Abstract
The development of Fusarium Basal Rot (FBR)-resistant onion cultivars through field and seedling screening approaches faces tremendous challenges due to non-uniform distribution of the disease pathogen and possible multiple mechanisms of host–plant resistance. This study compared the efficiencies of these two methods for increasing FBR resistance of short-day onion after a single selection cycle. Asymptomatic plants or bulbs of seven onion cultivars were selected using a seedling screen performed in a growth chamber or a field screening of mature bulbs. Original and selected populations were evaluated for their responses to FBR stress thereafter employing the same two methods used for screening. The field screening of mature bulbs was found unreliable in both selection and evaluation, likely due to a non-random distribution of the FBR pathogen and variable environmental factors present in the field. The seedling screening successfully increased FBR resistance in the selected cultivar populations revealed by a seedling evaluation. From the results, it is recommended to use a consistent method for both screening and evaluation to make the most selection progress.
1. Introduction
Fusarium Basal Rot (FBR), caused by Fusarium oxysporum Schlecht. emend. Snyder & Hansen f.sp. cepae (Hans.) (FOC) is the second most important soil-borne fungal disease of onion after pink root in New Mexico [1]. FBR results in an average 23% loss in the field and storage if grown on infested soils in the USA [2]. FOC is a soil saprophyte that can obtain nutrients from decaying organic matter in the rhizosphere [3] and survive for many years in an infested field as chlamydospores [3,4]. Crop rotation with a non-host crop for at least four years can reduce the inoculum population in the field [5]. However, with limited land availability due to urbanization, perennial plant production, water limitations, and soil salinity issues, crop rotation cycles become shorter leading to an increase in the pathogen levels in the soil with successive onion crops [6]. Soil fumigation can be an effective practice of reducing the levels of FOC in the soil, yet, it is cost prohibitive [7] and it destroys beneficial soil microorganisms [8,9]. Due to these limitations, the development of FBR-resistant cultivars is the most viable option to reduce onion losses due to FBR.
The two most important steps to develop disease resistant cultivars are screening for resistant plant material and evaluation of the resistance over successive generations. The most widely practiced method to develop FBR-resistant onion cultivars is a seedling screening in a controlled environment, such as a greenhouse or growth chamber. Using these conditions, FBR-resistant seedlings are selected either by artificially inoculating sterile onion seeds [10,11] or the growing media [12,13,14,15] with one [16], two [10], three [12,15], or multiple [13,14] virulent FOC isolates. Also, evaluation of mature bulbs for FBR resistance can be done using field inoculation that use natural FOC infestation [10] or artificial inoculation of a field plot [11]. In the USA, several long-day and intermediate-day, FBR-resistant onion lines have been developed in the past at the University of Wisconsin–Madison using screening methods involving both growth phases [17]. A strong correlation has been found between screenings conducted on seedlings and mature bulbs grown in fields [18,19]. However, there are several challenges associated with screening of different growth phases because of the molecular differences of defense response and environmental variability. Due to the lack of information of FOC-resistance in onion, Fusarium root rot and Fusarium head blight studies in monocot wheat may suggest a molecular basis for Fusarium-resistance in onion. In wheat, the defense response to Fusarium is summarized broadly by the activation of the mycotoxin (e.g., Deoxynivalenol) detoxification associated genes and the rapid initiation of Jasmonate signaling genes [20]. These genes, which are responsible for this systemic response against Fusarium, are expressed at different levels based upon plant growth stages [20]. Therefore, seedling, older plants, and mature bulbs of individual onion selections or lines of a cultivar may vary in their resistance responses to FOC [4], suggesting different types of plant defense mechanisms operate during various growth stages [21]. As a result, improvement could be limited depending upon which growth phase is used for selection and evaluation. Even if resistance gene (R) transcript levels remains the same in all growth stages, physiological aging could also affect disease resistance [22]. In addition to genetic background and aging, environmental factors could also change R-gene transcript levels and in turn disease resistance [20,22]. During an onion seedling screening, environmental variability and plant aging could be avoided when completed inside a growth chamber or greenhouse for a short period of time [13,14]. Nonetheless, in field screening, an optimum screening would not be possible due to a non-uniform field distribution of multiple pathogen isolates [23] in variable soil and nutritional conditions, which could influence susceptibility reaction to FOC [24]. These variable factors in the field may result in escaping infection and subsequently disease escape by mature bulbs.
In this study, using seven onion cultivars and one cycle of selection, we compared two methods of screening for FBR resistance: (a) seedlings (S) artificially inoculated and incubated in a growth chamber and (b) mature bulbs (MB) planted in a naturally FOC-infested field. A comparison of these methods may be useful to select the one method to produce similar results across diverse onion populations. Since heterogeneous onion populations may not produce the same results, each cultivar served as a sub-study to compare the original and the selected population for FBR resistance achieved with each method.
2. Materials and Methods
2.1. Plant Materials
Seven autumn-sown, overwintered, short-day, Grano-type onion cultivars with a differing order of maturity from earliest to latest, viz., “NuMex Camino” [25], “NuMex Sweetpak” [26]. “NuMex Mesa” [27], “NuMex Chaco” [28], “NuMex Crispy” [29], “NuMex Vado” [30], and “NuMex Luna” [30], were selected for this study. Released by the New Mexico State University onion breeding program, these cultivars are adapted to southern New Mexico growing conditions and tend to exhibit a higher FBR disease incidence than winter-sown cultivars when grown in FOC-infested soils at the Fabian Garcia Science Center (FGSC) in Las Cruces, NM [16,31]. “NuMex Camino” has a similar genetic background as “NuMex Sweetpak” while “NuMex Vado” and “NuMex Luna” share a similar genetic background to each other. All cultivars produced a yellow dry outer scale except “NuMex Crispy”, which produced a white dry outer scale. Every cultivar was considered to be a sub-study and studied in separate fields or growth chamber trials.
2.2. Design of the Experiment
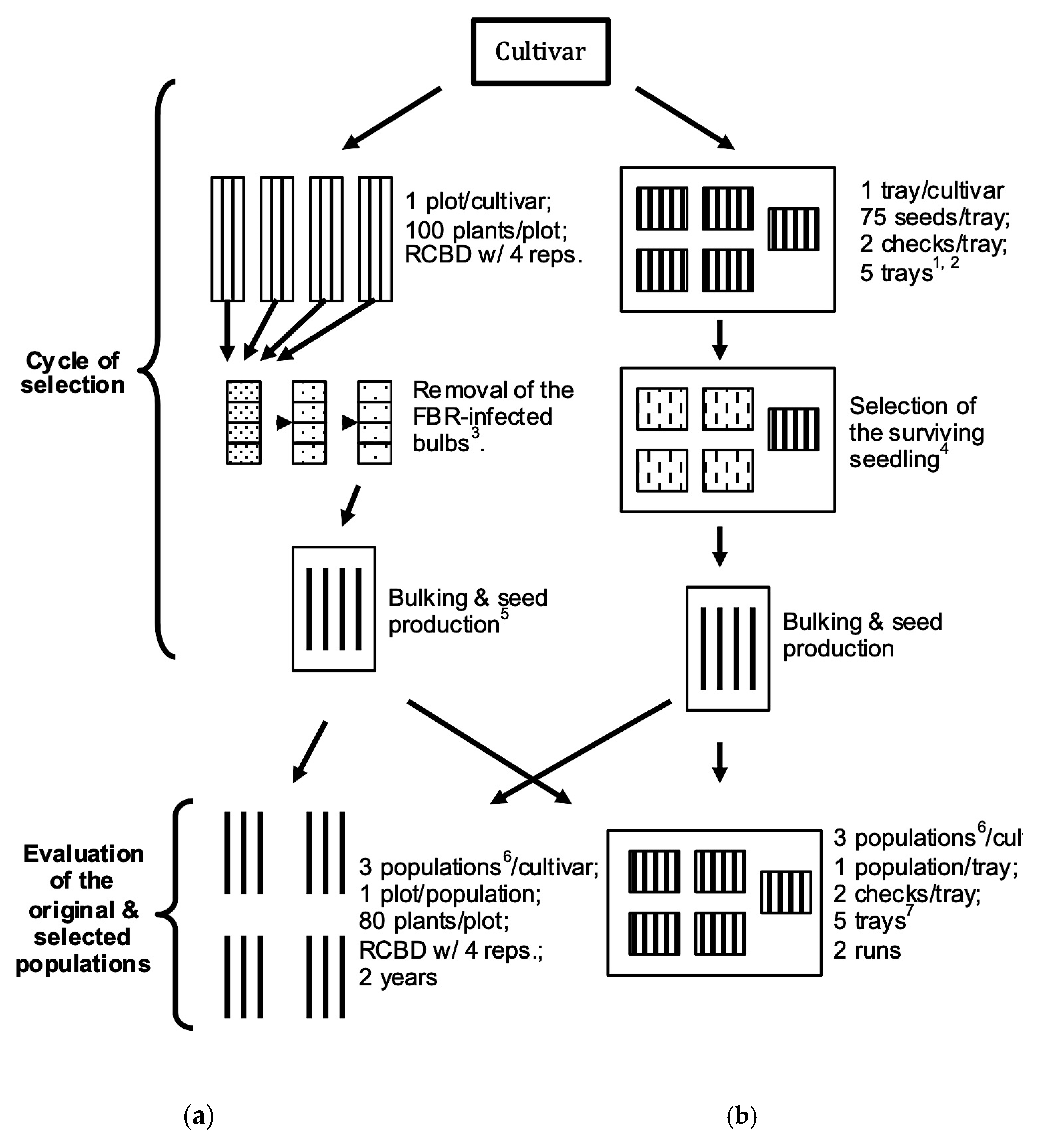
For each cultivar mentioned above, two steps were performed to complete the experiment, namely a cycle of selection for FBR resistance; and evaluation of the selected and the original populations for FBR reactions (Figure 1). The cycle of selection was started by employing S and MB screening methods in FOC-infested field and a FOC-inoculated growth chamber, respectively, followed by bulking of the selected materials and seed production (Figure 1). The evaluation compared the S and MB screening methods by comparing the Mature Bulb-Selected (MBS) and Seedling-Selected (SS) populations with the original population (Figure 1). Due to limitations in the quantity of seed available for the original populations during the 2004–2007 study period, the two screening procedures were initiated in two different years.
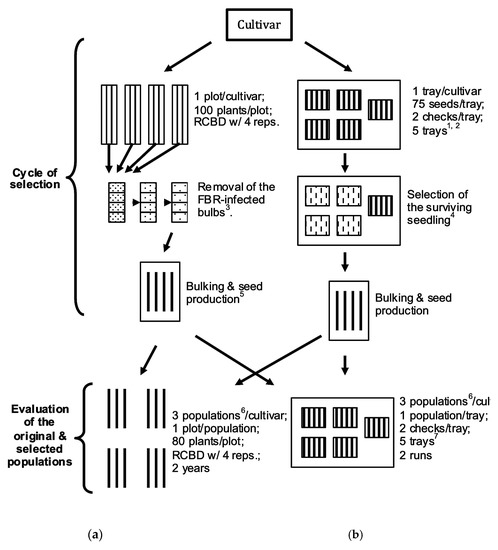
Figure 1.
Flow chart of selection cycle and evaluation of a cultivar population via (a) Mature Bulb Selection (MBS) in a naturally FOC-infested field, and (b) Seedling Selection (SS) in a growth chamber for improving FBR resistance in onion. 1 Four trays were inoculated with fungal inoculum; 2 A fifth tray was irrigated with equal amount of distilled water. 3 FBR-infected bulbs were discarded at harvest, 4 weeks after harvest, and before seed production. 4 Small bulbs were produced before being transplanted into the field. 5 Onion is a biennial crop that requires bulb production in between seed production to complete its life cycle. All the selected bulbs were bulked into a single cage for seed production. 6 In both evaluations original, mature bulb-screened and seedling-screened populations were planted in each replication. 7 Four trays were inoculated, and a fifth tray was uninoculated.
2.3. Screening of FBR-Resistant MB Populations Via Field Inoculation
During Sept. 2004, seeds of each cultivar were sown at the FGSC fields, which had a reputation of FBR disease incidence as high as 64% to 99% for susceptible cultivars [32]. High levels of disease propagules were ensured in these fields by monocropping over decades and incorporating diseased bulbs during field preparation [32]. Plots of 5.5 m in length were prepared in raised beds, which were distanced 1 m apart, contained two rows of plants in 20 cm between each other. The number of plants were maintained at 100 per plot after thinning at the 4–5 vegetative leaf stage with a plant spacing of 10 cm. Four plots per cultivar were arranged in a randomized complete block (RCBD) design. A pre-plant application of triple superphosphate (0.0N–20.1P–0.0K; Helena Chemical Co., Collierville, TN, USA) was made at a rate of 282.5 kg/ha. Standard cultural practices for growing onions in southern New Mexico were followed [33]. In each bed, subsurface drip irrigation tape (T-Tape; T-Systems International, San Diego, CA, USA), with 20 cm spaced emitters were placed 10 cm below the surface for fertigation. Acid-based liquid fertilizer (26N–0P–0K–6S; Western Blend, Inc., Las Cruces, NM, USA) was applied with drip irrigation until 3–4 weeks before harvest. In the summer of 2005, when 80% plant tops within a plot had lodged, the MB were harvested. For the bulbs, which were infected at harvest time, at least four weeks of storage were necessary for the development of disease symptoms [32,34]. To ensure selection of truly FBR-resistant bulbs post-harvest, FBR-infected bulbs were discarded at three stages, specifically, at harvest, after four weeks of storage, and after completion of storage before seed production (Figure 1a). A thin section of dried top portion of the basal plate tissue was cut transversely from bulbs and those that showed symptoms of FBR were discarded. During the autumn of 2005, disease-free bulbs for each cultivar were placed into separate crossing cages for seed multiplication.
2.4. Selection of FBR-Resistant S Populations Via Growth Chamber Inoculation
In the spring of 2006, FOC isolate “CSC-515”, described as the most virulent across eight genetically divergent onion cultivars [35], was used as the pathogenic material for this FBR seedling screening [16]. Long-term-stored chlamydospores of “CSC-515” were cultured onto one third strength potato dextrose agar (PDA) (13.0 g in 1 L) media for seven days at 25 °C [16]. A 1 cm2 size PDA plug with actively growing mycelium was then transferred to a 250 mL sterilized Erlenmeyer flask containing 100 mL of potato dextrose broth. Flasks were placed on a rotary shaker at 90 rpm for five days. Afterwards, the resulting mycelial mass was removed, blended, and sieved through cheesecloth to obtain a solution of mostly macro and micro conidia fungal spores. The solution was centrifuged at 3400 rpm for 10 min and the resulting pellet was resuspended into diH2O.
Three growth chambers were used to perform the seedling screening method. Within one growth chamber, four plastic trays, which containing 13 kg of sterilized silica sand each, were inoculated individually with 1 L of conidial suspension in diH2O to achieve a final concentration of 1 × 104 spores/g of sand. A fifth tray was kept uninoculated to measure seed germination in the absence of the pathogen. Seventy-five surface sterilized seeds (washed with 15% sodium hypochlorite solution followed by water) of each cultivar along with one susceptible check-Rumba (Nunhems USA, Inc., Parma, ID, USA) and one resistant check-Sierra Blanca (Seminis Vegetable Seeds, St. Louis, MO, USA) were sown in all five trays at 1.5 cm depth. The initial temperatures of the growth chambers were set at 22 °C to stimulate seedling growth before the disease symptoms appeared and a photoperiod of 16 h was maintained throughout the experiment. After two weeks, the temperature was raised to 28 °C to facilitate disease development [4]. At that moment, the number of germinated seeds was determined for all cultivars and those studies exhibiting one or more trays with poor germination were discarded and repeated later. After four weeks, germination rates of the resistant and susceptible checks were compared to assess the effectiveness of the screening protocol. For those trays in which the resistant check exhibited a high survival rate and the susceptible check exhibited a low survival rate, surviving seedlings of each cultivar were selected. These seedlings were transplanted to black plastic trays containing Metro Mix 300 (Sungro Horticulture, Bellevue, WA, USA) in a lathhouse to produce small bulbs. Osmocote 14–14–14 (Scotts–Sierra Horticulture, Marysville, OH, USA) was added to promote plant growth. In the autumn of 2006, the small bulbs were transplanted to a field to produce seeds of each cultivar selection in the following summer.
2.5. Seed Production of the MBS Cultivar Populations
During the autumn of 2005 (Figure 1), all selected bulbs from each cultivar were placed in a seed production field at the Leyendecker Plant Science Research Center (LPSRC) in Las Cruces, NM, USA. In April 2006, metal frames with insect-proof mesh surrounding them were assembled to form isolation cages (20 m length × 3 m width × 2 m height) around selected bulbs and to prevent cross-pollination among unrelated selected bulbs. Starting in May 2006, honeybee (Apis mellifera L.) colonies were placed for one month (during flowering time) inside each cage for successful pollination. During June 2006, mature umbels were harvested when the seed capsules turned brown and transferred to a well-ventilated shade to dry. Umbel threshing and seed cleaning (with H2O, 15% NaOCl solution) was done to remove any external inoculum of seed-borne pathogens. The selected populations were stored at 15.6 °C with 40% RH.
2.6. Seed Production of the SS Cultivar Populations
During the autumn of 2006 (Figure 1), small bulbs selected for FBR resistance were placed inside several small, insect-proof crossing cages (0.6 m length × 0.6 m width × 1 m height) at LPSRC. Within each cage five to six bulbs were planted to minimize the genetic depression caused by inbreeding. Starting in May 2006, three to four batches each containing ~30–40 recently hatched bluebottle flies (Calliphora vomitoria L.) were placed weekly for one month inside each cage for successful pollination. Mature umbels were harvested during July 2007 and placed into well-aerated conditions for complete drying. Dried seeds of the same cultivar that came from different cages were combined and processed as described in the Section 2.6. The quantity of seed from “NuMex Mesa” was too low to conduct any evaluation, while “NuMex Chaco” did not produce any seedstalks. Therefore, the unbolted bulbs of “NuMex Chaco” were planted again in September 2007 for seed production (Figure 1).
2.7. Field Inoculation Evaluation of the Original, MBS, and SS Populations
Seeds of the original, SS and MBS populations for each cultivar were planted during September of 2007 and 2008 at the same FGSC fields that possessed a high pathogen pressure previously used to screen them (Figure 1). Individual plots were 3 m in length with two rows of plant. Bed, row, and plant-to-plant distances were remained the same as mentioned during the original field screening. The number of plants per plot was 80 after thinning. A RCBD with four replications per cultivar population was used. Field preparation and cultural practices were performed in the same manner as mentioned before for the field inoculation screening method. Upon harvest in May and Jun. of 2008 and 2009 (Figure 1), the basal plates of 20 randomly selected bulbs were transversely cut and disease severity was rated on an ordinal scale of 1–9, in which a rating of “one” represented no decay of basal plate tissue and “nine” represented more than 70% tissue decayed [32]. Scores 2–8 were assigned to every 10% increase of disease development in the basal plate area (e.g., 2 = 1–10% basal plate area infected, 3 = 11–20% etc.) [32]. Disease incidence was calculated as the percentage of susceptible individuals out of the total number of individuals observed. Bulbs rated as a “nine” at harvest were discarded and the remaining bulbs from the same plot were kept in storage. After a period of four weeks, basal plates of 20 randomly selected bulbs per plot were again cut and disease severity was rated.
2.8. Seedling Inoculation Evaluation of the Original, MBS, and SS Populations
Seeds of the original, SS, and MBS populations of each cultivar were sown within a growth chamber during Jun. 2008 along with a susceptible check, “Rumba” (Nunhems USA, Inc., Parma, ID, USA), and a resistant check, “Sierra Blanca” (Seminis Vegetable Seeds, St. Louis, MO, USA) in each tray (Figure 1). The performance of the two checks across different inoculum batches and growth chambers were used to test any inconsistent behavior. The silica sand media preparation and successive inoculation was performed as described earlier for the seedling inoculation screening method. The experimental design of the seedling evaluation was kept consistent with that of the screening design. Each of the five trays (Four inoculated and one uninoculated) contained 75 surface sterilized seeds of original, SS, and MBS populations of each cultivar along with one susceptible check and one resistant check sown at a 1.5 cm depth. At the end of four weeks, the percentage of survived seedlings was calculated for all the populations and compared with the checks. For consistency, this evaluation procedure was repeated (two runs) once again in Sept. 2008 for each cultivar (Figure 1) except for “NuMex Mesa”, for which there was insufficient seed.
2.9. Data Collection and Statistical Analysis
The statistical analysis was performed using a software application SAS® Studio in a Web-based environment called SAS® OnDemand for Academics (Copyright © 2015, SAS Institute Inc., Cary, NC, USA). In the field evaluation, average FBR severity and percentage of FBR incidence of 20 MB within one replication (one plot) and among four replications were used to calculate mean FBR severity and incidence (%) per cultivar population. In the seedling evaluation, mean seedling survival was calculated per replication (one tray) and across four replications to calculate mean seedling survival (%) per cultivar population. To access the increased FBR resistance by each screening method statistical analyses were performed on a cultivar basis, where disease parameters of the original, SS, and MBS populations were compared separately for each cultivar. The Proc GLM statement along with Dunnett’s multiple comparison procedure was used to measure the differences in seedling survival (%), FBR severity, and FBR incidence (%) of the SS and MBS populations from their original population. The estimate of the effects due to different batches and growth chambers was calculated using the two checks tested over both runs separately. The Proc Mixed procedure was used to measure the consistency of the seedling inoculation evaluation procedure. Batch, growth chamber, and their interaction were considered to be fixed effects. There were two batches of inoculum and a single batch used for three growth chamber inoculations. The batch by replication (tray) was modeled as random effects and used to identify differences within batches. The batch by growth chamber by replication term was also modeled as a random term and used as a denominator to identify differences within growth chamber, and the interaction between batch and growth chamber.
3. Results
3.1. Field Inoculation Evaluation of the Original, MBS, and SS Populations
Mature bulbs of the MBS and SS populations did not show any considerable reductions in FBR severity and incidence ratings compared to their original populations for all the cultivars in both the seasons (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7).
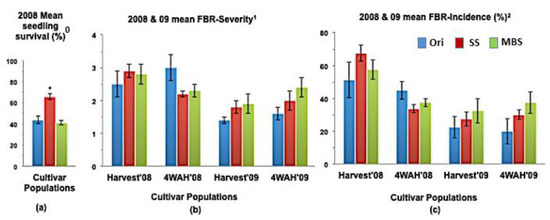
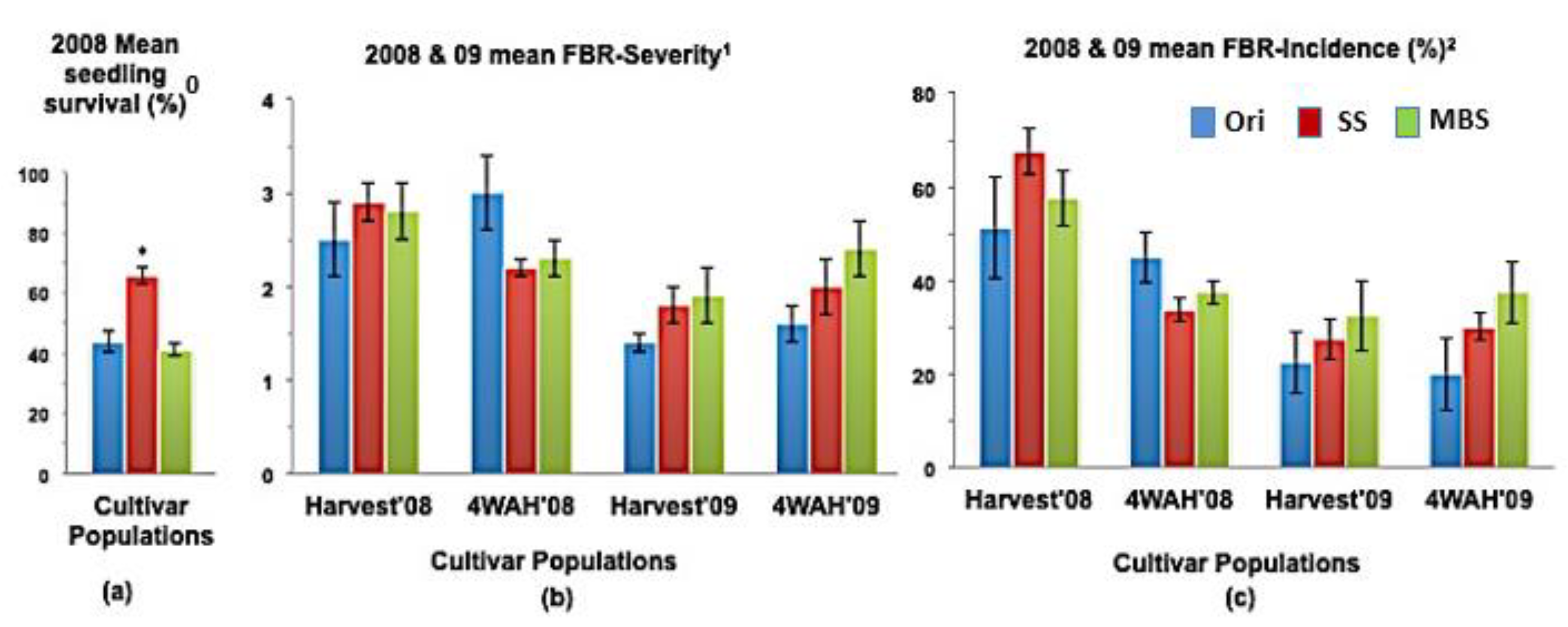
Figure 2.
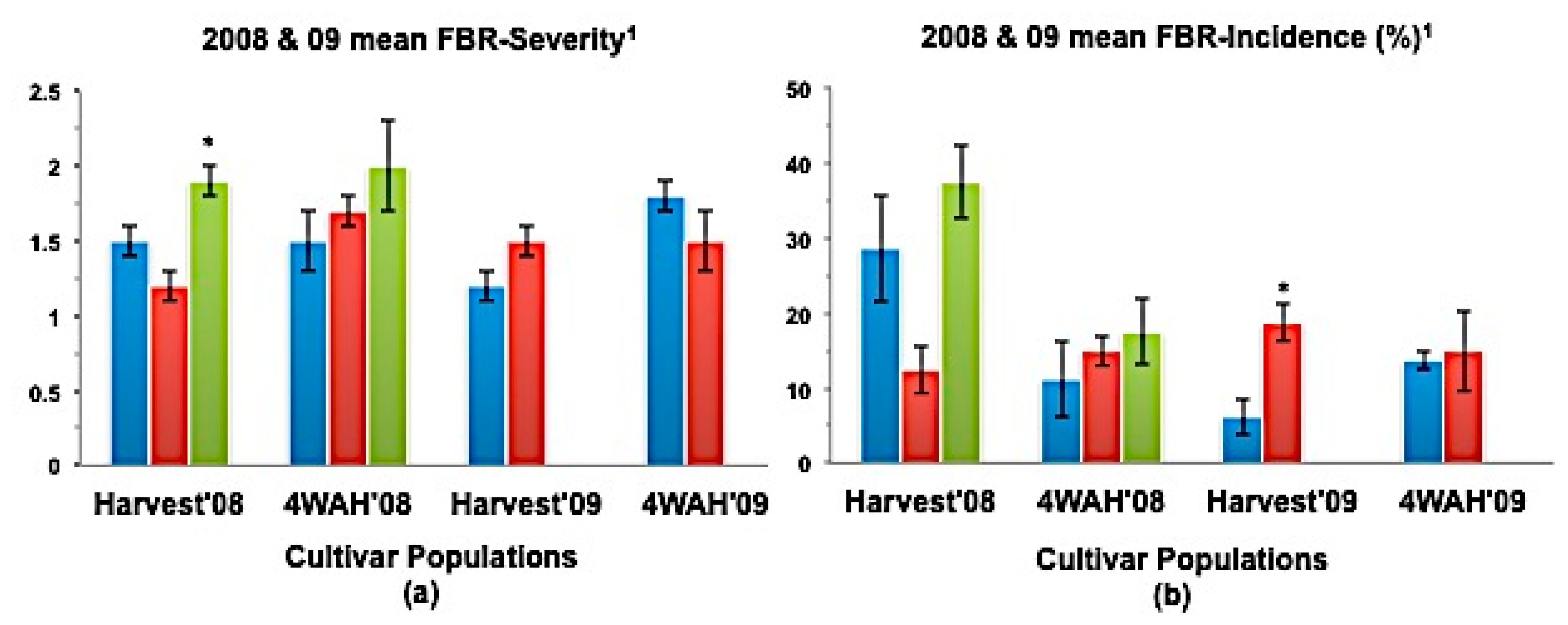
(a) Mean seedling survival, (b) Mean bulb FBR severity, and (c) Mean bulb FBR incidence (%) of NuMex Camino Ori, SS, and MBS populations in 2008 and 2009 evaluations. Std. error is shown on top of each bar. Grouping legend is provided in (c). (*) Significantly different from original at P = 0.05. 0 75 seeds of each population were sown in each tray for seedling evaluation. 1 Disease severity of 20 arbitrarily selected bulbs was rated at harvest (Harvest ’08 and ’09) and again four weeks after harvest (4WAH ’08 and ’09) on an scale of 1 to 9 [32]. 2 Percentage of susceptible individuals out of the total number of individuals of 20 arbitrarily selected bulbs was observed at harvest (Harvest ’08 and ’09) and again four weeks after harvest (4WAH ’08 and ’09).
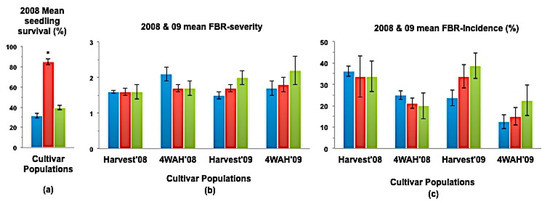
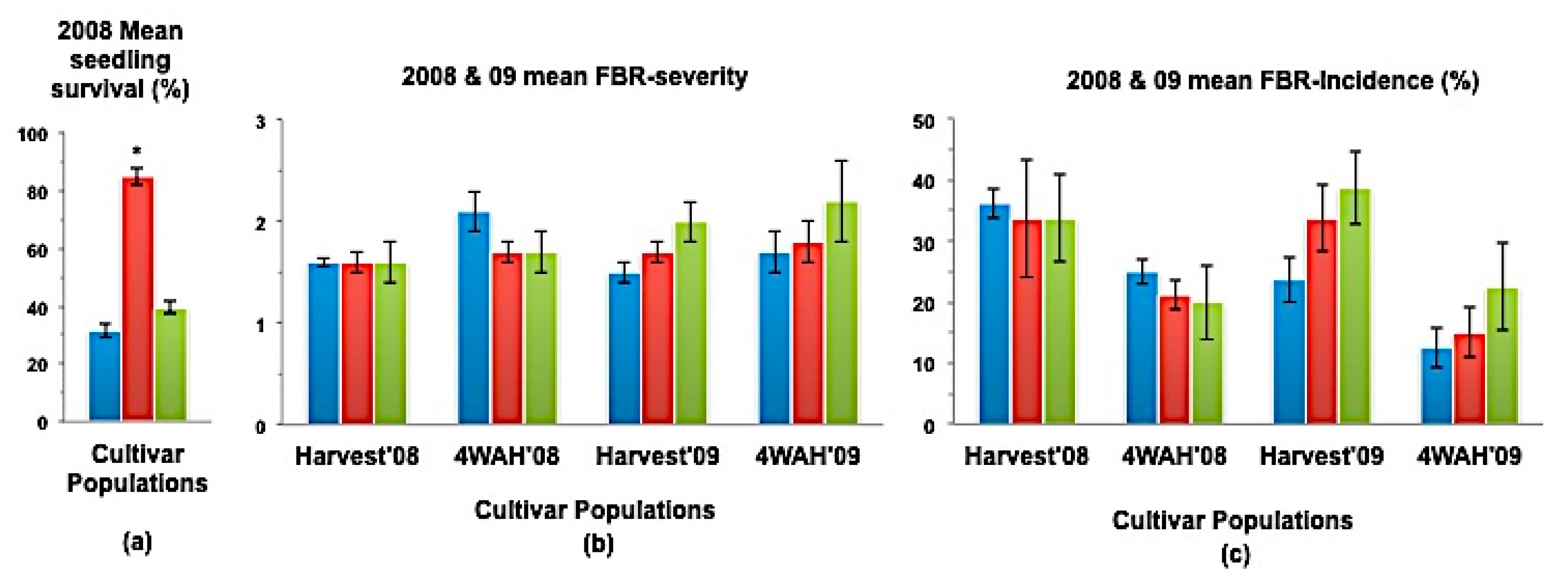
Figure 3.
(a) Mean seedling survival, (b) Mean bulb FBR severity, and (c) Mean bulb FBR incidence (%) of NuMex Crispy Ori, SS, and MBS populations in 2008 and 2009 evaluations. Std. error is shown on top of each bar. Grouping legend is provided in Figure 2. (*) Significantly different from original at P = 0.05.
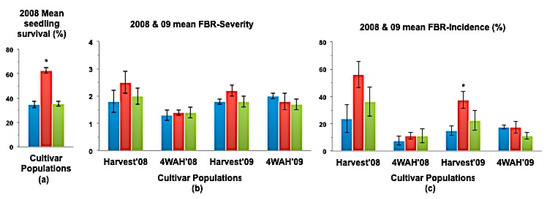
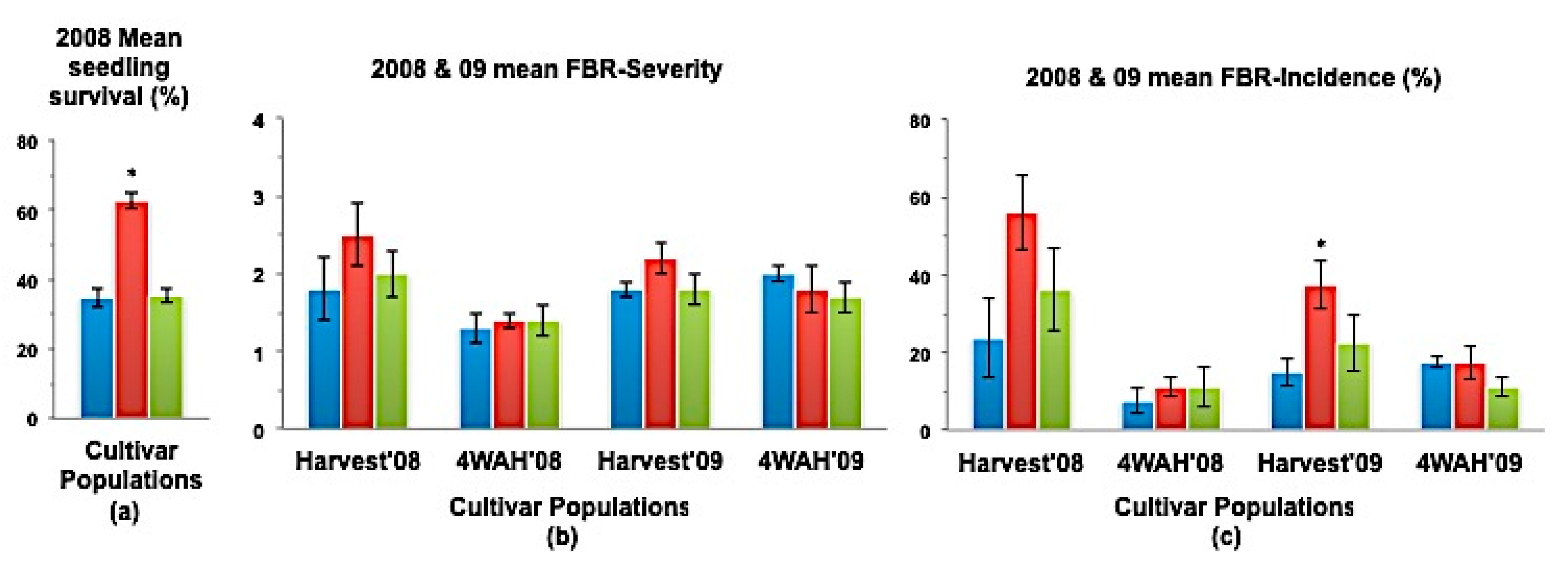
Figure 4.
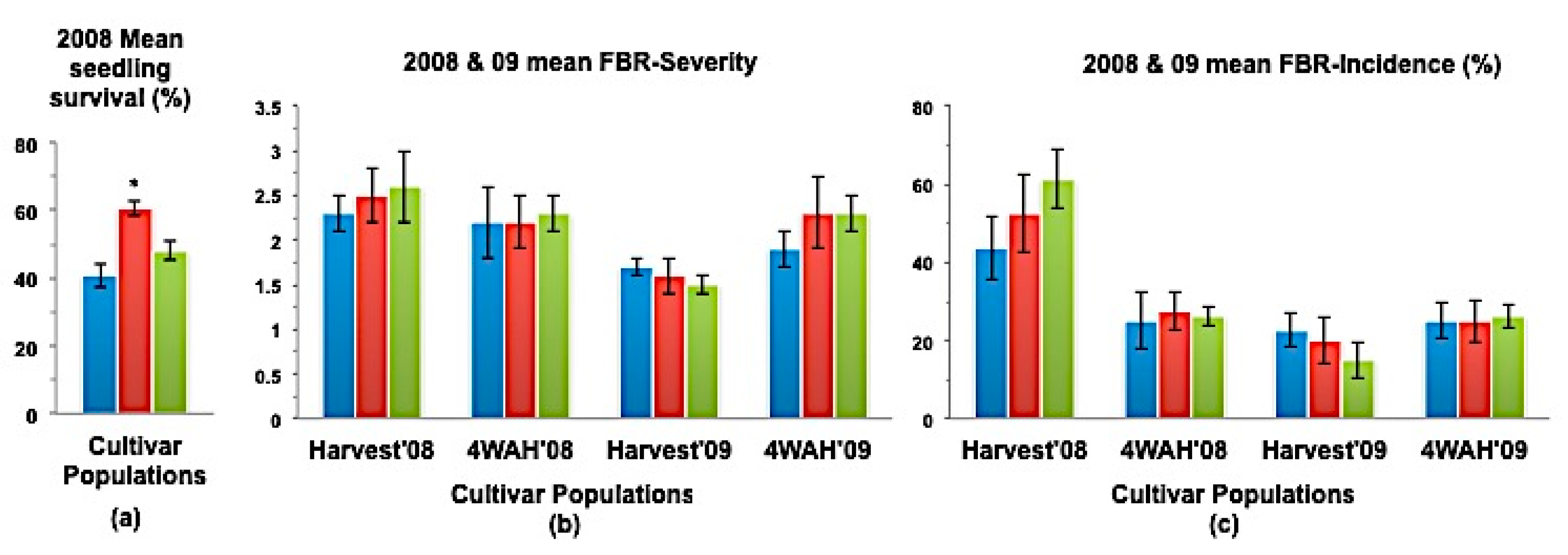
(a) Mean seedling survival; (b) Mean bulb FBR severity; and (c) Mean bulb FBR incidence (%) of NuMex Luna Ori, SS, and MBS populations in 2008 and 2009 evaluations. Std. error is shown on top of each bar. Grouping legend is provided in Figure 2. (*) Significantly different from original at P = 0.05.
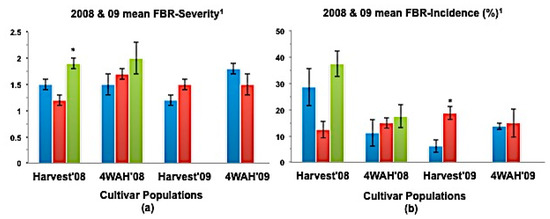
Figure 5.
(a) Mean bulb FBR severity; and (b) Mean bulb FBR incidence (%) of NuMex Mesa Ori, SS, and MBS populations in 2008 and 2009 evaluations. Std. error is shown on top of each bar. Grouping legend is provided in Figure 2. (*) Significantly different from original at P = 0.05. 1 Seeding evaluation and part of the bulb evaluation were not possible due to lack of sufficient seed.
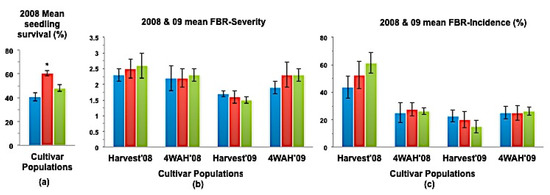
Figure 6.
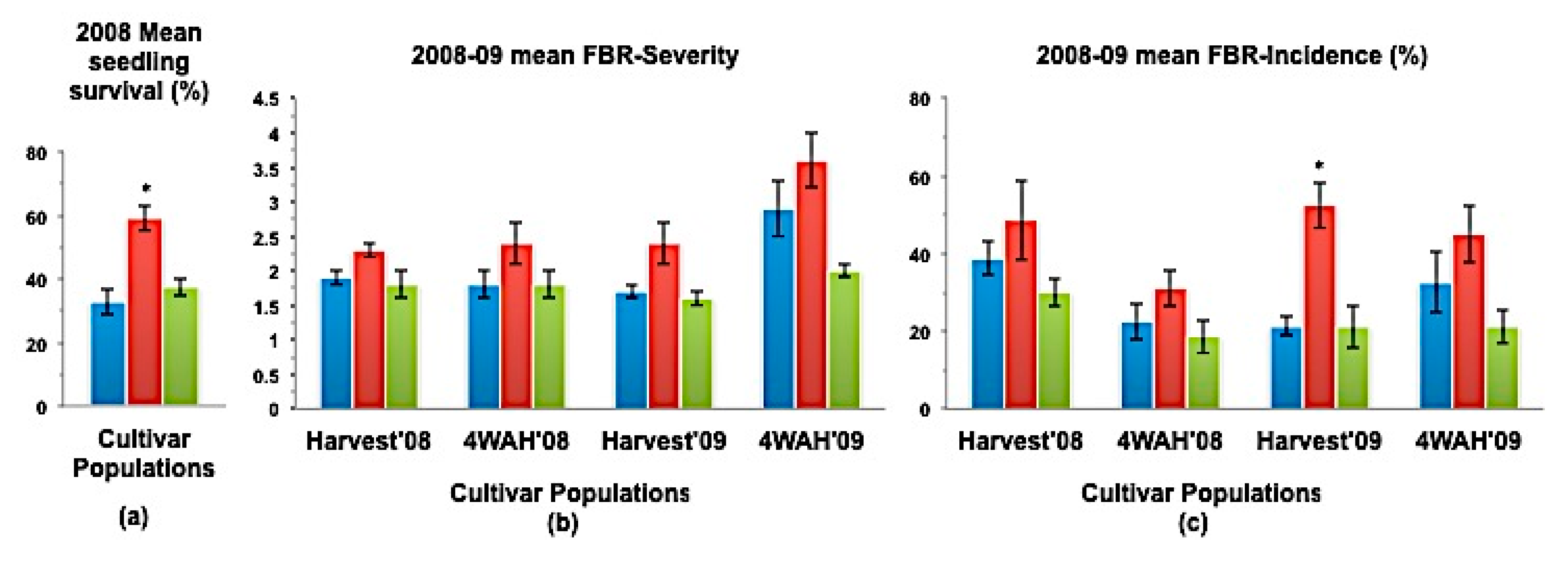
(a) Mean seedling survival; (b) Mean bulb FBR severity; and (c) Mean bulb FBR incidence (%) of NuMex Sweetpak Ori, SS, and MBS populations in 2008 and 2009 evaluations. Std. error is shown on top of each bar. Grouping legend is provided in Figure 2. (*) Significantly different from original at P = 0.05.
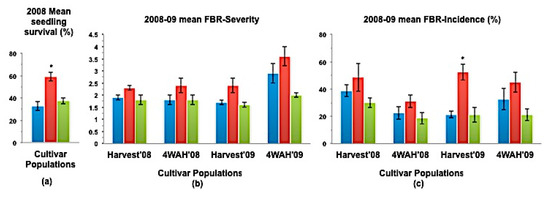
Figure 7.
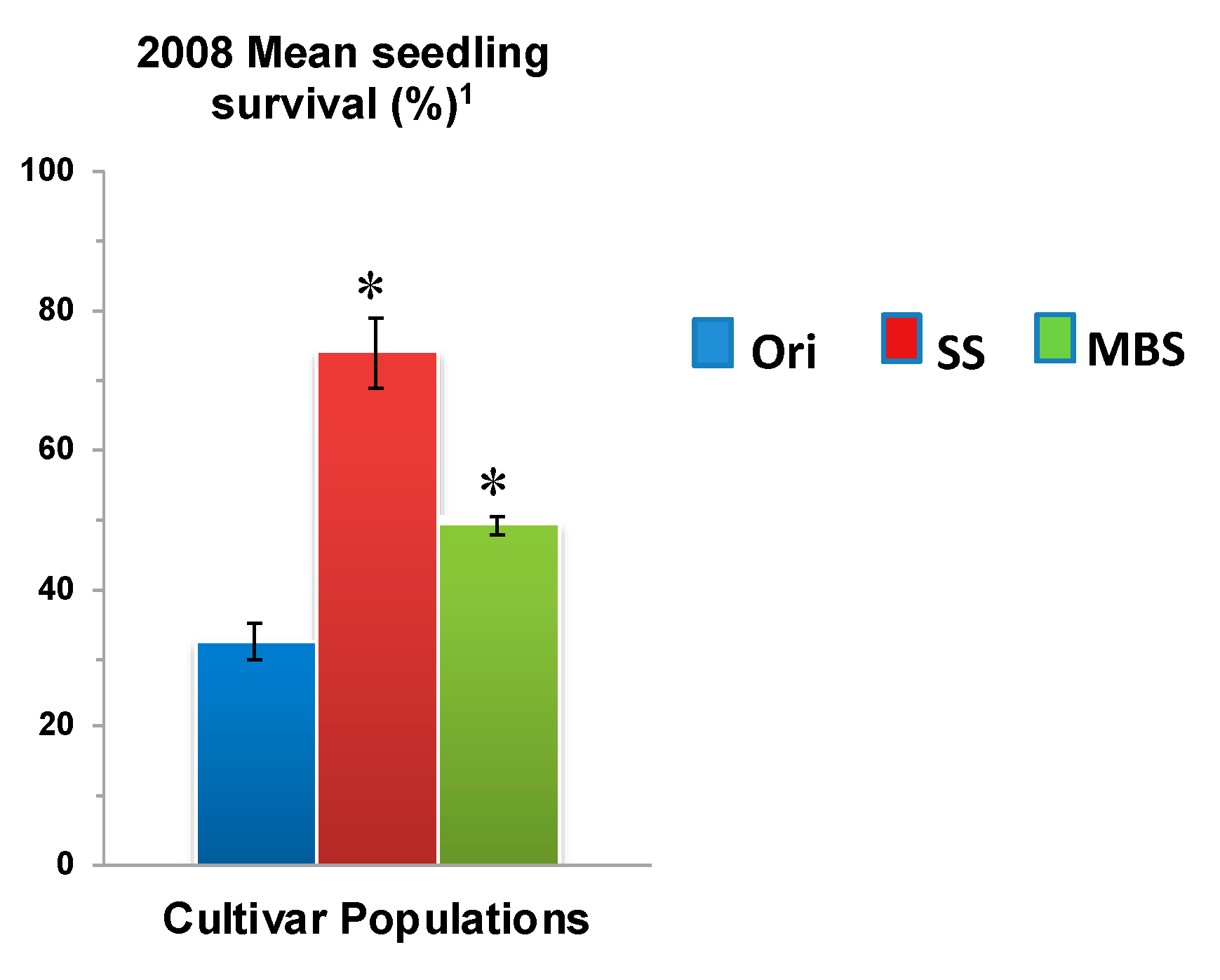
(a) Mean seedling survival; (b) Mean bulb FBR severity; and (c) Mean bulb FBR incidence (%) of NuMex Vado Ori, SS, and MBS populations in 2008 and 2009 evaluations. Std. error is shown on top of each bar. Grouping legend is provided in Figure 2. (*) Significantly different from original at P = 0.05.
In a few cases, severity and incidence of the selected populations were even higher than their respective original populations (P < 0.05). These were demonstrated in the FBR severity of the MBS population of NuMex Mesa; FBR incidence (%) of the SS populations of NuMex Luna, NuMex Mesa, and NuMex Vado (Figure 4, Figure 5 and Figure 7). Disease severity and incidence (%) were lower at four weeks after harvest for several cultivar populations. This phenomenon was observed in both FBR parameters for NuMex Luna (Figure 4b,c); FBR incidence (%) for NuMex Camino (Figure 2c), NuMex Crispy (Figure 3c), NuMex Mesa (Figure 5b), NuMex Sweetpak (Figure 6c), and NuMex Vado (Figure 7c).
3.2. Seedling Inoculation Evaluation of the Original, MBS, and SS Populations
The initial growth chamber runs with only the resistant and susceptible controls, “Sierra Blanca” and “Rumba”, revealed that neither the inoculum batches nor the growth chambers had any significant effect on the percent survival of their seedlings (Data not presented). The percentage of seedling survival for “Sierra Blanca” (57.7% to 84.8%) and “Rumba” (24.5% to 38.0%) in the inoculated trays implied higher and lower survival rates as expected for the resistant and susceptible control, respectively. However, seed germination percentages ranges for “Sierra Blanca” and “Rumba’ were relatively lower in the uninoculated trays, i.e., 65%–79% and 64%–82%, respectively (Data not presented).
Seedling survival rate (%) of six SS populations were significantly greater (P < 0.05) than their original populations (Figure 2a, Figure 3a, Figure 4a, Figure 6a, Figure 7a and Figure 8).
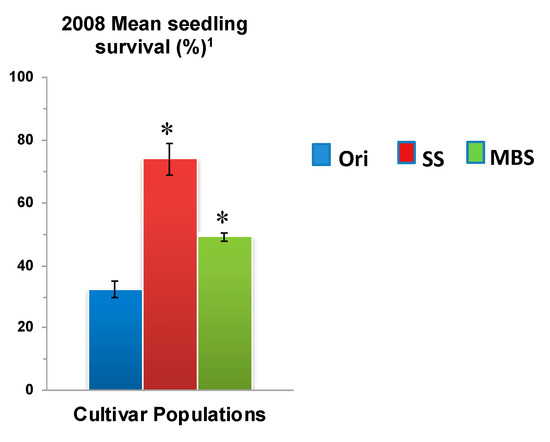
Figure 8.
Mean seedling survival of NuMex Chaco Original (Ori), Seedling-selected (SS) and Mature Bulb-selected (MBS) populations for two runs in 2008 evaluation. 75 seeds of each population were sown in each tray for seedling evaluation. Std. error is shown on top of each bar. Grouping legend is provided in the right. (*) Significantly different from original at P = 0.05. 1 Field evaluation was not possible for NuMex Chaco due to lack of sufficient seed.
The greatest increase in survival was observed for “NuMex Crispy” (85.1%, P < 0.05), which had one of the lowest survival rates in its original population (31.4%) (Figure 3a). However, seedling survival rate of the MBS populations were not significantly higher than their original populations except “NuMex Chaco”, which exhibited an increase (original—32.4%, selected—49.3%, P < 0.05) in seedling survival (Figure 8). These results again indicate that a large amount of progress has been made in seeding stage with just one cycle of selection using the SS method.
4. Discussion
This study revealed that the field screening method is not an efficient method either to increase FBR resistance among the selected cultivar populations or to evaluate the progress that was made using either screening method (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7). A lack of progress among the field-selected populations was confirmed by their low seedling survival for most of the cultivars during seedling evaluations (Figure 2a, Figure 3a, Figure 4a, Figure 6a and Figure 7a). Disease severity and incidence results of the selected cultivar populations through the field inoculation screening were very inconsistent at harvest, four weeks after storage, and between two consecutive years. These inconsistent results make it difficult to determine if the selection was ineffective for making progress or if the evaluation method was ineffective for assessing the level of FBR resistance found in this germplasm. Even though a very high amount of FBR incidence was reported earlier in the FGSC fields [32], the FOC spore distribution has been shown to be non-random and not uniform throughout a field [23]. With this field distribution of the pathogen, it is unknown whether bulbs are coming in contact with the pathogen or not. Additionally, the lack of favorable disease-causing environments under field conditions in different years could also result in inconsistent FBR infestation and generate a false interpretation of resistant germplasm. The lack of repeatability over years has been observed in our earlier FGSC field trials [32] suggesting that the pathogen is not coming in contact with the host on a consistent basis. Similar difficulties in field screenings for disease resistant materials have also been observed in other crops, e.g., in cotton for not uniform distribution of V. dahliae Kleb. (causes Verticillium wilt) and seasonal environmental variation [36]; in common bean for lack of viruliferous leafhopper (vector of Beet curly top virus) infestation in early season field test [37], in potato for uneven distribution of Spongospora subterranean (Wallr.) Lagerheim f.sp. subterranean Tomlinson (causes potato tuber scab) and absence of favorable environmental conditions at an early growth period [38].
Fusarium oxysporum Schlecht. emend. Snyder & Hansen is a complex soil saprophyte with several formae speciales (f.sp.) depending upon the host crop [39]. They comprise both pathogenic and non-pathogenic isolates that are prevalent in natural soils. A single pathogenic isolate of FOC could create variable susceptibility reaction to different onion cultivars [35]. On the other hand, a single cultivar could also produce variable FBR reactions to different FOC isolates [35]. A valuable insight of these pathogenic interactions to cause basal stem rot could be found in monocot oil palm studies, where the disease development in the field depends on pathogen species, isolate types, competition with other microbes, nutrition level of the field, and shading [24,40]. Adding to this complexity, in several cases, non-pathogenic isolates have been found to be microbial antagonists that prevent other pathogenic rhizospheric microbes to proliferate and cause disease. Microbial antagonists can create suppressive soils for pathogenic rhizospheric microbes under repeated cultivation [41,42,43]. The fields of the FGSC were also used for onion monoculture for the last three decades, and hence suggest the possible existence of suppressive soils formation. Currently, a substantial amount of FBR incidence is generally observed every year during the evaluation of multiple breeding lines. However, a future study to determine the formation of suppressive soils might be advantageous to determine the future usefulness of the field inoculation bulb evaluation.
During the field evaluation of the original and selected cultivar populations, insignificant differences in FBR severity and incidence four weeks after storage essentially reaffirming that FOC causes the most damage during storage [4]. A variable quantity of FOC spores that might come in contact with the bulbs under field conditions could manifest a variable amount of disease progression between bulbs during storage. Prior studies conducted at New Mexico State University suggest that a waiting period of four weeks after harvest is necessary to allow for the development of FBR symptoms in those non-symptomatic bulbs that were grown in naturally infested fields [4]. In contrast, the reduction in disease severity and incidence after four weeks of storage could be explained by the fact that two sets of 20 bulbs each, which were picked randomly to record the two disease parameters, were different at harvest and at four weeks after storage. FBR disease continues to progress in any infected bulb until the entire basal plate tissue is consumed, leading towards secondary contamination at the later stage [4]. Therefore, the infected bulbs with an advanced stage of infection, which were rated after harvest, would have been rotten and decayed four weeks after storage. At this stage, the rater could have picked the healthy-looking bulbs for the second scoring. If an FBR rating must be recorded both at harvest and four weeks after storage, then repeated measurements on the same set of bulbs should be followed. In this way an onion breeder could also measure FBR resistance, which often times results into slower progression in the resistant bulbs [4]. Otherwise, a disease rating of mature onion bulbs could be taken at least four weeks after storage.
During the initial runs of the seedling screening evaluation with only the resistant and susceptible controls, the higher and lower seedling survival rates of the resistant and susceptible checks in the inoculated trays, respectively, were in line with the fact that absolute resistance and susceptibility for FBR has not yet been achieved in onion [4]. The relatively lower germination of them in the uninoculated trays could be linked to reduced viability of the seed lots or the evaluation process itself. Uneven and lower seed germination in a seedling evaluation of onion cultivars was observed in earlier seedling screening studies [13,35] and could be an inherent characteristic of this method, as all of the seeds of each check in our study came from a single lot and the environmental conditions were uniform inside all of the growth chambers throughout the study. To account for the poor seed viability, seedling survival of any cultivar population, whether original or selected, was calculated with respect to the non-inoculated trays. This allowed us not to make an experimental error by falsely interpreting any susceptible and resistant lines [13]. Nevertheless, the seedling survival rate of the susceptible check “Rumba” was lower than the SS or MBS populations survival and at par with the original population survival for most of the cultivars (Data not presented). This result suggested that one cycle of selection can be effective at increasing the survival rate to a level that is greater than that observed in a susceptible check. Further selections using the seedling inoculation method are needed to increase the level of FBR resistance for all cultivars tested. However, the amount of gain per cycle for subsequent selections would be expected to be less than the gain made from the initial selection.
The most important observation from the seedling evaluation was higher seedling survival percentages of the seedling-selected populations than the original populations for all cultivars (Figure 2a, Figure 3a, Figure 4a, Figure 6a, Figure 7a, and Figure 8). This improvement of FBR resistance in the seedling-selected populations was not reflected when they were evaluated by field evaluation (Figure 2b,c, Figure 3b,c, Figure 4b,c, Figure 5, Figure 6b,c; and Figure 7b,c), much similar to what was observed by Holz and Knox-Davies in 1974 [21] and Ozer et al. in 2004 [10]. Resistance reaction differences in two growth stages of plants could be linked to the genetic, physiological or morphological differences between onion seedlings and adult plants apart from random distribution of the pathogen in the field. Fusarium root rot and Fusarium head blight studies in wheat revealed that the resistance or susceptibility against Fusarium graminaearum depends on a systematic activation or deactivation of mycotoxin detoxification genes, which restricts the spread of the infection, and rapid up-regulation of jasmonic acid signaling genes, respectively. Expressions of these genes depends upon the growth stages of wheat, resulting in seedling resistance and adult susceptibility or vice versa [20]. As this expression paradigm changes with genotype [20], it could result in variable progress of FBR resistance among the cultivars under selection. Susceptibility of the adult plants of onion could also happen due to changes in the plant metabolism as the plants form bulbs even if the transcription level of the resistance-related genes remains the same at all the developmental stages. This could be demonstrated by a foliar late blight study of potato, where the susceptibility of the adult plants was due to using metabolic energy for tuber production rather than plant defense, with consistent R-gene transcript levels in all the physiological ages [22]. This phenomenon is similar in onion as it forms bulb, which is a major sink for the plant and requires a lot of metabolic energy. As a result, the mature plants will not be able to withstand the attack of FOC as they would in much younger stage. In addition to the metabolic energy diversion, root cortex cell death of an adult onion plant due to physiological aging could harbor saprophytic fungi, such as Fusarium and result in a higher inoculum pressure than seedling roots [4,20].
The discussion above emphasizes the importance of choosing the same growth stage for both screening and evaluation. In our case, the selections were performed at two different growth stages. Resistance in the seedling stage may not necessarily be observed if germplasm is evaluated at an adult stage and vice versa. Seedling selection has an added advantage over field inoculation due to a single, highly virulent FOC isolate (CSC–515) [35] that is used to inoculate artificially. As a result, there is no competition between multiple isolates and less chance of a disease escape in contrast to field inoculation. These reasons support why the seedling inoculation method might be more effective for making progress than the field inoculation method.
5. Conclusions and Recommendations
This study demonstrated that the seedling inoculation screening method performed better than the field inoculation screening method in increasing the level of FBR resistance among the NM commercial short-day onion cultivars used in this study. However, this resistance was only confined to the seedling stage, which could be due to the difference in FBR resistance influenced by physiological aging. The field inoculation exhibited highly inconsistent results due to randomly distributed multiple FOC isolates and variable environmental conditions. A permanently infested field with more uniform distribution of FOC inoculum could be used as a final evaluation practice rather than making initial screenings, because of its resemblance to a commercial onion producer’s field condition. Maximum gain of increasing FBR resistance could be achieved when the same method was performed for both during the selection as well as in the evaluation stage. In the context of possible multiple FBR resistance mechanisms existing at different growth stages, another feasible screening method could be achieved by artificially inoculating the mature onion bulbs and looking for FBR-resistant bulbs, as most damage by FOC occurs in this stage [4].
Author Contributions
Data curation, formal analysis, visualization, writing—original draft preparation, writing—review and editing, S.M.; investigation, methodology, data curation, formal analysis, A.S.; conceptualization, supervision, investigation, methodology, data curation, writing—review and editing, C.S.C.; Formal analysis, R.L.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the New Mexico Agricultural Experiment Station and the New Mexico Dry Onion Commission for their funding support.
Acknowledgments
The authors would like to thank Ray Muhyi and the undergraduate students for their assistance in managing plants in the greenhouse and the field.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cramer, C.S. Current and Future Objectives of NMSU’s Onion Breeding Program. In Onion Production and Marketing in New Mexico; Cooperative Extension Service: Las Cruces, NM, USA, 2002; p. 577. [Google Scholar]
- Bacher, J.W.; Pan, S.; Ewart, L. Inheritance of Resistance to Fusarium Oxysporum f. sp. cepae in Cultivated Onions. In Proceedings of the National Onion Research Conference, Boise, ID, USA, 7–8 December 1989; pp. 85–91. [Google Scholar]
- Deacon, J. Fungi as Plant Pathogens. In Fungal Biology; Blackwell Publishing Limited: Malden, MA, USA, 2006; pp. 279–308. [Google Scholar]
- Cramer, C.S. Breeding and Genetics of Fusarium Basal Rot Resistance in Onion. Euphytica 2000, 115, 159–166. [Google Scholar] [CrossRef]
- Havey, M.J. Onion and Other Cultivated Alliums. In Evolution of Crop Plants; Smart, J., Simmonds, N.W., Eds.; Wiley: New York, NY, USA, 1995; pp. 344–350. [Google Scholar]
- Bennett, A.J.; Bending, G.D.; Chandler, D.; Hilton, S.; Mills, P. Meeting the Demand for Crop Production: The Challenge of Yield Decline in Crops Grown in Short Rotations. Biol. Rev. 2012, 87, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Thornton, M.K.; Mohan, S.K. Response of Sweet Spanish Onion Cultivars to Basal Rot and Pink Root. Plant Dis. 1996, 80, 660–663. [Google Scholar] [CrossRef]
- Brown, B.D. Onion Response to Fumigation and P Placement. Onion World 2001, 17, 8–9. [Google Scholar]
- Jawson, M.D.; Franzluebbers, A.J.; Galusha, D.K.; Aiken, R.M. Soil Fumigation within Monoculture and Rotations: Response of Corn and Mycorrhizae. Agron. J. 1993, 85, 1174–1180. [Google Scholar] [CrossRef]
- Özer, N.; Köycü, N.D.; Chilosi, G.; Magro, P. Resistance to Fusarium Basal Rot of Onion in Greenhouse and Field and Associated Expression of Antifungal Compounds. Phytoparasitica 2004, 32, 388–394. [Google Scholar] [CrossRef]
- Esfahani, M.N.; Hosseini, M.; Nasehi, A.; Golkhandan, E. Screening of Onion Seed Sets for Resistance against New Iranian Isolates of Fusarium Oxysporum f.sp. cepa. Arch. Phytopath. Plant Prot. 2013, 46, 1864–1873. [Google Scholar] [CrossRef]
- Galván, G.A.; Koning-Boucoiran, C.F.S.; Koopman, W.J.M.; Burger-Meijer, K.; González, P.H.; Waalwijk, C.; Kik, C.; Scholten, O.E. Genetic Variation among Fusarium Isolates from Onion, and Resistance to Fusarium Basal Rot in Related Allium Species. Eur. J. Plant Pathol. 2008, 121, 499–512. [Google Scholar] [CrossRef]
- Taylor, A.; Vagany, V.; Barbara, D.J.; Thomas, B.; Pink, D.A.C.; Jones, J.E.; Clarkson, J.P. Identification of Differential Resistance to Six Fusarium oxysporum f.sp. cepae Isolates in Commercial Onion Cultivars through the Development of a Rapid Seedling Assay. Plant Pathol. 2013, 62, 103–111. [Google Scholar] [CrossRef]
- Caligiore Gei, P.F.; Valdez, J.G.; Piccolo, R.J.; Galmarini, C.R. Influence of Fusarium Spp.. Isolate and Inoculum Density on Resistance Screening Tests in Onion. Trop. Plant Pathol. 2014, 39, 19–27. [Google Scholar] [CrossRef]
- Rout, E.; Tripathy, P.; Nanda, S.; Nayak, S.; Joshi, R.K. Evaluation of Cultivated and Wild Allium Accessions for Resistance to Fusarium oxysporum f.sp. cepae. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2016, 86, 643–649. [Google Scholar] [CrossRef]
- Lopez, J.A.; Cramer, C.S. Screening Short-Day Onion Varieties for Resistance to Fusarium Basal Rot. Acta Hortic. 2004, 637, 169–173. [Google Scholar] [CrossRef]
- Goldman, I.L. A List of Germplasm Releases from the University of Wisconsin Table Beetbreeding Program, 1964–1992. HortScience 1996, 31, 878–879. [Google Scholar] [CrossRef]
- Retig, N.; Kust, A.F.; Gabelman, W.H. Greenhouse and Field Tests for Determining the Resistance of Onion Lines to Fusarium Basal Rot. J. Am. Soc. Hort. Sci. 1970, 95, 422–424. [Google Scholar]
- Somkuwar, R.G.; Veere Gowda, R.; Singh, T.H.; Pathak, C.S. Screening of Onion for Resistance to Onion Basal Rot. Madras Agric. J. 1996, 83, 273–275. [Google Scholar]
- Wang, Q.; Shao, B.; Shaikh, F.I.; Friedt, W.; Gottwald, S. Wheat Resistances to Fusarium Root Rot and Head Blight Are Both Associated with Deoxynivalenol- and Jasmonate-Related Gene Expression. Phytopathology 2018. [Google Scholar] [CrossRef] [PubMed]
- Holz, G.; Davies, P.S. Resistance of Onion Selections to Fusarium oxysporum f.sp. cepae. Phytophylactica 1974, 6, 153–156. [Google Scholar]
- Millett, B.P.; Mollov, D.S.; Iorizzo, M.; Carputo, D.; Bradeen, J.M. Changes in Disease Resistance Phenotypes Associated with Plant Physiological Age Are Not Caused by Variation in R Gene Transcript Abundance. Mol. Plant Microbe Interact. 2009. [CrossRef]
- Saxena, A. Screening of Onion Cultivars for Fusarium Basal Rot and Spatial Distribution of Fusarium oxysporum f.sp. cepae. Master’s Thesis, New Mexico State University, Las Cruces, NM, USA, 2007. [Google Scholar]
- Pilotti, C.A. Stem Rots of Oil Palm Caused by Ganoderma Boninense: Pathogen Biology and Epidemiology. Mycopathologia 2005, 159, 129–137. [Google Scholar] [CrossRef]
- Cramer, C.S.; Corgan, J.N. “NuMex Camino” Onion. HortScience 2003, 38, 1251–1252. [Google Scholar] [CrossRef]
- Wall, M.; Corgan, J. “NuMex Sweetpak” Onion. HortScience 1999, 34, 1303–1304. [Google Scholar] [CrossRef]
- Corgan, J.N. ‘NuMex Mesa’ Onion; University of Agricultural Experiment Station: Las Cruces, NM, USA, 1996. [Google Scholar]
- Cramer, C.S.; Corgan, J.N. “Numex Chaco” Onion. HortScience 2001, 38, 1337–1338. [Google Scholar] [CrossRef]
- Corgan, J.N. ‘NuMex Crispy’ Onion; University of Agricultural Experiment Station: Las Cruces, NM, USA, 1996. [Google Scholar]
- Corgan, J.N. ‘NuMex Vado’ and ‘NuMex Luna’ Onion Varieties; University of Agricultural Experiment Station: Las Cruces, NM, USA, 1995. [Google Scholar]
- Lopez, J.A.; Cramer, C.S. Screening Intermediate-Day Onion Lines for Fusarium Basal Rot Resistance. In Proceedings of the National Onion Research Conference, Pasco, WA, USA, 11–14 December 2002; pp. 82–86. [Google Scholar]
- Gutierrez, J.A.; Cramer, C.S. Screening Short-Day Onion Cultivars for Resistance to Fusarium Basal Rot. HortScience 2005, 40, 157–160. [Google Scholar] [CrossRef]
- Corgan, J.N.; Wall, M.M.; Cramer, C.S.; Sammis, T.; Lewis, B.; Schroeder, J. Bulb Onion Culture and Management; New Mexico State University: Las Cruces, NM, USA, 2000; p. 563. [Google Scholar]
- Gutierrez, J.A.; Molina-Bravo, R.; Cramer, C.S. Screening Winter-Sown, Intermediate-Day Onion Cultivars for Resistance to Fusarium Basal Rot. Horttechnology 2006, 16, 177–181. [Google Scholar] [CrossRef]
- Saxena, A.; Cramer, C.S. Screening of Onion Seedlings for Resistance against New Mexico Isolates of Fusarium oxysporum f.sp. cepae. J. Plant Pathol. 2009, 91, 197–200. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, H.; Zhou, H.; Sanogo, S.; Ma, Z. Genetics, Breeding, and Marker-Assisted Selection for Verticillium Wilt Resistance in Cotton. Crop Sci. 2014, 54, 1289–1303. [Google Scholar] [CrossRef]
- Singh, S.P.; Schwartz, H.F. Breeding Common Bean for Resistance to Diseases: A Review. Crop Sci. 2010, 50, 2199–2223. [Google Scholar] [CrossRef]
- Merz, U.; Falloon, R.E. Review: Powdery Scab of Potato-Increased Knowledge of Pathogen Biology and Disease Epidemiology for Effective Disease Management. Potato Res. 2009, 52, 17–37. [Google Scholar] [CrossRef]
- Gordon, T.R.; Martyn, R.D. The Evolutionary Biology Of Fusarium oxysporum. Annu. Rev. Phytopathol. 1997, 35, 111–128. [Google Scholar] [CrossRef]
- Rees, R.W.; Flood, J.; Hasan, Y.; Cooper, R.M. Effects of Inoculum Potential, Shading and Soil Temperature on Root Infection of Oil Palm Seedlings by the Basal Stem Rot Pathogen Ganoderma Boninense. Plant Pathol. 2007. [Google Scholar] [CrossRef]
- Busby, P.E.; Ridout, M.; Newcombe, G. Fungal Endophytes: Modifiers of Plant Disease. Plant Mol. Biol. 2016, 90, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Wissuwa, M.; Mazzola, M.; Picard, C. Novel Approaches in Plant Breeding for Rhizosphere-Related Traits. Plant Soil 2009, 321, 409–430. [Google Scholar] [CrossRef]
- Nel, B.; Steinberg, C.; Labuschagne, N.; Viljoen, A. The Potential of Nonpathogenic Fusarium Oxysporum and Other Biological Control Organisms for Suppressing Fusarium Wilt of Banana. Plant Pathol. 2006, 55, 217–223. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).