An Organic–Inorganic Hybrid Semiconducting Quantum Spin Liquid Candidate: (BEDT-TTF)3[Cu2(μ-C2O4)3·CH3CH2OH·1.2H2O]
Abstract
1. Introduction
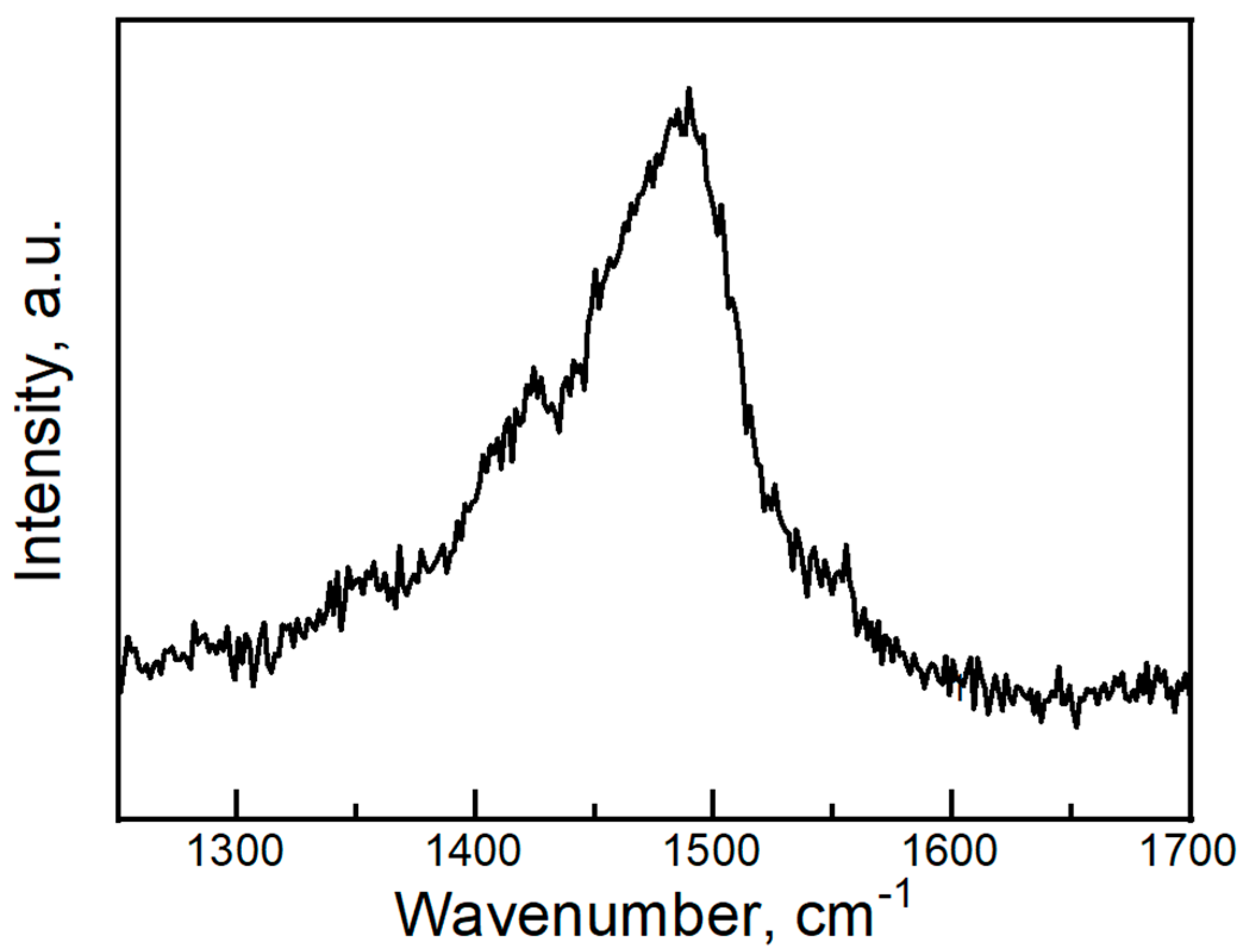
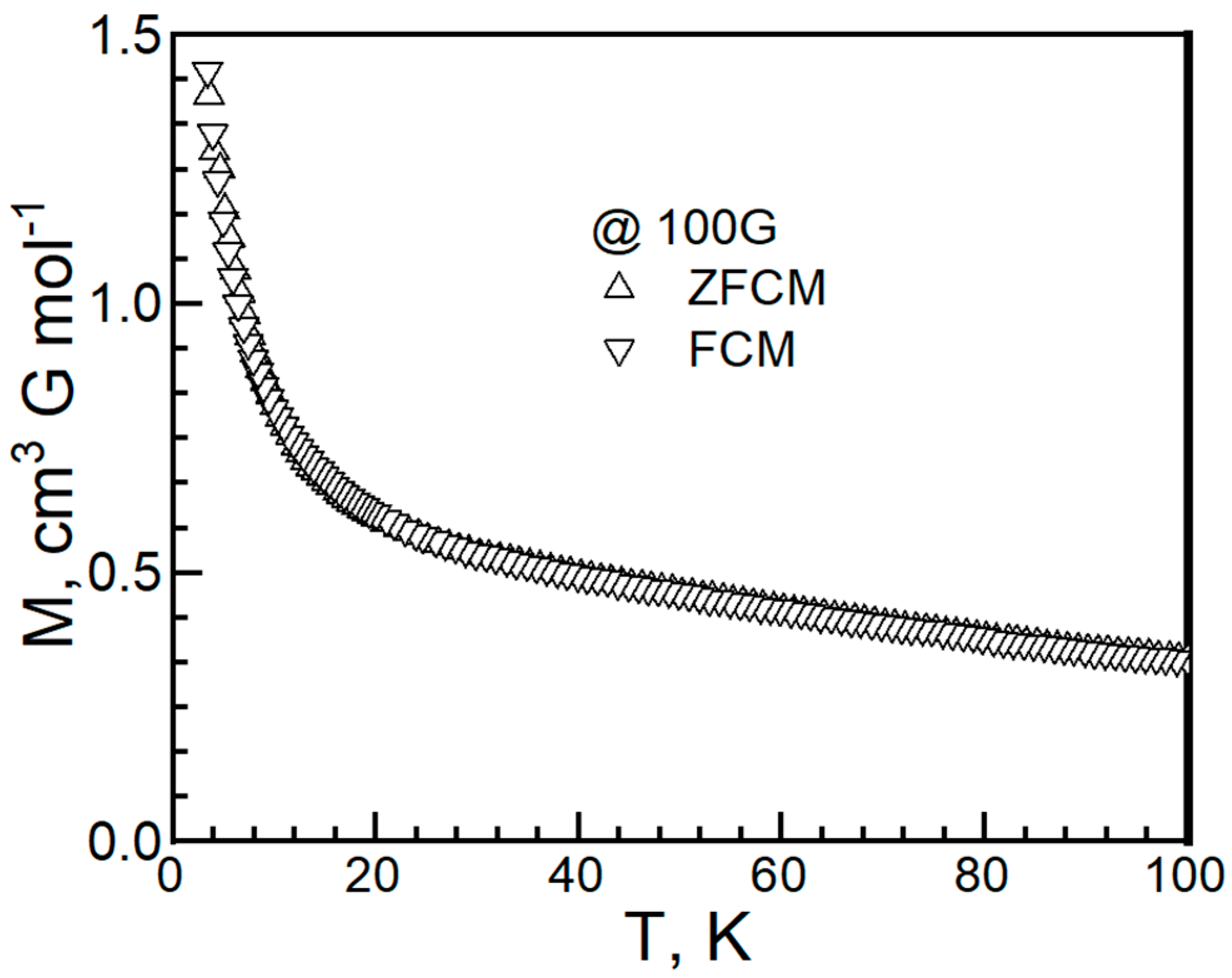
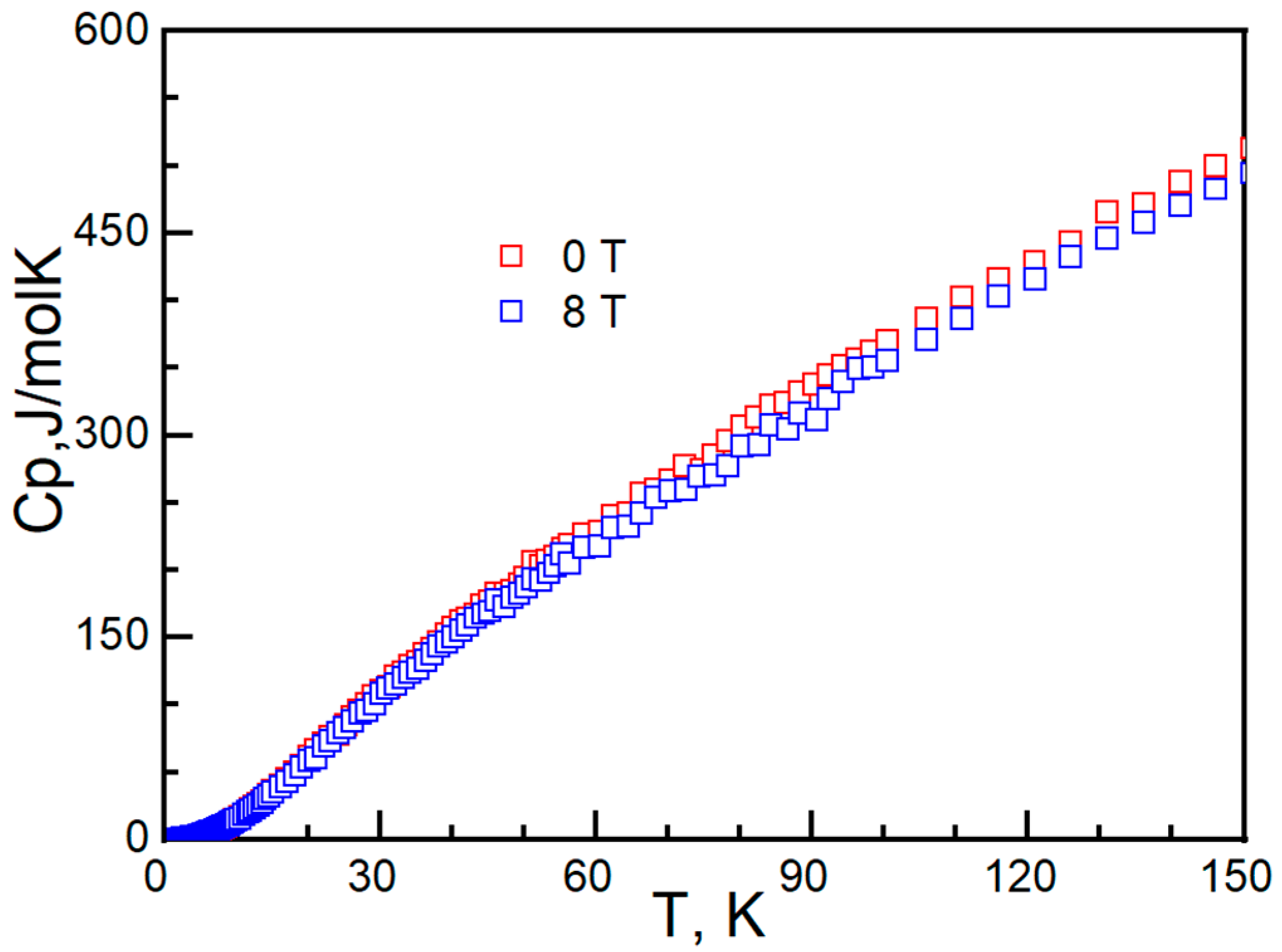
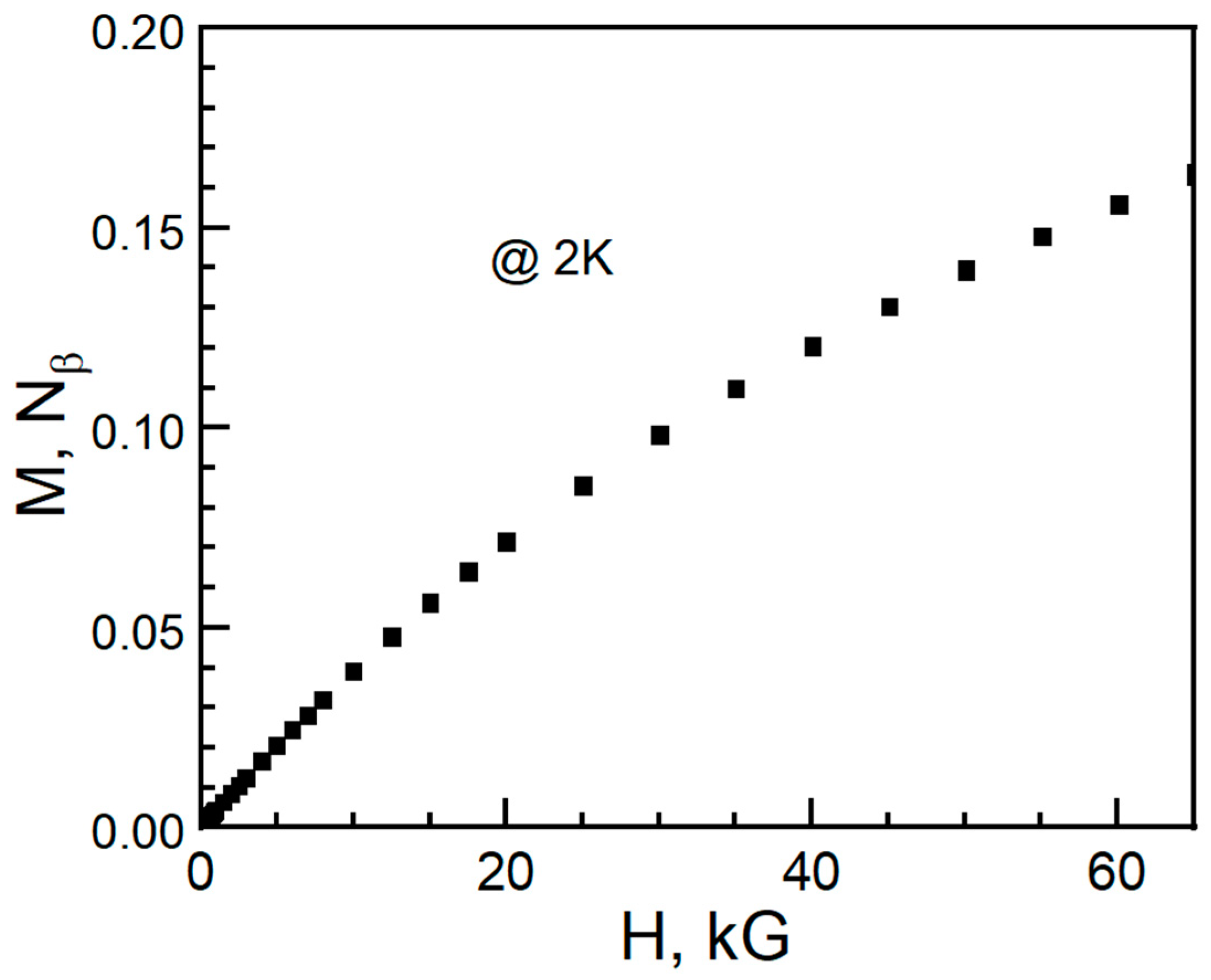
2. Experiment and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, P.W. Resonating Valence Bonds: A New Kind of Insulator. Mater. Res. Bull. 1973, 8, 153–160. [Google Scholar] [CrossRef]
- Bednorz, J.G.; Muller, K.A. Possible High Tc Superconductivity in the Ba−La−Cu−O System. Z. Phys. B. Condensed Matter 1986, 64, 189–193. [Google Scholar] [CrossRef]
- Anderson, P.W. The Resonating Valence Bond State in La2CuO4 and Superconductivity. Science 1987, 235, 1197–1198. [Google Scholar] [CrossRef] [PubMed]
- Balents, L. Spin liquids in frustrated magnets. Nature 2010, 464, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Broholm, C.; Cava, R.J.; Kivelson, S.A.; Nocera, D.G.; Norman, M.R.; Senthil, T. Quantum Spin Liquids. Science 2020, 367, eaay0668. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Schultz, A.J.; Geiser, U.; Carlson, K.D.; Kini, A.M.; Wang, H.H.; Kwok, W.; Whangbo, M. Organic Superconductors—New Benchmarks. Science 1991, 252, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Pratt, F.L.; Baker, P.J.; Blundell, S.J.; Lancaster, T.; Ohira-Kawamura, S.; Baines, C.; Shimizu, Y.; Kanoda, K.; Watanabe, I.; Saito, G. Magnetic and non-magnetic phase of quantum spin liquid. Nature 2011, 471, 612–616. [Google Scholar] [CrossRef]
- Shimizu, Y.; Hiramatsu, T.; Maesato, M.; Otsuka, A.; Yamochi, H.; Ono, A.; Itoh, M.; Yoshida, M.; Takigawa, M.; Yoshida, Y.; et al. Pressure-Tuned Exchange Coupling of q Quan Spin Liquid in the Molecular Triangular Lattice κ-(ET)2Ag2(CN)3. Phys. Rev. Lett. 2016, 117, 107203. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Nakata, N.; Senshu, Y.; Nagata, M.; Yamamoto, H.M.; Kato, R.; Shibauchi, T.; Matsuda, Y. Highly Mobile Gapless Excitations in a Two-Dimensional Candidate Quantum Spin Liquid. Science 2010, 328, 1246–1268. [Google Scholar] [CrossRef]
- Isono, T.; Kamo, H.; Ueda, A.; Takahashi, K.; Kimata, M.; Tajima, H.; Tsuchiya, S.; Terashima, T.; Uji, S.; Mori, H. Gapless Quantum Spin Liquid in an Organic Spin-1/2 Triangular-Lattice κ-H3(cat-EDT-TTF)2. Phys. Rev. Lett. 2014, 112, 177201. [Google Scholar] [CrossRef] [PubMed]
- Baudron, S.A.; Batail, P.; Coulon, C.; Clérac, R.; Canadell, E.; Laukhin, V.; Melzi, R.; Wzietek, P.; Jérome, D.; Auban-Senzier, P.; et al. (EDT-TTF-CONH2)6[Re6Se8(CN)6], a Metallic Kagome-Type Organic−Inorganic Hybrid Compound: Electronic Instability, Molecular Motion, and Charge Localization. J. Am. Chem. Soc. 2005, 127, 11785–11797. [Google Scholar] [CrossRef]
- Cano, J.; Alemany, P.; Alvarez, S.; Verdaguer, M.; Ruiz, E. Exchange Coupling in Oxalato-Bridged Copper(II) Binuclear Compounds: A Density Functional Study. Chem. Eur. J. 1998, 4, 476–484. [Google Scholar] [CrossRef]
- Hursthouse, M.B.; Light, M.E.; Price, D.J. One-dimensional Magnetism in Anhydrous Iron and Cobalt Ternary Oxalates with Rare Trigonal-Prismatic Metal Coordination Environment. Angew. Chem. Int. Ed. 2004, 43, 472–475. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Z.; Liu, T.; Gao, S. Synthesis, Structure, and Magnetic Properties of (A)[FeIII(oxalate)Cl2] (A = Alkyl Ammonium Cations) with Anionic 1D [FeIII(oxalate)Cl2]− Chains. Inorg. Chem. 2007, 46, 3089–3096. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Zhang, Y.; Zhang, B.; Zhu, D. Co(C2O4)(HO(CH2)3OH): An Antiferromagnetic Neutral Zigzag Chain Compound Showing Long-Range Ordering of Spin Canting. Inorg. Chem. 2008, 47, 9152–9154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Chang, G.; Wang, Z.; Zhu, D. Crystal-to-Crystal Transformation from K2[Co(C2O4)2(H2O)2]·4H2O to K2[Co(µ-C2O4)(C2O4)]. Magnetochemistry 2021, 7, 77. [Google Scholar] [CrossRef]
- Mathoniere, C.; Nuttall, C.J.; Carling, G.; Day, P. Ferrimagnetic Mixed-Valency and Mixed-Metal Tris(oxalato)iron(III) Compounds: Synthesis, Structure, and Magnetism. Inorg. Chem. 1996, 35, 1201–1206. [Google Scholar] [CrossRef]
- Tamaki, H.; Zhong, Z.J.; Matsumoto, N.; Kida, S.; Koikawa, M.; Achiwa, N.; Hashimoto, Y.; Okawa, H. Design of Metal-Complex Magnets. Syntheses and Magnetic Properties of Mixed-Metal Assemblies {NBu4[MCr(ox)3]} (NBu4+ = Tetra(n-buty1)ammonium Ion; ox2− = Oxalate Ion; M = Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+). J. Am. Chem. Soc. 1992, 114, 6974–6979. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Zhang, J.; Li, J.; Zhu, D. A neutral molecular-based layered magnet [Fe(C2O4)(CH3OH)]n exhibiting magnetic ordering at TN ≈ 23 K. Dalton Trans. 2008, 5037–5040. [Google Scholar] [CrossRef]
- Rousse, G.; Rodriguez-Carvajal, J. Oxalate-mediated long-range antiferromagnetism order in Fe2(C2O4)3·4H2O. Dalton Trans. 2016, 45, 14311–14319. [Google Scholar] [CrossRef] [PubMed]
- Decurtins, S.; Schmalle, H.W.; Schneuwly, P.; Ensling, J.; Gutlich, P. A Concept for the Synthesis of 3-Dimensional Homo- and Bimetallic Oxalate-Bridged Networks [M2(ox)3]n. Structural, Mössbauer, and Magnetic Studies in the Field of Molecular-Based Magnets. J. Am. Chem. Soc. 1994, 116, 9521–9528. [Google Scholar] [CrossRef]
- Hernandez-Molina, M.; Lloret, F.; Ruiz-Perez, C.; Julve, M. Weak Ferromagnetism in Chiral 3-Dimensional Oxalato-Bridged Cobalt(II) Compounds. Crystal Structure of Co(bpy)3][Co2(ox)3]ClO4. Inorg. Chem. 1998, 37, 4131–4145. [Google Scholar] [CrossRef]
- Coronado, E.; Galan-Mascaros, J.R.; Gomez-Garzia, C.J.; Martinez-Agudo, J.M. Molecule-Based Magnets Formed by Bimetallic Three-Dimensional Oxalate Networks and Chiral Tris(bipyridyl) Complex Cations. The Series Tris(bipyridyl) Complex Cations. The Series [ZII(bpy)3][ClO4][MIICrIII(ox)3] (ZII = Ru, Fe, Co, and Ni; MII = Mn, Fe, Co, Ni, Cu, and Zn; ox = Oxalate Dianion). Inorg. Chem. 2001, 40, 113–120. [Google Scholar]
- Mon, M.; Grancha, T.; Verdaguer, M.; Train, C.; Armentano, D.; Pardo, E. Solvent-Dependent Self-Assembly of an Oxalato-Based ThreeDimensional Magnet Exhibiting a Novel Architecture. Inorg. Chem. 2016, 55, 6845–6847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Zhang, J.; Xiang, H.; Zhu, D. Mn(C2O4)(H2O)0.25: An antiferromagnetic oxalato-based cage compound. Dalton Trans. 2011, 40, 5430–5432. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Galan-Mascaros, J.R.; Marti-Gastaldo, C. Single chain magnets based on the oxalate ligand. J. Am. Chem. Soc. 2008, 130, 14987–14989. [Google Scholar] [CrossRef]
- Thomas, T.W.; Che-Hsiung, H.; Labes, M.M.; Gomm, P.S.; Underhill, A.E.; Watkins, D.M. Temperature Dependence of Conductivity of Some Partly-Oxidised Platinum Complexes. J. Chem. Soc. Dalton 1972, 2050–2053. [Google Scholar] [CrossRef]
- Braude, A.; Carneiro, K.; Jacobsen, C.S.; Mortensen, K.; Turner, D.J.; Underhill, A.E. Solid-state properties of one-dimensional metals based on bis(oxalate)platinate anions with divalent cations. Phys. Rev. B 1987, 36, 7835–7846. [Google Scholar] [CrossRef] [PubMed]
- Gartner, S.; Heinen, I.; Schveitzer, D.; Nuber, B.; Keller, H.J. BEDT-TTF salts with square platinates (II) as counterions: (BEDT-TTF)4[Pt(C2O4)2]. A new organic metal. Synth. Met. 1989, 31, 199–213. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Ge, C.; Xing, X.; Wang, P.; Zhang, D.; Wu, P.; Zhu, D. Preparation and properties of a new organic conductor (BEDT-TTF)4Cu(C2O4)2. Synth. Met. 1991, 39, 355–358. [Google Scholar] [CrossRef]
- Wang, P.; Bandow, S.; Maruyama, Y.; Wang, X.; Zhu, D. Physical and structure properties of a new organic conductor (BEDT-TTF)4Cu(C2O4)2. Synth. Met. 1991, 44, 147–157. [Google Scholar] [CrossRef]
- Kurmoo, M.; Graham, A.W.; Day, D.; Simon, J.C.; Hursthouse, M.B.; Caufield, J.L.; Singleton, J.; Pratt, F.L.; Hayes, W.; Ducasse, L.; et al. Superconducting and Semiconducting Magnetic Charge Transfer Salts: (BEDT-TTF)4AFe(C2O4)3·C6H5CN. J. Am. Chem. Soc. 1995, 117, 12209–12217. [Google Scholar] [CrossRef]
- Cassoux, P. A Molecular Paramagnetic Superconductor. Science 1996, 272, 1277–1278. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Fujiwara, H.; Kobayashi, H.; Kurmoo, M.; Inoue, K.; Mori, T.; Gao, S.; Zhang, Y.; Zhu, D. Tetrathiafulvalene [FeIII(C2O4)Cl2]: An organic-inorganic hybrid exhibiting canted antiferromagnetism. Adv. Mater. 2005, 17, 1988–1991. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Zhang, Y.; Takahashi, K.; Okano, Y.; Cui, H.; Kobayashi, H.; Inoue, K.; Kurmoo, M.; Pratt, F.; et al. Hybrid organic-inorganic conductor with a magnetic chain anion: κ-BETS2[FeIII(C2O4)Cl2] [BETS = bis(ethylenedithio)tetraselenafulvalene]. Inorg. Chem. 2006, 45, 3275–3280. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Gao, Z.; Chang, G.; Su, S.; Wang, D.; Guo, Y.; Zhu, D. Synthesis, Crystal Structure, and Characterization of the Charge-transfer Salt: (BEDT-TTF)[Fe(C2O4)Cl2](CH2Cl2), (BEDT-TTF = bis(ethylenedithio)tetrathiafulvalene). Eur. J. Inorg. Chem. 2014, 2014, 4028–4032. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Wang, Z.; Yang, D.; Gao, Z.; Wang, D.; Guo, Y.; Zhu, D.; Mori, T. [BEDT-TTF][Fe(C2O4)Cl2]: An organic–inorganic hybrid semiconductive antiferromagnet. Dalton Trans. 2016, 45, 16561–16565. [Google Scholar] [CrossRef] [PubMed]
- Palacio, F.; Miller, J.S. A dual-function material. Nature 2000, 408, 421–422. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Galan-Mascaros, J.R.; Gomez-Garcia, C.J.; Laukin, V. Coexistence of ferromagnetism and metallic conductivity in a molecular-based layered compound. Nature 2000, 408, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Alberola, A.; Coronado, E.; Galan-Mascaros, J.R.; Gimenez-Saiz, C.; Gimene-Garcia, C.J. A Molecular Metal Ferromagnet from the Organic Donor Bis(ethylenedithio)tetraselenafulvalene and Bimetallic Oxalate Complexes. J. Am. Chem. Soc. 2003, 125, 10774–10775. [Google Scholar] [CrossRef] [PubMed]
- Jahn, H.A.; Teller, E. Stability of polyatomic molecules in degenerate electronic states-I—Orbital degeneracy. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1937, 161, 220–235. [Google Scholar]
- Zhang, B.; Zhang, Y.; Wang, Z.; Sun, Y.; Liang, T.; Liu, M.; Zhu, D. Two One-dimensional Copper-Oxalate Frameworks with the Jahn–Teller Effect: [(CH3)3NH]2[Cu(μ-C2O4)(C2O4]·2.5H2O (I) and [(C2H5)3NH]2[Cu(μ-C2O4)(C2O4)]·H2O. Magnetochemistry 2023, 9, 120. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Wang, Z.; Wang, D.; Baker, P.; Pratt, F.; Zhu, D. Candidate Quantum Spin Liquid due to Dimensional Reduction of a Two-Dimensional Honeycomb Lattice. Sci. Rep. 2014, 4, 6451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Zhu, D. (BEDT-TTF)3[Cu2(C2O4)3](CH3OH)2: An organic-inorganic hybrid antiferromagnetic semiconductor. Chem. Commun. 2012, 48, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Wang, Z.; Gao, S.; Guo, Y.; Liu, F.; Zhu, D. BETS3[Cu2(C2O4)3](CH3OH)2: An organic–inorganic hybrid antiferromagnetic metal (BETS = bisethylene(tetraselenfulvalene)). CrystEngComm 2013, 15, 3529–3535. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Zhu, D. [(C2H5)3NH]2Cu2(C2O4)3: A three-dimensional metal-oxalato framework showing structurally related dielectric and magnetic transitions at around 165 K. Dalton Trans. 2012, 14, 8509–8511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Baker, P.; Zhang, Y.; Wang, D.; Wang, Z.; Su, S.; Zhu, D. Quantum Spin Liquid from a Three-Dimensional Copper-Oxalate Framework. J. Am. Chem. Soc. 2018, 140, 122–125. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXL-2012/7, University of Göttingen: Göttingen, Germany, 2014.
- Guionneau, P.; Kepert, C.J.; Bravic, G.; Chasseau, D.; Truter, M.R.; Kurmoo, M.; Day, P. Determing the charge distribution in BEDT-TTD salts. Synth. Met. 1997, 86, 1973–1974. [Google Scholar] [CrossRef]
- Wang, H.H.; Ferraro, J.R.; Williams, J.M.; Geiser, U.; Schlueter, J.A. Rapid Raman Spectroscopic Determination of the Stoichiometry of Microscopic Quantities of BEDT-TTF-based Organic Conductors and Superconductors. J. Chem. Soc. Chem. Commun. 1994, 1893–1894. [Google Scholar] [CrossRef]
- Price, C.C.; Perkins, N.B. Critical Properties of the Kitaev-Heisenberg Model. Phy. Rev. Lett. 2012, 109, 187201. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; John Wiley & Sons Inc.: New York, NY, USA, 1993. [Google Scholar]
- Ramirez, A.P. Strongly geometrically frustrated magnets. Annu. Rev. Mater. Sci. 1994, 24, 453–480. [Google Scholar] [CrossRef]
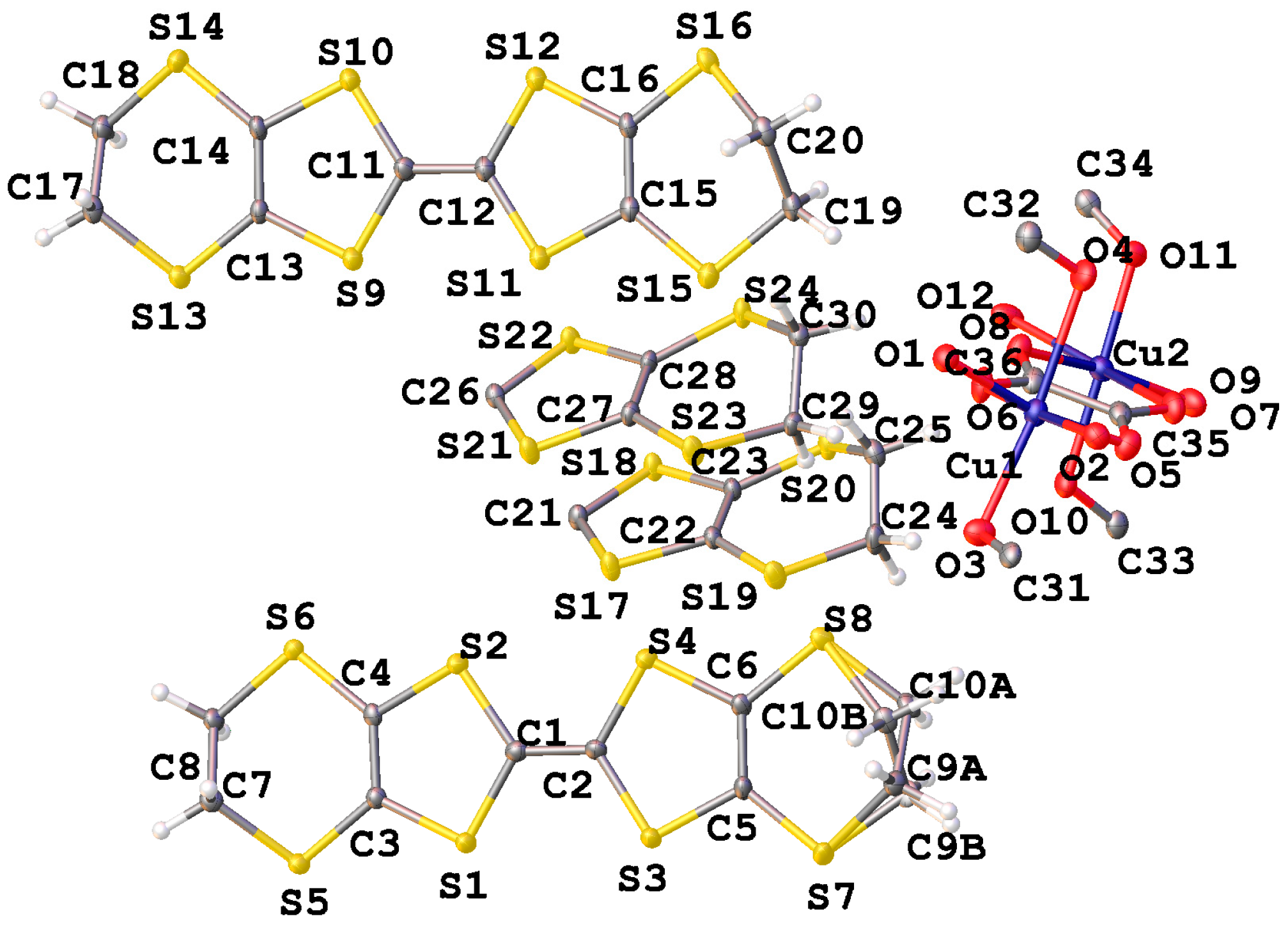
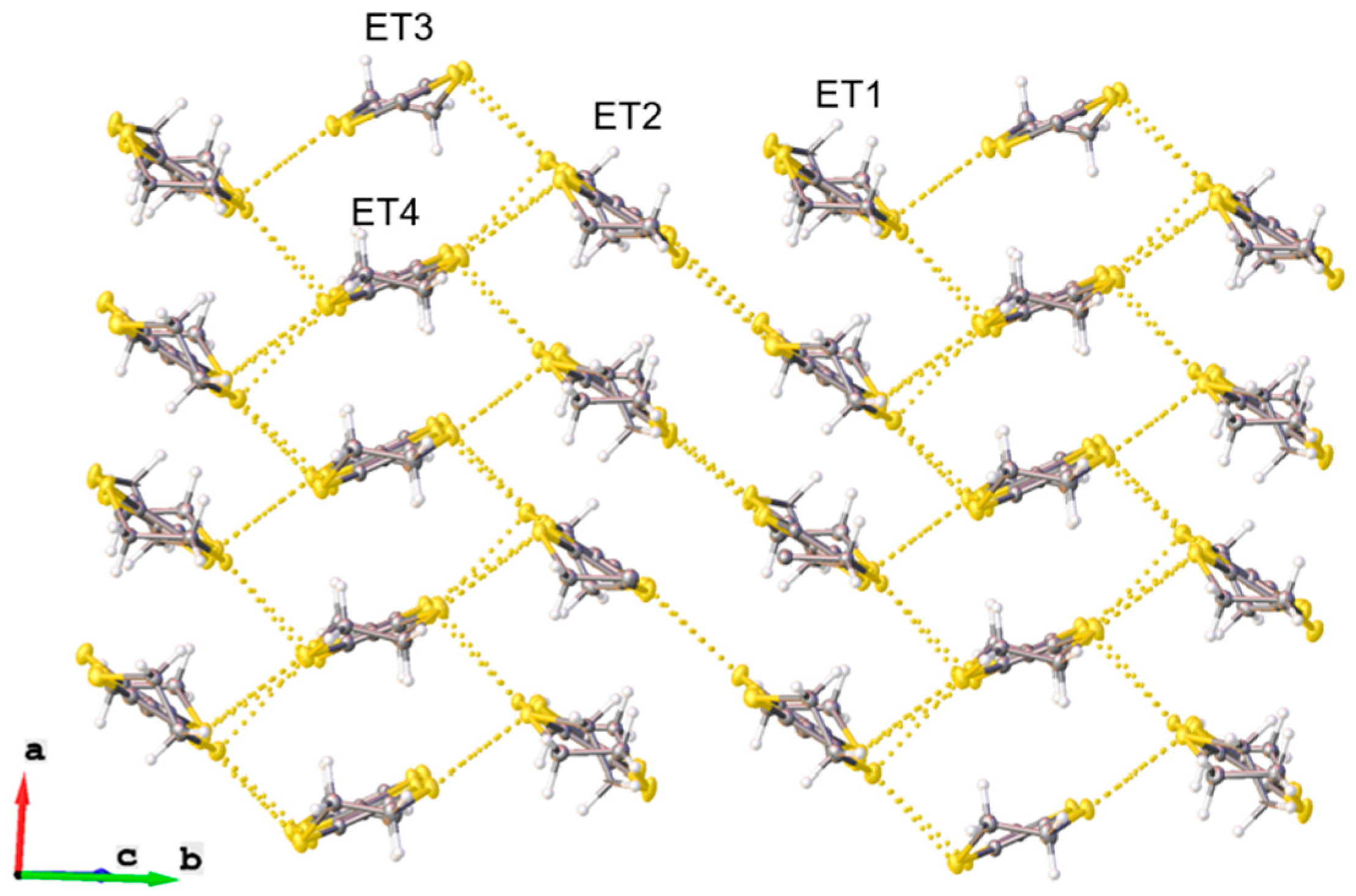
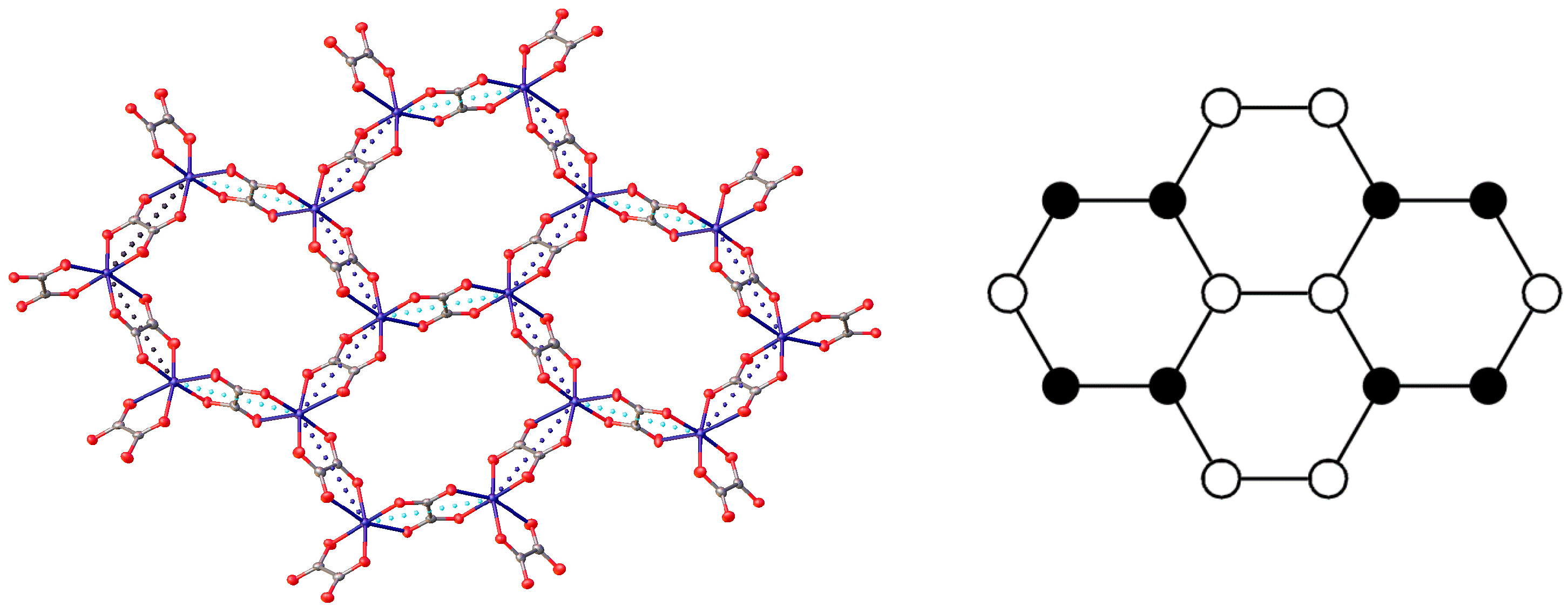



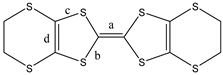 | ||||||
| δ = (b + c) − (a + d) | ||||||
| Q = 6.347 − 7.463δ | ||||||
| a | b | c | d | δ | Q | |
| ET1 | 1.370 ~1.370 * | 1.741 1.740 1.735 1.746 ~1.741 | 1.748 1.756 1.750 1.758 ~1.753 | 1.363 1.359 ~1.361 | 0.763 | 0.65 |
| ET2 | 1.379 ~1.370 | 1.737 1.734 1.733 1.734 ~1.735 | 1.748 1.746 1.752 1.741 ~1.747 | 1.362 1.355 ~1.359 | 0.744 | 0.80 |
| ET3 | 1.382 ~1.382 | 1.722 1.728 ~1.725 | 1.744 1.746 ~1.745 | 1.352 ~1.352 | 0.736 | 0.85 |
| ET4 | 1.354 ~1.354 | 1.753 1.756 ~1.755 | 1.756 1.765 ~1.761 | 1.337 ~1.337 | 0.824 | 0.20 |
| Total | 1.975 ** | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Zhang, Y.; Wang, D.; Wang, Z.; Chang, G.; Gao, Z.; Guo, Y.; Liu, F.; Zhao, Z.; Zhang, X.; et al. An Organic–Inorganic Hybrid Semiconducting Quantum Spin Liquid Candidate: (BEDT-TTF)3[Cu2(μ-C2O4)3·CH3CH2OH·1.2H2O]. Magnetochemistry 2025, 11, 12. https://doi.org/10.3390/magnetochemistry11020012
Zhang B, Zhang Y, Wang D, Wang Z, Chang G, Gao Z, Guo Y, Liu F, Zhao Z, Zhang X, et al. An Organic–Inorganic Hybrid Semiconducting Quantum Spin Liquid Candidate: (BEDT-TTF)3[Cu2(μ-C2O4)3·CH3CH2OH·1.2H2O]. Magnetochemistry. 2025; 11(2):12. https://doi.org/10.3390/magnetochemistry11020012
Chicago/Turabian StyleZhang, Bin, Yan Zhang, Dongwei Wang, Zheming Wang, Guangcai Chang, Zengqiang Gao, Yanjun Guo, Fen Liu, Zhijuan Zhao, Xiaoyu Zhang, and et al. 2025. "An Organic–Inorganic Hybrid Semiconducting Quantum Spin Liquid Candidate: (BEDT-TTF)3[Cu2(μ-C2O4)3·CH3CH2OH·1.2H2O]" Magnetochemistry 11, no. 2: 12. https://doi.org/10.3390/magnetochemistry11020012
APA StyleZhang, B., Zhang, Y., Wang, D., Wang, Z., Chang, G., Gao, Z., Guo, Y., Liu, F., Zhao, Z., Zhang, X., Qu, B., Xu, P., Wang, J., Dong, F., Liang, T., Sun, Y., Yang, D., Li, Q., Luo, X., ... Zhang, X. (2025). An Organic–Inorganic Hybrid Semiconducting Quantum Spin Liquid Candidate: (BEDT-TTF)3[Cu2(μ-C2O4)3·CH3CH2OH·1.2H2O]. Magnetochemistry, 11(2), 12. https://doi.org/10.3390/magnetochemistry11020012






