The Effect of Changing Climatic Conditions on the Morphological Traits and Chemical Composition of Almond Kernels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Almond Samples and Location Characteristics
2.2. Oil Extraction
2.3. Tocopherol Determination
2.4. The Analysis of the Minerals
2.5. Statistical Analysis
3. Results and Discussion
3.1. Meteorological Data
3.2. The Kernel Morphological Traits
3.3. The Oil Content and the Tocopherol Composition
3.4. Mineral Composition
3.5. The Genotype and Year Interactions
3.6. Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richardson, D.P.; Astrup, A.; Cocaul, A.; Ellis, P. The Nutritional and Health Benefits of Almonds: A Healthy Food Choice. Food Sci. Technol. Bull. Funct. Foods 2009, 6, 41–50. [Google Scholar] [CrossRef]
- Beyhan, Ö.; Aktaş, M.; Yilmaz, N.; Şimşek, N.; Gerçekçioǧlu, R. Determination of Fatty Acid Compositions of Some Important Almond (Prunus amygdalus L.) Varieties Selected from Tokat Province and Eagean Region of Turkey. J. Med. Plant Res. 2011, 5, 4907–4911. [Google Scholar]
- Jenkins, D.J.A.; Kendall, C.W.C.; Marchie, A.; Josse, A.R.; Nguyen, T.H.; Faulkner, D.A.; Lapsley, K.G.; Singer, W. Effect of Almonds on Insulin Secretion and Insulin Resistance in Nondiabetic Hyperlipidemic Subjects: A Randomized Controlled Crossover Trial. Metabolism 2008, 57, 882–887. [Google Scholar] [CrossRef]
- Vadivel, V.; Kunyanga, C.N.; Biesalski, H.K. Health Benefits of Nut Consumption with Special Reference to Body Weight Control. Nutrition 2012, 28, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Ouzir, M.; El Bernoussi, S.; Tabyaoui, M.; Taghzouti, K. Almond Oil: A Comprehensive Review of Chemical Composition, Extraction Methods, Preservation Conditions, Potential Health Benefits, and Safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3344–3387. [Google Scholar] [CrossRef]
- Socias i Company, R.; Alonso, J.M.; Kodad, O. Fruit Quality in Almond: Physical Aspects for Breeding Strategies. Acta Hortic. 2009, 814, 475–480. [Google Scholar] [CrossRef]
- Godini, A. Almond Fruitfulness and Role of Self-Fertility. In Acta Horticulturae; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2002; pp. 191–203. [Google Scholar] [CrossRef]
- Gradziel, T.M. Almond (Prunus dulcis) Breeding BT—Breeding Plantation Tree Crops: Temperate Species; Gradziel, T.M., Ed.; Springer: New York, NY, USA, 2009; pp. 1–31. [Google Scholar] [CrossRef]
- Kester, D.E.; Kader, A.A.; Cunningham, S.A. Almonds. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Finglas, P.M., Trugo, L.C., Eds.; Academic Press: Oxford, UK, 2003; pp. 150–155. [Google Scholar] [CrossRef]
- Kester, D.E.; Micke, W.C.; Rough, D.; Morrison, D.; Curtis, R. Almond Variety Evaluation. Calif. Agric. 1980, 34, 4–7. [Google Scholar]
- Socias i Company, R.; Ansón, J.M.; Espiau, M.T. Taxonomy, Botany and Physiology. Almonds Bot. Prod. Uses 2017, 1–42. [Google Scholar] [CrossRef]
- Şimşek, M.; Kizmaz, V. Determination of Chemical and Mineral Compositions of Promising Almond (Prunus amygdalus L.) Genotypes from Beyazsu (Mardin) Region. Int. J. Agri. Wild Sci. 2017, 3, 6–11. [Google Scholar]
- Rabadán, A.; Álvarez-Ortí, M.; Gómez, R.; Pardo-Giménez, A.; Pardo, J.E. Suitability of Spanish Almond Cultivars for the Industrial Production of Almond Oil and Defatted Flour. Sci. Hortic. 2017, 225, 539–546. [Google Scholar] [CrossRef]
- Roncero, J.M.; Álvarez-Ortí, M.; Pardo-Giménez, A.; Rabadán, A.; Pardo, J.E. Review about Non-Lipid Components and Minor Fat-Soluble Bioactive Compounds of Almond Kernel. Foods 2020, 9, 1646. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, K.M.; Yasin, R.T. Quantitative Analysis of Some Metals in Almond Kernel in Erbil City. Int. J. Pharma Sci. Res. 2016, 7, 32–37. [Google Scholar]
- He, F.J.; MacGregor, G.A. Beneficial Effects of Potassium on Human Health. Physiol. Plant. 2008, 133, 725–735. [Google Scholar] [CrossRef]
- Bird, R.P.; Eskin, N.A.M. Chapter Two—The Emerging Role of Phosphorus in Human Health. In The Latest Research and Development of Minerals in Human Nutrition; Eskin, N.A.M., Ed.; Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2021; Volume 96, pp. 27–88. [Google Scholar] [CrossRef]
- Yada, S.; Huang, G.; Lapsley, K. Natural Variability in the Nutrient Composition of California-Grown Almonds. J. Food Compos. Anal. 2013, 30, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar] [CrossRef]
- Moodley, R.; Kindness, A.; Jonnalagadda, S.B. Elemental Composition and Chemical Characteristics of Five Edible Nuts (Almond, Brazil, Pecan, Macadamia and Walnut) Consumed in Southern Africa. J. Environ. Sci. Health -Part B Pestic. Food Contam. Agric. Wastes 2007, 42, 585–591. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lapsley, K.; Blumberg, J. A Nutrition and Health Perspective on Almonds. J. Sci. Food Agric. 2006, 86, 2245–2250. [Google Scholar] [CrossRef]
- Stuetz, W.; Schlörmann, W.; Glei, M. B-Vitamins, Carotenoids and α-/γ-Tocopherol in Raw and Roasted Nuts. Food Chem. 2017, 221, 222–227. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The Role of Vitamin E in Human Health and Some Diseases. Sultan Qaboos Univ. Med. J. 2014, 14, 157–165. [Google Scholar]
- Kodad, O.; Socias i Company, R.; Alonso, J.M. Genotypic and Environmental Effects on Tocopherol Content in Almond. Antioxidants 2018, 7, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodad, O. Chemical Composition of Almond Nuts. In Almonds Botany, Production and Uses; Socias i Company, R., Gradziel, T.M., Eds.; CAB International: Wallingford, UK, 2017; pp. 428–448. [Google Scholar]
- Čolić, S.; Zec, G.; Natić, M.; Fotirić-Akšić, M. Almond (Prunus dulcis) Oil. In Fruit Oils: Chemistry and Functionality; Ramadan, M.F., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 149–180. [Google Scholar] [CrossRef]
- Zacheo, G.; Cappello, M.S.; Gallo, A.; Santino, A.; Cappello, A.R. Changes Associated with Post-Harvest Ageing in Almond Seeds. LWT-Food Sci. Technol. 2000, 33, 415–423. [Google Scholar] [CrossRef]
- García-Pascual, P.; Mateos, M.; Carbonell, V.; Salazar, D.M. Influence of Storage Conditions on the Quality of Shelled and Roasted Almonds. Biosyst. Eng. 2003, 84, 201–209. [Google Scholar] [CrossRef]
- Aslanta, R.; Güleryüz, M.; Turan, M. Some Chemical Contents of Selected Almond (Prunus amygdalus Batsch) Types. Cah. Options Méditerranéennes 2001, 56, 93–96. [Google Scholar]
- Drogoudi, P.D.; Pantelidis, G.; Bacchetta, L.; De Giorgio, D.; Duval, H.; Metzidakis, I.; Spera, D. Protein and Mineral Nutrient Contents in Kernels from 72 Sweet Almond Cultivars and Accessions Grown in France, Greece and Italy. Int. J. Food Sci. Nutr. 2013, 64, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Goldhamer, D.A.; Fereres, E. Establishing an Almond Water Production Function for California Using Long-Term Yield Response to Variable Irrigation. Irrig. Sci. 2017, 35, 169–179. [Google Scholar] [CrossRef]
- López-López, M.; Espadador, M.; Testi, L.; Lorite, I.J.; Orgaz, F.; Fereres, E. Water Use of Irrigated Almond Trees When Subjected to Water Deficits. Agric. Water Manag. 2018, 195, 84–93. [Google Scholar] [CrossRef]
- Čolić, S.D.; Fotirić Akšić, M.M.; Lazarević, K.B.; Zec, G.N.; Gašić, U.M.; Dabić Zagorac, D.; Natić, M.M. Fatty Acid and Phenolic Profiles of Almond Grown in Serbia. Food Chem. 2017, 234, 455–463. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, A.P. Determination of Tocopherols in Vegetable Oils. J. Am. Oil Chem. Soc. 1979, 56, 668–671. [Google Scholar] [CrossRef]
- Rabrenović, B.B.; Demin, M.A.; Basić, M.G.; Pezo, L.L.; Paunović, D.M.; Sovtić, F.S. Impact of Plum Processing on the Quality and Oxidative Stability of Cold-Pressed Kernel Oil. Grasas Aceites 2021, 72, 4–11. [Google Scholar] [CrossRef]
- Bingöl, M.; Yentür, G.; Er, B.; Öktem, A.B. Determination of Some Heavy Metal Levels in Soft Drinks from Turkey Using ICP-OES Method. Czech J. Food Sci. 2010, 28, 213–216. [Google Scholar] [CrossRef] [Green Version]
- Čolić, S.D.; Bakić, I.V.; Dabić Zagorac, D.; Natić, M.M.; Smailagić, A.T.; Pergal, M.V.; Pešić, M.B.; Milinčić, D.D.; Rabrenović, B.B.; Fotirić Akšić, M.M. Chemical Fingerprint and Kernel Quality Assessment in Different Grafting Combinations of Almond Under Stress Condition. Sci. Hortic. 2021, 275, 109705. [Google Scholar] [CrossRef]
- Imani, A.; Shamili, M. Almond Nut Weight Assessment by Stepwise Regression and Path Analysis. Int. J. Fruit Sci. 2018, 18, 338–343. [Google Scholar] [CrossRef]
- Sorkheh, K.; Shiran, B.; Khodambashi, M.; Moradi, H.; Gradziel, T.M.; Martínez-Gómez, P. Correlations between Quantitative Tree and Fruit Almond Traits and Their Implications for Breeding. Sci. Hortic. 2010, 125, 323–331. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Gutiérrez-Gordillo, S.; Ortega-Arévalo, C.; Iglesias-Contreras, M.; Moreno, J.M.; Souza-Ferreira, L.; Durán-Zuazo, V.H. Thermal Imaging to Monitor the Crop-Water Status in Almonds by Using the Non-Water Stress Baselines. Sci. Hortic. 2018, 238, 91–97. [Google Scholar] [CrossRef]
- Gutiérrez-Gordillo, S.; Durán-Zuazo, V.H.; García-Tejero, I. Response of Three Almond Cultivars Subjected to Different Irrigation Regimes in Guadalquivir River Basin. Agric. Water Manag. 2019, 222, 72–81. [Google Scholar] [CrossRef]
- Abaspour, M.; Imani, A.; Hassanlo, T. Effects of Almond Genotype and Growing Location on Oil Percentage and Fatty Acid Composition of Its Seeds. J. Nuts 2012, 3, 5–12. [Google Scholar]
- Maestri, D.; Martínez, M.; Bodoira, R.; Rossi, Y.; Oviedo, A.; Pierantozzi, P.; Torres, M. Variability in Almond Oil Chemical Traits from Traditional Cultivars and Native Genetic Resources from Argentina. Food Chem. 2015, 170, 55–61. [Google Scholar] [CrossRef]
- Zhu, Y.; Taylor, C.; Sommer, K.; Wilkinson, K.; Wirthensohn, M. Influence of Deficit Irrigation Strategies on Fatty Acid and Tocopherol Concentration of Almond (Prunus dulcis). Food Chem. 2015, 173, 821–826. [Google Scholar] [CrossRef]
- Zamany, A.J.; Samadi, G.R.; Kim, D.H.; Keum, Y.S.; Saini, R.K. Comparative Study of Tocopherol Contents and Fatty Acids Composition in Twenty Almond Cultivars of Afghanistan. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 805–817. [Google Scholar] [CrossRef]
- Kodad, O.; Estopañán, G.; Juan, T.; Alonso, J.M.; Espiau, M.T.; Socias i Company, R. Oil Content, Fatty Acid Composition and Tocopherol Concentration in the Spanish Almond Genebank Collection. Sci. Hortic. 2014, 177, 99–107. [Google Scholar] [CrossRef]
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Silva, A.S.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, İ.; et al. Almonds (Prunus dulcis Mill. D. A. Webb): A Source of Nutrients and Health-Promoting Compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munné-Bosch, S.; Alegre, L. The Function of Tocopherols and Tocotrienols in Plants. CRC Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Yada, S.; Lapsley, K.; Huang, G. A Review of Composition Studies of Cultivated Almonds: Macronutrients and Micronutrients. J. Food Compos. Anal. 2011, 24, 469–480. [Google Scholar] [CrossRef]
- Ros, E. Health Benefits of Nut Consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef] [Green Version]
- Amorello, D.; Orecchio, S.; Pace, A.; Barreca, S. Discrimination of Almonds (Prunus dulcis) Geographical Origin by Minerals and Fatty Acids Profiling. Nat. Prod. Res. 2016, 30, 2107–2110. [Google Scholar] [CrossRef]
- Lipan, L.; Cano-Lamadrid, M.; Hernández, F.; Sendra, E.; Corell, M.; Vázquez-Araújo, L.; Moriana, A.; Carbonell-Barrachina, Á.A. Long-Term Correlation between Water Deficit and Quality Markers in Hydrosostainable Almonds. Agronomy 2020, 10, 1470. [Google Scholar] [CrossRef]
- Onianwa, P.C.; Adetola, I.G.; Iwegbue, C.M.A.; Ojo, M.F.; Tella, O.O. Trace Heavy Metals Composition of Some Nigerian Beverages and Food Drinks. Food Chem. 1999, 66, 275–279. [Google Scholar] [CrossRef]
- Qi, Z.; Xiao, J.; Ye, L.; Chuyun, W.; Chang, Z.; Shugang, L.; Fenghong, H. The Effect of the Subcritical Fluid Extraction on the Quality of Almond Oils: Compared to Conventional Mechanical Pressing Method. Food Sci. Nutr. 2019, 7, 2231–2241. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Morad-Talab, N.; Abd-Allah, E.F.; Ahmad, P.; Hajiboland, R. Plant Growth under Drought Stress: Significance of Mineral Nutrients. Water Stress Crop Plants A Sustain. Approach 2016, 2, 649–668. [Google Scholar] [CrossRef]
- Carbonell-Barrachina, Á.A.; Memmi, H.; Noguera-Artiaga, L.; del Carmen Gijón-López, M.; Ciapa, R.; Pérez-López, D. Quality Attributes of Pistachio Nuts as Affected by Rootstock and Deficit Irrigation. J. Sci. Food Agric. 2015, 95, 2866–2873. [Google Scholar] [CrossRef]
- Gutiérrez-Gordillo, S.; Lipan, L.; Durán-Zuazo, V.H.; Carbonell-Barrachina, Á.A.; Cárceles Rodríguez, B.; Rubio-Casal, A.E.; Carbonell-Bojollo, R.; Ordoñez-Fernández, R.; García-Tejero, I.F. Linking Sustainability and Competitiveness of Almond Plantations under Water Scarcity and Changing Climate. In Resources Use Efficiency in Agriculture; Kumar, S., Meena, R.S., Jhariya, M.K., Eds.; Springer Nature: Singapore, 2020. [Google Scholar] [CrossRef]
- Rahimi Dvin, S.; Gharaghani, A.; Pourkhaloee, A. Genetic Diversity, Population Structure, and Relationships among Wild and Domesticated Almond (Prunus Spp.) Germplasms Revealed by Issr Markers. Adv. Hortic. Sci. 2020, 34, 287–300. [Google Scholar] [CrossRef]
- Kodad, O.; Estopañán, G.; Juan, T.; Mamouni, A. Tocopherol Concentration in Almond Oil: Genetic Variation and Environmental Effects under Warm Conditions. J. Agric. Food Chem. 2011, 59, 6137–6141. [Google Scholar] [CrossRef] [PubMed]
- Ibourki, M.; Bouzid, H.A.; Bijla, L.; Aissa, R.; Sakar, E.H.; Ainane, T.; Gharby, S.; El Hammadi, A. Physical Fruit Traits, Proximate Composition, Fatty Acid and Elemental Profiling of Almond [Prunus dulcis Mill. DA Webb] Kernels from Ten Genotypes Grown in Southern Morocco. OCL-Oilseeds Fats Crop. Lipids 2022, 29, 9. [Google Scholar] [CrossRef]

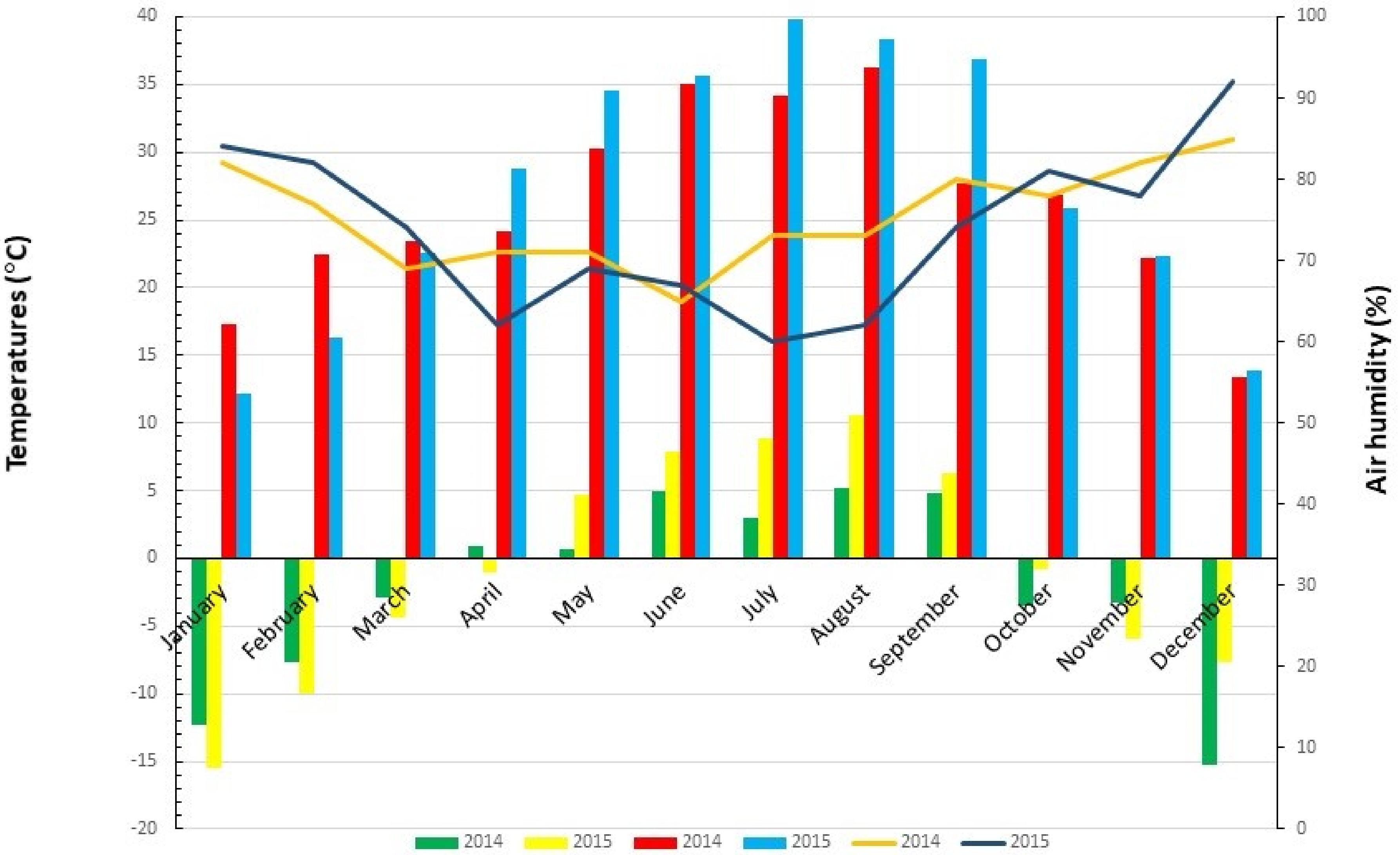
| Year | Temperature (°C) | Total Precipitation (mm) | Sum of Daily Average Air Humidity (%) | |||
|---|---|---|---|---|---|---|
| Period Average | Sum of Daily Average | Min | Max | |||
| 2014 | 16.3 | 3497 | −2.9 | 36.3 | 685.6 | 502 |
| 2015 | 17.8 | 3981 | −4.4 | 39.8 | 369.4 | 468 |
| Year | Temperature (°C) | Total Precipitation (mm) | Sum of Daily Average Air Humidity (%) | |||
|---|---|---|---|---|---|---|
| Period Average | Sum of Daily Average | Min | Max | |||
| 2014 | 12.9 | 4418 | −15.2 | 36.3 | 841.6 | 906 |
| 2015 | 12.1 | 4715 | −15.5 | 39.8 | 609.6 | 885 |
| Genotype/Year | Weight (g) | Length (mm) | Width (mm) | Thickness (mm) | Kernel Ratio (%) |
|---|---|---|---|---|---|
| ‘Texas’ | 1.25 a 1 | 27.0 bc | 13.6 ab | 7.8 a | 32.7 a |
| 15/03 | 0.76 e | 26.7 bc | 11.7 c | 5.4 c | 18.2 cd |
| 18/03 | 1.08 b | 27.8 ab | 12.7 bc | 5.9 bc | 17.2 d |
| 23/03 | 1.00 c | 28.1 a | 12.0 bc | 6.4 b | 21.3 b |
| 24/03 | 1.01 c | 25.7 cd | 14.2 a | 6.0 bc | 19.6 c |
| 25/03 | 0.92 d | 24.1 d | 11.6 c | 6.6 b | 21.1 b |
| A/04 | 0.92 d | 23.4 d | 13.0 b | 6.5 b | 20.1 bc |
| 2014 | 1.02 | 25.3 | 12.6 | 6.7 | 23.7 a |
| 2015 | 0.97 | 26.9 | 12.7 | 6.1 | 19.3 b |
| ‘Texas’ 2014 | 1.29 a | 26.5 bc | 13.9 | 7.9 a | 33.5 a |
| 15/03 2014 | 0.85 d | 26.7 bc | 12.3 c | 5.6 c | 21.7 e |
| 18/03 2014 | 1.12 b | 26.7 bc | 12.8 bc | 6.1 bc | 18.2 g |
| 23/03 2104 | 1.05 bc | 27.8 ab | 12.0 c | 6.6 b | 22.9 e |
| 24/03 2014 | 0.97 c | 23.7 cd | 13.2 b | 6.7 b | 24.1 c |
| 25/03 2014 | 0.85 d | 22.3 cd | 10.8 d | 7.0 ab | 23.2 d |
| A/04 2014 | 1.00 c | 23.4 cd | 13.4 b | 6.8 b | 22.1 de |
| ‘Texas’ 2015 | 1.22 a | 27.4 bc | 13.3 b | 7.7 a | 31.9 b |
| 15/03 2015 | 0.68 e | 26.8 bc | 11.1 d | 5.2 c | 14.8 i |
| 18/03 2015 | 1.04 c | 28.8 a | 12.6 c | 5.7 c | 16.1 h |
| 23/03 2015 | 0.96 c | 28.3 a | 12.0 c | 6.2 bc | 19.8 f |
| 24/03 2015 | 1.04 c | 27.8 ab | 15.2 a | 5.4 c | 15.1 |
| 25/03 2015 | 0.99 c | 25.8 d | 12.4 c | 6.2 bc | 19.1 f |
| A/04 2015 | 0.83 d | 23.3 cd | 12.6 c | 6.3 b | 18.1 g |
| Genotype/Year | α-Tocopherol | β + γTocopherol | δ-Tocopherol | Total Tocopherols | OilContent |
|---|---|---|---|---|---|
| ‘Texas’ | 247.4 c 1 | 17.4 a | 3.9 d | 268.7 c | 59.4 a |
| 15/03 | 404.4 a | 15.1 b | 4.6 b | 424.1 a | 55.4 b |
| 18/03 | 212.4 d | 17.3 a | 6.2 a | 235.9 d | 50.3 c |
| 23/03 | 39.4 f | 8.5 e | 3.9 d | 51.8 f | 54.9 b |
| 24/03 | 39.8 f | 7.9 e | 2.5 e | 50.2 f | 52.4 c |
| 25/03 | 206.4 e | 10.3 d | 4.1 c | 220.8 e | 52.6 bc |
| A/04 | 269.4 b | 12.9 c | 2.5 e | 284.8 b | 59.0 a |
| 2014 | 185.1 b | 11.6 b | 2.9 b | 199.6 b | 55.8 a |
| 2015 | 220.4 a | 13.9 a | 5.1 a | 239.4 a | 54.1 b |
| ‘Texas’ 2014 | 235.8 e | 18.1 b | 4.2 d | 258.1 e | 60.7 a |
| 15/03 2014 | 387.5 b | 18.5 b | 1.3 g | 407.3 b | 56.3 b |
| 18/03 2014 | 218.7 f | 14.1 e | 3.3 d | 236.1 f | 51.3 d |
| 23/03 2104 | 32.5 j | 5.8 i | 3.3 e | 41.6 j | 55.8 bc |
| 24/03 2014 | 26.5 j | 5.4 j | 0.00 | 31.9 k | 53.2 c |
| 25/03 2014 | 204.2 g | 10.6 g | 4.9 c | 219.7 g | 53.6 c |
| A/04 2014 | 190.1 h | 8.4 i | 3.0 e | 201.5 h | 59.7 a |
| ‘Texas’ 2015 | 258.9 d | 16.6 d | 3.7 de | 279.2 d | 59.4 a |
| 15/03 2015 | 421.4 a | 11.6 f | 7.9 b | 440.9 a | 54.5 bc |
| 18/03 2015 | 206.1 g | 20.4 a | 9.1 a | 235.6 f | 49.3 d |
| 23/03 2015 | 45.9 i | 11.2 f | 4.6 cd | 61.7 i | 54.0 bc |
| 24/03 2015 | 52.9 i | 10.5 g | 4.9 c | 68.5 i | 51.6 d |
| 25/03 2015 | 208.6 g | 9.9 h | 3.3 e | 221.8 g | 51.7 d |
| A/04 2015 | 348.6 c | 17.5 c | 2.1 f | 368.2 c | 58.4 a |
| Genotype/Year | Potassium | Sodium | Magnesium | Calcium | Phosphorus |
|---|---|---|---|---|---|
| ‘Texas’ | 1006.0 b 1 | 156.0 b | 317.5 e | 354.5 a | 592.0 d |
| 15/03 | 1104.5 a | 180.0 a | 339.5 c | 308.5 d | 614.5 c |
| 18/03 | 973.5 c | 91.5 d | 348.5 b | 296.0 e | 606.5 c |
| 23/03 | 972.5 c | 156.5 b | 371.0 a | 298.0 e | 575.0 b |
| 24/03 | 983.0 bc | 182.0 a | 350.5 b | 328.0 b | 656.5 b |
| 25/03 | 988.0 bc | 147.5 c | 327.0 d | 276.0 f | 702.5 a |
| A/04 | 882.0 d | 148.0 c | 297.5 f | 319.0 c | 538.0 e |
| 2014 | 1033.0 a | 177.6 a | 347.9 a | 351.6 a | 629.6 a |
| 2015 | 941.1 b | 125.7 b | 324.0 b | 271.3 b | 594.7 b |
| ‘Texas’ 2014 | 1130 a | 156 c | 321 d | 349 ef | 563 ij |
| 15/03 2014 | 1058 c | 205 b | 350 b | 398 h | 644 cd |
| 18/03 2014 | 978 d | 84 f | 364 b | 335 e | 634 de |
| 23/03 2104 | 1096 b | 206 b | 460 a | 373 g | 593 g |
| 24/03 2014 | 1088 b | 235 a | 335 c | 365 fg | 682 b |
| 25/03 2014 | 987 d | 158 c | 319 d | 279 c | 752 a |
| A/04 2014 | 894 e | 199 b | 286 f | 362 f | 539 k |
| ‘Texas’ 2015 | 882 e | 156 c | 314 de | 360 f | 621 f |
| 15/03 2015 | 1151 a | 155 b | 329 cd | 219 a | 585 gh |
| 18/03 2015 | 969 d | 99 e | 333 c | 257 b | 579 hi |
| 23/03 2015 | 849 f | 107 e | 282 f | 223 a | 557 j |
| 24/03 2015 | 878 ef | 129 d | 366 b | 291 d | 631 ef |
| 25/03 2015 | 989 d | 137 d | 335 c | 273 c | 653 c |
| A/04 2015 | 870 ef | 97 e | 309 e | 276 c | 537 k |
| Genotype/Year | Chromium | Copper | Iron | Manganese | Zink | Molybdenum |
|---|---|---|---|---|---|---|
| 2014 | 0.026 | 0.922 b | 3.523 a | 1.658 a | 3.302 | 0.101 |
| 2015 | 0.024 | 1.026 a | 3.340 b | 1.349 b | 3.363 | 0.092 |
| ‘Texas’ 2014 | 0.031 b | 0.965 e | 0.031 e | 1.181 f | 2.829 j | 0.038 g |
| 15/03 2014 | 0.026 c | 1.036 cd | 4.178 a | 2.510 a | 5.047 a | 0.133 d |
| 18/03 2014 | 0.018 d | 0.949 e | 3.725 b | 1.637 d | 3.177 g | 0.225 a |
| 23/03 2104 | 0.021 d | 1.050 bc | 3.453 d | 1.590 d | 3.255 f | 0.035 g |
| 24/03 2014 | 0.031 b | 0.557 h | 3.523 c | 2.198 b | 2.987 h | 0.099 e |
| 25/03 2014 | 0.035 a | 1.017 d | 3.444 d | 1.002 i | 3.424 d | 0.048 fg |
| A/04 2014 | 0.019 d | 0.878 f | 3.247 g | 1.489 e | 2.392 k | 0.131 d |
| ‘Texas’ 2015 | 0.028 bc | 1.070 b | 0.028 ef | 1.419 c | 2.778 h | 0.033 e |
| 15/03 2015 | 0.018 d | 0.994 d | 3.238 e | 1.457 ef | 4.483 b | 0.155 c |
| 18/03 2015 | 0.020 d | 0.925 ef | 3.103 f | 0.935 i | 3.320 e | 0.178 b |
| 23/03 2015 | 0.026 c | 1.246 a | 3.275 e | 1.296 g | 3.023 h | 0.021 h |
| 24/03 2015 | 0.028 bc | 0.815 g | 3.658 c | 1.913 c | 3.767 c | 0.102 e |
| 25/03 2015 | 0.032 ab | 1.231 a | 3.730 b | 1.155 h | 3.282 f | 0.057 f |
| A/04 2015 | 0.016 d | 0.904 f | 2.943 fg | 1.268 g | 2.889 i | 0.101 e |
| Heavy Metals | RDA per Day | UL per Day | AI per Day |
|---|---|---|---|
| Chromium | 20–35 μg 1 | - | |
| Copper | 900 μg | 10 mg | |
| Iron | 12–18 mg | 45 mg | |
| Manganese | - | 11 mg | 1.8–2.3 mg |
| Zink | 8–11 mg | 40 mg | |
| Molybdenum | 45 μg | 2 mg | |
| Chromium | 20–35 μg | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čolić, S.; Basić, Z.; Zec, G.; Bakić, I.; Rahović, D.; Akšić, M.F.; Mickovski Stefanović, V. The Effect of Changing Climatic Conditions on the Morphological Traits and Chemical Composition of Almond Kernels. Horticulturae 2022, 8, 487. https://doi.org/10.3390/horticulturae8060487
Čolić S, Basić Z, Zec G, Bakić I, Rahović D, Akšić MF, Mickovski Stefanović V. The Effect of Changing Climatic Conditions on the Morphological Traits and Chemical Composition of Almond Kernels. Horticulturae. 2022; 8(6):487. https://doi.org/10.3390/horticulturae8060487
Chicago/Turabian StyleČolić, Slavica, Zorica Basić, Gordan Zec, Ivana Bakić, Dragan Rahović, Milica Fotirić Akšić, and Violeta Mickovski Stefanović. 2022. "The Effect of Changing Climatic Conditions on the Morphological Traits and Chemical Composition of Almond Kernels" Horticulturae 8, no. 6: 487. https://doi.org/10.3390/horticulturae8060487
APA StyleČolić, S., Basić, Z., Zec, G., Bakić, I., Rahović, D., Akšić, M. F., & Mickovski Stefanović, V. (2022). The Effect of Changing Climatic Conditions on the Morphological Traits and Chemical Composition of Almond Kernels. Horticulturae, 8(6), 487. https://doi.org/10.3390/horticulturae8060487






