Efficacy of Lippia alba Essential Oil in Alleviating Osmotic and Oxidative Stress in Salt-Affected Bean Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of Halotolerance and Seed-Priming Treatment
2.2. Plant Growth Conditions
2.3. Water Content of Soil, Tolerance Index and Collection of Plants
2.4. Analyses of Calcium, Chlorophylls, Proline and Carbohydrates in Plants
2.5. Enzymatic Activities
2.6. Statistical Analysis
3. Results
3.1. Threshold of Halotolerance During Seed Germination
3.2. Increase in Water Uptake and Salt Tolerance Index of Bean Plants
3.3. Effect of Salt Stress on Chlorophylls and Osmolytes Synthesis
3.4. Ca2+ Translocation and Activation of Salt Stress Responses
3.5. Activation of Enzymatic Defence System in Response to Salinity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oğuz, M.Ç.; Oğuz, E.; Güler, M. Seed priming with essential oils for sustainable wheat agriculture in semi-arid region. PeerJ 2023, 11, e15126. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, B.; Cen, Z.; Fu, Y.; Zhu, X.; He, H.; Kong, D.; Wu, H. The variation in essential oils composition, phenolic acids and flavonoids is correlated with changes in antioxidant activity during Cinnamomum loureirii bark growth. Arab. J. Chem. 2021, 14, 103249. [Google Scholar] [CrossRef]
- Terzić, D.; Tabaković, M.; Oro, V.; Poštić, D.; Štrbanović, R.; Filipović, V.R. Impact of essential oils on seed quality and seed-borne pathogens of Althea officinalis seeds of different ages. Chem. Biol. Technol. Agric. 2023, 10, 33. [Google Scholar] [CrossRef]
- Borromeo, I.; De Luca, A.; Domenici, F.; Giordani, C.; Rossi, L.; Forni, C. Antioxidant properties of Lippia alba essential oil: A potential treatment for oxidative stress-related conditions in plants and cancer cells. Int. J. Mol. Sci. 2024, 25, 8276. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Mikallou, M.; Petropoulos, S.; Tzortzakis, N. Profiling of essential oils components and polyphenols for their antioxidant activity of medicinal and aromatic plants grown in different environmental conditions. Agronomy 2020, 10, 727. [Google Scholar] [CrossRef]
- Garzoli, S.; Vaglia, V.; Iriti, M.; Vitalini, S. Vapor and liquid phase profiles of essential oils from Abies, Picea and Pinus species and their phytotoxic interactions with weed growth in pre- and post-emergence conditions. Plants 2023, 12, 1172. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Jaramillo, B.E.; Martínez, J.R. Comparison of different extraction methods for the analysis of volatile secondary metabolites of Lippia alba (Mill.) NE Brown, grown in Colombia, and evaluation of its in vitro antioxidant activity. J. Chromatogr. A 2004, 1025, 93–103. [Google Scholar] [CrossRef]
- Tucker, A.O.; DeBaggio, T. The Encyclopedia of Herbs: A Comprehensive Reference to Herbs of Flavor and Fragrance; Timber Press: Portland, OR, USA, 2009. [Google Scholar]
- Duke, J.A.; Bogenschutz-Godwin, M.J.; Ottesen, A.R. Duke’s Handbook of Medicinal Plants of Latin America; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Pezo-Pérez, N.L.; Gonzales-Coral, A. Caracterización agronómica de pampa orégano Lippia alba (Mill). Folia Amaz. 1998, 9, 179–190. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Giordani, C.; Casettari, L.; Curzi, G.; Cappellacci, L.; Petrelli, R.; Maggi, F. Acute and sub-lethal toxicity of eight essential oils of commercial interest against the filariasis mosquito Culex quinquefasciatus and the housefly Musca domestica. Ind. Crop. Prod. 2018, 112, 668–680. [Google Scholar] [CrossRef]
- Idouraine, A.; Weber, C.W.; Kohlhepp, E.A. Composition of tepary bean (Phaseolus acutifolius) of the southwestern US and northern Mexico. Ecol. Food Nutr. 1995, 33, 139–147. [Google Scholar] [CrossRef]
- Wolf, M. Plant Guide for Tepary Bean (Phaseolus acutifolius); USDA-Natural Resources Conservation Service, Tucson Plant Materials Center: Tucson, AZ, USA, 2018. [Google Scholar]
- Suárez, J.C.; Contreras, A.T.; Anzola, J.A.; Vanegas, J.I.; Rao, I.M. Physiological characteristics of cultivated tepary bean (Phaseolus acutifolius A. Gray) and its wild relatives grown at high temperature and acid soil stress conditions in the amazon region of Colombia. Plants 2022, 11, 116. [Google Scholar] [CrossRef]
- Ketehouli, T.; Idrice Carther, K.F.; Noman, M.; Wang, F.W.; Li, X.W.; Li, H.Y. Adaptation of plants to salt stress: Characterization of Na+ and K+ transporters and role of CBL gene family in regulating salt stress response. Agronomy 2019, 9, 687. [Google Scholar] [CrossRef]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S.; et al. Impacts of salinity stress on crop plants: Improving salt tolerance through genetic and molecular dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ response mechanisms to salinity stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, D.; Feng, N.; Zhou, H.; Mu, D.; Zhao, L.; Shen, X.; Rao, G.; Meng, F.; Huang, A. Physiological mechanisms of ABA-induced salinity tolerance in leaves and roots of rice. Sci. Rep. 2022, 12, 8228. [Google Scholar] [CrossRef]
- Rai, G.K.; Khanday, D.M.; Choudhary, S.M.; Kumar, P.; Kumari, S.; Martínez-Andújar, C.; Martinez-Melgarejo, P.A.; Rai, P.K.; Pérez-Alfocea, F. Unlocking nature’s stress buster: Abscisic acid’s crucial role in defending plants against abiotic stress. Plant Stress 2024, 11, 100359. [Google Scholar] [CrossRef]
- Mahmood, M.Z.; Odeibat, H.A.; Ahmad, R.; Gatasheh, M.K.; Shahzad, M.; Abbasi, A.M. Low apoplastic Na+ and intracellular ionic homeostasis confer salinity tolerance upon Ca2SiO4 chemigation in Zea mays L. under salt stress. Front. Plant Sci. 2024, 14, 1268750. [Google Scholar] [CrossRef]
- Hao, S.; Wang, Y.; Yan, Y.; Liu, Y.; Wang, J.; Chen, S. A review on plant responses to salt stress and their mechanisms of salt resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Zaman, W.; Ayaz, A.; Park, S. Nanomaterials in Agriculture: A pathway to enhanced plant growth and abiotic stress resistance. Plants 2025, 14, 716. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Plant responses and tolerance to salt stress: Physiological and molecular interventions. Int. J. Mol. Sci. 2022, 23, 4810. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Yang, Y. How plants tolerate salt stress. Curr. Issues Mol. Biol. 2023, 45, 5914–5934. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- Borromeo, I.; Domenici, F.; Giordani, C.; Del Gallo, M.; Forni, C. Enhancing Bean (Phaseolus vulgaris L.) resilience: Unveiling the role of halopriming against saltwater stress. Seeds 2024, 3, 228–250. [Google Scholar] [CrossRef]
- Stassinos, P.M.; Rossi, M.; Borromeo, I.; Capo, C.; Beninati, S.; Forni, C. Enhancement of Brassica napus tolerance to high saline conditions by seed priming. Plants 2021, 10, 403. [Google Scholar] [CrossRef]
- Santangeli, M.; Capo, C.; Beninati, S.; Pietrini, F.; Forni, C. Gradual exposure to salinity improves tolerance to salt stress in rapeseed (Brassica napus L.). Water 2019, 11, 1667. [Google Scholar] [CrossRef]
- Ji, Y.; Hu, W.; Guan, Y.; Saren, G. Effects of plant essential oil treatment on the growth of pathogenic fungi and the activity of defense-related enzymes of fungi-inoculated blueberry. Horticulturae 2024, 10, 318. [Google Scholar] [CrossRef]
- Idrees, S.; Shabir, S.; Ilyas, N.; Batool, N.; Kanwal, S. Assessment of cadmium on wheat (Triticum aestivum L.) in hydroponics medium. Agrociencia 2015, 49, 917–929. [Google Scholar]
- Borromeo, I.; Domenici, F.; Del Gallo, M.; Forni, C. Role of polyamines in the response to salt stress of tomato. Plants 2023, 12, 1855. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigment of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Rossi, M.; Borromeo, I.; Capo, C.; Glick, B.R.; Del Gallo, M.; Pietrini, F.; Forni, C. PGPB improve photosynthetic activity and tolerance to oxidative stress in Brassica napus grown on salinized soils. Appl. Sci. 2021, 11, 11442. [Google Scholar] [CrossRef]
- Chun, Y.; Yin, Z.D. Glycogen assay for diagnosis of female genital Chlamydia trachomatis infection. J. Clin. Microbiol. 1998, 36, 1081–1082. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Orzali, L.; Forni, C.; Riccioni, L. Effect of chitosan seed treatment as elicitor of resistance to Fusarium graminearum in wheat. Seed Sci. Technol. 2014, 42, 132–149. [Google Scholar] [CrossRef]
- Yang, L.; Xi, Y.; Luo, X.Y.; Ni, H.; Liet, H.H. Preparation of peroxidase and phenolics using discarded sweet potato old stems. Sci. Rep. 2019, 9, 3769. [Google Scholar] [CrossRef]
- Iwase, T.; Tajima, A.; Sugimoto, S.; Okuda, K.I.; Hironaka, I.; Kamata, Y.; Takada, K.; Mizunoe, Y. A simple assay for measuring catalase activity: A visual approach. Sci. Rep. 2013, 3, 3081. [Google Scholar] [CrossRef]
- Sible, C.N.; Seebauer, J.R.; Below, F.E. Plant biostimulants: A categorical review, their implications for row crop production, and relation to soil health indicators. Agronomy 2021, 11, 1297. [Google Scholar] [CrossRef]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef]
- Ben Saad, R.; Ben Romdhane, W.; Wiszniewska, A.; Baazaoui, N.; Taieb Bouteraa, M.; Chouaibi, Y.; Alfaifi, M.Y.; Kačániová, M.; Čmiková, N.; Ben Hsouna, A.; et al. Rosmarinus officinalis L. essential oil enhances salt stress tolerance of durum wheat seedlings through ROS detoxification and stimulation of antioxidant defense. Protoplasma 2024, 261, 1207–1220. [Google Scholar] [CrossRef]
- Lu, Y.; Fricke, W. Salt stress-regulation of root water uptake in a whole-plant and diurnal context. Int. J. Mol. Sci. 2023, 24, 8070. [Google Scholar] [CrossRef]
- Gururani, M.A.; Atteya, A.K.; Elhakem, A.; El-Sheshtawy, A.N.A.; El-Serafy, R.S. Essential oils prolonged the cut carnation longevity by limiting the xylem blockage and enhancing the physiological and biochemical levels. PLoS ONE 2023, 18, e0281717. [Google Scholar] [CrossRef]
- El-Sayed, I.M.; El-Ziat, R.A. Utilization of environmentally friendly essential oils on enhancing the postharvest characteristics of Chrysanthemum morifolium Ramat cut flowers. Heliyon 2021, 7, e05909. [Google Scholar] [CrossRef]
- Hameed, A.; Ahmed, M.Z.; Hussain, T.; Aziz, I.; Ahmad, N.; Gul, B.; Nielsen, B.L. Effects of salinity stress on chloroplast structure and function. Cells 2021, 10, 2023. [Google Scholar] [CrossRef]
- Dyadiuchenko, L.; Taranenko, V.; Muraviev, V.; Dmitrieva, I. The study of natural essential oils as growth regulators of winter wheat. BIO Web Conf. 2020, 21, 00023. [Google Scholar] [CrossRef]
- Jeandet, P.; Formela-Luboińska, M.; Labudda, M.; Morkunas, I. The role of sugars in plant responses to stress and their regulatory function during development. Int. J. Mol. Sci. 2022, 23, 5161. [Google Scholar] [CrossRef]
- Wang, Y.; Diao, P.; Kong, L.; Yu, R.; Zhang, M.; Zuo, T.; Fan, Y.; Niu, Y.; Yan, F.; Wuriyanghan, H. Ethylene enhances seed germination and seedling growth under salinity by reducing oxidative stress and promoting chlorophyll content via ETR2 pathway. Front. Plant Sci. 2021, 11, 639869. [Google Scholar] [CrossRef]
- Gupta, A.K.; Kaur, N. Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J. Biosci. 2005, 30, 761–776. [Google Scholar] [CrossRef]
- Cao, H.; Guo, S.; Xu, Y.; Jiang, K.; Jones, A.M.; Chong, K. Reduced expression of a gene encoding a Golgi localized monosaccharide transporter (OsGMST1) confers hypersensitivity to salt in rice (Oryza sativa). J. Exp. Bot. 2011, 62, 4595–4604. [Google Scholar] [CrossRef] [PubMed]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Vyas, S. Comparative bio-accumulation of osmoprotectants in saline stress tolerating plants: A review. Plant Stress 2023, 9, 100177. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Das Bhowmik, S.; Long, H.; Cheng, Y.; Mundree, S.; Hoang, L.T.M. Rapid accumulation of proline enhances salinity tolerance in australian wild rice Oryza australiensis Domin. Plants 2021, 10, 2044. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Jin, L.; Peng, R. Crosstalk between Ca2+ and other regulators assists plants in responding to abiotic stress. Plants 2022, 11, 1351. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A.; GolezaniGhassemi, M. Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (Glycin max L.). Plant Omics J. 2012, 5, 60–67. [Google Scholar]

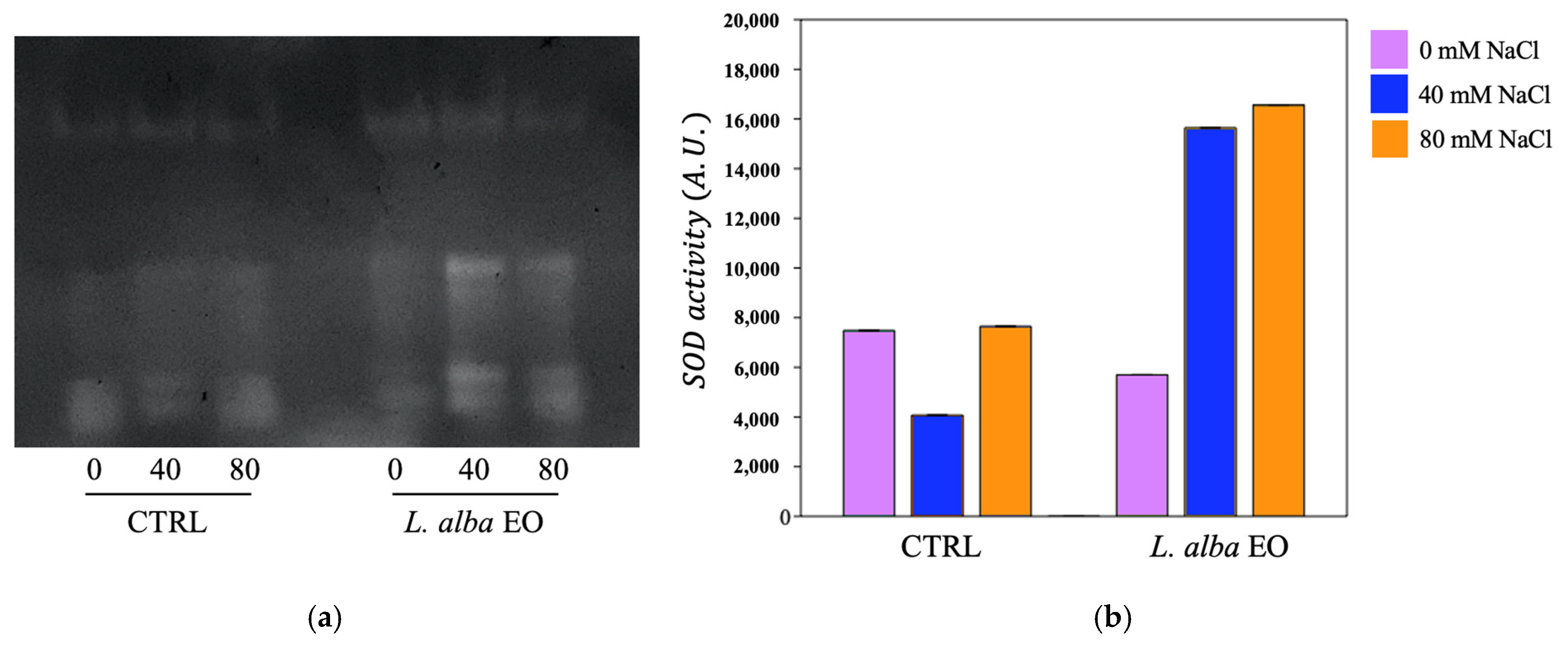
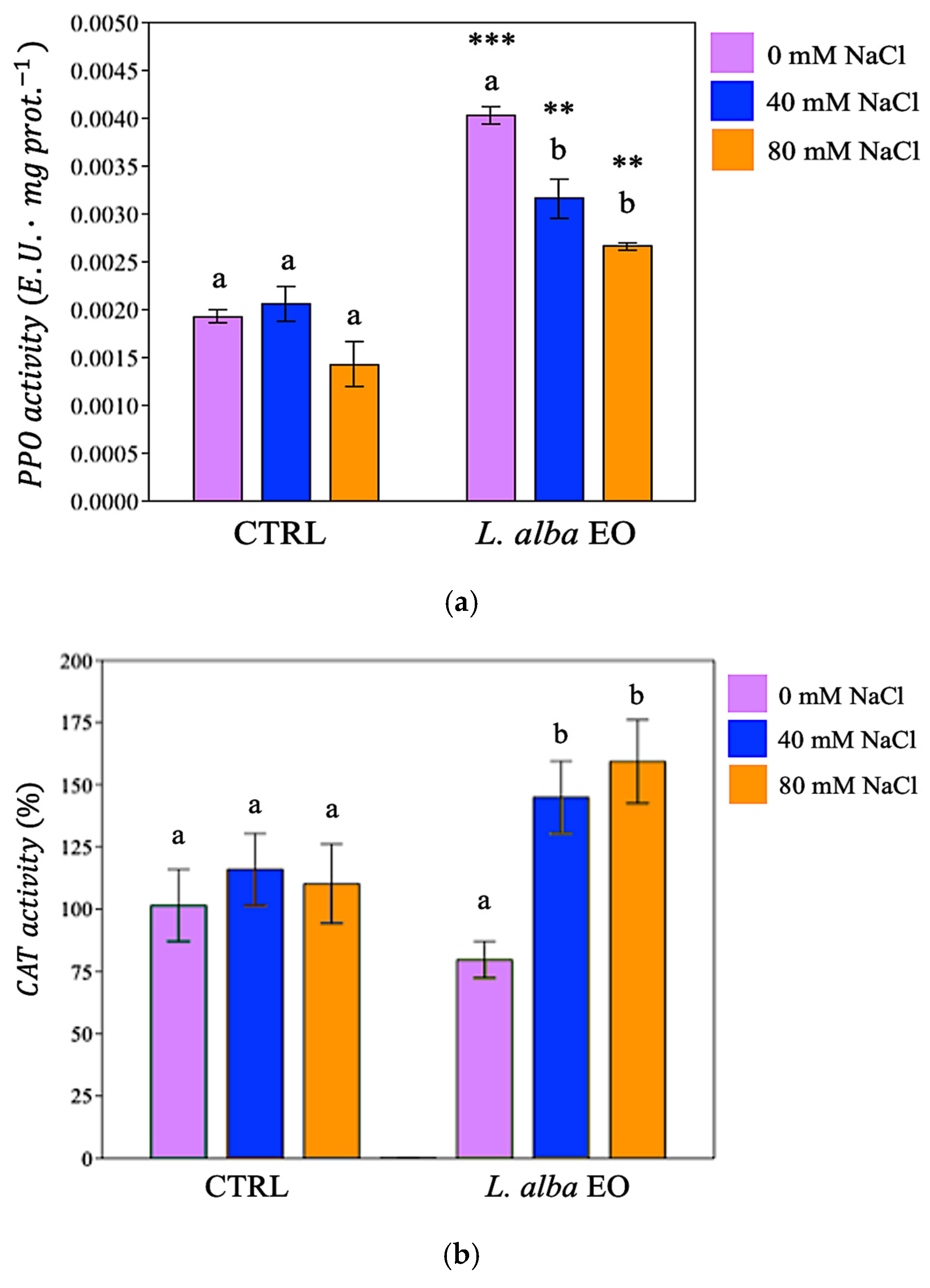
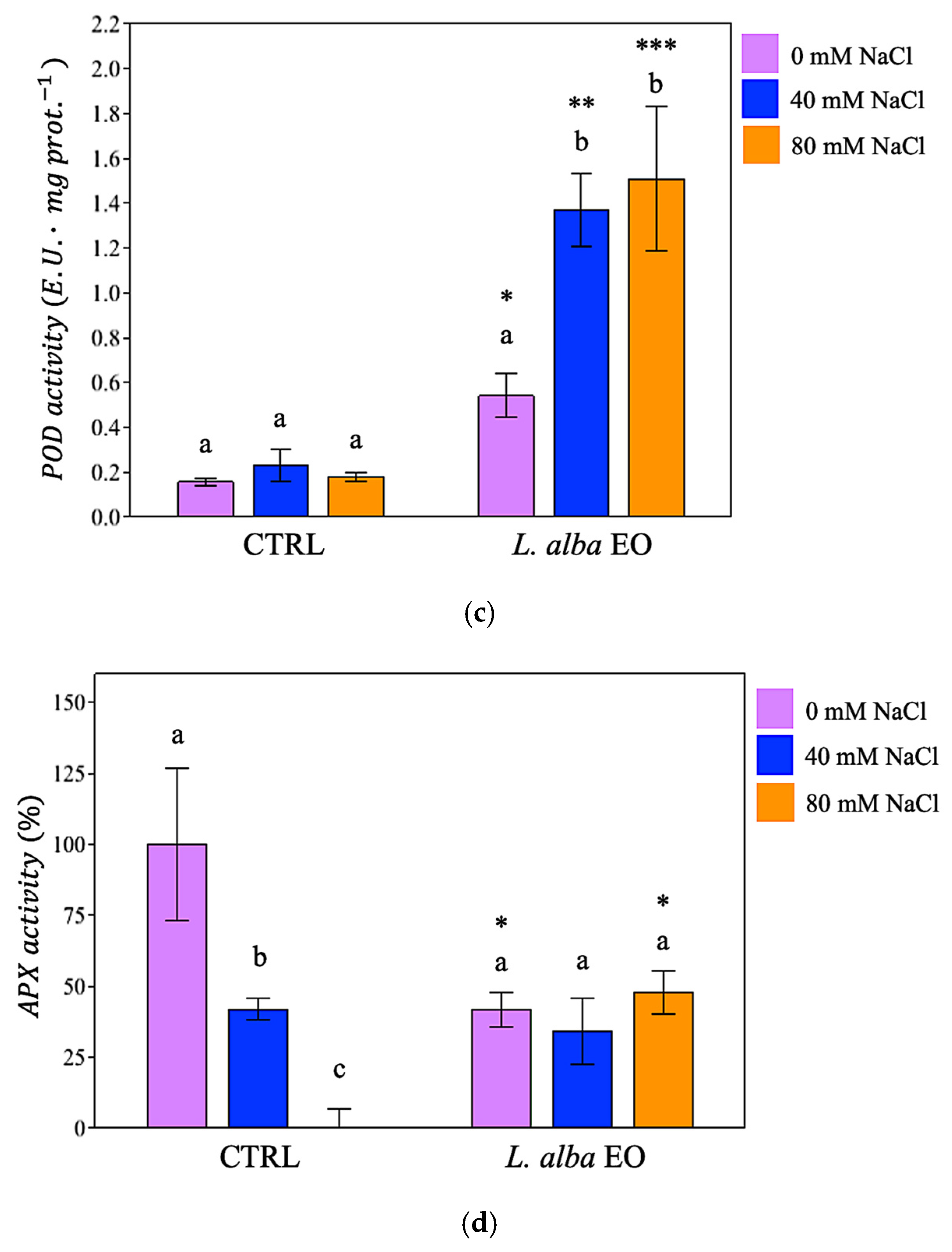
| NaCl (mM) | CTRL | Lippia alba EO |
|---|---|---|
| 0 | 92% ± 4% a | 88% ± 3% a |
| 40 | 89% ± 3% ab | 80% ± 2% a |
| 80 | 79% ± 6% b | 69% ± 6% ab |
| 160 | 55% ± 8% c | 68% ± 7% b |
| Priming Solution | NaCl (mM) | GWC of Soil (%) | STI (%) |
|---|---|---|---|
| CTRL | 0 | 32.6% ± 1.7% a | 100 |
| 40 | 32.9% ± 2.0% a | 81 | |
| 80 | 56.8% ± 3.1% b | 77 | |
| Lippia alba EO | 0 | 32.2% ± 1.8% a | 107 (+7%) |
| 40 | 24.3% ± 2.1% b ** | 109 (+28%) | |
| 80 | 45.6% ± 1.9% c ** | 91 (+14%) |
| Priming Solution | NaCl (mM) | Total Chl (μg·g f.w.−1) | Carbohydrates (mg glucose eq.·g f.w.−1) | Proline (μg·g f.w.−1) |
|---|---|---|---|---|
| CTRL | 0 | 45.6 ± 4.4 a | 0.131 ± 0.010 a | 59.7 ± 9.9 a |
| 40 | 37.7 ± 2.3 ab | 0.160 ± 0.003 ab | 49.7 ± 5.5 a | |
| 80 | 23.1 ± 3.7 b | 0.205 ± 0.016 b | 117.6 ± 18.3 b | |
| Lippia alba EO | 0 | 43.2 ± 0.8 a | 0.229 ± 0.019 a *** | 67.0 ± 6.6 a |
| 40 | 93.9 ± 4.6 b *** | 0.163 ± 0.012 b | 91.8 ± 3.2 a *** | |
| 80 | 95.8 ± 4.6 b *** | 0.141 ± 0.012 b * | 485.7 ± 41.3 b *** |
| Priming Solution | NaCl (mM) | Root (μg Ca2+·mg f.w.−1) | Shoot (μg Ca2+·mg f.w.−1) |
|---|---|---|---|
| CTRL | 0 | 0.13 ± 0.03 a | 2.13 ± 0.05 a |
| 40 | 0.19 ± 0.04 a | 2.04 ± 0.04 a | |
| 80 | 0.20 ± 0.03 a | 1.91 ± 0.09 a | |
| Lippia alba EO | 0 | 0.28 ± 0.05 a | 1.81 ± 0.03 a * |
| 40 | 0.27 ± 0.04 a | 1.91 ± 0.01 b | |
| 80 | 0.19 ± 0.04 a | 2.17 ± 0.07 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borromeo, I.; Giordani, C.; Forni, C. Efficacy of Lippia alba Essential Oil in Alleviating Osmotic and Oxidative Stress in Salt-Affected Bean Plants. Horticulturae 2025, 11, 457. https://doi.org/10.3390/horticulturae11050457
Borromeo I, Giordani C, Forni C. Efficacy of Lippia alba Essential Oil in Alleviating Osmotic and Oxidative Stress in Salt-Affected Bean Plants. Horticulturae. 2025; 11(5):457. https://doi.org/10.3390/horticulturae11050457
Chicago/Turabian StyleBorromeo, Ilaria, Cristiano Giordani, and Cinzia Forni. 2025. "Efficacy of Lippia alba Essential Oil in Alleviating Osmotic and Oxidative Stress in Salt-Affected Bean Plants" Horticulturae 11, no. 5: 457. https://doi.org/10.3390/horticulturae11050457
APA StyleBorromeo, I., Giordani, C., & Forni, C. (2025). Efficacy of Lippia alba Essential Oil in Alleviating Osmotic and Oxidative Stress in Salt-Affected Bean Plants. Horticulturae, 11(5), 457. https://doi.org/10.3390/horticulturae11050457









