1. Conversely, at a high DLI of 21.6 mol m−2 d−1, a reduced N concentration of 7.5 mmol L−1 sufficed [23]. The findings of our current study support this, as the biomass accumulation in bell pepper increased with higher DLI, contingent upon appropriate nutrient supply via high-EC nutrient solutions (2.4 dS m−1) (Figure 5), whereas tomato exhibited similar responses with solutions of 2.0 dS m−1 (Figure 4).
Despite the improved growth of seedlings produced in the IVF, there were significant decreases in nutrient concentration. Currey et al. [64] reported similar reductions in greenhouse-grown parsley [Petroselinum crispum (Mill.) Fuss], cilantro (Coriandrum sativum L.), and dill (Anethum graveolens L.), where K decreased by 15%, 16%, and 20%, respectively, under high-DLI conditions (18.0 mol m−2 d−1) when compared to a low DLI (7.0 mol m−2 d−1). However, increases in other nutrients, such as Ca, Cu, and P, were also noted. Likewise, lettuce cultivated in indoor systems under LED lighting had reduced NO3− concentrations when DLI increased from 5.04 or 7.56 mol m−2 d−1 to 15.12 mol m−2 d−1 [65]. This reduction was attributed to an enhancement in the activity of nitrate reductase [65,66], a key enzyme in N metabolism for NO3− assimilation. Walters and Currey [67] demonstrated that increasing the DLI had a diminishing effect on nutrient concentration in three species of basil (Ocimium sp.). However, the authors noted that the total nutrient content in plants grown under a high DLI was greater than that of plants grown under a low DLI. Currey et al. [64] found that biomass and tissue “nutrient content” for all micro- and macronutrients increased with DLI, so that the observed decrease in K and Mn concentrations at high DLIs reflects a “dilution effect” where nutrient content and growth increase, but not in a proportional manner.
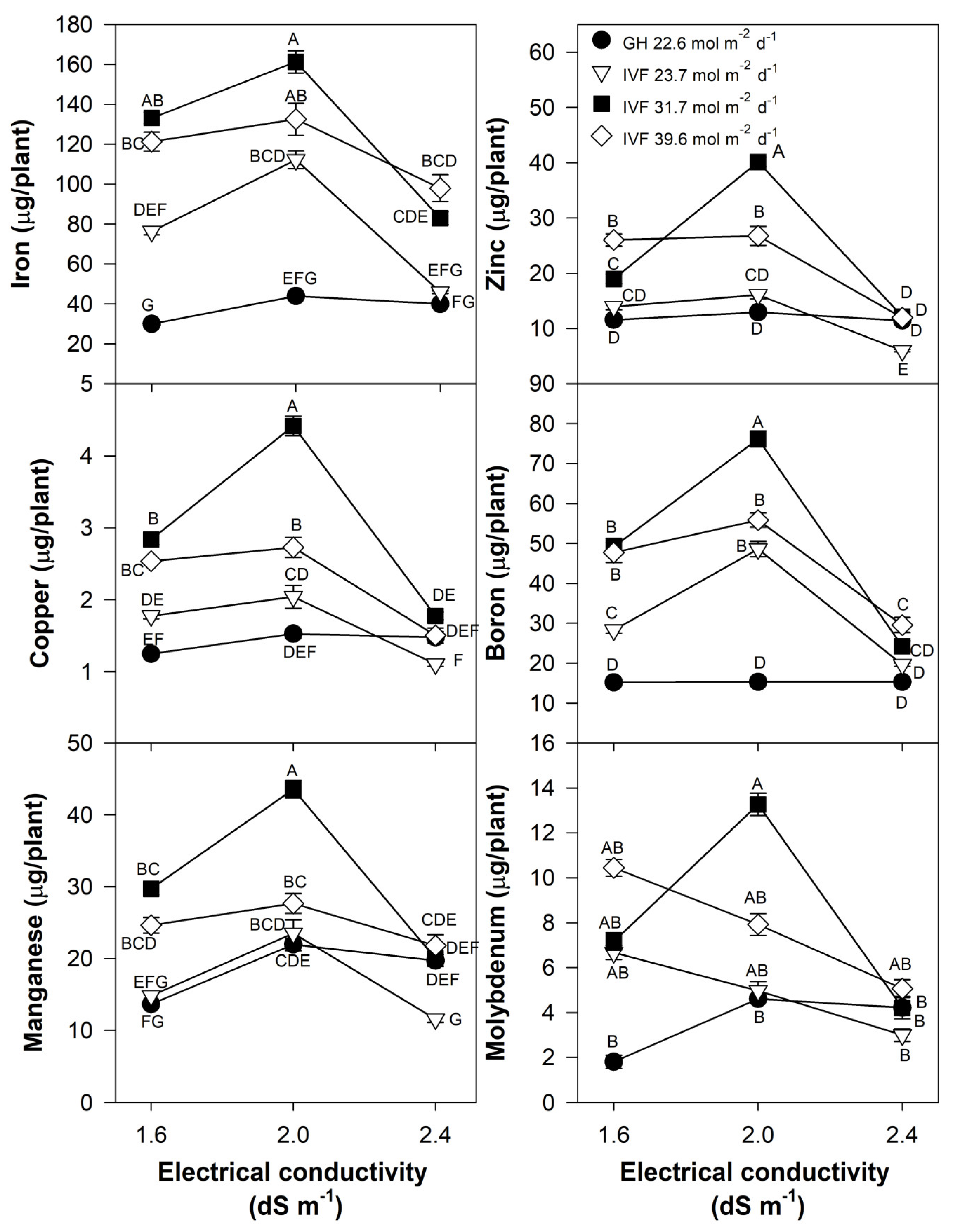
The present findings align with previous studies, indicating that the reduced nutrient concentration in seedlings grown in the IVF was linked to their increased growth under favorable environmental conditions, resulting in the dilution of nutrients within plant tissues. The total nutrient content of seedlings indicated that tomato plants exhibited the highest contents of N, P, Ca, and Mg when cultivated in the IVF, irrespective of the DLI, except under conditions of the highest EC in the nutrient solution (Figure 8). A similar trend was observed for K, but only at a DLI of 31.7 or 39.6 mol m−2 d−1. In bell pepper, a comparable pattern occurred, although N and Ca contents were similar between greenhouse plants and those grown under the lowest DLI (Figure 9). The highest macronutrient content was recorded in transplants irrigated with solutions at 2.0 dS m−1 and a DLI of 31.7 mol m−2 d−1 for both tomato (Figure 8) and bell pepper (Figure 9). Micronutrients such as Fe, Zn, Cu, B, and Mn followed a similar trend, reaching peak concentrations when plants were irrigated with solutions at 2.0 dS m−1 and a DLI of 31.7 mol m−2 d−1 (Figure 10 and Figure 11).
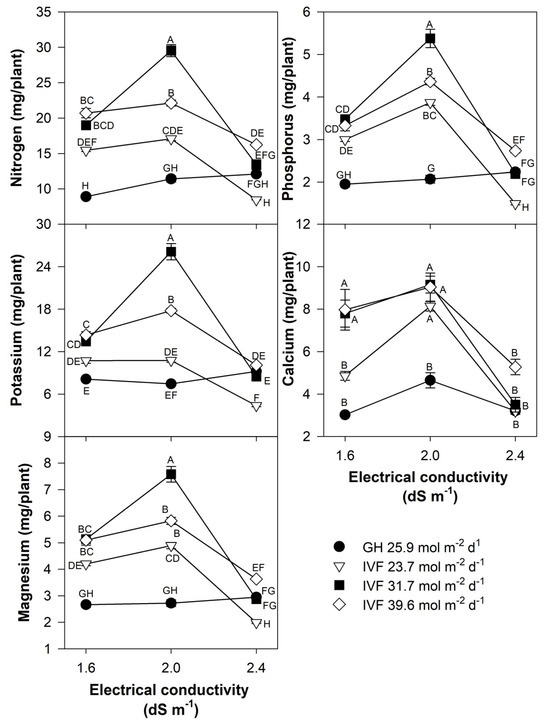
Figure 8.
Effects of the daily light integral, provided through natural light under greenhouse conditions (GH) or through light-emitting diode lamps in an indoor vertical farm (IVF), and electrical conductivity of the nutrient solution on macronutrient (nitrogen, potassium, magnesium, phosphorus, and calcium) content in tomato (Solanum lycopersicum L.) seedlings. Bars represent the standard error of the mean. Letters represent mean separation according to Tukey’s multiple comparison test with p < 0.05.
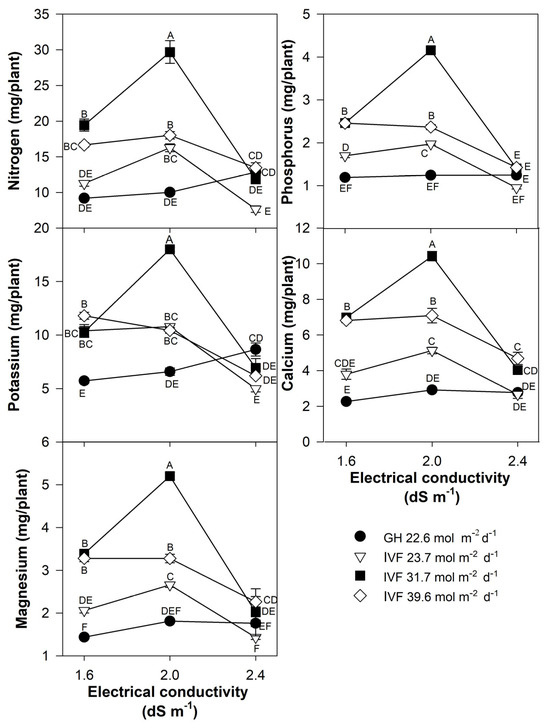
Figure 9.
Effects of the daily light integral, provided through natural light under greenhouse conditions (GH) or through light-emitting diode lamps in an indoor vertical farm (IVF), and electrical conductivity of the nutrient solution on macronutrient (nitrogen, potassium, magnesium, phosphorus, and calcium) content in bell pepper (Capsicum annuum L.) seedlings. Bars represent the standard error of the mean. Letters represent mean separation according to Tukey’s multiple comparison test with p < 0.05.
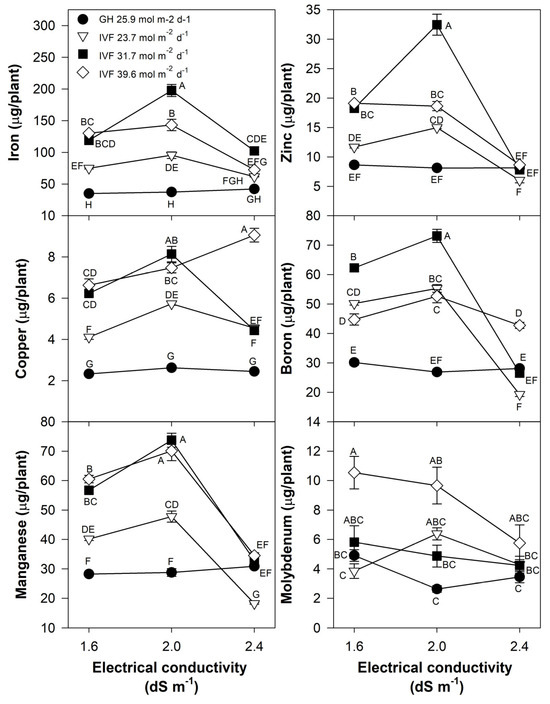
Figure 10.
Effects of the daily light integral, provided through natural light under greenhouse conditions (GH) or through light-emitting diode lamps in an indoor vertical farm (IVF), and electrical conductivity of the nutrient solution on micronutrient (iron, copper, manganese, zinc, boron, and molybdenum) content in tomato (Solanum lycopersicum L.) seedlings. Bars represent the standard error of the mean. Letters represent mean separation according to Tukey’s multiple comparison test with p < 0.05.
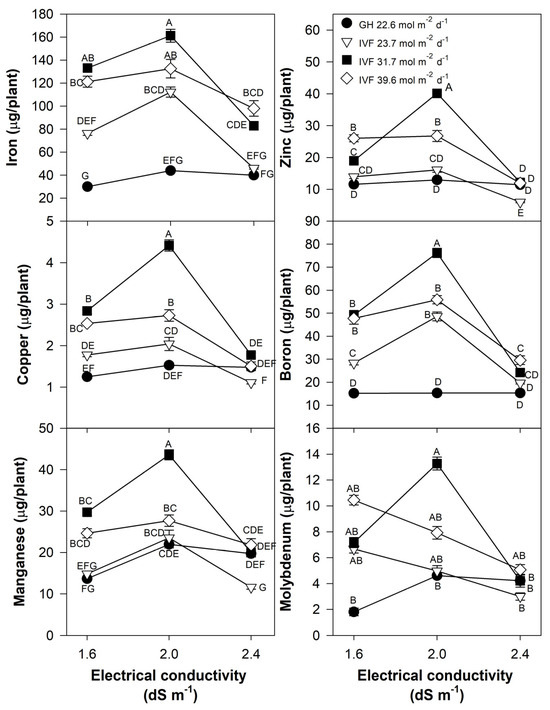
Figure 11.
Effects of the daily light integral, provided through natural light under greenhouse conditions (GH) or through light-emitting diode lamps in an indoor vertical farm (IVF), and electrical conductivity of the nutrient solution on micronutrient (iron, copper, manganese, zinc, boron, and molybdenum) content in bell pepper (Capsicum annuum L.) seedlings. Bars represent the standard error of the mean. Letters represent mean separation according to Tukey’s multiple comparison test with p < 0.05.
4. Conclusions
Tomato and bell pepper seedlings cultivated in the IVF exhibited higher biomass accumulation. Specifically, for tomato, the shoot-to-root ratio decreased in seedlings grown indoors. The DLI significantly influenced seedling growth, with optimal DLI levels of 31.7 mol m−2 d−1 for tomato and 39.6 mol m−2 d−1 for bell pepper. However, a DLI of 23.7 mol m−2 d−1 was enough to cause an average total dry weight increase of 153% and 264% in tomato and bell pepper seedlings, respectively, compared to those from the greenhouse. Nutrient requirements were met when tomato seedlings were irrigated with solutions of 2.0 dS m−1, whereas bell pepper seedlings showed enhanced growth even at 2.4 dS m−1. Although nutrient concentrations decreased in the IVF-grown seedlings due to a dilution effect, total nutrient content per plant was higher in IVF-grown seedlings than in those cultivated in the greenhouse.
Author Contributions
Conceptualization, D.Y.A.-A., D.A.-C. and L.A.V.-A.; Data curation, D.Y.A.-A. and L.A.V.-A.; Formal analysis, D.Y.A.-A., L.A.V.-A. and L.d.A.A.S.-M.; Funding acquisition, D.A.-C. and A.D.C.; Investigation, D.Y.A.-A., D.A.-C., L.A.V.-A. and L.d.A.A.S.-M.; Methodology, D.Y.A.-A., D.A.-C., L.A.V.-A., D.L.C. and L.d.A.A.S.-M.; Project administration, D.A.-C. and L.A.V.-A.; Resources, D.A.-C., A.D.C. and D.L.C.; Software, A.D.C. and D.L.C.; Supervision, D.A.-C. and L.A.V.-A.; Validation, A.D.C. and D.L.C.; Visualization, D.A.-C., L.A.V.-A., A.D.C. and L.d.A.A.S.-M.; Writing—original draft, D.Y.A.-A., D.A.-C., L.A.V.-A. and L.d.A.A.S.-M.; Writing—review and editing, A.D.C., D.L.C. and L.d.A.A.S.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We gratefully thank the T.R. Ellett Agricultural Research Trust for the financial support of Andrew D. Cartmill and Karma Verde Fresh for material donations for conducting the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Agehara, S.; Leskovar, D.I. Growth suppression by exogenous abscisic acid and uniconazole for prolonged marketability of bell pepper transplants in commercial conditions. Sci. Hortic. 2015, 194, 118–125. [Google Scholar] [CrossRef]
- Agehara, S.; Leskovar, D.I. Growth suppression by exogenous abscisic acid and uniconazole for prolonged marketability of tomato transplants in commercial conditions. HortScience 2017, 52, 606–611. [Google Scholar] [CrossRef]
- Carrera, C.G.; Calleja, J.C.; Pernas, M.; Gómez, L.; Oñate, S.L. An updated overview on the regulation of seed germination. Plants 2020, 9, 703. [Google Scholar] [CrossRef]
- Garcia, C.; Lopez, R.G. Supplemental radiation quality influences cucumber, tomato, and pepper transplant growth and development. HortScience 2020, 55, 804–811. [Google Scholar] [CrossRef]
- Liriano, G.R.; Terán, R.M.A.; Núñez, S.D.B.; Ibañez, M.D.; Pérez, R.J. Worm humus in the production of seedlings of Lycopersicon esculentum Mill in a community of Cojedes State, Venezuela. Cent. Agrícola 2017, 44, 23–29. Available online: http://scielo.sld.cu/pdf/cag/v44n4/cag04417.pdf (accessed on 27 January 2025).
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 2021, 41, 742–780. [Google Scholar] [CrossRef]
- Avgoustaki, D.D.; Xydis, G. Plant factories in the water-food-energy Nexus era: A systematic bibliographical review. Food Secur. 2020, 12, 253–268. [Google Scholar] [CrossRef]
- Van Delden, S.H.; SharathKumar, M.; Butturini, M. Current status and future challenges in implementing and upscaling vertical farming systems. Nat. Food 2021, 2, 944–956. [Google Scholar] [CrossRef]
- de Carbonnel, M.; Stormonth-Darling, J.M.; Liu, W.; Kuziak, D.; Jones, M.A. Realising the environmental potential of vertical farming systems through advances in plant photobiology. Biology 2022, 11, 922. [Google Scholar] [CrossRef]
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Avendaño-Abarca, V.H.; Alvarado-Camarillo, D.; Valdez-Aguilar, L.A.; Sánchez-Ortíz, E.A.; González-Fuentes, J.A.; Cartmill, A.D. Response of strawberry to the substitution of blue light by green light in an indoor vertical farming system. Agronomy 2023, 13, 99. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Agarwal, A. Artificial lighting system for plant growth and development: Chronological advancement, working principles, and comparative assessment. In Light Emitting Diodes for Agriculture: Smart Lighting; Dutta Gupta, S., Ed.; Springer: Singapore, 2017; pp. 1–25. [Google Scholar] [CrossRef]
- Hernandez, R.; Kubota, C. Tomato seedling growth and morphological responses to supplemental LED lighting red: Blue ratios under varied daily solar light integrals. Acta Hortic. 2012, 956, 187–194. [Google Scholar] [CrossRef]
- Alvarado-Camarillo, D.; Valdez-Aguilar, L.A.; Cartmill, D.L.; Cartmill, A.D. Strawberry grown in an indoor vertical farm responds to increased photosynthetic photon flux density when calcium is supplied at higher concentrations. HortScience 2024, 59, 1806–1814. [Google Scholar] [CrossRef]
- Torres, A.P.; Lopez, R.G. Photosynthetic daily light integral during propagation of Tecoma stans influences seedling rooting and growth. HortScience 2011, 46, 282–286. [Google Scholar] [CrossRef]
- Faust, J.E.; Logan, J. Daily light integral: A research review and high-resolution maps of the United States. HortScience 2018, 53, 1250–1257. [Google Scholar] [CrossRef]
- Poorter, H.; Fiorani, F.; Pieruschka, R.; Wojciechowski, T.; van der Putten, W.H.; Kleyer, M.; Uli, S.; Postma, J. Pampered inside, pestered outside? Differences and similarities between plants growing in controlled conditions and in the field. New Phytol. 2016, 212, 838–855. [Google Scholar] [CrossRef]
- Mayorga-Gomez, A.M.; Van Iersel, M.W.; Ferrarezi, R.S. Lowering the target daily light integrals following days with excessive lighting can reduce lettuce production costs. Front. Plant Sci. 2024, 15, 1467443. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, L.; Dai, J.; Liu, Y.; Lin, D.; Yang, Y. Morphological and physiological responses of cucumber seedlings to different combinations of light intensity and photoperiod with the same daily light integral. HortScience 2021, 56, 1430–1438. [Google Scholar] [CrossRef]
- Torres, A.P.; Lopez, R.G. Measuring Daily Light Integral in a Greenhouse; Purdue Extension: West Lafayette, IN, USA, 2010; p. 7. Available online: www.extension.purdue.edu/extmedia/ho/ho-238-w.pdf (accessed on 15 January 2025).
- Kosai, T. Plant production process, floor plan, and layout of PFAL. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Kosai, T., Niu, G., Takagaki, M., Eds.; Academic Press: Amsterdam, The Netherlands, 2020; pp. 261–271. [Google Scholar] [CrossRef]
- Aguirre-Becerra, H.; García, T.F.; Vázquez, H.C.; Alvarado, M.A.; Feregrino, P.A.A.; Guevara, G.G.R.; Contreras, M.M. Effect of extended photoperiod with a fixed mixture of light wavelengths on tomato seedlings. Hortscience 2020, 55, 1832–1839. [Google Scholar] [CrossRef]
- Yan, Z.; Cao, X.; Bing, L.; Lin, D.; Cheng, F.; Wang, K.; Qi, Y.; Yang, Y. Assessment of the growth and quality of pepper seedlings under the combinations of daily light integral and nitrogen concentration. Hortic. Environ. Biotechnol. 2025, 66, 331–346. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, J.; Ju, J.; Hu, Y.; Liu, X.; He, R.; Song, J.; Huang, Y.; Liu, H. The impact of daily light integral from artificial lighting on tomato seedling cultivation in plant factory. Agronomy 2024, 15, 70. [Google Scholar] [CrossRef]
- Morgan, L. Daily Light Integral (DLI) and Greenhouse Tomato Production. Available online: www.spring-lake.net/pdfs/light-temp/DLI-tomato.pdf (accessed on 15 March 2025).
- Avgoustaki, D.D.; Vatsika, G.; Giakoumatos, A.; Bartzanas, T. How different daily light integrals and spectral treatments influence the development of Valerianella locusta plants grown in an indoor vertical farm. Sci. Horticul. 2024, 332, 113044. [Google Scholar] [CrossRef]
- Gavhane, K.P.; Hasan, M.; Singh, D.K.; Kumar, S.N.; Sahoo, R.N.; Alam, W. Determination of optimal daily light integral (DLI) for indoor cultivation of iceberg lettuce in an indigenous vertical hydroponic system. Sci. Rep. 2023, 13, 10923. [Google Scholar] [CrossRef]
- Hosseini, H.; Mozafari, V.; Roosta, H.R.; Shirani, H.; van de Vlasakker, P.C.; Farhangi, M. Nutrient use in vertical farming: Optimal electrical conductivity of nutrient solution for growth of lettuce and basil in hydroponic cultivation. Horticulturae 2021, 7, 283. [Google Scholar] [CrossRef]
- Soltanpour, P.N.; Johnson, G.W.; Workman, S.M.; Jones, J.B.; Miller, R.O. Inductively coupled plasma emission spectrometry and inductively coupled plasma mass spectrometry. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of North America: Madison, WI, USA, 1996; pp. 91–139. [Google Scholar]
- Bremner, J.M. Total nitrogen. In Methods of Soil Analysis. Part II. Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1085–1086. [Google Scholar]
- Kozai, T. Closed systems with lamps for high quality transplant production at low costs using minimum resources. In Plant Tissue Culture Engineering; Gupta, S.D., Ibaraki, Y., Eds.; Spinger: Dordrecht, The Netherlands, 2008; pp. 275–312. [Google Scholar] [CrossRef]
- Chen, L.L.; Zhang, K.; Gong, X.C.; Wang, H.Y.; Gao, Y.H.; Wang, X.Q.; Zeng, Z.; Hu, Y.G. Effects of different LEDs light spectrum on the growth, leaf anatomy, and chloroplast ultrastructure of potato plantlets in vitro and minituber production after transplanting in the greenhouse. J. Integr. Agric. 2020, 19, 108–119. [Google Scholar] [CrossRef]
- Ke, X.; Yoshida, H.; Hikosaka, S.; Goto, E. Optimization of photosynthetic photon flux density and quality for increasing radiation- use efficiency in dwarf tomato under led light at the vegetative growth stage. Plants 2022, 11, 121. [Google Scholar] [CrossRef]
- Bonarota, M.S.; Kosma, D.K.; Barrios-Masias, F.H. Salt tolerance mechanisms in the Lycopersicon clade and their trade-offs. AoB Plants 2022, 14, plab072. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.M.; Fontes, P.C.R.; Milagres, C.D.C.; de Abreu, J.A.A. Yield and nitrogen use efficiency of bell pepper grown in SLAB fertigated with different nitrogen rates. J. Plant Nutr. 2020, 43, 2833–2843. [Google Scholar] [CrossRef]
- Savvas, D.; Ntatsi, G.; Passam, H.C. Plant nutrition and physiological disorders in greenhouse grown tomato, pepper and eggplant. Eur. J. Plant Sci. Biotechnol. 2008, 2, 45–61. Available online: http://www.globalsciencebooks.info/Online/GSBOnline/images/0812/EJPSB_2(SI1)/EJPSB_2(SI1)45-61o.pdf (accessed on 12 February 2025).
- Molinero-Rosales, N.; Latorre, A.; Jamilena, M.; Lozano, R. SINGLE FLOWER TRUSS regulates the transition and maintenance of flowering in tomato. Planta 2004, 218, 427–434. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.I.; Heuvelink, E.; van Ieperen, W.; Vreugdenhil, D.; Millenaar, F.F. Continuous Light as a Way to Increase Greenhouse Tomato Production: Expected Challenges. Acta Hortic. 2012, 956, 51–57. [Google Scholar] [CrossRef]
- Lanoue, J.; Thibodeau, A.; Little, C.; Zheng, J.; Grodzinski, B.; Hao, X. Light spectra and root stocks affect response of greenhouse tomatoes to long photoperiod of supplemental lighting. Plants 2021, 10, 1674. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.; Gómez, C. Effects of daily light integral on compact tomato plants grown for indoor gardening. Agronomy 2022, 12, 1704. [Google Scholar] [CrossRef]
- Steed, G.; Ramirez, D.C.; Hannah, M.A.; Webb, A.A. Chronoculture, harnessing the circadian clock to improve crop yield and sustainability. Science 2021, 372, eabc9141. [Google Scholar] [CrossRef]
- Xiang, Y.; Sapir, T.; Rouillard, P.; Ferrand, M.; Jiménez-Gómez, J.M. Interaction between photoperiod and variation in circadian rhythms in tomato. BMC Plant Biol. 2022, 22, 187. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.A.; Wijnen, C.L.; Srinivasan, A.; Ryngajllo, M.; Ofner, I.; Lin, T.; Ranjan, A.; West, D.; Maloof, J.N.; Sinha, R.; et al. Domestication selected for deceleration of the circadian clock in cultivated tomato. Nat. Genet. 2016, 48, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Veléz-Ramírez, A.I. Continuous Light in Tomato. From Gene to Yield. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 19 September 2014. [Google Scholar] [CrossRef]
- Lanoue, J.; St. Louis, S.; Little, C.; Hao, X. Photosynthetic adaptation strategies in peppers under continuous lighting: Insights into photosystem protection. Front. Plant Sci. 2024, 15, 1372886. [Google Scholar] [CrossRef]
- Hwang, H.; An, S.; Pham, M.D.; Cui, M.; Chun, C. The combined conditions of photoperiod, light intensity, and air temperature control the growth and development of tomato and red pepper seedlings in a closed transplant production system. Sustainability 2020, 12, 9939. [Google Scholar] [CrossRef]
- Miyama, Y. Effects of different light sources on shoot to root ratio and intumescence incidence in tomato seedlings grown in a commercial closed seedling production system. Int. J. Hortic. Sci. Technol. 2022, 10, 1–8. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Sutulienė, R.; Laužikė, K.; Duchovskis, P.; Małek, S. Effect of different ratios of blue and red led light on brassicaceae microgreens under a controlled environment. Plants 2021, 10, 801. [Google Scholar] [CrossRef]
- Lanoue, J.; Leonardos, E.D.; Grodzinski, B. Effects of light quality and intensity on diurnal patterns and rates of photo-assimilate translocation and transpiration in tomato leaves. Front. Plant Sci. 2018, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Miotto, Y.E.; da Costa, C.T.; Offringa, R.; Kleine-Vehn, J.; dos Santos, M.F. Effects of light intensity on root development in a D-root growth system. Front. Plant Sci. 2021, 12, 778382. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ding, Y.; Wang, S.; Song, C.; Wang, F. Growth and development responses of the rhizome-root system in (Pleioblastus pygmaeus) to light intensity. Plants 2022, 11, 2204. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Li, J.; Xie, J.; Li, N.; Bakpa, E.P.; Han, K.; Yang, Y.; Wang, C. Exogenous zeaxanthin alleviates low temperature combined with low light induced photosynthesis inhibition and oxidative stress in pepper (Capsicum annuum L.) Plants. Curr. Issues Mol. Biol. 2022, 44, 2453–2471. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.X.; Xu, Z.G.; Liu, X.Y.; Tang, C.M.; Wang, L.W.; Han, X.L. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Kim, H.; Hwang, S. The growth and development of ‘mini chal’ tomato plug seedlings grown under various wavelengths using light emitting diodes. Agronomy 2019, 9, 157. [Google Scholar] [CrossRef]
- Carballo, M.F.J.; Urrestarazu, M.; Rodríguez, O.J.C.; Morales, I. Electrical conductivity of the nutrient solution on the vegetative propagation of bell pepper and tomato. Cienc. Rural. 2021, 3, 2. [Google Scholar] [CrossRef]
- Utasi, L.; Kovacs, V.; Gulyas, Z.; Marcek, T.; Janda, T.; Darko, E. Threshold or not: Spectral composition and light-intensity dependence of growth and metabolism in tomato seedlings. Sci. Hortic. 2023, 313, 111946. [Google Scholar] [CrossRef]
- Kalaitzoglou, P.; Taylor, C.; Calders, K.; Hogervorst, M.; Van, W.; Harbinson, J.; Visser, P.; Nicole, C.C.S.; Marcelis, L.F.M. Unraveling the effects of blue light in an artificial solar background light on growth of tomato plants. Environ. Exp. Bot. 2021, 184, 104377. [Google Scholar] [CrossRef]
- Jin, D.; Su, X.; Li, Y.; Shi, M.; Yang, B.; Wan, W.; Wen, X.; Yang, S.; Ding, X.; Zou, J. Effect of red and blue light on cucumber seedlings grown in a plant factory. Horticulturae 2023, 9, 124. [Google Scholar] [CrossRef]
- Izzo, L.G.; Mickens, M.A.; Aronne, G.; Gómez, C. Spectral effects of blue and red light on growth, anatomy, and physiology of lettuce. Physiol. Plant. 2021, 172, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Vaštakaitė-Kairienė, V.; Brazaitytė, A.; Miliauskienė, J.; Runkle, E.S. Red to blue light ratio and iron nutrition influence growth, metabolic response, and mineral nutrients of spinach grown indoors. Sustainability 2022, 14, 12564. [Google Scholar] [CrossRef]
- Son, K.H.; Kim, E.Y.; Oh, M.M. Growth and development of cherry tomato seedlings grown under various combined ratios of red to blue LED lights and fruit yield and quality after transplanting. J. Bio-Environ. Control. 2018, 27, 54–63. [Google Scholar] [CrossRef]
- Nasiri, Z.; Ghasemi, K.; Bahmanyar, M.A.; Agehara, S. Effects of different LED light spectra and nutrient solution strength on the growth, photosynthetic parameters, and nutrients uptake in tomato seedlings. J. Plant Nutr. 2025, 48, 864–875. [Google Scholar] [CrossRef]
- Gupta, M.K.; Chandra, P.; Samuel, D.V.K.; Singh, B.; Singh, A.; Garg, M.K. Modeling of tomato seedling growth in greenhouse. Agric. Res. 2012, 1, 362–369. [Google Scholar] [CrossRef]
- Currey, C.J.; Walters, K.J.; Flax, N.J. Nutrient solution strength does not interact with the daily light integral to affect hydroponic cilantro, dill, and parsley growth and tissue mineral nutrient concentrations. Agronomy 2019, 9, 389. [Google Scholar] [CrossRef]
- Yan, Z.; He, D.; Niu, G.; Zhou, Q.; Qu, Y. Growth, nutritional quality, and energy use efficiency of hydroponic lettuce as influenced by daily light integrals exposed to white versus white plus red light-emitting diodes. HortScience 2019, 54, 1737–1744. [Google Scholar] [CrossRef]
- He, J.; Gan, J.H.S.; Qin, L. Productivity, photosynthetic light-use efficiency, nitrogen metabolism and nutritional quality of C4 halophyte Portulaca oleracea L. grown indoors under different light intensities and durations. Front. Plant Sci. 2023, 14, 1106394. [Google Scholar] [CrossRef]
- Walters, K.J.; Currey, C.J. Effects of nutrient solution concentration and daily light integral on growth and nutrient concentration of several basil species in hydroponic production. HortScience 2018, 53, 1319–1325. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).