Magnetic Substrates for Tissue Engineering—A Review
Abstract
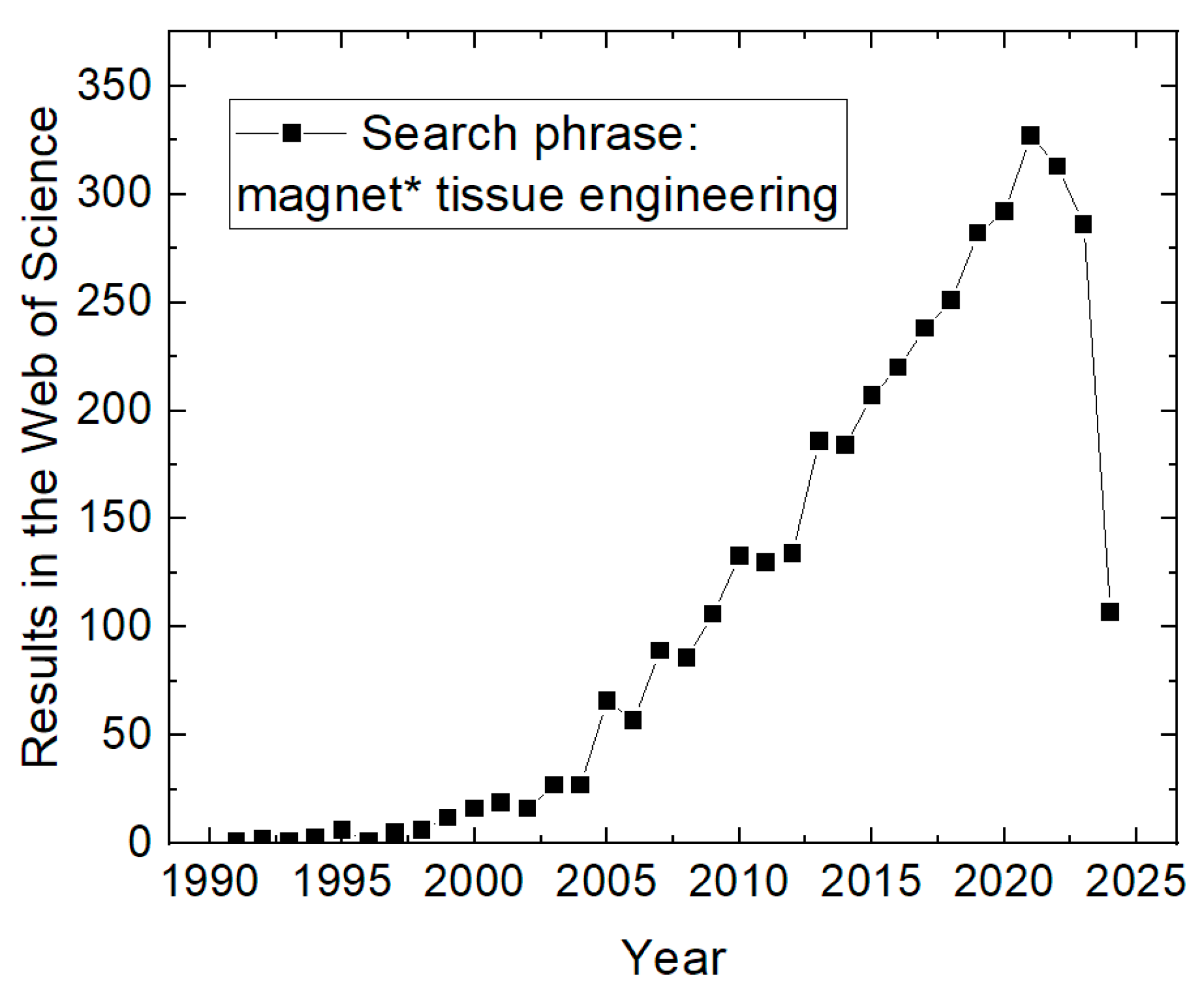
:1. Introduction
2. Integration of Magnetic Material in Tissue Engineering Systems—Methods and Outcomes
3. Production Methods of Magnetic Scaffolds
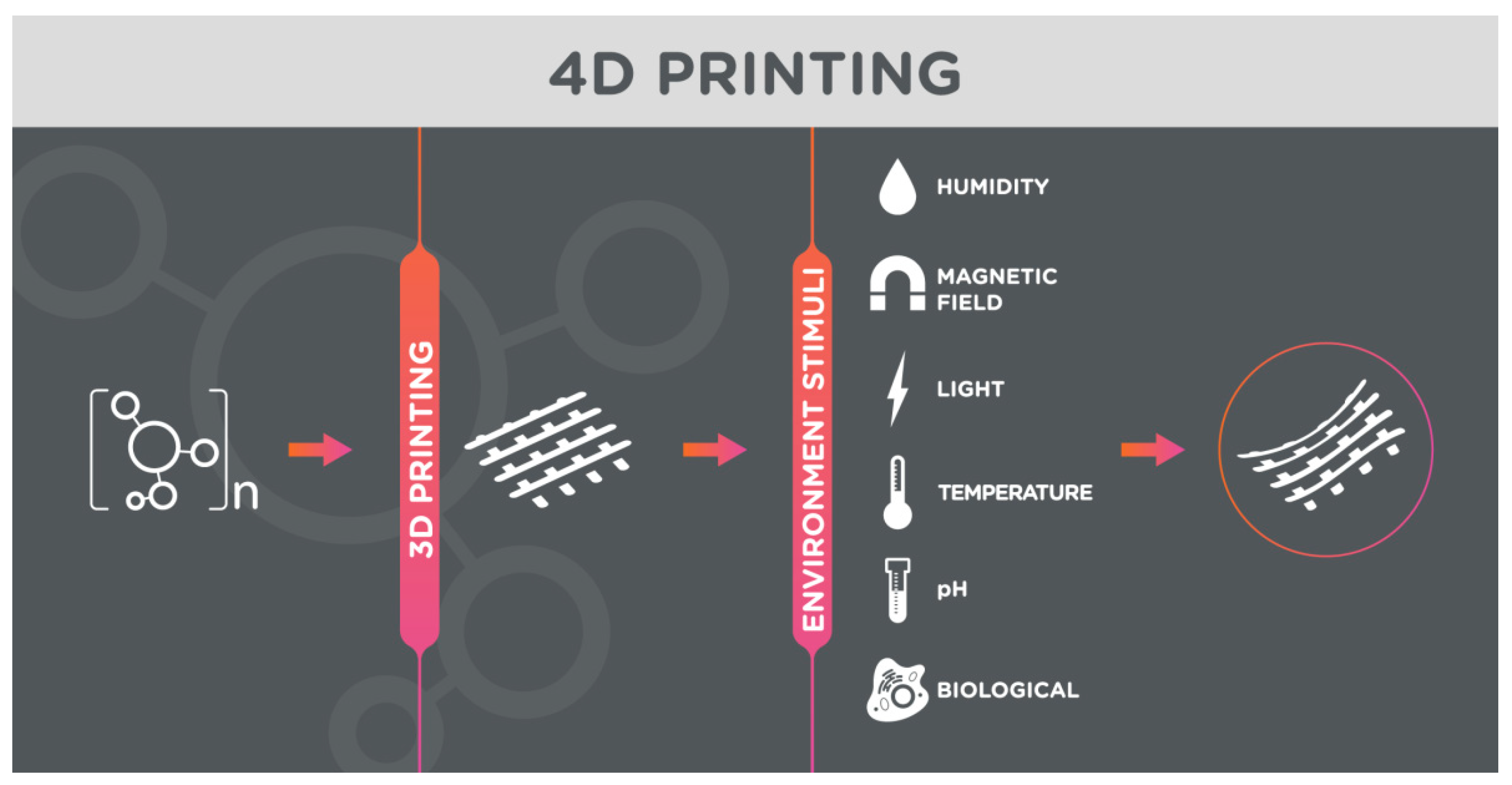
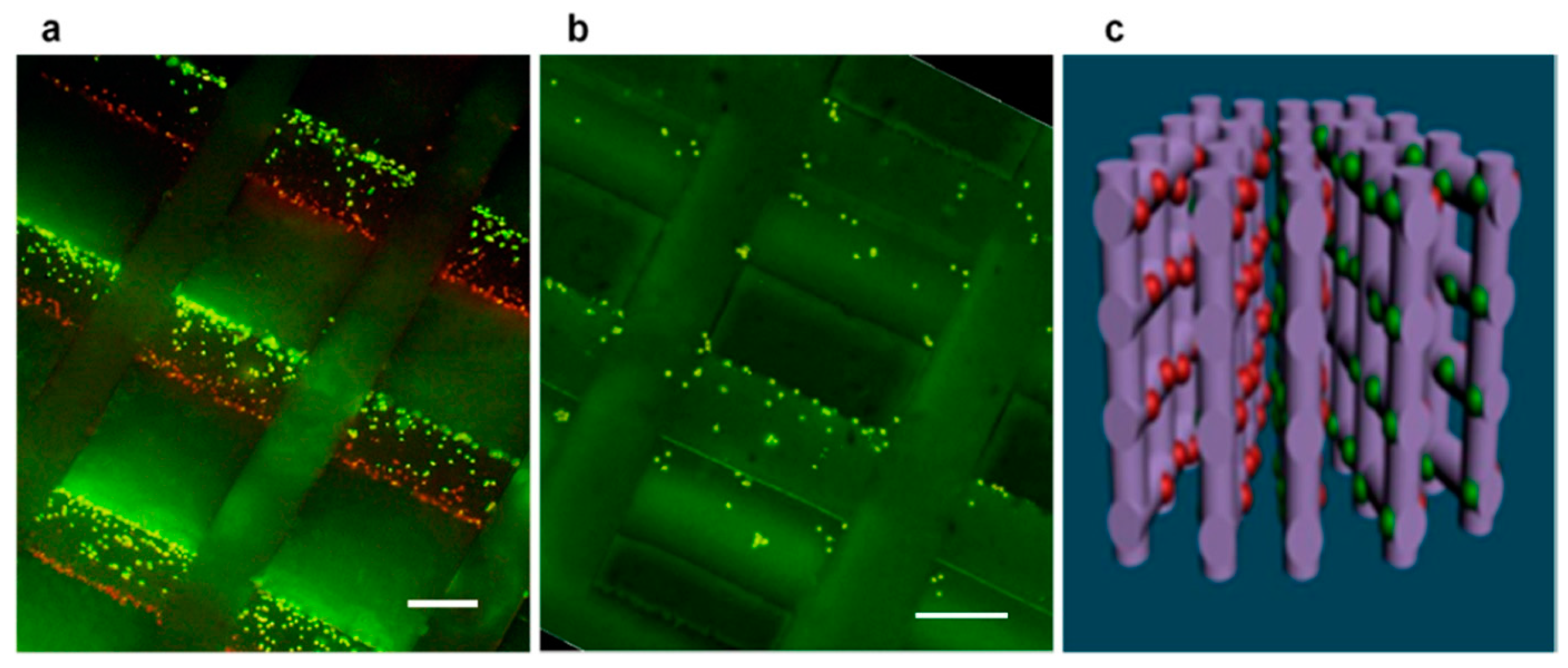
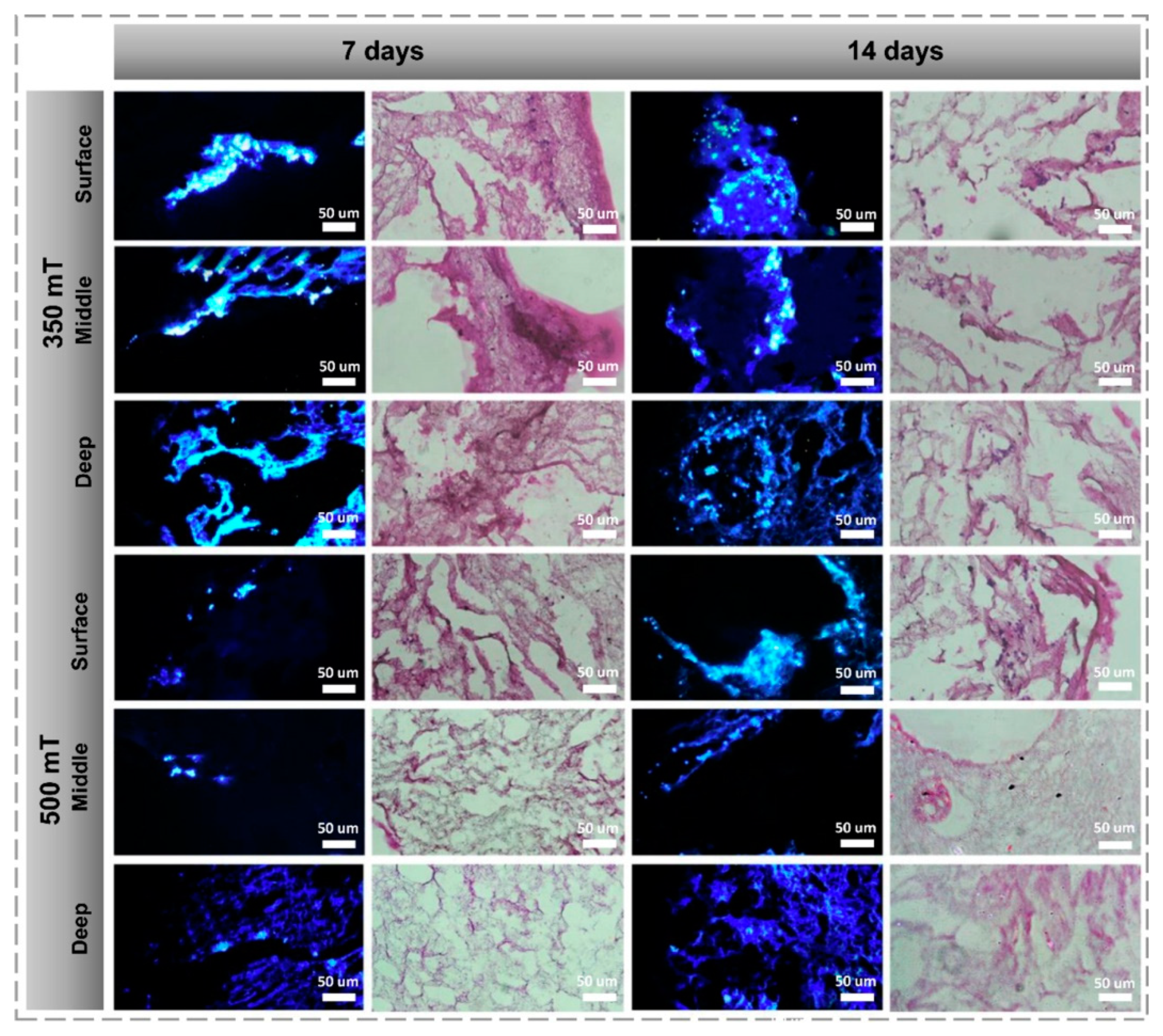
3.1. 3D/4D Printing
3.2. Electrospinning
3.3. Hydrogels
4. Applications of Magnetic Scaffolds
4.1. Bone Tissue Engineering
4.2. Nerve Tissue Engineering
4.3. Other Tissue Engineering Scaffolds
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Giwa, S.; Lewis, J.K.; Alvarez, L.; Langer, R.; Roth, A.E.; Church, G.M.; Markmann, J.F.; Sachs, D.H.; Chandraker, A.; Wertheim, J.A.; et al. The promise of organ and tissue preservation to transform medicine. Nat. Biotechnol. 2017, 35, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.M.; Mendicino, M.; Au, P. An FDA perspective on preclinical development of cell-based regenerative medicine products. Nat. Biotechnol. 2014, 32, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.L.; Li, Q.; Rokosh, G.; Sanganalmath, S.K.; Chen, N.; Ou, Q.; Stowers, H.; Hunt, G.; Bolli, R. Long-term outcome of administration of c-kitPOS cardiac progenitor cells after acute myocardial infarction: Transplanted cells do not become cardiomyocytes, but structural and functional improvement and proliferation of endogenous cells persist for at least one year. Circ. Res. 2016, 118, 1091–1105. [Google Scholar]
- Li, C.C.; Ouyang, L.L.; Armstrong, J.P.K.; Stevens, M.M. Advances in the Fabrication of Biomaterials for Gradient Tissue Engineering. Trends Biotechnol. 2021, 39, 150–164. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Deng, Y.H.; Su, J.C. Recent Advances in Design of Functional Biocompatible Hydrogels for Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2009432. [Google Scholar] [CrossRef]
- Mabrouk, M.; Beherei, H.H.; Das, D.B. Recent progress in the fabrication techniques of 3D scaffolds for tissue engineering. Mater. Sci. Eng. C 2020, 110, 110716. [Google Scholar] [CrossRef]
- Kraeutler, M.J.; Belk, J.W.; Purcell, J.M.; McCarty, E.C. Microfracture versus autologous chondrocyte implantation for articular cartilage lesions in the knee: A systematic review of 5-year outcomes. Am. J. Sports Med. 2017, 46, 995–999. [Google Scholar] [CrossRef]
- Guex, A.G.; Kocher, F.M.; Fortunato, G.; Körner, E.; Hegemann, D.; Carrel, T.; Tevaearai, H.; Giraud, M. Fine-tuning of substrate architecture and surface chemistry promotes muscle tissue development. Acta Biomater. 2012, 8, 1481–1489. [Google Scholar] [CrossRef]
- Guan, X.; Avci-Adali, M.; Alarcin, E.; Cheng, H.; Kashaf, S.S.; Li, Y.; Chawla, A.; Jang, H.L.; Khademhosseini, A. Development of hydrogels for regenerative engineering. Biotechnol. J. 2017, 12, 1600394. [Google Scholar] [CrossRef] [PubMed]
- Alves da Silva, M.; Martins, A.; Costa-Pinto, A.R.; Monteiro, N.; Faria, S.; Reis, R.L.; Neves, N.M. Electrospun nanofibrous meshes cultured with Wharton’s jelly stem cell: An alternative for cartilage regeneration, without the need of growth factors. Biotechnol. J. 2017, 12, 1700073. [Google Scholar] [CrossRef]
- Pina, S.; Canadas, R.F.; Jiménez, G.; Perán, M.; Marchal, J.A.; Reis, R.L.; Oliveira, J.M. Biofunctional ionic doped calcium phosphates: Silk fibroin composites for bone tissue engineering scaffolding. Cells Tissues Organs 2017, 204, 150–163. [Google Scholar] [CrossRef]
- Dzobo, K.; Turnley, T.; Wishart, A.; Rowe, A.; Kallmeyer, K.; van Vollenstee, F.A.; Thomford, N.E.; Dandara, C.; Chopera, D.; Pepper, M.S.; et al. Fibroblast-derived extracellular matrix induces, chondrogenic differentiation in human adipose-derived mesenchymal stromal/stem cells in vitro. Int. J. Mol. Sci. 2016, 17, 1259. [Google Scholar] [CrossRef]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021, 17, 100721. [Google Scholar] [CrossRef]
- Zulkifli, M.Z.A.; Nordin, D.; Shaari, N.; Kamarudin, S.K. Overview of Electrospinning for Tissue Engineering Applications. Polymers 2023, 15, 2418. [Google Scholar] [CrossRef]
- Chung, J.J.; Im, H.J.; Kim, S.H.; Park, J.W.; Jung, Y.M. Toward Biomimetic Scaffolds for Tissue Engineering: 3D Printing Techniques in Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 586406. [Google Scholar] [CrossRef]
- Zaszczynska, A.; Moczulska-Helja, M.; Gradys, A.; Sajkiewicz, P. Advances in 3D Printing for Tissue Engineering. Materials 2021, 14, 3149. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Al-Thebaiti, M.A.; Hashmi, M.U.; Aftab, S.; Abd Razak, S.I.; Abu Hassan, S.; Kadir, M.R.A.; Amin, R. Synthesis of Silver-Coated Bioactive Nanocomposite Scaffolds Based on Grafted Beta-Glucan/Hydroxyapatite via Freeze-Drying Method: Anti-Microbial and Biocompatibility Evaluation for Bone Tissue Engineering. Materials 2020, 13, 971. [Google Scholar] [CrossRef]
- Li, T.-T.; Zhang, Y.; Ren, H.-T.; Peng, H.-K.; Lou, C.-W.; Lin, J.-H. Two-step strategy for constructing hierarchical pore structured chitosan–hydroxyapatite composite scaffolds for bone tissue engineering. Carbohydr. Polym. 2021, 260, 117765. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Jodati, H.; Yılmaz, B.; Evis, Z. A review of bioceramic porous scaffolds for hard tissue applications: Effects of structural features. Ceram. Int. 2020, 46, 15725. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Hasan, M.S.; Ahmed, I.; Parsons, A.J.; Rudd, C.D.; Walker, G.S.; Scotchford, C.A. Investigating the use of coupling agents to improve the interfacial properties between a resorbable phosphate glass and polylactic acid matrix. J. Biomater. Appl. 2013, 28, 354. [Google Scholar] [CrossRef]
- Kargozar, S.; Montazerian, M.; Fiume, E.; Baino, F. Multiple and Promising Applications of Strontium (Sr)-Containing Bioactive Glasses in Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 161. [Google Scholar] [CrossRef]
- Pieralli, S.; Kohal, R.J.; Jung, R.E.; Vach, K.; Spies, B.C. Clinical Outcomes of Zirconia Dental Implants: A Systematic Review. J. Dent. Res. 2016, 96, 38. [Google Scholar] [CrossRef]
- Carlström, I.E.; Rashad, A.; Campodoni, E.; Sandri, M.; Syverud, K.; Bolstad, A.I.; Mustafa, K. Cross-linked gelatin-nanocellulose scaffolds for bone tissue engineering. Mater. Lett. 2020, 264, 127326. [Google Scholar] [CrossRef]
- Ehrmann, A. Non-Toxic Crosslinking of Electrospun Gelatin Nanofibers for Tissue Engineering and Biomedicine—A Review. Polymers 2021, 13, 1973. [Google Scholar] [CrossRef]
- Hernandez-Gonzalez, A.C.; Tellez-Jurado, L.; Rodriguez-Lorenzo, L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar] [CrossRef]
- Tanzli, E.; Ehrmann, A. Electrospun Nanofibrous Membranes for Tissue Engineering and Cell Growth. Appl. Sci. 2021, 11, 6929. [Google Scholar] [CrossRef]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef] [PubMed]
- Souness, A.; Zamboni, F.; Walker, G.M.; Collins, M.N. Influence of scaffold design on 3D printed cell constructs. J. Biomed. Mater. Res. B 2018, 106, 533. [Google Scholar] [CrossRef] [PubMed]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2019, 226, 119536. [Google Scholar] [CrossRef]
- Lizarraga-Valderrama, L.R.; Taylor, C.S.; Claeyssens, F.; Haycock, J.W.; Knowles, J.C.; Roy, I. Unidirectional neuronal cell growth and differentiation on aligned polyhydroxyalkanoate blend microfibres with varying diameters. J. Tissue Eng. Regen. Med. 2019, 13, 1581. [Google Scholar] [CrossRef]
- Constantinides, C.; Basnett, P.; Lukasiewicz, B.; Carnicer, R.; Swider, E.; Majid, Q.A.; Srinivas, M.; Carr, C.A.; Roy, I. In vivo tracking and 1H/19F magnetic resonance imaging of biodegradable polyhydroxyalkanoate/polycaprolactone blend scaffolds seeded with labeled cardiac stem cells. ACS Appl. Mater. Interfaces 2018, 10, 25056. [Google Scholar] [CrossRef]
- Liao, J.; Xu, B.; Zhang, R.H.; Fan, Y.B.; Xie, H.Q.; Li, X.M. Applications of decellularized materials in tissue engineering: Advantages, drawbacks and current improvements, and future perspectives. J. Mater. Chem. B 2020, 8, 10023. [Google Scholar] [CrossRef]
- Alizadeh, P.S.M.; Tutar, R.; Apu, E.H.; Unluturk, B.; Contag, C.H.; Ashammakhi, N. Use of electroconductive biomaterials for engineering tissues by 3D printing and 3D bioprinting. Essays Biochem. 2021, 65, 441. [Google Scholar]
- Du, V.; Luciani, N.; Richard, S.; Mary, G.; Gay, C.; Mazuel, F.; Reffay, M.; Menasché, P.; Agbulut, O.; Wilhelm, C. A 3D magnetic tissue stretcher for remote mechanical control of embryonic stem cell differentiation. Nat. Commun. 2017, 8, 400. [Google Scholar] [CrossRef]
- Lodi, M.B. Recent Advances and Challenges of Magnetic Scaffolds for Tumor Hyperthermia and Tissue Engineering. In Proceedings of the 2022 IEEE 22nd International Conference on Nanotechnology (NANO), Palma de Mallorca, Spain, 4–8 July 2022; pp. 317–320. [Google Scholar]
- Dulinska-Litewka, J.; Lazarczyk, A.; Halubiec, P.; Szafranski, O.; Karnas, K.; Karewicz, A. Superparamagnetic Iron Oxide Nanoparticles—Current and Prospective Medical Applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.S.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef]
- Unterweger, H.; Dézsi, L.; Matuszak, J.; Janko, C.; Poettler, M.; Jordan, J.; Bäuerle, T.; Szebeni, J.; Fey, T.; Boccaccini, A.R.; et al. Dextran-coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging: Evaluation of size-dependent imaging properties, storage stability and safety. Int. J. Nanomed. 2018, 13, 1899–1915. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Le, W.; Mej, T.; Wang, T.; Chen, L.; Lei, Y.; Cui, S.; Chen, B.; Cui, Z.; Shao, C. In vitro and in vivo targeting imaging of pancreatic cancer using a Fe3O4@SiO2 nanoprobe modified with anti-mesothelin antibody. Int. J. Nanomed. 2016, 11, 2195–2207. [Google Scholar]
- Tutkun, L.; Gunaydin, E.; Turk, M.; Kutsal, T. Anti-Epidermal Growth Factor Receptor Aptamer and Antibody Conjugated SPIONs Targeted to Breast Cancer Cells: A Comparative Approach. J. Nanosci. Nanotechnol. 2017, 17, 1681–1697. [Google Scholar] [CrossRef]
- Azhdarzadeh, M.; Atyabi, F.; Saei, A.A.; Varnamkhasti, B.S.; Omidi, Y.; Fateh, M.; Ghavami, M.; Shanehsazzadeh, S.; Dinarvand, R. Theranostic MUC-1 aptamer targeted gold coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging and photothermal therapy of colon cancer. Colloids Surf. B 2016, 143, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Li, D.G.; Li, Q.T.; Cao, X.D.; Dong, H. Microgel assembly: Fabrication, characteristics and application in tissue engineering and regenerative medicine. Bioact. Mater. 2022, 9, 105–119. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef] [PubMed]
- Municoy, S.; Álvarez Echazú, M.I.; Antezana, P.E.; Galdopórpora, J.M.; Olivett, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M.V.; Alvarez, G.S.; Hardy, J.G.; et al. Stimuli-Responsive Materials for Tissue Engineering and Drug Delivery. Int. J. Mol. Sci. 2020, 21, 4724. [Google Scholar] [CrossRef] [PubMed]
- Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Melnikov, M.Y. Magnetic Nanoparticles for Biomedical Purposes: Modern Trends and Prospects. Magnetochemistry 2020, 6, 30. [Google Scholar] [CrossRef]
- Castro, A.L.; Vedaraman, S.; Haraszti, T.; Barbosa, M.A.; Goncalves, R.M.; de Laporte, L. Engineering Anisotropic Cell Models: Development of Collagen Hydrogel Scaffolds with Magneto-Responsive PEG Microgels for Tissue Engineering Applications. Adv. Mater. Technol. 2024, 9, 2301391. [Google Scholar] [CrossRef]
- Filippi, M.; Dasen, B.; Guerrero, J.; Garello, F.; Isu, G.; Born, G.; Ehrbar, M.; Martin, I.; Scherberich, A. Magnetic nanocomposite hydrogels and static magnetic field stimulate the osteoblastic and vasculogenic profile of adipose-derived cells. Biomaterials 2019, 223, 119468. [Google Scholar] [CrossRef]
- Hou, R.; Zhang, G.; Du, G.; Zhan, D.; Cong, Y.; Cheng, Y.; Fu, J. Magnetic nanohydroxyapatite/PVA composite hydrogels for promoted osteoblast adhesion and proliferation. Colloids Surf. B Biointerfaces 2013, 103, 318–325. [Google Scholar] [CrossRef]
- Armstrong, J.P.K.; Stevens, M.M. Using Remote Fields for Complex Tissue Engineering. Trends Biotechnol. 2020, 38, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, A.A.; Lee, J.; Bharadwaj, N.A.; Ewoldt, R.H.; Kilian, K.A. Temporal modulation of stem cell activity using magnetoactive hydrogels. Adv. Healthcare Mater. 2016, 5, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Simchi, A.; Imani, M.; Shokrgozar, M.A.; Milani, A.S.; Häfelif, U.O.; Stroeve, P. A new approach for the in vitro identification of the cytotoxicity of superparamagnetic iron oxide nanoparticles. Colloids Surf. B Biointerfaces 2010, 75, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Simchi, A.; Imani, M.; Milani, A.S.; Stroeve, P. An in vitro study of bare and poly(ethylene glycol)-co-fumarate-coated superparamagnetic iron oxide nanoparticles: A new toxicity identification procedure. Nanotechnology 2009, 20, 225104. [Google Scholar] [CrossRef]
- Unterweger, H.; Subatzus, D.; Tietze, R.; Janko, C.; Poettler, M.; Stiegelschmitt, A.; Schuster, M.; Maake, C.; Boccaccini, A.R.; Alexiou, C. Hypericin-bearing magnetic iron oxide nanoparticles for selective drug delivery in photodynamic therapy. Int. J. Nanomed. 2015, 10, 6985–6996. [Google Scholar] [CrossRef] [PubMed]
- Altanerova, U.; Babincova, M.; Babinec, P.; Benejova, K.; Jakubechova, J.; Altanerova, V.; Zduriencikova, M.; Repiska, V.; Altaner, C. Human mesenchymal stem cell-derived iron oxide exosomes allow targeted ablation of tumor cells via magnetic hyperthermia. Int. J. Nanomed. 2017, 12, 7923–7936. [Google Scholar] [CrossRef]
- Du, S.; Li, J.; Du, C.; Huang, Z.; Chen, G.; Yan, W. Overendocytosis of superparamagnetic iron oxide particles increases apoptosis and triggers autophagic cell death in human osteosarcoma cell under a spinning magnetic field. Oncotarget 2017, 8, 9410–9424. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.D.; Babo, P.S.; Costa-Almeida, R.; Domingues, R.M.A.; Mendes, B.B.; Paz, E.; Freitas, P.; Rodrigues, M.T.; Granja, P.L.; Gomes, M.E. Multifunctional magnetic-responsive hydrogels to engineer tendon-to-bone interface. Nanomedicine 2018, 14, 2375–2385. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Memarzadeh, K.; Stephen, A.S.; Allaker, R.P.; Brown, R.A.; Huang, J. Development of a 3D collagen model for the in vitro evaluation of magnetic-assisted osteogenesis. Sci. Rep. 2018, 8, 16270. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, D.; Li, Y.; Xie, W.; Wang, X.; Tao, L.; Wei, Y.; Wang, X.; Zhao, L. Effect of nanoheat stimulation mediated by magnetic nanocomposite hydrogel on the osteogenic differentiation of mesenchymal stem cells. Sci. China Life Sci. 2018, 61, 448–456. [Google Scholar] [CrossRef]
- Zamboni, F.; Beaucamp, A.; Serafin, A.; Collins, M.N. Electrical/magnetic stimulation in musculoskeletal tissue engineering and regenerative medicine. In Multiscale Cell-Biomaterials Interplay in Musculoskeletal Tissue Engineering and Regenerative Medicine; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2024; pp. 161–180. [Google Scholar]
- Liu, Z.Y.; Liu, J.H.; Cui, X.; Wang, X.; Zhang, L.C.; Tang, P.F. Recent Advances on Magnetic Sensitive Hydrogels in Tissue Engineering. Front. Chem. 2020, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Gómez-Florit, M.; Barbosa, S.; Taboada, P.; Domingues, R.M.A.; Gomes, M.E. Magnetic Nanocomposite Hydrogels for Tissue Engineering: Design Concepts and Remote Actuation Strategies to Control Cell Fate. ACS Nano 2021, 15, 175–209. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.P.; Cicha, I.; Alexiou, C. Iron Oxide Nanoparticles in Regenerative Medicine and Tissue Engineering. Nanomaterials 2021, 11, 2337. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Y.; Zhu, M.R.; Gao, Q.M. Functionalized magnetic nanosystems for tissue engineering. In Functionalized Magnetic Nanosystems for Diagnostic Tools and Devices; Elsevier: Amsterdam, The Netherlands, 2024; pp. 413–443. [Google Scholar]
- Wan, Z.Q.; Zhang, P.; Liu, Y.S.; Lv, L.W.; Zhou, Y.S. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2020, 101, 26–42. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Ahmed, W.; Arshad, H. A review on four-dimensional (4D) bioprinting in pursuit of advanced tissue engineering applications. Bioprinting 2022, 27, e00203. [Google Scholar] [CrossRef]
- Mushtaq, A.; Zhao, R.B.; Luo, D.D.; Dempsey, E.; Wang, X.M.; Iqbal, M.Z.; Kong, X.D. Magnetic hydroxyapatite nanocomposites: The advances from synthesis to biomedical applications. Mater. Des. 2021, 197, 109269. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, J.Y.; Pang, X.L.; Zhao, M.; Wang, B.Q.; Yang, L.L.; Wan, H.S.; Wu, J.B.; Fu, S.Z. Magnetic nanoparticle-loaded electrospun polymeric nanofibers for tissue engineering. Mater. Sci. Eng. C 2017, 73, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Brito-Pereira, R.; Correia, D.M.; Ribeiro, C.; Francesko, A.; Etxebarria, I.; Pérez-Àlvarez, L.; Vilas, J.L.; Martins, P.; Lanceros-Mendez, S. Silk fibroin-magnetic hybrid composite electrospun fibers for tissue engineering applications. Comp. B Eng. 2018, 141, 70–75. [Google Scholar] [CrossRef]
- Jia, Y.F.; Yang, C.Y.; Chen, X.Y.; Xue, W.Q.; Hutchins-Crawford, H.J.; Yu, Q.Q.; Topham, P.D.; Wang, L.G. A review on electrospun magnetic nanomaterials: Methods, properties and applications. J. Mater. Chem. C 2021, 9, 9042–9082. [Google Scholar] [CrossRef]
- Levy, I.; Sher, I.; Corem-Salkmon, E.; Ziv-Polat, O.; Meir, A.; Treves, A.J.; Nagler, A.; Kalter-Leibovici, O.; Margel, S.; Rotenstreich, Y. Bioactive magnetic near Infra-Red fluorescent core-shell iron oxide/human serum albumin nanoparticles for controlled release of growth factors for augmentation of human mesenchymal stem cell growth and differentiation. J. Nanobiotechnol. 2015, 13, 34. [Google Scholar] [CrossRef]
- Li, C.; Armstrong, J.P.; Pence, I.; Kit-Anan, W.; Puetzer, J.L.; Carreira, S.C.; Moore, A.; Stevens, M.M. Glycosylated superparamagnetic nanoparticle gradients for osteochondral tissue engineering. Biomaterials 2018, 176, 24–33. [Google Scholar] [CrossRef]
- Hu, S.; Zhou, Y.; Zhao, Y.; Xu, Y.; Zhang, F.; Gu, N.; Ma, J.; Reynolds, M.A.; Xia, Y.; Xu, H.H. Enhanced bone regeneration and visual monitoring via superparamagnetic iron oxide nanoparticle scaffold in rats. J. Tissue Eng. Regen. Med. 2018, 12, e2085–e2098. [Google Scholar] [CrossRef]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Rahgozar, S.; Zarrabi, A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neurosci. 2017, 18, 51. [Google Scholar] [CrossRef]
- Van De Walle, A.; Fromain, A.; Sangnier, A.P.; Curcio, A.; Lenglet, L.; Motte, L.; Lalatonne, Y.; Wilhelm, C. Real-time in situ magnetic measurement of the intracellular biodegradation of iron oxide nanoparticles in a stem cell-spheroid tissue model. Nano Res. 2020, 13, 467–476. [Google Scholar] [CrossRef]
- Van de Walle, A.; Perez, J.; Abou-Hassan, A.; Hémadi, M.; Luciani, N.; Wilhelm, C. Magnetic nanoparticles in regenerative medicine: What of their fate and impact in stem cells? Mater. Today Nano 2020, 11, 100084. [Google Scholar] [CrossRef]
- Andreas, K.; Georgieva, R.; Ladwig, M.; Mueller, S.; Notter, M.; Sittinger, M.; Ringe, J. Highly efficient magnetic stem cell labeling with citrate-coated superparamagnetic iron oxide nanoparticles for MRI tracking. Biomaterials 2012, 33, 4515–4525. [Google Scholar] [CrossRef]
- Wu, M.; Gu, L.; Gong, Q.; Sun, J.; Ma, Y.; Wu, H.; Wang, Y.; Guo, G.; Li, X.; Zhu, H. Strategies to reduce the intracellular effects of iron oxide nanoparticle degradation. Nanomedicine 2017, 12, 555–570. [Google Scholar] [CrossRef]
- Duan, L.; Zuo, J.; Zhang, F.; Li, B.; Xu, Z.; Zhang, H.; Yang, B.; Song, W.; Jiang, J. Magnetic Targeting of HU-MSCs in the Treatment of Glucocorticoid-Associated Osteonecrosis of the Femoral Head Through Akt/Bcl2/Bad/Caspase-3 Pathway. Int. J. Nanomed. 2020, 15, 3605–3620. [Google Scholar] [CrossRef] [PubMed]
- Mocanu-Dobranici, A.-E.; Costache, M.; Dinescu, S. Insights into the Molecular Mechanisms Regulating Cell Behavior in Response to Magnetic Materials and Magnetic Stimulation in Stem Cell (Neurogenic) Differentiation. Int. J. Mol. Sci. 2023, 24, 2028. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; GhavamiNejad, A.; Tutar, R.; Fricker, A.; Roy, I.; Chatzistavrou, X.; Apu, E.H.; Nguyen, K.L.; Ahsan, T.; Pountos, I.; et al. Highlights on Advancing Frontiers in Tissue Engineering. Tissue Eng. B Rev. 2021, 28, 12. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Q.; Krishaa, L.; Fong, E.L.S. Magnetic force-based cell manipulation for in vitro tissue engineering. APL Bioeng. 2023, 7, 031504. [Google Scholar] [CrossRef] [PubMed]
- Imashiro, C.; Shimizu, T. Fundamental Technologies and Recent Advances of Cell-Sheet-Based Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 425. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Kim, E.M.; Yamamoto, M.; Park, H.; Shin, H.S. Engineering Multi-Cellular Spheroids for Tissue Engineering and Regenerative Medicine. Adv. Healthc. Mater. 2020, 9, 2000608. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, S.; Vijayavenkataraman, S. Design of 3D printed scaffolds for bone tissue engineering: A review. Bioprinting 2021, 24, e00167. [Google Scholar] [CrossRef]
- Bauer, L.; Brandstäter, L.; Letmate, M.; Palachandran, M.; Wadehn, F.O.; Wolfschmidt, C.; Grothe, T.; Güth, U.; Ehrmann, A. Electrospinning for the Modification of 3D Objects for the Potential Use in Tissue Engineering. Technologies 2022, 10, 66. [Google Scholar] [CrossRef]
- Mirkhalaf, M.; Men, Y.H.; Wang, R.; Zreiqat, Y.N.H. Personalized 3D printed bone scaffolds: A review. Acta Biomater. 2023, 156, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Davis, B.; Jin, Y.F.; Huang, Y. Effects of printing-induced interfaces on localized strain within 3D printed hydrogel structures. Mater. Sci. Eng. C 2018, 89, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Saska, S.; Pilatti, L.; Blay, A.; Shibli, J.A. Bioresorbable Polymers: Advanced Materials and 4D Printing for Tissue Engineering. Polymers 2021, 13, 563. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Ang, Q.Y.; Yu, S.Z.; Lin, Z.; Wang, C.Y.; Chen, Z.P.; Jiang, L.L. 4D Printing of Magnetoactive Soft Materials for On-Demand Magnetic Actuation Transformation. ACS Appl. Mater. Interfaces 2021, 13, 4174–4184. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Li, X.J.; Jiang, L.M.; Tang, D.F.; Xu, X.; Zhao, P.; Fu, J.Z.; Zhou, Q.F.; Chen, Y. 3D Printing of Functional Magnetic Materials: From Design to Applications. Adv. Funct. Mater. 2021, 31, 2102777. [Google Scholar] [CrossRef]
- Ehrmann, G.; Blachowicz, T.; Ehrmann, A. Magnetic 3D-Printed Composites—Production and Applications. Polymers 2022, 14, 3895. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Bakht, S.M.; Gomez-Florit, M.; Rial, R.; Monteiro, R.F.; Teixeira, S.P.B.; Taboada, P.; Reis, R.L.; Domingues, R.M.A.; Gomes, M.E. Magnetically-Assisted 3D Bioprinting of Anisotropic Tissue-Mimetic Constructs. Adv. Funct. Mater. 2022, 32, 2208940. [Google Scholar] [CrossRef]
- Gertz, F.; Khitun, A. Biological cell manipulation by magnetic nanoparticles. AIP Adv. 2016, 6, 025308. [Google Scholar] [CrossRef]
- Zwi-Dantsis, L.; Wang, B.; Marijon, C.; Zonetti, S.; Ferrini, A.; Massi, L.; Stuckey, D.J.; Terracciano, C.M.; Stevens, M.M. Remote Magnetic Nanoparticle Manipulation Enables the Dynamic Patterning of Cardiac Tissues. Adv. Mater. 2019, 32, 1904598. [Google Scholar] [CrossRef] [PubMed]
- Bongaerts, M.; Aizel, K.; Secret, E.; Jan, A.; Nahar, T.; Raudzus, F.; Neumann, S.; Teling, N.; Heumann, R.; Siaugue, J.-M.; et al. Parallelized Manipulation of Adherent Living Cells by Magnetic Nanoparticles-Mediated Forces. Int. J. Mol. Sci. 2020, 21, 6560. [Google Scholar] [CrossRef] [PubMed]
- Goranov, V.; Shelyakova, T.; de Santis, R.; Haranava, Y.; Makaniok, A.; Gloria, A.; Tampieri, A.; Russo, A.; Kon, E.; Marcacci, M.; et al. 3D Patterning of cells in Magnetic Scaffolds for Tissue Engineering. Sci. Rep. 2020, 10, 2289. [Google Scholar] [CrossRef] [PubMed]
- Keramaris, N.C.; Kaptanis, S.; Moss, H.L.; Loppini, M.; Pneumaticos, S.; Maffulli, N. Endothelial Progenitor Cells (EPCs) and Mesenchymal Stem Cells (MSCs) in Bone Healing. Curr. Stem Cell Res. Ther. 2012, 7, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Ksouri, R.; Odabas, S.; Seda Yar Saglam, A. Exosome loaded 3D printed magnetic PLA constructs: A candidate for bone tissue engineering. Prog. Addit. Manuf. 2024. online first. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Liu, C.Y.; Chen, B.R.; Lui, Y.X. Magnetically-driven drug and cell on demand release system using 3D printed alginate based hollow fiber scaffolds. Int. J. Biol. Macromol. 2021, 168, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Najafabadi, F.M.; Karbasi, S.; Benisi, S.Z.; Shojaei, S.; Poursamar, S.A.; Azadani, R.N. Evaluation of the effects of alumina nanowire on 3D printed polycaprolactone / magnetic mesoporous bioactive glass scaffold for bone tissue engineering applications. Mater. Chem. Phys. 2023, 303, 127616. [Google Scholar] [CrossRef]
- Hanif, M.; Zhang, L.; Shah, A.H.; Chen, Z.W. Mechanical analysis and biodegradation of oxides-based magneto-responsive shape memory polymers for material extrusion 3D printing of biomedical scaffolds. Addit. Manuf. 2024, 86, 104174. [Google Scholar] [CrossRef]
- Choi, Y.T.; Kim, C.G.; Kim, H.S.; Moon, C.W.; Lee, K.Y. 3D Printing of dynamic tissue scaffold by combining self-healing hydrogel and self-healing ferrogel. Colloids Surf. B Biointerfaces 2021, 208, 112108. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.S.; Kim, C.G.; Choi, Y.T.; Lee, K.Y. 3D printing of self-healing ferrogel prepared from glycol chitosan, oxidized hyaluronate, and iron oxide nanoparticles. Carbohydr. Polym. 2020, 245, 116496. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, L.J.; Tai, G.P.; Yan, F.F.; Cai, L.; Xin, C.X.; Al Islam, S. Graphene Oxide-loaded magnetic nanoparticles within 3D hydrogel form High-performance scaffolds for bone regeneration and tumor treatment. Compos. Part A Appl. Sci. Manuf. 2022, 152, 106672. [Google Scholar] [CrossRef]
- Monks, P.; Wychowaniec, J.K.; McKiernan, E.; Clerkin, S.; Crean, J.; Rodriguez, B.J.; Reynaud, E.G.; Heise, A.; Brougham, D.F. Spatiotemporally Resolved Heat Dissipation in 3D Patterned Magnetically Responsive Hydrogels. Small 2021, 17, 2004452. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, J.D.; Cheng, H.; Li, G.Q.; Cho, H.J.; Jiang, M.J.; Gao, Q.; Zhang, X.W. Developments of Advanced Electrospinning Techniques: A Critical Review. Adv. Mater. Technol. 2021, 6, 2100410. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.B.; Mo, J.M.; Deng, B.L.; Wang, J.X.; Zhu, Y.Q.; Xiao, X.D.; Xu, G. Construction of hierarchical structure of Co3O4 electrode based on electrospinning technique for supercapacitor. J. Alloys Compd. 2021, 853, 157271. [Google Scholar] [CrossRef]
- Li, X.Y.; Chen, W.C.; Qian, Q.R.; Huang, H.T.; Chen, Y.M.; Wang, Z.Q.; Chen, Q.H.; Yang, J.; Li, J.; Mai, Y.-W. Electrospinning-Based Strategies for Battery Materials. Adv. Energy Mater. 2020, 11, 2000845. [Google Scholar] [CrossRef]
- Li, M.; Yu, H.; Xie, Y.F.; Guo, Y.H.; Cheng, Y.L.; Qian, H.; Yao, W.R. Fabrication of eugenol loaded gelatin nanofibers by electrospinning technique as active packaging material. LWT 2021, 139, 110800. [Google Scholar] [CrossRef]
- Castro Coelho, S.; Nogueiro Estevinho, B.; Rocha, F. Encapsulation in food industry with emerging electrohydrodynamic techniques: Electrospinning and electrospraying—A review. Food Chem. 2021, 339, 127850. [Google Scholar] [CrossRef]
- Russo, F.; Castro-Munoz, R.; Santoro, S.; Galiano, F.; Figoli, A. A review on electrospun membranes for potential air filtration application. J. Environ. Chem. Eng. 2022, 10, 108452. [Google Scholar] [CrossRef]
- Margarita Valencia-Osorio, L.; Álvarez-Láinez, M.L. Global View and Trends in Electrospun Nanofiber Membranes for Particulate Matter Filtration: A Review. Macromol. Mater. Eng. 2021, 306, 2100278. [Google Scholar] [CrossRef]
- Partheniadis, I.; Nikolakakis, I.; Laidmäe, I.; Heinämäki, J. A Mini-Review: Needleless Electrospinning of Nanofibers for Pharmaceutical and Biomedical Applications. Processes 2020, 8, 673. [Google Scholar] [CrossRef]
- Garkal, A.; Kulkarni, D.; Musale, S.; Mehta, T.; Giram, P. Electrospinning nanofiber technology: A multifaceted paradigm in biomedical applications. New J. Chem. 2021, 46, 21508–21533. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Ghayour, H.; Fauzi Ismail, A.; Nur, H.; Berto, F. Electrospun Nano-Fibers for Biomedical and Tissue Engineering Applications: A Comprehensive Review. Materials 2020, 13, 2153. [Google Scholar] [CrossRef] [PubMed]
- Blachowicz, T.; Ehrmann, A. Most recent developments in electrospun magnetic nanofibers: A review. J. Eng. Fibers Fabr. 2020, 15, 1558925019900843. [Google Scholar] [CrossRef]
- Choe, J.A.; Uthamaraj, S.; Dragomir-Daescu, D.; Sandhu, G.S.; Tefft, B.J. Magnetic and Biocompatible Polyurethane Nanofiber Biomaterial for Tissue Engineering. Tissue Eng. A 2022, 29, 224. [Google Scholar] [CrossRef]
- Khalili, M.; Keshvari, H.; Imani, R.; Naderi Sohi, A.; Esmaeili, E.; Tajabadi, M. Study of osteogenic potential of electrospun PCL incorporated by dendrimerized superparamagnetic nanoparticles as a bone tissue engineering scaffold. Polym. Adv. Technol. 2021, 33, 782–794. [Google Scholar] [CrossRef]
- Zhang, J.B.; Zhang, M.; Lin, R.C.; Du, Y.G.; Wang, L.M.; Yao, Q.Q.; Zannettino, A.; Zhang, H. Chondrogenic preconditioning of mesenchymal stem/stromal cells within a magnetic scaffold for osteochondral repair. Biofabrication 2022, 14, 025020. [Google Scholar] [CrossRef]
- Bakhtiary, N.; Pezeshki-Modaress, M.; Najmoddin, N. Wet-electrospinning of nanofibrous magnetic composite 3-D scaffolds for enhanced stem cells neural differentiation. Chem. Eng. Sci. 2022, 264, 118144. [Google Scholar] [CrossRef]
- Chen, X.; Ge, X.M.; Qian, Y.; Tang, H.Z.; Song, J.L.; Qu, X.H.; Yue, B.; Yuan, W.-E. Electrospinning Multilayered Scaffolds Loaded with Melatonin and Fe3O4 Magnetic Nanoparticles for Peripheral Nerve Regeneration. Adv. Funct. Mater. 2020, 30, 2004537. [Google Scholar] [CrossRef]
- Estévez, M.; Montalbano, G.; Gallo-Cordova, A.; Ovejero, J.G.; Izquierdo-Barba, I.; González, B.; Tomasina, C.; Moroni, L.; Vallet-Regí, M.; Vitale-Brovarone, C.; et al. Incorporation of Superparamagnetic Iron Oxide Nanoparticles into Collagen Formulation for 3D Electrospun Scaffolds. Nanomaterials 2022, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Funnell, J.L.; Ziemba, A.M.; Nowak, J.F.; Awada, H.; Prokopiou, N.; Samuel, J.; Guari, Y.; Nottelet, B.; Gilbert, R.J. Assessing the combination of magnetic field stimulation, iron oxide nanoparticles, and aligned electrospun fibers for promoting neurite outgrowth from dorsal root ganglia in vitro. Acta Biomater. 2021, 131, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Reizabal, A.; Brito-Pereira, R.; Fernandes, M.M.; Castro, N.; Correia, V.; Ribeiro, C.; Costa, C.M.; Perez, L.; Vilas, J.L.; Lanceros-Méndez, S. Silk fibroin magnetoactive nanocomposite films and membranes for dynamic bone tissue engineering strategies. Materialia 2020, 12, 100709. [Google Scholar] [CrossRef]
- Moradian, E.; Rabiee, S.M.; Haghighipour, N.; Salimi-Kenari, H. Fabrication and physicochemical characterization of a novel magnetic nanocomposite scaffold: Electromagnetic field effect on biological properties. Mater. Sci. Eng. C 2020, 116, 111222. [Google Scholar] [CrossRef] [PubMed]
- Pryadko, A.S.; Botvin, V.V.; Mukhortova, Y.R.; Pariy, I.; Wagner, D.V.; Laktionov, P.P.; Chernonosova, V.S.; Chelobanov, B.P.; Chernozem, R.V.; Surmeneva, M.A.; et al. Core-Shell Magnetoactive PHB/Gelatin/Magnetite Composite Electrospun Scaffolds for Biomedical Applications. Polymers 2022, 14, 529. [Google Scholar] [CrossRef] [PubMed]
- Pryadko, A.S.; Mukhortova, Y.R.; Chernozem, R.V.; Pariy, I.; Alipkina, S.I.; Zharkova, I.I.; Dudun, A.A.; Zhuikov, V.A.; Moisenovich, A.M.; Bonartseva, G.A.; et al. Electrospun Magnetic Composite Poly-3-hydroxybutyrate/Magnetite Scaffolds for Biomedical Applications: Composition, Structure, Magnetic Properties, and Biological Performance. ACS Appl. Bio Mater. 2022, 5, 3999–4019. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, H.L.; Matera, D.L.; Rose, M.J.; Kent, R.N.; Todd, C.W.; Stout, M.E.; Wank, A.E.; Schiavone, M.C.; DePalma, S.J.; Zarouk, A.A.; et al. Magnetic Alignment of Electrospun Fiber Segments Within a Hydrogel Composite Guides Cell Spreading and Migration Phenotype Switching. Front. Bioeng. Biotechnol. 2021, 9, 679165. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, T.; Wang, Z.H.; Hou, J.D.; Liu, S.T.; Yang, Q.; Yu, L.; Guo, W.H.; Wang, Y.J.; Guo, B.L.; et al. Injectable remote magnetic nanofiber/hydrogel multiscale scaffold for functional anisotropic skeletal muscle regeneration. Biomaterials 2022, 285, 121537. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; El-Dakroury, W.A.; Zewail, M.B.; Noshy, M.; Abdelfatah, A.M.; Doghish, A.S. Smart/stimuli-responsive hydrogels: State-of-the-art platforms for bone tissue engineering. Appl. Mater. Today 2022, 29, 106560. [Google Scholar] [CrossRef]
- Guo, Z.W.; Dong, L.; Xia, J.J.; Mi, S.L.; Sun, W. 3D Printing Unique Nanoclay-Incorporated Double-Network Hydrogels for Construction of Complex Tissue Engineering Scaffolds. Adv. Healthc. Mater. 2021, 10, 2100036. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Wang, S.C.; Chen, X.; Jiang, Y.Y.; Su, J.C. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Bonhome-Espinosa, A.B.; Campos, F.; Durand-Herrera, D.; Sánchez-López, J.D.; Schau, S.; Durán, J.D.G.; Lopez-Lopez, M.T.; Carriel, V. In vitro characterization of a novel magnetic fibrin-agarose hydrogel for cartilage tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103619. [Google Scholar] [CrossRef] [PubMed]
- Sakr, M.A.; Sakthivel, K.; Hossain, T.; Shin, S.R.; Siddiqua, S.; Kim, J.W.; Kim, K.Y. Recent trends in gelatin methacryloyl nanocomposite hydrogels for tissue engineering. J. Biomed. Mater. Res. A 2021, 110, 708–724. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B.; Liu, M.; Zhang, Y.J.; Yin, J.B.; Pei, R.J. Nanocomposite hydrogels for tissue engineering applications. Nanoscale 2020, 12, 14976–14995. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.P.; Flores, M.; Madureira, S.; Zanotto, F.; Monteiro, F.J.; Laranjeira, M.S. Magnetic Bone Tissue Engineering: Reviewing the Effects of Magnetic Stimulation on Bone Regeneration and Angiogenesis. Pharmaceutics 2023, 15, 1045. [Google Scholar] [CrossRef] [PubMed]
- Bekhite, M.M.; Figulla, H.R.; Sauer, H.; Wartenberg, M. Static magnetic fields increase cardiomyocyte differentiation of Flk-1+ cells derived from mouse embryonic stem cells via Ca2+ influx and ROS production. Int. J. Cardiol. 2013, 167, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ding, C.; Shang, P. Alterations of Mineral Elements in Osteoblast During Differentiation Under Hypo, Moderate and High Static Magnetic Fields. Biol. Trace Elem. Res. 2014, 162, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Lew, W.Z.; Huang, Y.C.; Huang, K.Y.; Lin, C.T.; Tsai, M.T.; Huang, H.M. Static magnetic fields enhance dental pulp stem cell proliferation by activating the p38 mitogen-activated protein kinase pathway as its putative mechanism. J. Tissue Eng. Regen. Med. 2018, 12, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Qian, A.R.; Gao, X.; Zhang, W.; Li, J.B.; Wang, Y.; Di, S.M.; Hu, L.F.; Shang, P. Large Gradient High Magnetic Fields Affect Osteoblast Ultrastructure and Function by Disrupting Collagen I or Fibronectin/αβ1 Integrin. PLoS ONE 2013, 8, e51036. [Google Scholar] [CrossRef]
- Martino, C.F.; Perea, H.; Hopfner, U.; Ferguson, V.L.; Wintermantel, E. Effects of weak static magnetic fields on endothelial cells. Bioelectromagnetics 2010, 31, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, J.; Wang, X.; Xu, Y.; Liang, Z.; Gu, X.; He, C. Coupling induction of osteogenesis and type H vessels by pulsed electromagnetic fields in ovariectomy-induced osteoporosis in mice. Bone 2022, 154, 116211. [Google Scholar] [CrossRef] [PubMed]
- Eivazzadeh-Keihan, R.; Noruzi, E.B.; Chenab, K.K.; Jafari, A.; Radinekiyan, F.; Hashemi, S.M.; Ahmadpour, F.; Behboudi, A.; Mosafer, J.; Mokhtarzadeh, A.; et al. Metal-based nanoparticles for bone tissue engineering. J. Tissue Eng. Regen. Med. 2020, 14, 1687–1714. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, Y.N.; Xu, J.W.; Wang, J.X.; Gu, X.N.; Li, P.; Fan, Y.B. Three-dimensional magnetic fibrous scaffold with icariin expanded by supercritical CO2 for bone tissue engineering under static magnetic field. Comp. B Eng. 2021, 226, 109304. [Google Scholar] [CrossRef]
- Fu, C.; Bai, H.; Hu, Q.; Gao, T.; Bai, Y. Enhanced proliferation and osteogenic differentiation of MC3T3-E1 pre-osteoblasts on graphene oxide-impregnated PLGA-gelatin nanocomposite fibrous membranes. RSC Adv. 2017, 7, 8886–8897. [Google Scholar] [CrossRef]
- Petretta, M.; Gambardella, A.; Desando, G.; Cavallo, C.; Bartolotti, I.; Shelyakova, T.; Goranov, V.; Brucale, M.; Dediu, V.A.; Fini, M.; et al. Multifunctional 3D-Printed Magnetic Polycaprolactone/Hydroxyapatite Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 3825. [Google Scholar] [CrossRef] [PubMed]
- Kaliannagounder, V.K.; Hossain, M.A.; Kim, J.-H.; Thangavelu, M.; Adithan, A. Magnetic Hydroxyapatite Composite Nanoparticles for Augmented Differentiation of MC3T3-E1 Cells for Bone Tissue Engineering. Mar. Drugs 2023, 21, 85. [Google Scholar] [CrossRef] [PubMed]
- Jasemi, A.; Moghadas, B.K.; Khandan, A.; Saber-Samandari, S. A porous calcium-zirconia scaffolds composed of magnetic nanoparticles for bone cancer treatment: Fabrication, characterization and FEM analysis. Ceram. Int. 2022, 48, 1314–1325. [Google Scholar] [CrossRef]
- Wu, D.; Chang, X.; Tian, J.; Kang, L.; Wu, Y.H.; Liu, J.Y.; Wu, X.D.; Huang, Y.; Gao, B.; Wang, H.; et al. Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: Release of exosomal miR-1260a improves osteogenesis and angiogenesis. J. Nanobiotechnol. 2021, 19, 209. [Google Scholar] [CrossRef]
- Cojocaru, F.D.; Balan, V.; Popa, M.I.; Lobiuc, A.; Antoniac, A.; Antoniac, I.V.; Verestiuc, L. Biopolymers–Calcium phosphates composites with inclusions of magnetic nanoparticles for bone tissue engineering. Int. J. Biol. Macromol. 2019, 125, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, M.; Alvarez Primo, F.; Anil Kumar, S.; Mudloff, J.A.; Dominguez, E.; Fregoso, G.; Ortiz, N.; Weiss, W.M.; Jaddar, B. Bone tissue engineering techniques, advances, and scaffolds for treatment of bone defects. Curr. Opin. Biomed. Eng. 2021, 17, 100248. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.T. An Introduction to the Basic Principles of Magnetic Nerve Stimulation. J. Clin. Neurophysiol. 1991, 8, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Cheng, Y.; Cai, J.Q.; Zhao, X.T.; Quyang, Y.M.; Yuan, W.-E.; Fan, C.Y. Advances in Electrical and Magnetic Stimulation on Nerve Regeneration. Regen. Med. 2019, 14, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.P.; Yang, Y.J.; Deng, J.Y.; Ur Rahman, M.S.; Sun, C.M.; Xu, S.S. Physical Stimulation Combined with Biomaterials Promotes Peripheral Nerve Injury Repair. Bioengineering 2022, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Zuidema, J.M.; Provenza, C.; Caliendo, T.; Dutz, S.; Gilbert, R.J. Magnetic NGF-releasing PLLA/iron oxide nanoparticles direct extending neurites and preferentially guide neurites along aligned electrospun microfibers. ACS Chem. Neurosci. 2015, 6, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Glaser, T.; Bueno, V.B.; Cornejo, D.R.; Petri, D.F.; Ulrich, H. Neuronal adhesion, proliferation and differentiation of embryonic stem cells on hybrid scaffolds made of xanthan and magnetite nanoparticles. Biomed. Mater. 2015, 10, 045002. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, L.; Liu, L.; Luo, B.; Liang, M.; Sun, Z.; Zhu, S.; Quan, X.; Yang, Y.; Ma, T. Activation of Schwann cells in vitro by magnetic nanocomposites via applied magnetic field. Int. J. Nanomed. 2015, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.D.; Ganguly, D.; Zuidema, J.M.; Cardinal, T.J.; Ziemba, A.M.; Kearns, K.R.; McCarthy, S.M.; Thompson, D.M.; Ramanath, G.; Borca-Tasciuc, D.A. Injectable, magnetically orienting electrospun fiber conduits for neuron guidance. ACS Appl. Mater. Interfaces 2018, 11, 356–372. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, F.; Zamanian, A.; Shams, A.; Shamoosi, A.; Aidun, A. Fabrication and characterisation of super-paramagnetic responsive PLGA-gelatine-magnetite scaffolds with the unidirectional porous structure: A physicochemical, mechanical and in vitro evaluation. IET Nanobiotechnol. 2019, 13, 860–867. [Google Scholar] [CrossRef]
- Dai, Y.; Lu, T.W.; Shao, M.H.; Lyu, F.Z. Recent advances in PLLA-based biomaterial scaffolds for neural tissue engineering: Fabrication, modification, and applications. Front. Biogn. Biotechnol. 2022, 10, 1011783. [Google Scholar] [CrossRef]
- Arzaghi, H.; Adel, B.; Jafari, H.; Askarian-Amiri, S.; Dezfuli, A.S.; Akbarzadeh, A.; Pazoki-Toroudi, H. Nanomaterial integration into the scaffolding materials for nerve tissue engineering: A review. Rev. Neurosci. 2020, 31, 843–872. [Google Scholar] [CrossRef]
- Hu, Y.N.; Zhang, H.; Wie, H.; Cheng, H.; Cai, J.Y.; Chen, X.Y.; Xia, L.; Wang, H.; Chai, R.J. Scaffolds with anisotropic structure for neural tissue engineering. Eng. Regen. 2022, 3, 154–162. [Google Scholar] [CrossRef]
- Frese, N.; Gölzhäuser, A.; Wortmann, M. Helium ion microscopy. In Imaging Modalities for Biological and Preclinical Research: A Compendium; IOP Publishing: Bristol, UK, 2021; Volume 1, pp. I.7-1–I.7-9. [Google Scholar]
- Ghaderinejad, P.; Najmoddin, N.; Bagher, Z.; Saeed, M.; Karimi, S.; Simorgh, S.; Pezeshki-Modaress, M. An injectable anisotropic alginate hydrogel containing oriented fibers for nerve tissue engineering. Chem. Eng. J. 2021, 420, 130465. [Google Scholar] [CrossRef]
- Lacko, C.S.; Singh, I.; Wall, M.A.; Garcia, A.R.; Porvasnik, S.L.; Rinaldi, C.; Schmidt, C.E. Magnetic particle templating of hydrogels: Engineering naturally derived hydrogel scaffolds with 3D aligned microarchitecture for nerve repair. J. Neural Eng. 2020, 17, 016057. [Google Scholar] [CrossRef] [PubMed]
- Scherer, F.; Anton, M.; Schillinger, U.; Henke, J.; Bergemann, C.; Krüger, A.; Gänsbacher, B.; Plank, C. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002, 9, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Aadil, K.R.; Ranjan, S.; Kumar, V.B. Advances in nanotechnology and nanomaterials based strategies for neural tissue engineering. J. Drug Deliv. Sci. Technol. 2020, 57, 101617. [Google Scholar] [CrossRef]
- Vinhas, A.; Goncalves, A.I.; Rodrigues, M.T.; Gomes, M.E. Human tendon-derived cell sheets created by magnetic force-based tissue engineering hold tenogenic and immunomodulatory potential. Acta Biomater. 2021, 131, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.; Kumar, A.; Han, S.S. Polysaccharide-based magnetically responsive polyelectrolyte hydrogels for tissue engineering applications. J. Mater. Sci. Technol. 2018, 34, 1371–1377. [Google Scholar] [CrossRef]
- Ito, A.; Kamihira, M. Tissue Engineering Using Magnetite Nanoparticles. Prog. Mol. Biol. Transl. Sci. 2011, 104, 355–395. [Google Scholar] [PubMed]
- Alam, F.; Varadarajan, K.M.; Kumar, S. 3D printed polylactic acid nanocomposite scaffolds for tissue engineering applications. Polym. Test. 2020, 81, 106203. [Google Scholar] [CrossRef]
- Ajiteru, O.; Choi, K.Y.; Lim, T.H.; Kim, D.Y.; Hong, H.S.; Lee, Y.J.; Lee, J.S.; Lee, H.; Suh, Y.J.; Sultan, T.; et al. A digital light processing 3D printed magnetic bioreactor system using silk magnetic bioink. Biofabrication 2021, 13, 034102. [Google Scholar] [CrossRef] [PubMed]
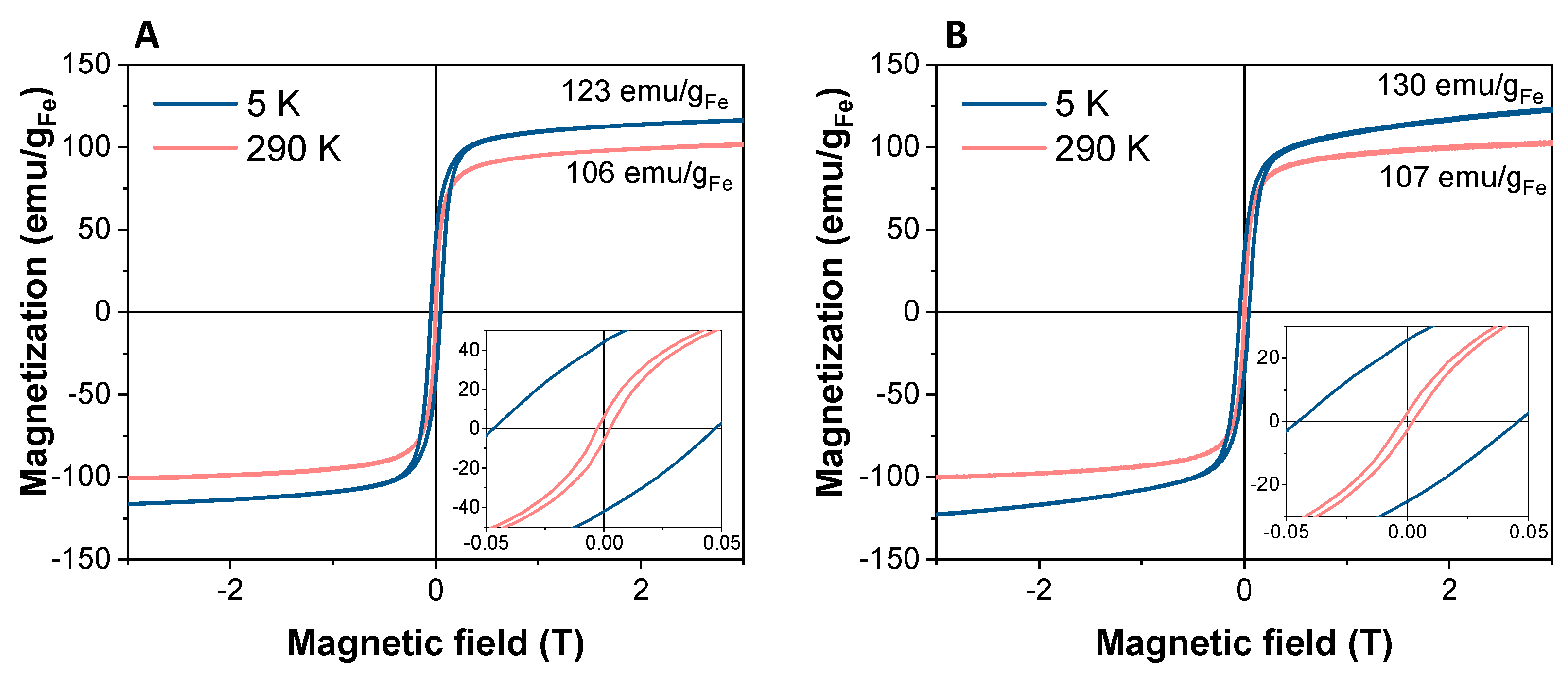

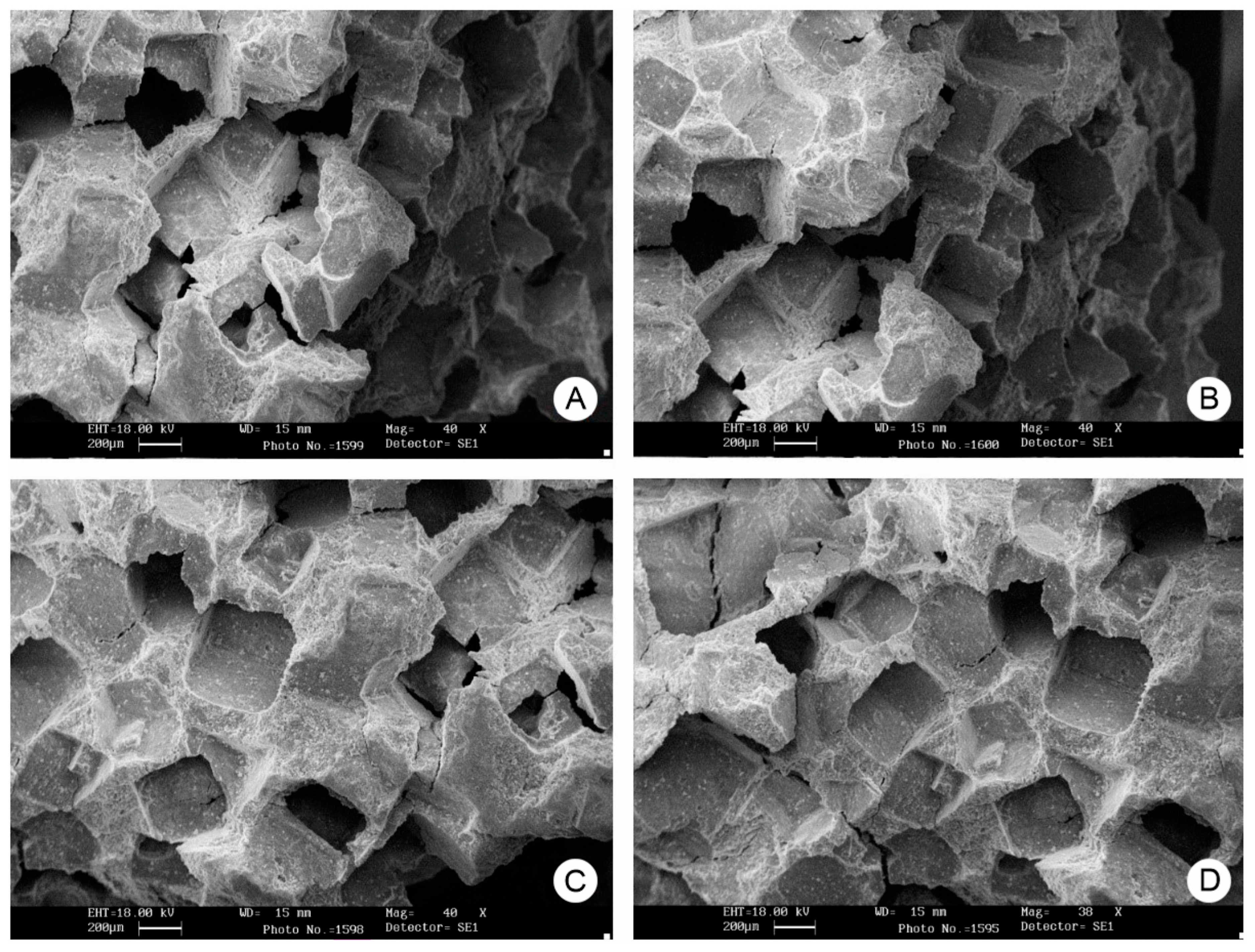
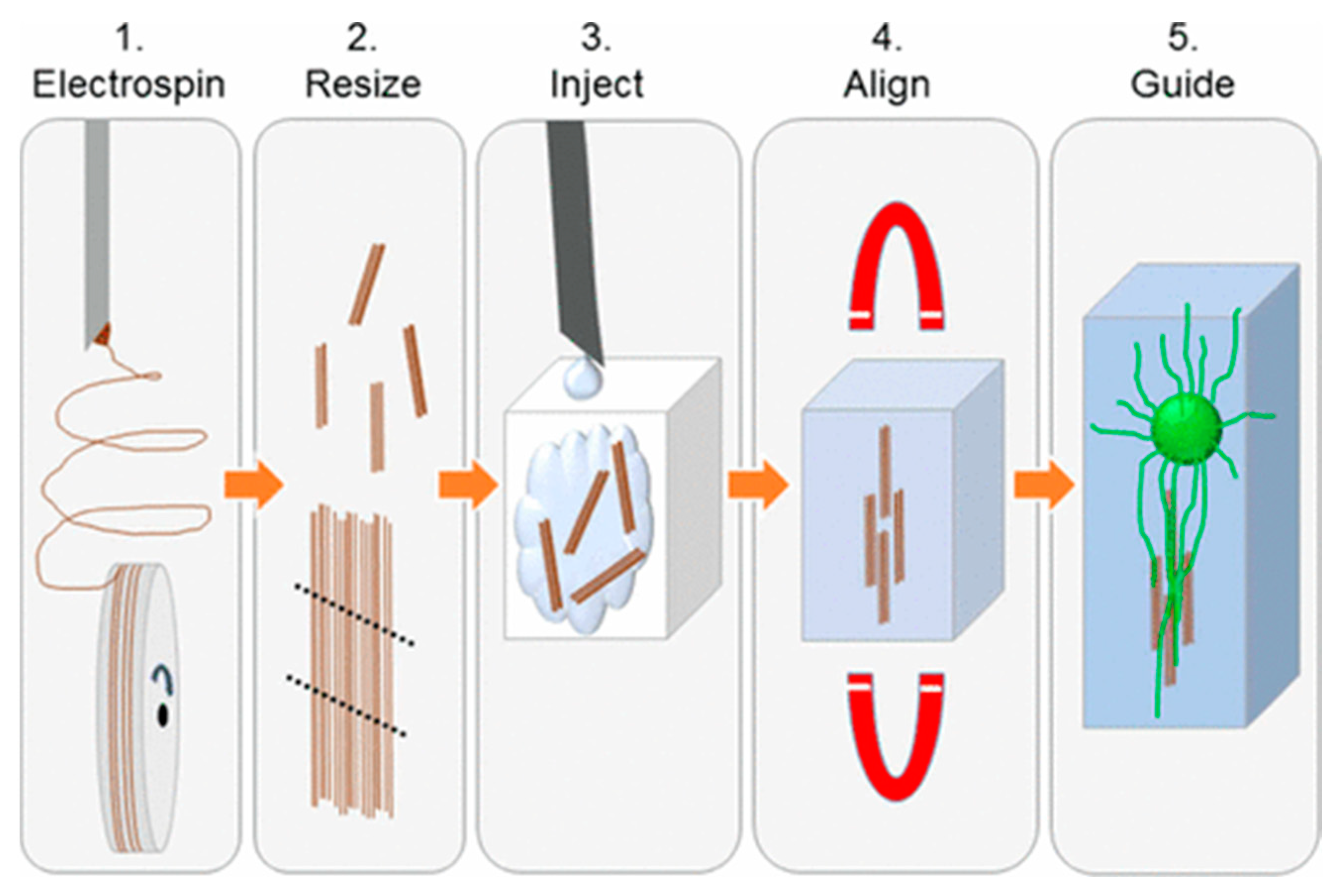
| Method | Materials | Ref. |
|---|---|---|
| 4D-printed hydrogels | Agarose, alginate, chitosan, collagen, gelatin, hyaluronic acid, PEG, PLA, PLGA, PCL, poloxamer with magnetic nanoparticles, ferrogel | [92,106,107,108,109] |
| 4D-printed polymers | PNIPAM, PDA | [92] |
| 3D-printed anisotropic fibrous microstructures | Low-viscosity magnetic bioinks | [96] |
| 3D bioprinting | PCL/Fe-doped HAP | [100] |
| FDM printing | Magnetic PLA | [102] |
| LDM printing | Magnetic bioactive glass | [104] |
| FDM printing | PCL/Fe3O4 | [105] |
| Electrospinning on 3D-printed scaffolds | Magnetic nanofibers | [121,122,123] |
| Magnetically assisted wet electrospinning | Gelatin/PCL/iron oxide | [124] |
| Electrospinning | Melatonin/magnetite-loaded fibers | [125] |
| Electrospinning-aligned fibers | SPIONs in nanofibers | [126] |
| Needle electrospinning | SPIONs in collagen nanofibers | [127] |
| Electrospinning | Cobalt–zinc ferrite/PCL | [129] |
| Core–shell electrospinning | PHB/magnetite/gelatin | [130] |
| Hydrogel | PVA, collagen, gelatin, PEG, agarose and other polymers with magnetic nanoparticles | [134,137,139] |
| Application | Production | Materials | Ref. |
|---|---|---|---|
| Bone tissue engineering | Electrospinning | PCL/magnetite/icariin | [148] |
| Bone tissue engineering | Bioprinting | PCL/HAP/SPIONs | [150] |
| Bone tissue engineering | Wet chemical co-precipitation | HAP/magnetic nanoparticles | [151] |
| Nerve tissue engineering | Electrospinning | PLA/oleic acid-coated iron oxide nanoparticles | [162] |
| Nerve tissue engineering | Hydrogel | Injected short PCL/SPION nanofibers | [168] |
| Nerve tissue engineering | Hydrogel | Aligned magnetic alginate microparticles | [169] |
| Tendon therapy | Cultivation in static magnetic field | Confluent cell monolayer with magnetic nanoparticles | [172] |
| Skin, cartilage, muscle or connective tissue engineering | Hydrogel | Magnetic polysaccharides | [173] |
| Diverse tissues | Filling scaffolds | Magnetic nanoparticles | [175,176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blachowicz, T.; Ehrmann, A. Magnetic Substrates for Tissue Engineering—A Review. Magnetochemistry 2024, 10, 52. https://doi.org/10.3390/magnetochemistry10080052
Blachowicz T, Ehrmann A. Magnetic Substrates for Tissue Engineering—A Review. Magnetochemistry. 2024; 10(8):52. https://doi.org/10.3390/magnetochemistry10080052
Chicago/Turabian StyleBlachowicz, Tomasz, and Andrea Ehrmann. 2024. "Magnetic Substrates for Tissue Engineering—A Review" Magnetochemistry 10, no. 8: 52. https://doi.org/10.3390/magnetochemistry10080052








