Impact of Chitosan, Sucrose, Glucose, and Fructose on the Postharvest Decay, Quality, Enzyme Activity, and Defense-Related Gene Expression of Strawberries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
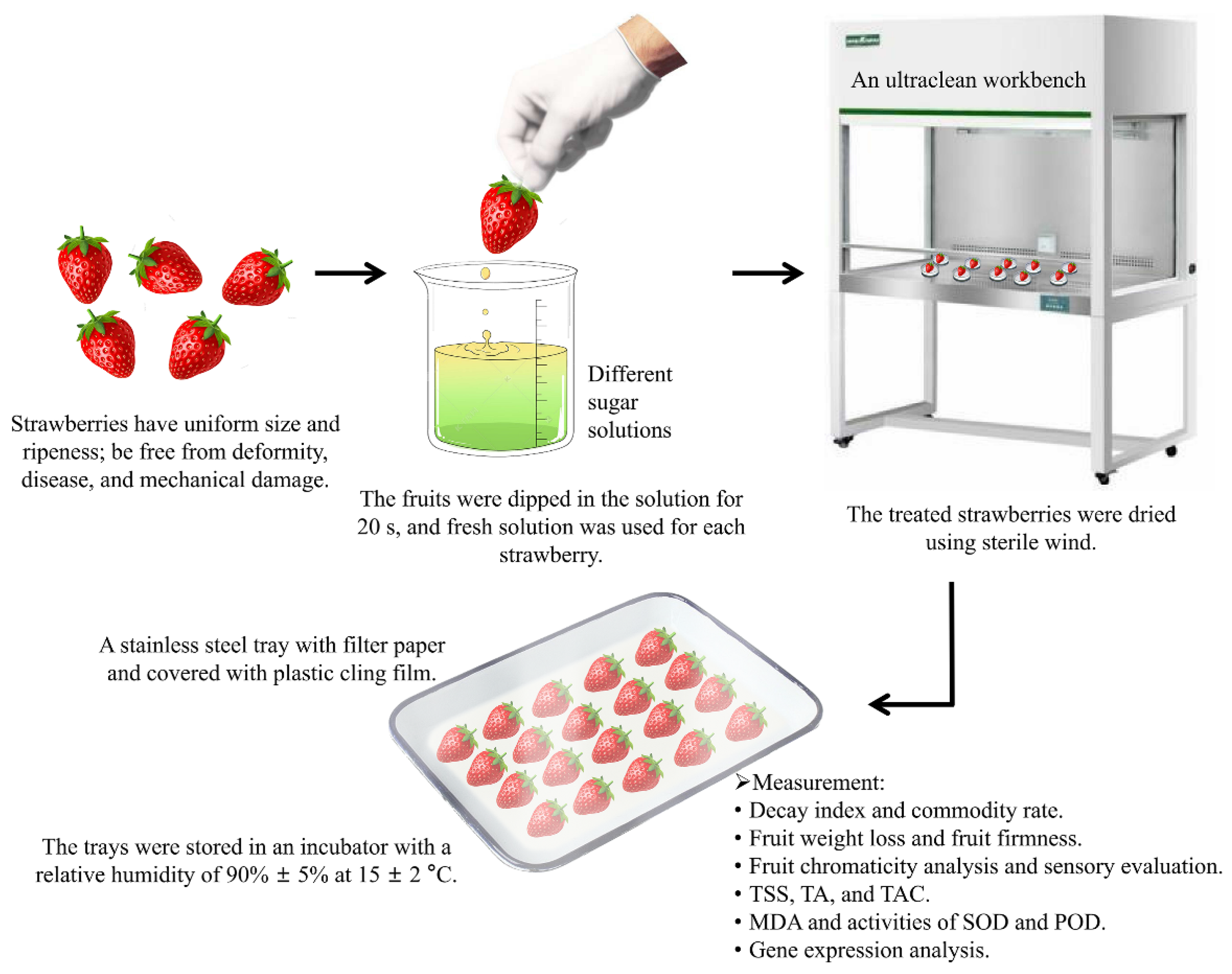
2.2. Experimental Design
2.3. Analytical Methods
2.3.1. Evaluation of Decay Index and Commodity Rate
grade × total number of fruits)] × 100%
with Grade 1 decay)/total number of fruits × 100%
2.3.2. Measurement of Weight Loss
storage intervals)/weight of fruit before storage] × 100
2.3.3. Fruit Firmness Measurement
2.3.4. Fruit Chromaticity Analysis
2.3.5. Sensory Evaluation
2.3.6. Determination of Total Soluble Solids (TSS), Titratable Acidity (TA), and Total Anthocyanin Content (TAC)
2.3.7. Determination of Malondialdehyde (MDA) Content and Activities of Superoxide Dismutase and Catalase
2.3.8. Analysis of Gene Expression
2.4. Statistical Analysis
3. Results
3.1. Sugar Solution Concentration Screening Experiment
3.2. Effects of Treatment on Decay Index and Commodity Rate
3.3. Effects of Treatments on Firmness, Weight Loss, and Color after Storage
3.4. Effects of Treatments on Sensory Scores of Strawberries after Storage
3.5. Effects of Treatments on Total Soluble Solid (TSS), Titratable Acid (TA), and Total Anthocyanins Content (TAC)
3.6. Effects of Treatments on Malondialdehyde (MDA) Content and Antioxidant Enzyme Activity
3.7. Effects of Treatments on the Expression of Genes Related to Fruit Senescence, Quality, and Disease Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Campaniello, D.; Bevilacqua, A.; Sinigaglia, M.; Corbo, M.R. Chitosan: Antimicrobial activity and potential applications for preserving minimally processed strawberries. Food Microbiol. 2008, 25, 992–1000. [Google Scholar] [CrossRef]
- Yosefi, A.; Mozafari, A.; Javadi, T. Jasmonic acid improved in vitro strawberry (Fragaria × ananassa Duch.) resistance to PEG-induced water stress. Plant Cell Tiss. Organ. Cult. 2020, 142, 549–558. [Google Scholar] [CrossRef]
- Blanch, M.; Goñi, O.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. Characterisation and functionality of fructo-oligosaccharides affecting water status of strawberry fruit (Fragraria vesca cv. Mara de Bois) during postharvest storage. Food Chem. 2012, 134, 912–919. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Siebeneichler, T.J.; Crizel, R.L.; Camozatto, G.H.; Paim, B.T.; Messias, R.S.; Rombaldi, C.V.; Galli, V. The postharvest ripening of strawberry fruits induced by abscisic acid and sucrose differs from their in vivo ripening. Food Chem. 2020, 317, 126407. [Google Scholar] [CrossRef]
- Vighi, I.L.; Crizel, R.L.; Perin, E.C.; Rombaldi, C.V.; Galli, V. Crosstalk during fruit ripening and stress response among abscisic acid, calcium-dependent protein kinase and phenylpropanoid. Crit. Rev. Plant Sci. 2019, 38, 99–116. [Google Scholar] [CrossRef]
- Sallato, B.V.; Torres, R.; Zoffoli, J.P.; Latorre, B.A. Effect of boscalid on postharvest decay of strawberry caused by Botrytis cinerea and Rhizopus stolonifer. Span. J. Agric. Res. 2007, 5, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Osorio, L.F.; Pattison, J.A.; Peres, N.A.; Whitaker, V.M. Genetic variation and gains in resistance of strawberry to Colletotrichum gloeosporioides. Phytopathology 2014, 104, 67. [Google Scholar] [CrossRef] [Green Version]
- Cagnon, T.; Méry, A.; Chalier, P.; Guillaume, C.; Gontard, N. Fresh food packaging design: A requirement driven approach applied to strawberries and agro-based materials. Innov. Food Sci. Emerg. Technol. 2013, 20, 288–298. [Google Scholar] [CrossRef]
- Li, X.; Xie, X.; Xing, F.; Xu, L.; Zhang, J.; Wang, Z. Glucose oxidase as a control agent against the fungal pathogen Botrytis cinerea in postharvest strawberry. Food Control 2019, 105, 277–284. [Google Scholar] [CrossRef]
- Petrasch, S.; Knapp, S.J.; van Kan, J.A.L.; Blanco-Ulate, B. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 877–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellitto, V.M.; Zara, S.; Fracchetti, F.; Capozzi, V.; Nardi, T. Microbial biocontrol as an alternative to synthetic fungicides: Boundaries between pre- and postharvest applications on vegetables and fruits. Fermentation 2021, 7, 60. [Google Scholar] [CrossRef]
- Han, C.; Zhao, Y.; Leonard, S.W.; Traber, M.G. Edible coatings to improve storability and enhance nutritional value of fresh and frozen strawberries (Fragaria × ananassa) and raspberries (Rubus ideaus). Postharvest Biol. Technol. 2004, 33, 67–78. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, H.; Dong, C.; Ji, H.; Zhang, X.; Li, L.; Ban, Z.; Zhang, N.; Xue, W. Effect of ozone treatment on the phenylpropanoid biosynthesis of postharvest strawberries. RSC Adv. 2019, 9, 25429. [Google Scholar] [CrossRef] [Green Version]
- Civello, P.M.; Martínez, G.A.; Chaves, A.R.; Añón, M.C. Heat treatments delay ripening and postharvest decay of strawberry fruit. J. Agric. Food Chem. 1997, 45, 4589–4594. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Z.; Li, J.; Tao, C.; Feng, Y.; Han, Y. A novel endophytic strain of Lactobacillus plantarum CM-3 with antagonistic activity against Botrytis cinerea on strawberry fruit. Biol. Control 2020, 148, 104306. [Google Scholar] [CrossRef]
- He, Y.; Bose, S.K.; Wang, M.; Li, T.; Wang, W.; Lu, H.; Yin, H. Effects of chitosan oligosaccharides postharvest treatment on the quality and ripening related gene expression of cultivated strawberry fruits. J. Berry Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Ali, M.R.; Darwish, O.S.; Rogers, H.J. Impact of salicylic acid, abscisic acid, and methyl jamonate on postharvest quality and bioactive compounds of cultivated strawberry fruit. J. Berry Res. 2019, 9, 333–348. [Google Scholar] [CrossRef]
- Zhao, J.; Pan, L.; Zhou, M.; Yang, Z.; Meng, Y.; Zhang, X. Comparative physiological and transcriptomic analyses reveal mechanisms of improved osmotic stress tolerance in annual ryegrass by exogenous chitosan. Genes 2019, 10, 853. [Google Scholar] [CrossRef] [Green Version]
- Pongprayoon, W.; Roytrakul, S.; Pichayangkura, R.; Chadchawan, S. The role of hydrogen peroxide in chitosan-induced resistance to osmotic stress in rice (Oryza sativa L.). Plant Growth Regul. 2013, 70, 159–173. [Google Scholar] [CrossRef]
- Virgen-Ortiz, J.J.; Morales-Ventura, J.M.; Colín-Chávez, C.; Esquivel-Chávez, F.; Vargas-Arispuro, I.; Aispuro-Hernández, E.; Martínez-Téllez, M.A. Postharvest application of pectic-oligosaccharides on quality attributes, activities of defense-related enzymes, and anthocyanin accumulation in strawberry. J. Sci. Food Agric. 2020, 100, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; Rabea, E.I.; El-Nouby, M.A.M.; Ismail, R.I.A.; Taktak, N.E.M. Strawberry shelf life, composition, and enzymes activity in response to edible chitosan coatings. Int. J. Fruit Sci. 2017, 17, 117–136. [Google Scholar] [CrossRef]
- Xu, F.; Tang, Y.; Dong, S.; Shao, X.; Wang, H.; Zheng, Y.; Yang, Z. Reducing yellowing and enhancing antioxidant capacity of broccoli in storage by sucrose treatment. Postharvest Biol. Technol. 2016, 112, 39–45. [Google Scholar] [CrossRef]
- Fei, L.; Yuan, X.; Chen, C.; Wan, C.; Fu, Y.; Chen, J.; Gan, Z. Exogenous Application of Sucrose Promotes Postharvest Ripening of Kiwifruit. Agronomy 2020, 10, 245. [Google Scholar] [CrossRef] [Green Version]
- Molinett, S.A.; Alfaro, J.F.; Sáez, F.A.; Elgueta, S.; Moya-León, M.A.; Figueroa, C.R. Postharvest treatment of hydrogen sulfide delays the softening of chilean strawberry fruit by downregulating the expression of key genes involved in pectin catabolism. Int. J. Mol. Sci. 2021, 22, 10008. [Google Scholar] [CrossRef]
- Kobayashi, S.; Goto-Yamamoto, N.; Hirochika, H. Retrotransposon-induced mutations in grape skin color. Science 2004, 304, 982. [Google Scholar] [CrossRef]
- Hernández-Muñoz, P.; Almenar, E.; Del Valle, V.; Velez, D.; Gavara, R. Effect of chitosan coating combined with postharvest calcium treatment on strawberry (Fragaria × ananassa) quality during refrigerated storage. Food Chem. 2008, 110, 428–435. [Google Scholar] [CrossRef]
- Nguyen, V.; Nguyen, D.; Nguyn, H. Combination effects of calcium chloride and nano-chitosan on the postharvest quality of strawberry (Fragaria × ananassa duch.). Postharvest Biol. Technol. 2020, 162, 111103. [Google Scholar] [CrossRef]
- Lu, H.; Wang, K.; Wang, L.; Li, D.; Yan, J.; Ban, Z.; Luo, Z.; Li, L.; Yang, D. Effect of superatmospheric oxygen exposure on strawberry (Fragaria × ananassa Fuch.) volatiles, sensory and chemical attributes. Postharvest Biol. Technol. 2018, 142, 60–71. [Google Scholar] [CrossRef]
- Vieira, J.M.; Flores-López, M.L.; de Rodríguez, D.J.; Sousa, M.C.; Vicente, A.A.; Martins, J.T. Effect of chitosan—Aloe vera coating on postharvest quality of blueberry (Vaccinium corymbosum) Fruit. Postharvest Biol. Technol. 2016, 116, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.; Yang, S.; Xu, Q.; Xu, L.; Zhu, D.; Li, X.; Shui, Y.; Liu, X.; Bi, X. Effect of chitosan/nano-TiO2 composite coating on the postharvest quality of blueberry fruit. Coatings 2021, 11, 512. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Wu, K.; Zhang, Q.; Feng, Y.; Miao, Y.; Yan, Z. Exogenous application of chitosan alleviate salinity stress in lettuce (Lactuca sativa L.). Horticulturae 2021, 7, 342. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principaldye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Patel, C.; Panigrahi, J. Starch glucose coating-induced postharvest shelf-life extension of cucumber. Food Chem. 2019, 288, 208–214. [Google Scholar] [CrossRef]
- Zheng, F.; Zheng, W.; Li, L.; Pan, S.; Liu, M.; Zhang, W.; Liu, H.; Zhu, C. Chitosan controls postharvest decay and elicits defense response in kiwifruit. Food Bioproc. Technol. 2017, 10, 1937–1945. [Google Scholar] [CrossRef]
- Gol, N.B.; Patel, P.R.; Rao, T.R. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol. Technol. 2013, 85, 185–195. [Google Scholar] [CrossRef]
- Eshghi, S.; Hashemi, M.; Mohammadi, A.; Badii, F.; Mohammadhoseini, Z.; Ahmadi, K. Effect of nanochitosan-based coating with and without copper loaded on physicochemical and bioactive components of fresh strawberry fruit (Fragaria × ananassa Duchesne) during storage. Food Bioproc. Technol. 2014, 7, 2397–2409. [Google Scholar] [CrossRef]
- Zhang, P.; Jia, H.; Gong, P.; Sadeghnezhad, E.; Pang, Q.; Dong, T.; Li, T.; Jin, H.; Fang, J. Chitosan induces jasmonic acid production leading to resistance of ripened fruit against botrytis cinerea infection. Food Chem. 2021, 337, 127772. [Google Scholar]
- Chen, F.; Liu, H.; Yang, H.; Lai, S.; Cheng, X.; Xin, Y.; Yang, B.; Hou, H.; Yao, Y.; Zhang, S.; et al. Quality attributes and cell wall properties of strawberries (Fragaria annanassa Duch.) under calcium chloride treatment. Food Chem. 2011, 126, 450–459. [Google Scholar] [CrossRef]
- Chisari, M.; Barbagallo, R.N.; Spagna, G. Characterization of polyphenol oxidase and peroxidase and influence on browning of cold stored strawberry fruit. J. Agric. Food Chem. 2007, 55, 3469–3476. [Google Scholar] [CrossRef] [PubMed]
- Benítez, S.; Soro, L.; Achaerandio, I.; Sepulcre, F.; Pujolà, M. Combined effect of a low permeable film and edible coatings or calcium dips on the quality of fresh-cut pineapple. J. Food Process. Eng. 2014, 37, 91–99. [Google Scholar] [CrossRef]
- Hassan, J.; Anwar, R.; Khan, A.S.; Ahmad, S.; Malik, A.U.; Nafees, M.; Hussain, Z.; Inam-ur-Raheem, M. Chitosan-based edible coating delays fungal decay and maintains quality of strawberries during storage. Intl. J. Agric. Biol. 2020, 24, 486–492. [Google Scholar]
- Nie, Z.; Huang, Q.; Chen, C.; Wan, C.; Chen, J. Chitosan coating alleviates postharvest juice sac granulation by mitigating ROS accumulation in harvested pummelo (Citrus grandis L. Osbeck) during room temperature storage. Postharvest Biol. Technol. 2020, 169, 111309. [Google Scholar] [CrossRef]
- Bal, E.; Bahtiyar, A.Ü. Effects of chitosan coating with putrescine on bioactive compounds and quality of strawberry cv. san andreas during cold storage. Erwerbs-Obstbau 2021, 63, 1–8. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Saba, M.K.; Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Li, Z.; Wei, Y.; Xu, Y.; Han, P.; Jiang, S.; Xu, F.; Wang, H.; Tao, N.; Shao, X. Terpinen-4-ol treatment maintains quality of strawberry fruit during storage by regulating sucrose-induced anthocyanin accumulation. Postharvest Biol. Technol. 2021, 174, 111461. [Google Scholar] [CrossRef]
- Kalt, W.; Forney, C.F.; Martin, A.; Prior, R.L. Antioxidant capacity, vitamin C, phenolics, and anthocyanins after fresh storage of small fruit. J. Agric. Food Chem. 1999, 47, 4638–4644. [Google Scholar] [CrossRef]
- Nunes, M.C.N.; Brecht, J.K.; Morais, A.M.B.; Sargent, S.A. Possible influences of water loss and polyphenol oxidase activity on anthocyanin content and discoloration in fresh ripe strawberry (cv. Oso Grande) during storage at 1 °C. J. Food Sci. 2005, 70, S79–S84. [Google Scholar] [CrossRef]
- Ben-Yehoshua, S.; Rodov, V. Transpiration and water stress. Postharvest Physiol. Pathol. Veg. 2003, 2, 111–159. [Google Scholar]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of chitosan coating on the postharvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.V.H.; Nguyen, D.H.H. Effects of nano-chitosan and chitosan coating on the postharvest quality, polyphenol oxidase activity and malondialdehyde content of strawberry (Fragaria × ananassa Duch.). J. Hort. Postharvest Res. 2020, 3, 11–24. [Google Scholar]
- Jia, H.; Chai, Y.; Li, C.; Lu, D.; Luo, J.; Qin, L.; Shen, Y. Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol. 2011, 157, 188–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pareek, S. Calcium treatments. In Novel Postharvest Treatments of Fresh Produce; CRC Press: Boca Raton, FL, USA, 2017; pp. 79–106. [Google Scholar]
- Wang, S.Y.; Chen, C.S.; Yin, J.J. Effect of allyl isothiocyanate on antioxidants and fruit decay of blueberries. Food Chem. 2010, 120, 199–204. [Google Scholar] [CrossRef]
- Safari, Z.S.; Ding, P.; Juju Nakasha, J.; Yusoff, S.F. Combining chitosan and vanillin to retain postharvest quality of tomato fruit during ambient temperature storage. Coatings 2020, 10, 1222. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Lu, H.; Ye, C.; Guo, S.; Sheng, K.; Shao, L.; Zhou, T.; Yu, T.; Zheng, X. Preharvest application of antagonistic yeast Rhodosporidium paludigenum induced resistance against postharvest diseases in mandarin orange. Biol. Control 2013, 67, 130–136. [Google Scholar] [CrossRef]
- Petriccione, M.; Pagano, L.; Forniti, R.; Zampella, L.; Mastrobuoni, F.; Scortichini, M.; Mencarelli, F. Postharvest treatment with chitosan affects the antioxidant metabolism and quality of wine grape during partial dehydration. Postharvest Biol. Technol. 2018, 137, 38–45. [Google Scholar] [CrossRef]
- Lastdrager, J.; Hanson, J.; Smeekens, S. Sugar signals and the control of plant growth and development. J. Exp. Bot. 2014, 65, 799–807. [Google Scholar] [CrossRef]
- Shangguan, L.; Sun, X.; Zhang, C.; Mu, Q.; Leng, X.; Fang, J. Genome identification and analysis of genes encoding the key enzymes involved in organic acid biosynthesis pathway in apple, grape, and sweet orange. Sci. Hortic. 2015, 185, 22–28. [Google Scholar] [CrossRef]
- Tang, N.; An, J.; Deng, W.; Gao, Y.; Chen, Z.; Li, Z. Metabolic and transcriptional regulatory mechanism associated with postharvest fruit ripening and senescence in cherry tomatoes. Postharvest Biol. Technol. 2020, 168, 111274. [Google Scholar] [CrossRef]
- Rose, J.K.C.; Lee, H.H.; Bennett, A.B. Expression of a divergent expansin gene is fruit-specific and ripening-regulated. Proc. Natl. Acad. Sci. USA 1997, 94, 5955–5960. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Sun, C.; Wang, P.; Shan, L.; Cai, C.; Zhang, B.; Zhang, W.; Li, X.; Ferguson, I.; Chen, K. Expression of expansin genes during postharvest lignification and softening of ‘Luoyangqing’ and ‘Baisha’ loquat fruit under different storage conditions. Postharvest Biol. Technol. 2008, 49, 46–53. [Google Scholar] [CrossRef]
- Elomaa, P.; Uimari, A.; Mehto, M.; Albert, V.A.; Laitinen, R.A.E.; Teeri, T.H. Activation of anthocyanin biosynthesis in Gerbera hybrida (Asteraceae) suggests conserved protein-protein and protein-promoter interactions between the anciently diverged monocots and eudicots. Plant Physiol. 2003, 133, 1831–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravaglia, D.; Espley, R.V.; Henry-Kirk, R.A.; Andreotti, C.; Ziosi, V.; Hellens, R.P.; Costa, G.; Allan, A.C. Transcriptional regulation of flavonoid biosynthesis in nectarine (Prunus persica) by a set of R2R3 MYB transcription factors. BMC Plant Biol. 2013, 13, 68–81. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhao, F.; Zhang, G.; Jia, S.; Yan, Z. FaWRKY11 transcription factor positively regulates resistance to Botrytis cinerea in strawberry fruit. Sci. Hortic. 2021, 279, 109893. [Google Scholar] [CrossRef]
- Saavedra, G.M.; Sanfuentes, E.; Figueroa, P.M.; Figueroa, C.R. Independent preharvest applications of methyl jasmonate and chitosan elicit differential upregulation of defense-related genes with reduced incidence of gray mold decay during postharvest storage of Fragaria chiloensis fruit. Int. J. Mol. Sci. 2017, 18, 1420. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Ge, L.; Chen, K.; Zhao, L.; Zhang, X. Enhanced biocontrol activity of Rhodotorula mucilaginosa cultured in media containing chitosan against postharvest diseases in strawberries: Possible mechanisms underlying the effect. J. Agric. Food Chem. 2014, 62, 4214–4224. [Google Scholar] [CrossRef] [PubMed]







| Storage Time (d) | Treatment | Firmness (N) | Weight Loss (%) | a* Value | L* Value |
|---|---|---|---|---|---|
| 0 | CK | 4.49 ± 0.09 a | - | 25.51 ± 1.25 a | 53.83 ± 4.05 a |
| CTS (3 g/L) | 4.51 ± 0.54 a | - | 25.58 ± 2.95 a | 53.01 ± 4.81 a | |
| Suc (5 g/L) | 4.55 ± 0.31 a | - | 24.85 ± 3.64 a | 54.95 ± 3.21 a | |
| Glc (15 g/L) | 4.46 ± 0.50 a | - | 25.74 ± 5.64 a | 53.19 ± 4.01 a | |
| Fru (15 g/L) | 4.54 ± 0.49 a | - | 25.52 ± 3.77 a | 53.57 ± 3.74 a | |
| 9 | CK | 2.61 ± 0.36 b | 19.51 ± 0.11 a | 32.33 ± 2.55 a | 30.27 ± 2.65 b |
| CTS (3 g/L) | 2.87 ± 0.37 ab | 9.51 ± 0.05 c | 34.18 ± 2.60 a | 33.08 ± 4.81 ab | |
| Suc (5 g/L) | 2.94 ± 0.26 ab | 17.15 ± 2.11 ab | 34.67 ± 1.87 a | 31.56 ± 1.69 b | |
| Glc (15 g/L) | 3.21 ± 0.16 a | 18.74 ± 3.11 a | 35.78 ± 2.00 a | 37.18 ± 2.67 a | |
| Fru (15 g/L) | 2.67 ± 0.17 b | 14.62 ± 0.10 b | 33.20 ± 2.58 a | 30.30 ± 1.33 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yan, Z.; Tang, W.; Zhang, Q.; Lu, B.; Li, Q.; Zhang, G. Impact of Chitosan, Sucrose, Glucose, and Fructose on the Postharvest Decay, Quality, Enzyme Activity, and Defense-Related Gene Expression of Strawberries. Horticulturae 2021, 7, 518. https://doi.org/10.3390/horticulturae7120518
Wang Y, Yan Z, Tang W, Zhang Q, Lu B, Li Q, Zhang G. Impact of Chitosan, Sucrose, Glucose, and Fructose on the Postharvest Decay, Quality, Enzyme Activity, and Defense-Related Gene Expression of Strawberries. Horticulturae. 2021; 7(12):518. https://doi.org/10.3390/horticulturae7120518
Chicago/Turabian StyleWang, Yuanhua, Zhiming Yan, Weihua Tang, Qing Zhang, Bei Lu, Qiong Li, and Geng Zhang. 2021. "Impact of Chitosan, Sucrose, Glucose, and Fructose on the Postharvest Decay, Quality, Enzyme Activity, and Defense-Related Gene Expression of Strawberries" Horticulturae 7, no. 12: 518. https://doi.org/10.3390/horticulturae7120518
APA StyleWang, Y., Yan, Z., Tang, W., Zhang, Q., Lu, B., Li, Q., & Zhang, G. (2021). Impact of Chitosan, Sucrose, Glucose, and Fructose on the Postharvest Decay, Quality, Enzyme Activity, and Defense-Related Gene Expression of Strawberries. Horticulturae, 7(12), 518. https://doi.org/10.3390/horticulturae7120518





