Er(III) and Yb(III) Complexes with a Tripodal Nitroxyl Radical: Magnetochemical Study and Ab Initio Calculations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Compounds
2.2. X-Ray Structure Determination
2.3. Computational Details
3. Results and Discussion
3.1. Characterization
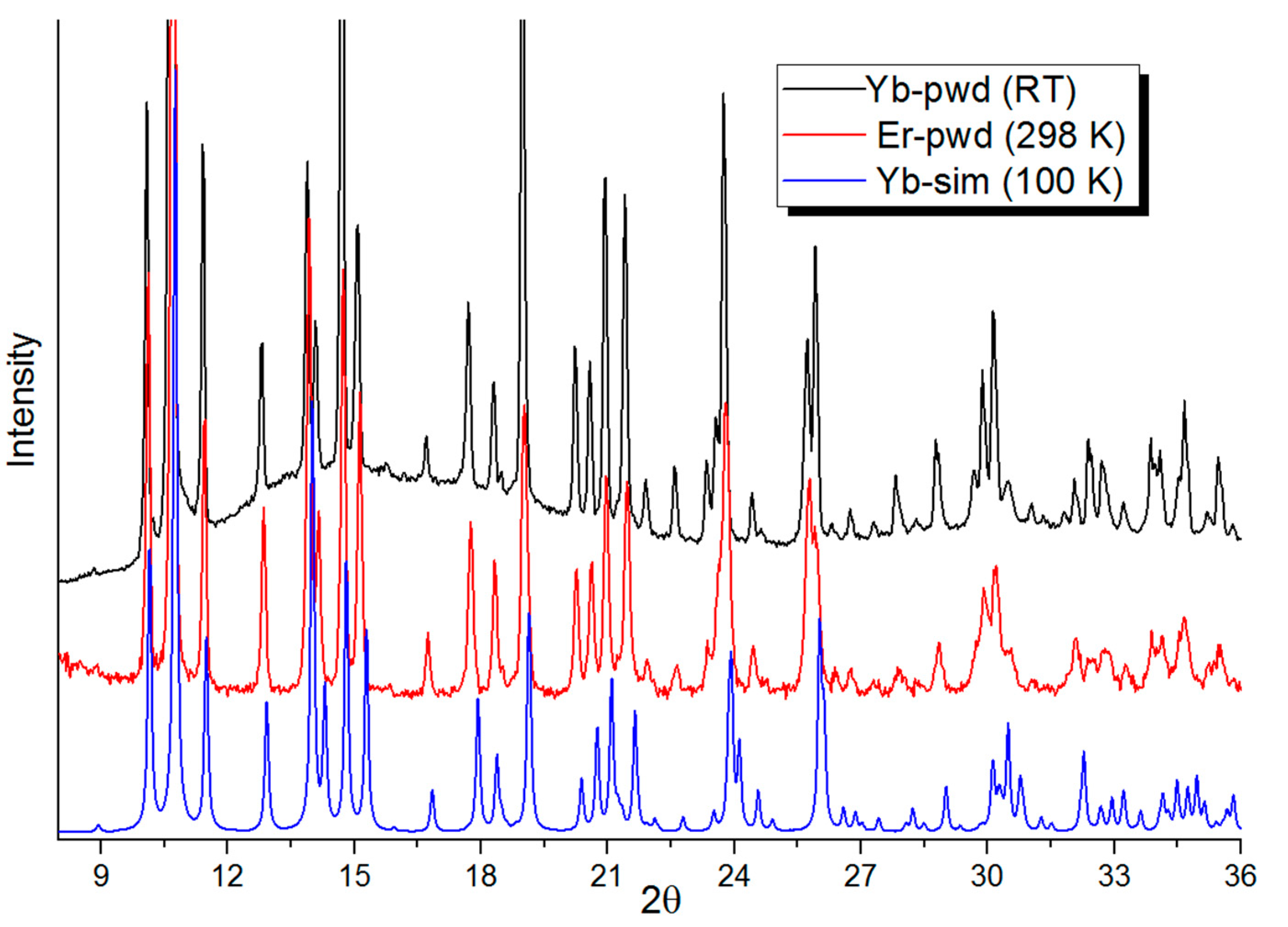
3.1.1. Crystal and Molecular Structure
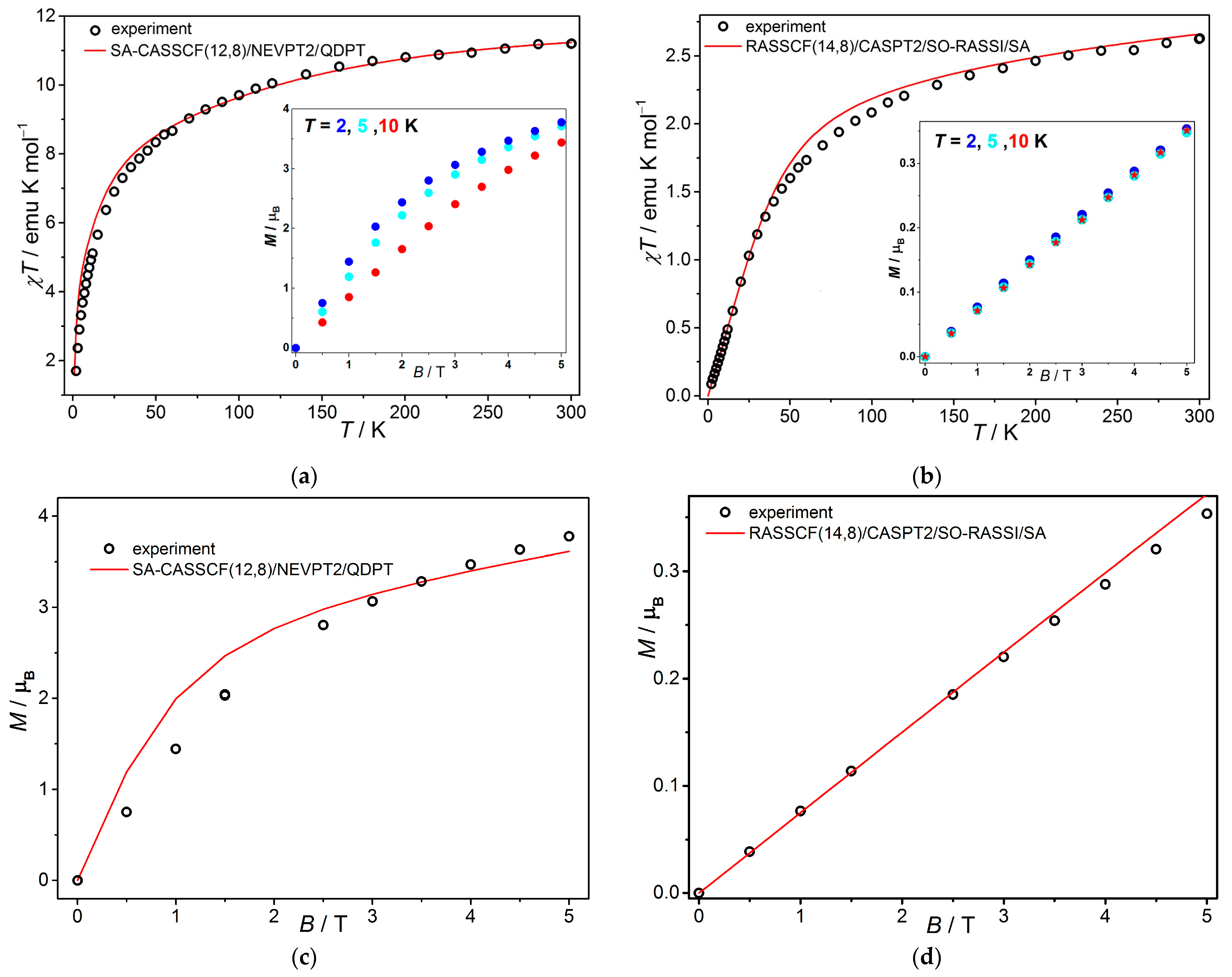
3.1.2. SQUID Magnetometry
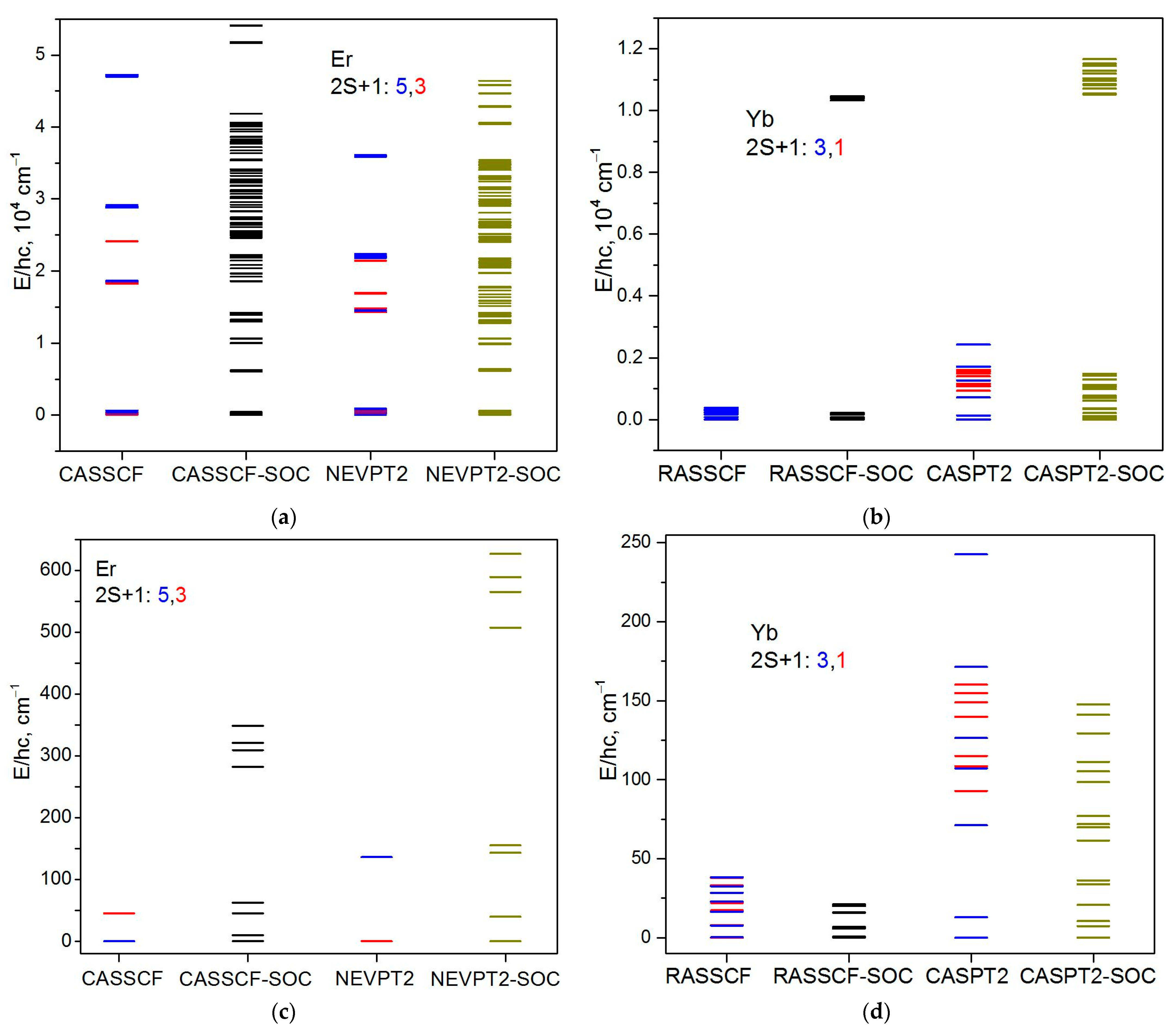
3.2. Theoretical Calculations and Magnetic Behavior Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kammerer, C.; Rapenne, G. Scorpionate Hydrotris(Indazolyl)Borate Ligands as Tripodal Platforms for Surface-Mounted Molecular Gears and Motors. Eur. J. Inorg. Chem. 2016, 2016, 2214–2226. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Ivashenko, O.; Carroll, G.T.; Robertus, J.; Kistemaker, J.C.M.; London, G.; Browne, W.R.; Rudolf, P.; Feringa, B.L. Control of Surface Wettability Using Tripodal Light-Activated Molecular Motors. J. Am. Chem. Soc. 2014, 136, 3219–3224. [Google Scholar] [CrossRef]
- Rabinovich, D. Synthetic Bioinorganic Chemistry: Scorpionates Turn 50 BT—50 Years of Structure and Bonding—The Anniversary Volume; Mingos, D.M.P., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 139–157. ISBN 978-3-319-35138-4. [Google Scholar]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide Luminescence for Functional Materials and Bio-Sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]
- Kurşunlu, A.N.; Özmen, M.; Güler, E. A Fluorescent Sensor-Based Tripodal-Bodipy for Cu (II) Ions: Bio-Imaging on Cells. Turkish J. Chem. 2021, 45, 31. [Google Scholar] [CrossRef]
- Yu, T.; Zhao, Y.; Fan, D.; Hong, Z.; Su, W. Preparation, Photo- and Electro-Luminescent Properties of a Novel Complex of Tb (III) with a Tripod Ligand. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 69, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Perfetti, M.; Caneschi, A.; Sukhikh, T.S.; Vostrikova, K.E. Lanthanide Complexes with a Tripodal Nitroxyl Radical Showing Strong Magnetic Coupling. Inorg. Chem. 2020, 59, 16591–16598. [Google Scholar] [CrossRef]
- Lyubov, D.M.; Mahrova, T.V.; Cherkasov, A.V.; Fukin, G.K.; Fedorov, Y.V.; Trifonov, A.A. Synthesis, Structures, and Luminescent Properties of Chloride and Nitrate LnIII Complexes Coordinated by Tris(pyrazolyl)methane. Eur. J. Inorg. Chem. 2023, 26, e202300292. [Google Scholar] [CrossRef]
- Szczepura, L.F.; Witham, L.M.; Takeuchi, K.J. Tris(2-Pyridyl) Tripod Ligands. Coord. Chem. Rev. 1998, 174, 5–32. [Google Scholar] [CrossRef]
- Natrajan, L.; Pécaut, J.; Mazzanti, M.; LeBrun, C. Controlled Hydrolysis of Lanthanide Complexes of the N-Donor Tripod Tris(2-pyridylmethyl)amine versus Bisligand Complex Formation. Inorg. Chem. 2005, 44, 4756–4765. [Google Scholar] [CrossRef] [PubMed]
- Bellemin-Laponnaz, S.; Gade, L.H. A Modular Approach to C1 and C3 Chiral N-Tripodal Ligands for Asymmetric Catalysis. Angew. Chem. Int. Ed. 2002, 41, 3473–3475. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chen, H.-H. 1,1,1-Tris(hydroxymethyl)ethane as a New, Efficient, and Versatile Tripod Ligand for Copper-Catalyzed Cross-Coupling Reactions of Aryl Iodides with Amides, Thiols, and Phenols. Org. Lett. 2006, 8, 5609–5612. [Google Scholar] [CrossRef] [PubMed]
- Eckert, M.; Brethon, A.; Li, Y.-X.; Sheldon, R.A.; Arends, I.W.C.E. Study of the Efficiency of Amino-Functionalized Ruthenium and Ruthenacycle Complexes as Racemization Catalysts in the Dynamic Kinetic Resolution of 1-Phenylethanol. Adv. Synth. Catal. 2007, 349, 2603–2609. [Google Scholar] [CrossRef]
- Andrez, J.; Bozoklu, G.; Nocton, G.; Pécaut, J.; Scopelliti, R.; Dubois, L.; Mazzanti, M. Lanthanide(II) Complexes Supported by N,O-donor Tripodal Ligands: Synthesis, Structure, and Ligand-dependent Redox Behavior. Chem.—Eur. J. 2015, 21, 15188–15200. [Google Scholar] [CrossRef] [PubMed]
- Wietzke, R.; Mazzanti, M.; Latour, J.-M.; Pécaut, J.; Cordier, P.-Y.; Madic, C. Lanthanide(III) Complexes of Tripodal N-donor Ligands: Structural Models for the Species Involved in Solvent Extraction of Actinides(III). Inorg. Chem. 1998, 37, 6690–6697. [Google Scholar] [CrossRef]
- Dai, Z.; Canary, J.W. Tailoring Tripodal Ligands for Zinc Sensing. New J. Chem. 2007, 31, 1708–1718. [Google Scholar] [CrossRef]
- Kuswandi, B.; N/a, N.; Verboom, W.; Reinhoudt, D.N. Tripodal Receptors for Cation and Anion Sensors. Sensors 2006, 6, 978–1017. [Google Scholar] [CrossRef]
- Zhu, L.; Tang, H.; Harima, Y.; Yamashita, K.; Hirayama, D.; Aso, Y.; Otsubo, T. Electrochemical Properties of Self-assembled Monolayers of Tripod-Shaped Molecules and Their Applications to Organic Light-emitting Diodes. Chem. Commun. 2001, 18, 1830–1831. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-L.; Liu, Y.; Pan, M.; Lü, X.-Q.; Zhang, J.-Y.; Zhao, C.-Y.; Tong, Y.-X.; Su, C.-Y. Bright Blue-Emitting Ce3+ Complexes with Encapsulating Polybenzimidazole Tripodal Ligands as Potential Electroluminescent Devices. Angew. Chem. Int. Ed. 2007, 46, 7399–7403. [Google Scholar] [CrossRef] [PubMed]
- Vostrikova, K.E. The Tripodal Ligand’s 4f Complexes: Use in Molecular Magnetism. Inorganics 2023, 11, 307. [Google Scholar] [CrossRef]
- Hay, M.A.; Boskovic, C. Lanthanoid Complexes as Molecular Materials: The Redox Approach. Chem. Eur. J. 2021, 27, 3608–3637. [Google Scholar] [CrossRef] [PubMed]
- Vostrikova, K.E.; Sukhikh, T.S.; Lavrov, A.N. The Synthesis, Crystal Structure, and Magnetic Properties of Mono-scorpionate Eu(III) Complexes. Inorganics 2023, 11, 418. [Google Scholar] [CrossRef]
- Ito, A.; Nakano, Y.; Urabe, M.; Tanaka, K.; Shiro, M. Structural and Magnetic Studies of Copper(II) and Zinc(II) Coordination Complexes Containing Nitroxide Radicals as Chelating Ligands. Eur. J. Inorg. Chem. 2006, 2006, 3359–3368. [Google Scholar] [CrossRef]
- Gass, I.A.; Lu, J.; Ojha, R.; Asadi, M.; Lupton, D.W.; Geoghegan, B.L.; Moubaraki, B.; Martin, L.L.; Bond, A.M.; Murray, K.S. [FeII(L•)2][TCNQF4•−]2: A Redox-active Double Radical Salt. Aust. J. Chem. 2019, 72, 769–777. [Google Scholar] [CrossRef]
- Gass, I.A.; Tewary, S.; Nafady, A.; Chilton, N.F.; Gartshore, C.J.; Asadi, M.; Lupton, D.W.; Moubaraki, B.; Bond, A.M.; Boas, J.F.; et al. Observation of Ferromagnetic Exchange, Spin Crossover, Reductively Induced Oxidation, and Field-induced Slow Magnetic Relaxation in Monomeric Cobalt Nitroxides. Inorg. Chem. 2013, 52, 7557–7572. [Google Scholar] [CrossRef] [PubMed]
- Gass, I.A.; Tewary, S.; Rajaraman, G.; Asadi, M.; Lupton, D.W.; Moubaraki, B.; Chastanet, G.; Létard, J.-F.; Murray, K.S. Solvate-Dependent Spin Crossover and Exchange in Cobalt(II) Oxazolidine Nitroxide Chelates. Inorg. Chem. 2014, 53, 5055–5066. [Google Scholar] [CrossRef] [PubMed]
- Gass, I.A.; Asadi, M.; Lupton, D.W.; Moubaraki, B.; Bond, A.M.; Guo, S.-X.; Murray, K.S. Manganese(II) Oxazolidine Nitroxide Chelates: Structure, Magnetism, and Redox Properties. Aust. J. Chem. 2014, 67, 1618. [Google Scholar] [CrossRef]
- Pedersen, A.H.; Geoghegan, B.L.; Nichol, G.S.; Lupton, D.W.; Murray, K.S.; Martínez-Lillo, J.; Gass, I.A.; Brechin, E.K. Hexahalorhenate(IV) Salts of Metal Oxazolidine Nitroxides. Dalt. Trans. 2017, 46, 5250–5259. [Google Scholar] [CrossRef]
- Gass, I.A.; Lu, J.; Asadi, M.; Lupton, D.W.; Forsyth, C.M.; Geoghegan, B.L.; Moubaraki, B.; Cashion, J.D.; Martin, L.L.; Bond, A.M.; et al. Use of the (TCNQF4)2− Dianion in the Spontaneous Redox Formation of [FeIII(L−)2][TCNQF4−]. Chempluschem 2018, 83, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Rey, P.; Caneschi, A.; Sukhikh, T.S.; Vostrikova, K.E. Tripodal Oxazolidine-N-oxyl Diradical Complexes of Dy3+ and Eu3+. Inorganics 2021, 9, 91. [Google Scholar] [CrossRef]
- Vogel, R.; Müntener, T.; Häussinger, D. Intrinsic Anisotropy Parameters of a Series of Lanthanoid Complexes Deliver New Insights into the Structure-Magnetism Relationship. Chem 2021, 7, 3144–3156. [Google Scholar] [CrossRef]
- Brzozowska, M.; Handzlik, G.; Kurpiewska, K.; Zychowicz, M.; Pinkowicz, D. Pseudo-tetrahedral vs. Pseudo-octahedral Er(III) Single Molecule Magnets and the Disruptive Role of Coordinated TEMPO Radical. Inorg. Chem. Front. 2021, 8, 2817–2828. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, L.; Wang, C.; Xue, S.; Lin, S.-Y.; Tang, J. Equatorially Coordinated Lanthanide Single Ion Magnets. J. Am. Chem. Soc. 2014, 136, 4484–4487. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Pinkowicz, D.; Saber, M.R.; Dunbar, K.R. A Trigonal-Pyramidal Erbium(III) Single-Molecule Magnet. Angew. Chem. Int. Ed. 2015, 54, 5864–5868. [Google Scholar] [CrossRef]
- Zhang, H.; Nakanishi, R.; Katoh, K.; Breedlove, B.K.; Kitagawa, Y.; Yamashita, M. Low Coordinated Mononuclear Erbium(III) Single-Molecule Magnets with C3v Symmetry: A Method for Altering Single-Molecule Magnet Properties by Incorporating Hard and Soft Donors. Dalt. Trans. 2018, 47, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-M.; Wen, L.-M.; Liu, F.-F.; Wang, S.-P.; Zhang, J.-J.; Liu, Z.-Y. Magnetic Slow Relaxation of Pseudo-Binuclear Ln(III)-Nitronyl Nitroxide Radical Complexes Involving Non-Covalent O⋯O Interactions. J. Mol. Struct. 2020, 1207, 127735. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, Z.; Yin, X.; Wang, S.; Yang, S.; Zhang, J.; Geng, L.; Shi, S. Structure and Magnetic Investigation of a Series of Rare Earth Heterospin Monometallic Compounds of Nitronyl Nitroxide Radical. Z. Für Anorg. Und Allg. Chem. 2016, 642, 148–154. [Google Scholar] [CrossRef]
- Zhao, Q.-H.; Ma, Y.-P.; Du, L.; Fang, R.-B. Three Novel Rare-Earth Complexes with Nitronyl Nitroxide Radical [RE(Hfac)3 (NITPhOCH3)2] (RE = GdIII, YIII and ErIII): Syntheses, Crystal Structures and Magnetic Properties. Transit. Met. Chem. 2006, 31, 593–597. [Google Scholar] [CrossRef]
- Xiao, Z.-X.; Miao, H.; Shao, D.; Wei, H.-Y.; Zhang, Y.-Q.; Wang, X.-Y. A Family of Lanthanide Compounds with Reduced Nitronyl Nitroxide Diradical: Syntheses, Structures and Magnetic Properties. Dalt. Trans. 2018, 47, 7925–7933. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L. Four Tri-Spin Lanthanide–Nitronyl Nitroxide (LnIII = GdIII, DyIII, ErIII, and HoIII) Complexes: Syntheses, Structures, and Magnetic Properties. J. Coord. Chem. 2011, 64, 4334–4343. [Google Scholar] [CrossRef]
- Lv, X.-H.; Yang, S.-L.; Li, Y.-X.; Zhang, C.-X.; Wang, Q.-L. A Family of Lanthanide Compounds Based on Nitronyl Nitroxide Radicals: Synthesis, Structure, Magnetic and Fluorescence Properties. RSC Adv. 2017, 7, 38179–38186. [Google Scholar] [CrossRef]
- Wang, X.-L. Five New Tri-spin Lanthanide–Nitronyl Nitroxide (LnIII= GdIII, TbIII, DyIII, HoIII, ErIII) Complexes: Structures and Magnetic Properties. Inorganica Chim. Acta 2012, 387, 20–24. [Google Scholar] [CrossRef]
- Xie, J.; Li, C.; Sun, G.; Yang, M.; Li, L.-C. New 2p-3d-4f Chain Compounds [LnZn(Hfac)5(NIT-Pyrim)2] Constructed from Pyrimidine Based Nitronyl Nitroxides. Z. Für Anorg. Und Allg. Chem. 2018, 644, 827–832. [Google Scholar] [CrossRef]
- Dong, H.; Ma, S.; Zhong, Q.; Zhu, M. Crystal Structure, Magnetic Properties and Luminescent Behavior of Four Mononuclear Lanthanide-Radical Complexes. J. Mol. Struct. 2022, 1252, 132195. [Google Scholar] [CrossRef]
- Wang, J.; Miao, H.; Xiao, Z.-X.; Zhou, Y.; Deng, L.-D.; Zhang, Y.-Q.; Wang, X.-Y. Syntheses, Structures and Magnetic Properties of the Lanthanide Complexes of the Pyrimidyl-Substituted Nitronyl Nitroxide Radical. Dalt. Trans. 2017, 46, 10452–10461. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, M.; Wang, J.; Li, L. Unusual Gd—Nitronyl Nitroxide Antiferromagnetic Coupling and Slow Magnetic Relaxation in the Corresponding Tb Analogue. Dalt. Trans. 2015, 44, 13890–13896. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Li, H.-Q.; Shen, F.-X.; Wei, H.-Y.; Wang, B.-L.; Wang, X.-Y. A Family of Lanthanide Complexes with a Bis-Tridentate Nitronyl Nitroxide Radical: Syntheses, Structures and Magnetic Properties. Dalt. Trans. 2019, 48, 10337–10345. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Yao, B.; Wang, R.; Wang, W.; Wang, Q.; Ma, Y.; Li, L. Structures and Magnetic Properties of Five Lanthanide-Radical Complexes Constructed by 8-Methoxyquinoline Substituted Tridentate Chelating Nitronyl Nitroxide Radical. J. Solid State Chem. 2021, 298, 122115. [Google Scholar] [CrossRef]
- Huang, G.; Daiguebonne, C.; Calvez, G.; Suffren, Y.; Guillou, O.; Guizouarn, T.; Le Guennic, B.; Cador, O.; Bernot, K. Strong Magnetic Coupling and Single-Molecule-Magnet Behavior in Lanthanide-TEMPO Radical Chains. Inorg. Chem. 2018, 57, 11044–11057. [Google Scholar] [CrossRef] [PubMed]
- Zheludev, A.; Barone, V.; Bonnet, M.; Delley, B.; Grand, A.; Ressouche, E.; Rey, P.; Subra, R.; Schweizer, J. Spin Density in a Nitronyl Nitroxide Free Radical. Polarized Neutron Diffraction Investigation and ab initio Calculations. J. Am. Chem. Soc. 1994, 116, 2019–2027. [Google Scholar] [CrossRef]
- Schweizer, J.; Ressouche, E. Neutron Scattering and Spin Densities in Free Radicals. In Magnetism: Molecules to Materials I; Miller, J.S., Drillon, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2001; pp. 325–355. ISBN 9783527600847. [Google Scholar]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]
- Apex3 Software Suite: Apex3, SADABS-2016/2 and SAINT 8.40a; Bruker AXS Inc.: Madison, WI, USA, 2017.
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.-F.; Yin, B. The Interpretation and Prediction of Lanthanide Single-Ion Magnets from ab initio Electronic Structure Calculation: The Capability and Limit. Dalt. Trans. 2022, 51, 14793–14816. [Google Scholar] [CrossRef] [PubMed]
- Chibotaru, L.F. Ab Initio Investigation of Anisotropic Magnetism and Magnetization Blocking in Metal Complexes BT—Computational Modelling of Molecular Nanomagnets; Rajaraman, G., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–62. ISBN 978-3-031-31038-6. [Google Scholar]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. Natural Bond Orbital Methods. WIREs Comput. Mol. Sci. 2012, 2, 1–42. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. NBO 7.0: New Vistas in Localized and Delocalized Chemical Bonding Theory. J. Comput. Chem. 2019, 40, 2234–2241. [Google Scholar] [CrossRef]
- Roos, B.O.; Taylor, P.R.; Sigbahn, P.E.M. A Complete Active Space SCF Method (CASSCF) Using a Density Matrix Formulated Super-CI Approach. Chem. Phys. 1980, 48, 157–173. [Google Scholar] [CrossRef]
- Angeli, C.; Bories, B.; Cavallini, A.; Cimiraglia, R. Third-order Multireference Perturbation Theory: The n-Electron Valence State Perturbation-Theory Approach. J. Chem. Phys. 2006, 124, 054108. [Google Scholar] [CrossRef] [PubMed]
- Angeli, C.; Cimiraglia, R.; Evangelisti, S.; Leininger, T.; Malrieu, J.-P. Introduction of n-Electron Valence States for Multireference Perturbation Theory. J. Chem. Phys. 2001, 114, 10252–10264. [Google Scholar] [CrossRef]
- Angeli, C.; Cimiraglia, R.; Malrieu, J.-P. n-Electron Valence State Perturbation Theory: A Fast Implementation of the Strongly Contracted Variant. Chem. Phys. Lett. 2001, 350, 297–305. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Li Manni, G.; Fdez. Galván, I.; Alavi, A.; Aleotti, F.; Aquilante, F.; Autschbach, J.; Avagliano, D.; Baiardi, A.; Bao, J.J.; Battaglia, S.; et al. The OpenMolcas Web: A Community-driven Approach to Advancing Computational Chemistry. J. Chem. Theory Comput. 2023, 19, 6933–6991. [Google Scholar] [CrossRef] [PubMed]
- Aquilante, F.; Autschbach, J.; Baiardi, A.; Battaglia, S.; Borin, V.A.; Chibotaru, L.F.; Conti, I.; De Vico, L.; Delcey, M.; Fdez.Galván, I.; et al. Modern Quantum Chemistry with [Open]Molcas. J. Chem. Phys. 2020, 152, 214117. [Google Scholar] [CrossRef]
- Hess, B.A. Relativistic Electronic-Structure Calculations Employing a Two-component No-pair Formalism with External-Field Projection Operators. Phys. Rev. A 1986, 33, 3742–3748. [Google Scholar] [CrossRef]
- Wolf, A.; Reiher, M.; Hess, B.A. The Generalized Douglas–Kroll Transformation. J. Chem. Phys. 2002, 117, 9215–9226. [Google Scholar] [CrossRef]
- Aravena, D.; Neese, F.; Pantazis, D.A. Improved Segmented All-electron Relativistically Contracted Basis Sets for the Lanthanides. J. Chem. Theory Comput. 2016, 12, 1148–1156. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef]
- Pantazis, D.A.; Chen, X.-Y.; Landis, C.R.; Neese, F. All-electron Scalar Relativistic Basis Sets for Third-Row Transition Metal Atoms. J. Chem. Theory Comput. 2008, 4, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. A Fully Direct RI-HF Algorithm: Implementation, Optimised Auxiliary Basis Sets, Demonstration of Accuracy and Efficiency. Phys. Chem. Chem. Phys. 2002, 4, 4285–4291. [Google Scholar] [CrossRef]
- Weigend, F. Hartree–Fock Exchange Fitting Basis Sets for H to Rn. J. Comput. Chem. 2008, 29, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Roos, B.O.; Lindh, R.; Malmqvist, P.-Å.; Veryazov, V.; Widmark, P.-O. Main Group Atoms and Dimers Studied with a New Relativistic ANO Basis Set. J. Phys. Chem. A 2004, 108, 2851–2858. [Google Scholar] [CrossRef]
- Neese, F. Efficient and Accurate Approximations to the Molecular Spin-orbit Coupling Operator and Their Use in Molecular g-Tensor Calculations. J. Chem. Phys. 2005, 122, 034107. [Google Scholar] [CrossRef] [PubMed]
- Malmqvist, P.Å.; Roos, B.O.; Schimmelpfennig, B. The Restricted Active Space (RAS) State Interaction Approach with Spin–Orbit Coupling. Chem. Phys. Lett. 2002, 357, 230–240. [Google Scholar] [CrossRef]
- Roos, B.O.; Malmqvist, P.-Å. Relativistic Quantum Chemistry: The Multiconfigurational Approach. Phys. Chem. Chem. Phys. 2004, 6, 2919. [Google Scholar] [CrossRef]
- Chibotaru, L.F.; Ungur, L. Ab initio Calculation of Anisotropic Magnetic Properties of Complexes. I. Unique Definition of Pseudospin Hamiltonians and Their Derivation. J. Chem. Phys. 2012, 137, 064112. [Google Scholar] [CrossRef] [PubMed]
- Ungur, L.; Chibotaru, L.F. Computational Modelling of the Magnetic Properties of Lanthanide Compounds. In Lanthanides and Actinides in Molecular Magnetism; Wiley: Hoboken, NJ, USA, 2015; pp. 153–184. [Google Scholar]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE, Version 2.1, Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools; University of Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Sorace, L.; Dmitriev, A.A.; Perfetti, M.; Vostrikova, K.E. Slow Magnetic Relaxation and Strong Magnetic Coupling in the Nitroxyl Radical Complexes of Lanthanide(III) with Diamagnetic Ground State (Ln = Lu, Eu). Chem. Sci. 2025, 16, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Briganti, M.; Lucaccini, E.; Chelazzi, L.; Ciattini, S.; Sorace, L.; Sessoli, R.; Totti, F.; Perfetti, M. Magnetic Anisotropy Trends along a Full 4f-Series: The f(n+7) Effect. J. Am. Chem. Soc. 2021, 143, 8108–8115. [Google Scholar] [CrossRef]



| Anion | Cation | ||||
|---|---|---|---|---|---|
| KD | ΔE, cm−1 | |Jz| (Contribution, %) | KD | ΔE, cm−1 | |Jz| (Contribution, %) |
| 1 | 0 | 13/2 (54.7), 11/2 (12.1) | 1 | 0 | 13/2 (60.6), 11/2 (16.5) |
| 2 | 66.9 | 11/2 (32.2), 1/2 (19.8), 3/2 (14.1), 13/2 (11.6) | 2 | 108.6 | 1/2 (34.3), 11/2 (34.1), 9/2 (9.7) |
| 3 | 209.4 | 3/2 (23.4), 13/2 (19.3), 1/2 (15.3), 9/2 (13.7), 5/2 (11.3) | 3 | 286.7 | 15/2 (40.4), 9/2 (18.0), 13/2 (17.1), 1/2 (9.6) |
| 4 | 268.6 | 1/2 (38.9), 11/2 (18.9), 3/2 (11.3) | 4 | 353.7 | 3/2 (30.5), 11/2 (18.2), 5/2 (14.9), 15/2 (12.3), 9/2 (10.6) |
| 5 | 381.3 | 15/2 (33.4), 9/2 (23.4), 7/2 (16.8), 5/2 (14.4) | 5 | 402.5 | 3/2 (33.7), 11/2 (19.7), 1/2 (13.5), 5/2 (11.5) |
| 6 | 467.5 | 3/2 (26.4), 9/2 (22.6), 1/2 (19.5), 5/2 (13.3) | 6 | 521.6 | 7/2 (36.0), 5/2 (23.4), 15/2 (13.5) |
| 7 | 513.6 | 15/2 (35.7), 7/2 (21.2), 11/2 (12.8), 5/2 (10.8), 3/2 (10.2) | 7 | 574.3 | 1/2 (21.0), 5/2 (20.6), 9/2 (19.9), 7/2 (15.0), 11/2 (14.7) |
| 8 | 636.5 | 7/2 (30.3), 5/2 (27.5), 9/2 (16.5), 15/2 (13.9) | 8 | 691.2 | 7/2 (27.1), 9/2 (24.8), 5/2 (16.7), 3/2 (15.5) |
| Anion | Cation | ||||
|---|---|---|---|---|---|
| KD | ΔE, cm−1 | |Jz| (Contribution, %) | KD | ΔE, cm−1 | |Jz| (Contribution, %) |
| 1 | 0 | 7/2 (65.6), 3/2 (20.2), 1/2 (10.8) | 1 | 0 | 7/2 (89.8) |
| 2 | 169.3 | 5/2 (49.8), 1/2 (25.6), 3/2 (17.6) | 2 | 224.7 | 5/2 (65.5), 3/2 (24.8) |
| 3 | 385.7 | 5/2 (36), 3/2 (25.8), 7/2 (20.7), 5/2 (17.3) | 3 | 438.8 | 1/2 (64.5), 3/2 (18.8), 5/2 (13.2) |
| 4 | 537.9 | 1/2 (46.2), 3/2 (36.5), 5/2 (10.8) | 4 | 565.7 | 3/2 (52.9), 1/2 (29.9), 5/2 (15.3) |
| 1 Anion | 1 Cation | 2 Anion | 2 Cation | |
|---|---|---|---|---|
| g | (0.79, 0.54, 13.4) | (0.30, 0.61, 14.2) | (1.07, 3.05, 5.55) | (1.18, 1.66, 6.90) |
| J, cm−1 | 6 | 7 | 16 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perfetti, M.; Dmitriev, A.A.; Vostrikova, K.E. Er(III) and Yb(III) Complexes with a Tripodal Nitroxyl Radical: Magnetochemical Study and Ab Initio Calculations. Magnetochemistry 2025, 11, 16. https://doi.org/10.3390/magnetochemistry11020016
Perfetti M, Dmitriev AA, Vostrikova KE. Er(III) and Yb(III) Complexes with a Tripodal Nitroxyl Radical: Magnetochemical Study and Ab Initio Calculations. Magnetochemistry. 2025; 11(2):16. https://doi.org/10.3390/magnetochemistry11020016
Chicago/Turabian StylePerfetti, Mauro, Alexey A. Dmitriev, and Kira E. Vostrikova. 2025. "Er(III) and Yb(III) Complexes with a Tripodal Nitroxyl Radical: Magnetochemical Study and Ab Initio Calculations" Magnetochemistry 11, no. 2: 16. https://doi.org/10.3390/magnetochemistry11020016
APA StylePerfetti, M., Dmitriev, A. A., & Vostrikova, K. E. (2025). Er(III) and Yb(III) Complexes with a Tripodal Nitroxyl Radical: Magnetochemical Study and Ab Initio Calculations. Magnetochemistry, 11(2), 16. https://doi.org/10.3390/magnetochemistry11020016






